Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Amarantus Bioscience Holdings, Inc. | v356539_8k.htm |

Michael J. Fox Foundation for Parkinson’s Research
Grant Award Progress Report
Comparisons and Actions of MANF and GDNF in
Rodent Models Parkinson’s Disease
MJFF Program: Neurotrophic Factors Program
Final Report: Phases 1 and 2
5th May 2013
Principal Investigator: J.W. Commissiong PhD, CSO
Amarantus Biosocience Holdings Inc.
| This report is based on the results of experiments carried out by Amarantus Bioscience with support from the Michael J. Fox Foundation, under the supervision of John W. Commissiong, the company’s chief scientific officer. Amarantus engaged the Swiss Consulting Firm NeuroAssets (CEO: David A. Lowe, PhD, assisted by Roman Urfer, PhD) to perform an independent review of the results, and prepare a written report based on the data. |
| CONFIDENTIAL 1 |
EXECUTIVE SUMMARY
| · | The objectives of this study were (1) to confirm MANF’s activity in the 6-OHDA model of Parkinson’s disease (PD), (2) to evaluate striatal and nigral administration of MANF, (3) to administer MANF in neuroprotection and neuroregeneration protocols, (4) to asses different dose levels of MANF, (5) to compare MANF with GDNF under identical experimental conditions, (6) to apply an array of behavioral, structural and functional measures, and (7) to measure diffusion of MANF after convection enhanced delivery. |
| · | MANF displayed strong neuroprotective activity when administered to the striatum as evidenced by normalized ipsilateral rotational behavior evoked by amphetamine and protection of TH+ cell bodies in the substantia nigra. |
| · | MANF prevented the striatal 6-OHDA-induced decrease of striatal dopaminergic terminals when administered to the substantia nigra. |
| · | MANF’s activity is dependent on its location of administration and MANF’s effects manifest themselves distal to the administration site. Striatal administration of MANF protects nigral cell bodies while nigral administration of MANF protects striatal dopaminergic fiber densities. |
| · | MANF may display effects contralateral to the growth factor administration site. |
| · | MANF could be delivered to the striatum by convection enhanced delivery and MANF diffusion and distribution volumes could be measured by immunohistochemistry. |
| · | Continued MANF development for the treatment of PD is warranted based on the results of this present study, the known mechanism of action and published literature, and will involve the following elements: |
| o | Rodent PD studies to investigate mechanistic hypotheses on site-specific pathways activated by MANF, distal action of MANF (including activation of contralateral circuits), protection from 6-OHDA-induced apoptosis and reactive oxygen species formation, and kinetics of dopamine levels in the striatum of freely behaving animals. |
| o | Non-human primate PK study to optimize MANF dose, site of administration, and dosing regimen to optimally cover target tissues. |
| o | Non-human primate pharmacodynamic study in a model of Parkinson’s disease as preclinical proof-of-concept and in support of the human clinical study design. |
| CONFIDENTIAL 2 |
Table of Contents
| 1 | Introduction | 5 | ||
| 1.1 | MANF biology and structure in relation to Parkinson’s disease therapy | 5 | ||
| 1.2 | In vivo activities of MANF with focus on PD | 8 | ||
| 1.3 | Grant background and objectives | 10 | ||
| 1.3.1 | Grant framework | 10 | ||
| 1.3.2 | Study timelines | 10 | ||
| 1.3.3 | Study objectives | 10 | ||
| 2 | Methods | 12 | ||
| 2.1 | MANF and GDNF protein source and characterization | 12 | ||
| 2.2 | Animal housing | 13 | ||
| 2.3 | Administration of 6-OHDA, MANF and GDNF | 13 | ||
| 2.3.1 | Phase 1: Striatal administration of 6-OHDA, MANF and GDNF | 13 | ||
| 2.3.2 | Phase 2: Striatal administration of 6-OHDA, nigral administration of MANF and GDNF | 14 | ||
| 2.4 | Amphetamine-induced rotational behavior | 14 | ||
| 2.5 | Transcardiac perfusion and tissue collection | 15 | ||
| 2.6 | Quantification of TH+ cells in the substantia nigra (Phase 1) | 16 | ||
| 2.7 | Embedding and sectioning of rat brains (Phase 2) | 16 | ||
| 2.8 | Quantification of TH+ neurons in the substantia nigra by stereology (Phase 2) | 16 | ||
| 2.9 | Quantification of dopaminergic terminals in the striatum (Phase 2) | 17 | ||
| 2.10 | Determination of striatal levels of dopamine, DOPAC and HVA | 18 | ||
| 2.11 | MANF striatal diffusion by convection enhanced delivery (CED) | 19 | ||
| 2.12 | Statistical analyses | 21 | ||
| 3 | Results | 22 | ||
| 3.1 | Overall study design | 22 | ||
| 3.2 | Phase 1: Striatal administration of growth factors | 23 | ||
| 3.2.1 | Neuroprotection protocol | 24 | ||
| 3.2.2 | Neuroregeneration protocol | 27 | ||
| 3.3 | Phase 2: Nigral administration of growth factors | 31 | ||
| 3.3.1 | Neuroprotection protocol | 31 | ||
| 3.3.2 | Neuroregeneration protocol | 40 | ||
| CONFIDENTIAL 3 |
| 3.4 | Diffusion of MANF with convection-enhanced delivery | 47 | ||
| 4 | Discussion | 50 | ||
| 5 | Conclusions and Outlook | 56 | ||
| 6 | References | 57 | ||
| CONFIDENTIAL 4 |
1 Introduction
1.1 MANF biology and structure in relation to Parkinson’s disease therapy
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta. The lack of dopamine causes the classical motor symptoms of bradykinesia, rigidity and resting tremors. Current PD therapy is strictly symptomatic and is focused on dopamine replacement strategies. PD therapeutic agents include various formulations of L-DOPA, dopamine receptor agonists, monoamine oxidase (MAO) B inhibitors and catechol O-methyltransferase (COMT) inhibitors. Much of the scientific and clinical efforts on the discovery and development of new compounds and agents for treatment of PD symptoms attempt to address motor dysfunction including dyskinesia and gait disorders, hallucinations / psychosis, depression / anxiety, autonomic failure but few aim to achieve disease modification or neuroprotection (Meissner et al., 2011). The intended application of mesencephalic, astrocyte-derived neurotrophic factor (MANF) falls into this latter category offering the possibility of a neuroprotective (i.e., halting disease progression) and a neuroregenerative (i.e., reversal of neurodegeneration) treatment of PD.
MANF (Petrova et al., 2003) and cerebral dopamine neurotrophic factor (CDNF) (Lindholm et al., 2007) form a distinct family of evolutionary conserved trophic factors with a unique domain organization. MANF was initially purified from conditioned media from an immortalized ventral mesencephalic astrocytic cell line (Petrova et al., 2003). Biochemical analyses combined with bioinformatics revealed that the MANF protein is encoded by the gene for human arginine-rich protein (ARP) also known as human arginine-rich, mutated in early stage tumors (ARMET) (Shridhar et al., 1996).
The three-dimensional structures of MANF and CDNF were solved by NMR (Hoseki et al., 2010, Hellman et al., 2011) and X-ray crystallography (Parkash et al., 2009) and offer important insight into the function of these growth factors. The NMR-structure of mature MANF identified two distinct domains joined by a linker. The N-terminal domain (N-domain) of MANF is entirely helical and contains three disulfide bonds. A cluster of positively charged residues in the p and 310 helices of the structure are conserved among MANF homologues and may indicate functionally important residues. A weak but significant structural similarity to the N-domain was found with saposin-like proteins (i.e., Saposin D). Saposins are required for the degradation of plasma membrane-derived glycosphingolipids in the lysosome. However, the charged surface of MANF suggests that interacting molecules and the biological function may differ considerably between MANF and saposins but the similarity between the two may indicate a function of MANF at intracellular or extracellular membranes.
| CONFIDENTIAL 5 |
The C-terminal domain (C-domain) of MANF is also entirely helical and contains one disulfide bond between conserved cysteines in the CXXC motif between a-helices 5 and 6. The CXXC motif is a consensus sequence of proteins of the thiol-protein oxidoreductase superfamily. No enzymatic oxidoreductase activity has been detected for MANF so far (Mizobuchi et al, 2007, data not shown). The MANF C-domain is structurally similar to SAP-domains (SAF-A/B, Acinus, PLAS) and most similar to the SAP-domain of Ku70, a cytoplasmic protein with anti-apoptotic activity (Hellman et al., 2011). Based on structural considerations it is thus conceivable that MANF displays functions related to apoptosis.
MANF expression is widespread in the nervous system and in non-neuronal tissues (Lindholm et al., 2008). mRNA levels in human brain tissues was highest in the cerebral frontal cortex, optic nerve, cerebellum, dentate nucleus and pons. High levels were also detected in medulla, cerebellum white matter, cerebral pedunculi, colliculi, corpus callosum and hippocampus. Low levels of mRNA were detected in many additional brain tissues, including the substantia nigra. MANF protein expression in the substantia nigra was only partially co-localized with tyrosine hydroxylase (TH) (Lindholm et al., 2008). MANF is thus expressed in potential target tissues relevant to the treatment of PD.
MANF in vivo biology has been studied in Drosophila (Palgi et al., 2009) and zebrafish (Chen et al., 2012). DmMANF was required for the development of the Drosophila nervous system. Maternal and zygotic DmMANF null mutants led to a complete loss of dopaminergic neurites and a drastic reduction of dopamine levels. These events were followed by a degeneration of axonal bundles in the embryonic central nervous system with subsequent cell death. MANF is widely expressed during embryonic development and in adult organs of zebrafish (Chen et al., 2012). In the brain, MANF-positive cells were located close to TH-positive cells in preoptic, ventral and dorsal thalamic regions and only few MANF-containing cells were found to co-express TH. Knockdown of MANF expression during development with antisense oligonucleotides resulted in no apparent phenotype. However, the level of dopamine was reduced by about 50% and the expression of the two TH genes, th1 and th2, was reduced in some brain regions. MANF is thus involved in the development of the dopaminergic system in Drosophila and zebrafish. Since developmental processes are sometimes re-activated in response to neuronal injury it is conceivable that MANF could have regenerative activity in PD.
The regulation of expression and secretion of MANF was extensively studied in the context of the cellular stress response. MANF expression is induced by the unfolded protein response (UPR) (Apostolou et al., 2008). The MANF promoter contains an ER stress response element, ERSE-II, that is activated by known ER stressors like tunicamycin and thapsigargin (Apostolou et al., 2008; Tadimalla et al., 2009). Consequently, induction of MANF expression by ER stressors was demonstrated in several independent studies (NIH3T3 cells / tunicamycin, thapsigargin, Mizobuchi et al., 2007; U2OS, 293, SHST5Y cells / tunicamycin, thapsigargin, Apostolou et al., 2008; Primary cultured neurons / tunicamycin, Yu et al., 2010; Cardiac myocytes, HeLa cells / tunicamycin, thapsigargin, DTT, Glembotski et al., 2012; Neuro2a cells / thapsigargin, Oh-hashi et al., 2012). It is thus well established that ER stress induces MANF expression in many different cell types.
| CONFIDENTIAL 6 |
The endoplasmic reticulum (ER) is a major site of protein synthesis. ER quality control mechanisms monitor protein folding and prevent the transport and secretion of immature proteins. When ER stress overwhelms the capacity of the quality control system, unfolded or misfolded proteins accumulate in the ER. ER stress sensor proteins, PERK, IRE1 and ATF6 activate an intracellular signal transduction pathway called the unfolded protein response (UPR). The UPR increases the expression of several target genes to restore ER homeostasis. The functions of UPR target genes vary broadly and include protein folding helpers (i.e., chaperones) and proteins involved in glycosylation, oxidative stress response, protein trafficking, lipid biosynthesis and ER-associated degradation. Aspects of ER stress and the UPR have been linked to the development of several neurodegenerative disorders (Lindholm et al., 2006). In the context of PD, it is noteworthy that a prominent feature of this disease is the presence of intraneuronal cytoplasmic inclusion bodies, known as Lewy bodies. Studies of families with rare autosomal recessive PD identified several genes coding for mutated proteins that could be causative for PD. Among them, aggregated alpha-synuclein is found in Lewy bodies. In the transgenic mouse line A53TaS aggregated alpha-synuclein was associated with abnormal UPR that could promote neuronal death (Colla et al., 2012). It is thus conceivable that a growth factor such as MANF whose expression is induced by ER stress and the UPR could counteract degenerative mechanisms caused by protein aggregation.
The question arises whether MANF could have activities that manifest themselves intracellularly that would not require secretion of MANF and the subsequent activation of a receptor-mediated signaling pathway. Knock-down of MANF expression by siRNA rendered HeLa cells more sensitive to cell death induced by ER stress. Moreover, overexpression of MANF in U2OS cells protected cells from ER-stress induced cell death (Apostolou et al., 2008). Knock-down of MANF expression by micro-RNA increased cell death of cardiomyocytes after simulated ischemia / reperfusion while overexpression of MANF protected these cells from serum-deprivation induced caspase-3 activation and ischemia-induced cell death (Tadimalla et al., 2008). Overexpression or microinjection of MANF and the C-terminal domain of MANF prevented apoptotic cell death of sympathetic neurons (Hellman et al., 2011). Both siRNA-mediated knockdown and overexpression of MANF will affect ER-resident MANF as well as secreted MANF. The observed effects could thus be explained by extracellular MANF binding to a receptor or by ER-resident MANF performing an intracellular function. Hence, intracellular and possibly exogenous MANF protein protects cells from stress induced by ischemia, serum-deprivation and more specifically, ER stress.
Apoptosis induced by ER stress or other mechanisms may play a role in the progress of nigral dopaminergic neurodegeneration in PD. Therefore, anti-apoptotic activities of MANF could be important for its therapeutic potential in PD. Recombinant MANF selectively increased the survival of dopaminergic (i.e., TH+) neurons (Petrova et al., 2003) in mixed neuronal cultures. Recombinant MANF decreased caspase-3 activation in a dose-dependent manner in cardiomyocytes that were serum-starved (Tadimalla et al., 2009). Recombinant ARMET fully protected primary mixed cortical and hippocampal neuronal cultures exposed to tunicamycin using quantification of TUNEL-positive cells as a marker of apoptosis (Yu et al., 2010). MANF thus seems to have anti-apoptotic activity when administered as an exogenous protein.
| CONFIDENTIAL 7 |
MANF increased the frequency of spontaneous and miniature gamma-aminobutyric acid (GABA)-receptor mediated inhibitory postsynaptic currents (IPSCs) without changing the mean amplitudes in mechanically dissociated dopaminergic neurons (Zhou et al., 2006). In enzymatically dissociated neurons, MANF had no effect on currents induced by exogenous GABA.
1.2 In vivo activities of MANF with focus on PD
MANF was identified as a mesencephalic astrocyte-derived neurotrophic factor and displays a spectrum of cellular activities that could translate to neuroprotective or restorative effects in PD. Therefore, MANF was tested in the 6-hydroxy dopamine (6-OHDA) model of PD using differing administration protocols. A single intrastriatal injection of MANF was administered either 6 hours pre-6-OHDA (i.e., neuroprotection protocol) or 4 weeks after 6-OHDA (i.e., neuroregenereration protocol) and outcomes on amphetamine-induced rotational behavior and TH+ cells in the substantia nigra and TH+ fibres in the striatum were evaluated (Voutilainen et al., 2009). In the neuroprotection protocol, MANF (10 μg) reduced the 6-OHDA-induced deficit in rotational behavior two weeks after administration by about 80% and this effect was sustained at the four week time-point (90% reduction). The TH+ neurons in the substantia nigra were protected (70%) by MANF (10 mg) but the effect on TH+ fibers in the striatum was modest (10% protection). In the neuroregeneration protocol, MANF displayed a time dependent decrease of amphetamine-induced rotational behavior that led to a cumulative reduction of about 50% at week 12 compared to vehicle treated animals. This effect was observed at the same dose level of MANF (10 μg). TH+ cells were protected to some degree (25%) but the effects in this protocol were substantially smaller than the ones observed in the neuroprotection protocol. GDNF (Glia cell line-derived neurotrophic factor) was profiled using an identical treatment regimen and even though this growth factor displayed similar activities as MANF it was generally less active under the same conditions.
A second study investigated MANF activities in the rat 6-OHDA model in which the growth factor was applied by an osmotic mini-pump starting two weeks after 6-OHDA for two weeks with a two-site intrastriatal infusion. The total amounts of administered MANF were 21, 42 and 63 μg (Voutilainen et al., 2011). These amounts were considerably higher than the active dose in the previous study (i.e., 10 mg) and this difference could be of importance given the U-shaped dose-response curve of MANF. MANF did not display significant effects on amphetamine-induced rotational behavior or provide protection of TH+ cells and fibers in the substantia nigra and striatum, respectively. However, the vehicle control displayed very low cumulative rotation numbers indicating rapid spontaneous recovery. Moreover, GDNF was not different from vehicle even though in a parallel experiment in which GDNF and CDNF were investigated in an otherwise identical protocol GDNF showed a clear trend towards reduction of rotational behavior. Hence, conclusions on MANF activity after chronic intrastriatal infusion cannot be based on this study.
| CONFIDENTIAL 8 |
The profiling of GDNF in the 6-OHDA model of Parkinson’s disease has been the subject of many publications and study designs included neuroprotection and neuroregeneration protocols in which the growth factor was administered either to the striatum (Rosenblad et al., 1998; Kirik et al., 2000; Lindholm et al., 2007; Vouitilainen et al., 2009) or the substantia nigra (Sauer et al., 1995; Winkler et al., 1996; Kearns et al., 1996; Lapchak et al, 1997; Kirik et al., 2000). The functional and structural readouts in these studies included amphetamine-induced rotational behavior, assessments of the structural integrity and function of dopaminergic terminals and fibers (i.e., striatal TH+ fibers, dopaminergic terminal densities, dopamine levels) and the survival of dopaminergic neurons in the substantia nigra (i.e., TH+ nigral cell bodies). The reported activities of GDNF in these experimental systems and treatment paradigms are described in the result section of this report. However, GDNF did display distinct sets of neuroprotective and neuroregenerative activities depending on the location of GDNF injection (i.e., striatal versus nigral) and it is thus of interest to profile GDNF side-by-side with the novel neurotrophic growth factor MANF to understand commonalities and distinct features of these neurotrophic factors.
In conclusion, MANF displays a promising profile of cellular activities relevant to disease mechanisms of PD. Consequently, MANF in vivo activity was demonstrated in the 6-OHDA model of PD. However, there remain significant uncertainties as to the effects of MANF on behavior, cellular markers and biochemical read-outs in models of PD. Therefore, this present study was designed to further investigate MANF in the 6-OHDA model, to provide additional evidence and independent confirmation of MANF’s activities and to add further experimental support for the development of MANF for treatment of PD. Moreover, an additional object for this study was to understand how MANF and GDNF compare under the same experimental conditions.
| CONFIDENTIAL 9 |
1.3 Grant background and objectives
| 1.3.1 Grant framework | |
| MJFF Program: | Neurotrophic Factors Program |
| Award start date: | April 4, 2010 |
| Award duration: | 1 year (Extended by agreement with MJFF) |
| Project title: | Comparison and actions of MANF and GDNF in rodent models of |
| Parkinson’s disease | |
| Principal investigator: | John W. Commissiong, PhD |
| Organization: | Amarantus Bioscience Holdings Inc., c/o The Parkinson’s Institute, |
| 675 Almanor Ave., Sunnyvale, CA 94085, USA. |
1.3.2 Study timelines
Award start date was April 4, 2010.
Completion of Phase 1 experiments in October 2010.
Written report of Phase 1 results submitted to MJFF on October 10, 2010.
Oral presentation to MJFF in New York on November 9, 2011.
Follow-up teleconference between Jamie Ebeling (MJFF) and John Commissiong (AMBS) was held on December 4, 2011. A change of scope of the study was agreed on by MJFF and consequently densitometry and stereology methods were included in the Phase 2 of the grant.
Given delays in reporting data from the Phase 2 of this study, AMBS management decided to contract Drs. Lowe and Urfer of NeuroAssets, a Swiss-based consulting company, to collate and assist Dr. John Commissiong in writing this final report of Phase 1 and 2 of the study. The final report of this study was submitted to MJFF in May 2013.
1.3.3 Study objectives
(1) Confirm the activity of MANF in a well established model of PD (i.e., intrastriatal administration of 6-OHDA).
(2) Compare the activity of MANF with GDNF under identical experimental conditions.
(3) Assess and compare the activities of MANF and GDNF after single injections into the striatum versus the substantia nigra.
(4) Assess and compare the activities of MANF and GDNF when applied prior to 6-OHDA (i.e., neuroprotection) or weeks after the administration of the toxin (i.e., neuroregeneration).
| CONFIDENTIAL 10 |
(5) Assess activities of different dose levels of MANF.
(6) Measure the diffusion of MANF by convection enhanced delivery after administration to the striatum.
(7) Apply an array of well established and accepted read-outs including behavioral (i.e, amphetamine-induced rotations), functional (i.e., dopamine and dopamine metabolites levels) and structural (i.e., TH+ cell counts in the substantia nigra; TH+ terminal densities in the striatum) measures.
The study was conducted in two phases. In Phase 1, the growth factors were applied to the striatum and in Phase 2 to the substantia nigra. The detailed study design is described in section 3.1.
| CONFIDENTIAL 11 |
2 Methods
2.1 MANF and GDNF protein source and characterization
The gene of a human MANF variant (R155P; Petrova et al., 2003) with a C-terminal 6xHis tag and an enterokinase cleavage site was inserted into the kanamycin resistant expression vector pJexpress 411. E. coli BL21(DE3) cells transformed with the resulting expression vector were grown in a 5 liter fermenter in fortified LB medium containing a phosphate buffer and glucose. Cells were grown at 37°C until the glucose was nearly exhausted at which time a glucose feed was started. The glucose concentration was maintained at or below 1 g/l. MANF expression was induced by addition of Isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 1 mM and cells were harvested 4 h post induction using a continuous flow centrifuge. Cell paste was stored at -80° C.
The chromatography system and the columns for MANF purification were sanitized by soaking in 0.5 M NaOH, rinsed with low endotoxin water, and equilibrated in buffers prepared with low endotoxin water. One hundred grams of cells from the fermentor run were resuspended in 1 liter of 20 mM NaH2PO4, 0.25 M NaCl, pH 8 with a hand held homogenizer and passed through a microfluidizer three times at approximately 15,000 psi. The lysate was clarified by centrifugation and filtration. The clarified lysate was applied to a 35 ml IMAC fast flow (FF) column (2.6 cm by 6.3 cm) equilibrated in Buffer NA (20 mM NaH2PO4, 5 mM imidazole, 0.5 M NaCl, pH 8). The column was washed with 2 column volumes (CV) Buffer NA, 2 CV Buffer NA containing 2 M urea and 1 % Triton X-100, 2 CV Buffer NA, and Buffer NA containing 25 mM imidazole. The protein was eluted with Buffer NA containing 200 mM imidazole and the column was purged with Buffer NB (20 mM NaH2PO4, 500 mM imidazole, 0.5 M NaCl, pH 8). Fractions were collected in sterile 125 ml capacity PETG bottles. The pooled fractions containing MANF were dialyzed against 2 liters of 20 mM NaH2PO4, 50 mM NaCl, 0.1 % Tween 20, pH 7.5 at room temperature. The dialysate was diluted to OD280 ~2 with 20 mM NaH2PO4, 50 mM NaCl, 0.1 % Tween 20, pH 7.5, CaCl2 was added to 2 mM, and 160 units enterokinase (EKMax, Invitrogen) was added (to approximately 1 unit/ml). Digestion proceeded at room temperature for 8 hours and terminated by addition of EDTA to 5 mM final concentration. The solution was stored at 4°C overnight. The pH of the EK-digested MANF was adjusted to 6 with HCl and filtered through a 0.22 μm cellulose acetate filter (Corning). The entire 80 ml of EK-digested MANF preparation was applied to a sanitized prepacked 5 mL SP HP HiTrap column (GE Healthcare) equilibrated in Buffer SA (10 mM NaH2PO4, pH 6) containing 50 mM NaCl, followed by a wash with several CV of Buffer SA containing 50 mM NaCl. Bound proteins were eluted by an initial step to 150 mM NaCl, followed by a continuous gradient to 0.6 M NaCl, and a final step to 1 M NaCl. Fractions containing MANF were combined and stored at 4°C. This pool was combined with the pool from a previous purification run using a similar protocol and was dialyzed against 10 mM Na citrate, 150 mM NaCl, pH 6.0. The dialysate was passed through a Mustang E filter and concentrated using a sanitized Amicon Ultra-15 (10 kDa molecular weight cut off) to an OD280 of 10. The endotoxin level of this protein preparation was less than 10 EU per mg of protein using a Pyrosate® LAL clot assay kit (Cape Cod Associates). The biological activity of MANF was verified in a dopaminergic cell culture assay (Takeshima et al., 1994; Takeshima et al, 1996).
| CONFIDENTIAL 12 |
Recombinant human GDNF was obtained from Peprotech (Catalog # 450-10). The mature sequence of human GDNF with a methionine residue at the N-terminal with no tags was expressed in E.coli. The purity of the recombinant material was reported as greater than 98% by SDS-PAGE and HPLC analyses. The biological activity of GDNF was tested in a rat C6 cell proliferation assay with an ED50 of <0.1 ng/ml (Peprotech technical information). For administration to animals, MANF and GDNF were diluted in filter sterilized 10 mM Na citrate buffer, pH 6.8. The solutions were prepared in siliconized, 1.5 ml Eppendorf tubes just prior to use.
2.2 Animal housing
Adult male Wistar rats were housed in groups of four per cage in a temperature-controlled environment on a 12h:12h light:dark cycle. Animals were given free access to food and water. Animals used in the research studies were handled, housed, and sacrificed in accord with the current NIH guidelines regarding the use and care of laboratory animals, and all applicable local, state, and federal regulations and guidelines.
The in vivo part of this study was performed in the laboratory of Dr. Nigel Maidment, UCLA.
2.3 Administration of 6-OHDA, MANF and GDNF
2.3.1 Phase 1: Striatal administration of 6-OHDA, MANF and GDNF
The Phase 1 of this study consisted of a neuroprotection and neuroregeneration protocol and each protocol included 5 experimental groups (6-OHDA / Vehicle; 6-OHDA / MANF 3 μg; 6-OHDA / MANF 10 μg; 6-OHDA / MANF 36 μg; 6-OHDA / GDNF 10 μg) with 12 animals assigned to each group.
In preparation of 6-OHDA and growth factor administration, Wistar rats underwent stereotaxic surgery in a Kopf stereotaxic apparatus under isoflurane anesthesia for implantation of a unilateral injection guide cannula above the striatum. Animals were allowed to recover for one week before administration of growth factors or 6-OHDA was initiated.
In the neuroprotection protocol, MANF (3, 10, 36 μg; 4 μl filter sterilized 10 mM Na citrate, pH 6.8), GDNF (10 μg; 4 μl filter sterilized 10 mM Na citrate, pH 6.8) or phosphate-buffered saline (PBS) were injected unilaterally via the previously implanted injection guide cannula with a 10 μl Hamilton syringe over a 3 minutes period to the middle of the right or left striatum (Stereotaxic coordinates relative to Bregma: Rostral +1.0 mm, lateral = ±2.7 mm, ventral = 6.0 mm). The needle was left in place for a further 5 min, withdrawn slowly to Z = -2.5 mm, left in place for a further 5 min, and then slowly withdrawn. The wound was closed loosely with surgical clips to regain access later. Administration of growth factors was performed 6 hours prior to 6-OHDA administration.
| CONFIDENTIAL 13 |
For the 6-OHDA administration, animals were re-anesthetized with isoflurane and injected with desipramine (15 mg/kg, i.p.) to protect noradrenergic neurons. 6-OHDA (8 μg free base dissolved in 4 μl of filter sterilized PBS / 0.02% ascorbic acid) was injected using the same procedure and to the same sterotaxic location as described for the growth factor administration. Animals were allowed to recover from anesthesia and were then returned to their home cages.
The neuroregeneration protocol followed the same surgical procedures but differed in two important aspects. (1) The amount of striatally administered 6-OHDA was 20 μg. (2) MANF and GDNF were administered four weeks after the 6-OHDA injection.
2.3.2 Phase 2: Striatal administration of 6-OHDA, nigral administration of MANF and GDNF
The Phase 2 of this study consisted of a neuroprotection and neuroregeneration protocol and each protocol included 6 experimental groups (Vehicle / Vehicle; 6-OHDA / Vehicle; 6-OHDA / MANF 3 μg; 6-OHDA / MANF 10 μg; 6-OHDA / MANF 36 μg; 6-OHDA / GDNF 10 μg) with 12 animals assigned to each group. The administration of 6-OHDA, MANF and GDNF in this Phase 2 of the study followed the same general surgical procedures as presented in the previous section. The amount of striatally administered 6-OHDA was 8 μg for the neuroprotection protocol and 20 μg for the neuroregeneration protocol. In the neuroregeneration protocol, MANF (3, 10 or 36 μg, single administration) or GDNF (10 μg, single administration) were administered two weeks after the 6-OHDA injection while in the neuroprotection protocol the growth factors were administered 6 hours prior to 6-OHDA. The growth factors were administered to the substantia nigra using the following coordinates relative to bregma and the skull surface: Caudal 4.9 mm, lateral ±2.0 mm, ventral 8.3 mm.
2.4 Amphetamine-induced rotational behavior
In order to asses unilateral neuronal damage induced by 6-OHDA injection and effects of treatment by growth factors, D-amphetamine sulphate (2.5 mg/kg free base, i.p.) was administered to animals of the treatment groups in Phases 1 and 2 of this study and circling behavior was monitored over a 2-h period using a video tracking system.
In the Phase 1 neuroprotection protocol, rotational behavior was assessed at 2, 4 and 8 weeks after administration of 6-OHDA / MANF / GDNF. Due to the neuroprotection design of this study there was no mechanism available to identify and exclude animals with minimal 6-OHDA-induced damage.
| CONFIDENTIAL 14 |
In the Phase 1 neuroregeneration protocol, the initial rotational behavior was assessed 3 weeks after 6-OHDA administration (1 week prior to planned growth factor administration). A threshold of 150 unilateral rotations / 2 h after amphetamine treatment was applied to include animals in the study. Based on this data, 13 animals (6 in vehicle group, 3 in MANF 3 μg group, 2 in MANF 36 μg group, 2 in GDNF 10 μg group) were removed from further testing and were not included in the analysis of the results.
In the Phase 2 neuroprotection protocol, rotational behavior was assessed 2 and 4 weeks after administration of 6-OHDA / MANF / GDNF. Due to the neuroprotection design of this study there was no mechanism available to identify and exclude animals with minimal 6-OHDA-induced damage.
In the Phase 2 neuroregeneration protocol, the initial rotational behavior was assessed 1 week after 6-OHDA administration (1 week prior to planned growth factor administration). A threshold of 150 unilateral rotations / 2 h after amphetamine treatment was applied to include animals in the study. Based on this data 7 animals (2 vehicle; 2 MANF 10 μg; 2 MANF 36 μg; 1 GDNF 10 μg) were excluded from further testing and were not included in the analysis of the results.
2.5 Transcardiac perfusion and tissue collection
The day following the last behavioral test (Phase 1: 8 weeks after growth factor administration; Phase 2: 4 weeks after growth factor administration) half of the animals from each treatment group were euthanized by decapitation, the heads rapidly frozen in liquid nitrogen, stored at -80 ºC until the brains were removed and the striata dissected for neurochemical analysis (i.e., determination of striatal dopamine (DA), 3,4-di-hydroxyphenyl acetic acid (DOPAC) and homovanillic acid (HVA) levels). The other half of the animals from each treatment group were euthanized by deep anesthesia with a lethal dose of pentobarbital (100 mg/kg, i.p.), transcardially perfused with cold phosphate buffered saline and further perfused with 200 ml of cold 4% paraformaldehyde in PBS, pH 7.4 (Phase 1: Quantification of TH+ neurons in the substantia nigra; Phase 2: Quantification of nigral TH+ cells by stereology; Density of striatal dopaminergic (TH+) terminals by densitometry). After euthanasia, the brains were removed and cryopreserved in 25 ml 30 % cold sucrose for 24 hours and stored at -80ºC until analyzed.
| CONFIDENTIAL 15 |
| 2.6 | Quantification of TH+ cells in the substantia nigra (Phase 1) |
A total of 18 sections (40 μm thickness) were cut through the substantia nigra and mounted on pre-cleaned, Superfrost Plus glass slides (6 sections per slide). The slides were air dried on the lab bench for at least 4 hours prior to staining. The sections were hydrated (1x PBS, 3x 10 min), endogenous peroxidase quenched (0.3% H2O2 in 50% MeOH, 20 min), washed (PBS, 3x 10min) and blocked (5% normal horse serum in PBS/ 0.3% Tween for 2 hrs, at room temperature). The primary anti-tyrosine hydroxylase (TH) monoclonal antibody (Sigma, T-2928, 1:2000, in 5% normal horse serum/1x PBS), was applied over night, at 4 ºC (in the refrigerator), followed by washing (3x PBS, 3x 10 min). The secondary Ab, biotinylated anti-Mouse IgG, raised in horse (Vector Lab), 40 μl Ab/10 ml 1% horse serum/1X PBS was applied for 2 hrs at room temperature, with the sections protected from light with aluminum foil. After washing (0.1% tween/1x PBS, 4x 10 min), the peroxidase complex (ABC: Vector Lab), made up 45 mins previously according to the instructions of the manufacturer, was applied for 1 hr, at room temperature, followed by washing (4x 0.1% tween in 1x PBS). Finally, the freshly prepared diaminobenzadiene (DAB) substrate was applied, and the dark deposit in the substantia nigra developed within 5 to 8 minutes. The sections were rinsed in tap water, and dehydrated through 65%, 80 %, 95% and 100% changes of ethanol. The sections were then cleared in HistoClear, mounted in VectMount (Vector Labs), cover slipped, and dried in the hood for 3 hours, before microscopic analysis. Using a 10x objective, the number of TH+ cells per field in the dorsolateral region of the substantia nigra was determined by counting. This region was selected because the density of neurons is lower in this region and accurate cell counts can be made.
| 2.7 | Embedding and sectioning of rat brains (Phase 2) |
Rat brains were treated overnight with 20% glycerol and 2% dimethylsulfoxide to prevent freeze-artifacts, trimmed to yield the region from substantia nigra through striatum and embedded into six blocks of 12 brains each into a gelatin matrix using MultiBrain® Technology (NeuroScience Associates, Knoxville, TN). After curing, each block was rapidly frozen by immersion in isopentane, chilled to -70ºC with crushed dry ice and mounted on an AO 860 sliding microtome. Each MultiBrain® block was cut coronally to generate sections of 40 μm thickness. All sections were collected sequentially in containers filled with antigen preserve solution (50% PBS pH 7.0, 50% ethylene glycol, 1% polyvinyl pyrrolidone). For the stereological analysis of TH+ cells in the substantia nigra every 8th section was selected and stained. For the striatal densitometry analysis every 8th section (320 μm spacing) was selected and stained yielding data on a total of four rostral to caudal levels per animal.
| 2.8 | Quantification of TH+ neurons in the substantia nigra by stereology (Phase 2) |
TH+ neurons of the substantia nigra were quantified by stereology. Sections were stained with a TH-specific antibody and nucleoli were used as the basic counting unit to quantify neurons. Nucleoli were stained using a commercially available, proprietary method to stain the argyrophilic, acidic proteins of the nucleolar organizing region referred to as “AgNORs” (Switzer et al., 2011). Sections stained for TH and AgNOR were incubated in HCl to enhance permeabilization, bleached to avoid non-specific silver staining, and incubated with a one-quarter strength concentration of the TH antibody to provide optimal contrast with the AgNOR stain while retaining robust pigmentation of TH-positive structures (Switzer et al., 2011).
| CONFIDENTIAL 16 |
The stereological analysis and quantification of TH+ neurons in the substantia nigra was performed as described (Healy-Stoffel et al., 2012). Every 8th section containing the substantia nigra was selected and the substantia nigra was carefully outlined by using an atlas. TH-AgNOR-stained cells within these boundaries were quantified using the optical fractionator method and the Stereologer software package (Stereology Resource Center, Chester MD & Tampa-St.Petersburg, FL). A Nikon Eclipse 80i microscope was used which was coupled to a Sony 3CCD color digital video camera and operated an Advanced Scientific Instrumentation MS-2000 motorized stage with input into a Dell Precision 650 server and a high-resolution plasma monitor. The areas of interest were first identified using 4× / 1.3 aperture dry lenses and the stereology was performed at high magnification with 100× / 1.4 aperture oil immersion lenses which allowed for clear visualization of the nucleoli and precise definition of the cell boundaries.
Sectioning of the substantia nigra and staining for TH and AgNORs were performed at Neuroscience Associates (NSA) (Knoxville, Tennessee). The stereology analysis was performed by Dr. S. Omar Ahmad (St. Louis University) under contract with NSA.
| 2.9 | Quantification of dopaminergic terminals in the striatum (Phase 2) |
Dopaminergic (TH+) terminals in the striatum were quantified by densitometry of TH-stained brain sections. For each animal, four 40 μm thick sections of the ipsilateral and contralateral sides of the substantia nigra / striatum were analyzed from rostral to caudal, spaced by 320 μm. The sections were then stained free-floating. The dopaminergic terminals were stained using a TH-specific antibody (Pel-Freez Biologicals; 1:1600 in cold TBS) and the Vectastain Elite ABC kit (Vector Laboratories). The immunohistochemical staining for TH was followed by a thionine Nissl counterstain and sections were then mounted on gelatinized glass slides. A 3 x 3 grid was placed on each section relative to a landmark (Figure 1) to collect densitometry data from the temporal (positions 1, 2, 3 (left) and 7, 8, 9 (right)), medial (positions 4, 5, 6 (left and right)) and basal (positions 7, 8, 9 (left) and 1, 2, 3 (right)) striatum (Figure 1). Similarly, data for the dorsal (positions 1, 4, 7 (left and right)) and ventral (positions 3, 6, 9 (left and right)) striata were collected. The global striatal data combined all values obtained from the four rostrocaudal levels (4 x 9 = 36 data points) from the ipsilateral or contralateral sides, respectively. The optical densities were measured at the 4 rostrocaudal levels for the temporal, medial and basal striatum with a digital camera and a constant illumination table. To estimate the specific TH staining density, the optical density readings were corrected for the non-specific density as measured on the completely denervated parts of the striatum.
| CONFIDENTIAL 17 |

Figure 1: 6-OHDA-induced dopaminergic denervation in the striatum. Brain sections (40 μm thickness) stained for TH+ and Nissl, mounted on glass slides.
(A) Densitometry data were acquired from rostral to caudal at four levels spaced by 320 μm. A 3x3 grid was applied to define areas for spatial resolution (i.e., temporal, medial, basal; dorsal, ventral) of densitometry data. Shown is a brain section generated from a vehicle/vehicle animal. (B) 6-OHDA treatment leads to partial denervation in the dorsomedial region. (C) 6-OHDA-induced full denervation of the left striatum.
2.10 Determination of striatal levels of dopamine, DOPAC and HVA
Striatal levels of DA and dopamine metabolites DOPAC and HVA were determined by negative chemical ionization / mass spectrometry. The frozen brains were allowed to thaw on ice, the striata were removed and placed on a pre-cooled piece of aluminum foil and quickly placed back on dry ice. The striatal samples were weighed using a microbalance with a sensitivity of 10 μg, transferred to 1.6 ml Eppendorf tubes, then sonicated on ice in 500 μl 0.1 N HCl, and centrifuged at 15,000x g for 10 min. The supernatants were transferred to micro centrifuge tubes and frozen at -80ºC. To initiate the analysis, the samples were thawed and centrifuged at 21,000x g for 5 min in an IEC Micromax RF Refrigerated Microfuge (Thermo Fisher Scientivic, Asheville, NC). Two 50 μl aliquots were removed from the supernatant and transferred to 0.8 ml amber glass autosampler vials.
Stock solutions of DA and 2H5-DA, DOPAC and 2H5-DOPAC, and HVA and 2H5-HVA each at a concentration of 2.0 mg/ml, were diluted by serial dilution and standard curves prepared to match the anticipated concentrations of DA, DOPAC and HVA in the striatum. All calibration samples were prepared and analyzed in duplicate.
2H5 Internal Standards (IS) for each of the three analytes were added to each sample. The samples were dried under a stream of N2 in a 96-well format dryer operated at 60°C and further dried in a vacuum (25 mm Hg) at 70°C for 30 min. For derivatization, pentafluoropropionic anhydride (PFPA) (50 μl) and hexafluoroisopropanol (HFIP) (25 μl) were added to each dried sample. The samples were capped and incubated at 65°C for 90 min. The caps were removed and the samples were dried under a stream of N2 in a 96-well format dryer operated at 60 °C. The samples were reconstituted in 25 μl toluene and recapped.
| CONFIDENTIAL 18 |
The reconstituted samples (1 μl) were injected on a 15 M RXi®-5MS capillary column (0.25 mm ID, 0.25 μm film thickness) interfaced to a Thermo TSQ 7000 mass spectrometer. The gas chromatography was performed at a heating rate of 20°C/min from 100 to 200°C with hydrogen (1 ml/min) as the carrier gas. The injector temperature was 250°C. The mass spectrometer was run in single quad mode using chemical ionization with methane reagent gas for negative ions to detect molecular ions and fragments. Co-eluting negative ions were observed at m/z 463 for DOPAC and 468 for the 2H5-DOPAC which correspond to the loss of C2F5CO (Mw=147) from the derivatized precursors. Similarly, negative ions were observed for HVA and 2H5-HVA at m/z 330 and 334, respectively, and for DA and 2H5-DA at m/z of 571 and 576, respectively. Product ion (MS/MS) spectra of each analyte were generated at various collision energies using argon (1 mTorr) as the collision gas. Major product ions were observed at m/z 343 and 347 for DOPAC and 2H5-DOPAC, 163 and 166 for HVA and its 2H5-HVA, and 376 and 380 for DA and 2H5-DA. The intensities of the transitions were optimized to 15 eV by varying the collision energy. Samples were analyzed in selected reaction monitoring mode using time segments for the corresponding precursor/product ion transitions for the IS/analyte pairs.
The concentration of DA in samples was calculated from the equation DA (nmol/g) = PADA/PA2H5-DA x (VH+Wt)/VA x 1000/Wt, whereas PADA = ADC counts for m/z 571, PA2H5-DA = ADC counts for m/z 576, VH = homogenization volume, VA = volume analyzed, Wt = tissue weight. Concentrations for DOPAC and HVA were calculated using the same method but adapted to their specific molecular ion (m/z): DOPAC m/z 463, 2H5-DOPAC m/z 468; HVA m/z 330, 2H5-HVA m/z 334.
2.11 MANF striatal diffusion by convection enhanced delivery (CED)
All animal work was performed in accordance with the United Kingdom (UK) Animal Scientific Procedures Act 1986 and was covered by both project and personal licenses that were issued by the Home Office and these were also reviewed and approved by the University of Bristol ethical committee (project licenses 30/2353 & 30/2902). Adult male Wistar rats (Charles River, Margate, UK, 225 to 275g) were anaesthetised with intraperitoneal ketamine (Ketaset; 60mg/kg, Pfizer Animal Health, Sandwich, UK) and medetomidine (Dormitor; 0.4mg/kg, Pfizer) and then placed in a stereotactic frame (Stoelting, Illinois, USA). A midline skin incision was made from glabella to occiput to expose bregma. Bilateral burr holes were drilled using a 2 mm drill. All convection enhanced delivery (CED) procedures were performed using a custom-made catheter with an outer diameter of 0.22 mm and inner diameter of 0.15 mm, composed of fused silica with a laser cut tip. The cannula was attached to a 1 ml syringe (Hamilton, Bonaduz, Switzerland) connected to a rate-controlled microinfusion pump (World Precision Instruments Inc., Sarasota, FL, USA) and the tip placed at stereotactic co-ordinates derived from the Paxinos and Watson stereotactic rat brain atlas (0.5 mm anterior and 3 mm lateral to bregma, depth 4.5 mm), in order to target the striatum.
| CONFIDENTIAL 19 |
10 μg of MANF or GDNF in a total volume of 2 μl phosphate buffered saline (PBS) or PBS (vehicle) alone, were delivered into the striatum. CED procedures were performed at an infusion rate of 0.1 μl/min, 1.25 μl/min, 2.5 μl/min, or 5.0 μl/min. On completion of CED the cannula was left in situ for 10 min to minimise reflux, then withdrawn at a rate of 1mm/ min. The wound was closed with 4/0 Vicryl, and a dose of intramuscular buprenorphine (Centaur Services, Castle Cary, UK) was administered (30 μg/kg). The anaesthetic was reversed with 0.1 mg/kg i.p. atipamezole hydrochloride (Pfizer) during the recovery procedures. Rats were euthanised by anaesthetic overdose with an intraperitoneal injection of 1 ml pentobarbital (Euthatal; Merial Animal Health, Harlow, UK) at pre-defined time-points following CED (0, 3, 24 hours or 7 days). For immunohistochemical analysis (IHC), animals were transcardially perfused with 4% paraformaldehyde. Brains were removed and placed in 4% paraformaldehyde for 24 h, then cryoprotected in 30% sucrose.
Rat brains were cut into 35 μm thick coronal sections using a Leica CM1850 cryostat (Leica Microsystems, Wetzlar, Germany) at -20 °C. For fluorescent immunohistochemistry, fixed sections were mounted on gelatine-subbed slides. Once dry, the sections were washed with PBS for 5 min x 3. Sections were blocked in PBS plus 0.1% triton-x-100 containing 10% normal donkey serum (Sigma Aldrich, UK) for 1 hour at room temperature (RT). They were then washed with 0.1% triton-x-100 in PBS for 5 min. Following washing, sections were incubated in goat anti-GDNF primary antibody (1:250; R&D Systems, Abingdon, UK) or goat anti-MANF (1:200; R&D Systems, Abingdon, UK) in order to determine the presence and distribution of infused GDNF/ MANF.
The next day, the primary antibody was removed and sections were washed with 0.1% triton-x-100 in PBS for 5 min x 3. Sections were incubated in Donkey Anti-Goat Alexa Fluor® 488 (1:300, Life Technologies, Paisley, UK) at RT for 2 hours in the dark and then washed with PBS for 5 min x 3. Sections were mounted in FluorsaveTM Reagent (Calbiochem®, Merck Millipore, Billerica, MA, USA) before viewing. Images were captured using the Stereo Investigator platform (MicroBrightField Bioscience, Williston, VT, USA) with a Leica DM5500 microscope (Leica Microsystems, Germany) and digital camera (Microbrightfield Bioscience, Williston, VT, USA).
Fluorescent imaging was undertaken using a Leica DM5500 microscope (Leica Microsystems) and digital camera (Leica Microsystems). The volume of distribution of MANF or GDNF recombinant proteins was calculated by tracing contours around the outer margins of the visualised protein using ImageJ software at 2-12 section intervals. Infusions that were associated with obvious reflux of protein into the white matter were excluded from further analysis.
This section of the study was performed at the University of Bristol in the laboratory of Prof. Steven Gill.
| CONFIDENTIAL 20 |
2.12 Statistical analyses
The amphetamine-induced rotations data of the Phase 1 neuroprotection, Phase 2 neuroprotection and Phase 2 neuroregeneration protocols were analyzed by repeated measures two-way ANOVA. The Phase 1 neuroregeneration data was analyzed with simple two-way ANOVA. The TH+ neuron cell counts in the substantia nigra (Phase 1), the substantia nigra TH+ cell counts determined by stereology (Phase 2), the density of dopaminergic terminals determined by striatal densitometry (Phase 2) and striatal dopamine (Phases 1 and 2) and dopamine metabolites levels (Phase 2) data were analyzed using one-way ANOVA.
If the ANOVA resulted in a P value less than 0.05, a post-hoc analysis was performed with the Fisher’s Least Significant Difference (LSD) test to assess differences between treatment groups (one-way ANOVA, two-way ANOVA) or between different time points within a treatment group (two-way ANOVA). No adjustments for multiple comparisons were made. Significant differences were defined as P<0.05. Trends (P<0.1) of treatment effect differences were detected and are indicated as such in the figures.
All statistical analyses were performed with Prism Version 6 software.
| CONFIDENTIAL 21 |
| 3 | Results |
| 3.1 | Overall study design |
The in vivo activities of MANF and GDNF were evaluated in rats in the 6-OHDA model of PD. The study consisted of two phases with administration of the growth factors to the striatum (Phase 1) or the substantia nigra (Phase 2), respectively. Both phases included a neuroprotection and a neuroregeneration protocol in which the growth factors were administered shortly before (6h) or weeks after the 6-OHDA administration, respectively (Figure 2).
This temporal and spatial variation of growth factor administration was paired with a comprehensive test battery of behavioral, biochemical and morphological assessments (Figure 3).


Figure 2: Study design. Striatal 6-OHDA administration at time = 0. Unit of time is weeks. Single administration of growth factors at the indicated time-points. In the neuroprotection protocols the growth factors were administered 6h pre-6-OHDA. Behavioral testing in amphetamine-induced rotations at the indicated time-points. Tissue collections for biochemical and cell biological analyses immediately after the last behavioral test.
| CONFIDENTIAL 22 |
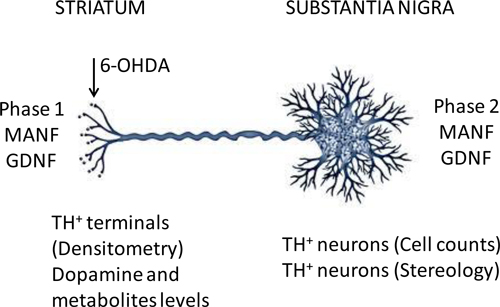
Figure 3: Biochemical and cell biological analyses. The 6-OHDA and growth factor administration schedules were as described in Figure 2. Phase 1 tested MANF and GDNF activities after administration to the striatum. Phase 2 tested MANF and GDNF activities after administration to the substantia nigra. Phase 1 analyses included counts of TH+ neurons in the substantia nigra and measurement of dopamine levels in the striatum. Phase 2 analyses included densitometry of TH+ staining in the striatum, levels of dopamine and metabolites in the striatum and determination of the number of dopaminergic neurons in the substantia nigra by stereology.
| 3.2 | Phase 1: Striatal administration of growth factors |
The experimental design of this Phase 1 of the study is described in the previous section (Figures 2 and 3). In brief, 6-OHDA lesions were introduced by intrastriatal administration of the toxin. This Phase 1 consisted of a neuroprotection and a neuroregeneration protocol in which the growth factors (i.e., MANF or GDNF) were administered by a single injection to the striatum either 6h prior to (Neuroprotection) or 4 weeks after (Neuroregeneration) of the 6-OHDA. The effects of growth factor treatment on behavior were investigated in the amphetamine-induced rotations test at weeks 2, 4 and 8 after growth factor administration. These behavioral assessments were complemented by counting dopaminergic neurons (i.e., TH+ neurons) in the substantia nigra and measuring levels of dopamine in the striatum. The former is a measure of surviving dopaminergic cell bodies while the latter provides for a measure of functionality of dopaminergic terminals in the striatum.
| CONFIDENTIAL 23 |
This Phase 1 of the study included five treatment groups (i.e., 6-OHDA/vehicle, 6-OHDA/MANF 3 μg, 6-OHDA/MANF 10 μg, 6-OHDA/MANF 36 μg, 6-OHDA/GDNF 10 μg) with a planned inclusion of 12 animals per group with a total number of animals of 60.
3.2.1 Neuroprotection protocol
The amphetamine-induced rotations test was performed on weeks 2, 4 and 8 after the administration of 6-OHDA and growth factors (Figure 2A). Net ipsilateral rotations were recorded over a 2 hours period and data was analyzed by 2-way ANOVA followed by Fisher’s test post hoc. A preliminary analysis of the data revealed that four animals displayed ipsilateral rotations with more than 2 standard deviations from the mean of all animals. These animals, one each in the 6-OHDA/veh, 6-OHDA/MANF 3μg, 6-OHDA/MANF 36μg and 6-OHDA/GDNF 10μg, were removed from the further behavioral analysis and the final animal count for this experiment is listed in Figure 4.

Figure 4: Amphetamine-induced rotational behavior. Net ipsilateral rotations (mean ± SEM) during a 2h observation period. Treatment groups as indicated (Vehicle (N=11), MANF 3μg (N=11), MANF 10μg (N=12), MANF 36μg (N=10), GDNF 10μg (N=11)). Time points as indicated (2 weeks, 4 weeks and 8 weeks post 6-OHDA / MANF / GDNF). Statistical analysis with repeated measures two-way ANOVA followed by Fisher’s LSD.
A) Amphetamine-induced rotations by time-point (week 2, week 4, week 8); (**) P<0.01, (*) P<0.05, (x) P<0.1 (trend) compared to vehicle. B) Amphetamine-induced rotations by treatment group for each time-point. (#) P<0.05 compared to prior time-point.
At week 2 after administration of 6-OHDA and growth factors, a significant reduction in ipsilateral net rotations compared to vehicle treatment was observed for the MANF 3μg and GDNF 10 μg treatment groups (Figure 4A). This normalization of the rotational behavior was sustained at week 4 in the GDNF and MANF 3 μg treatment groups and occurred also in the MANF 10 μg group. The MANF 36 μg group also improved at this time point compared to vehicle but this difference did not reach statistical significance. No significant differences between treatment groups were observed at week 8. This might be due to the fact that the vehicle group displayed substantial recovery at this time point as opposed to weeks 2 and 4 when there was still a significant behavioral impairment observed in the vehicle group. This is further substantiated by the fact that the vehicle group displayed a statistically significant improvement at week 8 compared to week 4 (Figure 4B).
| CONFIDENTIAL 24 |
MANF has been tested in an almost identically designed protocol (Voutilainen et al., 2009) and a significant improvement compared to vehicle was demonstrated at weeks 2 and 4 after the 6-OHDA lesion. The strongest response to treatment was seen with the MANF 10 μg dose level. Moreover, GDNF has been tested in similar protocols (Kirik et al., 2000; Lindholm et al., 2007) and a reduction in net ipsilateral rotations was observed at weeks 2 and 4 (Lindholm et al., 2007) and week 6 (Kirik et al., 2000) post-6-OHDA. Hence, this current study yielded results for MANF and GDNF that resemble closely the effects reported in the literature.
Having observed significant treatment effects with MANF and GDNF in the behavioral assessment, an investigation of the underlying cellular effects may lead to an understanding of how these growth factors mediate their neuroprotective activity. To this end, the TH+ cells were counted in the substantia nigra at week 8 after administration of 6-OHDA and compared between the different treatment groups (Figure 5). The TH+ cell counts were analyzed for the ipsilateral (Figure 5A) and contralateral (Figure 5B) sides of the lesion, and ratios between ipsilateral and contralateral sides (Figure 5C) were calculated. The treatment with 6-OHDA led to a reduction of TH+ cell counts to about 55% on the ipsalateral side compared to the contralateral side, which is in very good agreement with similarly designed studies (30%, Kirik et al., 2000; 65%, Voutilainen et al. 2009; 65% Lindholm et al., 2007).

Figure 5: Dopaminergic neurons in the substantia nigra. Number of TH+ neurons per field (mean ± SEM). Treatment groups as indicated (Vehicle (N=5), MANF 3μg (N=5), MANF 10μg (N=5), MANF 36μg (N=6), GDNF 10μg (N=6)). 1-wayANOVA followed by Fisher’s LSD. (*) P<0.05, (**) P<0.01 compared to vehicle; (o) P<0.05 compared to GDNF 10μg.
(A) Ipsilateral TH+ neurons, (B) Contralateral TH+ neurons, (C) Ratio ipsilateral / contralateral
| CONFIDENTIAL 25 |
On the ipsilateral side, a significant increase in TH+ cells was observed in the MANF 3 μg and 10 μg treatment groups compared to 6-OHDA/vehicle suggesting that growth factor treatment in the striatum protected a significant proportion of dopaminergic neurons (Figure 5A). These effects were not observed on the contralateral side as none of the treatment groups was significantly different from 6-OHDA/vehicle (Figure 5B). The ratios between ipsilateral and contralateral TH+ counts were significantly increased compared to 6-OHDA/vehicle in the MANF 3 μg and GDNF 10 μg groups. The MANF 3 μg effect was solely dependent on the increased TH+ counts on the ipsilateral side as the contralateral TH+ counts were almost identical between MANF 3 μg and 6-OHDA/vehicle.
The neuroprotective effect on TH+ cells in the substantia nigra was demonstrated in a similarly designed study (Voutilainen et al., 2009) and a similar degree of protection by MANF was observed as in this present study. However, the most active dose was MANF 10 μg while in this present study a significant neuroprotective effect with MANF treatment was observed already at the 3 μg dose level. GDNF neuroprotective effects on TH+ cells in the substantia nigra after administration to the striatum in a neuroprotection protocol have been demonstrated in several studies (Kirik et al., 2000; Lindholm et al., 2007; Voutilainen et al., 2009) and are in good agreement with observations in this present study.
In order to assess the functionality of dopaminergic neurons and in particular their axonal projections to the striatum, levels of dopamine in the striatum were measured on the ipsilateral and contralateral sides. It is apparent that 6-OHDA leads to a strong reduction of dopamine levels on the ipsilateral sides compared to the contralateral sides in vehicle treated animals (Figure 6A and B). Treatment with MANF or GDNF did not result in any differences compared to vehicle in striatal dopamine levels on the ipsilateral or contralateral sides.

| CONFIDENTIAL 26 |
Figure 6: Striatal dopamine (DA) levels. Amounts of dopamine shown as nmol/g wet tissue weight (mean ± SD). Treatment groups as indicated. Statistical analysis by 1-way ANOVA. None of the treatment groups were different from 6-OHDA/vehicle. (A) Ipsilateral, (B) Contralateral.
The absolute amounts of dopamine detected in the striatum are similar to levels reported in the literature (Kearns et al., 1997) in a similarly designed study. Therefore, the methodology to detect and quantify dopamine employed in this study yielded reliable results similar to the ones of an independently conducted study. The effects of MANF on the integrity of striatal projections were studied in a similarly designed study (Voutilainen et al., 2009). MANF at the 10 μg dose level protected about 70% of ipsilateral TH+ fibers but dopamine levels were not measured in that study. Conversely, this present study did not investigate the integrity of TH+ fibers and thus a comparison of MANF effects between these studies is difficult. GDNF administration to the striatum in a neuroprotection protocol has shown mixed results on striatal TH+ fiber density with two studies showing significant protection (Kirik et al., 2000; Lindholm et al., 2007) and another study showing no protection (Voutilainen et al., 2009).
3.2.2 Neuroregeneration protocol
In this neuroregeneration protocol, MANF or GDNF were administered by a single striatal injection 4 weeks after the unilateral 6-OHDA lesion. This design allowed for an amphetamine-induced rotations test prior to MANF or GDNF administration at week 3 post 6-OHDA to identify and exclude animals that did not display the expected rotational behavior. This procedure led to the exclusion of 6 animals in the 6-OHDA/vehicle, 3 animals in the 6-OHDA/MANF 3 μg, 2 animals in the 6-OHDA/MANF 36 μg and 2 animals in the 6-OHDA/GDNF 10 μg groups.
The amphetamine-induced rotations test for assessments of treatment effects was performed on weeks 2, 4 and 8 after the administration of growth factors (Figure 2) (i.e., weeks 6, 8 and 12 after administration of 6-OHDA). Net ipsilateral rotations were recorded over a 2 hours period and data was analyzed by 2-way ANOVA followed by Fisher’s LSD test post hoc (Figure 7).
| CONFIDENTIAL 27 |

Figure 7: Amphetamine-induced rotational behavior. Net ipsilateral rotations (mean ± SEM). Treatment groups as indicated (Vehicle (N=5), MANF 3μg (N=7), MANF 10μg (N=11), MANF 36μg (N=9), GDNF 10μg (N=9)). Time points as indicated (1 week pre, 2 weeks, 4 weeks and 8 weeks post MANF / GDNF administration). Statistical analysis with two-way ANOVA followed by Fisher’s LSD.
A) Amphetamine-induced rotations by time-point (week -1, week 2, week 4, week 8); None of the treatment groups was statistically different from vehicle treatment at any time point. (x) P<0.1 (trend) versus GDNF 10 μg. B) Amphetamine-induced rotations by treatment group for each time-point. (#) P<0.05, (+) P<0.1 (trend) compared to prior time-point (within treatment group comparisons).
At week -1 relative to growth factor treatment all groups displayed similar rotational behavior indicating that the treatment groups were well balanced prior to the initiation of growth factor treatment (Figure 7A). At week 2 after growth factor or vehicle treatment, all groups, including the vehicle group, displayed equally improved ipsilateral rotational behavior. At week 4, a further improvement was observed in the vehicle treated group but the MANF 3 μg and 10 μg groups tended to perform better than vehicle even though this difference did not reach statistical significance. Finally, at week 8, a further improvement in the vehicle group and a sustained normalization of the rotational behavior was observed with MANF 3 μg. None of the comparisons between treatment groups were statistically significant. A time-dependent improvement in all treatment groups, including vehicle, was apparent (Figure 7B).
Striatal administration of MANF and GDNF within a neuroregeneration protocol has been reported previously (Rosenblad et al., 1998; Lindholm et al., 2007; Voutilainen et al. 2009). Spontaneous recovery in the vehicle treated group was negligible up to 10 weeks post 6-OHDA in two independent studies (Lindholm et al., 2007; Voutilainen et al., 2009) facilitating the detection of growth factor therapeutic effects. MANF treatment with a 10 μg single dose led to a time-dependent improvement of ipislateral rotations reaching statistical significance versus vehicle in cumulative rotations over a 12 week observation period (Voutilainen et al., 2009).
| CONFIDENTIAL 28 |
Similarly, GDNF decreased amphetamine-induced ipsilateral rotations compared to vehicle over a 12 week observation period (Voutilainen et al., 2009; Lindholm et al., 2007). The lack of a significant treatment effect by MANF or GDNF in the neuroregeneration protocol of this present study may be due to the comparatively rapid and almost complete spontaneous recovery of the vehicle treated animals.
In order to assess the potential of MANF and GDNF to restore the TH+ phenotype of neurons in the substantia nigra, the number of nigral TH+ neurons was quantified for all treatment groups for the ipsilateral and contralateral sides (Figure 8A and B) and ratios of ipsilateral to contralateral TH+ counts were calculated (Figure 8C). The number of TH+ neurons of vehicle treated animals on the ipsilateral side was reduced to about 60% of the number counted on the contralateral side. This extent of nigral cell death is in agreement with the results obtained in the neuroprotection protocol of this study (Figure 5) and with values reported in the literature (Kirik et al., 2000; Voutilainen et al. 2009; Lindholm et al., 2007). None of the MANF treatment groups on the ipsilateral or the contralateral sides was significantly different from the vehicle treated group and thus MANF did not show a regenerative effect on nigral neurons. This result is in agreement with observations in a similarly designed study (Voutilainen et al., 2009) in which only a modest non-significant effect was shown for MANF 10 μg at 12 weeks post 6-OHDA. GDNF treatment significantly increased the TH+ counts on both the ipsilateral and the contralateral sides compared to 6-OHDA/vehicle (Figure 8A and B). However, when the ratios of ipsilateral and contralateral TH+ counts were compared between GDNF and 6-OHDA/vehicle treatments no statistically significant difference was observed (Figure 8C). TH+ protective effects induced by GDNF in neuroregeneration protocols were reported in similarly designed studies (Rosenblad et al., 1998; Lindholm et al., 2007). The effects on TH+ phenotype restoration by MANF and GDNF are thus similar in this present study and in the literature.

| CONFIDENTIAL 29 |
Figure 8: Dopaminergic neurons in the substantia nigra. Number of TH+ neurons per field (mean ± SEM). Treatment groups as indicated (Vehicle (N=7), MANF 3μg (N=4), MANF 10μg (N=4), MANF 36μg (N=4), GDNF 10μg (N=4)). 1-wayANOVA followed by Fisher’s LSD. (*) P<0.05 compared to vehicle; (o) P<0.05 compared to GDNF 10 μg.
(A) Ipsilateral TH+ neurons, (B) Contralateral TH+ neurons, (C) Ratio ipsilateral / contralateral
In order to assess the functionality of dopamineric axonal terminals in the striatum, the levels of dopamine were determined in the ipsilateral (Figure 9A) and contralateral (Figure 9B) striata for each of the treatment groups. In the vehicle group, the ipsilateral dopamine level was strongly reduced compared to the contralateral side, confirming the maintenance of a functional lesion present at the end of the evaluation period (i.e., 12 weeks after 6-OHDA treatment). Treatment with MANF or GDNF did not lead to any difference in dopamine levels compared to vehicle treated animals on the ipsilateral or the contralateral sides. Hence, none of the growth factor treatment regimens restored dopamine levels at the end of the observation period. This is remarkable in view of the complete functional recovery observed in the rotational behavior with several of the treatment groups (i.e., vehicle, MANF 3 μg, MANF 10 μg, MANF 36 μg).

Figure 9: Striatal dopamine (DA) levels. Amounts of dopamine shown as nmol/g wet tissue weight (mean ± SEM). Treatment groups as indicated (6-OHDA / Vehicle (N=4, ipsi; N=3, contra), 6-OHDA / MANF 3μg (N=3, ipsi; N=4, contra), 6-OHDA / MANF 10μg (N=4, ipsi; N=4, contra), 6-OHDA / MANF 36μg (N=5, ipsi; N=4, contra ), 6-OHDA / GDNF 10μg (N=4, ipsi; N=6, contra)). 1-way ANOVA followed by Fisher’s LSD. None of the treatment groups were different from 6-OHDA/veh. (A) Ipsilateral, (B) Contralateral, (C) Ratio ipsilateral / contralateral.
| CONFIDENTIAL 30 |
| 3.3 | Phase 2: Nigral administration of growth factors |
The experimental design of this Phase 2 of the study is described in the previous section (Figures 2 and 3). In brief, 6-OHDA lesions were introduced by intrastriatal administration of the toxin. This Phase 2 consisted of a neuroprotection and a neuroregeneration protocol in which the therapeutic growth factors (i.e., MANF or GDNF) were administered by a single injection to the substantia nigra either 6h prior to (Neuroprotection) or 2 weeks after (Neuroregeneration) the 6-OHDA injection. The effects of growth factor treatment on behavior were investigated in the amphetamine-induced rotations test at weeks 2 and 4 after growth factor administration. These behavioral assessments were complemented by counting dopaminergic neurons (i.e., TH+ neurons) in the substantia nigra using computer assisted stereology, quantification of striatal dopaminergic terminals by densitometry and measuring levels of dopamine and its metabolites DOPAC and HVA in the striatum. The stereology provides for a measure of surviving dopaminergic cell bodies in the substantia nigra while the densitometry and dopamine level quantification provides for a measure of functional and structural improvement, respectively, of dopaminergic terminals in the striatum.
This Phase 2 of the study included six treatment groups (i.e., Vehicle/vehicle, 6-OHDA/vehicle, 6-OHDA/MANF 3 μg, 6-OHDA/MANF 10 μg, 6-OHDA/MANF 36 μg, 6-OHDA/GDNF 10 μg) with a planned inclusion of 12 animals per group with a total number of animals of 60.
3.3.1 Neuroprotection protocol
The amphetamine-induced rotations test was performed on weeks 2 and 4 after the administration of 6-OHDA and growth factors (Figure 2). Net ipsilateral rotations were recorded over a 2 hours period and data was analyzed by repeated measures 2-way ANOVA followed by Fisher’s test post hoc. Incomplete data collection over the 2 hours period at the week 2 time point caused the exclusion of animals in the vehicle/vehicle (4 animals), 6-OHDA/vehicle (4 animals), 6-OHDA/MANF 3μg (2 animals), 6-OHDA/MANF 10 μg (4 animals), 6-OHDA/MANF 36 μg (4 animals) and GDNF 10 μg (2 animals) groups. The numbers of animals assessed, analyzed and included in the statistical analysis of the rotational behavior data is shown in Figure 10.
| CONFIDENTIAL 31 |

Figure 10: Amphetamine-induced rotational behavior. Net ipsilateral rotations (mean ± SEM). Treatment groups as indicated (Vehicle / vehicle (N=8), 6-OHDA / veh (N=8), 6-OHDA / MANF 3μg (N=10), 6-OHDA / MANF 10μg (N=8), 6-OHDA / MANF 36μg (N=8), 6-OHDA / GDNF 10μg (N=10)). Time points as indicated (2 weeks and 4 weeks post MANF / GDNF administration). Statistical analysis with repeated measures two-way ANOVA followed by Fisher’s LSD.
A) Amphetamine-induced rotations by time-point (week 2, week 4); (*) P<0.05, (x) P<0.1 (trend) compared to 6-OHDA/vehicle; (o) P<0.05 compared to GDNF 10μg. B) Amphetamine-induced rotations by treatment group for each time-point. None of the within treatment groups differences were statistically significant.
At the week 2 time point, the vehicle/vehicle and 6-OHDA/vehicle values were separated but this difference was not statistically significant (Figure 10A). This was most likely caused by the relatively low number of net ipsilateral rotations observed in the 6-OHDA/vehicle group. Due to the nature of the neuroprotection protocol in which 6-OHDA and the growth factors are administered at almost the same time it is not possible to know whether these low numbers were due to an ineffective lesion, a rapid recovery, or both. However, at this time point, MANF 10 μg displayed an increase in rotational behavior compared to 6-OHDA/vehicle. While the net ipsilateral rotations in the 6-OHDA/vehicle group decreased from week 2 to week 4, the values for MANF 10 μg and GDNF 10 μg remained elevated. The GDNF 10 μg values were significantly higher compared to 6-OHDA/vehicle while the values for MANF 10 μg displayed a trend towards a difference to 6-OHDA/vehicle. Since MANF has not been administered to the substantia nigra in any of the previous studies there is no literature comparison available. GDNF has been administered to the substantia nigra in several 6-OHDA studies in a neuroprotection design (Sauer et al., 1995; Winkler et al., 1996; Kearns et al., 1997; Kirik et al., 2000). Winkler et al. did not observe an effect on net ipsilateral rotations 5 months after GDNF treatment. In contrast, Kirik et al. reported an increase in net ipsialateral rotations at week 6 after GDNF was administered to the substantia nigra, similar to the effect observed in this study. Hence, this study and the literature provide evidence that GDNF and MANF increase net ipsilateral rotations in the 6-OHDA model when administered to the substantia nigra in a neuroprotection protocol.
| CONFIDENTIAL 32 |
In this Phase 2 of the study, the number of TH+ neurons in the substantia nigra was quantified by stereology. The numbers of TH+ neurons were determined from nigral sections and a computational model to reconstruct neurons in three-dimensional space for the ispilateral and contralateral sides of animals from all treatment groups (Figure 11A and B). The ipsilateral to contralateral ratios were calculated at the individual animal level (Figure 11C). There was no difference between the vehicle/vehicle and 6-OHDA/vehicle groups on the ipsilateral and the contralateral sides while the ratios were separated slightly but non-significantly. Inspection of the underlying raw data revealed a substantial variability in the computed cell counts with six instances of ipsilateral values substantially higher than the corresponding contralateral cell numbers. Moreover, due to technical difficulties, 13 of the 33 animals with ipsilateral stereology data did not have corresponding contralateral data, leaving the MANF 36 μg group with just one data point. Given these experimental uncertainties, no firm conclusions on the effect of growth factor treatments on protecting TH+ cells in the substantia nigra can be drawn.
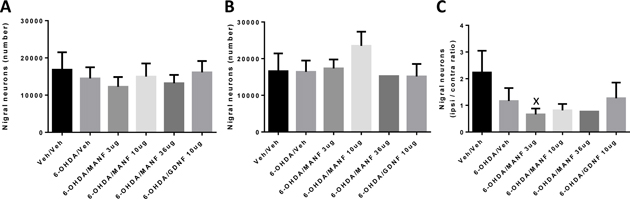
Figure 11: Dopaminergic neurons in the substantia nigra determined by stereology. Computed number of nigral TH+ neurons (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Vehicle / vehicle (N=5, ipsi, contra), 6-OHDA / vehicle (N=6, ipsi; N=4, contra), 6-OHDA / MANF 3μg (N=6, ipsi; N=4, contra), 6-OHDA / MANF 10μg (N=5, ipsi; N=4, contra), 6-OHDA / MANF 36μg (N=5, ipsi; N=1, contra ), 6-OHDA / GDNF 10μg (N=5, ipsi; N=3, contra)). 1-way ANOVA followed by Fisher’s LSD. None of the treatment groups were different from 6-OHDA/vehicle. (x) P<0.05 compared to vehicle/vehicle. (A) Ipsilateral, (B) Contralateral, (C) Ratio ipsilateral / contralateral
There are several studies in which GDNF was administered to the substantia nigra in a 6-OHDA model and in which survival of nigral TH+ cells was quantified. Generally, GDNF protected TH+ cells to a significant extent, ranging from 60% protection (Kirik et al., 2000) to complete protection (Sauer et al., 1995; Kearns et al., 1997). However, TH+ cells seem to be protected but remained in an atrophic state (Winkler et al., 1996) thereby preventing a substantial functional recovery.
| CONFIDENTIAL 33 |
In order to assess whether the dopaminergic axonal terminals of TH+ neurons were affected by 6-OHDA and prior treatment with growth factors, densitometry measurements of the ispilateral and contralateral striata of animals were performed at the end of the observation period (Figure 12). The numbers of dopaminergic (i.e., TH+) terminals in the striatum were significantly different between the vehicle/vehicle and 6-OHDA/vehicle groups on the ipsilateral side for the entire striatum (Figure 12A) and for the dorsal (Figure 12D) and ventral (Figure 12G) striata. Hence, as expected, 6-OHDA treatment significantly reduced the number of dopaminergic terminals in the striatum. However, there were subtle differences between the striatal compartments in that the ventral striatum (Figures 12G and I) was more resistant to 6-OHDA than the dorsal striatum (Figures 12D and F). Most importantly, treatment with MANF 36 μg almost completely restored the dopaminergic terminal densities on the ipsilateral side with significant differences to both 6-OHDA/vehicle and GDNF 10 μg treatments in the entire striatum (Figure 12A) and the dorsal (Figure 12D) and ventral (Figure 12G) striata. There was a MANF dose-dependent increase of dopaminergic terminals on the contralateral side (Figure 12B) both in the dorsal (Figure 12E) and ventral (Figure 12H) striata, which however did not reach statistical significance. The calculated ratios between the ipsilateral and contralateral densities yielded a significant difference between MANF 36 μg and GDNF 10 μg (Figures 12C, F and I). An analysis of further striatal spatial subgroups (Figure 13A-I; temporal, medial and basal striatum) revealed essentially the same MANF treatment effects as for the global data (Figure 12A, B and C). MANF 36 μg protected dopaminergic terminals significantly compared to 6-OHDA/vehicle and 6-OHDA/ GDNF 10 μg treatment in all three striatal compartments. However, the response to 6-OHDA differed between the temporal, medial and basal striata in that the temporal striatum displayed a marked degree of resistance to 6-OHDA-induced toxicity.
Densitometry quantifies the amount of TH+ immunoreactivity in the striata but does not yield information on the level of enzymatic activity present in the tissue. Further studies will investigate the functional consequences of MANF-induced increases of striatal TH+ immunoreactivity.
| CONFIDENTIAL 34 |

Figure 12: Dopaminergic terminals in the global, dorsal and ventral striatum determined by densitometry. Density of terminals (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Veh/veh (N=5 global, N=6 dorsal, ventral), 6-OHDA/veh (N=6), 6-OHDA/MANF 3μg (N=6), 6-OHDA/MANF 10μg (N=6 global, N=5 dorsal, ventral), 6-OHDA/MANF 36μg (N=5), 6-OHDA/GDNF 10μg (N=6)). 1-way ANOVA followed by Fisher’s LSD. (**) P<0.01, (*) P<0.05 compared to 6-OHDA/vehicle; (o) P<0.05 compared to 6-OHDA/GDNF 10 μg.
Global striatum, (A) ipsilateral, (B) contralateral, (C) ratio ipsilateral / contralateral;
Dorsal striatum, (D) ipsilateral, (E) contralateral, (F) ratio ipsilateral / contralateral;
Ventral striatum, (G) ipsilateral, (H) contralateral, (I) ratio ipsilateral / contralateral.
| CONFIDENTIAL 35 |

Figure 13: Dopaminergic terminals in the temporal, medial and basal striatum determined by densitometry. Density of terminals (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Veh/veh (N=6), 6-OHDA/veh (N=6), 6-OHDA/MANF 3μg (N=6), 6-OHDA/MANF 10μg (N=5), 6-OHDA/MANF 36μg (N=5), 6-OHDA/GDNF 10μg (N=6)). 1-way ANOVA followed by Fisher’s LSD. (***) P<0.001, (*) P<0.05, (x) P<0.1 (trend) compared to 6-OHDA/vehicle; (o) P<0.05 compared to 6-OHDA/GDNF 10 μg.
Temporal striatum, (A) ipsilateral, (B) contralateral, (C) ratio ipsilateral / contralateral;
Medial striatum, (D) ipsilateral, (E) contralateral, (F) ratio ipsilateral / contralateral;
Basal striatum, (G) ipsilateral, (H) contralateral, (I) ratio ipsilateral / contralateral.
| CONFIDENTIAL 36 |
Nigral administration of GDNF did not increase the density of dopaminergic terminals in the striatum (Figures 12 and 13). Several previous studies have investigated the effect of nigral administration of GDNF on striatal terminals in a 6-OHDA model. No effect on striatal fiber density was observed after GDNF treatment in a neuroprotection protocol (Sauer et al., 1995) and no effects on TH+ terminals by densitometry was observed (Winkler et al., 1996; Kirik et al., 2000). Moreover, there was no indication of sprouting in the striatum (Winkler et al., 1996). This current study is thus in agreement with published accounts of a lack of effect on striatal dopaminergic terminals by nigral administration of GDNF. Conversely, MANF significantly increased the dopaminergic (TH+) terminal density when administered to the substantia nigra.
Given the significant protective effect on striatal dopaminergic terminals by MANF treatment the determination of dopamine levels in the striatum would provide information on the functionality of these terminals. Therefore, levels of dopamine were determined in the ipsilateral (Figure 14A) and contralateral (Figure 14B) striata for each of the treatment groups. There was a significant difference between the vehicle/vehicle and 6-OHDA/vehicle groups on the ipsilateral side and the ipsilateral / contralateral ratios (Figure 14C), but not for the contralateral side. Treatment with MANF 36 μg or any other growth factor treatment, however, did not restore the reduced dopamine levels observed in the 6-OHDA/vehicle group. The MANF 36 μg treatment group displayed a significantly increased dopamine level compared to the GDNF 10 μg treatment in the ipsilateral, contralateral and ratio comparisons. Hence, none of the growth factor treatment regimens restored dopamine levels at the end of the observation period. This is remarkable in view of the significant protection of dopaminergic terminals by MANF 36 μg. There appears to be a dissociation between the protection of the TH+ phenotype in the striatal terminals and the dopamine levels in the striatum.

Figure 14: Striatal dopamine (DA) levels. Amounts of dopamine shown as nmol/g wet tissue weight (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Vehicle / vehicle (N=5, ipsi, contra), 6-OHDA / Vehicle (N=5 ipsi, contra), 6-OHDA / MANF 3μg (N=5, ipsi, contra), 6-OHDA / MANF 10μg (N=5, ipsi, contra), 6-OHDA / MANF 36μg (N=4, ipsi, contra ), 6-OHDA / GDNF 10μg (N=6, ipsi, contra)). 1-way ANOVA followed by Fisher’s LSD. (*) P<0.05, (x) P<0.1 (trend) compared to 6-OHDA / vehicle; (o) P<0.05 compared to 6-OHDA/GDNF 10 μg.
(A) Ipsilateral, (B) Contralateral, (C) Ratio ipsilateral / contralateral
| CONFIDENTIAL 37 |
GDNF administration to the substantia nigra and its effects on dopamine levels in the substantia nigra and the striatum were investigated in a 6-OHDA model (Kearns et al., 1997). Administration of 6-OHDA to the striatum led to a significant decrease of dopamine levels on the ipsilateral side in both the striatum and the substantia nigra which was almost completely prevented by prior administration of GDNF to the substantia nigra.
Dopamine is metabolized to DOPAC by MAO and then to HVA by COMT. An alternative metabolic pathway employs COMT first, yielding 3-methoxytyramine (3-MT) followed by oxidation to HVA by MAO. In this study, striatal levels of DOPAC (Figure 15) and HVA (Figure 16) were determined for each of the six treatment groups for the ipsilateral and the contralateral sides relative to the 6-OHDA lesion.
DOPAC levels were significantly different between the vehicle/vehicle and 6-OHDA/vehicle groups on the ipsilateral but not on the contralateral side, and for the ipsilateral/contralateral ratio. Neither MANF 3 μg nor MANF 36 μg were different from 6-OHDA/vehicle but MANF 10 μg and GDNF 10 μg had their DOPAC levels significantly reduced. Similar differences were also observed for the ipsilateral/contralateral ratios. The DOPAC levels largely paralleled the effects seen across the treatment groups for the dopamine levels. For both outcomes, the vehicle/vehicle and 6-OHDA/vehicle groups are significantly different, the MANF 10 μg and GDNF 10 μg differ from the 6-OHDA/vehicle group and the MANF 36 μg differs from the GDNF 10 μg group.
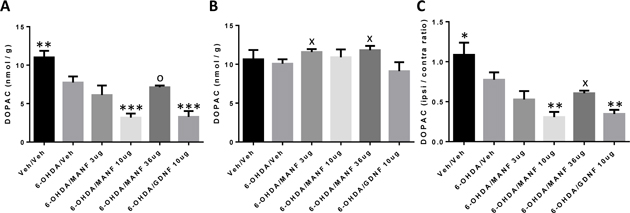
| CONFIDENTIAL 38 |
Figure 15: Striatal DOPAC levels. Amounts of DOPAC shown as nmol/g wet tissue weight (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Vehicle / vehicle (N=5, ipsi, contra), 6-OHDA / Vehicle (N=5 ipsi, contra), 6-OHDA / MANF 3μg (N=5, ipsi, contra), 6-OHDA / MANF 10μg (N=5, ipsi, contra), 6-OHDA / MANF 36μg (N=4, ipsi, contra ), 6-OHDA / GDNF 10μg (N=6, ipsi, contra)). 1-way ANOVA followed by Fisher’s LSD. (*) P<0.05, (**) P<0.01 and (***) P<0.001 compared to 6-OHDA/vehicle; (o) P<0.05, (x) P<0.1 (trend) compared to 6-OHDA/GDNF 10 μg. (A) Ipsilateral, (B) Contralateral, (C) Ratio ipsilateral / contralateral.
HVA levels were significantly different between the vehicle/vehicle and 6-OHDA/vehicle groups on the ipsilateral (Figure 16A) but not on the contralateral side (Figure 16B), and for the ipsilateral/contralateral ratio (Figure 16C). MANF 3 μg, MANF 10 μg and GDNF 10 μg had their HVA levels significantly reduced compared to the 6-OHDA/vehicle group. These differences were also observed for the ipsilateral/contralateral ratios but in addition, the MANF 36 μg level was also lower than 6-OHDA/vehicle. Again, the HVA levels largely parallel the effects seen across the treatment groups for the DOPAC and dopamine levels. For all outcomes, the vehicle/vehicle and 6-OHDA/vehicle groups are significantly different, the MANF 10 μg and GDNF 10 μg differ from the 6-OHDA/vehicle group and the MANF 36 μg differs from the GDNF 10 μg group.

Figure 16: Striatal HVA levels. Amounts of HVA shown as nmol/g wet tissue weight (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Vehicle / vehicle (N=5, ipsi, contra), 6-OHDA / Vehicle (N=5 ipsi, contra), 6-OHDA / MANF 3μg (N=5, ipsi, contra), 6-OHDA / MANF 10μg (N=5, ipsi, contra), 6-OHDA / MANF 36μg (N=4, ipsi, contra ), 6-OHDA / GDNF 10μg (N=6, ipsi, contra)). 1-way ANOVA followed by Fisher’s LSD.(*) P<0.05, (**) P<0.01 and (***) P<0.001 compared to 6-OHDA/vehicle; (o) P<0.05 , (+) P<0.1 (trend) compared to 6-OHDA/GDNF 10 μg.
(A) Ipsilateral, (B) Contralateral, (C) Ratio ipsilateral / contralateral.
| CONFIDENTIAL 39 |
3.3.2 Neuroregeneration protocol
In this neuroregeneration protocol, MANF or GDNF were administered by a single injection into the substantia nigra 2 weeks after the unilateral 6-OHDA lesion to the striatum. This design allowed for an amphetamine-induced rotations test prior to MANF or GDNF administration at week 1 post 6-OHDA to identify and exclude animals that did not display the expected rotational behavior. This procedure led to the exclusion of 1 animal in the vehicle/vehicle, 2 animals in the 6-OHDA/vehicle, 2 animals in the 6-OHDA/MANF 10 μg, 2 animals in the 6-OHDA/MANF 36 μg and 1 animal in the 6-OHDA/GDNF 10 μg groups.
The amphetamine-induced rotations test for assessments of treatment effects was performed on weeks 2 and 4 after the administration of growth factors (Figure 2) (i.e., weeks 4 and 6 after administration of 6-OHDA). Net ipsilateral rotations were recorded over a 2 hours period and data was analyzed by repeated measures 2-way ANOVA followed by Fisher’s LSD test post hoc (Figure 17).
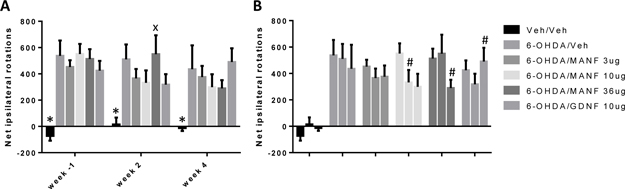
Figure 17: Amphetamine-induced rotational behavior. Net ipsilateral rotations (mean ± SEM). Treatment groups as indicated (Vehicle / vehicle (N=11), 6-OHDA / veh (N=10), 6-OHDA / MANF 3μg (N=12), 6-OHDA / MANF 10μg (N=10), 6-OHDA / MANF 36μg (N=10), 6-OHDA / GDNF 10μg (N=11)). Time points as indicated (1 week pre, 2 weeks and 4 weeks post MANF / GDNF administration). Statistical analysis with repeated measures two-way ANOVA followed by Fisher’s LSD. (*) P<0.05 compared to 6-OHDA/vehicle; (x) P<0.1 (trend) compared to 6-OHDA/GDNF 10 μg.
A) Amphetamine-induced rotations by time-point (week -1, week 2, week 4); None of the growth factor treatment groups was statistically different from 6-OHDA/vehicle treatment at any time point. No trends of treatment differences to 6-OHDA/vehicle were detected. B)
| CONFIDENTIAL 40 |
Amphetamine-induced rotations by treatment group for each time-point. (#) P<0.05 compared to prior time-point (within treatment group comparisons).
The vehicle/vehicle group was significantly different from the 6-OHDA/vehicle group at all time points (Figure 17A). Moreover, at the initial assessment of the rotational behavior at week -1 relative to growth factor administration, all treatment groups were similar, indicating that the groups were well balanced prior to the initiation of growth factor treatment. At weeks 2 and 4 after treatment initiation, none of the 6-OHDA groups differed significantly. Hence, there was no significant effect by MANF or GDNF treatment on ameliorating net ipsilateral rotations compared to vehicle treatment (Figure 17A). In within group comparisons, MANF 10 μg at week 2 was significantly better than at week -1, an effect that was sustained at week 4. Similarly, MANF 36 μg significantly improved between the week 2 and 4 assessments while the GDNF group appeared to get worse in the same period (Figure 17B). However, this time-dependent MANF treatment effect was relatively modest as it could not be separated statistically from the 6-OHDA/vehicle spontaneous recovery. Moreover, compared to the Phase 1 data at the same time-point relative to 6-OHDA administration, the spontaneous recovery by 6-OHDA/vehicle was less pronounced.
There is no literature on MANF administration to the substantia nigra in an 6-OHDA model and it is therefore not possible to compare the results of this current study with independently generated data. However, GDNF was administered to the substantia nigra in a model in which 6-OHDA was injected into the basal forebrain bundle (Lapchak et al., 1997). GDNF was given as single injections of 100 μg or 1000 μg, respectively, at 9 weeks post 6-OHDA. At week 10, the turning behavior was fully restored, an effect that was sustained until the end of the study at week 20. Given the vastly different GDNF dose levels between these studies and the different locations of the 6-OHDA injection, a comparison of the data of this study with Lapchak et al. is difficult.
As described in the neuroprotection section of this Phase 2 of the study, the number of TH+ neurons in the substantia nigra was quantified by stereology. The numbers of TH+ neurons were determined for the ispilateral and contralateral sides of animals from all treatment groups (Figure 18A and B). The ipsilateral to contralateral ratios were calculated at the individual animal level (Figure 18C). There was no difference between the vehicle/vehicle and 6-OHDA/vehicle groups on the ipsilateral and the contralateral sides while the ratios were separated slightly but non-significantly. While the variation of values observed in the neuroprotection protocol, were less pronounced in the neuroregeneration protocol, there were still three instances of ipsilateral values substantially higher than the corresponding contralateral cell numbers. Moreover, due to technical difficulties, 14 of the 36 animals with ipsilateral stereology data did not have corresponding contralateral data. Given these experimental uncertainties, no firm conclusions on the effect of growth factor treatments on regenerating TH+ cells in the substantia nigra can be drawn.
| CONFIDENTIAL 41 |

Figure 18: Dopaminergic neurons in the substantia nigra determined by stereology. Computed number of nigral TH+ neurons (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Vehicle / vehicle (N=6, ipsi; N=2, contra), 6-OHDA / Vehicle (N=6, ipsi; N=3, contra), 6-OHDA / MANF 3μg (N=6, ipsi; N=3, contra), 6-OHDA / MANF 10μg (N=6, ipsi; N=5, contra), 6-OHDA / MANF 36μg (N=6, ipsi; N=5, contra ), 6-OHDA / GDNF 10μg (N=6, ipsi; N=4, contra)). 1-way ANOVA followed by Fisher’s LSD. None of the treatment groups were different from 6-OHDA/vehicle. (x) P<0.05 compared to vehicle/vehicle. (A) Ipsilateral, (B) Contralateral, (C) Ratio ipsilateral / contralateral.
The survival of TH+ neurons in the substantia nigra was assessed after nigral administration of GDNF in a neuroregeneration protocol of the striatal 6-OHDA model (Sauer et al., 1995). A single treatment with GDNF 10 μg administered 1 week post 6-OHDA, increased the number of ipsilateral TH+ neurons by about 50% compared to vehicle treatment when assessed four weeks post 6-OHDA. In a long-term neuroregeneration study applying 6-OHDA to the basal forebrain bundle, a single dose of GDNF 100 μg and 1000 μg nine weeks post 6-OHDA restored TH enzymatic activity in the substantia nigra but not in the striatum (Lapchak et al., 1997).
| CONFIDENTIAL 42 |
As described in the neuroprotection section of this Phase 2 of the study, dopaminergic axonal terminals of TH+ neurons were assessed by TH immunoreactivity densitometry of the ispilateral and contralateral striata of animals in all treatment groups at the end of the observation period. The numbers of dopaminergic (i.e., TH+) terminals in the striatum were significantly different between the vehicle/vehicle and 6-OHDA/vehicle groups on the ipsilateral side for the entire striatum (Figure 19A) and the dorsal (Figure 19D) and ventral (Figure 19G) striata. As already seen in the neuroprotection protocol, 6-OHDA treatment significantly reduced the number of dopaminergic terminals in the striatum. The compartmental differentiation of dorsal and ventral striata, respectively, in response to 6-OHDA treatment was similar to the one observed in the neuroprotection protocol, although less pronounced. None of the treatment groups displayed significant differences in the number of dopaminergic terminals on the ipsilateral (Figures 19A, D and G) and the contralateral sides (Figures 19B, E and H). There was a trend towards a difference between MANF 3 μg and MANF 36 μg compared to GDNF 10 μg (Figure 19A). This trend became statistically significant when the ipsilateral / contralateral ratios of MANF 3 μg were compared to GDNF 10 μg (Figure 19C). Nevertheless, none of the growth factors treatments were different from the 6-OHDA/vehicle group. An analysis of further striatal spatial subgroups (Figure 20A-I; temporal, medial and basal striatum) revealed the same absence of MANF treatment effects as observed for the global data. Also, the differential response to 6-OHDA observed in the neuroprotection protocol was less pronounced in the neuroregeneration protocol.
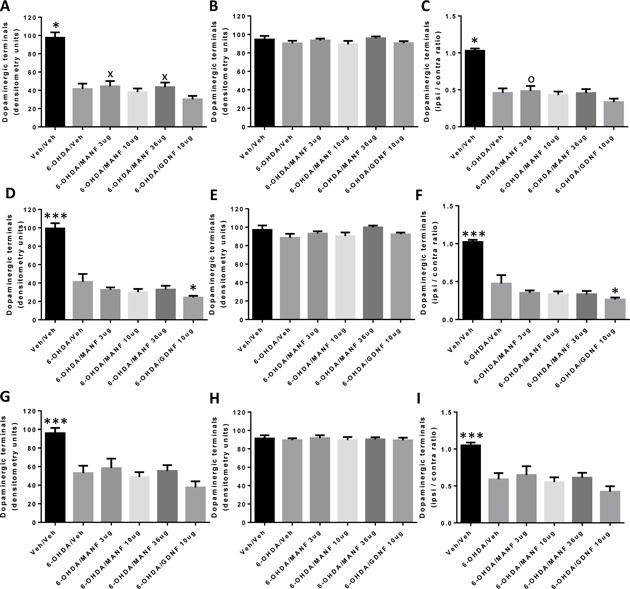
| CONFIDENTIAL 43 |
Figure 19: Dopaminergic terminals in the global, dorsal and ventral striatum determined by densitometry. Density of terminals (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Veh/veh (N=6), 6-OHDA/veh (N=6 dorsal, N=5 global, ventral), 6-OHDA/MANF 3μg (N=6), 6-OHDA/MANF 10μg (N=6 global, dorsal, N=5 ventral), 6-OHDA/MANF 36μg (N=6), 6-OHDA/GDNF 10μg (N=6)). 1-way ANOVA followed by Fisher’s LSD. (***) P<0.001, (*) P<0.05 compared to 6-OHDA/vehicle; (o) P<0.05, (x) P<0.1 (trend) compared to 6-OHDA/GDNF 10 μg.
Global striatum, (A) ipsilateral, (B) contralateral, (C) ratio ipsilateral / contralateral;
Dorsal striatum, (D) ipsilateral, (E) contralateral, (F) ratio ipsilateral / contralateral;
Ventral striatum, (G) ipsilateral, (H) contralateral, (I) ratio ipsilateral / contralateral.

| CONFIDENTIAL 44 |
Figure 20: Dopaminergic terminals in the temporal, medial and basal striatum determined by densitometry. Density of terminals (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Veh/veh (N=6), 6-OHDA/veh (N=4 basal, contralateral temporal, contralateral medial, all others N=6), 6-OHDA/MANF 3μg (N=6), 6-OHDA/MANF 10μg (N=6), 6-OHDA/MANF 36μg (N=6), 6-OHDA/GDNF 10μg (N=6)). 1-way ANOVA followed by Fisher’s LSD. (***) P<0.001 compared to 6-OHDA/vehicle; (o) P<0.05 compared to 6-OHDA/GDNF 10 μg.
Temporal striatum, (A) ipsilateral, (B) contralateral, (C) ratio ipsilateral / contralateral;
Medial striatum, (D) ipsilateral, (E) contralateral, (F) ratio ipsilateral / contralateral;
Basal striatum, (G) ipsilateral, (H) contralateral, (I) ratio ipsilateral / contralateral.
There are no published reports on MANF administration to the substantia nigra and therefore, these results cannot be compared to independently generated data. However, nigral administration of GDNF was assessed in neuroregenerative protocols of 6-OHDA lesions in the striatum (Sauer et al., 1995) or the medial forebrain bundle (Lapchak et al., 1997). GDNF did not have any effect on TH+ fiber density in the striatum (Sauer et al., 1995) or the striatal TH enzymatic activity (Lapchak et al., 1997). Hence, the results of the present study as they relate to GDNF are in agreement with published information.
In order to understand the functionality of dopaminergic terminals in the striatum in response to neuroregenerative growth factor therapy the levels of dopamine were determined in the ipsilateral (Figure 21A) and contralateral (Figure 21B) striata for each of the treatment groups, and the ipsilateral / contralateral ratios were calculated at the individual animal level (Figure 21C). There was a significant difference of dopamine levels between the vehicle/vehicle and 6-OHDA/vehicle groups on the ipsilateral side and for the ipsilateral / contralaetral ratios, but not the contralateral side. None of the growth factor treatments was significantly different from 6-OHDA/vehicle group on the ipsilateral side of the lesion. However, MANF 36 μg showed a trend towards normalization of dopamine levels in the striatum. The striatal dopamine levels of GDNF were not different from 6-OHDA/vehicle. This lack of striatal dopamine level restoration was also observed in an independent study of GDNF assessing its neuroregenerative potential in a 6-OHDA medial forebrain bundle lesion (Lapchak et al., 1997).
| CONFIDENTIAL 45 |
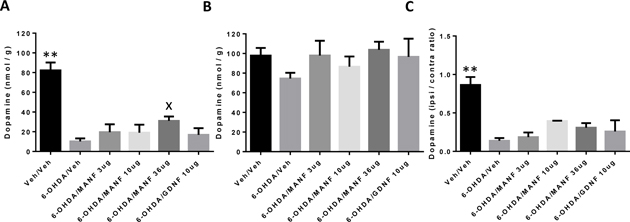
Figure 21: Striatal dopamine (DA) levels. Amounts of dopamine shown as nmol/g wet tissue weight (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Vehicle / vehicle (N=5, ipsi, contra), 6-OHDA / Vehicle (N=4 ipsi, contra), 6-OHDA / MANF 3μg (N=4, ipsi, contra), 6-OHDA / MANF 10μg (N=5, ipsi; N=3, contra), 6-OHDA / MANF 36μg (N=4, ipsi, contra ), 6-OHDA / GDNF 10μg (N=5, ipsi, contra)). 1-way ANOVA followed by Fisher’s LSD. (**) P<0.01, (x) P<0.1 (trend) compared to 6-OHDA/vehicle. (A) Ipsilateral, (B) Contralateral, (C) Ratio ipsilateral / contralateral.
As in the neuroprotection protocol, striatal levels of DOPAC (Figure 22) and HVA (Figure 23) were determined for each of the six treatment groups in this neuroregeneration protocol. DOPAC levels were significantly different between the vehicle/vehicle and 6-OHDA/vehicle groups on the ipsilateral (Figure 22A) and for the ipsilateral/contralateral ratio (Figure 22C), but not on the contralateral side (Figure 22B). The MANF 36 μg group showed a trend towards a difference from 6-OHDA/vehicle on the ipsilateral side but none of the other treatment groups was different from 6-OHDA/vehicle. The effect by MANF 36 μg on DOPAC mirrored the results obtained for dopamine.

| CONFIDENTIAL 46 |
Figure 22: Striatal DOPAC levels. Amounts of DOPAC shown as nmol/g wet tissue weight (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Vehicle / vehicle (N=5, ipsi, contra), 6-OHDA / Vehicle (N=4 ipsi, contra), 6-OHDA / MANF 3μg (N=4, ipsi, contra), 6-OHDA / MANF 10μg (N=6, ipsi; N=3, contra), 6-OHDA / MANF 36μg (N=4, ipsi, contra ), 6-OHDA / GDNF 10μg (N=5, ipsi, contra)). 1-way ANOVA followed by Fisher’s LSD. (**) P<0.01 compared to 6-OHDA/vehicle; (x) P<0.1 (trend) compared to 6-OHDA/GDNF 10μg.
(A) Ipsilateral, (B) Contralateral, (C) Ratio ipsilateral / contralateral.
HVA levels were significantly different between the vehicle/vehicle and 6-OHDA/vehicle groups on the ipsilateral (Figure 23A) and for the ipsilateral/contralateral ratio (Figure 23C), but not on the contralateral side (Figure 23B). None of growth factor treatment groups was different from the 6-OHDA/vehicle group.

Figure 23: Striatal HVA levels. Amounts of HVA shown as nmol/g wet tissue weight (mean ± SEM) at week 4 post MANF / GDNF administration. Treatment groups as indicated (Vehicle / vehicle (N=5, ipsi, contra), 6-OHDA / Vehicle (N=5 ipsi, contra), 6-OHDA / MANF 3μg (N=4, ipsi, contra), 6-OHDA / MANF 10μg (N=5, ipsi; N=3, contra), 6-OHDA / MANF 36μg (N=4, ipsi, contra ), 6-OHDA / GDNF 10μg (N=5, ipsi, contra)). 1-way ANOVA followed by Fisher’s LSD. (**) P<0.01 and (***) P<0.001 compared to 6-OHDA/vehicle. (A) Ipsilateral, (B) Contralateral, (C) Ratio ipsilateral / contralateral.
| 3.4 | Diffusion of MANF with convection-enhanced delivery |
Convection-enhanced delivery (CED) has emerged as a novel neurosurgical technique with the potential to achieve more effective coverage of the putamen. CED describes a direct method of drug delivery to the brain through very fine microcatheters. By establishing a pressure gradient at the tip of the infusion catheter, CED confers several advantages over conventional drug injection techniques, in particular, homogeneous drug distribution through large and clinically-relevant brain volumes (Bobo et al., 1994). The primary objective of this study was to determine whether a single infusion of MANF could be distributed via CED and whether MANF could be detected after 7 days.
| CONFIDENTIAL 47 |
MANF (10 μg) was delivered via CED at flow rates of 0.1 μl/ min, 1.25 μl/ min, and 5 μl/ min into the striatum. MANF was detected at 0 hours after the infusion at all flow rates by immunohistochemistry (Figure 24).

Figure 24: Distribution of MANF in the striatum at different flow rates on Day 0. 10 μg of MANF or GDNF in 2 μl of PBS were delivered by CED. MANF was detected by immunohistochemistry, scale bar = 250 μm. (A) 0.1 μl/min; (B) 1.25 μl/min; (C) 5 μl/min.
Following on from the 0 hour time-point results, the flow rates for MANF delivery by CED for the 7 day study were 1.25 μl/ min and 2.5 μl/ min. MANF could be detected by immunohistochemistry at 7 days post-infusion following CED at both flow rates (1.25 μl/ min and 2.5 μl/ min) (Figure 25).
| CONFIDENTIAL 48 |

Figure 25: Distribution of MANF and GDNF in the striatum at different flow rates after seven days. 10 μg of MANF or GDNF in 2 μl of PBS were delivered by CED. MANF and GDNF were detected by immunohistochemistry, scale bar = 250 μm. (A) GDNF 2.5 μl/min; (B) No infusion control; (C) MANF 2.5 μl/min; (D) No infusion control; (E) MANF 1.25 μl/min; (F) No infusion control.
A higher volume of MANF was detectable in the hemispheres infused with a flow rate of 1.25 μl/ min (0.0505 ± 0.028 mm3) compared to 2.5 μl/ min (0.0185 ± 0.008 mm3). MANF infusion with this flow rate resulted also in a higher volume of distribution compared to GDNF infusion at 2.5 μl/ min (0.028 ± 0.006 mm3) (Figure 26). However, these differences were not statistically significant and a larger number of animals will have to be tested to confirm the observed effects.

Figure 26: Calculated volumes of distribution in the striatum for MANF and GDNF infusions at different flow rates after 7 days (mean ± SEM). MANF (10 μg in 2 μl PBS) striatal infusions were performed at flow rates of 1.25 μl/min (N = 2) and 2.5 μl/min (N = 2). GDNF (10 μg in 2 μl PBS) striatal infusions were performed at a flow rate of 2.5 μl/min (N = 2). Statistical analysis by 1-way ANOVA followed by Fisher’s LSD. No statistically significant differences in distribution volumes between the treatment groups were found.
| CONFIDENTIAL 49 |
| 4 | Discussion |
The main objectives of this study were to assess the neurotrophic factor MANF in the 6-OHDA model of Parkinson’s disease in rats after single administration to either the striatum or the substantia nigra at different dose levels and to compare its activity to GDNF under the same experimental conditions. The inclusion of neuroprotection and neuroregeneration protocols, respectively, and its combination with a battery of behavioral, structural and functional assessments furthered the understanding of MANF’s mechanism of action and led to a set of testable hypotheses that will be the subject of future studies. The key results of the Phases 1 (i.e., striatal administration of growth factors) and 2 (i.e., nigral administration of growth factors) are summarized in Table 1.

Table 1: Summary of key data of Phases 1 and 2. Growth factors were administered to the striatum (Phase 1) or the substantia nigra (Phase 2). Both Phases consisted of a neuroprotection protocol and a neuroregeneration protocol. Growth factor activities were assessed by behavioral tests (amphetamine-induced rotations) and nigral / striatal effects on dopaminergic neurons by structural (TH+ cell counts, TH+ cell numbers by stereology; TH+ terminal densities by densitometry) and functional tests (Dopamine and dopamine metabolites levels). Active doses (P<0.05 versus vehicle) of MANF or GDNF are shown with the time-points at which they occurred. “No effect” means that no difference to vehicle treated animals was observed.
| CONFIDENTIAL 50 |
Administration of MANF to the striatum resulted in a robust behavioral affect most notably in the neuroprotection protocol, and to a lesser extent in the neuroregeneration protocol. MANF normalized the 6-OHDA-induced ipsilateral rotations as early as 2 weeks after a single administration and this effect was sustained at 4 weeks post growth factor treatment. The active doses ranged from 3 μg to 10 μg and are in good agreement with the literature. It appears likely that lower doses would be active in this paradigm; the minimally effective dose remains to be determined. These behavioral effects were paralleled by a significant protection of the dopaminergic cell bodies in the substantia nigra. MANF protected dopaminergic neurons in vitro (Petrova et al., 2003), similar to the effects observed in vivo in this present study. Hence, administration of MANF to the striatum protects the cell bodies of dopaminergic neurons with a concomitant normalization of the rotational behavior. MANF may counteract some of the known intracellular effects of 6-OHDA and testable hypotheses on how MANF protects neurons from 6-OHDA-induced death can be generated. 6-OHDA is relatively selective for monoaminergic neurons, resulting from preferential uptake by dopaminergic and noradrenergic transporters (Luthman et al., 1989). Once inside the neurons 6-OHDA accumulates in the cytosol, generates reactive oxygen species and quinones with the latter leading to the inactivation of biological macromolecules by attack of nucleophilic groups (Cohen and Werner, 1994). The mechanism of cell death in mesencephalic neurons involves caspase-dependent pathways (Han et al., 2003). 6-OHDA, but not MPP+, induced caspase-3 and caspase-9 enzymatic activity in the MN9D dopaminergic neuronal cell line. Application of a caspase inhibitor (zVAD-fmk) prevented 6-OHDA-induced TH+ cell death. The specific neuronal toxicity of 6-OHDA thus involves several mechanisms including the activation of apoptotic pathways and the generation of reactive oxygen species. MANF may counteract the toxicity of 6-OHDA on both levels. MANF decreased caspase-3 activation in serum starved cardiomyocytes (Tadimalla et al., 2009). It is thus conceivable that MANF could prevent 6-OHDA-induced caspase activation in dopaminergic neurons and thereby promote the survival of these cells. Moreover, MANF protected cardiomyocytes from reperfusion injury in vivo. It is well known that a key mechanism of reperfusion injury is the generation of reactive oxygen species (Verma et al., 2002). MANF could thus counteract the effects of 6-OHDA-generated reactive oxygen species. Finally, MANF might have a more general role in counteracting cellular stress and in particular endoplasmatic reticulum stress, as exemplified by its protective activity in thapsigargin- (Apostolou et al., 2008) and tunicamycin-induced (Yu et al., 2010) cell death. These anti-stress effects of MANF may contribute to the observed in vivo effects of MANF in the 6-OHDA model of PD. In future studies, MANF activities to counteract apoptosis induced by 6-OHDA and other mechanisms, including the formation of reactive oxygen species in nigrostriatal dopaminergic neurons, will be studied biochemically in vivo and in vitro.
| CONFIDENTIAL 51 |
MANF displayed a strong neuroprotective activity that manifested itself in a reduced rotational behavior and a rescue of TH+ neurons in the substantia nigra. Remarkably, this protective effect was not paralleled by a functional normalization of the striatal dopaminergic terminals as no differences in dopamine levels between the treatment groups were observed. A resolution of this apparent discrepancy between the effects on behavior and striatal dopaminergic neuronal health and the absence of striatal functional normalization will be the subject of a more detailed future investigation. In such a study, levels of dopamine and dopamine metabolites will be measured in real time using microdialysis in freely behaving animals with 6-OHDA lesions.
The effects of MANF on dopaminergic terminals in the striatum were assessed by densitometry in the Phase 2 of the current study. Administration of MANF 36 μg to the substantia nigra in the neuroprotection protocol resulted in a significant increase of the ipsilateral dopaminergic (TH+) terminal density at the end of the observation period compared to 6-OHDA/vehicle treatment. MANF protected dopaminergic terminals almost completely at the highest dose with no difference to vehicle / vehicle animals. It is noteworthy that there was also a non-significant MANF dose-dependent increase of dopaminergic terminal densities on the contralateral side. In contrast, dopamine levels in the striatum were not normalized by treatment with MANF. Therefore, the effect on terminal densities was dissociated from an effect on dopamine levels. In this context, it is important to note that densitometry detects the presence of TH in the dopaminergic terminals. An increase of the densitometry signal thus reflects an increase in the amount of TH protein but not necessarily an increase of active TH. Accordingly, nigral administration of MANF might thus increase or preserve the levels of TH protein in the striatum but may not prevent its inactivation. In order to elucidate this possibility further, the effects of MANF treatment on TH activity and activation status (i.e. phosphorylation state) will be investigated by biochemical and immunohistochemical methods in a future study. A further possibility to explain our results is that a non-linear relationship exists between innervating fiber density and dopamine levels, such that nigral administration of MANF in this experimental situation could increase the former but not the latter. Moreover, MANF could facilitate outgrowth of fibers from sick or surviving neurons. This could be because of a neuroprotective MANF effect on neurons and their projections, and/ or by a sprouting of new fibers into previously occupied striatal terminal fields from surviving neurons or those unaffected by the 6-OH-dopamine lesion.
| CONFIDENTIAL 52 |
In addition to the global densitometry analysis, an effort was made to understand region-specific effects on striatal structures by striatal 6-OHDA and after nigral administration of MANF. Striatal administration of 6-OHDA in the neuroprotection protocol led to a reduction of dopaminergic terminals in the striatum that was more pronounced in the dorsal than in the ventral striatum. Moreover, 6-OHDA administration in the same protocol affected the dopaminergic terminal density more in the medial striatum than in the temporal or the basal striata. These compartmental differences were also observed in the neuroregeneration protocol albeit to a lesser extent. It is noteworthy that the amounts of 6-OHDA applied in the neuroprotection and neuroregeneration protocols differed and were 8 μg and 20 μg, respectively. The relatively higher densities of dopaminergic terminals in the temporal and the ventral striata could be due to a higher resistance of these neurons to 6-OHDA-induced toxicity and/or a limited ability of 6-OHDA to reach these neurons. The reduced differential effects on striatal compartments in the neuroregeneration protocol might be due to the higher amount of 6-OHDA applied to the striatum. Finally, effects mediated by nigral administration of MANF in the neuroprotection protocol yielded similar results in all analyzed compartments. MANF (36 μg) treatment led to significantly higher ipsilateral terminal densities than 6-OHDA/vehicle and 6-OHDA/GDNF treatment in global, dorsal, ventral, temporal, medial and basal striata.
The increase of the ipsilateral rotational behavior induced by MANF (or GDNF) administration to the substantia nigra in the neuroprotection protocol was remarkable but not unprecedented in the literature (Kirik et al., 2000). Kirik et al. observed a reduction in TH+ fiber density in the striatum after administration of GDNF to the substantia nigra. In addition, aberrant local sprouting in the ventral thalamus was observed and the combination of these effects may have resulted in more severe motor impairments. In our study, we observed an increased TH density in the striatum but we have not collected data on aberrant sprouting close to the growth factor injection site. An alternative explanation for the increased behavioral abnormalities observed in this Phase 2 of the study could involve activation of contralateral circuits by striatal administration of MANF. Neuronal circuits that may be involved in this mechanism are the interhemispheric nigrostriatal and corticostriatal pathways (Lieu et al., 2012). Importantly, injection of 6-OHDA into the striatum spares these interhemispheric nigrostriatal neurons (Sauer and Oertel, 1994). It is thus conceivable that nigral administration of MANF could affect the activity of interhemispheric neurons by increasing the contralateral striatal dopaminergic terminals. Since those terminals are located in an environment conducive to neuronal activity, increased TH protein levels may represent increased active enzymatic activity resulting in increased striatal dopamine levels which could then lead to an increase of ipsilateral rotations. In this context it is interesting to note that both the terminal densities and the dopamine levels on the contralateral side of MANF treated animals were slightly increased. In a future study, the effects of MANF administration to the substantia nigra on the activity state of TH and the activation of contralateral circuits will be investigated.
| CONFIDENTIAL 53 |
This study investigated the activities of GDNF and MANF at doses previously shown to be active in the 6-OHDA model. However, the active doses and dosing regimens for GDNF vary considerably in the literature. Activities in the rodent 6-OHDA model were reported for GDNF after a single striatal injection of 10 μg (Sauer et al., 1995; Lindholm et al. 2007; Voutilainen et al. 2011) or 25 μg (Kirik et al., 2000), single nigral injection of 25 μg (Kirik et al., 2000), 100 or 1000 μg (Lapchak et al., 1997), multiple striatal injections totaling 50 μg (Rosenblad et al., 1998; 10 x 5 μg), multiple nigral injection totaling 90 μg (Winkler et al., 1996; 9 x 10 μg) or 140 μg (Sauer et al., 1995; 14 x 10 μg) and chronic infusion totaling 21, 42 or 63 μg (Voutilainen et al., 2011). In non-human primates, GDNF was administered by chronic infusion at 5 and 15 μg/day for three months (Gronding et al., 2002). The single 10 μg GDNF dose was chosen for this study because GDNF displayed neuroprotective activity in similarly designed recent studies (Lindholm et al., 2007; Voutilainen et al. 2009) that compared its activity with MANF. However, considering the totality of GDNF studies, the 10 μg dose level is at the lower end of active GDNF doses. MANF has been investigated previously at the 3, 10 and 30 μg dose levels with a single administration to the striatum (Voutilainen et al., 2009). The current study applied MANF at similar dose levels (i.e., 3, 10 and 36 μg). Striatal administration of MANF resulted in an improvement of the rotational behavior as well as a protection of TH+ cell bodies at the 3 and 10 μg doses, which is in close agreement with independently generated data (Voutilainen et al., 2009). In contrast to Voutilainen et al., this study did not identify a U-shaped dose-response in the behavioral outcome but rather an inverse dose-response in which the lowest dose (3 μg) resulted in the highest degree of protection (Figure 4). This behavioral data is consistent with the TH+ cell counts (Figure 5) in which both the 3 and 10 μg doses afforded protection while the highest dose (36 μg) did not. An exploration of lower MANF doses with the current MANF preparation would be necessary to resolve the discrepancy in MANF dose-response characteristics between Voutilainen et al. and this present study. The determination of the true dose-response relationship of MANF might shed light on the mechanism of receptor engagement. A better understanding of this issue is important as it has implications for MANF development in general and dose and dosing regimen selection in particular.
While single intrastriatal injections of MANF resulted in encouraging neuroprotective and neuroregenerative effects, chronic infusions of MANF into the striatum totaling 21, 42 or 63 μg (Voutilainen et al., 2011) yielded disappointing results with no difference to vehicle treated animals. However, it is noteworthy that in Vouitilainen et al. (2011) the vehicle control displayed unusually low cumulative rotation counts indicating rapid spontaneous recovery. Moreover, GDNF was not different from vehicle even though in a parallel experiment of the same study GDNF showed a clear trend towards reduction of rotational behavior. Therefore, both the negative (vehicle) and positive (GDNF) controls failed in the part of the study in which MANF was assessed. Therefore, conclusions on MANF activity after chronic intrastriatal infusion should not be based on the Voutilainen et al. (2011) study. The present study did not investigate the effects of chronic MANF administration and given the uncertainties in the published literature regarding this issue a future investigation should consider chronic or multiple striatal administration of MANF. In order to gain further experience with delivery of MANF to the striatum, we explored the use of CED in combination with immunohistochemical detection of MANF. Infusion of MANF via CED could be achieved, MANF was detectable in the striatum seven days after administration and distribution volumes could be calculated for the various experimental conditions. This delivery mechanism will thus be further explored in future rodent and non-human primate studies. The objectives of those studies will include the further optimization of the MANF doses and the CED flow rate with the goal of achieving full coverage of the target tissue with MANF protein.
| CONFIDENTIAL 54 |
A further objective of this study was to compare MANF and GDNF under identical experimental conditions. Administration of MANF or GDNF to the striatum in the neuroprotection protocol yielded similar results. Both MANF and GDNF reduced the 6-OHDA-induced ipsilateral rotations and protected nigral cell bodies of dopaminergic neurons to a similar extent. In the neuroregeneration protocol, GDNF displayed a protective effect on TH+ neuron cell bodies but, like MANF, did not restore the 6-OHDA-induced behavioral deficit. Hence, when administered to the striatum, MANF and GDNF display similar activities. Administration of GDNF or MANF to the substantia nigra under the neuroprotection protocol increased the ipsilateral rotational behavioral to a similar extent at the same time points. However, at the neuronal structural level, important differences between MANF and GDNF were detected. While MANF increased the number of TH+ striatal densities compared to 6-OHDA/vehicle or GDNF treated animals, GDNF had no such effect. Moreover, GDNF displayed significantly lower dopamine, DOPAC and HVA concentrations in the striatum compared to MANF 36 μg. These observations could point to a fundamentally different biology of these two growth factors that is likely rooted in the interaction with distinct receptors with differing signaling pathways and locations. Further studies will include a systematic investigation of MANF’s role in regulating known receptor pathways and enzymes important in biogenic amine metabolism, as well as MANF’s interaction with known neuronal receptors. In this respect, MANF has demonstrated a potential to modulate neurotransmission as shown by its activity on GABA-receptor mediated postsynaptic currents in dopaminergic neurons of the substantia nigra (Zhou et al., 2006).
The site of growth factor administration is an important consideration for future non-human primate and clinical studies. In this study we explored differences between MANF administration to the striatum and the substantia nigra, respectively. In the neuroprotection protocols, MANF administration to these tissues resulted in dramatically different behavioral effects. While striatal administration of MANF ameliorated the ipsilateral rotational behavior this same behavior was increased by nigral administration of the growth factor. Possible explanations of the latter effect involving the presence of interhemispheric nigrostriatal pathways were discussed in a previous section. TH+ cell bodies in the substantia nigra were protected in the neuroprotection protocol with striatal administration of MANF but paradoxically not with nigral administration. Conversely, nigral administration of MANF protected dopaminergic terminals in the striatum but not TH+ cells numbers in the substantia nigra. It appears thus that the manifestation of MANF effects occur distal to the site of administration. Receptor engagement in the substantia nigra and the striatum, respectively, could result in the activation of different intracellular mechanisms. While the latter may involve receptor-mediated retrograde transport, the former may lead to a more direct activation of a receptor signaling pathway with subsequent changes in gene expression or regulation of protein activity (i.e., TH phosphorylation). In any case, these are testable hypotheses and amenable to experimental investigation in a future study of MANF in the 6-OHDA model. In the neuroregeneration protocols, MANF administration to either location did not normalize the rotational behavior and we can thus not reach a firm conclusion of a preferred administration site in a regenerative treatment setting. However, a trend towards normalization of dopamine levels in the striatum was observed after nigral administration of MANF but not after striatal administration. This observation points again towards a distal effect by MANF as observed in the neuroprotection protocol. Nigral MANF may regulate the expression or activation of TH resulting in a distal, striatal effect on dopamine levels. In conclusion, given these observations with nigral and striatal administration of MANF, it might be important to administer this protein to both locations and possibly bilaterally.
| CONFIDENTIAL 55 |
| 5 | Conclusions and Outlook |
MANF’s potent neuroprotective activity was confirmed in this present study both at the behavioral and structural level. A single striatal administration of MANF prevented 6-OHDA-induced behavioral deficits and dopaminergic cell death in the substantia nigra. A comparison of striatal and nigral administration of MANF revealed an intriguing mechanism of distal action of this growth factor. When MANF was administered to the substantia nigra, striatal dopaminergic terminal densities increased and when the striatum was the target of MANF administration an increase of TH+ cells in the substantia nigra was detected. MANF (and GDNF) administration to the substantia nigra resulted in an increase of ipsilateral rotations and we hypothesize that this effect might be due to activation of contralateral circuits. Therefore, in conclusion, MANF is a potent neuroprotective agent, MANF activity is site specific and MANF may cause effects in nigral neurons of the ipsilateral and contralateral projections to the striatum.
The continued development of MANF will consist of two main components, mechanistic investigations in rodent models of PD and proof-of-concept studies in non-human primates (NHP).
Our mechanistic studies will further investigate the three areas identified in this study, site-specific pathways activated by MANF, distal action of MANF (including retrograde transport and activation of contralateral circuits) and protection from 6-OHDA (or MPTP)-induced apoptosis and reactive oxygen species formation. Moreover, a better understanding of the dynamics of dopamine and metabolites levels in the striatum after MANF treatment will be desirable and therefore, microdialysis experiments in behaving 6-OHDA-treated animals will be performed. A further area of study will be the effects of MANF in genetic mutation models.
| CONFIDENTIAL 56 |
Our proof-of-concept study in NHP will require careful planning and may be modeled after the study performed with GDNF and incorporate the human clinical experience with GDNF. The active MANF doses in rodents ranged from 3 to 10 μg administered by single injections. Scaling of equivalent NHP doses will be based on a pilot pharmacokinetics study in which tissue distribution of MANF will be measured after CED. A further consideration will include the dosing regimen by which MANF is applied to the NHP. Single administration of MANF has been effective in rodents but may not be optimal for a NHP model with a more chronic disease development. Therefore, alternatives such as intermittent applications or chronic infusion will be evaluated. Given the potential contralateral effects of MANF a bilateral administration of the growth factor may have to be considered.
The results of this study together with the literature on MANF warrant a further development of this growth factor for the treatment of PD. While the path forward is facilitated by experience gained in the GDNF development, MANF, based on its different and unexploited mechanism of action, offers a promising new approach towards therapy of PD.
| 6 | References |
Apostolou A, Shen Y, Liang Y, Luo J, Fang S. Armet, a UPR-upregulated protein, inhibits cell proliferation and ERstress-induced cell death. Exp Cell Res. 2008 Aug 1;314(13):2454-67.
Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2076-80.
Chen YC, Sundvik M, Rozov S, Priyadarshini M, Panula P. MANF regulates dopaminergic neuron development in larval zebrafish. Dev Biol. 2012 Oct 15;370(2):237-49.
Cohen, G., and Werner, P. (1994). Free radicals, oxidative stress, and neurodegeneration. In Neurodegenerative Diseases, D.B. Calne, ed. (Philadelphia: W.B. Saunders), pp. 139–161.
Colla E,Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, Schneider BL, Lee MK. Endoplasmic reticulum stress is important for the manifestations of a-synucleionopathy in vivo. J. Neurosci.;32(10):3306-3320.
Glembotski CC, Thuerauf DJ, Huang C, Vekich JA, Gottlieb RA, Doroudgar S. Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J Biol Chem. 2012 Jul 27;287(31): 25893-904.
Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, Elsberry DD, Klein MC, Gerhardt GA, Gash DM. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002 Oct;125(Pt 10):2191-201.
Han BS, Hong HS, Choi WS, Markelonis GJ, Oh TH, Oh YJ. Caspase-dependent and - independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J Neurosci. 2003 Jun 15;23(12):5069-78.
| CONFIDENTIAL 57 |
Hellman M, Arumäe U, Yu LY, Lindholm P, Peränen J, Saarma M, Permi P. Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons. J Biol Chem. 2011 Jan 28;286(4):2675-80.
Healy-Stoffel M, Ahmad SO, Stanford JA, Levant B. A novel use of combined tyrosine hydroxylase and silver nucleolar staining to determine the effects of a unilateral intrastriatal 6-hydroxydopamine lesion in the substantia nigra: a stereological study. J Neurosci Methods. 2012 Sep 30;210(2):187-94.
Hoseki J, Sasakawa H, Yamaguchi Y, Maeda M, Kubota H, Kato K, Nagata K. Solution structure and dynamics of mouse ARMET. FEBS Lett. 2010 Apr 16;584(8):1536-42.
Kearns CM, Cass WA, Smoot K, Kryscio R, Gash DM. GDNF protection against 6-OHDA: time dependence and requirement for protein synthesis. J Neurosci. 1997 Sep 15;17(18):7111-8.
Kirik D, Rosenblad C, Björklund A. Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur J Neurosci. 2000 Nov;12(11):3871-82.
Lapchak PA, Miller PJ, Collins F, Jiao S. Glial cell line-derived neurotrophic factor attenuates behavioural deficits and regulates nigrostriatal dopaminergic and peptidergic markers in 6-hydroxydopamine-lesioned adult rats: comparison of intraventricular and intranigral delivery. Neuroscience. 1997 May;78(1):61-72.
Lindholm P, Wootz H, Korhonen L. ER stress and neuroregenerative diseases. Cell Death and Differentiation 2006;13:385-392.
Lindholm P, Voutilainen MH, Laurén J, Peränen J, Leppänen VM, Andressoo JO, Lindahl M, Janhunen S, Kalkkinen N, Timmusk T, Tuominen RK, Saarma M. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature. 2007 Jul 5;448(7149):73-7.
Lindholm P, Peränen J, Andressoo JO, Kalkkinen N, Kokaia Z, Lindvall O, Timmusk T, Saarma M. MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol Cell Neurosci. 2008 Nov;39(3):356-71.
Lieu CA, Subramanian T. The interhemispheric connections of the striatum: Implications for Parkinson's disease and drug-induced dyskinesias. Brain Res Bull. 2012 Jan 4;87(1):1-9.
Luthman J, Fredriksson A, Sundström E, Jonsson G, Archer T. Selective lesion of central dopamine or noradrenaline neuron systems in the neonatal rat: motor behavior and monoamine alterations at adult stage. Behav Brain Res. 1989 Jul 1;33(3):267-77.
Meissner WG, Frasier M, Gasser T, Goetz CG, Lozano A, Piccini P, Obeso JA, Rascol O, Schapira A, Voon V, Weiner DM, Tison F, Bezard E. Priorities in Parkinson's disease research. Nat Rev Drug Discov. 2011 May;10(5):377-93.
| CONFIDENTIAL 58 |
Mizobuchi N, Hoseki J, Kubota H, Toyokuni S, Nozaki J, Naitoh M, Koizumi A, Nagata K. ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element. Cell Struct Funct. 2007;32(1):41-50.
Oh-Hashi K, Tanaka K, Koga H, Hirata Y, Kiuchi K. Intracellular trafficking and secretion of mouse mesencephalic astrocyte-derived neurotrophic factor. Mol Cell Biochem. 2012 Apr;363(1-2): 35-41.
Palgi M, Greco D, Lindström R, Auvinen P, Heino TI. Gene expression analysis of Drosophilaa Manf mutants reveals perturbations in membrane traffic and major metabolic changes. BMC Genomics. 2012 Apr 11;13:134.
Parkash V, Lindholm P, Peränen J, Kalkkinen N, Oksanen E, Saarma M, Leppänen VM, Goldman A. The structure of the conserved neurotrophic factors MANF and CDNF explains why they are bifunctional. Protein Eng Des Sel. 2009 Apr;22(4):233-41.
Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003 Apr;20(2):173-88.
Rosenblad C, Martinez-Serrano A, Björklund A. Intrastriatal glial cell line-derived neurotrophic factor promotes sprouting of spared nigrostriatal dopaminergic afferents and induces recovery of function in a rat model of Parkinson's disease. Neuroscience. 1998 Jan;82(1):129-37.
Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994 Mar;59(2):401-15.
Sauer H, Rosenblad C, Björklund A. Glial cell line-derived neurotrophic factor but not transforming growth factor beta 3 prevents delayed degeneration of nigral dopaminergic neurons following striatal 6-hydroxydopamine lesion. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8935-9.
Shridhar V, Rivard S, Shridhar R, Mullins C, Bostick L, Sakr W, Grignon D, Miller OJ, Smith DI. A gene from human chromosomal band 3p21.1 encodes a highly conserved arginine-rich protein and is mutated in renal cell carcinomas. Oncogene. 1996 May 2;12(9):1931-9.
Switzer RC, Brain J, Tipton B, Segovia C, Ahmad SO, Benkovic SA. A novel use of combined tyrosine hydroxylase and silver nucleolar staining to determine the effects of a unilateral intrastriatal 6-hydroxydopamine lesion in the substantia nigra: a stereological study. Society for Neuroscience Meeting 2011 Abstract 304.05/XX57
Tadimalla A, Belmont PJ, Thuerauf DJ, Glassy MS, Martindale JJ, Gude N, Sussman MA, Glembotski CC. Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ Res. 2008 Nov 21;103(11):1249-58.
| CONFIDENTIAL 59 |
Takeshima T, Johnston JM, Commissiong JW. (1994) Mesencephalic type 1 astrocytes rescue dopaminergic neurons from death induced by serum deprivation. J Neurosci 14: 4769-4779.
Takeshima T, Shimoda K, Johnston JM, Commissiong JW. (1996) Standardized methods to bioassay neurotrophic factors for dopaminergic neurons. J Neurosci Meth 67: 27-41.
Verma S, Fedak PW, Weisel RD, Butany J, Rao V, Maitland A, Li RK, Dhillon B, Yau TM. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002 May 21;105(20):2332-6.
Voutilainen MH, Bäck S, Pörsti E, Toppinen L, Lindgren L, Lindholm P, Peränen J, Saarma M, Tuominen RK. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson's disease. J Neurosci. 2009 Jul 29;29(30):9651-9.
Voutilainen MH, Bäck S, Peränen J, Lindholm P, Raasmaja A, Männistö PT, Saarma M,Tuominen RK. Chronic infusion of CDNF prevents 6-OHDA-induced deficits in a rat model of Parkinson's disease. Exp Neurol. 2011 Mar;228(1):99-108.
Winkler C, Sauer H, Lee CS, Björklund A. Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson's disease. J Neurosci. 1996 Nov 15;16(22):7206-15.
Yu YQ, Liu LC, Wang FC, Liang Y, Cha DQ, Zhang JJ, Shen YJ, Wang HP, Fang S, Shen YX. Induction profile of MANF/ARMET by cerebral ischemia and its implication for neuron protection. Cereb Blood Flow Metab. 2010 Jan; 30(1):79-91.
Zhou C, Xiao C, Commissiong JW, Krnjević K, Ye JH. Mesencephalic astrocyte-derived neurotrophic factor enhances nigral gamma-aminobutyric acid release. Neuroreport. 2006 Feb 27;17(3):293-7.
| CONFIDENTIAL 60 |
