Attached files
| file | filename |
|---|---|
| EX-10.1 - EX-10.1 - CUBIST PHARMACEUTICALS INC | a13-17558_5ex10d1.htm |
| EX-10.2 - EX-10.2 - CUBIST PHARMACEUTICALS INC | a13-17558_5ex10d2.htm |
| 8-K - 8-K - CUBIST PHARMACEUTICALS INC | a13-17558_58k.htm |
| EX-2.1 - EX-2.1 - CUBIST PHARMACEUTICALS INC | a13-17558_5ex2d1.htm |
|
|
Planned Acquisitions of Trius Therapeutics and Optimer Pharmaceuticals Accelerating Cubist’s Global Leadership in Acute Care July 30, 2013 Exhibit 99.1 |
|
|
Safe Harbor Statement 2 This presentation and statements in this conference call regarding the proposed transactions contain forward-looking statements. Any statements contained herein which do not describe historical facts, including but not limited to, statements regarding: the proposed transactions between Cubist and Trius and between Cubist and Optimer; the expected timetable for completing each transaction; Cubist’s future financial and operating results, including the expected impact of the anticipated transactions on Cubist’s earnings; Cubist’s forecast of peak year revenue contributions from DIFICID and tedizolid phosphate combined; strategic and other potential benefits of each transaction, including that each transaction supports Cubist’s Building Blocks of Growth long-range goals; Cubist’s ability and plans to finance each transaction; Trius’ product candidates, including regarding the therapeutic and commercial potential of, and the expected timing for filing U.S. and E.U. regulatory approval submissions for and commercial launches of, tedizolid phosphate; Optimer’s commercial product, DIFICID, including regarding its therapeutic and commercial potential; Cubist’s products and product candidates, including ceftolozane/tazobactam; Cubist’s plans to expand internationally; and any other statements about Cubist, Trius or Optimer managements’ future expectations, beliefs, goals, plans, or prospects, are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include: the possibility that certain closing conditions to either transaction will not be satisfied; that required regulatory approvals for either transaction may not be obtained in a timely manner, if at all; the ability to consummate either transaction and possibility that either transaction will not be completed; the ability of Cubist to successfully integrate Trius’ and/or Optimer’s operations and employees; the anticipated benefits of either transaction may not be realized; risks related to drug development and commercialization; the ability of Cubist to achieve its Building Blocks of Growth long-range goals, including as a result of Cubist’s ability to continue to grow revenues from the sale of CUBICIN® and ENTEREG®, competition, Cubist’s ability to successfully develop, gain marketing approval for and commercially launch its product candidates for their planned indications and on their expected timelines, and Cubist’s ability to discover, in-license or acquire new products and product candidates; and those additional factors discussed in Cubist’s, Trius’ and Optimer’s most recent Annual and Quarterly Reports on Forms 10-K and 10-Q filed with the Securities and Exchange Commission. Cubist, Trius and Optimer caution investors not to place considerable reliance on the forward-looking statements contained in this presentation and call. These forward-looking statements speak only as of the date of this call, and Cubist, Trius and Optimer undertake no obligation to update or revise any of these statements. |
|
|
Non-GAAP Financial Measures 3 Use of Non-GAAP Financial Measures Cubist uses non-GAAP financial measures, such as non-GAAP adjusted operating income to assess and analyze its operational results and trends and to make financial and operational decisions. Cubist believes these non-GAAP financial measures are also useful to investors because they provide greater transparency regarding Cubist’s operating performance. These non-GAAP financial measures should not be considered an alternative to measurements required by GAAP, such as operating income, and should not be considered measures of Cubist’s liquidity. In addition, these non-GAAP financial measures are unlikely to be comparable with non-GAAP information provided by other companies. Reconciliations between non-GAAP financial measures and GAAP financial measures are included at the end of this presentation. |
|
|
Non-GAAP Financial Measures Reconciliation of 2017 Non-GAAP Adjusted Operating Income to 2017 GAAP Operating Income Cubist’s Building Blocks of Growth include forward-looking information about the Company's future financial performance, including projected adjusted operating income in 2017 of $700 million, which is a non-GAAP financial measure. This non-GAAP financial measure would be derived by excluding certain amounts from operating income that would be determined in accordance with GAAP and should not be considered an alternative to measurements required by GAAP or a measure of Cubist’s future liquidity. The determination of the amounts that are excluded from non-GAAP financial measures is a matter of management judgment and depends upon, among other factors, the nature of the underlying expense or income amounts. The Company is unable to present a quantitative reconciliation of 2017 adjusted operating income to 2017 operating income, its most directly comparable forward-looking GAAP financial measure, because management cannot reasonably predict with sufficient reliability all of the necessary components of such GAAP measure. For its 2013 guidance, the Company has excluded from its projected non-GAAP adjusted operating income expected contingent consideration expense and amortization expense related to acquired intangible assets, which are included in projected GAAP operating income. The Company is likely to exclude these items from non-GAAP adjusted operating income in the future and may also exclude the following items, and similar items, the effect of which is uncertain but may be significant in amount: Other expenses related to completed and future acquisitions of other businesses, including amortization and/or impairment of acquired intangible and tangible assets, integration costs, and transaction costs; Expenses associated with any potential restructuring activities, including but not limited to, asset impairments, accelerated depreciation, severance costs, and lease abandonment charges; Adjustments related to the timing of income or expense recognition for potential collaboration activities under GAAP; Other one-time charges; and The tax implications of such activities or transactions. 4 |
|
|
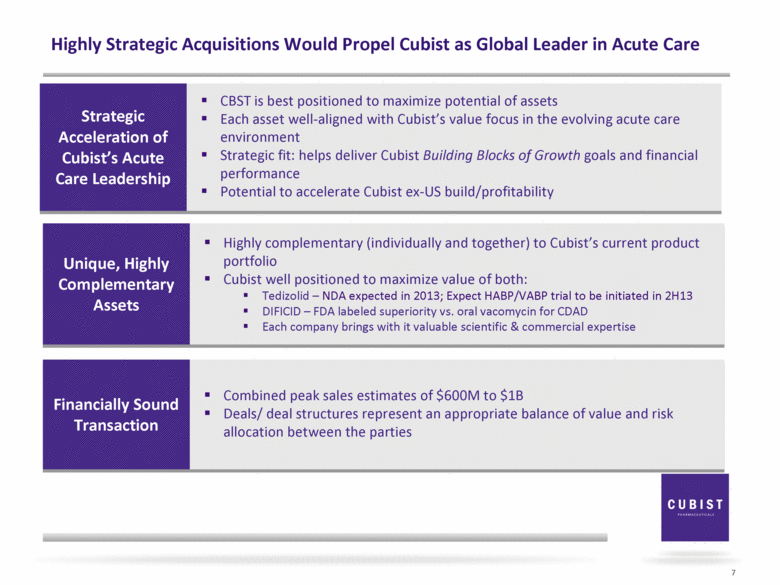
Highly Strategic Acquisitions Would Propel Cubist as Global Leader in Acute Care 5 Strategic Acceleration of Cubist’s Acute Care Leadership Unique, Highly Complementary Assets Financially Sound Transactions; Accretive in 2015 |
|
|
Cubist 5 Year Goals (2013 – 2017) 6 A Highly Differentiated Culture $2B in Global Revenue 4 Product Candidates in Late-Stage Clinical Trials $700M in Non-GAAP Adjusted Operating Income Building Blocks of Growth |
|
|
Highly Strategic Acquisitions Would Propel Cubist as Global Leader in Acute Care 7 Unique, Highly Complementary Assets Highly complementary (individually and together) to Cubist’s current product portfolio Cubist well positioned to maximize value of both: Tedizolid – NDA expected in 2013; Expect HABP/VABP trial to be initiated in 2H13 DIFICID – FDA labeled superiority vs. oral vacomycin for CDAD Each company brings with it valuable scientific & commercial expertise Financially Sound Transaction Combined peak sales estimates of $600M to $1B Deals/ deal structures represent an appropriate balance of value and risk allocation between the parties Strategic Acceleration of Cubist’s Acute Care Leadership CBST is best positioned to maximize potential of assets Each asset well-aligned with Cubist’s value focus in the evolving acute care environment Strategic fit: helps deliver Cubist Building Blocks of Growth goals and financial performance Potential to accelerate Cubist ex-US build/profitability |
|
|
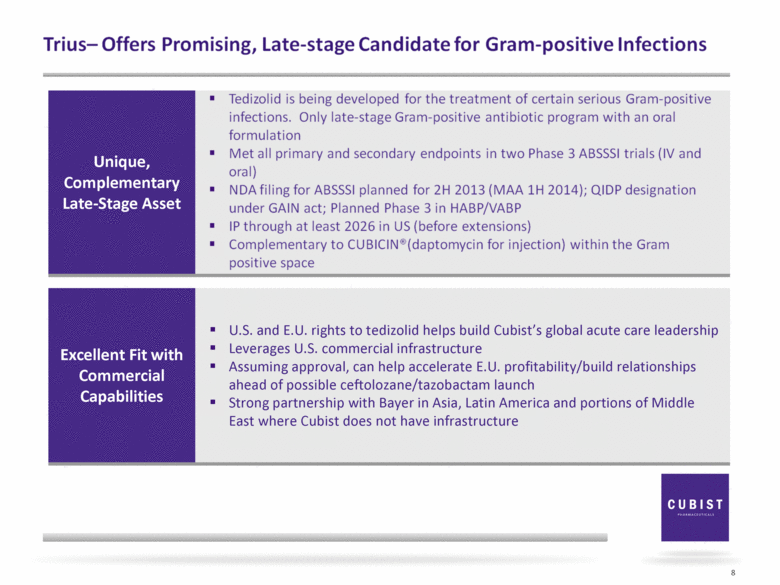
8 Unique, Complementary Late-Stage Asset Excellent Fit with Commercial Capabilities U.S. and E.U. rights to tedizolid helps build Cubist’s global acute care leadership Leverages U.S. commercial infrastructure Assuming approval, can help accelerate E.U. profitability/build relationships ahead of possible ceftolozane/tazobactam launch Strong partnership with Bayer in Asia, Latin America and portions of Middle East where Cubist does not have infrastructure Tedizolid is being developed for the treatment of certain serious Gram-positive infections. Only late stage Gram-positive antibiotic program with an oral formulation Met all primary and secondary endpoints two Phase 3 ABSSSI trials (IV and oral) NDA filing for ABSSSI planned for 2H 2013 (MAA 1H 2014); QIDP designation under GAIN act; Planned Phase 3 in HABP/NABP IP through at least 2026 in US (before extensions) Complementary to CUBICIN® (deptomycin for injection) within the Gram positive space |
|
|
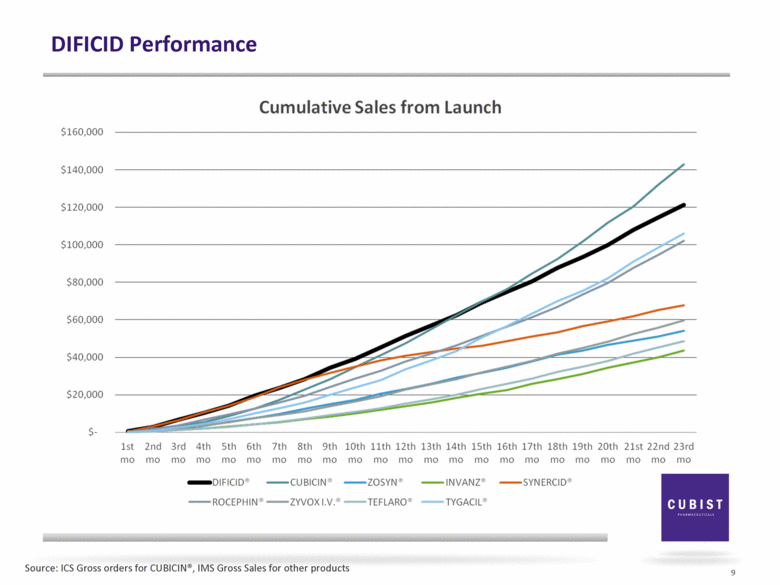
DIFICID Performance 9 Source: ICS Gross orders for CUBICIN®, IMS Gross Sales for other products |
|
|
Optimer – Marketed Product helping address need in large CDAD Market 10 Complementary to Commercial Infrastructure Leverage Cubist’s world-class acute care/hospital infrastructure to support growth in US Co-promote agreement extended; expect smooth transition post closing No substantial new investments needed to support growth Strong partnership with Astellas and AZ (Europe/Japan/parts of Middle East) Superior Acute Care Product Optimer’s DIFICID, approved to treat clostridium difficile-associated diarrhea (CDAD) Approved and launched in US in 2011 FDA-labeled superiority to standard of care in sustained response; established safety profile First product approved for CDAD in more than 25 years Narrow spectrum of activity, minimal impact on other normal gut flora CDAD is large, growing market: 600,000 cases annually in U.S. |
|
|
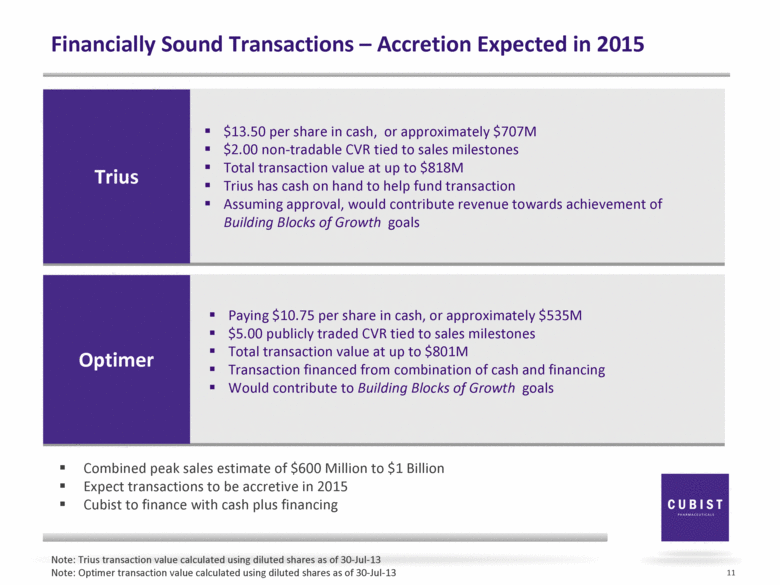
Financially Sound Transactions – Accretion Expected in 2015 11 Trius $13.50 per share in cash, or approximately $707M $2.00 non-tradable CVR tied to sales milestones Total transaction value at up to $818M Trius has cash on hand to help fund transaction Assuming approval, would contribute revenue towards achievement of Building Blocks of Growth goals Optimer Paying $10.75 per share in cash, or approximately $535M $5.00 publicly traded CVR tied to sales milestones Total transaction value at up to $801M Transaction financed from combination of cash and financing Would contribute to Building Blocks of Growth goals Note: Trius transaction value calculated using diluted shares as of 30-Jul-13 Note: Optimer transaction value calculated using diluted shares as of 30-Jul-13 Combined peak sales estimate of $600 Million to $1 Billion Expect transactions to be accretive in 2015 Cubist to finance with cash plus financing |
|
|
IDSA 10 X 20 Initiative IDSA Challenge to industry/ public policy makers: Find a way to deliver at least 10 new antibiotics by the year 2020. Assuming completion of planned acquisitions + success of combined late-stage pipeline: Cubist would ultimately be responsible for 4 of these new antibiotic therapies by 2020. 12 |
|
|
Product and Pipeline Candidates – (With Planned Acquisitions) 13 Therapy / Candidate Phase 1 Phase 2 Phase 3 Filing Market Milestones/Highlights CUBICIN® (daptomycin for injection) FY 2012 Net Revenues of $926.4 ENTEREG®(alvimopan) FY 2012 Net Revenues of $40.2M; sNDA filed: PDUFA 10/22/13 DIFICID®(fidaxomicin) FY 2012 Net US/Canada Sales of $62.4M (Optimer) Tedizolid phosphate ABSSSI (IV/Oral)** Expect to file NDA in H2 ‘13 & MAA in H1 ‘14 (Trius) Tedizolid phosphate HAP/VAP (IV/Oral)** Expect to initiate Phase 3 In 2H13 (Trius) Ceftolozane/tazobactam cUTI /cIAI** Expect Top-Line Data on Phase 3 trials 4Q13 Ceftolozane/tazobactam HABP/VABP** Open-label VABP trial in progress; Clarity re: Pathogen specific Ph. 3 protocol expected by YE Surotomycin in CDAD** Phase 3 in progress Bevenopran in OIC** Phase 3 in progress Adynxx* (Acute Pain**) Phase 2 in progress * Cubist has rights to AYX1 through its exclusive option to acquire Adynxx, Inc. ** Potential indications sought, pending outcomes of clinical trials. Information as of July 30, 2013. |
|
|
Strategic Acceleration of Cubist’s Global Acute Care Leadership Transactions are right in our wheelhouse Co-promote on DIFICID Helped pioneer Gram+ market over past decade Both products fit with importance of delivering value in evolving healthcare environment Should enable acceleration of International profitability and build-out Provide access to needed top talent and capabilities Accretive in 2015 14 |
|
|
Important Additional Information 15 Important Additional Information about the Potential Trius Transaction will be Filed with the U.S. Securities and Exchange Commission This presentation and the statements during this call are not a recommendation, an offer to purchase or a solicitation of an offer to sell shares of Trius stock. Cubist has not commenced the tender offer for shares of Trius’ stock described in this presentation. Upon commencement of the tender offer, Cubist will file with the Securities and Exchange Commission a tender offer statement on Schedule TO and related exhibits, including an offer to purchase, letter of transmittal, and other related documents. Following commencement of the tender offer, Trius will file with the Securities and Exchange Commission a solicitation/ recommendation statement on Schedule 14D-9. Shareholders should read the offer to purchase and solicitation/recommendation statement and the tender offer statement on Schedule TO and related exhibits when such documents are filed and become available, as they will contain important information about the tender offer. Shareholders can obtain these documents when they are filed and become available free of charge from the Securities and Exchange Commission's website at www.sec.gov, or by contacting the Investor Relations departments of Cubist or Trius at eileen.mcintyre@cubist.com or sloren@westwicke.com, respectively. |
|
|
Important Additional Information 16 Important Additional Information about the Proposed Optimer Transaction and Where to Find it. This presentation and the statements during this call shall not constitute an offer of any securities for sale. The acquisition will be submitted to Optimer’s stockholders for their consideration. In connection with the acquisition, Cubist and Optimer intend to file relevant materials with the Securities and Exchange Commission (SEC), including a registration statement on Form S-4, which will include a proxy statement/prospectus and other relevant documents concerning the merger. Investors and stockholders of Cubist and Optimer are urged to read the registration statement, the proxy statement/prospectus and other relevant documents filed with the SEC when they become available, as well as any amendments or supplements to the documents because they will contain important information about Cubist, Optimer and the merger. Stockholders of Cubist and Optimer can obtain more information about the proposed transaction by reviewing the Form 8-K to be filed by Cubist and Optimer in connection with the announcement of the entry into the merger agreement, and any other relevant documents filed with the SEC when they become available. The registration statement, the proxy statement/prospectus and any other relevant materials (when they become available), and any other documents filed by Cubist and Optimer with the SEC, may be obtained free of charge at the SEC's web site at www.sec.gov. In addition, investors and stockholders may obtain free copies of the documents filed with the SEC by directing a written request to: Cubist, 65 Hayden Avenue, Lexington, MA 02421, Attention: Investor Relations, or Optimer, 4755 Nexus Center Drive, San Diego, CA 92121, Attention: Investor Relations. Investors and stockholders are urged to read the registration statement, the proxy statement/prospectus and the other relevant materials when they become available before making any voting or investment decision with respect to the merger. Participants in Solicitations Optimer and its directors, executive officers and other members of their management and employees may be deemed to be participants in the solicitation of proxies from stockholders of Optimer in connection with the merger. Information regarding Optimer's directors and executive officers and their ownership of Optimer common stock is available in Optimer’s annual report on Form 10-K for the year ended December 31, 2012, filed with the SEC on March 18, 2013 and the proxy statement on Schedule 14A for Optimer’s 2013 annual meeting of stockholders, filed with the SEC on April 12, 2013 and as amended on April 19, 2013. Certain directors and executive officers of Optimer and other persons may have direct or indirect interests in the proposed merger due to securities holdings, pre-existing or future indemnification arrangements and rights to severance payments if their employment is terminated prior to or following the merger. If and to the extent that any of the Optimer participants will receive any additional benefits in connection with the merger, the details of those benefits will be described in the proxy statement/prospectus relating to the merger. Investors and security holders may obtain additional information regarding the direct and indirect interests of Optimer and its executive officers and directors in the merger by reading the proxy statement/prospectus regarding the Merger when it becomes available. |
|
|
Planned Acquisitions of Trius Therapeutics and Optimer Pharmaceuticals Accelerating Cubist’s Global Leadership in Acute Care July 30, 2013 |