Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - TG THERAPEUTICS, INC. | v355956_8k.htm |

NASDAQ: TGTX September 2013

This presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements are often, but not always, made through the use of words or phrases such as “anticipates”, “expects”, “plans”, “believes”, “intends”, and similar words or phrases. Such statements involve risks and uncertainties that could cause TG Therapeutics’ actual results to differ materially from the anticipated results and expectations expressed in these forward - looking statements. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks and uncertainties inherent in clinical trials, drug development, and commercialization. You are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date hereof. All forward - looking statements are qualified in their entirety by this cautionary statement and TG Therapeutics undertakes no obligation to update these statements, except as required by law. Forward Looking Safe Harbor Statement 2

3 ▪ Emerging biopharmaceutical company focused on cancer & autoimmune - related diseases ▪ Formed as a spin - out from LFB Biotechnologies, a French pharmaceutical company in January 2012 ▪ $0.5B sales ▪ Private company, sole shareholder the French government ▪ Developing two drugs for B - cell cancers – Leukemia and Lymphoma ▪ TG - 1101 – Novel Glyco - Engineered, Anti - CD20 monoclonal antibody Same class as Rituxan ®, which has ~$7BB WW Sales Enhanced ADCC profile to increase potency Activity demonstrated in CLL and NHL in Phase 1/2 studies ▪ TGR - 1202 – N ovel PI3K δ inhibitor Same class as CAL - 101 and IPI - 145 Currently in Phase 1 dose escalation study Potential best in class attributes TG Therapeutics, Inc.

4 Today… Treatment of B - cell cancers Evolving market dynamics Treatment Line of Therapy Sales Rituxan 1 st and 2 nd ~$5B Benda mustine 1 st and 2 nd ~$1B CHOP 1 st and 2 nd Nominal Chemotherapy 3 rd and Salvage Nominal Future…non - chemotherapy - based combinations, with: ▪ Anti - CD20’s continuing to be the backbone; and ▪ Novel kinase inhibitors • BTK inhibitors • PI3K Delta inhibitors Market opportunity could easily double in next 5 - 10 years

5 Anti - CD20 + Kinase Inhibitor Combination Therapy is the Future

6 Anti - CD20 Landscape mAb’s Company Attribute Status Rituxan (rituximab) Roche Type 1, Chimeric Approved NHL, CLL, RA, GPA, MPA Arzerra ( Ofatumumab ) Genmab /GSK Type 1, high CDC Approved R/R CLL GA101 ( Obinutuzumab ) Roche Type 2, high ADCC, PCD Phase III (primarily frontline settings) TG - 1101 ( ublituximab ) TG Therapeutics Type 1, high ADCC Ph I/II Single Agent and Combo w/ Revlimid ongoing in Rituxan Rel /Ref NHL, CLL
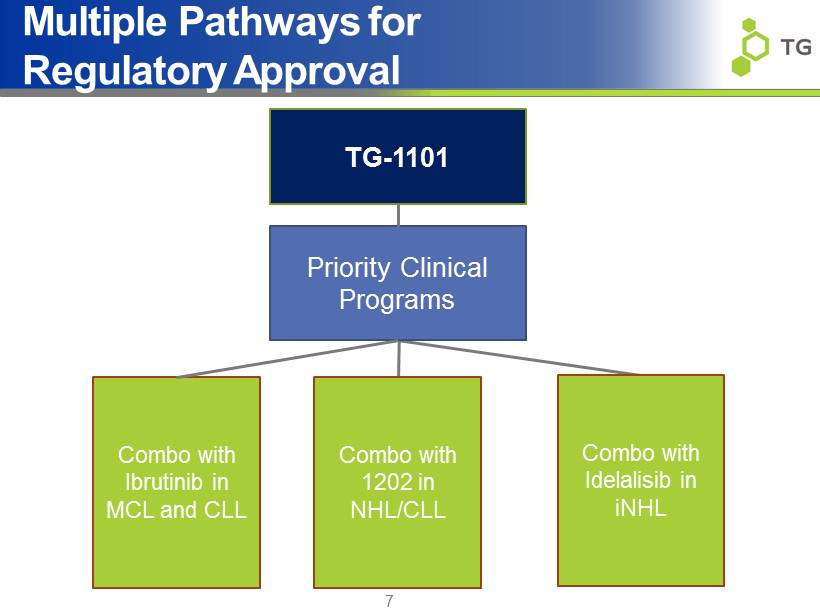
Multiple Pathways for Regulatory Approval 7 TG - 1101 Combo with 1202 in NHL/CLL Combo with Idelalisib in iNHL Priority Clinical Programs Combo with Ibrutinib in MCL and CLL

▪ Same target (CD20) as Rituxan ® but NOT a biogeneric or biosimilar ▪ Unique protein sequence ▪ Potential advantages over current standard of care: ▪ Glycoengineered to enhance the cell killing effects of the body’s immune response — (Antibody Dependent Cellular Cytotoxicity) ▪ Demonstrated activity in “low” CD20 expressing tumors, a characteristic of Rituxan ® resistance ▪ Binding to novel epitope on the CD20 antigen, which may also aid in overcoming Rituxan ® resistance 8 TG - 1101 ( ublituximab )

0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 50,000 0 1 2 3 4 5 6 7 8 9 10 11 12 Lymphocytes (109/L) Month 9 Part II Patients 12 Evaluable 11 * CR 0 PR at M4 7 (63.6%) SD at M4 4 PD at M4 0 PR at M6 5 (45.5%) In a pivotal study of rituximab monotherapy in patients with previously treated CLL/SLL, an overall response rate of 13% was observed . 1 *pt: premature withdrawal for secondary acute leukemia (1) McLaughlin et al, 1998 TG - 1101 in the Treatment of Rel /Ref CLL Phase 1b Efficacy Results 100% “Peripheral Response” seen at M4 TG - 1101 weekly x 8 No maintenance

o 10 / 12 patients were evaluable for efficacy ( 2 patients were too early for response assessment ) o 5 patients achieved an objective response ( 3 CRs, 2 PRs) 10 90% of evaluable patients had a reduction in target lesion ASCO 2013 Presentation: TG - 1101 Phase 1 Efficacy Results in Rel /Ref NHL

Upcoming Combination Trials 11 ▪ TG - 1101 in Combination with Ibrutinib ▪ Eligibility: CLL and MCL (as per ibrutinib label) ▪ Sites: 20 - 30 Centers ▪ Status : Site review/contracts ▪ Target Start Date: Prior to October 30, 2013; awaiting ibrutinib approval ▪ Lead Centers: USON and Columbia University ▪ TG - 1101 in Combination with TGR - 1202 ▪ Eligibility: all B - Cell Lymphomas and CLL ▪ Sites : 7 - 10 Centers ▪ Status : IRB Review ▪ Target Start Date: Before YE2013 ▪ Lead Center: MD Anderson

TGR - 1202 (PI3K - δ INHIBITOR) 12
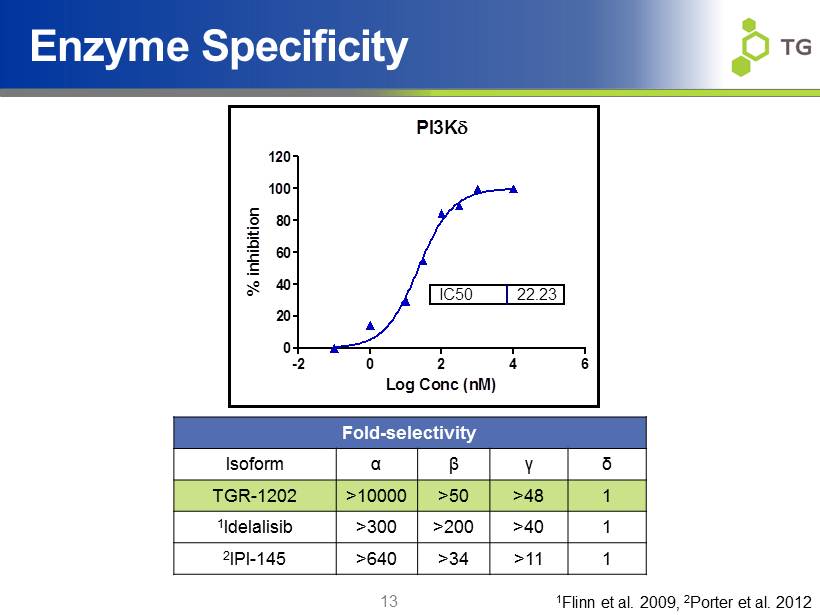
13 Enzyme Specificity PI3K -2 0 2 4 6 0 20 40 60 80 100 120 IC50 22.23 Log Conc (nM) % inhibition Fold - selectivity Isoform α β γ δ TGR - 1202 >10000 >50 >48 1 1 Idelalisib >300 >200 >40 1 2 IPI - 145 >640 >34 >11 1 1 Flinn et al. 2009, 2 Porter et al. 2012

TGR - 1202 vs. Idelalisib 14 Friedman et al 2012, ASH Poster ▪ Blinded in vitro study conducted at Duke University comparing TGR - 1202 and Idelalisib in CLL patient cells (n=7) Equivalent dose dependent cytotoxicity Equivalent suppression of pAKT Equivalent dose dependent induction of apoptosis
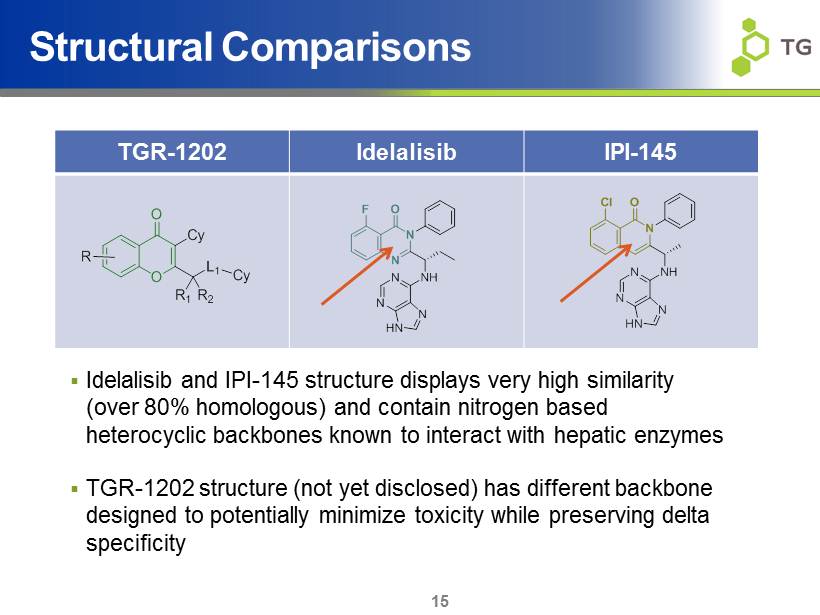
Structural Comparisons ▪ Idelalisib and IPI - 145 structure displays very high similarity (over 80% homologous ) and contain nitrogen based heterocyclic backbones known to interact with hepatic enzymes ▪ TGR - 1202 structure (not yet disclosed) has different backbone designed to potentially minimize toxicity while preserving delta specificity TGR - 1202 Idelalisib IPI - 145 N N O N N HN N NH F O Cy L 1 Cy O R 1 R 2 R N O N N HN N NH Cl 15

Pre - Clinical Pharmacokinetics of TGR - 1202 16 TGTX/ Rhizen Internal Data Parameter Units Mice Rat Dog Monkey Dose mg/kg 20 20 30 50 C max µM 7.81 6.08 4.21 2.68 AUC 0 - t µM.hr 33.96 59.41 94.86 29.29 AUC 0 - inf µM.hr 34.02 105.01 172.65 30.73 T max hr 0.50 1.50 7.00 3.00 t ½ hr 2.39 17.85 37.13 10.17 16

17 Phase I First - in - Human Study of TGR - 1202 Patients with Rel /Ref Hematologic Malignancies ▪ Includes Patients with Relapsed/Refractory Hematologic Malignancies ▪ No limit on prior therapies ▪ Continuous once daily oral dose (QD) Cohort 1 Cohort 2 Cohort 3 Cohort 4 Cohort 5 Cohort 6 Cohort 7 50 mg 100 mg 200 mg 400 mg 800 mg 1200 mg 1800 mg ▪ Study Chair: Michael Savona, MD, Sarah Cannon Research Institute Current dose level Dosing beyond 1800mg TBD
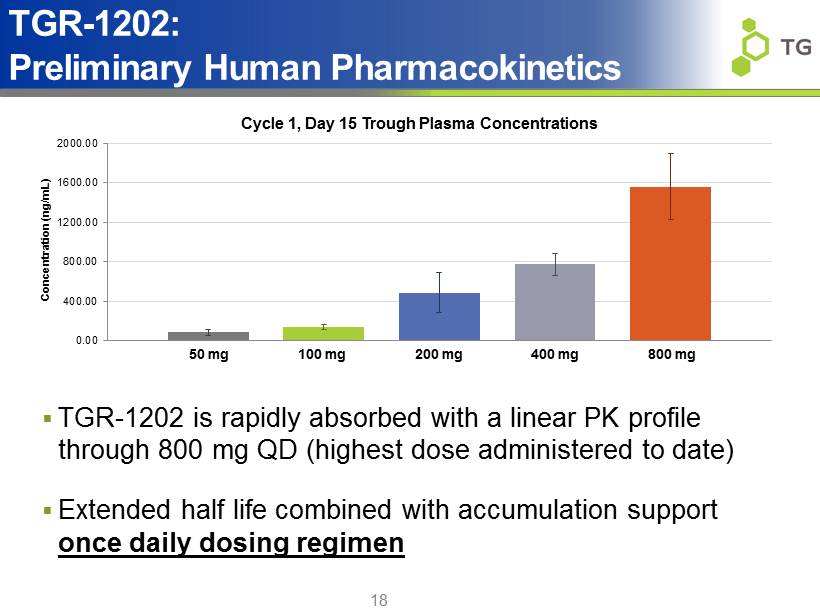
18 0.00 400.00 800.00 1200.00 1600.00 2000.00 Concentration (ng/mL) TGR - 1202: Preliminary Human Pharmacokinetics 50 mg 100 mg 200 mg 400 mg 800 mg Cycle 1, Day 15 Trough Plasma Concentrations ▪ TGR - 1202 is rapidly absorbed with a linear PK profile through 800 mg QD (highest dose administered to date) ▪ Extended half life combined with accumulation support once daily dosing regimen
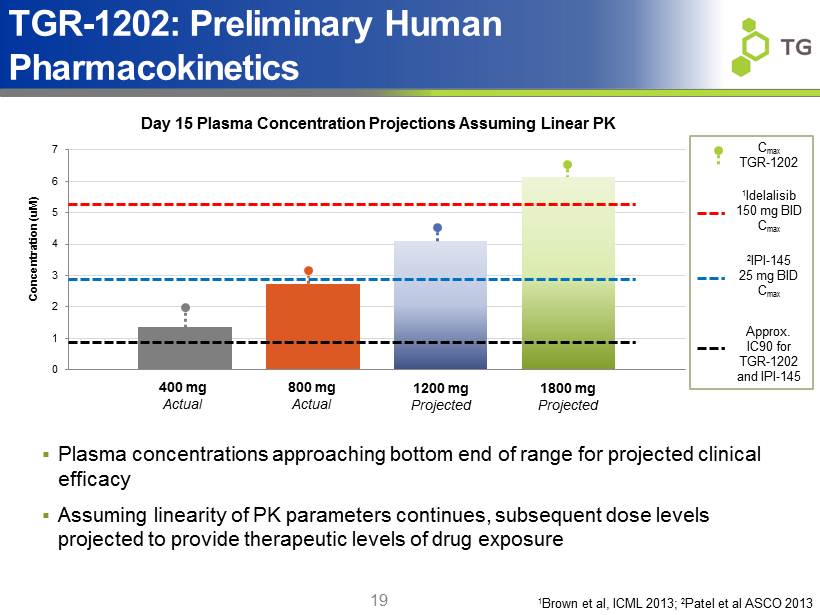
0 1 2 3 4 5 6 7 Concentration ( uM ) 19 TGR - 1202: Preliminary Human Pharmacokinetics 800 mg Actual 1200 mg Projected 1800 mg Projected Day 15 Plasma Concentration Projections Assuming Linear PK 1 Idelalisib 150 mg BID C max 2 IPI - 145 25 mg BID C max ▪ Plasma concentrations approaching bottom end of range for projected clinical efficacy ▪ Assuming linearity of PK parameters continues, subsequent dose levels projected to provide therapeutic levels of drug exposure 1 Brown et al, ICML 2013; 2 Patel et al ASCO 2013 Approx. IC90 for TGR - 1202 and IPI - 145 400 mg Actual C max TGR - 1202

20 TGR - 1202: Preliminary Findings ▪ Extended half - life supports 1x daily dosing ▪ Linear PK through 800mg qd ▪ Only one DLT observed: Gr. 3 rash at 800mg possibly related to study drug ▪ Rash resolved and patient restarted without recurrence ▪ No drug related hepatotoxicity observed to date ▪ Single incident of Gr . 1 elevated GGT at a lower dose, deemed unrelated ▪ Delta - like activity and disease control beyond 6 months observed
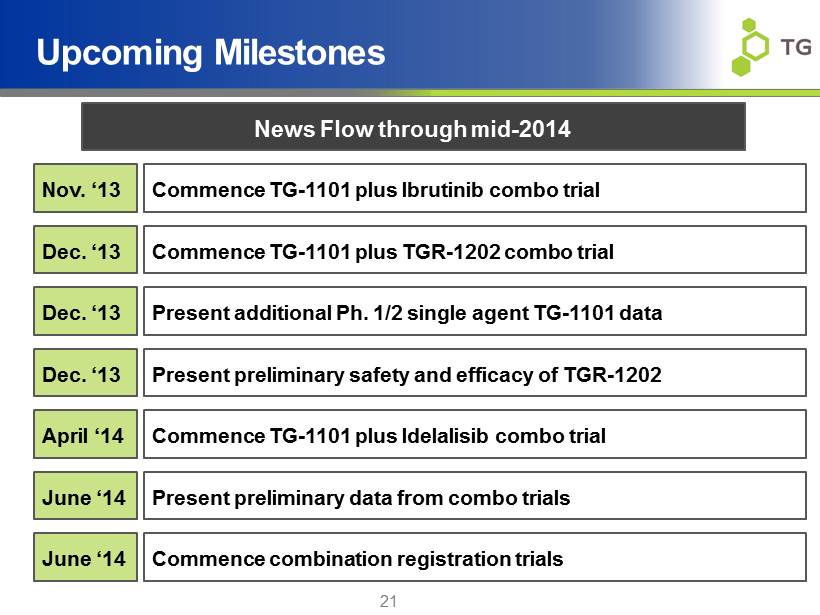
21 Upcoming Milestones Commence TG - 1101 plus Ibrutinib combo trial Commence TG - 1101 plus TGR - 1202 combo trial Present preliminary safety and efficacy of TGR - 1202 Present preliminary data from combo trials Commence combination registration trials Present additional Ph. 1/2 single agent TG - 1101 data News Flow through mid - 2014 Nov. ‘13 Dec. ‘13 Dec. ‘13 Dec. ‘13 June ‘14 June ‘14 Commence TG - 1101 plus Idelalisib combo trial April ‘14
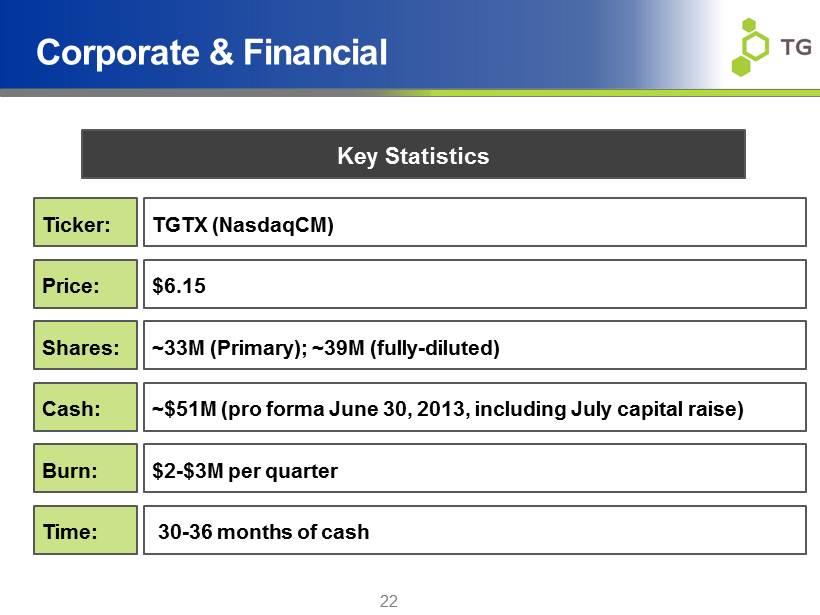
22 Corporate & Financial TGTX ( NasdaqCM ) $6.15 ~$51M (pro forma June 30, 2013, including July capital raise) $2 - $3M per quarter 30 - 36 months of cash ~33M (Primary); ~39M (fully - diluted) Key Statistics Ticker: Price: Shares: Cash: Burn: Time:

23 Key Takeaways ▪ Unequivocal activity of TG - 1101 in CLL and NHL ▪ Positioning TG - 1101 as the backbone of combination therapy in Rituxan ® relapsed/refractory patients, a multi - billion dollar opportunity ▪ Multiple combination regulatory pathways for TG - 1101 ▪ Novel PI3K Delta inhibitor with potential best in class attributes ▪ Dramatic results when PI3K Delta’s are combined with CD20’s, approaching 100% ORR

NASDAQ: TGTX
