Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - HARROW HEALTH, INC. | immy_8k.htm |
Exhibit 99.1

Imprimis Pharmaceuticals, Inc.
“A Unique Approach to 505(b)(2)”
July 2013
Mark L. Baum, C.E.O.
1

Safe Harbor Statement
This presentation contains "forward-looking statements" as defined in the Private Securities Litigation Reform Act
of 1995. You are cautioned not to rely on these forward-looking statements. These statements are based on
current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or
uncertainties materialize, actual results could vary materially from our expectations and projections.
of 1995. You are cautioned not to rely on these forward-looking statements. These statements are based on
current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or
uncertainties materialize, actual results could vary materially from our expectations and projections.
Some of the potential risks and uncertainties that could cause actual results to differ from those predicted include
the difficulties related to the Company’s ability to obtain regulatory approval to market Impracor™, capitalize on
its perceived potential benefits arising from its relationship with Professional Compounding Centers of America,
Inc., leverage compounded generic drugs to create a development pipeline and otherwise pursue its business
plan, and leverage its Accudel technology in the development of potential product candidates. In addition, the
outcome of the final analyses of the data from the past and future Phase 3 clinical trial may vary from the
Company’s initial conclusions, the FDA may not agree with the Company’s interpretation of such results or may
challenge the adequacy of the Company’s future Impracor clinical trial design or the execution of the same clinical
trials, the FDA may require the Company to complete additional clinical trials for Impracor before the Company
can submit a 505(b)(2) NDA application, the results of any future clinical trials may not be favorable and the
Company may never receive regulatory approval for Impracor™, the Company may be unable to raise additional
funding to complete its product development plans, or be unable to acquire, develop or commercialize new
products and or enter into strategic alliances and transactions. Other risks include uncertainties inherent in pre-
clinical studies and clinical trials, difficulties in conducting its clinical trials, unexpected new data, safety and
technical issues, competition and market conditions. More detailed information about the Company and the risk
factors that may affect the realization of forward-looking statements is set forth in the Company’s filings with the
Securities and Exchange Commission, including its Annual Report on Form 10-K and its Quarterly Reports on
Form 10-Q filed with the SEC. Such documents may be read free of charge on the SEC’s web site at
www.sec.gov.
the difficulties related to the Company’s ability to obtain regulatory approval to market Impracor™, capitalize on
its perceived potential benefits arising from its relationship with Professional Compounding Centers of America,
Inc., leverage compounded generic drugs to create a development pipeline and otherwise pursue its business
plan, and leverage its Accudel technology in the development of potential product candidates. In addition, the
outcome of the final analyses of the data from the past and future Phase 3 clinical trial may vary from the
Company’s initial conclusions, the FDA may not agree with the Company’s interpretation of such results or may
challenge the adequacy of the Company’s future Impracor clinical trial design or the execution of the same clinical
trials, the FDA may require the Company to complete additional clinical trials for Impracor before the Company
can submit a 505(b)(2) NDA application, the results of any future clinical trials may not be favorable and the
Company may never receive regulatory approval for Impracor™, the Company may be unable to raise additional
funding to complete its product development plans, or be unable to acquire, develop or commercialize new
products and or enter into strategic alliances and transactions. Other risks include uncertainties inherent in pre-
clinical studies and clinical trials, difficulties in conducting its clinical trials, unexpected new data, safety and
technical issues, competition and market conditions. More detailed information about the Company and the risk
factors that may affect the realization of forward-looking statements is set forth in the Company’s filings with the
Securities and Exchange Commission, including its Annual Report on Form 10-K and its Quarterly Reports on
Form 10-Q filed with the SEC. Such documents may be read free of charge on the SEC’s web site at
www.sec.gov.
You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the
date hereof, given these risks and uncertainties. All forward-looking statements are qualified in their entirety by
this cautionary statement and the Company undertakes no obligation to revise or update any forward-looking
statements as a result of new information or future events or developments.
date hereof, given these risks and uncertainties. All forward-looking statements are qualified in their entirety by
this cautionary statement and the Company undertakes no obligation to revise or update any forward-looking
statements as a result of new information or future events or developments.
© Imprimis Pharmaceuticals, Inc. | *
2

Imprimis Overview
© Imprimis Pharmaceuticals, Inc. | *
3

Imprimis Snapshot
© Imprimis Pharmaceuticals, Inc. | *
• Approx. $19.0M in cash as of 03/31/13
• Nominal debt; no preferred instruments
• Phase 3 topical NSAID pivotal to start in Q3 2013
• Exclusive commercial rights to PCCA development IP
• 10,000+ drug formulations
• 10+ drug delivery technologies
• Vast market “unmet need” database
• Exclusive access to review PCCA member IP
• Experienced science and management teams
4
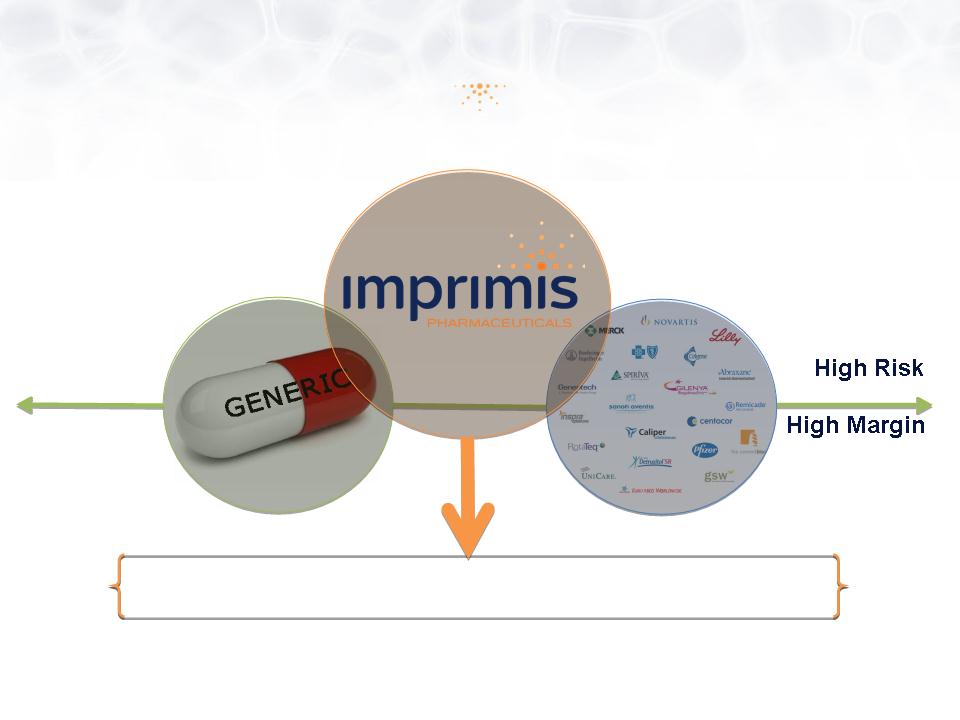
Imprimis Overview
© Imprimis Pharmaceuticals, Inc. | *
Low Risk
Low Margin
505(b)(2) … Lower Risk | Higher Margin
Our mission is to develop proprietary drugs using the
FDA 505(b)(2) drug development pathway
5
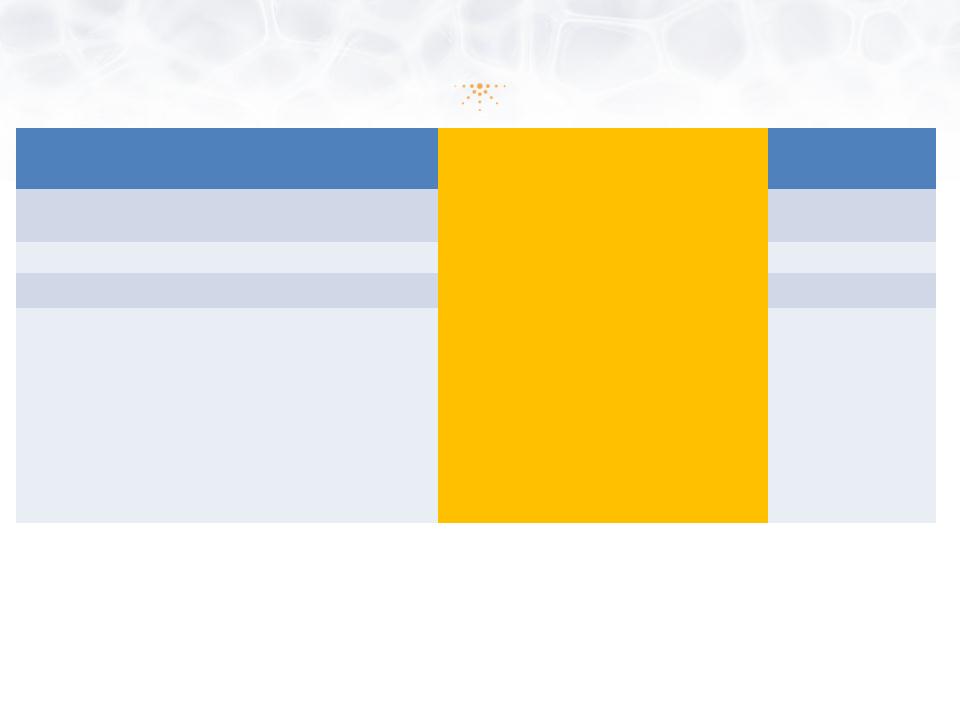
Less Development Time & Lower Cost
|
Application
|
505(b)(1) NDA
|
505(b)(2) NDA
|
505(j) ANDA
|
|
New Chemical Entity
(NCE) |
Yes
|
Yes/No
(Rely on RLD and Prior Investigation)
|
No
(RLD is off patent)
|
|
New Indication
|
Yes
|
Yes
|
No
|
|
New Form/Dose
|
Yes
|
Yes
|
No
|
|
Required Data for
Approval |
• Complete Pharmacology
• Complete Preclinical
Safety, including long term carcinogenicity in 2 species • Complete analytical
development and quality manufacturing • Complete Phase 1-3
clinical trials |
• Data from published literature
• FDA findings on efficacy/safety of
approved drug/formulation • Studies to support change
• Dermal/Eye Safety (topical drugs)
• Clinical Efficacy/Safety
• CMC (3 registration batches with
stability data) |
• Bioequivalence
|
• 505(b)(2) products can have Orange Book-listed patents, can enjoy 30-month protection against
generic competitors; NCE (5 yrs); Orphan Drug (7 yrs); Pediatric Extension (6 mos.)
generic competitors; NCE (5 yrs); Orphan Drug (7 yrs); Pediatric Extension (6 mos.)
• 505(b)(2) Development Budget Comparison: $2-7M versus $100M+ for (b)(1)
© Imprimis Pharmaceuticals, Inc. | 6
6
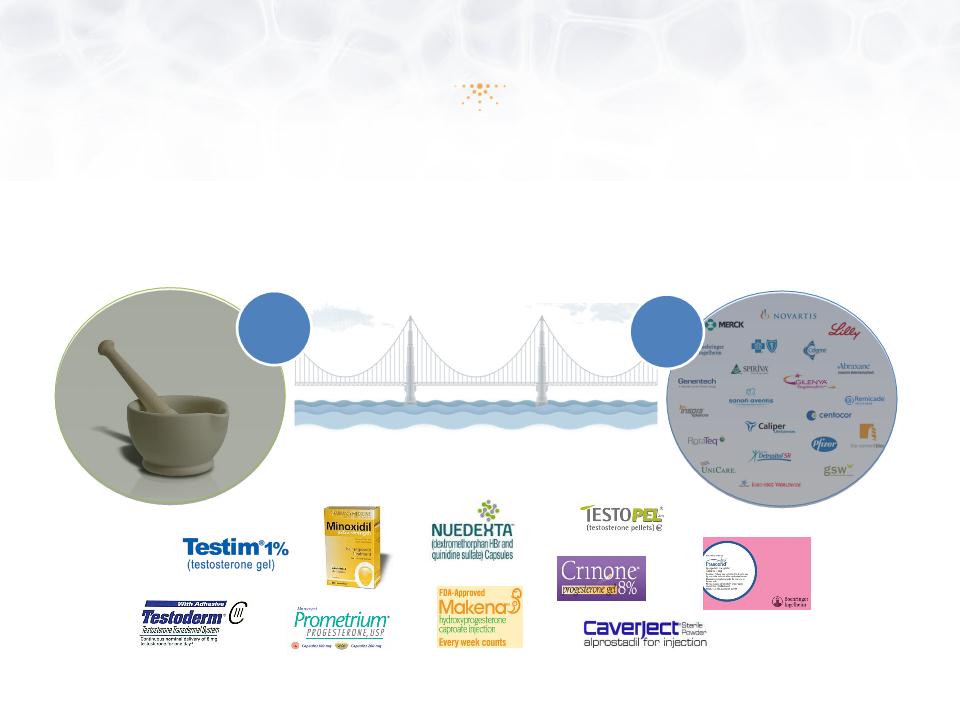
Imprimis Development Model
© Imprimis Pharmaceuticals, Inc. | *
Imprimis Brings Innovation
from Pharmaceutical Compounders
to the >$300B U.S. Pharmaceutical Industry
Pharmaceutical
Compounders
1%
99%
7
PCCA Strategic Relationship
• Professional Compounding Centers of America
(PCCA) is the largest compounding pharmacy
organization in North America
(PCCA) is the largest compounding pharmacy
organization in North America
1. Supply chemicals, equipment, accredited training, software,
and business/pharmacy consulting assistance
and business/pharmacy consulting assistance
• Over 4,000 pharmacy businesses/chains worldwide
• PCCA relationship gives Imprimis exclusive access to:
1. Proprietary and proven drug formulations
2. Proprietary and proven drug delivery technologies
(Lipoderm® and others)
(Lipoderm® and others)
3. Market data (>100,000 inbound calls per year)
4. Analytics (Eagle Analytics)
5. Exclusive access to review PCCA member IP
• Our strategic relationship is exclusive
• PCCA invested $4M into Imprimis at $4.80 per share
505(b)(2) Focused
Proprietary Drug Pipeline
+
© Imprimis Pharmaceuticals, Inc. | *
8
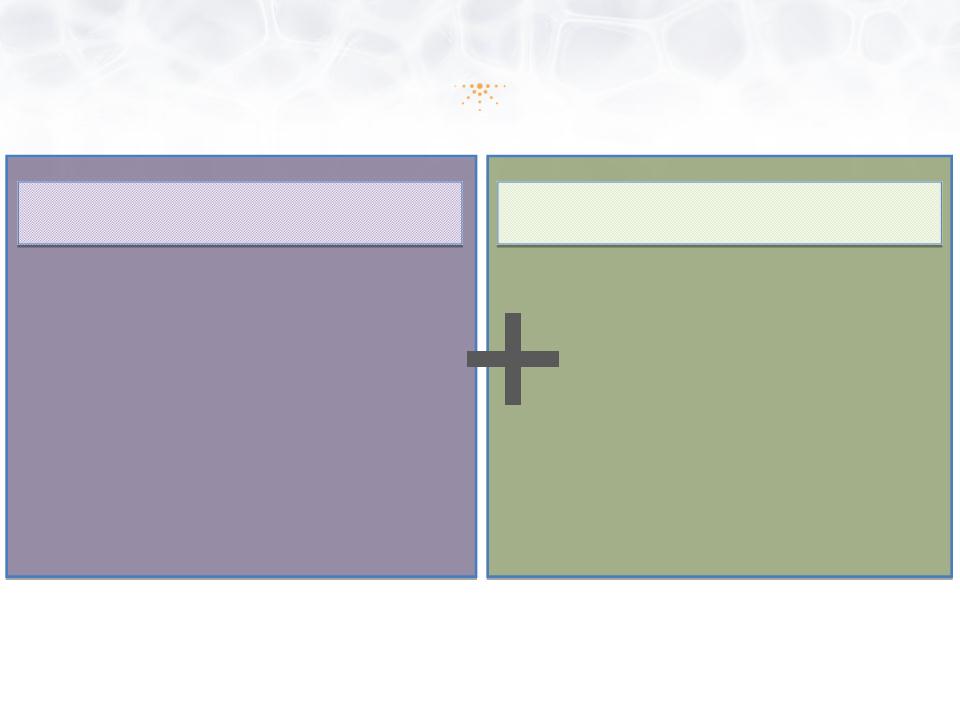
Imprimis Vision
© Imprimis Pharmaceuticals, Inc. | *
Drive Shareholder Value
•Monetize vast 505(b)(2)
development assets
development assets
• Selectively internal
development
development
• Partner
• Out-license
Improve Patient Care
•Novel drug administration
• Reduce or eliminate
negative side effect
profiles
negative side effect
profiles
• Increase therapeutic
benefit to patients
benefit to patients
9
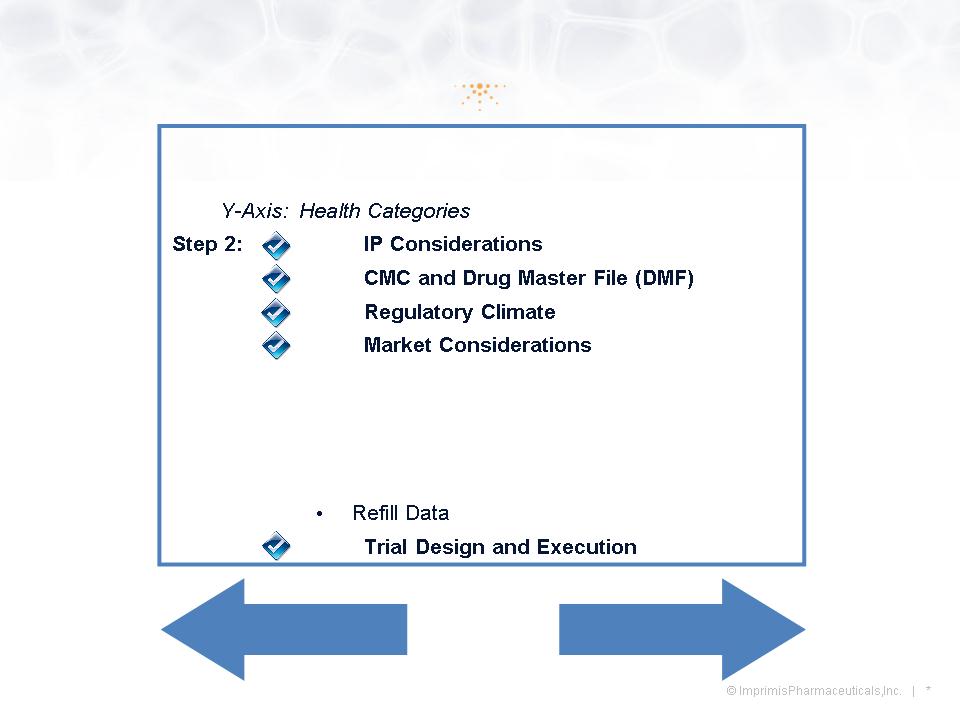
Monetizing the PCCA Relationship
Step 1: Opportunity Matrix
X-Axis: Drug Administration
• Competition
• Reimbursement Landscape
• Dollar Size
• Number of Annual RX
Internally Develop
Partner, Out-License
10
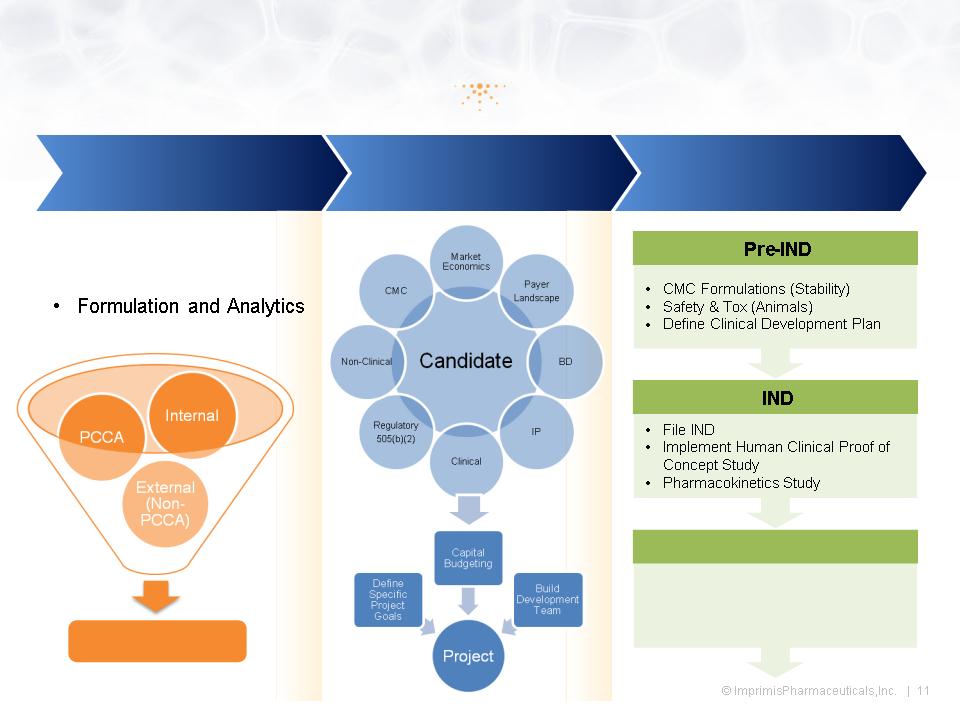
Imprimis Growth & Development Process
Ideas
Candidates
Projects
• Market Data
• Drug Master File
• Field Experience
• Out-License or Develop
• Complete Phase 3
• NDA via 505(b)(2)
• Market Launch/Partner
NDA & LAUNCH
Candidate
11

Imprimis Pipeline
© Imprimis Pharmaceuticals, Inc. | *
12
Imprimis Pipeline
|
Therapeutic
Area |
Compound
|
Indication
|
Market Analysis
|
|
PAIN
|
IPI110
Impracor™, Ketoprofen 10%
Cream |
Sprains, Strains and
Joint Pain |
$10B US NSAID Market
Transitioning to Topicals due to high incidence of AEs
Voltaren Gel has 75% Rx Share
Topical NSAID market may
exceed $1B by 2015
|
|
OPHTHALMOLOGY |
IPI004**
Corticosteroid + Antibiotic |
Prophylaxis of Post-
Operative Complication following Cataract, Glaucoma, and Retinal Surgery |
19M Global Cataract Surgeries (2011); $200-
$300/drugs/US surgery Growing Market due to Aging Pop. and Demand to have
surgery at an earlier age Combination (single injection) will minimize risk for injury
and infection versus multiple injections |
|
WOUND HEALING
|
IPI120
Tranexamic Acid + Antibiotic
|
Topical wound care
treatment of genetic and acquired bleeding disorders |
$9B Global Bleeding and Clotting Disorders Market
$17B US Wound Care Market
7M American suffering from chronic wound, annually
|
|
** Imprimis has an exclusive option and right to negotiate to acquire this drug formulation and intellectual property based on substantially prenegotiated terms. |
|||
© Imprimis Pharmaceuticals, Inc. | *
Additional compounds in late stage assessment are in the therapeutic areas of
Pain and Sexual Dysfunction
13

Improving Patient Care:
Impracor™ Phase 3
Topical NSAID
Impracor™ Phase 3
Topical NSAID
© Imprimis Pharmaceuticals, Inc. | *
14
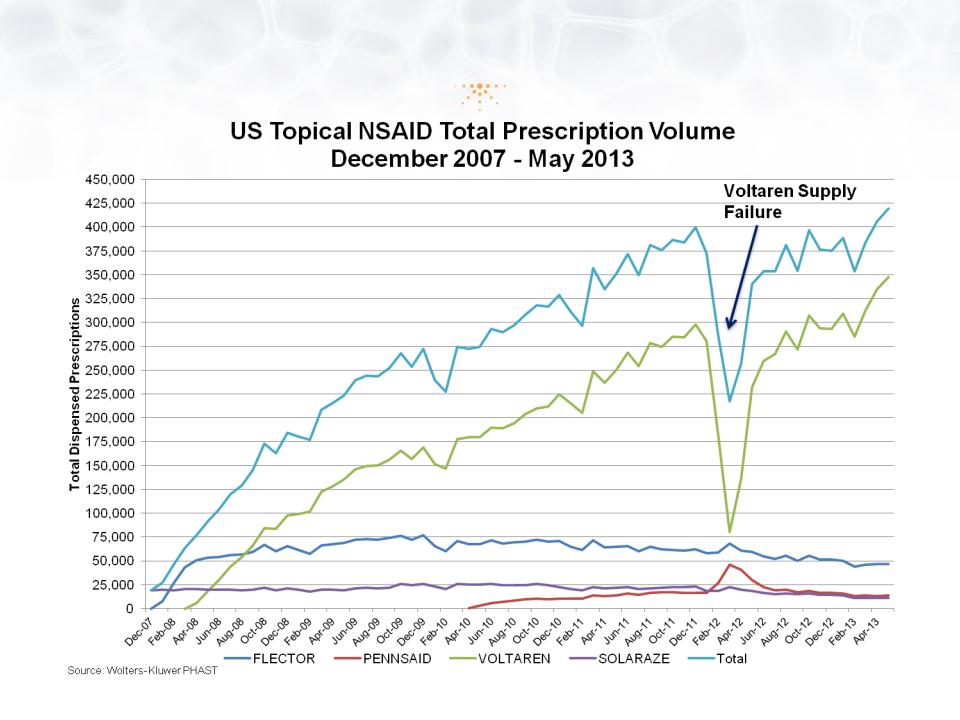
The Topical NSAID Market
© Imprimis Pharmaceuticals, Inc. | 15
15

The Case for Impracor™
© Imprimis Pharmaceuticals, Inc. | *
• Market Analysis:
• The $10B+ US NSAID Market is Transitioning to Topicals
• Voltaren Gel (1% diclofenac) has ~75% Rx share
• FDA Guidance: Voltaren™ generics must complete clinical trials prior to ANDA
• Despite Suboptimal Products, U.S. Topical NSAID Market is Growing
• 2016 Topical NSAID Market Possibly >$1B
• There is a compelling unmet need for an effective semi-solid NSAID
|
Factor
|
Impracor™
|
Voltaren®
|
|
Delivery Technology
|
Patented Accudel™ Micelles
|
None; Alcohol
|
|
API
|
10% Ketoprofen
|
1% Diclofenac
|
|
COX Selectivity
|
Cox 1
|
Cox 2
|
|
Smell
|
Neutral
|
Insect Repellant
|
|
Tactile
|
Smooth
|
Greasy
|
16

Impracor™ Phase 3 Program
Initial Phase 3 Trial
• mITT analysis: p=0.038
• Per protocol analysis: p=0.034
New Acute Pain Clinical Trials to Achieve FDA Approval
• Two adequate/well controlled acute pain trials (one in label)
• Leading pain trial experts have designed protocol
• Use proprietary tools to reduce placebo effect
• Seek “sprains, strains and joint pain” label
• Could be the only acute pain topical NSAID (if FDA approved)
• OA Flare trial protocol IRB Approved
© Imprimis Pharmaceuticals, Inc. | *
Phase 3 Clinical Trials Planned - Q3 2013
Trial Data - 1H 2014
17

Executive Team and
Select Financial Data
Select Financial Data
© Imprimis Pharmaceuticals, Inc. | *
18

Management Team Snapshot
Strong operational and management experience within our leadership group
Compensation weighted in equity
Chief Executive Officer: Mark L. Baum, J.D.
15+ Years of Senior Executive Experience; Founder/President, YesRx.com (1999)
Founder of 3 private investment funds; Restructured numerous companies (private-to-public)
Responsible for Restructuring Imprimis, including ~$24M new equity investment and PCCA transaction
Senior Advisor, Pre-Clinical Services: Balbir Brar, D.V.M., Ph.D.
25 Years of Senior Drug Development Experience
Senior Positions: Lederle/Wyeth, SmithKline & Beckman, and Allergan
Drugs: Botox, Ketorolac (Cataracts), Restasis (Dry Eye), Lumigam, Latisse, Alphagan and 8 other drugs
Chief Medical Officer: Joachim P.H. Schupp, M.D.
Senior Positions: Ciba-Geigy, Novartis, ProSanos, Adventrx, Apricus Biosciences
Senior Positions: Ciba-Geigy, Novartis, ProSanos, Adventrx, Apricus Biosciences
Drugs: Voltaren line extensions, Apligraf, Femara, Exjade and Sandoglobulin
VP, Accounting and Public Reporting: Andrew R. Boll
8+ years of experience in small capitalization company financial reporting; focus on restructured businesses;
Led forensic-type accounting and financial reporting of historical Imprimis records during restructuring
VP, Corporate Development: Gary Seelhorst, MS, MBA
15+ years of clinical and corporate development experience with both large-cap pharmaceutical companies
(e.g. Eli Lilly and Pfizer) as well as start-up ventures including extensive capital raising, licensing, and M&A
transactions
(e.g. Eli Lilly and Pfizer) as well as start-up ventures including extensive capital raising, licensing, and M&A
transactions
© Imprimis Pharmaceuticals, Inc. | *
19

Clinical and Regulatory Team Snapshot
Senior Regulatory Advisor: Lee S. Simon, M.D.
FDA Division Director of Analgesic, Anti-Inflammatory & Ophthalmologic Drug Products (2001-2003)
Served on multiple FDA advisory committees; 12 years as an NIH funded investigator
FDA Division Director of Analgesic, Anti-Inflammatory & Ophthalmologic Drug Products (2001-2003)
Served on multiple FDA advisory committees; 12 years as an NIH funded investigator
Senior consultant to Pharmacia/Searle on COX-2 development
Two terms on the BOD of the American College of Rheumatology; 110 Original Publications
Senior Clinical Advisor: Roy D. Altman, M.D.
Professor of Medicine, Division of Rheumatology/Immunology at UCLA ; 35+ yrs clinical experience
Founding Member/Past President of the Osteoarthritis Research Society International
Chairman for the Design and Conduct of Clinical Trials in Osteoarthritis as well as the Chairman on
Clinical Trials in Osteoarthritis; Over 200 juried manuscripts and over 60 books
Clinical Trials in Osteoarthritis; Over 200 juried manuscripts and over 60 books
Edited the 4th edition of Osteoarthritis: Diagnosis and Management.
Co-editor:Seminars in Arthritis and Rheumatism and Editor and Chief of Osteoarthritis and Cartilage
Senior Clinical Advisor: Marc C. Hochberg, M.D.
Faculty, The Johns Hopkins University SOM & University of Maryland SOM
Head of the Division of Rheumatology and Clinical Immunology at University of Maryland SOM
Focus on clinical epidemiology of musculoskeletal diseases, osteoarthritis and osteoporosis
PI of NIH and Dep’t Vet. Affairs funded studies, and is a Co-investigator on several other studies
Senior Regulatory Advisor: Allan M. Green, M.D., PhD, J.D.
Physician, Attorney, Inventor and Research Scientist
Operating and Management Experience with Numerous Biomedical Companies
Of Counsel to Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C.
Teaches Food and Drug Law at Boston College Law School
© Imprimis Pharmaceuticals, Inc. | *
20

Capital Structure
|
|
Capital Structure
March 31, 2013
(Unaudited) |
Percent
|
|
Common Shares
|
8,888,250
|
82.72%
|
|
Total Restricted Stock Units
|
200,000
|
1.86%
|
|
Total Options & Warrants - Weighted Avg. Ex. Price
$5.42 |
1,656,258
|
15.42%
|
|
Total Common Shares - Diluted
|
10,744,508
|
100.00%
|
|
|
|
|
© Imprimis Pharmaceuticals, Inc. | *
21

Summary
© Imprimis Pharmaceuticals, Inc. | *
• Imprimis is a Company with Vision
• Unique Drug Development Model
• Near Term Catalysts
• Robust and Compelling Development Assets
• Key Strategic Relationships
• Cash Resources to Execute
• Highly Capable Team
22

Questions & Discussion
Imprimis Pharmaceuticals, Inc.
Delivering Safe, Effective and Direct Solutions
FOR MORE INFORMATION CONTACT:
Mark L. Baum, J.D.
(858) 433-2816 - Direct
mark@imprimispharma.com
© Imprimis Pharmaceuticals, Inc. | *
23

Appendix - Investment
Summary & Support
Summary & Support
© Imprimis Pharmaceuticals, Inc. | A1
24

Accudel™ Topical
Delivery Technology
Delivery Technology
© Imprimis Pharmaceuticals, Inc. | A2
25
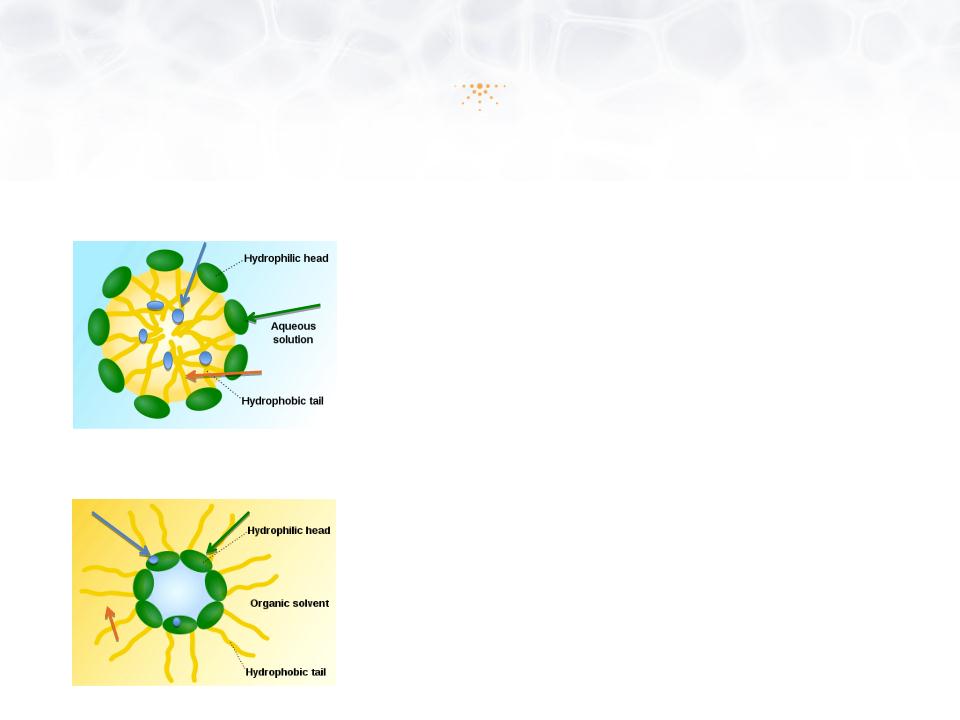
Introduction to Accudel™
Pluronic Lecithin Organogel (PLO) Platform
Lipophillic APIs
Hydrophillic APIs
Pluronic Phase
Lecithin Phase
• Accudel™ is a cream that “carries” drugs
through the skin, penetrating to the problem site
through the skin, penetrating to the problem site
• Pluronic Lecithin Organogel (PLO) drug carrier
• Accommodates different size molecules and large
quantities of active drugs
quantities of active drugs
• Works with drugs with different physicochemical
properties
properties
• Quickly absorbed and aesthetically pleasing
• Low toxicity and biodegradable; components are
non-immunogenic and are “Generally Regarded
As Safe” (GRAS) by the US FDA
non-immunogenic and are “Generally Regarded
As Safe” (GRAS) by the US FDA
• Thermodynamically stable, insensitive to moisture
and resistant to microbial contamination
and resistant to microbial contamination
Drug
© Imprimis Pharmaceuticals, Inc. | A3
Lecithin Phase
Drug
Pluronic Phase
26

Introduction to Accudel™
In Vitro Penetration Data for Impracor™ and
European Marketed Products (Fastum®, Ketum®, Oruvail®)
European Marketed Products (Fastum®, Ketum®, Oruvail®)
• 63% - 70% of ketoprofen in Impracor that was available for
release diffused through the membrane (0.45 m Nylon) of a
Franz Cell Apparatus within 4 hoursi.
release diffused through the membrane (0.45 m Nylon) of a
Franz Cell Apparatus within 4 hoursi.
• (Fastum, Ketum, Oruvail) 2.5% topical ketoprofen were tested
in a Franz Cell Apparatus (Silicon membrane). Less than 20%
of ketoprofen present in the formulation was made available to
diffuse out of the formulation into the receptor phase in the un-
ionized formii.
in a Franz Cell Apparatus (Silicon membrane). Less than 20%
of ketoprofen present in the formulation was made available to
diffuse out of the formulation into the receptor phase in the un-
ionized formii.
i. DPT Study Report TC.0706.01
ii. Thesis Tettey-Amlalo, Dec 2005
Faculty of Pharmacy Rhodes University, Grahamstown
© Imprimis Pharmaceuticals, Inc. | A4
27

Impracor™ Topical
NSAID
Competition and Market
NSAID
Competition and Market
© Imprimis Pharmaceuticals, Inc. | A5
28
The Problem With Oral NSAIDs
Solution is to deliver NSAIDs topically to the specific site of pain or inflammation
Fact: Extremely Large Population Uses NSAIDs
•70 Million prescriptions for NSAIDs each year in US (Wiegard in Medscape)
•Regularly used by more than 60M Americans (Arch Intern Med. 2005;165:171-177)
•70% of all 65+ Year Olds Take NSAIDs Weekly
•Usage of oral NSAIDs is increasing
Result: Toxicity to Gastro Intestinal (GI) Tract, Kidneys and Liver
•Over 100,000 per year are hospitalized from NSAID complications
•Hospitalizations alone cost more than $2B per year
•Over 16,000 deaths every year from GI NSAID complications
•NSAID GI Toxicity - the 15th most common cause of death in US
Widespread Usage With Serious Side Effects
© Imprimis Pharmaceuticals, Inc. | A6
29
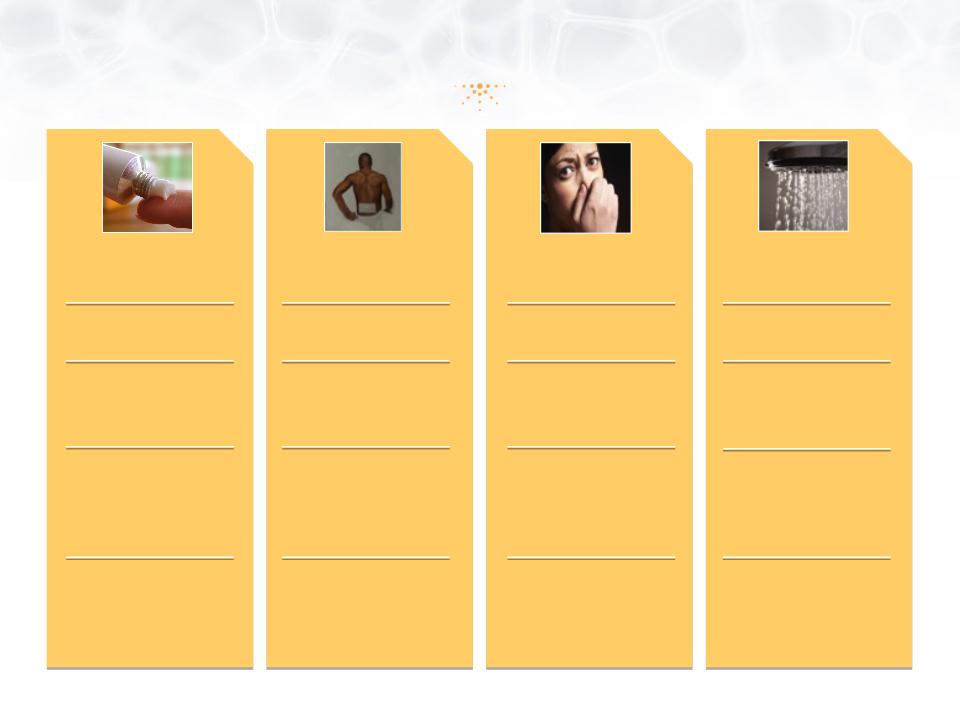
Competitive Landscape
10% Ketoprofen
Cream
Proprietary
Safe / Cutaneous
Elegant Formulation
Convenient / Cream
Accudel Delivery System
Local AEs 1-2%
Local AEs 1-2%
Seeking label sprains,
strains, and joint pain
strains, and joint pain
IMPRACOR
(Imprimis)
1.3% Diclofenac
epolamine
epolamine
10 x 14 cm patch
2 x per day
2 x per day
Fixed one size patch
Adherence issues
Not to be worn in water
Local AEs 11%
Acute soft tissue
injury (positive data
in ankle sprain)
injury (positive data
in ankle sprain)
FLECTOR PATCH
(Pfizer/IBSA)
1% Diclofenac
sodium
2-4 gram
4 x per day =
16 grams/day
16 grams/day
Large Quantities
Sticky / Greasy
Odor / Staining
Local AEs 7%
Chronic OA of hand
and knee
and knee
VOLTAREN GEL
(Endo/Novartis)
1.5% Diclofenac
sodium
40 drops of liquid
(10 drops to each of 4 sides of knee)
(10 drops to each of 4 sides of knee)
3-4 x per day =
160 drops/day
160 drops/day
Dimethyl sulfoxide
(DMSO); Safety concerns
(DMSO); Safety concerns
Complicated application
Causes garlic taste/breath
Local AEs 47%
Chronic OA of knee
PENNSAID
(Covidien/Nuvo)
© Imprimis Pharmaceuticals, Inc. | A7
30

Ketoprofen vs. Ibuprofen
“Meta-analysis of 26 trials (n=2,853) … showed that Ketoprofen was
significantly better than all other topical NSAIDs. In terms of efficacy,
Ketoprofen was significantly better than ibuprofen, felbinac, piroxicam and
indomethacin.”
significantly better than all other topical NSAIDs. In terms of efficacy,
Ketoprofen was significantly better than ibuprofen, felbinac, piroxicam and
indomethacin.”
Topical NSAIDs for acute pain: a meta-analysis
Lorna Mason, R Andrew Moore*, Jayne E Edwards, Sheena Derry and Henry J McQuay
BMC Family Practice 2004, 5:10
The Impracor Solution
Ketoprofen vs. Diclofenac
The proportion of participants experiencing successful treatment with topical
ketoprofen in seven clinical studies was 73% (251/346, range 57% to 89%)
ketoprofen in seven clinical studies was 73% (251/346, range 57% to 89%)
The proportion of participants experiencing successful treatment with topical
diclofenac in three clinical studies was 52% (166/319, range 39% to 92%)
diclofenac in three clinical studies was 52% (166/319, range 39% to 92%)
Ketoprofen is a Superior Active Ingredient
© Imprimis Pharmaceuticals, Inc. | A8
Topical NSAIDs for acute pain in adults.
Massey T, Derry S, Moore RA, McQuay HJ
Cochrane Database Syst Rev. 2010;6:CD007402
31

Additional Impracor™
Clinical Materials
Clinical Materials
© Imprimis Pharmaceuticals, Inc. | A9
32
Topical NSAIDs in Acute OA Knee Pain Model
© Imprimis Pharmaceuticals, Inc. | A10
|
|
Ketoprofen 20% Patch
(ENDO)
|
Ketoprofen Transfersome
Gel, Diractin™ (IDEA) |
Diclofenac Solution
Pennsaid™ (Nuvo) |
|
Phase
|
3
|
2/3
|
2
|
|
Study Dates
|
Aug 2006 - May 2007
|
Jul 2003 - Jan 2004
|
Jul 2010 - Mar 2011
|
|
# of Subjects/ Age
|
309 / above 18 years
|
397/ above 40 years
|
248 / 18 -80 years
|
|
Regimen/ Duration
|
Ketoprofen Patch applied o.d.
4 weeks
|
110 mg ketoprofen b.i.d. (n=138)
6 weeks
(1 placebo capsule b.i.d.
100 mg celecoxib capsule b.i.d.)
|
1.3 mL applied to front, back and sides of knee
b.i.d. (n=84) Vehicle and placebo controlled
4 weeks
|
|
Selection
|
Diagnosis of knee (unilateral or bilateral),
CRO: PPD |
Morning stiffness < 30’, crepitus, at least 3 on
Likert’s 5 point scale, not on NSAIDS |
Patients using NSAIDs underwent a 1-week
washout This was a non-flare study
|
|
Primary Endpoint
|
WOMAC (pain) week 2
|
WOMAC (pain ) week 6•
|
WOMAC (pain ) week 4
|
|
Secondary Endpoints
|
Pain Intensity/ relief (diary)
WOMAC (function), Rescue Medication,
quality of sleep, lost days of work. Pat./Phys. global assessment |
WOMAC (function)-week 6.
Patient global assessment (5 Point Likert)•
|
WOMAC (stiffness) , WOMAC (function), WOMAC
(pain on walking) - - week 4 Patient global assessment
Pain assessment 11 point scale
|
|
Conclusions
|
ITT Primary Endpoint met: Significant
differences vs placebo (p=0.014). All secondary endpoints met. Previously two Phase 3 sprain/strain trials failed, program discontinued. ENDO 10Q 2007 |
WOMAC pain LS mean reduction - 18.2 (-22.1 to -
14.3), -20.3 (-24.3 to -16.2) and -9.9 (-13.9 to - 5.8) osteoarthritis (p <0.01) All WOMAC subscale scores were normalized to
a scale of 0 to 100 by dividing the sum subscale score by the number of questions of each score. Ann Rheum Dis. 2007; 66(9): 1178-83. Swissmedic approval based on single study |
WOMAC pain reduction (5-Point Likert) from
baseline (-3.9 [- 4.8 to -2.9]) compared with vehicle -control solution (-2.5 [- 3.3 to -1.7]; p = 0.023) or the placebo solution (-2.5 [-3.3 to -1.7]; p = 0.016). CMAJ • AUG. 17, 2004; 171 (4) 5 Phase 3 trials have achieved all 3 primary end
points in OA. |
33
Initial Phase 3 Trial
|
Design:
|
Randomized, double-blind, placebo-controlled at 26 sites
|
|
Study Population:
|
Efficacy, n = 361
Uncomplicated acute soft tissue injuries
Ankle (n=97), Shoulder (n=87), Knee (n=59), Wrist (n=57), Elbow (n=30), Calf/Anterior Tibialis
(n=11), Hamstring/Quadriceps (n=8), Forearm (n=5), Biceps/Triceps (n=3), Hand (n=3) Safety, n = 364
Ranging in age from 18 - 75 years
|
|
Key Entry Criteria:
|
Injury occurred within 72 hours, pain intensity ≥ 60mm on 100 mm Visual Analogue Scale
(VAS); no intake of unallowable medication |
|
Dosing Regimen:
|
Impracor vs. Placebo (Vehicle) cream, 1g t.i.d. x 7 days
|
|
Primary Endpoint:
|
Change from baseline in pain intensity during daily activities on Day 3 office
visit (+1, +2 days) with 100 mm VAS measurement |
|
Secondary
Endpoints: |
• Change from baseline in three times daily pain intensity immediately prior to medication
• Various other treatment satisfaction and safety assessments
• Pharmacokinetics in subset of patients
|
Sprain-Strain Soft Tissue Study
© Imprimis Pharmaceuticals, Inc. | A11
34
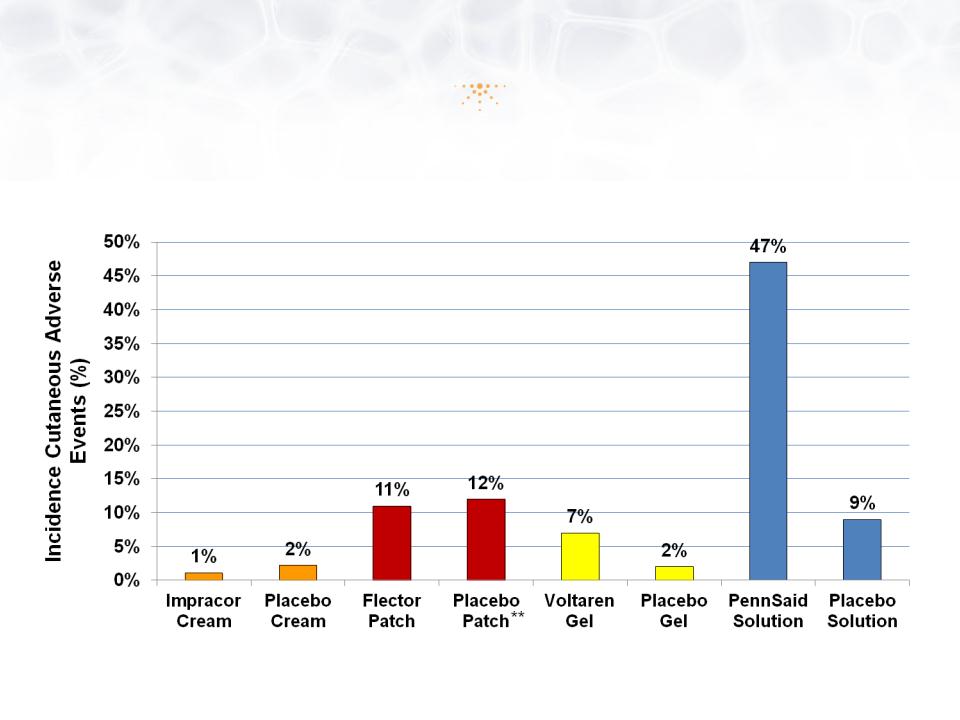
Safety: Low Incidence of Adverse Events
* Clinical Study Report: TDLP-110-001, September 2010
** Prescribing Information for Flector Patch, Voltaren Gel and Pennsaid Solution
• No related gastrointestinal (GI), cardiac, liver, or other serious AEs
• Low incidence of cutaneous AEs
© Imprimis Pharmaceuticals, Inc. | A12
1g, 3x daily
180mg, 2x daily
4g, 4x daily
40 drops, 4x daily
**
**
**
**
**
*
*
35
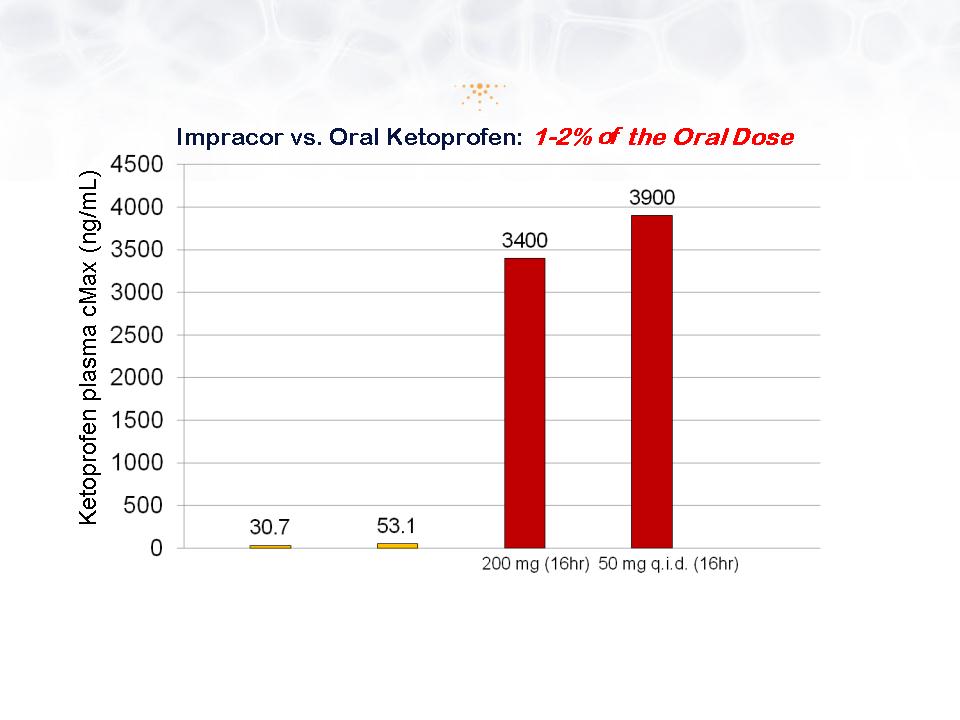
Pharmacokinetics: Low Systemic
Absorption
Absorption
* Cannavino, C. et al. Efficacy of Transdermal Ketoprofen in delayed onset muscular soreness,Clinical Journal of Sports Medicine, 13: 200-208,
2003 and Clinical Study Report Project No. 990808, Phase 1/2 Study Report Aug 2007
2003 and Clinical Study Report Project No. 990808, Phase 1/2 Study Report Aug 2007
**Orudis ketoprofen extended release capsule/ Oruvail capsule prescription information
Impracor*
10% ketoprofen cream
Oruvail**
1g t.i.d. (48hr)
2g t.i.d. (48hr)
© Imprimis Pharmaceuticals, Inc. | A13
Orudis**
36

Additional Corporate
Information
Information
© Imprimis Pharmaceuticals, Inc. | A14
37

Board of Directors
Robert J. Kammer, D.D.S. (Co-Founder)
Active Clinical Research & Consulting Practice; Diplomate, American Board of Orofacial Pain
Retired Associate Professor & Course Director - Orofacial Pain, University of Colorado
Mark L. Baum, J.D. (Co-Founder)
15+ Years of Senior Executive Experience
Founder of 3 private investment funds; Restructured numerous companies (private-to-public)
Responsible for Restructuring Imprimis, including $24M New Equity Investment and PCCA transaction
Paul Finnegan, M.D., M.B.A.
Senior Positions: Avalon Ventures, Alexion, Pharmacia/Searle); Univ. of Chicago MBA
Senior Positions: Avalon Ventures, Alexion, Pharmacia/Searle); Univ. of Chicago MBA
Drugs: Celebrex, Bextra, Arthrotec, Soliris, Inspra and Aldactone/Soldactone
Jeff Abrams, M.D.
Director since 1998; Co-developer of Accudel drug delivery technology and Impracor topical NSAID
Stephen Austin, C.P.A.
Audit Committee Chairman; Significant BOD Experience; Partner, Swenson Advisors, LLP since May
1998
1998
Manages audit, SEC, Sarbanes-Oxley and business consulting engagements with a focus on technology,
manufacturing, service, real estate, social media and non-profit organizations
manufacturing, service, real estate, social media and non-profit organizations
Gus S. Bassani, Pharm.D
Shareholder in PCCA; Vice-President of Consulting, R&D and Formulations at PCCA
Member of the 2010 - 2015 United States Pharmacopeia (USP) Council of Experts
© Imprimis Pharmaceuticals, Inc. | A15
38

Balance Sheet
|
ASSETS
|
|
March 31, 2013
|
||
|
Current Assets
|
|
|
||
|
|
Cash and short term investments
|
$
|
19,029,031
|
|
|
|
Other assets
|
|
217,540
|
|
|
|
|
TOTAL ASSETS
|
$
|
19,246,579
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
|
|
||
|
|
Accounts payable and other accruals
|
$
|
848,549
|
|
|
|
|
TOTAL LIABILITIES
|
|
848,549
|
|
Stockholder’s Equity
|
|
|
||
|
|
Common stock, $0.001 par value, 395,000,000 shares authorized,
|
|
|
|
|
|
|
8,888,250 shares issued and outstanding
|
|
8,888
|
|
|
Additional paid-in capital
|
|
43,958,891
|
|
|
|
Deficit accumulated during the development stage
|
|
(25,569,749)
|
|
|
|
|
TOTAL STOCKHOLDERS' EQUITY
|
|
18,398,030
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY
|
$
|
19,246,579
|
© Imprimis Pharmaceuticals, Inc. | A16
(Unaudited and Abbreviated)
39
