Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - QUESTCOR PHARMACEUTICALS INC | d580909d8k.htm |
 1
August 2013
NASDAQ:
QCOR
NASDAQ:
QCOR
Exhibit 99.1
1 |
 2
Safe Harbor Statement
Note: Except for the historical information contained herein, this press release contains
forward-looking statements that have been made pursuant to the Private Securities
Litigation Reform Act of 1995. These statements relate to future events or our future financial
performance. In some cases, you can identify forward-looking statements by terminology such
as "believes," "continue," "could," “ensuring,”
"estimates," "expects," "growth," "may," “momentum,” "plans," "potential," "remain," "should," “start,” "substantial,"
“sustainable” or "will" or the negative of such terms and other comparable
terminology. These statements are only predictions. Actual events or results may differ
materially. Factors that could cause or contribute to such differences include, but are not limited to, the
following: Our reliance on Acthar for substantially all of our net sales and profits; Reductions
in vials used per prescription resulting from changes in treatment regimens by physicians
or patient compliance with physician recommendations; Our ability to receive high
reimbursement levels from third party payers; The complex nature of our manufacturing process
and the potential for supply disruptions or other business disruptions; The lack of
patent protection for Acthar and the possible FDA approval and market introduction of
competitive products; Our ability to continue to generate revenue from sales of Acthar to treat on-label indications
associated with NS, MS, IS or rheumatology-related conditions, and our ability to develop
other therapeutic uses for Acthar; Research and development risks, including risks
associated with Questcor's work in the area of NS and Lupus and efforts to develop and obtain
FDA approval of Synacthen, our reliance on third-parties to conduct research and development
and the ability of research and development to generate successful results; The results
of any pending or future litigation, investigations or claims, including with respect to
the investigation by the United States Attorney’s Office for the Eastern District of Pennsylvania regarding the Company’s
promotional practices and litigation brought by certain shareholders arising from the federal
securities laws, currently pending in the United States District Court for the Central
District of California; Our ability to comply with federal and state regulations, including
regulations relating to pharmaceutical sales and marketing practices; Regulatory changes or
other policy actions by governmental authorities and other third parties in connection
with U.S. health care reform or efforts to reduce federal and state government deficits;
An increase in the proportion of our Acthar unit sales comprised of Medicaid-eligible patients and government entities; Our
ability to estimate reserves required for Acthar used by government entities and
Medicaid-eligible patients and the impact that unforeseen invoicing of historical
Medicaid prescriptions may have upon our results; Our ability to effectively manage our growth,
including the expansion of our sales forces, and our reliance on key personnel; Our ability to
integrate the BioVectra business with our business and to manage, and grow, a contract
manufacturing business; Our ability to comply with foreign regulations related to the
operation of BioVectra's business and the international sales of Synacthen; The impact to our
business caused by economic conditions; Our ability to protect our proprietary rights; The
risk of product liability lawsuits; Unforeseen business interruptions and security
breaches; Volatility in Questcor's monthly and quarterly Acthar shipments, estimated channel
inventory, and end-user demand, as well as volatility in our stock price; and Other
risks discussed in Questcor's annual report on Form 10-K for the year ended December
31, 2012 as filed with the Securities and Exchange Commission, or SEC, on February 27, 2013, and
other documents filed with the SEC.
The risk factors and other information contained in these documents should be considered in
evaluating Questcor's prospects and future financial performance.
|
 3
A biopharmaceutical company focused on the
treatment of patients with serious, difficult-to-
treat autoimmune and inflammatory disorders
A biopharmaceutical company focused on the
treatment of patients with serious, difficult-to-
treat autoimmune and inflammatory disorders
Questcor |
 4
Flagship product Acthar has a unique
Flagship product Acthar has a unique
therapeutic role and sustainable competitive
therapeutic role and sustainable competitive
advantages
advantages
Acthar is approved for 19 indications, many
Acthar is approved for 19 indications, many
in markets with sizable unmet need
in markets with sizable unmet need
Market penetration remains modest;
Market penetration remains modest;
sales increasing rapidly
sales increasing rapidly
Increasing investment in R&D to create
Increasing investment in R&D to create
future value and diversity
future value and diversity
Profitable, strong cash flow and balance
Profitable, strong cash flow and balance
sheet; strong commitment to creating
sheet; strong commitment to creating
shareholder value
shareholder value
Investment Highlights |
 5
Q2-2013 Financial Results
Q2 –
2013
Q2 –
2012
Change
Net Sales ($M)
$184.6
$112.5
64%
Net Sales ($M), Non-GAAP
$196.1
$112.5
74%
Fully Diluted, GAAP EPS
$1.12
$0.65
72%
Fully Diluted, Non-GAAP EPS
$1.35
$0.69
96%
Cash flow from operations ($M)
$81.5
$43.2
89%
Diluted shares outstanding
61.5
64.1
The reconciliation between GAAP and non-GAAP financial
measures is provided at the end of this presentation.
|
 Flagship
Product: Flagship Product:
•
19 approved indications
•
19 approved indications
Key Therapeutic Areas:
Key Therapeutic Areas:
•
Nephrotic Syndrome, Multiple Sclerosis Relapse, Infantile
Spasms, Rheumatology, Sarcoidosis
•
Significant areas of unmet need; large growth potential
•
Nephrotic Syndrome, Multiple Sclerosis Relapse, Infantile
Spasms, Rheumatology, Sarcoidosis
•
Significant areas of unmet need; large growth potential
Strategy:
Strategy:
•
Expand awareness, appropriate use of Acthar in key specialties
•
Develop
additional
on-label
and
new
indications
•
Expand use to international markets
•
Expand awareness, appropriate use of Acthar in key specialties
•
Develop
additional
on-label
and
new
indications
•
Expand use to international markets
*In this presentation, the terms “Nephrotic Syndrome,”
“Multiple Sclerosis Relapse,”
“Infantile Spasms,”
“Rheumatology,”
and
“Sarcoidosis,”
and
their
abbreviations,
refer
to
on-label
indications
for
Acthar
associated
with
such
conditions.
Investors
should
refer
to
the
FDA
approved
Acthar
label,
which
can
be
found
at
http://www.acthar.com/files/Acthar-PI.pdf
Acthar
6 |
 7
Acthar Distribution by Indication
Note: Questcor sells Acthar to a distributor and does not have complete data with
respect to end-use; allocation based on internal estimates (Q2
2013). Other
2%
Rheumatology
18%
Infantile Spasms
10%
Neurology
30%
Nephrology
40%
Based on Acthar Net Sales |
 8
Nephrotic Syndrome (NS)
•
Characterized by excessive spilling of protein
from the kidneys into the urine (proteinuria)
•
Caused by a number of underlying types of kidney
disease (e.g., iMN, FSGS, IgA nephropathy)
•
Can result in end-stage renal disease (ESRD), dialysis,
transplant
•
Significant unmet need; few FDA approved options
•
Acthar is indicated to induce a diuresis or a remission
of proteinuria in the nephrotic syndrome without
uremia of the idiopathic type or that due to lupus
erythematosus
•
Characterized by excessive spilling of protein
from the kidneys into the urine (proteinuria)
•
Caused by a number of underlying types of kidney
disease (e.g., iMN, FSGS, IgA nephropathy)
•
Can result in end-stage renal disease (ESRD), dialysis,
transplant
•
Significant unmet need; few FDA approved options
•
Acthar is indicated to induce a diuresis or a remission
of proteinuria in the nephrotic syndrome without
uremia of the idiopathic type or that due to lupus
erythematosus |
 9
•
A neurodegenerative disease occurring in about
400,000 patients in the US (>100,000 relapses/year)
•
Relapses range from mild to severe and can cause a
range of symptoms
–
Loss of sensation in the extremities
–
Loss of vision
–
Loss of ability to walk
•
Relapses have a measurable and sustained effect on
disability in MS patients
•
A neurodegenerative disease occurring in about
400,000 patients in the US (>100,000 relapses/year)
•
Relapses range from mild to severe and can cause a
range of symptoms
–
Loss of sensation in the extremities
–
Loss of vision
–
Loss of ability to walk
•
Relapses have a measurable and sustained effect on
disability in MS patients
Multiple Sclerosis (MS) Relapse
1
Lublin et al. Neurology
2003
1 |
 10
•
Rheumatology-related indications on the Acthar label*
–
Dermatomyositis/Polymyositis (DM/PM)
–
Systemic lupus erythematosus (Lupus)
–
Rheumatoid arthritis
–
Psoriatic arthritis
•
Each can pose a serious health risk if not adequately
controlled
•
Some cases difficult to manage; Acthar is an FDA-
approved treatment that may be appropriate for select
patients
•
Expanding Rheum sales force from 55 to 62 reps
•
Rheumatology-related indications on the Acthar label*
–
Dermatomyositis/Polymyositis (DM/PM)
–
Systemic lupus erythematosus (Lupus)
–
Rheumatoid arthritis
–
Psoriatic arthritis
•
Each can pose a serious health risk if not adequately
controlled
•
Some cases difficult to manage; Acthar is an FDA-
approved treatment that may be appropriate for select
patients
•
Expanding Rheum sales force from 55 to 62 reps
Rheumatology
*See http://www.acthar.com/files/Acthar-PI.pdf for specific
label information. |
 11
Dermatomyositis and Polymyositis (DM/PM)
•
Inflammatory neuromuscular diseases
that cause a loss of muscle strength and
mass
–
Significant quality of life issues; some patients
require walkers or wheelchairs
–
Can also cause significant lung impairment
–
Patients can experience acute exacerbations
(flares)
•
Commonly used DM/PM treatments
•
An estimated 30% of patients may need
an additional treatment alternative*
Alternative
Needed
~20K
* Questcor sponsored market research study (2012)
~70,000 Patients (US)
Prednisone, plaquenil, methotrexate,
azathioprine, IVIG, Rituxan
Adequately
Treated
~50K |
 12
Systemic Lupus Erythematosus (Lupus)
•
Chronic autoimmune disorder that can
effect virtually any area of the body
–
Patients can experience acute exacerbations
(flares)
•
Commonly used lupus treatments
•
An estimated 25% of patients may need
an additional treatment alternative*
~250K SLE Patients (US)
* Questcor sponsored market research study (2012)
Adequately
Treated
~50K
Alternative
Needed
~63K
-
Prednisone, plaquenil, methotrexate,
mycophenolate, azathioprine, Benlysta, Rituxan |
 13
Rheumatoid Arthritis (RA)
•
Chronic autoimmune disorder that causes
inflammation of the synovial joints
–
Can be debilitating and lead to join destruction
–
Patients can experience acute exacerbations (flares)
•
Commonly used RA treatments
-
Prednisone, methotrexate, azathioprine, Remicade,
Humira, Rituxan
•
An estimated 5% of patients may need an
additional treatment alternative*
Alternative
Needed
~65K
* Questcor sponsored market research study, 2012
~1.3 million patients (US)
Adequately
Treated
~1.23M |
 14
Psoriatic Arthritis (PsA)
•
Chronic autoimmune disorder manifesting as
both arthritis and psoriasis
–
Patients have both skin and joint manifestations
–
Patient can experience acute exacerbations (flares)
•
Commonly used PsA treatments
•
An estimated 5% of patients may need an
additional treatment alternative*
Alternative
Needed
~45K
* Questcor sponsored market research study, 2012
~900K patients (US)
-
Prednisone, methotrexate, azathioprine, Remicade,
Humira, Enbrel
Adequately
Treated
~855K |
 15
•
Symptomatic sarcoidosis involves inflammation and formation
of nodules in multiple organs, most commonly the lungs
•
Sarcoidosis fits our commercial model
–
Difficult-to-treat autoimmune/inflammatory condition
–
Limited treatment options
•
Previous successes in neurology, nephrology, rheumatology
•
150,000 total sarcoidosis patients
•
Some patients are well controlled but only with high-dose
steroids
•
Symptomatic sarcoidosis involves inflammation and formation
of nodules in multiple organs, most commonly the lungs
•
Sarcoidosis fits our commercial model
–
Difficult-to-treat autoimmune/inflammatory condition
–
Limited treatment options
•
Previous successes in neurology, nephrology, rheumatology
•
150,000 total sarcoidosis patients
•
Some patients are well controlled but only with high-dose
steroids
Sarcoidosis
-Half are symptomatic
-90% of symptomatic have pulmonary issues |
 16
•
Devastating, ultra-rare form of childhood epilepsy
•
Can cause permanent developmental disabilities,
increased mortality
•
Acthar
is
often
considered
the
“gold
standard”
and
is
currently
used
to
treat
40-50%
of
IS
patients
•
Devastating, ultra-rare form of childhood epilepsy
•
Can cause permanent developmental disabilities,
increased mortality
•
Acthar
is
often
considered
the
“gold
standard”
and
is
currently
used
to
treat
40-50%
of
IS
patients
Infantile Spasms (IS) |
 17
Advancing Our Understanding of the
Science of Acthar and Melanocortin Peptides
•
One of 9 families of hormones produced by the
pituitary, the “master gland”
•
Believed to modulate the immune system and
associated inflammatory response through
binding to melanocortin receptors
•
Activate up to 5 target melanocortin receptors
–
MC1R, MC2R, MC3R, MC4R, and MC5R
–
Differences in chemical structure influence binding affinity
•
ACTH is one of many melanocortin peptides
–
Acthar (ACTH 1-39); Synacthen (ACTH 1-24) |
 18
Acthar
Acthar
ar
Steroid-dependent
anti-inflammatory
effects (indirect)
MC2R
Corticosteroids
Adrenal cortical cells
References available upon request
Steroid-independent anti-inflammatory/immunomodulatory effects
MC1R
MC3R
MC4R
MC5R
Altered
tissue/organ &
cell function
Direct effect
on organ-
specific cells
(eg, CNS,
kidney,
muscle)
Effects on
immune cells
Immune cells, CNS cells, kidney cells, muscle cells,
many other cells
Melanocortin Peptides Activate
Up to Five Melancortin Receptors |
 19
Acthar Mechanism of Action
1:
Bomback
et
al,
Am
J
Nephrol
2012;36:58–67
2: Gong et al, Kidney International, 83, January 2013
o
Acthar has increased efficacy (>86% vs <30%) vs.
corticosteroids in infantile spasms
o
Acthar has been used with success when steroids
are ineffective in idiopathic nephrotic syndrome
(iMN, FSGS and IgA nephropathy)
1
2
Clinical observations:
•
Preclinical observations demonstrate steroid-
independent anti-inflammatory, immuno-modulatory
activity of ACTH & other MC peptides
|
 20
•
We now believe Acthar and other melanocortins
impact
–
Immune system
–
Inflammation
–
Some cell function
–
Homeostasis
•
Dozens of severe medical conditions may benefit from
Acthar or other melanocortin therapeutics
•
We now believe Acthar and other melanocortins
impact
–
Immune system
–
Inflammation
–
Some cell function
–
Homeostasis
•
Dozens of severe medical conditions may benefit from
Acthar or other melanocortin therapeutics
Implications of Acthar and Other
Melanocortins (Synacthen and new MCs) |
 21
•
Have funded or have approved funding for ~70 projects
–
Company sponsored pre-clinical and clinical studies
–
Independent physician sponsored studies
•
Gaining an understanding of the biological properties of Acthar
–
Specific biochemical pathways, cells, and tissues
–
Immunomodulation and anti-inflammatory effects
•
Expanding the body of evidence for on-label indications
•
Exploring possible new indications and targets
–
Autoimmune/inflammatory conditions
•
Initiating development of Synacthen
•
Have funded or have approved funding for ~70 projects
–
Company sponsored pre-clinical and clinical studies
–
Independent physician sponsored studies
•
Gaining an understanding of the biological properties of Acthar
–
Specific biochemical pathways, cells, and tissues
–
Immunomodulation and anti-inflammatory effects
•
Expanding the body of evidence for on-label indications
•
Exploring possible new indications and targets
–
Autoimmune/inflammatory conditions
•
Initiating development of Synacthen
Significantly Increasing Investment in R&D |
 22
•
Idiopathic Membranous Nephropathy
–
Phase 4 trial ongoing
–
Refractory, non-responsive, or have relapsed on standard therapies
•
Persistently Active Lupus
–
Phase 4 trial initiated 4Q 2012
–
Daily Acthar administration over a 6-month period
•
Lupus Exacerbations (flares)
–
Prospective investigator initiated
–
Study completed
–
Investigator preparing data publication
•
DM/PM Studies
–
ADAPT Patient Registry currently collecting data
•
Idiopathic Membranous Nephropathy
–
Phase 4 trial ongoing
–
Refractory, non-responsive, or have relapsed on standard therapies
•
Persistently Active Lupus
–
Phase 4 trial initiated 4Q 2012
–
Daily Acthar administration over a 6-month period
•
Lupus Exacerbations (flares)
–
Prospective investigator initiated
–
Study completed
–
Investigator preparing data publication
•
DM/PM Studies
–
ADAPT Patient Registry currently collecting data
Acthar: Ongoing Research in Approved
Indications |
 23
Acthar Label Enhancement Strategy
•
Diabetic Nephropathy
–
One of the most common causes of end-stage renal disease in the U.S.
–
Phase 2 IND trial; Approx 40% enrolled
•
Amyotrophic Lateral Sclerosis (ALS)
–
Fatal neurological disease caused by progressive loss of motor neurons
in the brain and spinal cord
•
Inflammatory component to ALS contributes to disease pathology/progression
•
Mean survival time from diagnosis is 3-5 years
•
Affects ~30K people in US and ~30K in Europe; Peak incidence 40-70 years of
age –
Phase 2 IND study patient enrollment underway
–
Orphan Drug Designation granted by FDA
–
Study results to determine whether to pursue ALS as a potential new
Acthar indication |
 24
Near Term
Near Term
Global Growth Strategy
•
Continue to develop knowledge about melanocortin
peptide pharmacology and potential clinical benefits
•
Continue the commercial and scientific development
programs related to on-label Acthar indications
•
Expand commercial effort internationally with
Synacthen |
 25
Medium
term
Medium
term
Global Growth Strategy
•
Develop new Acthar indications in the U.S.
•
Develop Synacthen for the U.S.
•
Investigate/pursue Acthar approvals ex U.S.
Longer Term
•
Develop new melanocortin therapeutics |
 26
•
Continued stewardship of Acthar
–
Identifying and expanding Acthar therapeutic role in existing and new indications
•
Demonstrated ability to execute
•
Long term investment in R&D --
doubled R&D spending in 2012
•
Highly selective, strategic diversification
•
Have returned $379 million to shareholders through share
repurchases and dividends*
–
22.2 million shares repurchased
–
6.3 million shares remain available for repurchase under share repurchase
program*
•
Quarterly dividend increased to $0.25 per share during Q2-2013
•
Continued stewardship of Acthar
–
Identifying and expanding Acthar therapeutic role in existing and new indications
•
Demonstrated ability to execute
•
Long term investment in R&D --
doubled R&D spending in 2012
•
Highly selective, strategic diversification
•
Have returned $379 million to shareholders through share
repurchases and dividends*
–
22.2 million shares repurchased
–
6.3 million shares remain available for repurchase under share repurchase
program*
•
Quarterly dividend increased to $0.25 per share during Q2-2013
Committed to Creating Long Term Value
for Shareholders
*Data as of 6/30/13 |
 27
27
August 2013
August 2013
NASDAQ:
QCOR
NASDAQ:
QCOR |
 28
Reconciliation of Non-GAAP Adjusted
Financial Disclosure
Net income per share –
basic and diluted may
not foot due to rounding.
Use of Non-GAAP Financial Measures
Our
“non-GAAP
adjusted
net
income”
excludes
the
following items from GAAP net income:
1. Share-based compensation expense.
2. Depreciation and amortization expense, including
amortization expense on our purchased intangibles.
3. Interest expense associated with the net present value
adjustment on our contingent consideration.
4. Compensation expense associated with the
BV Trust agreement.
5. Foreign currency transaction loss.
6. Medicaid adjustment for prior
period 2002 -
2009
7. BioVectra purchase price adjustment related to a labor
rebate received in the second quarter 2013
8. Impairment of purchased technology related
to our acquisition of Doral.
Three Months Ended
Six Months Ended
June 30,
June 30,
2013
2012
2013
2012
Adjusted net income
$83,323
$44,244
$128,987
$84,514
Share-based compensation expense (1)
(4,382)
(2,521)
(8,546)
(4,054)
Depreciation and amortization expense (2)
(1,882)
(218)
(3,131)
(412)
Interest expense associated with contingent consideration (3)
(194)
0
(391)
0
Compensation expense associated with BV Trust (4)
(193)
0
(339)
0
Foreign currency transaction loss (5)
0
0
(328)
0
Medicaid adjustment for 2002 -
2009 (6)
(7,717)
0
(7,751)
0
BioVectra purchase price adjustment (7)
168
0
169
0
Impairment of purchased technology (8)
0
0
(485)
0
Net income -
GAAP
$69,123
$41,505
$108,185
$80,048
Adjusted net income per share -
basic
$1.41
$0.72
$2.20
$1.36
Share-based compensation expense (1)
(0.07)
(0.04)
(0.15)
(0.07)
Depreciation and amortization expense (2)
(0.03)
0.00
(0.05)
(0.01)
Interest expense associated with contingent consideration (3)
0.00
—
(0.01)
—
Compensation expense associated with BV Trust (4)
0.00
—
(0.01)
—
Foreign currency transaction loss (5)
—
—
(0.01)
—
Medicaid adjustment for 2002 -
2009 (6)
(0.13)
—
(0.13)
—
BioVectra purchase price adjustment (7)
0.00
—
0.00
—
Impairment of purchased technology (8)
—
—
(0.01)
—
Net income per share -
basic
$1.17
$0.68
$1.86
$1.28
Adjusted net income per share -
diluted
$1.35
$0.69
$2.13
$1.29
Share-based compensation expense (1)
(0.07)
(0.04)
(0.14)
(0.06)
Depreciation and amortization expense (2)
(0.03)
0.00
(0.05)
(0.01)
Interest expense associated with contingent consideration (3)
0.00
—
(0.01)
—
Compensation expense associated with BV Trust (4)
0.00
—
(0.01)
—
Foreign currency transaction loss (5)
—
—
(0.01)
—
Medicaid adjustment for 2002 -
2009 (6)
(0.13)
—
(0.13)
—
BioVectra purchase price adjustment (7)
0.00
—
0.00
—
Impairment of purchased technology (8)
—
—
(0.01)
—
Net income per share -
diluted
$1.12
$0.65
$1.79
$1.23
Net sales -
Questcor
$177,045
$112,452
$303,817
$208,421
Net sales -
BioVectra
7,528
0
15,885
0
Consoldiated net sales
184,573
112,452
319,702
208,421
Medicaid adjustment
11,500
0
11,500
0
Adjusted consolidated net sales
$196,073
$112,452
$331,202
$208,421 |
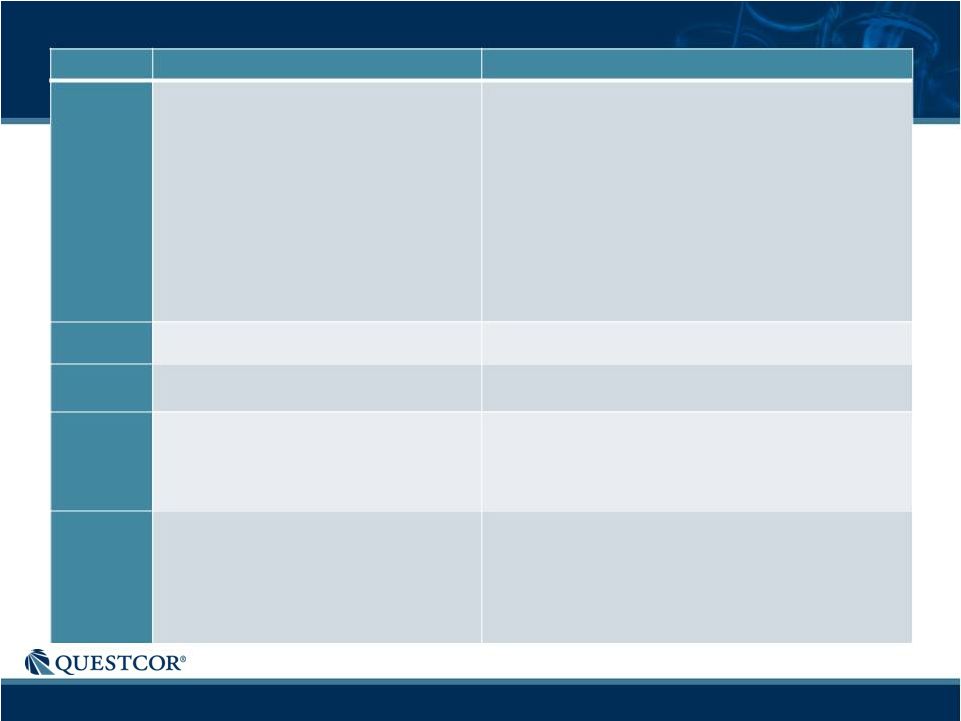 29
•
Immunomodulation
•
B cell signaling
•
Exocrine secretion
•
Ocular immunity
•
Lipid regulation
Central Nervous System
Exocrine Glands
Lymphocytes
MC5R
•
Regulation of neuroinflammation
•
Cerebral ischemic protection
•
Metabolic control
Podocytes
Renal Mesangial Cells
Endothelial Cells (Glomerular, Tubular)
Central Nervous System
MC4R
•
Protection from ischemia
•
Immunomodulation
Central Nervous System
Macrophages
MC3R
•
Steroidogenesis
Adrenal Cortex, Adipocytes, Testis
MC2R
•
Immunomodulation(including modulation of
antigen presentation; immune cell adhesion and
trafficking; dampen autoimmunity; NF-Kß
sequestration)
•
Cytoprotection (reduction of oxidative stress)
•
Ischemia-reperfusion protection Protection from
LPS-induced systemic inflammatory response
•
Cytoskeletal effects (regulate expression of
collagen, vimentin, podocyte specific proteins)
Podocytes
Renal Mesangial Cells
Endothelial Cells (Glomerular, Tubular,
Vascular)
Macrophages, Monocytes, Neutrophils
Melanocytes
Keratinocytes
Central Nervous System
Chondrocytes
Respiratory tract
GI tract
MC1R
MCR
Tissue/Cell expression
Potential biologic activity |
