Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Fibrocell Science, Inc. | d578216d8k.htm |
 August 5,
2013 Fibrocell Science
Exhibit 3.1 |
 2
This
presentation
includes
statements
that
are
“forward-looking
statements.”
Forward-looking
statements
include,
without limitation, our ability (i) to timely commence our clinical programs in 2013 to treat
patients with restrictive burn scars and vocal cord scars, and a timely
completion of these trial (ii) to successfully genetically modify the fibroblast cell
to treat patients who have collagen deficient diseases, (iii) to successfully leverage our relationships
with
UCLA
and
MIT
to
develop
new
applications,
and
(iv)
to
adopt
technologies
that
will
dramatically
reduce
manufacturing time, complexity, and labor costs for our cell therapies. While management has
based any forward- looking statements contained in the presentation on its current
expectations, the information on which such expectations were based may change. These
forward-looking statements rely on a number of assumptions concerning future events
and are subject to a number of risks, uncertainties, and other factors, many of which are outside of
Fibrocell Science’s control, that could cause actual results to materially differ from
such statements. Such risks, uncertainties, and other factors include, but are not
necessarily limited to, those set forth under the caption “Item 1A. Risk
Factors”
in
Fibrocell
Science’s
most
recent
Form
10-K
filing,
as
updated
in
“Item
1A.
Risk
Factors”
in
Fibrocell
Science’s most recent Form 10-Q filing. In addition, Fibrocell Science operates in a
highly competitive and rapidly changing environment, and new risks may arise.
Accordingly, you should not place any reliance on forward-looking statements as a
prediction of actual results. Fibrocell Science disclaims any intention to, and undertakes no obligation
to, update or revise any forward-looking statement. You are also urged to carefully review
and consider the various disclosures in Fibrocell Science’s most recent annual
report on Form 10-K, our most recent Form 10-Q as well as other public filings
with the SEC since the filing of Fibrocell Science’s most recent annual report.
Forward Looking Statement |
 Strategic Focus:
Unlocking the Potential of the Autologous Fibroblast Cell
3
•
Restrictive Burn Scarring
•
Vocal Cord Scarring
•
Acne Scarring
•
Recessive Dystrophic
Epidermolysis Bullosa (RDEB)
•
Morphea
•
Cutaneous Eosinophilias
•
Localized Treatment for
moderate to severe
Psoriasis
•
Focused on skin-derived
MSCs and more efficient
conversion of skin cells
to IPSCs
Autologous
Fibroblasts
Label Extension
for LAVIV®
(azficel-T)
Genetically-
Modified HDFs
for rare skin
diseases
Genetically-
Modified HDFs
for serious skin
diseases
UCLA/MIT
Collaboration |
 Aesthetics
|
 5
•
Positioned
as
premium
aesthetic
–
First
in
New
Class
–
Personalized
Aesthetics
–
Replaces
a
person’s
own
fibroblast
cells
•
LAVIV
has
received
national
recognition
–
Allure
magazine
‘Breakthrough
Award’
–
Wall
Street
Journal
Technology
Innovation
Awards
–
Edison
Awards
Silver
Winner
in
Aesthetics
of
Science/Medical
Category
LAVIV®
(azficel-T)
Please
refer
to
the
end
of
this
presentation
for
Important
Safety
Information
about
LAVIV®
(azficel-T). |
 The Only
Crème of Its Kind |
 •
Personalized
autologous
cream
•
Conditioned
complete
growth
media
from
the
azficel-T
production
process
is
collected,
concentrated
and
used
to
formulate
a
personalized
topical
cosmetic
Autologous Crème™
7 |
 Product
Pipeline 8
Restrictive Burn Scurring
Vocal Cord Scurring
Acne Scurring
Genetically Modified
Autologous Fibroblasts
Skin-derived MSC/IPSCs
•
Phase II study initiated
•
Efficacy data expected mid-2014
•
Positive pilot study published in Laryngoscope; abstract online:
http://www.ncbi.nlm.nih.gov/pubmed/21287562
•
Phase II protocol cleared by FDA
•
Estimated initiation of trial 2H 2013, once IRB approval is received
•
In end of Phase II discussions with FDA, regarding potential pathway forward
•
Results of Phase II study published in Dermatologic Surgery;
available online:
http://onlinelibrary.wiley.com/doi/10.1111/dsu.12204/pdf
•
Pre-clinical activity advancing in genetically modified fibroblasts for the treatment
of recessive dystrophic epidermolysis bullosa (RDEB); Orphan Indication
•
Pre-clinical activity advancing in genetically modified fibroblasts transfected ex
vivo with therapeutic
gene(s)
of
interest
under
the
control
of
the
RheoSwitch™
Gene
Expression
System for the
localized treatment of:
Moderate to Severe Psoriasis
Cutaneous Eosinophilias (Orphan Indication)
Morphea (Orphan Indication)
•
Reprogramming skin cells into induced pluripotent stem cells (IPSCs)
•
Identifying, purifying, and cultivating MSCs from human adult skin
Status |
 What is a
Fibroblast? |
 •
Most
common
cells
of
connective
tissue
•
Produce
collagen
and
extracellular
matrix
proteins,
which
form
infrastructure
of
tissue
•
Produce
growth
factors
and
other
cytokines
that
aid
in
cell
signaling
and
wound
repair
Fibroblast Cells
10 |
 Dermal
Fibroblasts 11 |
 Dermal
fibroblasts produce :
•
Collagen type I
•
Collagen type III
•
Collagen type VII
•
Elastin
•
Hyaluronic acid (hyaluronin)
•
Matrix metalloproteinases
(MMPs)
(1)
Artist’s interpretation
Dermal Fibroblasts
12
(1) Matrix metalloproteinases refers to a group of
proteins that play an important role in tissue remodeling associated with various physiological
and pathological processes such as morphogenesis, angiogenesis, tissue repair, cirrhosis,
arthritis, and metastasis. There is considerable recent research on MMPs and their
potential role in future therapeutics. |
 Addressable
Markets |
 14
Scarring: Burn, Vocal Cord, Acne
Fibrocell Scarring Pipeline:
Annual Addressable Market Opportunity
•
Restrictive Burn Scarring (U.S. only):
-
140,000 2 and 3 degree burn victims / year
(1)
-
Total excludes military burn injuries
-
20-30%
experience
restrictive
scarring
(2)
-
Assumption of 30% treated
-
Assumption of pool of patients available includes the
previous 3 years
(1)
American Burn Association http://www.ameriburn.org/
(2)
Fibrocell estimate based on discussion with key opinion leader
(3)
Fibrocell estimates based on current pricing and estimated average volumes of azficel-T
used to treat patients with these conditions; prices are subjected to change and variation
(4)
Cohen, (2010); Dailey et al. (2007); Roy et al. (2005); Poels et
al. (2003); Koufman and Isaacson (1991); Painter (1990); Bouchayer et al. (1985); Laguaite
(1972) (5)
Acne Resource Center. www.acne-resource.org •
Vocal Cord Scarring (U.S. only):
-
200,000-700,000
patients
with
VCS
(4)
-
Assumption of 10% treated
•
Acne Scarring (U.S. only):
-
20
million
patients
with
severe
acne
that
could
cause
scarring
(5)
-
Every 50,000 patients represents $750 million addressable market
Vocal Cord Scarring
$240
M
–
$840 M
Acne Scarring
$750 M
Restrictive Burn Scarring
$815 M
nd
rd
(3)
(3)
(3)
(3) |
 Restrictive Burn
Scarring
15 |
 •
No FDA approved therapeutics
•
Azficel-T
treatment:
potential
improvement
in
range
of
motion,
reduction
in
pain and aesthetic improvement
•
Potential indication for unmet medical need:
Pre-
Treatment
12 Months Post-
Treatment
•
Full range-clench
14 Months Post-
Treatment
•
Fine finger movement
(1)
www.ameriburn.org; Goodis. J and E.d. Schraga. Burns, thermal. eMedicine
Journal Restrictive Burn Scarring Overview
Before
After Six Months Post Treatment:
•
Full range of neck rotation, and pain
free
•
Patient has stopped all analgesics
•
Discarded cervical collar
16
Feb 1, 2006
August 11, 2006 –
45,000 yearly hospitalizations for severe burns
(1) (excluding military and existing victims)
–
Promising results in open-label case studies |
 •
Phase II Trial: Single site, double-blind, randomized, placebo-controlled,
21 subjects
•
Subjects:
-
Unilateral restrictive burn scars of a jointed area
-
20-50 percent restriction in normal range of motion
•
Measurement Scales:
-
Range of Motion Measurement
-
Brief Pain Index
-
Scar Appearance
•
Phase II Clinical Study initiated in 2Q 2013; efficacy data
expected mid-2014
Restrictive Burn Scarring Status
17 |
 Vocal Cord
Scarring |
 •
No
FDA
approved
therapeutics
(1)
Chhetri, Dinesh, Injection of Cultured Autologous Fibroblasts for Human Vocal Fold Scars.
The Laryngoscope 121(4) : 785-792, 2011. Vocal Cord Scarring Overview
19
•
Most
commonly
encountered
finding
is
loss
of
voice
-
Due
to
surgery,
radiation
therapy,
vocal
cord
trauma
and
idiopathic
causes
•
Positive
pilot
study
results
published
in
peer-reviewed
journal
The
Laryngoscope, 2011
(1)
-
Azficel-T
well-tolerated
when
injected
into
vocal
folds
-
Improvements
lasted
up
to
one
year
-
Findings
supported
by
mucosal
wave
grade,
voice
handicap
index,
and
voice
quality
questionnaire |
 •
Phase
II
protocol
cleared
by
FDA
•
Estimated
initiation
of
trial
2H
2013,
once
IRB
approval
is
received
•
Efficacy
data
expected
in
2H
2014
•
Proposed
Endpoints:
-
Absolute
change
from
baseline
in
mucosal
wave
grade
using
videostroboscopy
-
Absolute
and
percentage
change
from
baseline
in
the
Voice
Handicap
Index
(VHI)
-
Perceptual
analysis
using
the
GRBAS
scale
(Grade
of
dysphonia,
•
Proposed
trial
design:
Two-site,
double-blind,
randomized,
placebo-
controlled,
20
subjects
Vocal Cord Scarring Status
20
R
oughnes,
B
reathiness,
A
sthenia,
S
train) |
 Acne
Scarring |
 22
Acne Scarring Overview
•
No FDA approved therapeutics
•
Permanent discoloration and/or indentation of the skin as a result
of moderate to severe acne
•
Completed one Phase II study
-
Results published in peer-reviewed journal Dermatologic Surgery,
available online:
http://onlinelibrary.wiley.com/doi/10.1111/dsu.12204/pdf
•
Currently in discussions with FDA on path forward |
 23
•
Efficacy
-
Statistically
significant
number
of
responders
in
subject
and
evaluator
assessments
at
four
months
•
Safety
-
Most
common
adverse
events:
erythema
(11.1%),
swelling
(10.1%);
all
were
mild
or
moderate
in
severity
Acne Scarring Phase II Study Results
Includes
initial
treatment
only.
Note:
Photos
from
Fibrocell
clinical
studies.
Before
After |
 24
Acne Scarring
25 Month Photos Anecdotally Demonstrate Potential Long-Lasting Effect
Potential Long-Lasting Effect:
-
Reports anecdotally suggest >75% of patients demonstrate treatment benefit 9 and
12 months following treatment
-
Photos anecdotally demonstrate sustained improvement 25 months following last
treatment
Photos provided by Gilly S. Munavalli, MD, MHS, FACMS, Dermatology, Laser & Vein
Specialists of the Carolinas, Charlotte, NC |
 Intrexon
Collaboration: Genetically Modified Fibroblasts |
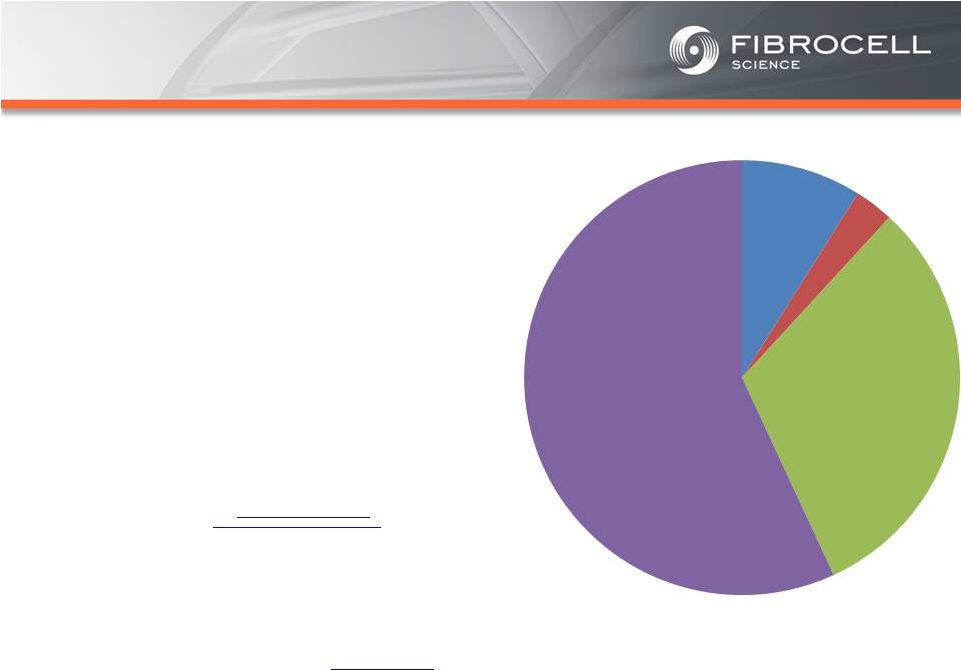 Intrexon
Collaboration: Executive Summary
Based on market analyses, the annual market potential
for each indication is as follows:
•
$1.7-$3.4 billion for a treatment for moderate to
severe psoriasis
(1,2,3,4)
•
$120-$140 million for a treatment for cutaneous
eosinophilias
(5,6)
•
$300-$350 million for a treatment for morphea
(localized scleroderma)
(7,8)
•
$560 million-$2.2 billion for a treatment for
Recessive Dystrophic Epidermolysis Bullosa
(RDEB)
(9,10,11, 12)
Cutaneous
Eosinophilias
$120-$140
million
Moderate to
Severe
Psoriasis
$1.7-$3.4 Billion
RDEB
$560million-
$2.2 billion
Morphea
$300-$500
million
(1)
National Psoriasis Foundation, “About Psoriasis” Available at:
http://www.psoriasis.org/about-psoriasis. Accessed June 27, 2013.
(2)
PhotoMedex, Inc., “Severity of Psoriasis” Available at:
http://xtracnow.com/physicians/psoriasis_stats.htm/ Accessed June 27, 2013.
(3)
Beyer et al. “Recent Trends in Systemic Psoriasis Treatment Costs” Arch
Dermatol2010;146(1):46-54. (4)
Stern et al. “Psoriasis Is Common, Carries a Substantial Burden Even When Not Extensive,
and Is Associated with Widespread Treatment Satisfaction” J InvestigDermatolzzz
Symp2004;9:136-139.
(5)
Cutaneous eosinophilias represent a family of more than 30 different conditions ranging from
eosinophilic cellulitis (Wells’ syndrome) to eosinophilicdermatosis and
eosinophilic fasciitis. The prevalence varies based on which conditions are targeted for
treatment. It could be as low as a few hundred patients (Wells’ syndrome and
eosinophilic fasciitis) to thousands of patients (Duhring disease, which affects 15% to 25% of
celiac patients). We chose 4,000 patients based on the Fibrocell press release
dated July 1, 2013.
(6)
Beyer et al. “Recent Trends in Systemic Psoriasis Treatment Costs” Arch Dermatol
2010;146(1):46-54. We assume that the cutaneous eosinophiliasindication will command
a higher price than the psoriasis indication due to the need for new treatment options, the
frequency of treatment, and the severity of the condition. (7)
Kaplan et al. “Localized Fibrosing Disorders – Linear Scleroderma,
Morphea, and Regional Fibrosis” eMedicine March 6, 2013.
(8)
Beyer et al. “Recent Trends in Systemic Psoriasis Treatment Costs” Arch
Dermatol2010;146(1):46-54. We assume that the morpheaindication will command a higher price
than the psoriasis indication due to the need for new treatment options, the frequency of
treatment, and the severity of the condition. (9)
Stanford School of Medicine, “EpidermolysisBullosa Clinic Frequently Asked Questions”
Available at: http://dermatology.stanford.edu/gsdc/eb_clinic/eb-faqs.html. Accessed
July 11, 2013. (10)
The Dystrophic EpidermolysisBullosa Research Association of America (DEBRA), “About
EB” http://www.debra.org/abouteb. Accessed July 11, 2013.
(11)
Herper, Matthew. “How A $440,000 Drug Is Turning Alexion Into Biotech‘s New
Innovation Powerhouse.” Forbes. 5 September 2012 (12)
The price range represents the price potential for a new therapy for a severe
ultra rare disease based on currently marketed rare disease therapies such as Soliris®
(eculizumab - ~$400,000/year), Elaprase® (idursulfase - ~$375,000/year),
Naglazyme® (galsulfase - ~$365,000/year), Myozyme® (alglucosidasealfa - ~$300,000/year), and
Fabrazyme® (agalsidase beta - ~$200,000/year). |
 Intrexon
Collaborations: Genetically Modified Fibroblasts
for Recessive Dystrophic Epidermolysis
Bullosa (RDEB), Cutaneous Eosinophilias,
Morphea, and Psoriasis |
 •
Leverage
Fibrocell’s
fibroblast
technology
with
Intrexon’s
synthetic
biology
platforms
to
genetically-modify
(GM)
the
patient’s
own
fibroblast
cells
•
GM
fibroblasts
for
recessive
dystrophic
epidermolysis
bullosa
(RDEB),
a
debilitating
rare
genetic
blistering
disease
•
GM
fibroblasts
transfected
ex
vivo
with
therapeutic
gene(s)
of
interest
under
the
control
of
the
RheoSwitch™
Gene
Expression
System
for
localized
treatment
of
Psoriasis,
Cutaneous
Eosinophilias,
and
Morphea,
which
are
serious
skin
diseases
Collaboration Approach
28 |
 •
Initial target: recessive dystrophic epidermolysis bullosa
(RDEB) –
devastating orphan disease, which in its most
severe form, blisters are present over the entire body
•
Developing a protein replacement therapy –
mutations
in COL7A1 gene cause this rare genetic disease
RDEB Overview
29
Stanford
School
of
Medicine,
“EpidermolysisBullosa
Clinic
Frequently
Asked
Questions”
Available
at:
http://dermatology.stanford.edu/gsdc/eb_clinic/eb-faqs.html.
The
Dystrophic
EpidermolysisBullosa
Research
Association
of
America
(DEBRA),
“About
EB”
Herper,
Matthew.
“How
A
$440,000
Drug
Is
Turning
Alexion
Into
Biotech‘s
New
Innovation
Powerhouse.”
Forbes.
5
September
2012
The
price
range
represents
the
price
potential
for
a
new
therapy
for
a
severe
ultra
rare
disease
based
on
currently
marketed
rare
disease
therapies
such
as
Soliris®
(eculizumab
-
~$400,000/year),
Elaprase®
(idursulfase
-
~$375,000/year), Naglazyme®
(galsulfase -
~$365,000/year), Myozyme®
(alglucosidasealfa -
~$300,000/year), and Fabrazyme®
(agalsidase beta -
~$200,000/year).
–
Gene encodes the alpha chain of type VII collagen
–
Type VII collagen forms the major anchoring fibril that
anchors the epidermis to the dermis
–
The incidence of RDEB is estimated to be about 1 to 2
per 1,000,000 people
(1)
,
with an estimated 2,800-5,600
RDEB patients in the U.S.
(2)
–
Rare disease price/patient estimate of >$200 to $400K
(3)
and estimated $560million-$2.2 billion addressable
market
(1,2,4)
(1)
(2)
(3)
(4)
http://www.debra.org/abouteb. Accessed
July
11,
2013.
Accessed
July
11,
2013. |
 Cutaneous
Eosinophilias Overview
The goal is to treat these systemic diseases locally using the autologous
fibroblast as a delivery vehicle
Cutaneous eosinophilias are a family of more than 30 diseases
characterized by inflammation due to the accumulation of eosinophils
(a form of white blood cell) in the layers of the skin
The total prevalence of cutaneous eosinophilias is estimated to be
approximately 4,000 patients in the U.S.
(1,2)
Treatment for cutaneous eosinophilias depends
on the exact nature of the disease and the
severity, but it typically includes topical therapies,
corticosteroids (including prednisone),
antimalarial drugs (including hydroxychloroquine),
and immunosuppressive medications
Cutaneous
eosinophilias
represent
a
family
of
more
than
30
different
conditions
ranging
from
eosinophilic
cellulitis
(Wells’
syndrome)
to
eosinophilicdermatosis
and
eosinophilic
fasciitis.
The
prevalence
varies
based
on
which
conditions
are
targeted
for
treatment.
It
could
be
as
low
as
a
few
hundred
patients
(Wells’
syndrome
and
eosinophilic
fasciitis)
to
thousands
of
patients
(Duhring
disease,
which
affects
15%
to
25%
of
celiac
patients).
We
chose
4,000
patients
based
on
the
Fibrocell
press
release
dated
July
1,
2013.
Beyer
et
al.
“Recent
Trends
in
Systemic
Psoriasis
Treatment
Costs”
Arch
Dermatol
2010;146(1):46-54.
We
assume
that
the
cutaneous
eosinophiliasindication
will
command
a
higher
price
than
the
psoriasis indication
due to the need for new treatment options, the frequency of treatment, and the severity of
the condition. (2)
•
•
•
•
(1) |
 Morphea
Overview
•
The
goal
is
to
treat
these
systemic
diseases
locally
using
the
autologous
fibroblast
as
a
delivery
vehicle
•
Morphea
is
a
rare
form
of
localized
scleroderma
characterized
by
the
thickening
and
hardening
of
the
skin
due
to
excessive
collagen
production;
other
organs
beyond
the
skin
are
typically
not
involved
•
The
total
prevalence
of
all
forms
of
morphea
is
thought
to
be
less
than
10,000
patients
in
the
U.S.
(1)
•
Morphea
treatment
typically
focuses
on
controlling
symptoms
and
slowing
disease
progression;
treatment
options
include
photo
(light)
therapy,
corticosteroids,
calcipotriene
cream,
antimalarial
drugs,
and
immunosuppressive
medications
(1)
Kaplan
et
al.
“Localized
Fibrosing
Disorders
–
Linear
Scleroderma,
Morphea,
and
Regional
Fibrosis”
eMedicine
March
6,
2013. |
 Psoriasis
Overview The goal is to treat these systemic diseases
locally using the autologous fibroblast as a
delivery vehicle Chronic skin disease characterized by the
overproduction of new skin cells, which
results in the formation of scaly patches Believed to be an
immune-mediated disease caused by a malfunction in the
immune system
No cure for Psoriasis: symptoms managed with topical agents,
phototherapy, and systemic therapies
U.S. prevalence approximately 4.5 million (25% with moderate to
severe disease, 10-20% also develop
psoriatic arthritis) (1,2,3)
U.S. psoriasis therapeutic market nearly $2
billion in 2012
Total direct and indirect healthcare costs
over $11 billion in 2008
(1) Stern
et
al.
“Psoriasis
Is
Common,
Carries
a
Substantial
Burden
Even
When
Not
Extensive,
and
Is
Associated
with
Widespread
Treatment
Satisfaction”
J
InvestigDermatolSymp2004;9:136
-139.
(2)
National
Psoriasis
Foundation,
“About
Psoriasis”
Available
at:
(3)
PhotoMedex,
Inc.,
“Severity
of
Psoriasis”
Available
at:
•
•
•
•
•
•
•
Accessed
June
27,
2013.
Accessed
June
27,
2013.
http://www.psoriasis.org/about-psoriasis.
http://xtracnow.com/physicians/psoriasis_stats.htm/ |
 UCLA
Collaboration: Skin-derived Stem Cell
and Skin-derived IPSCs |
 Identified a rare mesenchymal stem cell (MSC) population from adult
human skin
Published data -
“Vega-Crespo et al 2013, BioResearch Open Access, 1:25”
(http://online.liebertpub.com/doi/pdf/10.1089/biores.2012.0204)
Identified a small molecule BAY11 that can stabilize expression of OCT4
synthetic mRNA, key programming factor for generating induced
pluripotent stem cells
Published results -
“Awe et al 2013, Stem Cell Res Ther; 4:15”
(http://stemcellres.com/content/pdf/scrt163.pdf)
Exclusive license to both patent applications
Skin-derived Stem Cell and IPSC
Collaboration
34
•
•
•
•
• |
 Growing Momentum
of IPSCs (Induced Pluripotent Stem Cells)
35
Source: PubMed.gov |
 Growing Momentum
of MSCs (Mesenchymal Stem Cells)
36
Source: PubMed.gov |
 Financials
|
 •
Cash position of $26.1 million as of March 31, 2013
•
Large private capital raise completed in October 2012; institutional
investors include, among others, an affiliated fund of Third Security, LLC,
an investment firm founded by R.J. Kirk
•
No debt
•
Company’s capital structure greatly simplified
–
Outstanding preferred stock converted into common stock
–
Approximately 99% of warrants conceded anti-dilution provisions
Financial Information
38 |
 Commenced
trading on NYSE MKT on May 17, 2013 Capitalization Table
39
Common shares (post reverse split)
Warrants
Options
Fully diluted shares outstanding
Shareholder breakdown: Institutional vs. Retail
27.5M
6.3M
0.6M
34.4M
60% / 40% |
 Summary
|
 Summary
•
Cutting edge autologous cell therapy company developing first-in-class medical
applications using fibroblasts
•
Advancing
a
robust
pipeline
for
medical
applications
of
LAVIV®
(azficel-T):
–
Restrictive burn scarring, vocal cord scarring and acne scarring
•
Strategic collaboration with Intrexon focusing on genetically-modified autologous
fibroblast cells:
–
Developing therapeutic options for the localized treatment of moderate to severe psoriasis
and for rare skin diseases
•
Developing skin-derived MSCs and more efficient conversion of skin cells to IPSCs through
MIT/UCLA collaboration
•
LAVIV®
-
lead product
–
Indicated for improvement of the appearance of moderate to severe nasolabial fold
wrinkles in adults
–
One of only three FDA approved cell based products
–
LAVIV®
is positioned as a premium aesthetic
•
Strong cash position with $26.1 million at March 31, 2013
–
No debt
41 |
 42
Indication
LAVIV®
(azficel-T)
is
an
autologous
cellular
product
indicated
for
improvement
of
the
appearance
of
moderate
to
severe
nasolabial
fold
wrinkles
in
adults.
The
safety
and
efficacy
of
LAVIV
for
areas
other
than
the
nasolabial
folds
have
not
been
established.
The
efficacy
of
LAVIV
beyond
six
months
has
not
been
established. |
 43
Important Safety Information
LAVIV®(azficel-T) is an autologous cellular product for intradermal injection only.
LAVIV is contraindicated for allogeneic use, in patients with allergy to gentamicin,
amphotericin, dimethylsulfoxide (DMSO) or material of bovine origin and in
patients with active infection in the facial area. The following
reactions have been
reported following treatment with LAVIV: hypersensitivity reactions, bleeding and
bruising at the treatment site, vasculitis, herpes labialis, basal cell cancer; keloid
and hypertrophic scarring may occur following post-auricular skin biopsies or LAVIV
injections.
Additional
warnings
and
precautions
to
be
considered
include
the
use
of
LAVIV in patients with genetic disorders or formation of normal collagen matrices
and in immunosuppressed patients, or those patients undergoing chemotherapy
for malignancies or receive immunomodulatory therapies for autoimmune
diseases.
The most common adverse reactions, occurring in
1% of patients who receive
LAVIV, were injection-site redness, bruising, swelling, pain, hemorrhage, edema,
nodules, papules, irritation, dermatitis and pruritus.
For more information about LAVIV, please see the full Prescribing Information,
www.mylaviv.com.
> |
