Attached files
| file | filename |
|---|---|
| 8-K - 8-K - KYTHERA BIOPHARMACEUTICALS INC | a13-20822_18k.htm |
Exhibit 99.1
|
|
The beauty of science. September 17, 2013 |
|
|
Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. In some cases, you can identify forward-looking statements by the following words: “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “contemplate,” “believe,” “estimate,” “predict,” “project,” “seek,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements relate to future events or our future financial performance or condition and involve known and unknown risks, uncertainties and other factors that could cause our actual results, levels of activity, performance or achievement to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties and other factors are described more fully in our Annual Report on Form 10-K for the year ended December 31, 2012 filed with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” In light of the significant uncertainties in our forward-looking statements, you should not place undue reliance on these statements or regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all. The forward-looking statements contained in this presentation represent our estimates and assumptions only as of the date of this presentation and, except as required by law, we undertake no obligation to update or revise publicly any forward looking statements, whether as a result of new information, future events or otherwise after the date of this presentation. This presentation also contains estimates, projections and other information concerning our industry, our business, and the market for our drug candidate, as well as data regarding market research, estimates and forecasts prepared by our management. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. |
|
|
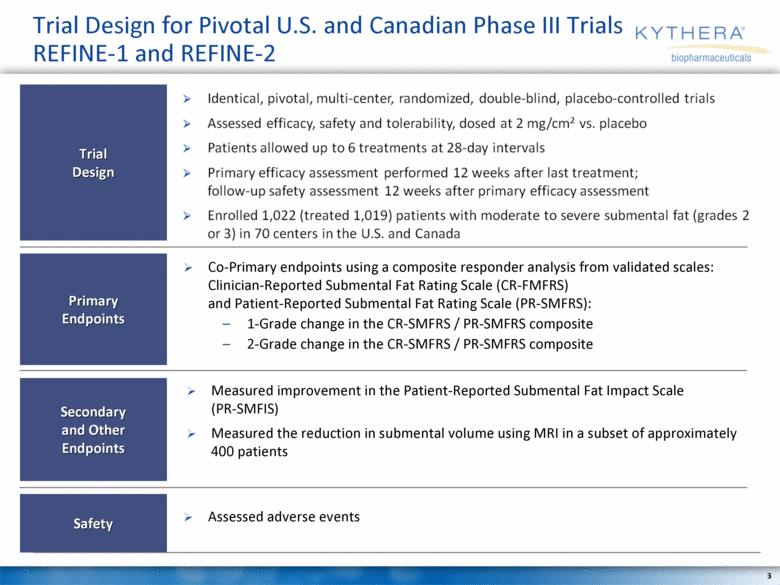
Trial Design for Pivotal U.S. and Canadian Phase III Trials REFINE-1 and REFINE-2 Assessed adverse events Co-Primary endpoints using a composite responder analysis from validated scales: Clinician-Reported Submental Fat Rating Scale (CR-FMFRS) and Patient-Reported Submental Fat Rating Scale (PR-SMFRS): 1-Grade change in the CR-SMFRS / PR-SMFRS composite 2-Grade change in the CR-SMFRS / PR-SMFRS composite Measured improvement in the Patient-Reported Submental Fat Impact Scale (PR-SMFIS) Measured the reduction in submental volume using MRI in a subset of approximately 400 patients Safety Trial Design Primary Endpoints Secondary and Other Endpoints |
|
|
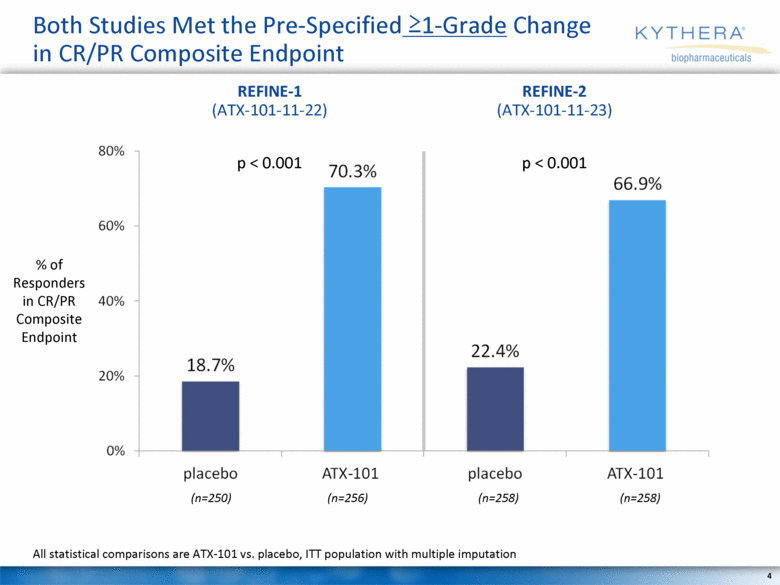
Both Studies Met the Pre-Specified >1-Grade Change in CR/PR Composite Endpoint (n=250) All statistical comparisons are ATX-101 vs. placebo, ITT population with multiple imputation (n=256) (n=258) (n=258) REFINE-1 (ATX-101-11-22) REFINE-2 (ATX-101-11-23) p < 0.001 p < 0.001 % of Responders in CR/PR Composite Endpoint |
|
|
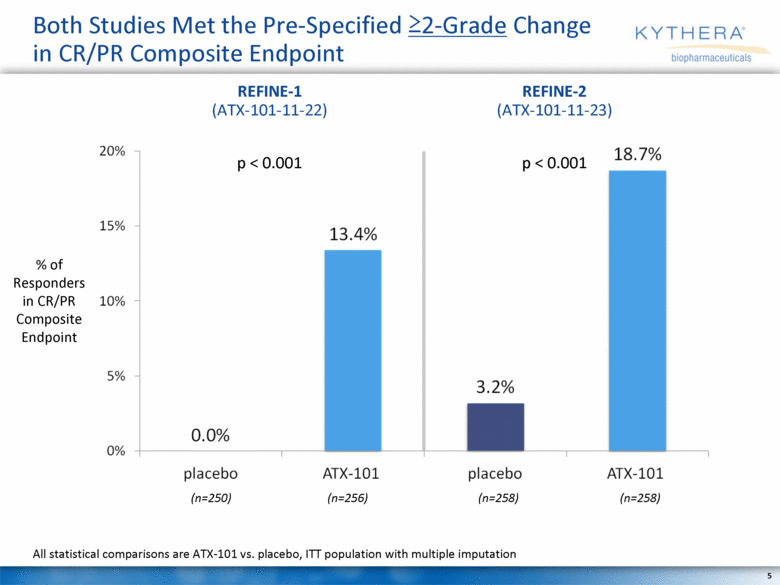
Both Studies Met the Pre-Specified >2-Grade Change in CR/PR Composite Endpoint % of Responders in CR/PR Composite Endpoint All statistical comparisons are ATX-101 vs. placebo, ITT population with multiple imputation REFINE-1 (ATX-101-11-22) REFINE-2 (ATX-101-11-23) p < 0.001 p < 0.001 (n=250) (n=256) (n=258) (n=258) |
|
|
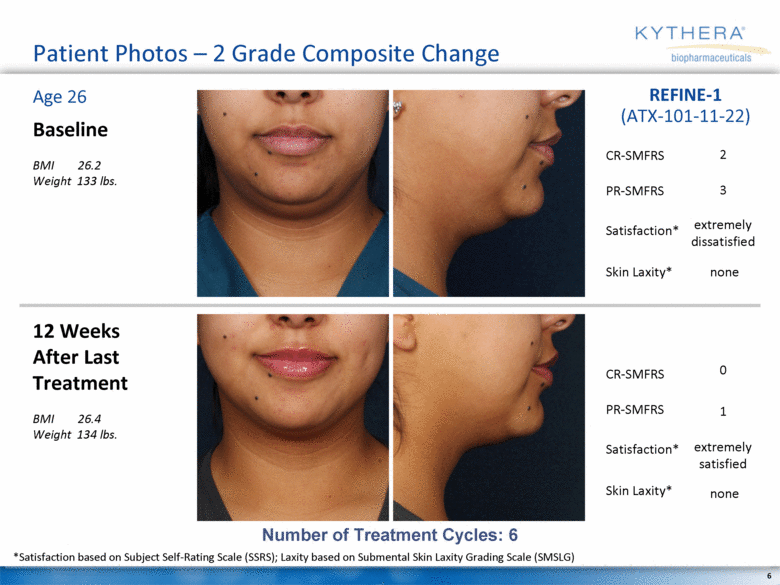
Patient Photos – 2 Grade Composite Change Baseline BMI 26.2 Weight 133 lbs. 12 Weeks After Last Treatment BMI 26.4 Weight 134 lbs. Age 26 REFINE-1 (ATX-101-11-22) Number of Treatment Cycles: 6 2 extremely dissatisfied 3 none *Satisfaction based on Subject Self-Rating Scale (SSRS); Laxity based on Submental Skin Laxity Grading Scale (SMSLG) 0 extremely satisfied 1 none CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* |
|
|
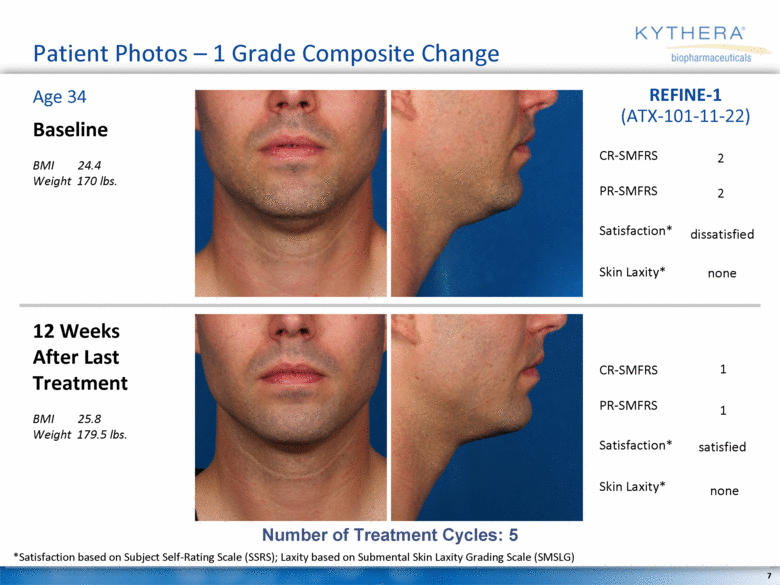
Patient Photos – 1 Grade Composite Change Baseline BMI 24.4 Weight 170 lbs. 12 Weeks After Last Treatment BMI 25.8 Weight 179.5 lbs. Age 34 REFINE-1 (ATX-101-11-22) Number of Treatment Cycles: 5 2 dissatisfied 2 none 1 satisfied 1 none *Satisfaction based on Subject Self-Rating Scale (SSRS); Laxity based on Submental Skin Laxity Grading Scale (SMSLG) CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* |
|
|
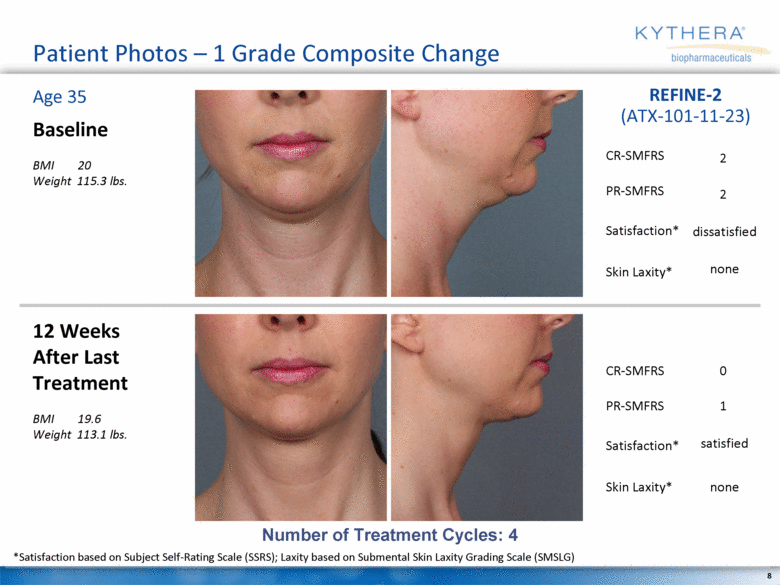
Patient Photos – 1 Grade Composite Change Baseline BMI 20 Weight 115.3 lbs. 12 Weeks After Last Treatment BMI 19.6 Weight 113.1 lbs. Age 35 REFINE-2 (ATX-101-11-23) Number of Treatment Cycles: 4 *Satisfaction based on Subject Self-Rating Scale (SSRS); Laxity based on Submental Skin Laxity Grading Scale (SMSLG) 2 dissatisfied 2 none 0 satisfied 1 none CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* |
|
|
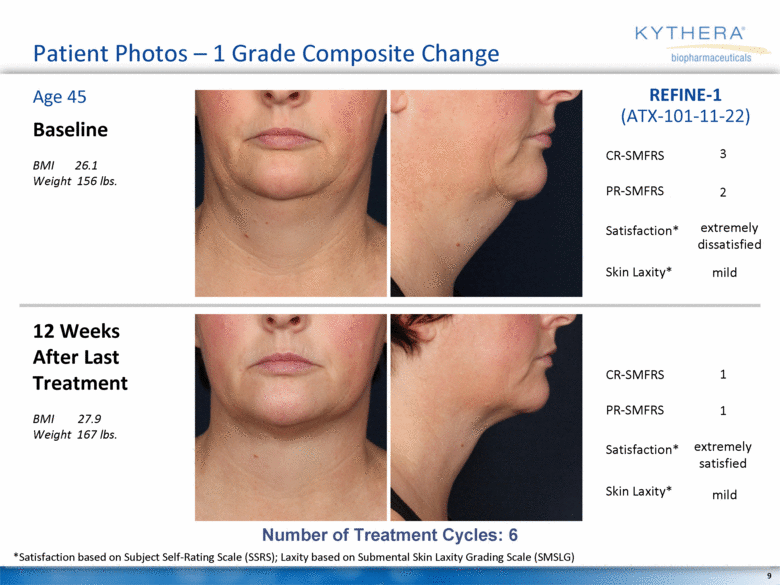
Patient Photos – 1 Grade Composite Change Baseline BMI 26.1 Weight 156 lbs. 12 Weeks After Last Treatment BMI 27.9 Weight 167 lbs. Age 45 REFINE-1 (ATX-101-11-22) Number of Treatment Cycles: 6 *Satisfaction based on Subject Self-Rating Scale (SSRS); Laxity based on Submental Skin Laxity Grading Scale (SMSLG) 3 2 mild 1 1 mild extremely dissatisfied extremely satisfied CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* |
|
|
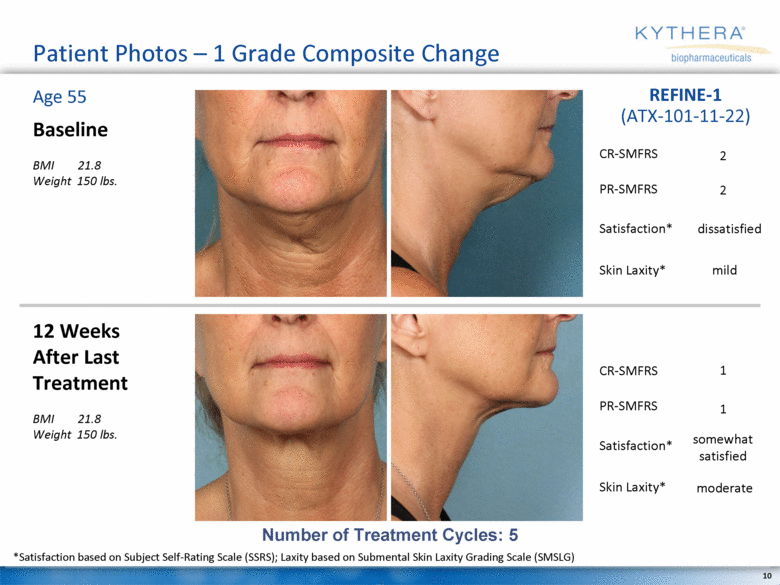
Patient Photos – 1 Grade Composite Change Baseline BMI 21.8 Weight 150 lbs. 12 Weeks After Last Treatment BMI 21.8 Weight 150 lbs. Age 55 REFINE-1 (ATX-101-11-22) Number of Treatment Cycles: 5 *Satisfaction based on Subject Self-Rating Scale (SSRS); Laxity based on Submental Skin Laxity Grading Scale (SMSLG) 2 2 mild dissatisfied 1 1 moderate somewhat satisfied CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* |
|
|
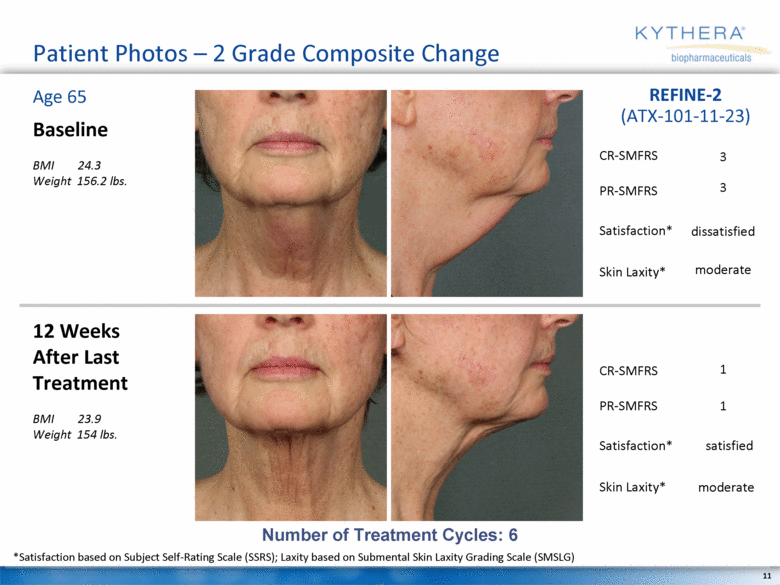
Patient Photos – 2 Grade Composite Change Baseline BMI 24.3 Weight 156.2 lbs. 12 Weeks After Last Treatment BMI 23.9 Weight 154 lbs. Age 65 REFINE-2 (ATX-101-11-23) Number of Treatment Cycles: 6 *Satisfaction based on Subject Self-Rating Scale (SSRS); Laxity based on Submental Skin Laxity Grading Scale (SMSLG) 3 3 moderate dissatisfied 1 1 satisfied moderate CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* CR-SMFRS Satisfaction* PR-SMFRS Skin Laxity* |
|
|
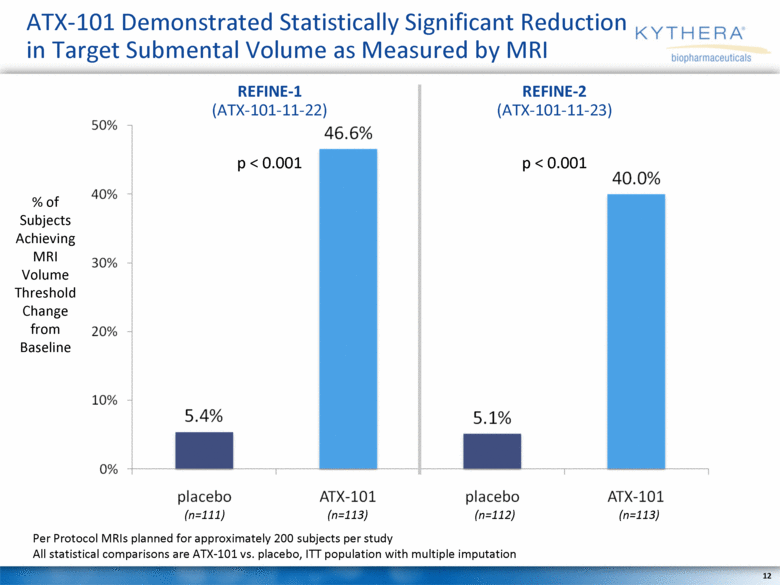
ATX-101 Demonstrated Statistically Significant Reduction in Target Submental Volume as Measured by MRI % of Subjects Achieving MRI Volume Threshold Change from Baseline (n=111) (n=113) (n=112) (n=113) Per Protocol MRIs planned for approximately 200 subjects per study All statistical comparisons are ATX-101 vs. placebo, ITT population with multiple imputation REFINE-1 (ATX-101-11-22) REFINE-2 (ATX-101-11-23) p < 0.001 p < 0.001 |
|
|
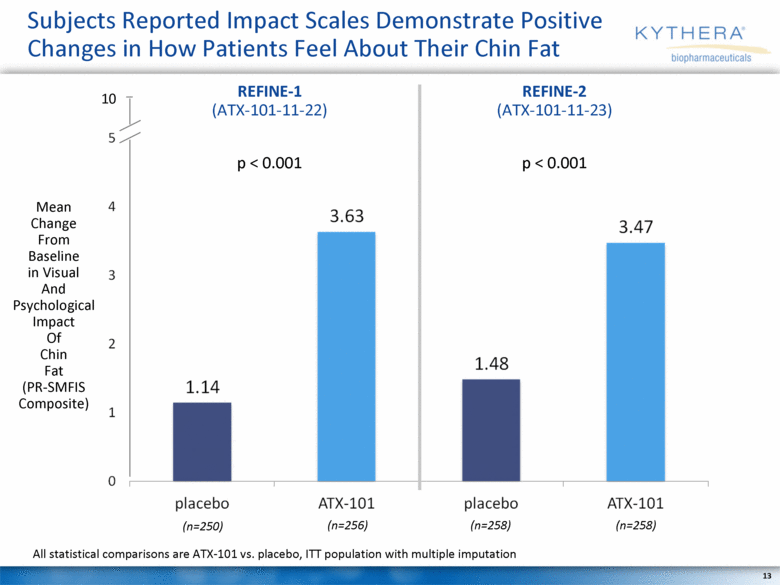
Subjects Reported Impact Scales Demonstrate Positive Changes in How Patients Feel About Their Chin Fat Mean Change From Baseline in Visual And Psychological Impact Of Chin Fat (PR-SMFIS Composite) (n=250) (n=256) (n=258) (n=258) All statistical comparisons are ATX-101 vs. placebo, ITT population with multiple imputation REFINE-1 (ATX-101-11-22) REFINE-2 (ATX-101-11-23) p < 0.001 p < 0.001 |
|
|
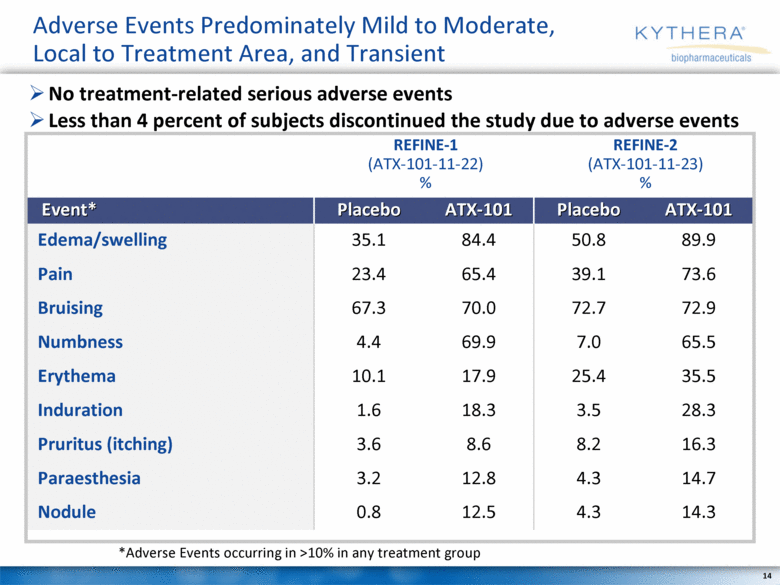
Adverse Events Predominately Mild to Moderate, Local to Treatment Area, and Transient *Adverse Events occurring in >10% in any treatment group No treatment-related serious adverse events Less than 4 percent of subjects discontinued the study due to adverse events Event* Placebo ATX-101 Placebo ATX-101 Edema/swelling 35.1 84.4 50.8 89.9 Pain 23.4 65.4 39.1 73.6 Bruising 67.3 70.0 72.7 72.9 Numbness 4.4 69.9 7.0 65.5 Erythema 10.1 17.9 25.4 35.5 Induration 1.6 18.3 3.5 28.3 Pruritus (itching) 3.6 8.6 8.2 16.3 Paraesthesia 3.2 12.8 4.3 14.7 Nodule 0.8 12.5 4.3 14.3 REFINE-1 (ATX-101-11-22) % REFINE-2 (ATX-101-11-23) % |
|
|
REFINE-1 and 2 Met Primary and Secondary Endpoints 1-grade on the Clinician-Reported Submental Fat Rating Scale (CR-SMFRS) and Patient-Reported Submental Fat Rating Scale (PR-SMFRS) composite 2-grade on the Clinician-Reported Submental Fat Rating Scale (CR-SMFRS) and Patient-Reported Submental Fat Rating Scale (PR-SMFRS) composite Statistically Significant Results on Primary Endpoints Statistically Significant Results on Secondary Endpoints Adverse Events Predominately Mild to Moderate Patient-Reported Submental Fat Impact Scale (PR-SMFIS) Submental Volume measure per MRI Consistent with previous studies, adverse events were predominately mild to moderate, transient and were local to the treatment area Additionally, these adverse events rarely resulted in discontinuation from treatment |
|
|
ATX-101 – Rigorous Development Program Non-clinical Studies and Scale Validation Robust Clinical Development Program Manufacturing Preparedness Full drug safety program including 6 and 9 month studies Characterization of mechanism of action Scale creation and validation per FDA Patient-Reported Outcomes (PRO) Guidance 7 Phase I studies – including PK, histology, lipid profiles 3 Phase II studies elucidating dosing paradigm 4 Phase III studies totaling more than 1,700 patients Successful QT/QTc study 1-year Phase IIIB open label study Ongoing 5-year, long term follow-up study Initial Drug Substance validation completed – 2nd manufacturer ongoing Drug product stability runs completed – stability ongoing |
|
|
ATX-101 – Potential to be First Submental Contouring Injectable Drug Approved for Reduction of Submental Fat or “Double Chin” Science ATX-101 is a proprietary formulation of a purified synthetic version of deoxycholic acid Causes focal adipocytolysis or destruction of fat cells where injected followed by a natural healing response Opportunity The submental region is visually important and impacts overall facial harmony and balance An undesirable submental profile resulting from excess submental fat is a hallmark of aging and can lead to negative self image If approved, ATX-101 will be a first in class injectable drug delivering the following Consistent, meaningful and durable results High patient satisfaction Favorable safety profile Low barrier to physician adoption with a ready group of patients who are already receiving facial injectables After Before |
|
|
The beauty of science. September 17, 2013 |