Attached files
| file | filename |
|---|---|
| 8-K - OPEXA THERAPEUTICS, INC. 8-K - Acer Therapeutics Inc. | a50690734.htm |
| EX-99.1 - EXHIBIT 99.1 - Acer Therapeutics Inc. | a50690734ex99_1.htm |
Exhibit 99.2

Opexa Therapeutics, Inc. Second Quarter 2013 Earnings Update Call 14 August 2013 NASDAQ: OPXA Precision Immunotherapy TM

2 Forward-Looking
Statements This earnings presentation contains forward-looking
statements which are made pursuant to the safe harbor provisions of
Section 27A of the Securities Act of 1933, as amended, and Section 21E
of the Securities Exchange Act of 1934, as amended. Statements contained
in this release, other than statements of historical fact, constitute
"forward-looking statements." The words "expects," "believes,"
"anticipates," "estimates," "may," "could," "intends," and similar
expressions are intended to identify forward-looking statements. The
forward-looking statements in this presentation do not constitute
guarantees of future performance. Investors are cautioned that
statements in this presentation which are not strictly historical
statements, including, without limitation, statements regarding the
development of the Company's product candidate, Tcelna (imilecleucel-T),
constitute forward-looking statements. Such forward-looking statements
are subject to a number of risks and uncertainties that could cause
actual results to differ materially from those anticipated. These risks
and uncertainties include, but are not limited to, risks associated
with: market conditions; our capital position; the rights and
preferences provided to the Series A convertible preferred stock and
investors in the convertible secured notes we issued in July 2012
(including a secured interest in all of our assets); our ability to
compete with larger, better financed pharmaceutical and biotechnology
companies; new approaches to the treatment of our targeted diseases; our
expectation of incurring continued losses; our uncertainty of developing
a marketable product; our ability to raise additional capital to
continue our development programs (including to undertake and complete
any ongoing or further clinical studies for Tcelna), including in this
regard our ability to satisfy various conditions required to access the
financing potentially available under the purchase agreements with
Lincoln Park Capital Fund, LLC (“Lincoln Park”) (such as the minimum
closing price for our common stock and the requirement for an ongoing
trading market for our stock); our ability to raise additional capital
through the sale of shares of our common stock under the purchase
agreements with Lincoln Park or under our at-the-market (ATM) facility;
our ability to maintain compliance with NASDAQ listing standards; the
success of our clinical trials (including the Phase IIb trial for Tcelna
in secondary progressive MS which, depending upon results, may determine
whether Ares Trading SA (“Merck”) elects to exercise its option for an
exclusive license to Tcelna for the treatment of MS (the “Option”));
whether Merck exercises its Option and, if so, whether we receive any
development or commercialization milestone payments or royalties from
Merck pursuant to the Option; our dependence (if Merck exercises its
Option) on the resources and abilities of Merck for the further
development of Tcelna; the efficacy of Tcelna for any particular
indication, such as for relapsing remitting MS or secondary progressive
MS; our ability to develop and commercialize products; our ability to
obtain required regulatory approvals; our compliance with all Food and
Drug Administration regulations; our ability to obtain, maintain and
protect intellectual property rights (including for Tcelna); the risk of
litigation regarding our intellectual property rights or the rights of
third parties; the success of third party development and
commercialization efforts with respect to products covered by
intellectual property rights that we may license or transfer; our
limitedmanufacturing capabilities; our dependence on third-party
manufacturers; our ability to hire and retain skilled personnel; our
volatile stock price; and otherrisks detailed in our filings with the
SEC. These forward-looking statements speak only as of the date made. We
assume no obligation or undertaking to update any forward-looking
statements to reflect any changes in expectations with regard thereto or
any change in events, conditions or circumstances on which any such
statement is based. You should, however, review additional disclosures
we make in our Annual Reports on Form 10 K, Quarterly Reports on Form
10-Q, and Current Reports on Form 8-K filed with the SEC.

3 Investment Thesis •
T-cell platform company • Esteemed Scientific Advisory Board • Precision
ImmunotherapyTM potentially optimizes benefit-risk profile • Targeting
an unmet medical need in a potentially substantial market • Option
Agreement with Merck Serono, a strong commercial partner • Replacement
value of company is multiples of present market cap • Attractive
potential risk-reward profile for long term/value investors •
Goal-oriented management team focused on value creation
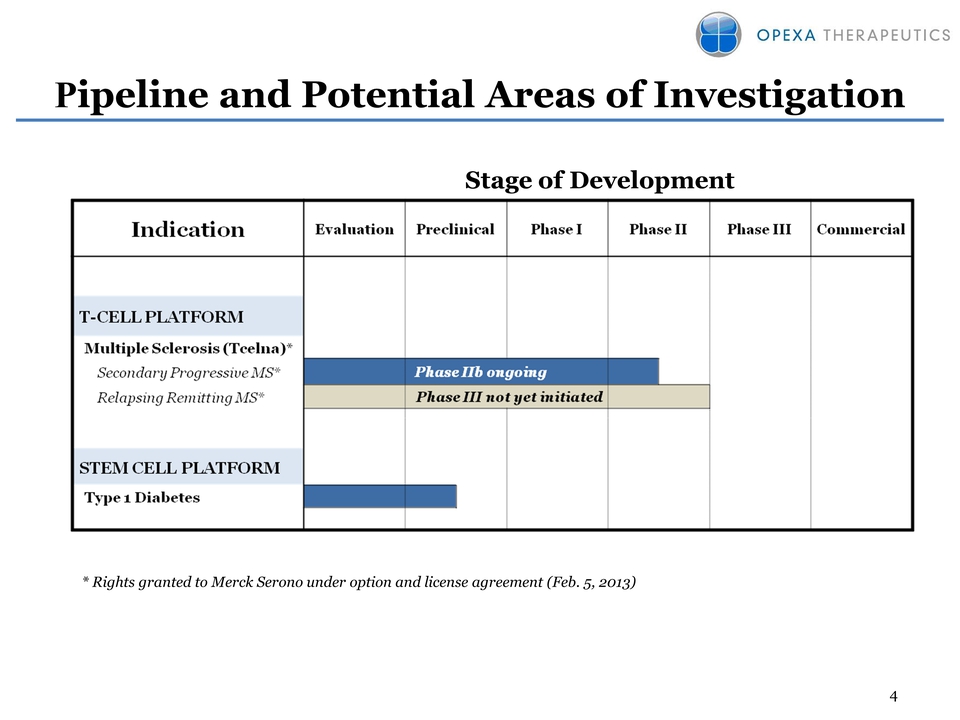
4 Pipeline and Potential
Areas of Investigation Stage of Development * Rights granted to Merck
Serono under option and license agreement (Feb. 5, 2013)
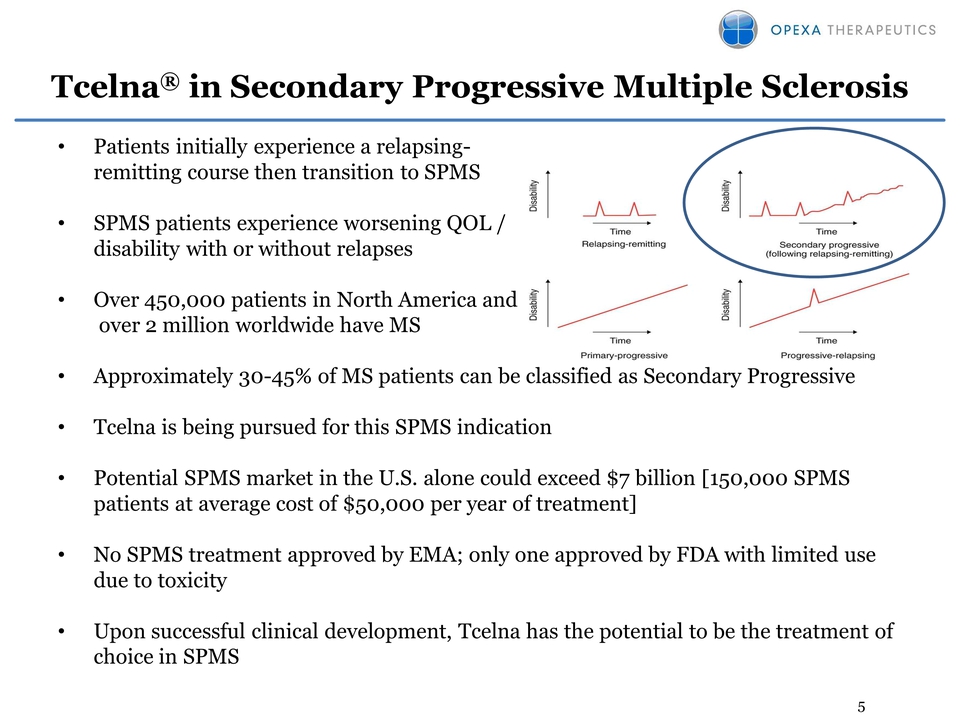
5 Tcelna® in Secondary
Progressive Multiple Sclerosis • Patients initially experience a
relapsingremitting course then transition to SPMS • SPMS patients
experience worsening QOL / disability with or without relapses • Over
450,000 patients in North America and over 2 million worldwide have MS •
Approximately 30-45% of MS patients can be classified as Secondary
Progressive • Tcelna is being pursued for this SPMS indication •
Potential SPMS market in the U.S. alone could exceed $7 billion [150,000
SPMS patients at average cost of $50,000 per year of treatment] • No
SPMS treatment approved by EMA; only one approved by FDA with limited
use due to toxicity • Upon successful clinical development, Tcelna has
the potential to be the treatment of choice in SPMS

6 Merck Serono Agreement
for MS indication • February 2013 option and license agreement with
Merck Serono – Up to $220 million in additional payments • Option
exercise $25 million for starting Phase III/ or $15 million if another
Phase II • $35 million FDA filing, approval and commercialized in US •
$30 million for EU filing, approval and commercialization in at least
three countries • RRMS development and commercialization of up to $40
million – One time commercial milestones of up to $85 million• Royalties
ranging from 8 % to 15% of annual net sales with step-ups occurring when
net sales exceed $500 million, $1 B & $2 B • Opexa maintains key rights:
– Development and commercialization rights to Tcelna in Japan – Certain
manufacturing rights – Co-development funding option in exchange for
increased royalties – Rights to all other disease indications
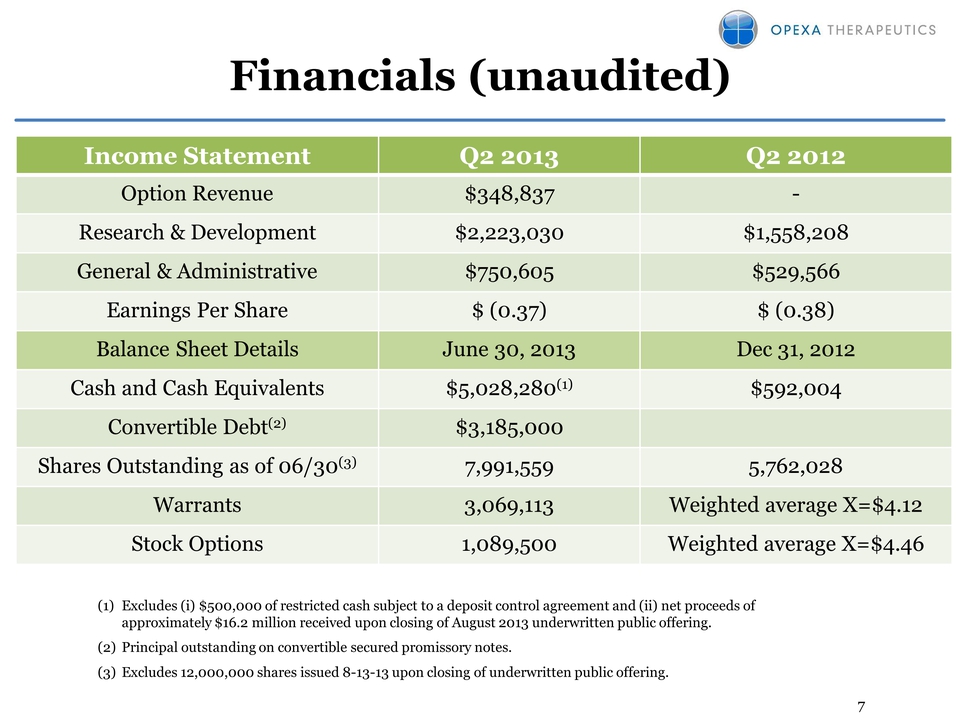
7 Financials (unaudited)
Income Statement Q2 2013 Q2 2012 Option Revenue $348,837 - Research &
Development $2,223,030 $1,558,208 General & Administrative $750,605
$529,566 Earnings Per Share $ (0.37) $ (0.38) Balance Sheet Details June
30, 2013 Dec 31, 2012 Cash and Cash Equivalents $5,028,280(1) $592,004
Convertible Debt(2) $3,185,000 Shares Outstanding as of 06/30(3)
7,991,559 5,762,028 Warrants 3,069,113 Weighted average X=$4.12 Stock
Options 1,089,500 Weighted average X=$4.46 (1) Excludes (i) $500,000 of
restricted cash subject to a deposit control agreement and (ii) net
proceeds of approximately $16.2 million received upon closing of August
2013 underwritten public offering. (2) Principal outstanding on
convertible secured promissory notes. (3) Excludes 12,000,000 shares
issued 8-13-13 upon closing of underwritten public offering.
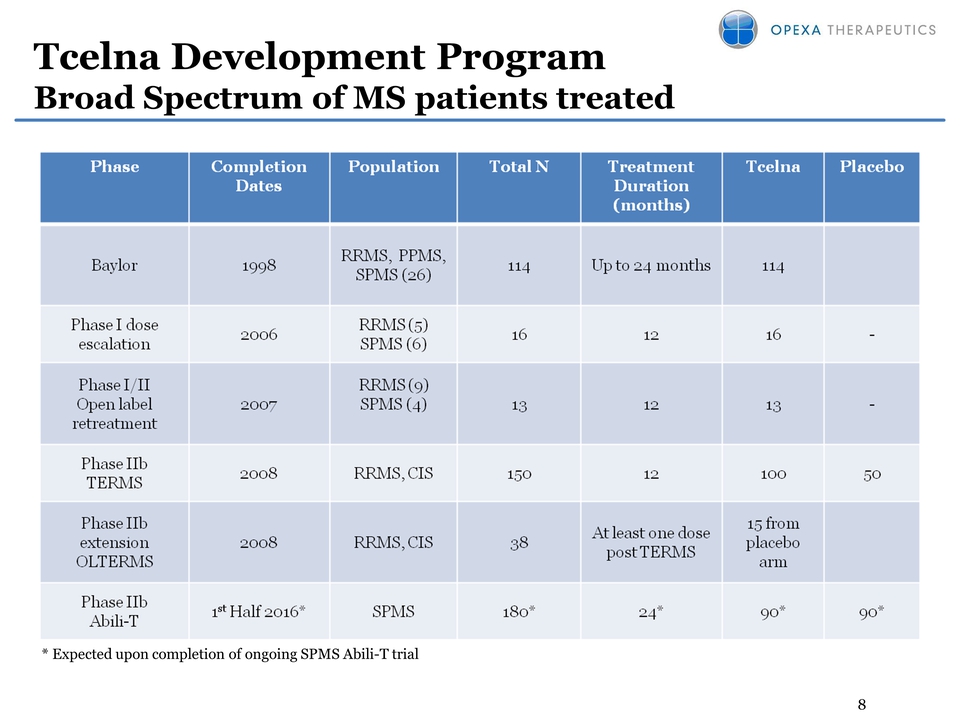
8 Tcelna Development
Program Broad Spectrum of MS patients treated * Expected upon completion
of ongoing SPMS Abili-T trial

9 Abili-T: Landmark trial
in SPMS • Abili-T Phase IIb clinical trial in SPMS is ongoing –
Double-blind, 1:1 randomized, placebo-controlled – Inclusion criteria:
Secondary Progressive MS with EDSS of 3 to 6 – 68 patients randomized as
of August 8, 2013 – Immune Monitoring program conducted on a blinded
basis• Fast Track designation granted by FDA for Tcelna in SPMS • 180
Patients expected to be enrolled – SPMS population – Approximately 30
sites in USA and Canada • Key Efficacy Endpoints: – Primary endpoint:
Whole Brain Atrophy – Secondary endpoint: Disability metrics including
EDSS, ARR, etc. – Exploratory endpoints: Quality of Life • 2 annual
courses of personalized therapy
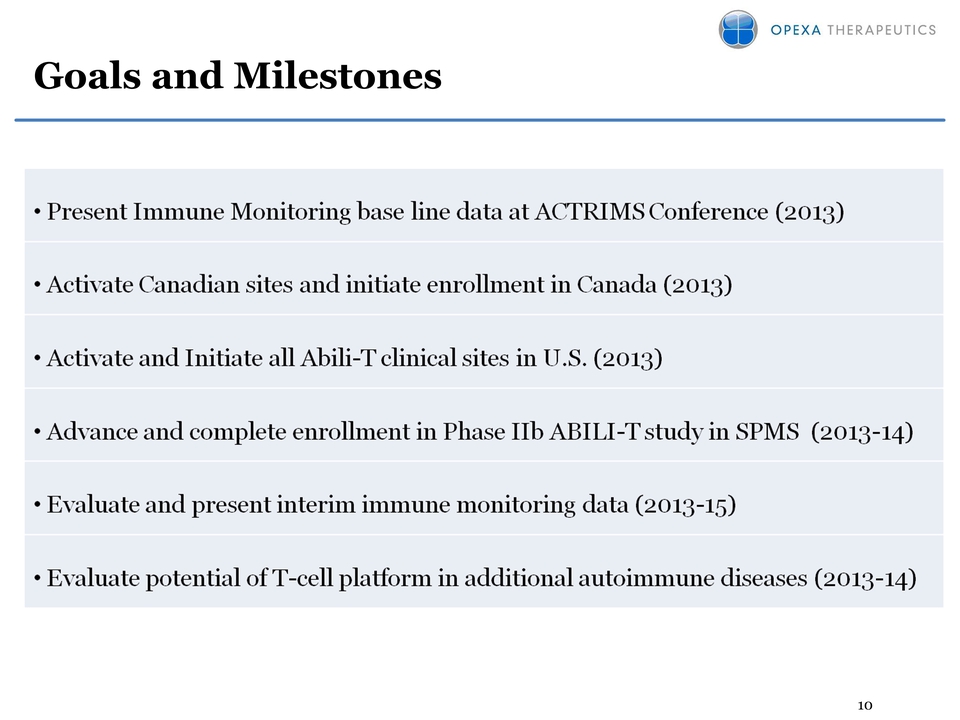
10 Goals and Milestones

11 Investment Highlights • Lead product, Tcelna®, a T-cell immunotherapy for Multiple Sclerosis (MS) • Currently conducting a Phase 2b clinical trial in Secondary Progressive MS (SPMS) • Limited treatment options currently available for SPMS • Potential SPMS market in North America alone could exceed $7 Billion • Tcelna positioned as potential first-to-market personalized T-cell immunotherapy • Option and license agreement secured with Merck Serono for Tcelna in MS indications only, worldwide excluding Japan • Opexa’s in-house cGMP manufacturing enables close control of process and COGS • In previous MS clinical studies, Tcelna has demonstrated good safety and potential indications of clinical efficacy• FDA has granted Opexa Fast Track Designation for Tcelna in SPMS
