Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REPROS THERAPEUTICS INC. | v355246_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - REPROS THERAPEUTICS INC. | v355246_ex99-1.htm |

Developing clinical stage small molecule therapeutics to treat hormonal and reproductive system disorders

Preliminary Summary of ZA - 300 • All subjects have completed 6 months of Androxal treatment and assessments for safety • Data not locked or fully audited

ZA - 300 Protocol • Secondary hypogonadal men • Morning Testosterone < 300 ng / dL • BMI between 25 to 42 • Initial dose of 12.5 mg Androxal – U p - titrated to 25.0 mg if Testosterone is < 450 ng / dL • 6 Months of treatment • 30 Sites
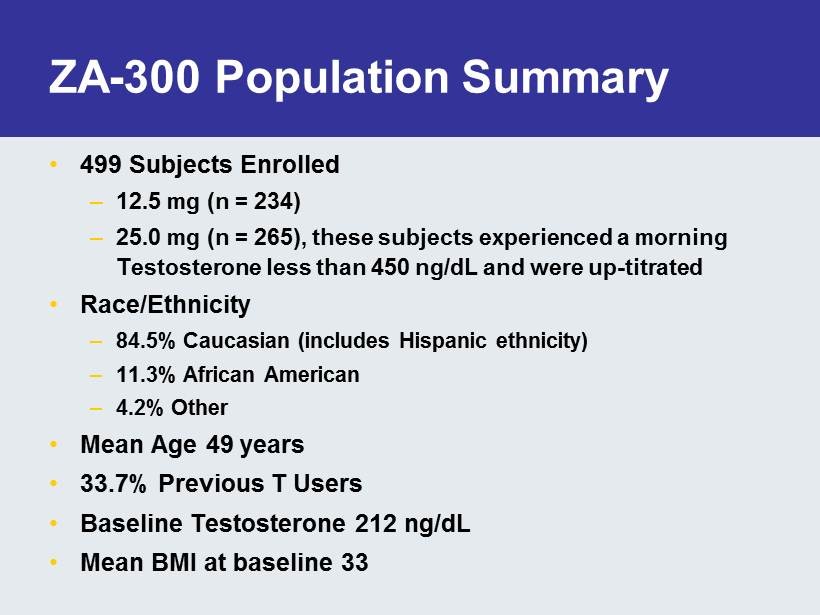
ZA - 300 Population Summary • 499 Subjects Enrolled – 12.5 mg (n = 234) – 25.0 mg (n = 265), these subjects experienced a morning Testosterone less than 450 ng / dL and were up - titrated • Race/Ethnicity – 84.5% Caucasian (includes Hispanic ethnicity) – 11.3% African American – 4.2% Other • Mean Age 49 years • 33.7% Previous T Users • Baseline Testosterone 212 ng / dL • Mean BMI at baseline 33
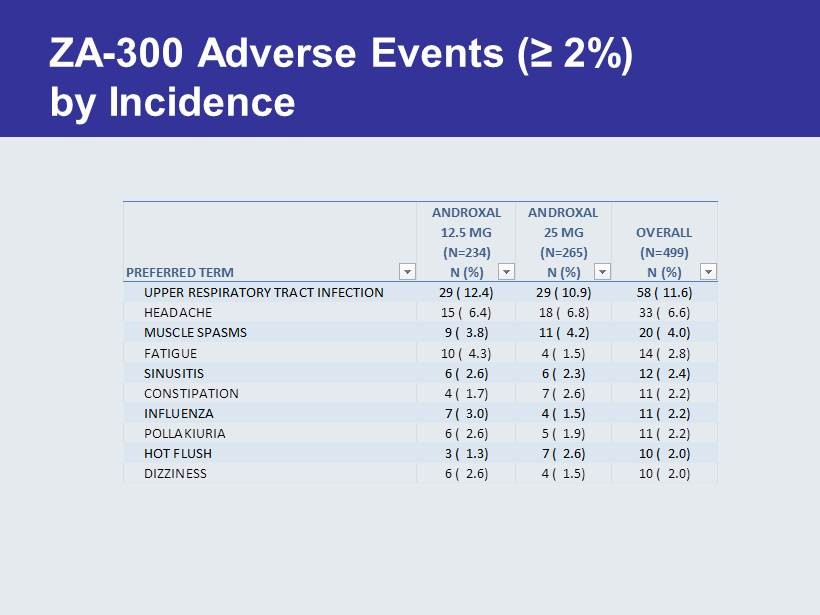
ZA - 300 Adverse Events (≥ 2 %) by Incidence PREFERRED TERM ANDROXAL 12.5 MG (N=234) N (%) ANDROXAL 25 MG (N=265) N (%) OVERALL (N=499) N (%) UPPER RESPIRATORY TRACT INFECTION 29 ( 12.4) 29 ( 10.9) 58 ( 11.6) HEADACHE 15 ( 6.4) 18 ( 6.8) 33 ( 6.6) MUSCLE SPASMS 9 ( 3.8) 11 ( 4.2) 20 ( 4.0) FATIGUE 10 ( 4.3) 4 ( 1.5) 14 ( 2.8) SINUSITIS 6 ( 2.6) 6 ( 2.3) 12 ( 2.4) CONSTIPATION 4 ( 1.7) 7 ( 2.6) 11 ( 2.2) INFLUENZA 7 ( 3.0) 4 ( 1.5) 11 ( 2.2) POLLAKIURIA 6 ( 2.6) 5 ( 1.9) 11 ( 2.2) HOT FLUSH 3 ( 1.3) 7 ( 2.6) 10 ( 2.0) DIZZINESS 6 ( 2.6) 4 ( 1.5) 10 ( 2.0)

ZA - 300 Drug Related Events by Incidence and Severity (> 1%) page 1 of 2 Investigator Assigned Relationship to Androxal of Definitely, Possibly or Probably - Related PREFERRED TERM ANDROXAL 12.5 MG (N=234) N (%) ANDROXAL 25 MG (N=265) N (%) OVERALL (N=499) N (%) HEADACHE 6 ( 2.6) 5 ( 1.9) 11 ( 2.2) MILD 4 ( 1.7) 5 ( 1.9) 9 ( 1.8) MODERATE 2 ( 0.9) 0 ( 0.0) 2 ( 0.4) HOT FLUSH 3 ( 1.3) 5 ( 1.9) 8 ( 1.6) MILD 3 ( 1.3) 4 ( 1.5) 7 ( 1.4) MODERATE 0 ( 0.0) 1 ( 0.4) 1 ( 0.2) MUSCLE SPASMS 4 ( 1.7) 2 ( 0.8) 6 ( 1.2) MILD 3 ( 1.3) 1 ( 0.4) 4 ( 0.8) MODERATE 1 ( 0.4) 1 ( 0.4) 2 ( 0.4) INCREASED APPETITE 4 ( 1.7) 1 ( 0.4) 5 ( 1.0) MILD 3 ( 1.3) 1 ( 0.4) 4 ( 0.8) MODERATE 1 ( 0.4) 0 ( 0.0) 1 ( 0.2) DIZZINESS 3 ( 1.3) 2 ( 0.8) 5 ( 1.0) MILD 2 ( 0.9) 2 ( 0.8) 4 ( 0.8) MODERATE 1 ( 0.4) 0 ( 0.0) 1 ( 0.2) MOOD SWINGS 4 ( 1.7) 1 ( 0.4) 5 ( 1.0) MILD 4 ( 1.7) 1 ( 0.4) 5 ( 1.0) ACNE 4 ( 1.7) 1 ( 0.4) 5 ( 1.0) MILD 3 ( 1.3) 1 ( 0.4) 4 ( 0.8) MODERATE 1 ( 0.4) 0 ( 0.0) 1 ( 0.2) FATIGUE 3 ( 1.3) 1 ( 0.4) 4 ( 0.8) MILD 2 ( 0.9) 0 ( 0.0) 2 ( 0.4) MODERATE 1 ( 0.4) 1 ( 0.4) 2 ( 0.4)

ZA - 300 Drug Related Events by Incidence and Severity (> 1%) page 2 of 2 Investigator Assigned Relationship to Androxal of Definitely, Possibly or Probably - Related PREFERRED TERM ANDROXAL 12.5 MG (N=234) N (%) ANDROXAL 25 MG (N=265) N (%) OVERALL (N=499) N (%) OEDEMA PERIPHERAL 3 ( 1.3) 0 ( 0.0) 3 ( 0.6) MILD 3 ( 1.3) 0 ( 0.0) 3 ( 0.6) GLYCOSYLATED HAEMOGLOBIN INCREASED 3 ( 1.3) 0 ( 0.0) 3 ( 0.6) MILD 1 ( 0.4) 0 ( 0.0) 1 ( 0.2) MODERATE 2 ( 0.9) 0 ( 0.0) 2 ( 0.4) PROSTATIC SPECIFIC ANTIGEN INCREASED 3 ( 1.3) 0 ( 0.0) 3 ( 0.6) MILD 1 ( 0.4) 0 ( 0.0) 1 ( 0.2) MODERATE 2 ( 0.9) 0 ( 0.0) 2 ( 0.4) WEIGHT INCREASED 3 ( 1.3) 0 ( 0.0) 3 ( 0.6) MILD 2 ( 0.9) 0 ( 0.0) 2 ( 0.4) MODERATE 1 ( 0.4) 0 ( 0.0) 1 ( 0.2) RASH 0 ( 0.0) 3 ( 1.1) 3 ( 0.6) MILD 0 ( 0.0) 3 ( 1.1) 3 ( 0.6)

ZA - 300 Events Causing Discontinuation Subject ID Androxal Dose Preferred Term Relationship 06-006 12.5mg Muscle Spasms Possibly 06-027 12.5mg Paraesthesia/Muscle Spasms Possibly 06-028 12.5mg Aggression Possibly 06-039 12.5mg Constipation Possibly 07-062 12.5mg Vitreous Floaters Defintley Not Related 12-045 12.5mg Abdominal Pain/Flank pain/Epididymitis/Prostatitis Possibly 12-075 12.5mg Visual Impairment Probably Not Related 12-083 12.5mg Weight increased/Fatigue/Libido Decreased/Myalgia/Vision Blurred Probably Not Related 16-001 12.5mg Vision blurred/Nausea/Chest Pain/Dizziness/Headache/Dyspnoe Possibly 17-017 12.5mg Pre-existing Seminoma Defintley Not Related 17-021 12.5mg Hot flush/ED/Weight increased/Mood swings Probably 17-048 12.5mg URTI Probably 18-011 12.5mg Prostatic specific antigen increased Not Specified 22-052 12.5mg Increased appetite/Headache Possibly 23-022 12.5mg Fatigue/Weight increased/Change in sustained attention Possibly 24-038 12.5mg Diplopia Probably 26-008 12.5mg HIV test positive Not Specified 30-041 12.5mg Vision blurred Possibly 02-001 25mg Pollakiuria Probably 02-029 25mg DVT/Pulmonary Embolism Possibly 15-004 25mg Weight increased Probably Not Related 15-037 25mg Myalgia/Paraesthesia Not Specified 16-023 25mg Lethargy Not Specified 17-016 25mg Depression/Weight increased Not Specified 19-005 25mg Hot flush/Breast Enlargement/ED/Testicular atrophy Possibly 30-047 25mg Hepatic enzyme increased Not Specified 43-024 25mg Rosacea/thrombophlebitis Possibly 43-042 25mg Irritability Possibly Queries are outstanding for events missing relationship determination.

ZA - 300 SAEs Subject Number Dose Event Term Relationship 06-042 12.5 mg Hypotension/Bradycardia post Knee Replacement Surgery Not Related 07-052 12.5 mg Gallstone/Cholecystectomy Not Related 12-071 12.5 mg Biliary Colic/Common Bile duct dilatation Not Related 14-080 12.5 mg TIA Not Related 15-003 12.5 mg Food Poisoning Not Related 16-001 12.5 mg Chest pain/Shortness of breath Not Related 16-029 12.5 mg Cellulitis Not Related 17-007 12.5 mg Deep vein thrombosis temporally related to flight Not Related 17-017 12.5 mg Pre-existing Seminoma Left Testicle Not Related 02-029 25 mg Deep vein thrombosis/Pulmonary embolism Possibly Related 06-034 25 mg Atrial flutter Not Related 07-002 25 mg Diverticulitis Not Related 19-007 25 mg Cellulitis secondary to dog bite Not Related 19-016 25 mg Diverticulitis Probably Not Related 22-001 25 mg Bladder diverticulum/Kidney infection Unlikely

ZA - 300 PSA • 37 subjects (7.4%) experienced an increase in PSA of more than 0.75 at the last assessment on Androxal – There was no difference in the incidence of PSA elevations by dose (p = 0.6438) • No subjects required biopsy • Most subject’s PSA decreased once therapy was discontinued and testosterone subsequently decreased • Elevated PSA is a pharmacological response to elevated T and not an adverse drug effect

ZA - 300 Testosterone Response • Over 90% of subjects had a maximum morning Testosterone above 300 ng / dL • Almost 67% of subjects had a maximum morning Testosterone above 450 ng / dL • 53% of subjects were up - titrated (T < 450 ng / dL ) • Only 3 subjects experienced a maximum morning Testosterone above 1,040 ng / dL ( 1720, 1479, 1264) – Two subjects reported concomitant testosterone replacement use – LH values of third subject suggest concomitant testosterone use • Subjects who required up - titration were less - likely to respond to treatment – 13.6% of subjects treated with 25 mg had a maximum morning Testosterone less than 300 ng / dL – 3.6% of subjects treated with 12.5 mg had a maximum morning Testosterone less than 300 ng / dL

ZA - 300 Summary • Androxal was found to be well - tolerated • Testosterone levels are restored to normal in a large majority of subjects • Only one SAE (DVT/Pulmonary Embolism) was deemed possibly related to study medication by the Investigator – Causality may be associated with increased Testosterone rather than Androxal – Subject had multiple risk factors: obesity, hypertension, race, tall stature, height, family history of clotting disorder • Overall safety profile is similar, if not superior, to testosterone replacements
