Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Sorrento Therapeutics, Inc. | d595928d8k.htm |
| EX-2.1 - EX-2.1 - Sorrento Therapeutics, Inc. | d595928dex21.htm |
| EX-99.2 - EX-99.2 - Sorrento Therapeutics, Inc. | d595928dex992.htm |
| EX-99.1 - EX-99.1 - Sorrento Therapeutics, Inc. | d595928dex991.htm |
 September 2013
Exhibit
99.3
Sorrento Therapeutics, Inc.
Next-Generation
Cancer Therapeutics |
 2
This presentation contains "forward-looking statements" as that term is
defined under the Private Securities Litigation Reform Act of 1995 (PSLRA),
including statements regarding expectations, beliefs or intentions regarding
our business, technologies and products strategies or prospects. Actual
results may differ from those projected due to a number of risks and
uncertainties, including, but not limited to, the possibility that some or all of
the pending matters and transactions being considered by the Company may not
proceed as contemplated, and by
all other matters specified in Company's filings with the Securities and Exchange
Commission, as well as risks inherent in funding, developing and obtaining
regulatory approvals of new, commercially-viable and competitive
products and product candidates. These statements are made based upon
current expectations that are subject to risk and uncertainty and information
available to the Company as of the date of this presentation. The company does not
undertake to update forward-looking statements in this presentation to
reflect actual results, changes in assumptions or changes in other factors
affecting such forward-looking information. Assumptions and other
information that could cause results to differ from those set forth in the
forward-looking information can be found in the Company's filings with the
Securities and Exchange Commission, including its most recent periodic
report. We intend that all forward- looking statements be subject to the
safe-harbor provisions of the PSLRA. Safe Harbor Statement
OTCQB: SRNE |
 Positioned to Become Oncology Leader
Cynviloq™
G-MAB
®
ADC/AfDC
Phase 3
Immuno-
therapy
mAb
candidates
Targeted
therapeutics |
 4
Sorrento’s Next-Generation Cancer Therapeutics
TUMOR
G-MAB
®
•
High-diversity human Ab library
•
10+ lead mAb programs, including
•
FTO and no stacking royalties
ADC
•
G-MAB
®
targets toxin to cancer cell
•
Programs include VEGFR2, c-Met
Cynviloq™
•
Bioequivalence (BE)
pathway for approval
•
Efficacy demonstrated
•
US and EU rights
•
G-MAB
®
targets approved oncolytics to the tumor
•
Effective against inherently target-heterogeneous tumors
Next-generation
Abraxane
®
PD-L1, PD-1, and CCR2 |
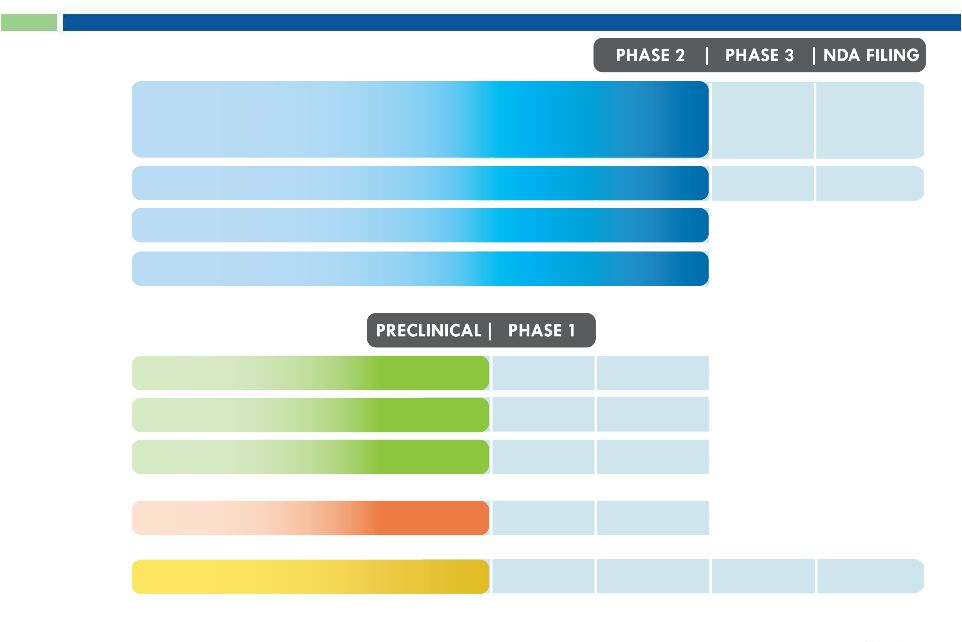 5
Pipeline
INDICATION
INDICATION > TARGET
G-MAB
®
ADC
AfDC
Cynviloq™
Oncology > PD-L1, PD-1, CXCR5
Inflammation > CCR2,
PD-L1, CXCR3
Infectious Disease > MRSA, C.diff
Oncology > VEGFR2,
c-Met, CXCR5, EGFR
Oncology > PD-L1, VEGFR2, c-Met
H1 2015
H2 2015
Metastatic Breast Cancer
Non-Small Cell Lung Cancer
Bladder Cancer (sNDA)
Ovarian Cancer (sNDA)
BE Trial
Q4 2013
Q4 2014 /
Q1 2015
505(b)(2) Pathway
}
Pancreatic Cancer (BE* or sNDA)
* Abraxane
®
orphan drug status (FDA approved: September 2013)
Multiple
Strategic
Partnership
Opportunities
5 |
 Seasoned Management Team
6
Henry Ji, Ph.D.
President, CEO & Director
•
Inventor of G-MAB
®
Technology
•
President & CEO of Stratagene Genomics
•
VP of CombiMatrix and Stratagene
Vuong Trieu, Ph.D.
Chief Scientific Officer
•
Founder and CEO of IgDraSol
•
Co-inventor of IP covering Abraxane
®
•
Instrumental
in
the
approval
of
Abraxane
®
•
Celgene acquired Abraxis Biosciences for > $3 billion
George Uy
Chief Commercial Officer
•
CCO of IgDraSol
•
Directed the launches of Abraxane
®
, Xeloda
®
& Fusilev
®
•
Built commercial infrastructures and organizations in startup
companies
Richard Vincent
CFO and Director
•
$430M sale of Elevation to Sunovion-Dainippon
•
Meritage Pharma option agreement with ViroPharma ($90M
upfront + milestones)
•
$310M sale of Verus asthma program to AstraZeneca
•
Elan: various acquisitions and divestitures with aggregate values
in excess of $300M |
 Oncology Franchise
Cynviloq™
Phase 3
7 |
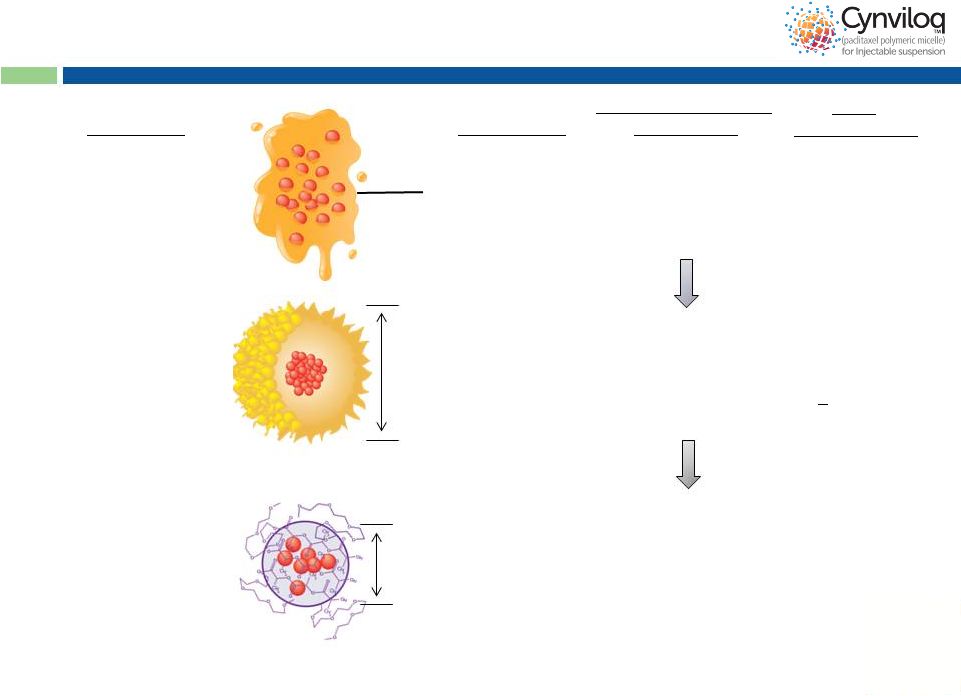 8
Cynviloq™
is the 3 Generation
Paclitaxel Therapy
Mean size
~25 nm
Cynviloq™
paclitaxel
polymeric micelle
Chemical
polymer:
Poly-lactide and
polyethylene glycol
diblock copolymer
3
>300 mg/m
(up to 435 mg/m )
Abraxane
®
nab-
Mean size
130 nm
Biological
polymer:
Donor-derived human
serum albumin (HSA)
2
260 mg/m
Taxol
®
paclitaxel
Cremophor EL
excipient:
Polyoxyethylated
castor oil
Formulation
Generation
1
175 mg/m
Maximum Tolerated
Dose (MTD)
Peak
Product
Sales
~ $1.6B (WW in 2000)
$430M
(2012, mostly MBC)
Est. >$1.7B* (US)
30-50% Abraxane
sales conversion +
new indications
(BC, OC etc)
*Analyst projection for MBC+NSCLC+PC
2
2
2
2
®
rd
nd
st
rd
paclitaxel |
 9
Why Cynviloq™?
1.
Abraxane sold to Celgene for > $3 billion
2.
Efficacy demonstrated in Phase 2 and Phase 3 studies
•
Phase 2 studies affirmed clinical activity in MBC, NSCLC, PC,
ovarian cancer (OC) and bladder cancer (BC)
•
Interim data from Phase 3 study in MBC patients shows activity comparable to
Abraxane
®
•
Approved and marketed in S. Korea and other countries
3.
FDA concurred that available data supports pursuing the 505(b)(2)
bioequivalence (BE) regulatory submission pathway
4.
Single BE study in MBC patients may be sufficient for approval for
both NSCLC and MBC indications with Abraxane
label
5.
Potential to expand Cynviloq™
label and increase market size
6.
US and EU Rights
7.
Multiple unique advantages over Abraxane
®
®
® |
 10
Cynviloq™
has Unique Advantages
vs. Abraxane
®
Cynviloq™
Abraxane
®
Taxol
®
Cynviloq™
Advantage
>300
260
175
Potential for
higher efficacy
Rapid reconstitution:
no foaming concerns
Convenience for busy
practices and pharmacies
No donor-derived human
serum albumin (HSA)
No viral / prion concerns
Convenient storage
conditions
No requirement for
controlled temp storage
No microbial growth
Chemical polymer
Cremophor-free
Reduced side effects
Dosing
q3w
q3w* &
weekly**
q3w & weekly
Exploits PK advantage
@ higher dose
* MBC; ** NSCLC & PC
Maximum Tolerated Dose
(mg/m
²
) |
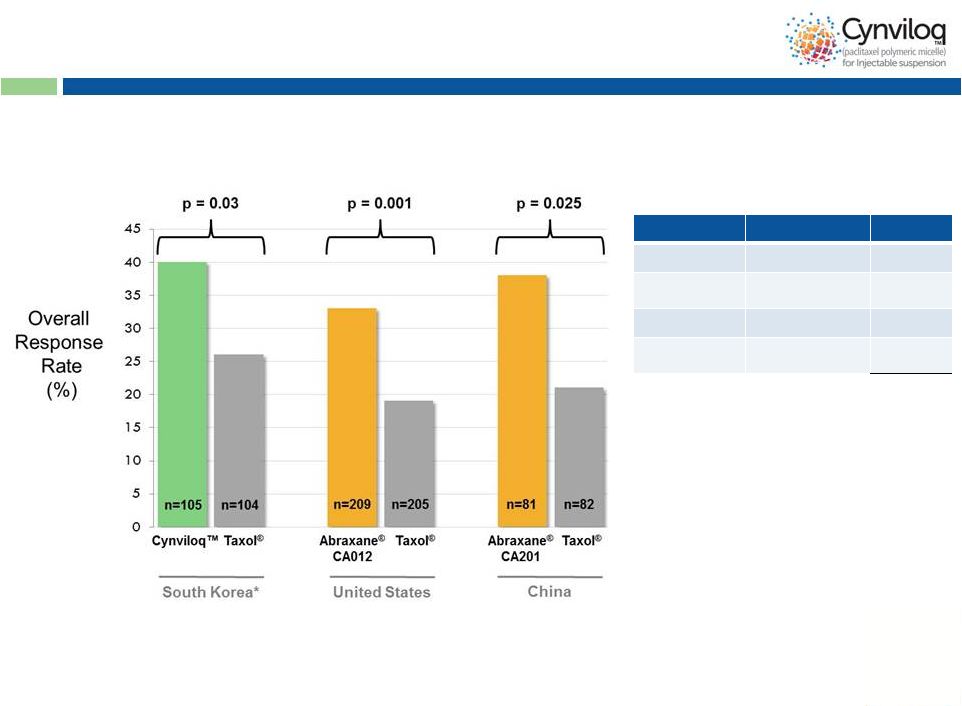 11
Cynviloq™
is Efficacious
Stage
Trial
Patients
Phase 1
MTD
80
Phase 2
MBC, NSCLC,
PC, OC, BC
259
Phase 3
MBC
105
Post Market
(Safety)
MBC, NSCLC
502
Total
946
Phase 3 study in
MBC Summary of
Clinical Exposures
11
US
approval
for
Abraxane
®
in
MBC
and
NSCLC
for
non-inferiority
against
Taxol
®
based
on
ORR
-
*
No obvious ethnic differences seen between ORR in trials
Interim data from trial; OS and PFS analyses ongoing
|
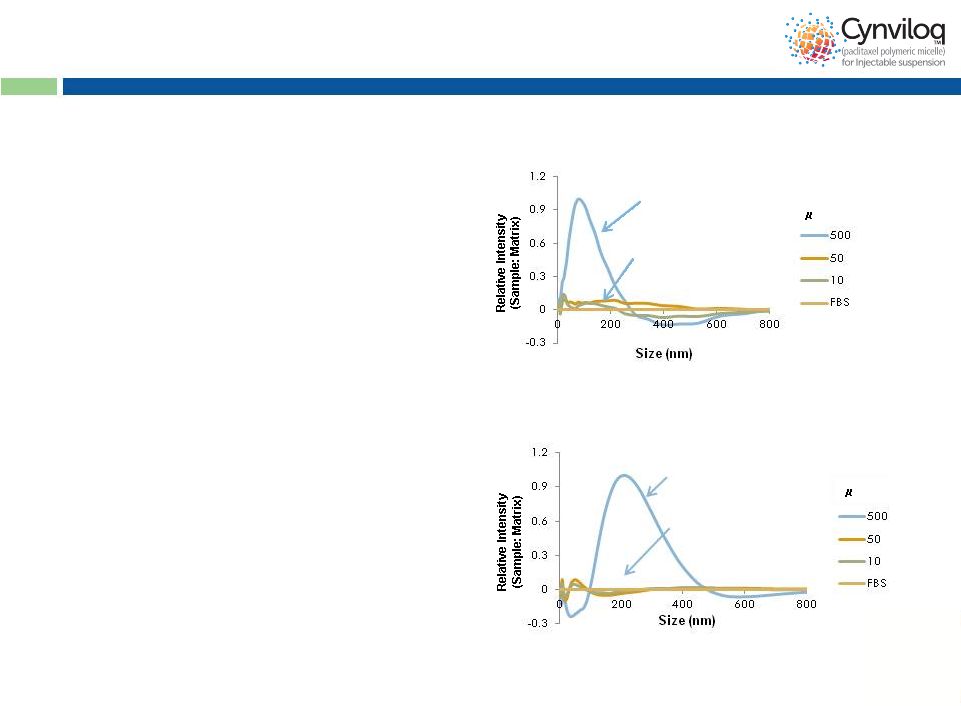 12
Common Mechanism Of Action
* Data on file
FBS = fetal bovine serum
•
Free paclitaxel exploits the
albumin-transport pathway
to preferentially accumulate
in tumor tissues
Intact nanoparticle
(in the vial)
Disintegration
upon dilution
(in blood)
Cynviloq™
Intact nanoparticle
(in the vial)
Disintegration
upon dilution
(in blood)
Abraxane
®
•
Cynviloq™
and Abraxane
®
nanoparticles disintegrate
rapidly in the bloodstream to
release free paclitaxel
g/mL
g/mL |
 13
Simulated PK Parameters Supportive of BE:
Cynviloq™
vs.
Abraxane
Note: Internal calculations done as 95% CI
Per FDA requirement, ratio T/R (Cmax and AUCinf) must be within 80-125%
(90% confidence interval, or CI)
Comparison of mean non-compartmental pharmacokinetic parameters of
Cynviloq™
(T) and Abraxane
®
(R) @ 260 mg/m
2
with 30 min infusion time:
Cmax
(ng/mL)
Ratio
Cmax(T)/Cmax(R)
AUCinf
(ng*h/mL)
Ratio
AUCinf(T)/AUCinf(R)
Cynviloq
TM
(Simulated PK)
19486
99.6%
22198
109.2%
Abraxane
®
(Actual PK)*
19556
20324
®
Cynviloq™
data on file
*
Gardner et al, 2008 |
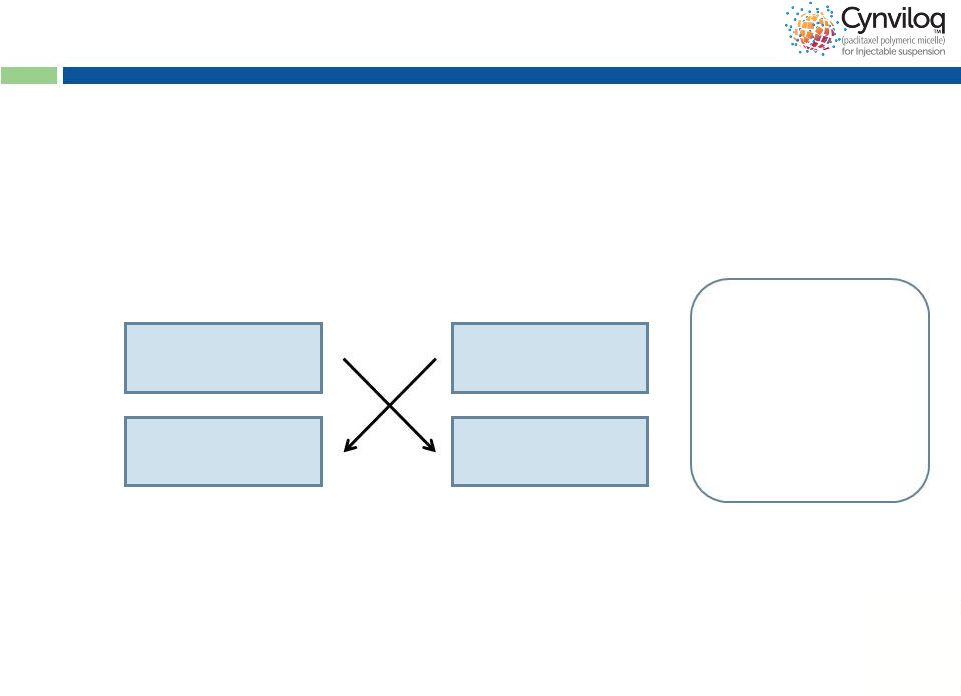 14
505(b)(2) Bioequivalence Dev’t Pathway
•
EOP2 meeting (July 2013): FDA concurrence on 505(b)2 BE as
appropriate regulatory submission pathway
•
Bioequivalence registration study in MBC (Q4 2013)
-
12 months of duration (including patient recruitment)
-
Trial cost less than $5M
•
NDA filing (Q4 2014 / Q1 2015), Approval (Q4 2015 / Q1 2016)
-
MBC and NSCLC and future (PC and MM) Abraxane
®
indications
•
Product launch for MBC and NSCLC (Q4 2015 / Q1 2016)
Abraxane
®
(50 patients)
Cynviloq™
(50 patients)
Cynviloq™
Abraxane
®
Cycle 1
Cycle 2
•
Dose 260 mg/m
•
30 min infusion time
•
3 weeks then cross
over for 3 weeks
•
Endpoint is AUC and
Cmax
2
(90% CI) |
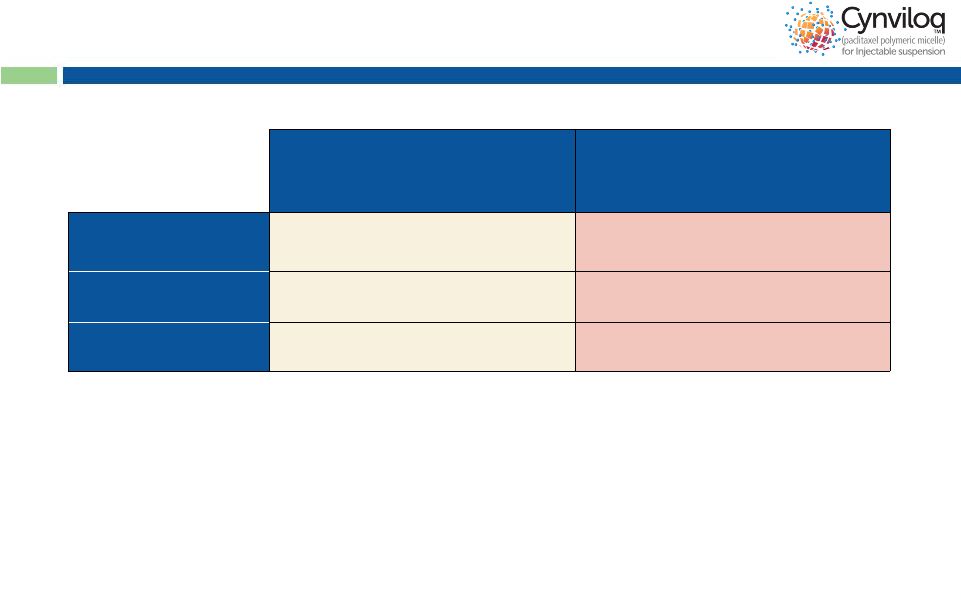 15
Potential to Expand Label Indications –
For
Example:
2
Line
Bladder
Cancer
Cynviloq™
Phase 2 (Korea)*
260-300 mg/m
q3w
n=34
#
Best Supportive Care
Phase 2 (Japan) **/ Phase 3 (EU)***
n=23** / n=108***
Overall Response
Rate (ORR)
21%
-
/ 0%***
Progression Free
Survival
2.7 M
-
/ 1.5 M***
Overall Survival
6.5 M
2.3 M** / 4.3 M***
*
Invest
New
Drugs
(2012)
30:1984–1990
# advanced urothelial carcinoma patients refractory to gemcitabine and
cisplatin ** AUA-
San Diego May 4th-8 ; *** JCO (2009): 4454-61
Summary:
•
High
unmet
need
with
no
FDA
approved
2
nd
line
drug
•
Generally well-tolerated and demonstrated clinical ORR
•
Phase
3-ready
for
development
as
2
nd
line
chemotherapy
in
patients
refractory to platinum-based therapy
nd
th
2 |
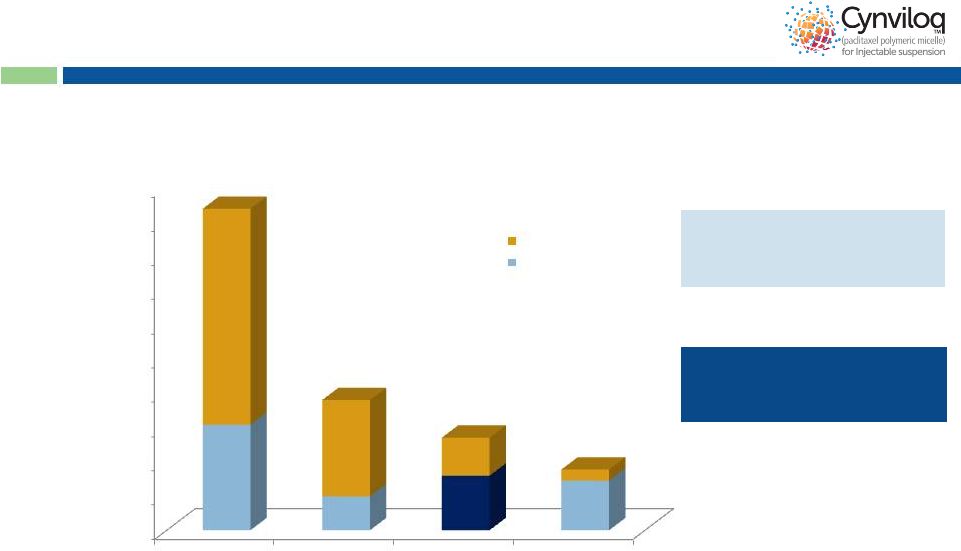 16
~55,000 patients with paclitaxel
as 1
st
line therapy in MBC,
NSCLC & OC
15,903 PC patients eligible for
treatment with Abraxane
®
+
Gemcitabine combination
Cynviloq™
Market Opportunity
Note: In PC, the blue portion represents
# pts treated with gem-based Rx in 2012 #
of
Patients
Treated
in
1
st
Line
(US
Only)
(2012)
Sources: US information, SEER Annual Cancer Review 1975-2006; US Census;
Mattson Jack; UHC and Medicare Claims; IntrinsiQ; Synovate Tandem. WHO
mortality database 2008 http://www.who.int/whosis/whosis/. World Population Prospects. The 2008 Revision. UN Population
Division 2009. http://esa.un.org/unpp/. Roche-Genentech Clinical, Patient Chart
Audits. Total patient numbers represent treatable population. 1
Line
patient
estimates
from
IntrinsiQ
2012
Monthly
LOT
Diag
Combo.
~70,000
patients
treated
with
paclitaxel-based
regimen
in
1
st
line
NSCLC
n = 93,800
MBC
n = 38,000
PC
n = 27,000
OC
n = 17,700
10,000
20,000
30,000
40,000
50,000
60,000
70,000
80,000
90,000
100,000
Other regimens
PAC+ treated
-
30,766
9,880
15,903
14,479
st
16 |
 Therapeutic Antibody Engine |
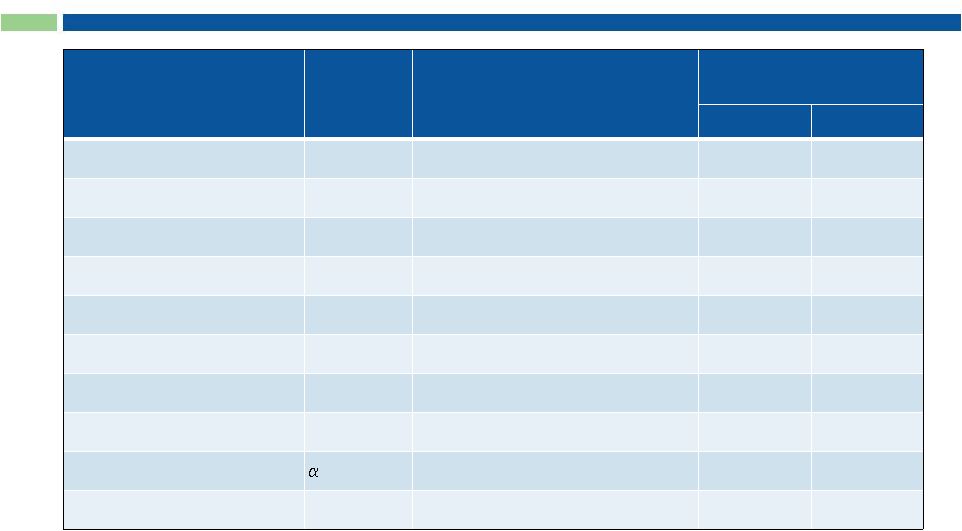 18
Top 10 Selling Therapeutic Antibodies (> $50B)
mAb
Target
Companies
2012 Sales
(US$ billions)*
US
Global
Humira (Adalimumab)
TNF
Abbott; Esai
4.4
9.3
Enbrel (Etanercept)
TNF
Amgen; Pfizer; Takeda
4.0
8.0
Rituxan (Rituximab)
CD20
Roche
3.3
7.0
Remicade (Infliximab)
TNF
J&J; Merck; Mitsubishi Tanabe
3.6
6.6
Herceptin (Trastuzumab)
HER2
Roche
1.7
6.2
Avastin (Bevacizumab)
VEGF
Roche
2.6
6.1
Lucentis (Ranibizumab)
VEGF
Novartis; Roche
1.6
4.0
Erbitux (Cetuximab)
EGFR
BMS; Merck-Serono
0.7
1.8
Tysabri (Natalizumab)
4 integrin
Biogen Idec
0.9
1.6
Xolair (Omalizumab)
IgE
Novartis; Roche
0.7
1.3
*Data Monitor
|
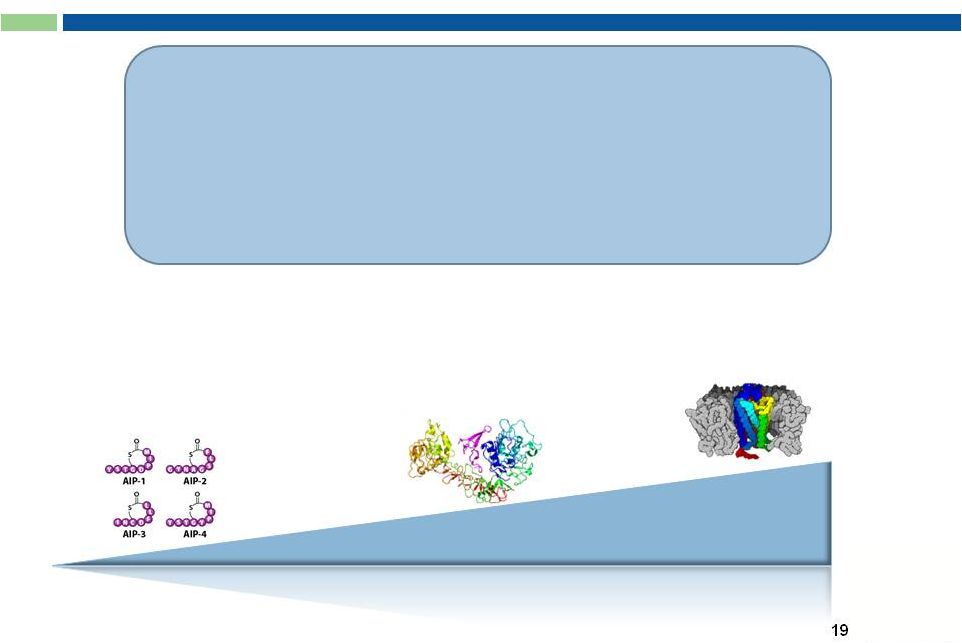 G-MAB
®
Library
Difficult Targets:
Small Molecules
Low molecular weight antigens
(< 2,500 Da; “haptens”)
Validated and/or Hot
Targets:
Medium-sized antigens
Soluble protein ligands
or cell surface receptors
Most Difficult Targets:
Large Complex Molecules
G protein-coupled receptors
(GPCRs)
Size of Target Antigen
•
Proprietary amplification
-
RNA amplification for variable
domains
•
Very high diversity:
V
H
: 7.1 x 10
7
•
Issued and pending patents
on G-MAB
®
library and
individual mAbs
•
Freedom-to-Operate (FTO)
•
No Stacking Royalties
V
L
: 2.9 x 10
8
Combined: 2.1 x 10
16 |
 20
Anti-PD-L1 mAbs Exhibit Potent Activity
In Vitro and In Vivo
In Vitro*
In Vivo**
* mAbs @ 0.05 mg/mL
** xenograft model using H1975 human NSCLC cells; % inhibition relative to control
mAb treatment *** p<0.05, mean tumor volumes are significantly reduced in
STI-A1010 group versus control groups as determined by Mann-Whitney u-test
Day
Competitor mAb
Sorrento mAb |
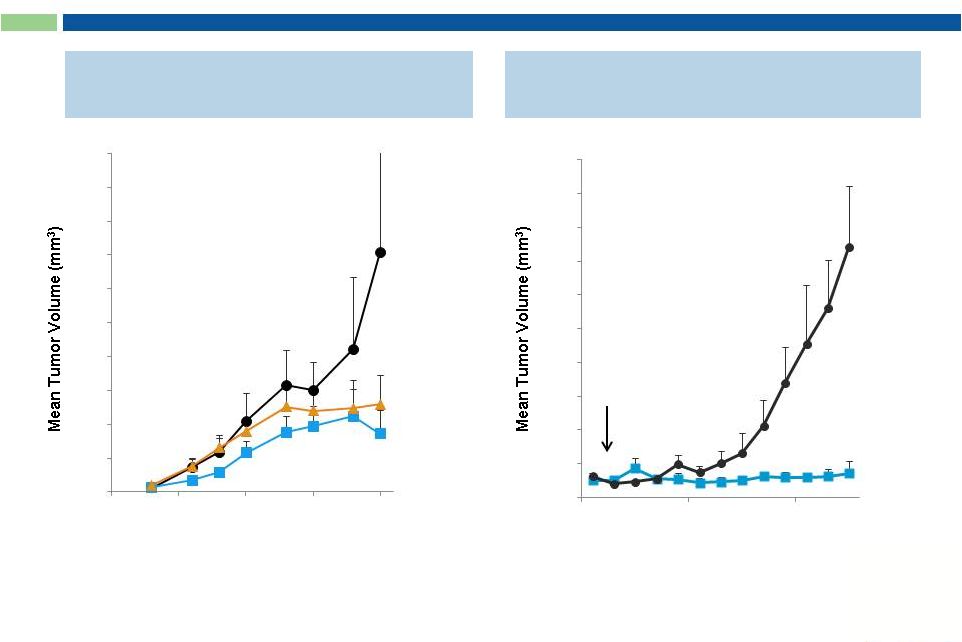 21
“Naked”
Anti-Cancer mAbs Exert Potent
Anti-Tumor Activity In Vivo
Anti VEGFR2 mAb*
Anti c-Met mAb**
treatment
initiated
Days After Tumor Inoculation
Sorrento mAb
PBS
* xenograft model –
human carcinoma cells A431
** xenograft model –
human glioblastoma cells U118
Days After Tumor Inoculation
Sorrento mAb
Anti-VEGF mAb
PBS
0
200
400
600
800
1000
1200
1400
1600
1800
2000
5
10
15
20
25
0
100
200
300
400
500
600
700
800
900
1000
7
14
21
28
35 |
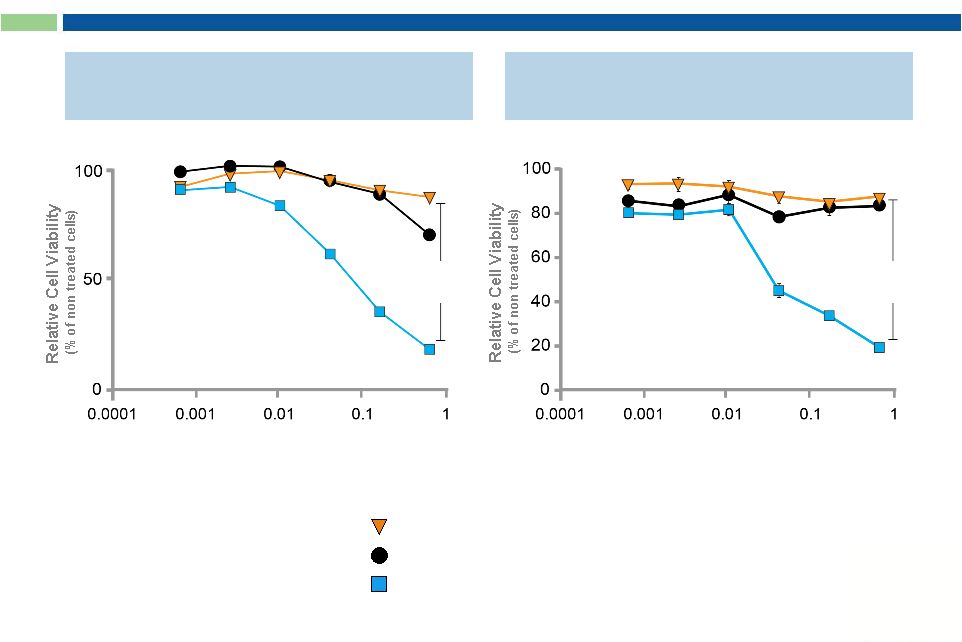 22
Anti-c-Met ADC**
IgG Concentration (nM)
Sorrento ADCs Possess Enhanced Cytotoxic Activity
Compared to “Naked”
mAbs
Leading Competitor mAb
Sorrento mAb + Toxin
Toxin Control
Anti-VEGFR2 ADC*
IgG Concentration (nM)
p value
< 0.0001
p value
< 0.0001
* Human Vascular Endothelial Cells (HUVECs)
** against Human A549 NSCLC Cells |
 23
Targeted Drug Formulation Platform
ADC/AfDC
Targeted
Therapeutics |
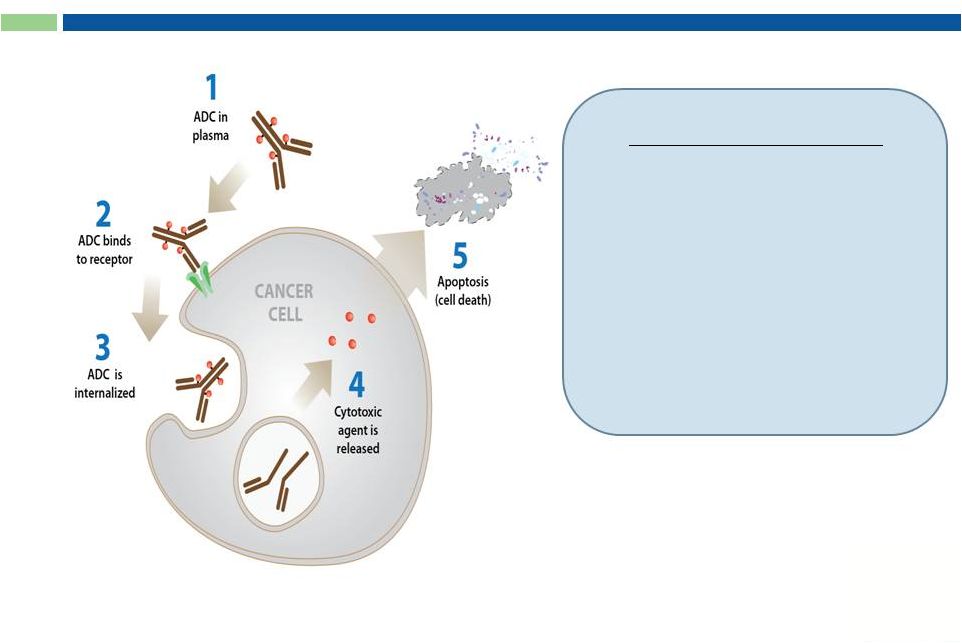 24
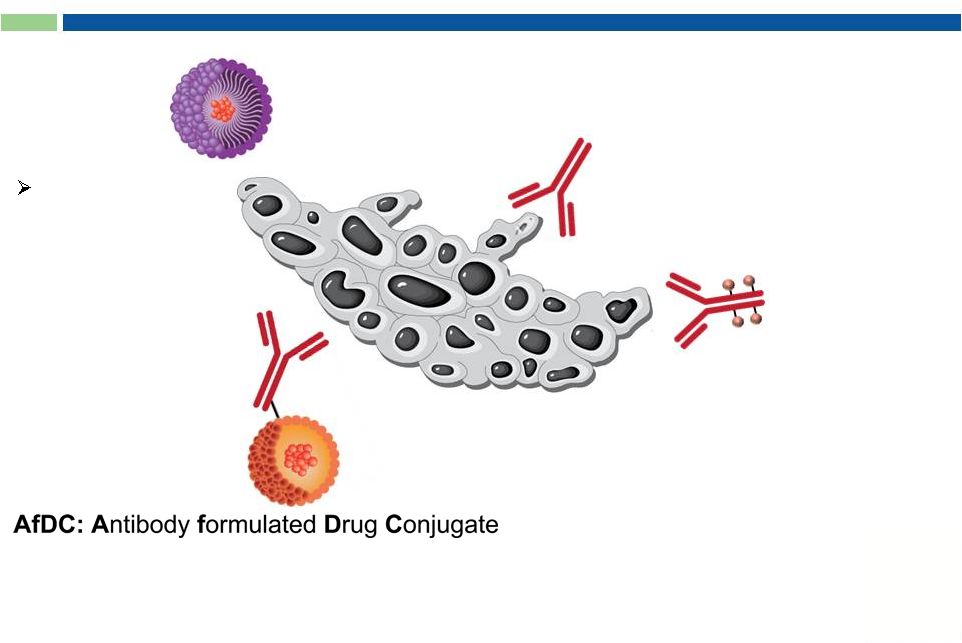
Antibody Drug Conjugates (ADC)
Key Components:
1.
Target-specific internalizing
antibody
2.
Potent cytotoxic prodrugs
3.
Linker and conjugation
chemistries
Drug released in CANCER CELL |
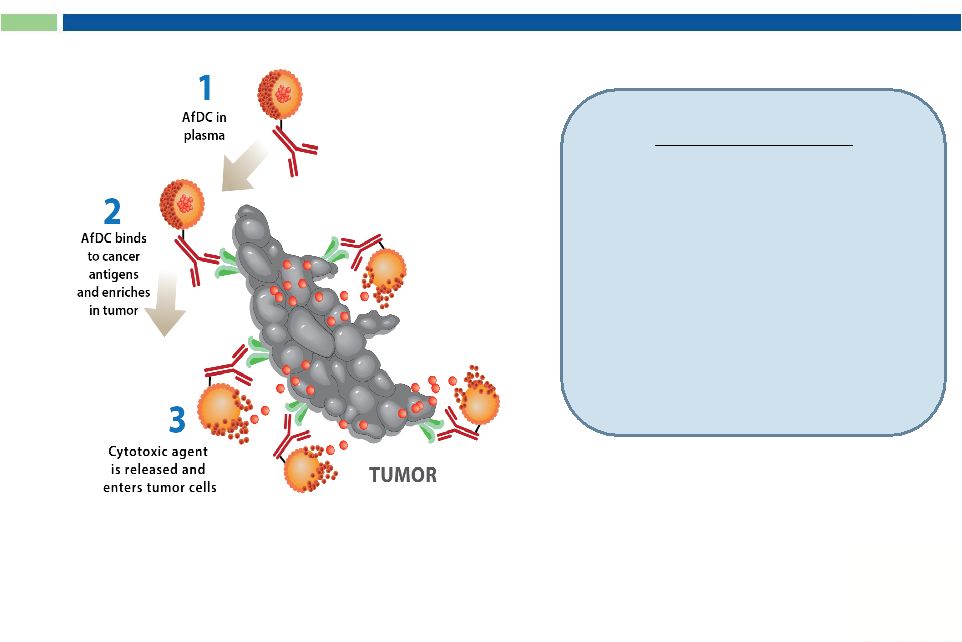 25
Key Features:
1.
2.
3.
Drug released in TUMOR
Antibody formulated Drug Conjugates (AfDC)
Approved oncolytic drug
with known safety profile
No internalization required
Flexibility in combining
mAbs and drugs |
 26
G-MAB
®
, ADC and AfDC Pipeline
G-MAB
®
Antibody
ONCOLOGY
PD-L1
INFLAMMATION
ONCOLOGY /
INFLAMMATION
(GPCR)
INFECTIOUS
DISEASE
PD-1
VEGFR2-ADC
EGFR
PD-L1
RAGE
CGRP
CCR2
CXCR3
CXCR5
MRSA
(STI-A100X)
(STI-A110X)
(STI-A020X)
(STI-B010X)
(STI-B120X)
(STI-B150X)
(STI-B020X)
(STI-A120X)
(STI-B030X)
(STI-C020X)
(STI-A0168)
H1 2015
H2 2015
c-Met
(STI-A060X)
ADC/AfDC
ADC
ADC
PD-L1
(STI-A100X)
AfDC
CCR2
(STI-B020X)
ADC
ADC/AfDC
H2 2015
Multiple
Strategic
Partnership
Opportunities |
 Positioned to Become Oncology Leader
Cynviloq™
Phase 3
G-MAB
®
Immuno-
therapy
mAb
candidates
ADC/AfDC
Targeted
therapeutics |
 28
Small Molecule Oncology Drug, Antibody Library
and ADC Company Valuations
Company
Small Molecule
Oncology Drug
Antibody
Platform
Targeted
Drug Delivery
Mkt Cap*
Sorrento: SRNE
NSCLC, MBC
(Ph3/Registration Trial)
Antibody
Library
ADC/
AfDC
$150M
Puma:
PBYI
MBC (Phase 3)
~$1.6B
Clovis:
CLVS
NSCLC, MBC (Phase 1)
~$2.0B
MorphoSys:
MOR.DE
Antibody
Library
~$1.6B
CAT:
Acquired (2006)
Antibody
Library
~$1.4B
Domantis:
Acquired (2007)
Antibody
Library
~$450M
Seattle Genetics:
SGEN
ADC
~$5.0B
ImmunoGen:
IMGN
ADC
~$1.6B
* based on publicly-available information (09/04/13)
|
 Sorrento Therapeutics
Next-Generation
Cancer Therapeutics
Contact:
Henry Ji
President and CEO
hji@sorrentotherapeutics.com
(858) 668-6923 |
