Attached files
| file | filename |
|---|---|
| 8-K - 8-K - MERRIMACK PHARMACEUTICALS INC | a13-20377_18k.htm |
| EX-99.1 - EX-99.1 - MERRIMACK PHARMACEUTICALS INC | a13-20377_1ex99d1.htm |
Exhibit 99.2
|
|
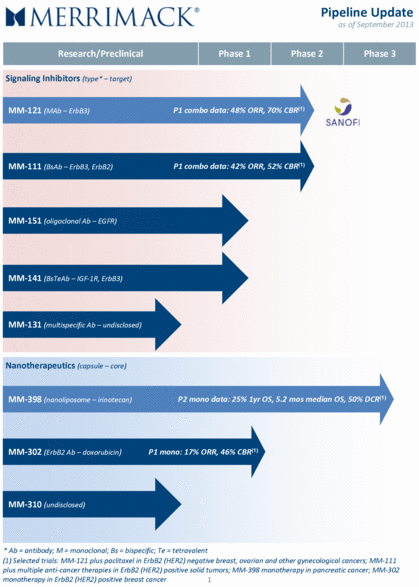
Research/Preclinical Phase 1 Phase 2 Phase 3 Signaling Inhibitors (type* – target) MM-121 (MAb – ErbB3) P1 combo data: 48% ORR, 70% CBR(1) MM-111 (BsAb – ErbB3, ErbB2) P1 combo data: 42% ORR, 52% CBR(1) MM-151 (oligoclonal Ab – EGFR) MM-141 (BsTeAb – IGF-1R, ErbB3) MM-131 (multispecific Ab – undisclosed) Nanotherapeutics (capsule – core) MM-398 (nanoliposome – irinotecan) P2 mono data: 25% 1yr OS, 5.2 mos median OS, 50% DCR(1) MM-302 (ErbB2 Ab – doxorubicin) P1 mono: 17% ORR, 46% CBR(1) MM-310 (undisclosed) * Ab = antibody; M = monoclonal; Bs = bispecific; Te = tetravalent 1 (1) Selected trials: MM-121 plus paclitaxel in ErbB2 (HER2) negative breast, ovarian and other gynecological cancers; MM-111 plus multiple anti-cancer therapies in ErbB2 (HER2) positive solid tumors; MM-398 monotherapy in pancreatic cancer; MM-302 monotherapy in ErbB2 (HER2) positive breast cancer Pipeline Update as of September 2013 |
|
|
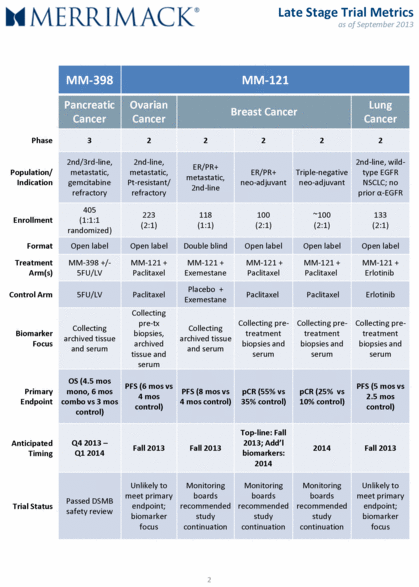
2 MM-398 MM-121 Pancreatic Cancer Ovarian Cancer Breast Cancer Lung Cancer Phase 3 2 2 2 2 2 Population/ Indication 2nd/3rd-line, metastatic, gemcitabine refractory 2nd-line, metastatic, Pt-resistant/ refractory ER/PR+ metastatic, 2nd-line ER/PR+ neo-adjuvant Triple-negative neo-adjuvant 2nd-line, wild-type EGFR NSCLC; no prior α-EGFR Enrollment 405 (1:1:1 randomized) 223 (2:1) 118 (1:1) 100 (2:1) ~100 (2:1) 133 (2:1) Format Open label Open label Double blind Open label Open label Open label Treatment Arm(s) MM-398 +/- 5FU/LV MM-121 + Paclitaxel MM-121 + Exemestane MM-121 + Paclitaxel MM-121 + Paclitaxel MM-121 + Erlotinib Control Arm 5FU/LV Paclitaxel Placebo + Exemestane Paclitaxel Paclitaxel Erlotinib Biomarker Focus Collecting archived tissue and serum Collecting pre-tx biopsies, archived tissue and serum Collecting archived tissue and serum Collecting pre-treatment biopsies and serum Collecting pre-treatment biopsies and serum Collecting pre-treatment biopsies and serum Primary Endpoint OS (4.5 mos mono, 6 mos combo vs 3 mos control) PFS (6 mos vs 4 mos control) PFS (8 mos vs 4 mos control) pCR (55% vs 35% control) pCR (25% vs 10% control) PFS (5 mos vs 2.5 mos control) Anticipated Timing Q4 2013 – Q1 2014 Fall 2013 Fall 2013 Top-line: Fall 2013; Add’l biomarkers: 2014 2014 Fall 2013 Trial Status Passed DSMB safety review Unlikely to meet primary endpoint; biomarker focus Monitoring boards recommended study continuation Monitoring boards recommended study continuation Monitoring boards recommended study continuation Unlikely to meet primary endpoint; biomarker focus Late Stage Trial Metrics as of September 2013 |