Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REPROS THERAPEUTICS INC. | v349636_8k.htm |

Developing clinical stage small molecule therapeutics to treat hormonal and reproductive system disorders

Repros Disclaimer Any statements made by the Company that are not historical facts contained in these slides (or in any oral accompanying discussion) are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from the results expressed or implied by such statements, including the ability to raise additional needed capital on a timely basis in order for it to continue to fund development of its Androxal ® and Proellex ® programs, the ability to have success in the clinical development of its technologies, the reliability of interim results to predict final study outcomes, and such other risks which are identified in the Company's most recent Annual Report on Form 10 - K and the subsequent quarterly report on Form 10 - Q and in the prospectus supplement and the accompanying prospectus included in the registration statement mentioned below. These documents are available on request from Repros Therapeutics or at www.sec.gov . Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise. In this presentation, we rely on and refer to information and statistics regarding the pharmaceutical industry. We obtained this information and these statistics from third - party sources, which we have supplemented where necessary with information from publicly available sources and our own internal estimates. Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information. While we believe that each of these studies and publications is reliable, we have not independently verified such data, and we make no any representation as to the accuracy of such information. Similarly, we believe our internal research is reliable, but it has not been verified by any independent sources. 1

Investment Highlights • Focused strategy: small molecule therapeutics for reproductive disorders • Two late stage clinical programs each with +$1B sales potential • Androxal ® : PHASE 3 (SPA) oral treatment for Low Testosterone – Patented and pending patent’s life to the mid 2020’s – Growing +$2B market – Restoration of testicular function and testosterone levels in treatment of 2º hypogonadism (most common cause of low T) • Proellex : PHASE 2 treatment for uterine fibroids and endometriosis – Pending patent/patent life to the mid 2020’s – +$5B market – Fibroid de - bulking and chronic relief of symptoms associated with uterine fibroids, endometriosis – Potential breast cancer intervention • Key late stage clinical & regulatory events driven news flow in 2013 2

Testosterone Market and Androxal Overview • US market for low testosterone exceeds $1.5 billion • Only approved non invasive therapies are hormone replacements • Repros believes 85% of hypogonadal men experience low T due to an endocrine disorder – These men have functional but un - stimulated testes – Hypothalamic - pituitary suppression due to estrogen • Androxal is the only oral medication in development that treats the underlying disorder for the majority of hypogonadal men 3
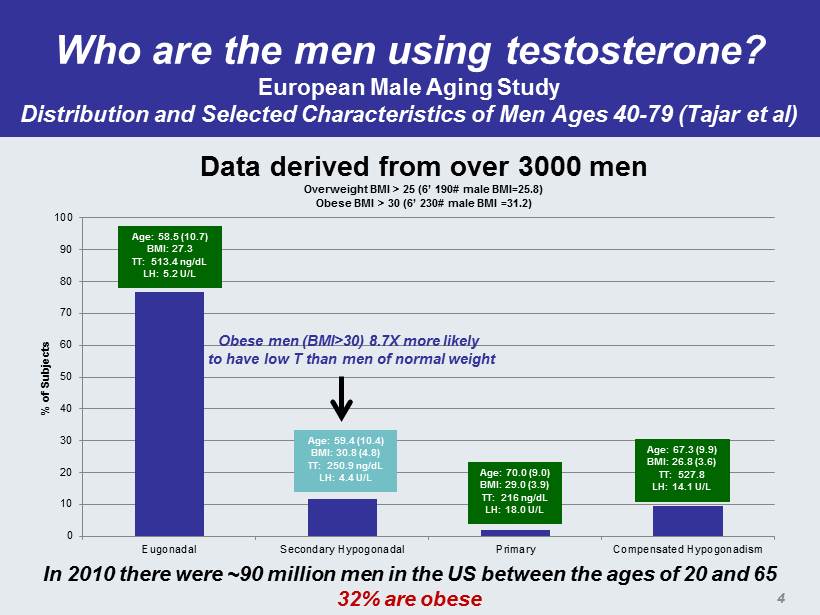
Who are the men using testosterone? European Male Aging Study Distribution and Selected Characteristics of Men Ages 40 - 79 ( Tajar et al) 0 10 20 30 40 50 60 70 80 90 100 Eugonadal Secondary Hypogonadal Primary Compensated Hypogonadism % of Subjects Age: 58.5 (10.7) BMI: 27.3 TT: 513.4 ng / dL LH: 5.2 U/L Age: 59.4 (10.4) BMI: 30.8 (4.8) TT: 250.9 ng / dL LH: 4.4 U/L Age: 70.0 (9.0) BMI: 29.0 (3.9) TT: 216 ng / dL LH: 18.0 U/L Age: 67.3 (9.9) BMI: 26.8 (3.6) TT: 527.8 LH: 14.1 U/L Data derived from over 3000 men Overweight BMI > 25 (6’ 190# male BMI=25.8) Obese BMI > 30 (6’ 230# male BMI =31.2) Obese men (BMI>30) 8.7X more likely to have low T than men of normal weight In 2010 there were ~90 million men in the US between the ages of 20 and 65 32% are obese 4

Androxal Exhibits Patient Reported Effects Equivalent to Androgel ZA - 003 Type C FDA Meeting Q4 - ’07 Unacceptable Endpoint for Phase 3 0 5 10 15 20 25 30 Androxal Androgel IIEF Score Baseline 6 mos. Androxal approaches statistical significance p=0.08 compared to baseline and Androgel change 0 2 4 6 8 10 Androxal Androgel IIEF Libido Score Baseline 6 mos. No statistical difference between changes from baseline for Androxal versus Androgel p=0.02 p=0.002 0 10 20 30 40 Androxal Androgel MSDS Score Baseline 6 mos. p=0.006 p=0.06 No statistical difference between changes from baseline for Androxal versus Androgel Lower Score Better 5

Type C Meetings in 1Q10, 3Q10 & 2Q12 ZA - 203 Lead to SPA on 1 st Pass Repros Convinces FDA Exogenous T Suppresses Pituitary Hormones and Testicular Function 0 2 4 6 8 10 12 14 12.5 mg 25 mg PL Testim FSH Treatment Effect of Treatment on Median FSH p versus Testim Before After p<0.00001 p=0.0004 0 10 20 30 40 50 60 70 80 90 100 12.5 mg 25 mg PL Testim [Sperm] in Millions/ml Treatment Effect of Treatment on Median Sperm Concentration p versus Testim Baseline EOS p=0.012 p=0.0021 p=0.0049 0 2 4 6 8 10 12 12.5 mg 25 mg PL Testim LH Treatment Effect of Treatment on Median LH p versus Testim Before After p=0.0004 0 50 100 150 200 250 300 350 400 450 500 12.5 mg 25 mg PL Testim TT in ng/dL Treatment Effect of Treatment on Median Serum TT p versus placebo Before After p<0.00001 p=0.0002 p<0.00001 6

FDA Accepts Phase 3 Protocols Under an SPA Goal to Submit NDA Mid 2014 • Phase 3 pivotal studies being conducted under SPA – 2 identical trials (BMI > 25, Age ≤ 60) • 152 subjects in each trial (114 on Androxal , 38 on placebo) – Men with morning T<300 ng / dL assessed twice on two separate days – Men with sperm concentration > 15 x10 6 per milliliter assessed on 2 separate days separated by at least 2 days • Up - titration from 12.5 to 25 • 3 month duration (men up - titrated on study for additional 6 weeks) – Co - primary endpoints • 75% of men achieve T in normal range (300 - 1040 ng / dL ) – 24 hr average at week 12 • Non inferior to placebo regarding change in sperm counts (average of week 12 & 13) – Up - titrated men assessed at weeks 18 & 19 for information only – All protocol changes to be reviewed with FDA before incorporating into study • Safety Requirements of Phase 3 program – >100 for one year – >800 for 6 months – 100 subject one year DEXA study (bone marker data suggests Androxal builds bone) 7

ZA - 301: Androxal Improves T without Affecting Sperm 0 50 100 150 200 250 300 350 400 450 Androxal Placebo Total T ( ng / dL ) Baseline End of Study 83 % in Normal Range 79% in ITT 0 10 20 30 40 50 60 Baseline End of Study Sperm Concentration X 106 Placebo Androxal Androxal Up-titration No statistical difference p<0.00001 1 Pbo <50% of baseline 15 Androxal <50% 8
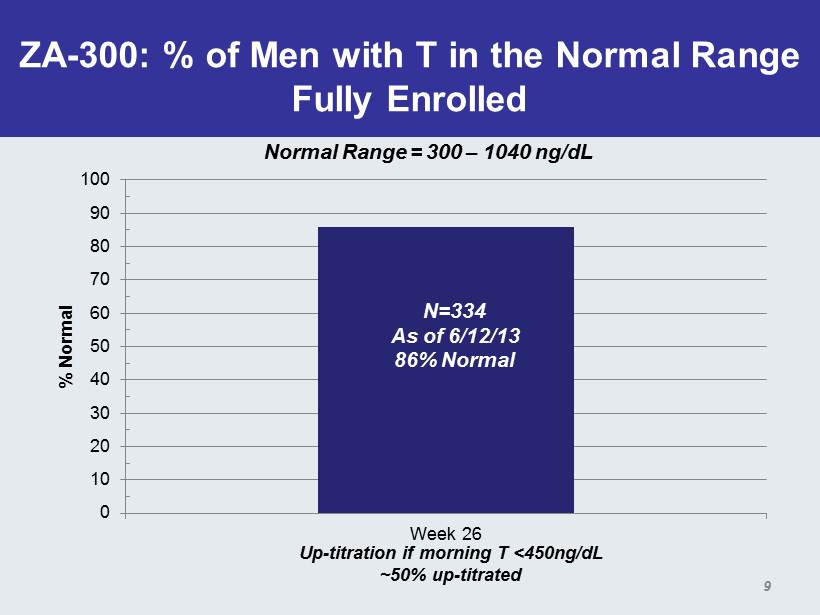
ZA - 300: % of Men with T in the Normal Range Fully Enrolled 0 10 20 30 40 50 60 70 80 90 100 Week 26 % Normal N=334 As of 6/12/13 86% Normal Normal Range = 300 – 1040 ng / dL Up - titration if morning T <450ng/ dL ~50% up - titrated 9

Phase III Androxal Program Status NDA Target: Mid 2014 Study Target Enrollment Study Duration Subjects Screened Subjects Enrolled Projected Full Enrollment Top Line Results ZA - 300 Safety 500 6 months 1288 (28 sites) 500 Fully Enrolled Q4 2013 ZA - 301 Pivotal 152 3 months (+ 6 weeks) 571 (17 sites) 151 Fully Enrolled Positive results on primary efficacy endpoints ZA - 302 Pivotal 180 3 months (+ 6 weeks) 395 (16 sites) 180 Fully Enrolled Q4 2013 ZA - 303 Safety 150 1 year 419 (13 sites) 150 Core Study Enrolled Q2 2014 (Core study of 150 subjects Q3 2013 (complete study) 10

Androxal Safety Treatment Emergent Adverse Events Studies 301, 300, and 303 Study 301 Study 300 Study 303 Preferred Term 12.5 mg (%) 25 mg (%) PBO (%) 12.5 mg (%) 25 mg (%) 12.5 mg (%) 25 mg (%) PBO (%) N 90 22 37 259 240 94 69 104 Any Event 28 23 24 38 44 19 39 30 Upper respiratory infection 6 5 0 6 5 4 6 5 Headache 3 9 5 5 3 1 1 2 Muscle twitch 2 0 0 0 0 0 0 0 Diarrhea 2 0 3 <1 1 2 1 0 Dyspepsia 2 0 0 1 1 0 0 0 Influenza 2 0 3 3 1 1 1 0 Lethargy 2 0 0 <1 0 0 0 0 Pollakiuria 2 0 0 1 1 0 0 0 Sinus congestion 2 0 0 <1 1 0 0 0 Data for 300 and 303 is interim and not fully monitored and validated Any event occurring in 2 or more subjects in pivotal Study 301. 11

Effects of Androxal on Bone Markers of Bone Turnover & Mineral Density Suggests Androxal Strengthens Bone 12

Androxal NDA Timeline Q4 2013 Q1 2014 Q2 2014 Q3 2014 NDA ZA - 302 ZA - 300 - F inal Safety Data for ISS ISE Pre - NDA Meeting 13

How do we maximize shareholder return? • All Repros Assets are Unencumbered • Androxal asset should be +90% de - risked during Q1 - ’14 • Androxal should open T treatment into primary care – License • Royalty rate commensurate with market penetration (20 – 30%) • Inevitable take - out by big pharma partner on partner’s terms – Sell the asset • Management target price: +$1 B • Deal on Repros terms if multiple bids – Difficult to project exact timing – Measured launch of Androxal into specialty space • KOL board established (first meeting scheduled for Oct.10, ‘13) • Recent follow - on offering enables this option – Scope of effort controlled by Company • Can lead to sale of Company 14

15

16

17

18

Proellex for the Treatment of Uterine Fibroids and Endometriosis • Over 30 million women of reproductive age in the US afflicted with symptomatic uterine fibroids or endometriosis • Over 300,000 hysterectomies performed every year in the US to treat these two disorders • No acceptable chronic therapeutic options available today 19
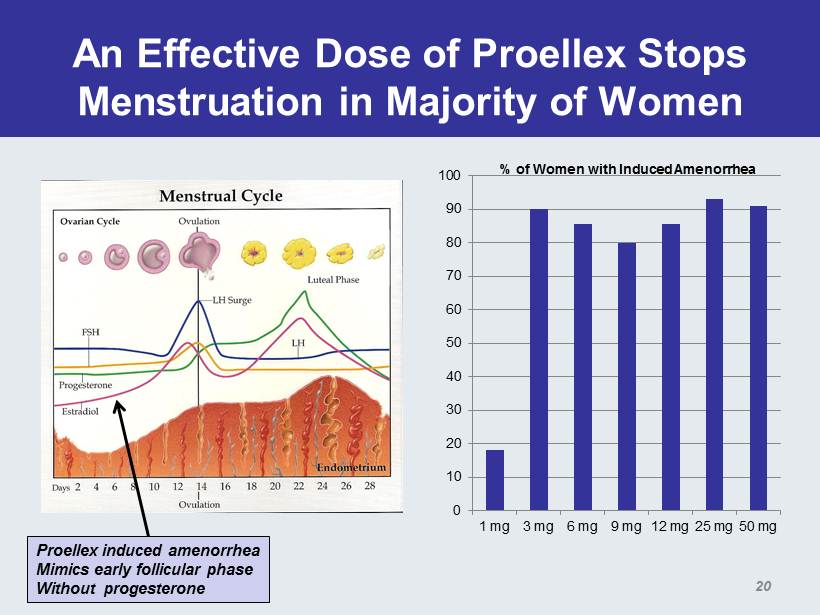
An Effective Dose of Proellex Stops Menstruation in Majority of Women 0 10 20 30 40 50 60 70 80 90 100 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg % of Women with Induced Amenorrhea Proellex induced amenorrhea Mimics early follicular phase Without progesterone 20

Proellex Eliminates the Pain and Need for Analgesics to Control the Pain of Endometriosis 0 10 20 30 40 50 60 70 Placebo Proellex 25mg Proellex 50 % of Baseline p - value versus placebo 25 mg = 0.001 50 mg = 0.017 55.5 22.2 13.6 0 10 20 30 40 50 60 70 80 90 100 Placebo 25mg Proellex 50mg Proellex % of Subjects Requiring Narcotics By End of Study Baseline End of Study P<0.01 21

ZPE - 202 Phase 2 Endometriosis Study • 90 subject double blind placebo controlled study balanced between placebo, 6 and 12 mg oral Proellex – Subject population (confirmed endometriosis) • Severe endometriosis as determined by BBSS score • Requiring narcotics or prescription analgesics to control endometriosis related pain – Study Duration: 4 months – Study endpoints: • Reduction in need for analgesics from baseline • Change from baseline in BBSS pain scores – Status: enrolling sites and subjects 22

Vaginal Proellex to Eliminate the Need for Hysterectomy in Most Situations • Initial Phase 2 study tested four doses of vaginal administration in the treatment of uterine fibroids completed – Assess reduction of fibroid size and elimination of symptoms – Top line data reported • FDA requires additional Phase 2b study before proceeding to Phase 3 to insure proper dose selection – Propose 90 subject Phase 2b study – Separate IND from low dose oral 23

Systemic Exposure to Oral Proellex Varies in a Dose Dependent Manner Significant reduction in exposure via vaginal delivery 0 200 400 600 800 1000 1200 1400 1600 1800 12 mg vaginal 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg Concentration ( ng /ml) Combined Cmax for Telapristone and Primary Metabolite Liver associated SAE’s limited to 50 mg dose 3.5% of women exposed 24

Vaginal Proellex (ZPV - 200) Uterine Fibroid Quality of Life – UFS QOL 25 12 mg dose significantly improves all symptoms of uterine fibroids

Vaginal Proellex (ZPV - 200) PBAC Scores Over Time PBAC Score >80 = menorrhagia 26 12 dose significantly improves excessive menstrual bleeding due to fibroids
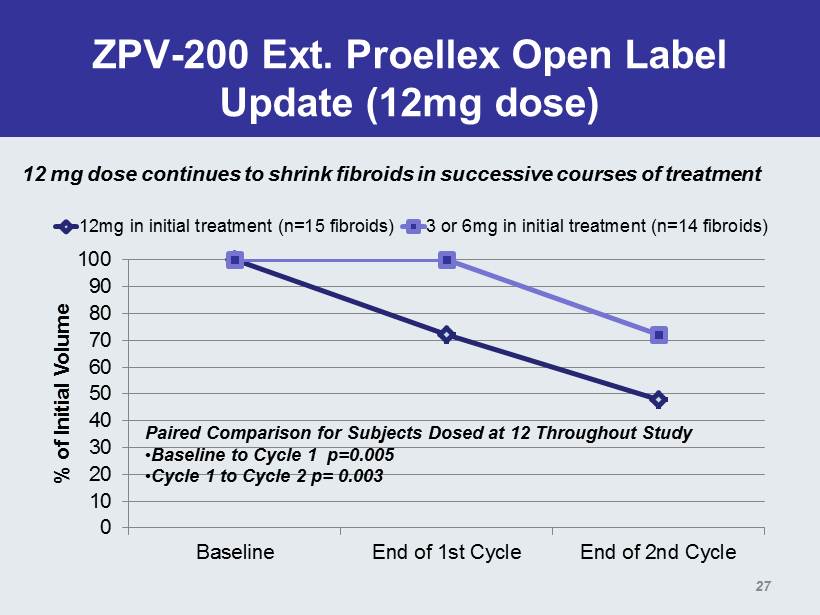
ZPV - 200 Ext. Proellex Open Label Update (12mg dose) 0 10 20 30 40 50 60 70 80 90 100 Baseline End of 1st Cycle End of 2nd Cycle % of Initial Volume 12mg in initial treatment (n=15 fibroids) 3 or 6mg in initial treatment (n=14 fibroids) Paired Comparison for Subjects Dosed at 12 Throughout Study • Baseline to Cycle 1 p=0.005 • Cycle 1 to Cycle 2 p= 0.003 27 12 mg dose continues to shrink fibroids in successive courses of treatment

Projected Income Through 2016 (in millions) 2014 2015 2016 Total Net Revenue - $5.0 $63.0 $68.0 Expenses: R&D Androxal $7.5 - - $7.5 R&D Proellex $8.0 $15.5 $14.5 $38.0 Sales & Marketing $2.0 $14.0 $23.0 $39.0 General Corporate $6.0 $10.0 $11.5 $27.5 Net Income (EBITDA) $(23.5) $(34.5) $14.0 $(44.0) Estimated Cash Balance $53.0 $18.5 $32.5 $32.5

Financial Summary • Cash and equivalents (as of 6/30/13 - unaudited) $87.7 M • Cash runway: 2016 – Both drugs’ NDAs filed – Anticipate Androxal approved • Current shares outstanding: 23.0 M shares – Warrants Outstanding – Series A – 877,137 (purchased in unit deal @ $2.45); Series B – 810,109 @ $2.49 exercise price. 29
