Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a13-22693_18k.htm |
| EX-99.1 - EX-99.1 - AMAG PHARMACEUTICALS, INC. | a13-22693_1ex99d1.htm |
| EX-99.3 - EX-99.3 - AMAG PHARMACEUTICALS, INC. | a13-22693_1ex99d3.htm |
Exhibit 99.2
|
|
Third Quarter 2013 Financial Results AMAG Pharmaceuticals, Inc. 1100 Winter Street Waltham, MA 02451 o 617.498.3300 www.amagpharma.com A SPECIALTY PHARMACEUTICAL COMPANY DEDICATED TO IMPROVING THE QUALITY OF PATIENTS’ LIVES. |
|
|
Forward-looking Statements 1 This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Any statements contained herein which do not describe historical facts, including but not limited to: improving Feraheme gross margins; our efforts to drive growth in the CKD segment; our plans for the third quarter AMAG launch of MuGard and MuGard commercial integration; the potential expansion of Feraheme’s labeled indication and our expectations regarding the FDA’s review of our sNDA for Feraheme; planning for the potential label expansion; expectations regarding our 2013 results, including our expected 2013 revenues and operating expenses; opportunities in the IV iron market; Feraheme market demand; competition; business development plans; expectations as to year-end cash; preparations for 2014 and positioning for success in 2013 and beyond are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include: (1) uncertainties regarding our and Takeda's ability to successfully compete in the intravenous iron replacement market both in the U.S. and outside the U.S., including the EU, (2) uncertainties regarding our ability to compete in the oral mucositis market in the U.S., (3) uncertainties regarding our ability to successfully and timely complete our clinical development programs and obtain regulatory approval for Feraheme/Rienso in the broader IDA indication both in the U.S. and outside of the US, including the EU, (4) the possibility that significant safety or drug interaction problems could arise with respect to Feraheme/Rienso, and in turn affect sales, regulatory approval or our ability to market the product both in the U.S. and outside of the U.S., including the EU, (5) uncertainties regarding the manufacture of Feraheme/Rienso or MuGard, (6) uncertainties relating to our patents and proprietary rights, both in the U.S. and outside of the U.S., (7) the risk of an Abbreviated New Drug Application (ANDA) filing following the FDA’s draft bioequivalence recommendation for ferumoxytol, (8) the risk that we may not realize the anticipated benefits of our licensing arrangement for and continued integration of MuGard and (9) other risks identified in our Securities and Exchange Commission filings, including our Quarterly Report on Form 10-Q for the quarter ended June 30, 2013 and subsequent filings with the SEC. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
Agenda Topic Speaker Opening Remarks Bill Heiden, CEO Financial Highlights & Outlook Frank Thomas, COO Commercial Performance Greg Madison, CCO Expansion Opportunities and Closing Remarks Bill Heiden, CEO 2 Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
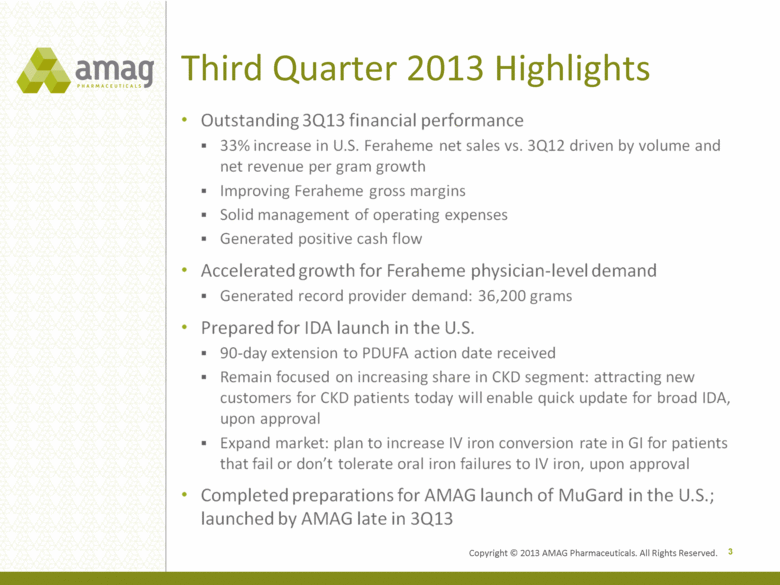
Third Quarter 2013 Highlights 3 Outstanding 3Q13 financial performance 33% increase in U.S. Feraheme net sales vs. 3Q12 driven by volume and net revenue per gram growth Improving Feraheme gross margins Solid management of operating expenses Generated positive cash flow Accelerated growth for Feraheme physician-level demand Generated record provider demand: 36,200 grams Prepared for IDA launch in the U.S. 90-day extension to PDUFA action date received Remain focused on increasing share in CKD segment: attracting new customers for CKD patients today will enable quick update for broad IDA, upon approval Expand market: plan to increase IV iron conversion rate in GI for patients that fail or don’t tolerate oral iron failures to IV iron, upon approval • Completed preparations for AMAG launch of MuGard in the U.S.; launched by AMAG late in 3Q13 Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
FINANCIAL OVERVIEW |
|
|
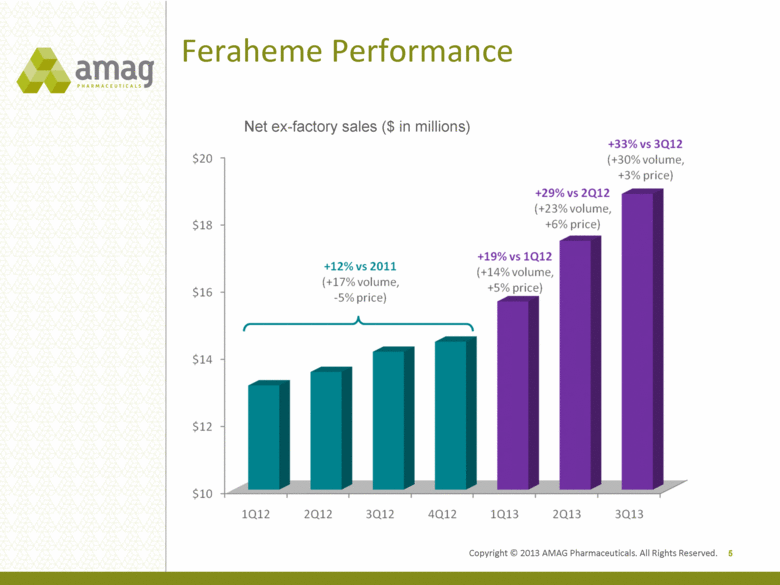
Feraheme Performance 5 Net ex-factory sales ($ in millions) Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
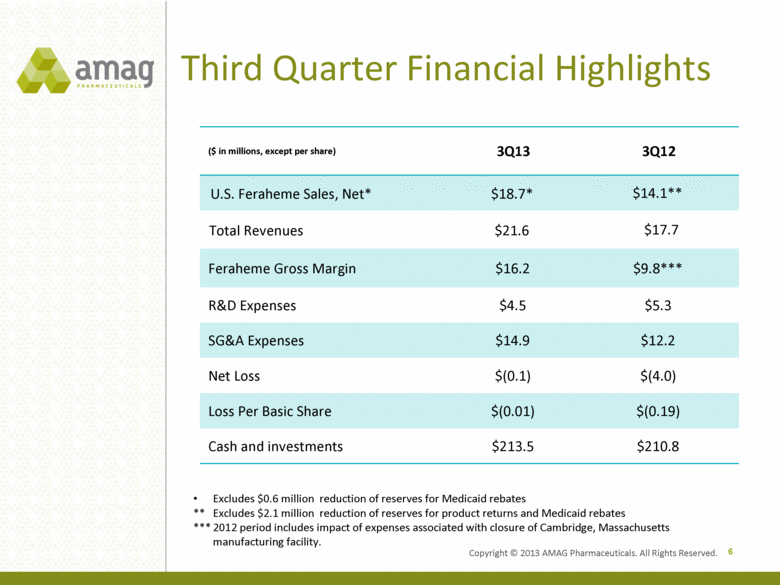
Third Quarter Financial Highlights ($ in millions, except per share) 3Q13 3Q12 U.S. Feraheme Sales, Net* $18.7* $14.1** Total Revenues $21.6 $17.7 Feraheme Gross Margin $16.2 $9.8*** R&D Expenses $4.5 $5.3 SG&A Expenses $14.9 $12.2 Net Loss $(0.1) $(4.0) Loss Per Basic Share $(0.01) $(0.19) Cash and investments $213.5 $210.8 6 Excludes $0.6 million reduction of reserves for Medicaid rebates ** Excludes $2.1 million reduction of reserves for product returns and Medicaid rebates *** 2012 period includes impact of expenses associated with closure of Cambridge, Massachusetts manufacturing facility. Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
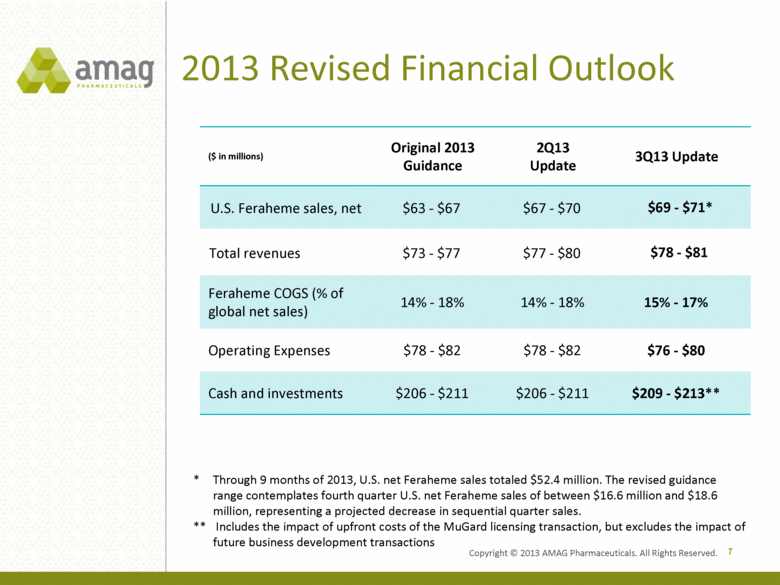
2013 Revised Financial Outlook ($ in millions) Original 2013 Guidance 2Q13 Update 3Q13 Update U.S. Feraheme sales, net $63 - $67 $67 - $70 $69 - $71* Total revenues $73 - $77 $77 - $80 $78 - $81 Feraheme COGS (% of global net sales) 14% - 18% 14% - 18% 15% - 17% Operating Expenses $78 - $82 $78 - $82 $76 - $80 Cash and investments $206 - $211 $206 - $211 $209 - $213** 7 * Through 9 months of 2013, U.S. net Feraheme sales totaled $52.4 million. The revised guidance range contemplates fourth quarter U.S. net Feraheme sales of between $16.6 million and $18.6 million, representing a projected decrease in sequential quarter sales. ** Includes the impact of upfront costs of the MuGard licensing transaction, but excludes the impact of future business development transactions Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
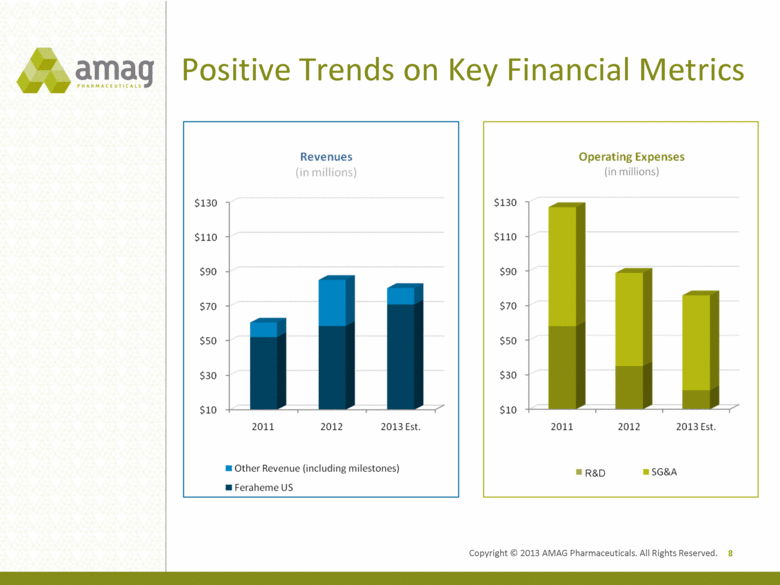
Positive Trends on Key Financial Metrics 8 R&D Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
U.S. COMMERCIAL PERFORMANCE |
|
|
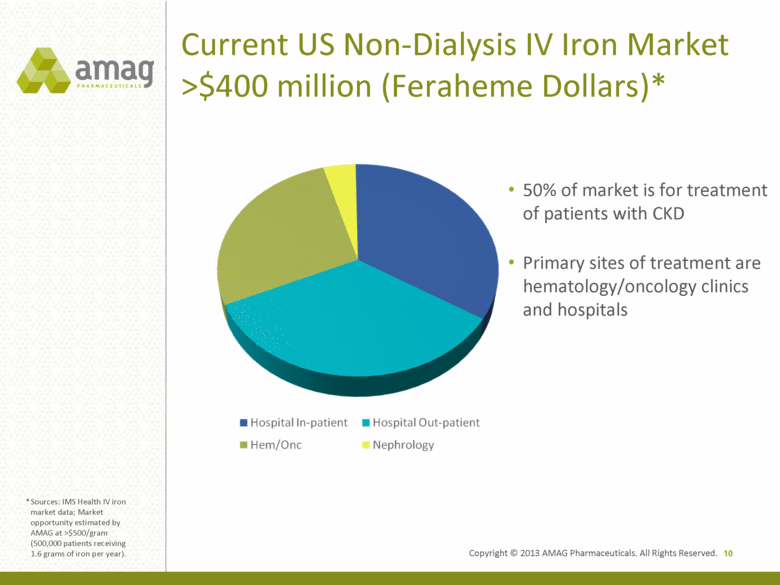
Current US Non-Dialysis IV Iron Market >$400 million (Feraheme Dollars)* 10 50% of market is for treatment of patients with CKD Primary sites of treatment are hematology/oncology clinics and hospitals * Sources: IMS Health IV iron market data; Market opportunity estimated by AMAG at >$500/gram (500,000 patients receiving 1.6 grams of iron per year). 50% of market is for treatment of patients with CKD Primary sites of treatment are hematology/oncology clinics and hospitals Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
Today’s Commercial Objective: Increase Share of Current IV Iron Market 11 Sources: U.S. Census; U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010: 41-42; Fishbane, S. et al. Iron Indices in CKD in the NHANES 1998-2004. Clin J Am Soc Nephrol. 2009 January; 4(1): 57–61; AMAG estimates market opportunity at $500/gram. 250,000 patients (400,000 grams) + $ 200 million Feraheme potential Today’s Opportunity Hematology/Oncology Strong Feraheme share in CKD Loyal and growing account base Hospitals Adoption and share increasing IDA IDA-CKD Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
Accelerating Feraheme Demand* Growth 12 * Source: IMS Health Data. Evolution Index (E.I.) of 120 Growing +20% above market growth and taking share from the competition 32% |
|
|
Feraheme Demand* Growth by Segment 13 3Q13 Market Share 17% of non-dialysis IV iron market (+2% point vs. 2Q13) 10% of hospital segment; E.I. 118 30% of hem/onc segment; E.I. 115 Contracting strategy drove increased hem/onc share in third quarter *Source: IMS Health Data. Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
MuGard Commercial Integration MuGard is a prescription oral rinse for the management of oral mucositis Approximately 40% of patients receiving chemotherapy and radiation therapy at risk of developing oral mucositis (~400,000 patients/year) MuGard has ~1% share of OM patients ~$1,000 net revenue per MuGard Rx (6 bottle treatment course) Fully prepared for AMAG’s MuGard launch Revised promotional messaging; developed new promotional materials Trained the field force; finalized incentive compensation plan Commercial synergy => direct overlap with current Feraheme physician audience Developed comprehensive target lists Began promoting to current Feraheme customers late in 3Q13 Significant growth opportunity with limited downside risk 14 Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
EXPANSION OPPORTUNITIES |
|
|
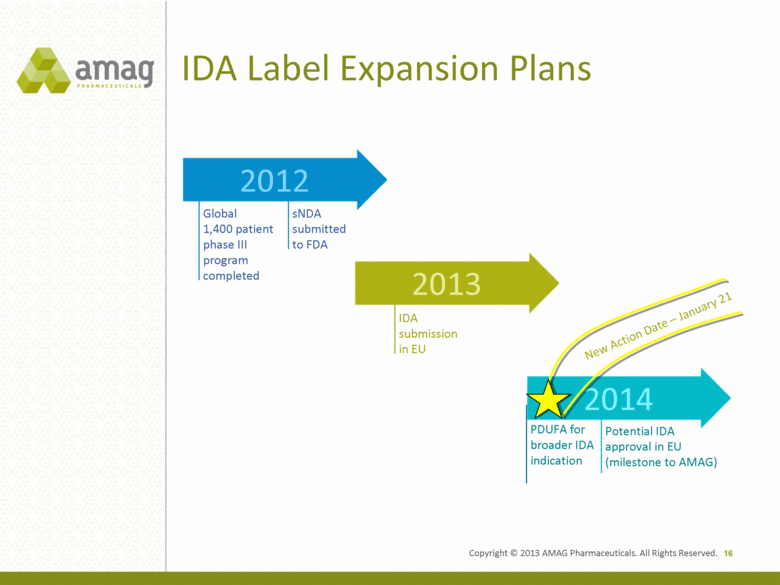
Potential IDA approval in EU (milestone to AMAG) PDUFA for broader IDA indication IDA submission in EU sNDA submitted to FDA Global 1,400 patient phase III program completed IDA Label Expansion Plans 16 2013 2012 2014 New Action Date – January 21 Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
Planning for 2014 with potential label expansion Opportunity: Gain Additional Share of Existing Market 17 Sources: U.S. Census; U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010: 41-42; Fishbane, S. et al. Iron Indices in CKD in the NHANES 1998-2004. Clin J Am Soc Nephrol. 2009 January; 4(1): 57–61; AMAG estimates market opportunity at $500/gram. 500,000 patients (800,000 grams) + $ 400 million Feraheme potential IDA IDA-CKD Label Expansion Opportunity Post approval Same call audience: Hematology/Oncology Expand use to all IDA patients in current accounts Convert single IV iron stockers to Feraheme Hospitals Expand use to all IDA patients in current accounts Continue to gain new accounts Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
18 IV Iron IDA IV Iron IDA-CKD Oral Iron IDA Oral Iron IDA-CKD 4,000,000 patients diagnosed with IDA placed on oral iron therapy IV Iron IDA-CKD IV Iron IDA Planning for 2014 with potential label expansion Opportunity: Grow IV Iron Market Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
Targeting Business Development 19 Financial Similarly sized (or smaller) specialty company Eliminate overlapping infrastructure and increase EBITDA Bulls-Eye Hem/onc or hospital company or product $10–$60 MM/yr. revenue potential IP runway Immediate accretive Opportunity for growth Strategic Opportunities aligned with Feraheme growth strategy (e.g., GI, Rheum) Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
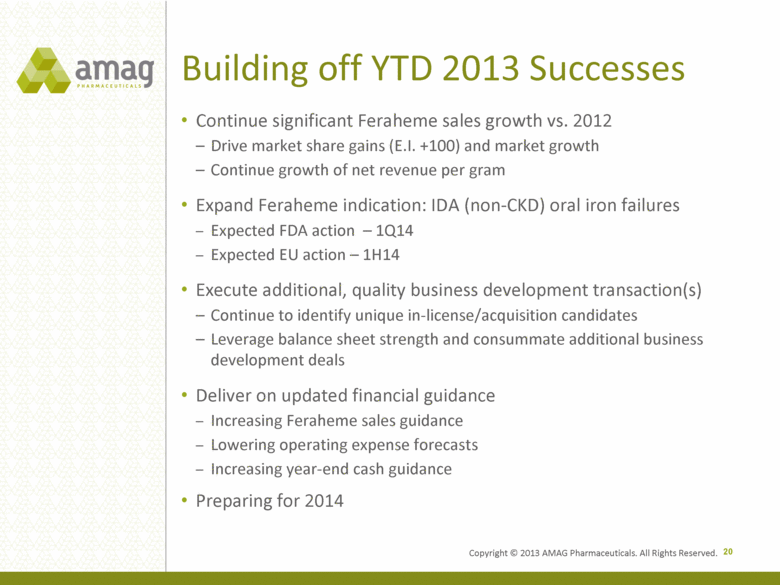
Building off YTD 2013 Successes Continue significant Feraheme sales growth vs. 2012 Drive market share gains (E.I. +100) and market growth Continue growth of net revenue per gram Expand Feraheme indication: IDA (non-CKD) oral iron failures Expected FDA action – 1Q14 Expected EU action – 1H14 Execute additional, quality business development transaction(s) Continue to identify unique in-license/acquisition candidates Leverage balance sheet strength and consummate additional business development deals Deliver on updated financial guidance Increasing Feraheme sales guidance Lowering operating expense forecasts Increasing year-end cash guidance Preparing for 2014 20 Copyright © 2013 AMAG Pharmaceuticals. All Rights Reserved. |
|
|
Well positioned for success in 2013. . . and beyond. AMAG Pharmaceuticals, Inc. 1100 Winter Street Waltham, MA 02451 o 617.498.3300 www.amagpharma.com A SPECIALTY PHARMACEUTICAL COMPANY DEDICATED TO IMPROVING THE QUALITY OF PATIENTS’ LIVES. |