Attached files
| file | filename |
|---|---|
| EX-4.9 - EX-4.9 - Q Therapeutics, Inc. | d545757dex49.htm |
| EX-23.1 - EX-23.1 - Q Therapeutics, Inc. | d545757dex231.htm |
Table of Contents
As filed with the Securities and Exchange Commission on July 5, 2013
File No: 333-189495
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
to
FORM S-1
REGISTRATION
UNDER
THE SECURITIES ACT OF 1933
Q THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
Delaware
(State or other jurisdiction of
incorporation or organization)
2834
(Primary Standard Industrial
Classification Code Number)
20-3708500
(I.R.S. Employer
Identification Number)
615 Arapeen Drive, Suite 102
Salt Lake City, UT 84108
Phone: (801) 582-5400
Fax: (801) 582-5401
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Steven Borst
Chief Financial Officer
615 Arapeen Drive, Suite 102
Salt Lake City, UT 84108
Phone: (801) 582-5400
Fax: (801) 582-5401
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With a copy to:
Kevin J. Ontiveros, Esq.
Life Science Law, PC
1885 West 2100 South.
Salt Lake City, UT 84119
T: (801) 839-3502
F: (801) 503-9255
As soon as practicable after this Registration Statement is declared effective.
(Approximate date of commencement of proposed sale to the public)
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” “non-accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x | |||
CALCULATION OF REGISTRATION FEE
|
| ||||||||
| Title of Each Class of Securities to be Registered |
Amount to be Registered |
Proposed Maximum Offering Price Per Share |
Proposed Maximum Aggregate Offering Price |
Amount of Registration Fee (1) | ||||
| Common stock, par value $0.0001 per share |
Up to 5,000,000 shares | |||||||
| Warrants to purchase shares of common stock (2) |
Up to 1,250,000 warrants | |||||||
| Common stock issuable upon exercise of warrants |
Up to 1,250,000 shares | |||||||
| Total |
$ 5,000,000 | $682.00 (3) | ||||||
|
| ||||||||
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933. |
| (2) | The securities registered also include such indeterminate number of shares of common stock as may be issued upon exercise of warrants pursuant to the antidilution provisions of the warrants. |
| (3) | Previously paid. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act, or until this Registration Statement shall become effective on such date as the Securities and Exchange Commission (or the “SEC”), acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the Registration Statement filed with the United States Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
DATED JULY 5, 2013
PROSPECTUS (Subject to Completion)
Q THERAPEUTICS, INC.
5,000,000 Shares of Common Stock
and
Warrants to Purchase up to 1,250,000 Shares of Common Stock
This is Q Therapeutics, Inc.’s initial public offering of Units, consisting of 5,000,000 shares of common stock and warrants to acquire an aggregate of up to 1,250,000 shares of common stock of Q Therapeutics. The warrants will be immediately exercisable and will expire on the fourth anniversary of their issuance date. We expect the initial public offering price to be between $[ ] and $[ ] per Unit. Each Unit consists of one share of common stock and one warrant exercisable for a quarter (.25) of a share of common stock. No Units will be issued, however, and purchasers will receive only shares of common stock and warrants.
Our common stock is presently not traded or quoted on any market or securities exchange, but we intend to apply to have our common stock quoted on the OTC Bulletin Board under the symbol “QCEL”. Public trading of our common stock may never materialize or even if materialized, be sustained.
WE ARE AN “EMERGING GROWTH COMPANY” UNDER THE FEDERAL SECURITIES LAWS AND WILL BE SUBJECT TO REDUCED PUBLIC COMPANY REPORTING REQUIREMENTS. THESE SECURITIES ARE SPECULATIVE AND INVOLVE A HIGH DEGREE OF RISK AND SHOULD BE CONSIDERED ONLY BY PERSONS WHO CAN AFFORD THE LOSS OF THEIR ENTIRE INVESTMENT. PLEASE REFER TO “RISK FACTORS” BEGINNING ON PAGE 6.
| Public offering price per Unit |
$ | $ | ||||||
| Underwriting discounts and commissions payable by us (1) (2) |
$ | $ | ||||||
| Proceeds, before expenses, to us |
$ | $ |
| (1) | For the purpose of estimating the underwriter’s fees, we have assumed that the underwriter will receive its maximum commission on all sales made in the offering. The underwriter will also be entitled to reimbursement of expenses up to a maximum of $[ ]. |
| (2) | We estimate the total expenses of this offering, excluding the underwriter fees, will be approximately $[ ]. Because there is no minimum offering amount required as a condition to closing in this offering, the actual public offering amount, underwriter fees, and proceeds to us, if any, are not presently determinable and may be substantially less than the total maximum offering set forth above. See “Underwriting” beginning on page 27 of this prospectus for more information on this offering and the underwriter arrangements. |
MLV & Co. LLC has agreed to act as our underwriter in connection with this offering. The underwriter is not purchasing the securities offered by us, and is not required to sell any specific number or dollar amount of securities, but will use its best efforts to arrange for the sale of the securities offered by us.
All costs associated with the registration will be borne by us. As there is no minimum purchase requirement, no funds are required to be placed in escrow, trust or similar arrangement and all net proceeds will be available to us at closing for use as set forth in “Use of Proceeds” beginning on page 27.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Sole Book-Running Manager

The date of this prospectus is , 2013.
Table of Contents
Table of Contents
ABOUT THIS PROSPECTUS
You should rely only on the information contained in or incorporated by reference in this prospectus and any applicable prospectus supplement. We have not authorized anyone to provide you with different or additional information. If anyone provides you with different or inconsistent information, you should not rely on it. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of securities described in this prospectus. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus or any prospectus supplement, as well as information we have previously filed with the Securities and Exchange Commission and incorporated by reference herein, is accurate as of the date on the front of those documents only. Our business, financial condition, results of operations and prospects may have changed since those dates.
CAUTIONARY NOTICE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. All forward-looking statements are inherently uncertain as they are based on current expectations and assumptions concerning future events or our future performance. Readers are cautioned not to place undue reliance on these forward-looking statements, which are only predictions and speak only as of the date hereof. Forward-looking statements usually contain the words “estimate,” “anticipate,” “believe,” “expect,” or similar expressions, and are subject to numerous known and unknown risks and uncertainties. In evaluating such statements, prospective investors should carefully review various risks and uncertainties identified in this prospectus, including the matters set forth under the captions “Risk Factors” and in our other filings with the Securities and Exchange Commission (SEC). These risks and uncertainties could cause our actual results to differ materially from those indicated in the forward-looking statements. We undertake no obligation to update or publicly announce revisions to any forward-looking statements to reflect future events or developments.
Although forward-looking statements in this prospectus reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties, and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, those specifically addressed under the heading “Risks Related to the Company’s Business” above, as well as those discussed elsewhere in this prospectus. Readers are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this prospectus. We file reports with the SEC. You can read and copy any materials we file with the SEC at the SEC’s Public Reference Room, 100 F. Street, NE, Washington, D.C. 20549 on official business days during the hours of 10 a.m. to 3 p.m. You can obtain additional information about the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, including us.
Table of Contents
This prospectus summary highlights selected information contained elsewhere in this prospectus and does not contain all the information that you should consider before investing in our common stock and warrants to purchase common stock. You should carefully read the entire prospectus, including “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and notes thereto before making an investment decision. In this prospectus, unless the context requires otherwise, references to “Q,” “we,” “us,” or “the Company” refer to Q Therapeutics, Inc. and its consolidated subsidiaries.
Company Overview
Q Therapeutics, Inc. is a biotechnology company focused on the field of regenerative medicine. We are developing novel and proprietary cell-based therapies to treat severe, often life-threatening neurodegenerative diseases, with a first clinical program to be launched in Amyotrophic Lateral Sclerosis (ALS, also known as Lou Gehrig’s disease) in early 2014.
Upon successful completion of our ALS program, we anticipate broadening our clinical program to additional neurodegenerative indications including Transverse Myelitis, Spinal Cord Injury, Stroke, Parkinson’s, Multiple Sclerosis and Alzheimer’s disease.
Q Therapeutics’ differentiated approach leverages on the scientific findings and intellectual property developed by our co-founder Mahendra Rao, M.D., Ph.D., during his tenure at the University of Utah and as Head of the Stem Cell Section at the National Institutes of Health (NIH). A global leader in glial stem cell biology, Dr. Rao was one of the first scientists to identify and seek patent coverage on stem cells and their progeny cells found in the Central Nervous System (CNS).
The Rationale for Q-Cells®
Neurodegenerative diseases are by nature complex and treatment modalities that have historically focused on single mechanisms of action have failed to show meaningful clinical benefits.
Understanding the multi-factorial nature of these processes, Q Therapeutics’ patented cell-based platform takes a more holistic approach and leverages on utilizing human glial restricted progenitor cells (GRPs) and their progeny cells in the treatment of neurodegenerative diseases.
Glial cells are the naturally occurring cells in the brain and spinal cord that serve to protect the health and function of neurons. In many neurodegenerative diseases, these native glial cells become diseased and subsequently die. When this occurs, the myriad repair functions that glial cells normally provide are lost, and the result is neuronal damage and destruction.
Q Therapeutics has trademarked the glial restricted progenitor cells (GRPs) together with their progeny, under the name of Q-Cells®.
By enhancing the natural cellular machinery that enables healthy CNS systems to function, the administration of Q-Cells® is expected to maintain and / or restore the integrity of existing neurons and protect their function and survival.
We own exclusive worldwide rights to issued patents and numerous patent applications covering Q-Cells® and their production through an agreement with the University of Utah, augmented by internally developed intellectual property. The patent portfolio includes 16 issued patents whose claims cover composition of matter, methods of production and methods of use of multiple cell types of the CNS (including Q-Cells®) as well as cells of the peripheral nervous system, giving us a strong intellectual property position. This patent portfolio provides proprietary protection until 2030.
The Process and Differentiation
Q-Cells® are administered into the brain and spinal cord. Once administered, Q-Cells® predictably replicate and migrate within the CNS before fully differentiating into two mature glial cell subtypes, specifically:
| • | Oligodendrocytes, which form the insulating myelin layer around the axons of neurons, enabling their normal function for signal transmission, and |
| • | Astrocytes, which protect and support neurons and axons through several pathways including the production of essential growth factors, other trophic factors and the removal of destructive toxins. |
1
Table of Contents
Our pre-clinical research has demonstrated the ability of newly formed astrocytes and oligodendrocytes, derived from glial or Q-Cells®, to repair and / or salvage neurons by tapping into the CNS’ natural support mechanism. The resulting effect has been modifying the course of these diseases, improving function and extending life span, in some instances.
Specifically, glial / Q-Cells® have been shown to halt neuronal degeneration and support neuronal health (demonstrated in ALS models; relevant to Parkinson’s disease); as well as provide myelin repair functions for diseases or injuries involving demyelination (relevant to MS, Transverse Myelitis, Cerebral Palsy and Spinal Cord Injury).
We believe that our approach, which leverages on salvaging and bolstering the function of the natural cellular mechanism of the CNS and existing neurons, represents a faster and more efficient approach to treat acutely debilitating neurodegenerative diseases than do alternative stem cell approaches. Specifically, Q-Cells® are mature progenitor cells at an advanced stage of differentiation versus the more primitive, less differentiated, neural stem cells, which carry the risk of producing aberrant neuronal connections and harmful neuron formation.
Also, Q-Cells® are native to the normal central nervous system, act locally and remain in the CNS. This is in contrast to Mesenchymal Stem Cells (derived from bone marrow blood or fat) that act systemically and are short-lived after transplantation. Finally, Q-Cells® appear to produce lasting benefits in animal models of disease.
Clinical Trial Plans – ALS
We have chosen ALS as the lead clinical indication for Q-Cells® for several reasons:
| • | Animal and human ALS disease data have shown glial cells to be defective in affected subjects |
| • | Benefits have been obtained in animal studies with transplanted healthy glial cells |
| • |
Q-Cells® produce both astrocytes and oligodendrocytes (glial cells) in animal models |
| • |
Q-Cells® may be able to functionally replace diseased astrocytes and oligodendrocytes, thereby reducing motor neuron death and slowing or halting disease progression |
| • | The unmet medical need for an effective treatment for this fatal disease is substantial. |
In January 2012, we held a pre-Investigational New Drug (IND) meeting with the Food and Drug Administration (FDA) to discuss Q-Cells® for the treatment of ALS. The FDA required that we complete definitive GLP safety studies in preparation for our first-in-man clinical trial.
In December 2012, we initiated these studies which will serve to demonstrate the local biodistribution of Q-Cells® within the CNS, among other parameters. Final data from these ongoing GLP safety studies is expected during the fourth quarter of 2013. To date, we have seen no safety issues associated with the administration of Q-Cells®. We plan to complete all requisite GLP pre-clinical safety and device studies during the second half of 2013 with the intention of submitting our IND to the FDA shortly thereafter.
Phase 1/2a Design
In early 2014, we intend to initiate a Phase 1/2a open label study at Emory University School of Medicine and Johns Hopkins University School of Medicine; both institutions with a leadership position in ALS clinical research.
We anticipate commencing enrollment in 12-15 ALS patients. It is anticipated that the two-site trial will be completed in 18-24 months pursuant to the initiation of enrollment.
The endpoints of the trial include safety of Q-Cells® administration as a primary measure, with surrogate measures of efficacy as secondary endpoints. These secondary endpoints will be measured using tests for muscle integrity (electrical impedance myography), muscle strength (handheld dynamometry), and respiratory function (forced vital capacity), as well as overall physical condition and function (ALSFRS-R).
2
Table of Contents
Given the open label nature of the trial, we expect to read out data starting in second half of 2014.
Upon completion of the initial Phase 1/2a portion of the trial, we plan to meet with FDA to discuss an extended Phase 2a trial consisting of up to 20 additional patients. We have had ongoing discussions with investigators at the University of California at San Francisco and the University of California at San Diego, as both have demonstrated interest in participating in an expanded trial.
Future Clinical Trial and Product Development Efforts
Following the initiation of our clinical trial with Q Cells® in ALS, we plan to pursue additional orphan indications, to demonstrate Q-Cells®’ benefit in Transverse Myelitis and Spinal Cord Injury. We believe focusing on demonstrating Q Cells® in severe diseases with orphan populations (and exclusivity) will enable us to attain an accelerated commercialization path while maintaining capital efficiency. We expect that this strategy will also enable us to enter into strategic partnerships with larger biotechnology, pharmaceutical or cell therapy companies to conduct trials for larger indications, including Multiple Sclerosis, Stroke, Parkinson’s and Alzheimer’s disease.
Historical Financing
Since inception, we have raised approximately $18 million in equity financing. In 2008, Invitrogen, Inc. (now Life Technologies, Inc.) invested $2.6 million into our platform. More recently, in 2011, Cephalon (now Teva Pharmaceutical Industries Ltd.), conducted extensive diligence prior to making an equity investment of $3.7 million into Q, following their 2010 investment in Mesoblast Ltd., another cell therapy company.
We have also benefitted from over $4 million of non-dilutive grant funding, predominantly from the National Institutes of Health (NIH).
These funds have enabled us to conduct studies in 5 distinct pre-clinical models of neurodegenerative disease including ALS, Multiple Sclerosis, Transverse Myelitis, Spinal Cord Injury and Myelination. The results of this work have been published in peer-reviewed journals including Nature Neuroscience, Cell Stem Cell, Regenerative Medicine, Glia, and the Journal of Neurotrauma. In addition, we have expanded our IP portfolio both through in house development and in-licensing, developed GLP/GMP manufacturing processes and worked closely with FDA to support our proposed IND filing in ALS.
Emerging Growth Company Status
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act (the “JOBS Act”) enacted into law on April 5, 2012, and we are eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not applicable to emerging growth companies. Many of these exemptions are also available to smaller reporting companies like us that have less than $75 million of worldwide common equity held by non-affiliates. Absent unforeseeable changed circumstances, however, we will not take advantage of those exemptions that are available to us solely as a result of our status as an emerging growth company, except with respect to allowable pre-offering communications in any future securities offerings.
Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended (the Securities Act) for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision not to take advantage of the extended transition period for complying with new or revised accounting standards is irrevocable.
As an emerging growth company, the Company is exempt from Section 404(b) of the Sarbanes-Oxley Act of 2002. Section 404(a) requires issuers to publish information in their annual reports concerning the scope and adequacy of the internal control structure and procedures for financial reporting. This statement shall also assess the effectiveness of such internal controls and procedures. Section 404(b) requires that the independent registered public accounting firm shall, in the same report, attest to and report on the assessment on the effectiveness of the internal control structure and procedures for financial reporting.
3
Table of Contents
As an emerging growth company, the Company is also exempt from Section 14A and B of the Exchange Act which require shareholder approval of executive compensation and golden parachutes.
We could remain an emerging growth company for up to five years, or until the earliest of (i) the last day of the first fiscal year in which our annual gross revenues exceed $1.0 billion, (ii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our Common Stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (iii) the date on which we have issued more than $1.0 billion in non-convertible debt during the preceding three year period.
Corporate History and Information
Q Therapeutics, Inc. was incorporated in the state of Delaware on March 28, 2002. On October 13, 2011, Q Therapeutics merged with Q Acquisition, Inc., a Delaware corporation and a wholly owned subsidiary of Grace 2, Inc. On November 2, 2011, Grace 2 changed its name to Q Holdings, Inc. and on December 10, 2012 it changed its name to Q Therapeutics, Inc. Our headquarters are located at 615 Arapeen Drive, Suite 102, Salt Lake City, Utah 84108. Our telephone number is (801) 582-5400. Our website is http://www.qthera.com. The information contained on or accessible though our website is not part of this prospectus, other than documents that we file with the SEC that are incorporated by reference into this prospectus.
4
Table of Contents
| The Issuer: | Q Therapeutics, Inc., a Delaware corporation | |
| Securities Being Offered: | Up to 5,000,000 shares of our common stock, par value $0.0001 per share and warrants to purchase up to 1,250,000 shares of common stock. Each warrant may be exercised at any time on or after the date that the warrant is issued until the fourth anniversary date at an exercise price of $ per share of common stock, subject to adjustment. This prospectus also relates to the offering of shares of common stock issuable upon exercise of warrants. | |
| Common stock outstanding as of July 5, 2013: | 24,811,833 shares | |
| Common stock to be outstanding after the offering assuming the sale of all shares covered hereby and no exercise of warrants for the shares covered by this prospectus: | 29,811,833 shares | |
| Common stock to be outstanding after the offering assuming the sale of all shares covered hereby and the exercise of all warrants for the shares covered by this prospectus: | 31,061,833 shares | |
| Use of Proceeds: | Assuming we sell [ ] units, we estimate that we will receive up to [$ ] million in net proceeds from the sale of the securities in this offering, assuming an initial public offering price of [$ ] per unit (which is the midpoint of the price range set forth on the cover page of this prospectus) and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. However, this is a best efforts offering, with no maximum or minimum, and there is no assurance that we will receive significant proceeds or enough proceeds to continue as a going concern and we may need to curtail or cease our operations. We intend to use the net proceeds from the sale of the securities for the clinical development of our Phase 1/2a study, for working capital needs, capital expenditures, and other general corporate purposes. See “Use of Proceeds” for more information. | |
| Dividend policy: | We currently intend to retain any future earnings to fund the development and growth of our business. Therefore, we do not currently anticipate paying cash dividends on our common stock. | |
| Risk Factors: | See “Risk Factors” and the other information in this prospectus for a discussion of the factors you should consider before deciding to invest in shares of our common stock. | |
| Proposed OTC trading symbol | QCEL | |
5
Table of Contents
An investment in our common stock and our warrants to purchase common stock involves a high degree of risk. In addition to the other information in this prospectus, you should carefully consider the following factors in evaluating us and our business before purchasing the shares of common stock and our warrants to purchase common stock offered hereby. This prospectus contains, in addition to historical information, forward-looking statements that involve risks and uncertainties. Our actual results could differ materially. Factors that could cause or contribute to such differences include, but are not limited to, those discussed below, as well as those discussed elsewhere in this prospectus, including the documents incorporated by reference.
RISKS RELATED TO THE COMPANY’S BUSINESS
Our business is at an early stage of development, and we may fail to develop and commercialize our potential product candidates.
Our business is at an early stage of development in that we do not yet have potential product candidates in clinical trials or on the market and we have not filed an Investigational New Drug, or IND, application with the FDA for any of our potential product candidates. Our ability to develop potential product candidates that progress to and through clinical trials is subject to our ability to, among other things:
| • | succeed in our research and development efforts; |
| • | select therapeutic compounds or cell therapies for development that have acceptable safety and efficacy profiles; |
| • | obtain required regulatory approvals; |
| • | finance, or obtain additional financing for, our clinical trials; |
| • | manufacture potential product candidates; and |
| • | collaborate successfully with clinical trial sites, academic institutions, physician investigators, clinical research organizations and other third parties; and achieve adequate enrollment of subjects. |
We have a history of losses and anticipate future losses, and continued losses could impair our ability to sustain operations.
We have incurred operating losses every year since our incorporation in 2002. As of March 31, 2013, our accumulated deficit was $20,991,557. Losses have resulted principally from costs incurred in connection with our research and development activities and from general and administrative expenses associated with our operations. We expect to incur additional operating losses and, as our development efforts continue and clinical testing activities, if any, commence our operating losses may increase in size.
Substantially all of our revenues to date have been from grants or license/research agreements. We may be unsuccessful in obtaining any new grants or entering into new license agreements that result in revenues. We do not expect that the revenues generated from any of these arrangements will be sufficient alone to continue or expand our research or development activities and otherwise sustain our operations.
Our ability to continue or expand our research and development activities and otherwise sustain our operations is dependent on our ability, alone or with others, to, among other things, manufacture and market therapeutic products. We also expect to experience negative cash flows from operating activities for the foreseeable future as we fund our operating losses and capital expenditures. This will result in decreases in our working capital, total assets and stockholders’ equity, which may not be offset by future financings. We will need to generate significant revenues to achieve profitability. In order to achieve profitability, we must
6
Table of Contents
develop products that can be commercialized by us or through our future collaborations. Our ability to generate revenues and become profitable will depend on our ability, alone or with potential collaborators, to successfully complete the development of our products in a timely and capital efficient manner. We may not be able to generate these revenues, and we may never achieve profitability. Our failure to achieve profitability could negatively impact the market price of our common stock. Even if we do become profitable, we cannot assure you that we would be able to sustain or increase profitability on a quarterly or annual basis.
We will need additional capital to conduct our operations and develop our potential product candidates, and our ability to obtain the necessary funding is uncertain.
We will require substantial capital resources in order to conduct our operations and develop our potential product candidates. The timing and degree of any future capital requirements will depend on many factors, including:
| • | the accuracy of the assumptions underlying our estimates for our capital needs for the remainder of the 2013 year end and beyond; |
| • | the magnitude and scope of our research and development programs; |
| • | the progress we make in our research and development programs, pre-clinical development and clinical trials; |
| • | our ability to establish, enforce and maintain strategic arrangements for research, development, clinical testing, manufacturing and marketing; |
| • | the number and type of potential product candidates that we pursue; |
| • | the time and costs involved in obtaining regulatory approvals and clearances; and |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims. |
We do not have any committed sources of capital. Additional financing through strategic collaborations, public or private equity financings, debt financing, capital lease transactions or other financing sources may not be available on acceptable terms, or at all. The receptivity of the public and private equity markets to proposed financings is substantially affected by the general economic, market and political climate and by other factors which are unpredictable and over which we have no control. Additional equity financings, if we obtain them, could result in significant dilution to our shareholders. Further, in the event that additional funds are obtained through arrangements with collaborative partners, these arrangements may require us to relinquish rights to some of our technologies, potential product candidates or proposed products that we would otherwise seek to develop and commercialize ourselves. If sufficient capital is not available, we may be required to delay, reduce the scope of or eliminate one or more of our programs, any of which could have a material adverse effect on our business.
Our business is dependent on limited potential product candidates.
At present, our ability to progress as an entity is significantly dependent on our first product candidate for neurodegenerative diseases, with the initial clinical indication for ALS, which is, currently in pre-clinical development. If we are successful with filing an IND and the FDA approves the application, we do not expect to commence human clinical trials before 2014. Any pre-clinical, regulatory or other development that significantly delays or prevents us from commencing or subsequently completing any of our trials, any material safety issue or adverse side effect to any study participant in these future trials, or the failure of trials to show the results expected would likely depress our stock price significantly and could prevent us from raising the additional capital we will need to further develop our cellular technologies. Moreover, any material adverse occurrence in our first clinical trials
7
Table of Contents
could substantially impair our ability to initiate clinical trials to test our cell therapies in other potential indications. Also, any material adverse event in clinical trials from other companies using cell therapy products could have a material adverse impact on our ability to carry out clinical trials. This, in turn, could adversely impact our ability to raise additional capital and pursue our planned research and development efforts.
Our business relies on cell therapy technologies that we may not be able to commercially develop.
We have concentrated the majority of our research on cell therapy technologies. Our ability to generate revenue and operate profitably will depend on being able to develop these technologies for human applications. These are emerging technologies and have limited human applications. We cannot guarantee that we will be able to develop our technologies or that such development will result in products with any commercial utility or value. We anticipate that the commercial sale of such products and royalty/licensing fees related to the technology will be our future primary sources of revenues. If we are unable to develop our technologies, we may never realize significant revenue.
Our product development programs are based on novel technologies and are inherently risky.
We are subject to the risks of failure inherent in the development of products based on new technologies. The novel nature of these therapies creates significant challenges in regard to product development and optimization, manufacturing, government regulation, third-party reimbursement and market acceptance. For example, the pathway to regulatory approval for cell-based therapies, including our potential product candidates, may be more complex and lengthy than the pathway for conventional drugs. These challenges may prevent us from developing and commercializing products on a timely or profitable basis, or at all.
We currently have no internal manufacturing capability and we intend to rely upon third-party manufacturers for our cells.
We currently have no internal manufacturing capability, and will rely extensively on licensees, strategic partners or third-party contract manufacturers or suppliers. Should we be forced to manufacture our cell therapy product, we cannot give you any assurance that we will be able to develop an internal manufacturing capability or procure alternative third-party suppliers. Moreover, we cannot give you any assurance that any contract manufacturers or suppliers we procure will be able to supply our product in a timely or cost effective manner or in accordance with applicable regulatory requirements or our specifications. We are presently working with the University of Utah’s Cell Therapy & Regenerative Medicine Facility (CTRM) in the production of Q-Cells. We will need to change manufacturers at some point in the future in order to manufacture a product for commercial sale, among other things. When that change to a new manufacturer is made, it will likely entail additional expense to achieve technology transfer and validation, which could cause delays in manufacturing and product development activities.
Restrictions on the use/development of human cell technology may adversely affect our business.
We base our research and development on the use of human cells obtained from human tissue. The U.S. federal and state governments and other jurisdictions impose restrictions on the acquisition and use of human tissue, including those incorporated in federal Good Tissue Practice (GTP) regulations. These regulatory and other constraints could prevent us from obtaining cells and other components of our products in the quantity or of the quality needed for their development or commercialization. These restrictions change from time to time and may become more onerous. Additionally, we may not be able to identify or develop reliable sources for the cells necessary for our potential products - that is, sources that follow all state and federal laws and guidelines for cell procurement. Certain components used to manufacture our cell potential product candidates will need to be manufactured in compliance with the FDA’s Good Manufacturing Practices (GMP). Accordingly, we will need to enter into supply agreements with companies that manufacture these components to GMP standards. There is no assurance that we will be able to enter into any such agreements.
8
Table of Contents
Noncompliance with applicable requirements both before and after approval, if any, can subject us, our third-party suppliers and manufacturers and our other collaborators to administrative and judicial sanctions, such as, among other things, warning letters, fines and other monetary payments, recall or seizure of products, criminal proceedings, suspension or withdrawal of regulatory approvals, interruption or cessation of clinical trials, total or partial suspension of production or distribution, injunctions, limitations on or the elimination of claims we can make for our products, refusal of the government to enter into supply contracts or fund research, or government delay in approving or refusal to approve new drug applications.
Our inability to complete pre-clinical and clinical testing and receive regulatory approvals will impair our viability.
No assurances can be given that we will be able to commence clinical trials for any of our potential product candidates or that any clinical trials will be completed or result in a successful outcome. If regulatory authorities do not approve our products or if we fail to maintain regulatory compliance, we would be unable to commercialize our therapeutic products, and our business and results of operations would be materially harmed.
Positive results from pre-clinical studies should not be relied upon as evidence that our clinical trials will succeed. Even if our potential product candidates achieve positive results in pre-clinical studies, we will be required to demonstrate through clinical trials that the potential product candidates are safe and effective for use in a diverse population before we can seek regulatory approvals for their commercial sale. There is typically an extremely high rate of attrition from the failure of potential product candidates as they proceed through clinical trials. If any product candidate fails to demonstrate sufficient safety and efficacy in any clinical trial, then we would experience potentially significant delays in, or be required to abandon, development of that product candidate. If we delay or abandon our development efforts of any of our potential product candidates, then we may not be able to generate sufficient revenues to become profitable, and our operations could be materially harmed.
Potential lead drug compounds or other potential product candidates and technologies require significant pre-clinical and clinical testing prior to regulatory approval in the United States and other countries. Our potential product candidates may prove to have undesirable and unintended side effects or other characteristics adversely affecting their safety, efficacy or cost-effectiveness that could prevent or limit their commercial use. In addition, our potential product candidates may not prove to be more effective for treating disease or injury than current therapies. Accordingly, we may have to delay or abandon efforts to research, develop or obtain regulatory approvals to market our potential product candidates. In addition, we will need to determine whether any of our potential products can be manufactured in commercial quantities at an acceptable cost. Our research and development efforts may not result in a product that can be or will be approved by regulators or marketed successfully. Competitors may have proprietary rights that prevent us from developing and marketing our products or they may sell similar, superior or lower cost products. Adverse results in research or clinical trials of others with cell therapy products may adversely affect regulatory or financial areas that could hinder our ability to continue with our clinical development. Because of the significant scientific, regulatory and commercial milestones that must be reached for any of our development programs or potential product candidates to be successful, any program or product candidate may be abandoned, even after we have expended significant resources, such as our investments or prospective investments in cell therapy technologies, which could adversely affect our business and materially and adversely affect our stock price.
The science and technology of stem and progenitor cell biology are relatively new. There is no precedent for the successful commercialization of therapeutic potential product candidates being developed by Q Therapeutics based on these technologies. Therefore, our development programs are particularly risky and uncertain. In addition, we, and/or our collaborators must undertake significant research and development activities to develop potential product candidates based on these technologies, which will require additional funding that may take years to accomplish, and may never result in successfully commercializing any of our potential product candidates.
9
Table of Contents
Our business is subject to ethical and social concerns and restrictions on use of stem or progenitor cells could prevent us from developing or gaining acceptance for commercially viable products based upon such technology and adversely affect the market price of our common stock.
The use of human cells for research and therapy has been the subject of debate regarding ethical, legal and social issues. Negative public attitudes toward cell therapy could result in greater governmental regulation of such therapies, which could harm our business. For example, concerns regarding such possible regulation could impact our ability to attract collaborators and investors. Existing and potential U.S. government regulation of human tissue may lead researchers to leave the field of stem and progenitor cell research or the country altogether, in order to assure that their careers will not be impeded by restrictions on their work. Similarly, these factors may induce graduate students to choose other fields less vulnerable to changes in regulatory oversight, thus exacerbating the risk that we may not be able to attract and retain the scientific personnel we need in the face of competition among pharmaceutical, biotechnology and health care companies, universities and research institutions for what may become a shrinking class of qualified individuals.
Some of our most important programs involve the use of progenitor cells that are derived from human fetal cadaver tissue. The use of these progenitor cells can give rise to ethical and social issues regarding elective termination of pregnancy and the appropriate use of tissue derived therefrom. Some political and religious groups have voiced opposition to these technologies and practices. We use progenitor cells, and may use stem cells, derived from fetal cadaver tissue. Our research related to these derivatives may become the subject of adverse commentary or publicity, which could significantly harm the market price of our common stock. Changes in federal or state laws regulating rights to elective terminations of pregnancy may adversely impact our ability to procure tissue for manufacturing our products.
We are not using cells derived from embryonic stem cells. Government imposed restrictions with respect to use of embryos in research and development still could have a negative material effect on our business, including reducing interest in the cell therapy field overall and reducing interest in financing our business, impairing our ability to establish critical partnerships and collaborations.
These potential effects and others may result in a decrease in the market price of our common stock.
We operate in leased facilities and cannot be assured that these facilities will be available to us upon expiration of our current lease.
We occupy laboratory and office space in facilities leased from a commercial landlord, and we have added improvements to these facilities at our own expense to meet our laboratory needs. Our lease agreement for this space has expired and we are presently in negotiations with the landlord on the terms of a new lease agreement. The landlord has continued to accept lease payments which we have been paying at the rate we expect to be charged in the new lease. We cannot be assured that we will be able to agree on terms with the landlord for continuing in the space and that we will remain in the same space. If we need to move to a new facility, this will require additional expense to add the improvements needed for our operations, as well as delay our development activities until we are fully operational in a new space.
We may engage in acquisitions or strategic transactions or make investments that could result in significant changes or management disruption and fail to enhance stockholder value.
We may engage in acquisitions or strategic transactions or make investments with the goal of maximizing stockholder value. We may acquire businesses, in-license new technologies for development, enter into joint
10
Table of Contents
ventures or other strategic transactions, and purchase equity and debt securities, including minority interests in publicly traded and private companies, non-investment-grade debt securities, equity and debt mutual and exchange-traded funds, and corporate bonds/notes. Some of our strategic investments may entail a high degree of risk and will not become liquid until more than one year from the date of investment, if at all. Any or all of our acquisitions or strategic investments that we may undertake in the future may not generate financial returns or result in increased adoption or continued use of our technologies. In addition, our other investments may not generate financial returns or may result in losses due to market volatility, the general level of interest rates and inflation expectations. In some cases, we may be required to consolidate or record our share of the earnings or losses of those companies. Our share of any losses will adversely affect our financial results until we exit from or reduce our exposure to these investments. Achieving the anticipated benefits of acquisitions depends in part upon our ability to integrate the acquired businesses in an efficient and effective manner. The integration of companies that have previously operated independently may result in significant challenges, and we may be unable to accomplish the integration smoothly or successfully. We cannot assure you that the integration of acquired businesses with our business will result in the realization of the full benefits anticipated by us to result from the acquisition. We may not derive any commercial value from the acquired technology, products and intellectual property or from future technologies and products based on the acquired technology and/or intellectual property, and we may be subject to liabilities that are not covered by indemnification protection we may obtain.
Compliance with changing regulation of corporate governance and public disclosure may result in additional expenses.
Changing laws, regulations and standards relating to corporate governance and public disclosure may create uncertainty regarding compliance matters. New or changed laws, regulations and standards are subject to varying interpretations in many cases. As a result, their application in practice may evolve over time. We are committed to maintaining high standards of corporate governance and public disclosure. Complying with evolving interpretations of new or changed legal requirements may cause us to incur higher costs as we revise current practices, policies and procedures, and may divert management time and attention from product development and revenue generation to compliance activities. If our efforts to comply with new or changed laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, our reputation might also be harmed.
We are subject to the reporting requirements of federal securities laws, which can be expensive and may divert management’s time and Company resources from other projects, thus impairing our ability to grow.
We are an SEC reporting company and, accordingly, subject to the information and reporting requirements of the Securities Exchange Act of 1934, as amended, and other federal securities laws, including compliance with the Sarbanes-Oxley Act of 2002 (the Sarbanes-Oxley Act). The costs of preparing and filing annual, quarterly and current reports, proxy statements and other information with the SEC, and furnishing audited reports to shareholders are high and will put pressure on our cash resources.
It may be time consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act. We may need to hire additional financial reporting, internal controls and other finance personnel in order to develop and implement appropriate internal controls and reporting procedures.
11
Table of Contents
We are an “emerging growth company,” and any decision on our part to comply only with certain reduced reporting and disclosure requirements applicable to emerging growth companies could make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups, or JOBS, Act enacted in April 2012, and, for as long as we continue to be an “emerging growth company,” we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies but not to “emerging growth companies,” including, but not limited to, not being required to have our independent registered public accounting firm audit our internal control over financial reporting under Section 404, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an “emerging growth company” for up to five years following the completion of this offering, although, if we have more than $1.0 billion in annual revenue, if the market value of our common stock that is held by non-affiliates exceeds $700 million as of June 30 of any year, or we issue more than $1.0 billion of non-convertible debt over a three-year period before the end of that five-year period, we would cease to be an “emerging growth company” as of the following December 31. We cannot predict if investors will find our common stock less attractive if we choose to rely on these exemptions. If some investors find our common stock less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our common stock and our stock price may be more volatile. As an “emerging growth company” the JOBS Act allows us to delay adoption of new or revised accounting pronouncements applicable to public companies until such pronouncements are made applicable to private companies. We have elected to use this extended transition period under the JOBS Act. As a result, our financial statements may not be comparable to the financial statements of issuers who are required to comply with the effective dates for new or revised accounting standards that are applicable to public companies, which may make our common stock less attractive to investors.
If we fail to establish and maintain an effective system of internal control, we may not be able to report our financial results accurately or to prevent fraud. Any inability to report and file our financial results accurately and timely could harm our reputation and adversely impact the trading price of our common stock.
Effective internal control is necessary for us to provide reliable financial reports and prevent fraud. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as we would if an effective control environment existed, and our business and reputation with investors may be harmed. As a result, our small size and any current internal control deficiencies may adversely affect our financial condition, results of operations and access to capital. We have not performed an in-depth analysis to determine if historical un-discovered failures of internal controls exist, and may in the future discover areas of our internal control that need improvement.
Public company compliance may make it more difficult to attract and retain officers and directors.
The Sarbanes-Oxley Act and new rules subsequently implemented by the SEC have required changes in corporate governance practices of public companies. As a public company, we expect these new rules and regulations to increase our compliance costs in 2013 and beyond and to make certain activities more time consuming and costly. As a public company, we also expect that these new rules and regulations may make it more difficult and expensive for us to obtain director and officer liability insurance in the future and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve on our board of directors or as executive officers.
12
Table of Contents
Compliance with changing regulation of corporate governance and public disclosure, and our management’s inexperience with such regulations will result in additional expenses and creates a risk of non-compliance.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 and related SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the public markets and public reporting. Our management team expects to invest significant management time and financial resources to comply with both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion of management time and attention from revenue generating activities to compliance activities. Management’s inexperience may cause us to fall out of compliance with applicable regulatory requirements, which could lead to enforcement action against us and a negative impact on our stock price.
RISKS RELATING TO OUR COMMON STOCK AND THIS OFFERING
There is currently no trading market for our common stock and we cannot ensure that one will ever develop or be sustained.
To date there has been no trading market for our common stock. We cannot predict how liquid the market for our common stock might become. We intend to apply to have our common stock quoted for trading on the OTC Bulletin Board, however, we cannot be sure that such quotation will be obtained promptly, if at all.
We currently do not satisfy the initial listing standards for any national securities exchange, and cannot ensure that we will be able to satisfy such listing standards or that our common stock will be accepted for listing on any such exchange. Should we fail to satisfy the initial listing standards of such exchanges, or our common stock is otherwise rejected for listing and remains listed on the OTC Bulletin Board or suspended from the OTC Bulletin Board, the trading price of our common stock could suffer, the trading market for our common stock may be less liquid and our common stock price may be subject to increased volatility.
Furthermore, for companies whose securities are traded on the OTC Bulletin Board, it is more difficult (1) to obtain accurate quotations, (2) to obtain coverage for significant news events because major wire services generally do not publish press releases about such companies, and (3) to obtain needed capital.
There is no minimum amount required to be raised in the offering, and if we cannot raise sufficient funds from this offering, we may need to curtail or cease operations.
There is not a minimum amount of units that need to be sold in this offering for the Company to access the funds. Therefore, the proceeds of this offering will be immediately available for use by us and we do not have to wait until a minimum number of units have been sold to keep the proceeds from any sales. We cannot assure you that subscriptions for the entire offering will be obtained. We have the right to terminate this offering at any time, regardless of the number of securities we have sold since there is no minimum subscription requirement. Our ability to meet our financial obligations and cash needs, and to achieve our objectives, could be adversely affected if the entire offering is not fully subscribed for and as a result we could be forced to curtail or cease our operations.
A FINRA-registered broker-dealer unaffiliated with the underwriter has submitted an application to FINRA to have our common stock quoted on the OTC Bulletin Board. The application may not be approved by FINRA and as a result our common stock may be difficult for our investors to trade.
A FINRA-registered broker-dealer unaffiliated with the underwriter has submitted an application to FINRA to have our common stock quoted on the OTC Bulletin Board. This FINRA-registered broker-dealer is
13
Table of Contents
expected to act as a market-maker for our common stock. We cannot give any assurances that the application to FINRA submitted by this FINRA broker-dealer will be approved. If our common stock is not quoted on the OTCBB, we intend to have our common stock listed on the OTC Markets, which might make it more difficult for investors to trade their shares.
If and when our common stock becomes publicly traded, our common stock may be deemed a “penny stock,” which would make it more difficult for our investors to sell their shares.
If and when our common stock becomes publicly traded, our common stock may be subject to the “penny stock” rules adopted under Section 15(g) of the Exchange Act. The penny stock rules generally apply to companies whose common stock is not listed on an exchange such as NYSE or NASDAQ. These rules require, among other things, that brokers who trade penny stocks to persons other than established customers complete certain documentation, make suitability inquiries of investors and provide investors with certain information concerning trading in the security, including a risk disclosure document and quote information under certain circumstances. Many brokers have decided not to trade penny stocks because of the requirements of the penny stock rules and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. If our securities are subject to the penny stock rules, investors will find it more difficult to dispose of our securities.
Investor relations activities, nominal float and supply and demand factors may affect the price of our common stock.
We have engaged an investor relations firm and financial advisory firm to create investor awareness for our Company. These campaigns may include personal, video and telephone conferences with investors and prospective investors in which our business practices are described. We may provide compensation to investor relations and financial advisory firms and pay for newsletters, websites, mailings and email campaigns that are produced by third-parties based upon publicly available information concerning us. We will not be responsible for the content of analyst reports and other writings and communications by investor relations firms not authored by us or from publicly available information. We do not intend to review or approve the content of such analysts’ reports or other materials based upon analysts’ own research or methods. Investor relations firms should generally disclose when they are compensated for their efforts, but whether such disclosure is made or complete is not under our control. Our investors may be willing, from time to time, to encourage investor awareness through similar activities. Investor awareness activities may also be suspended or discontinued which may impact the trading market of our common stock.
The SEC and FINRA enforce various statutes and regulations intended to prevent manipulative or deceptive devices in connection with the purchase or sale of any security and carefully scrutinize trading patterns and company news and other communications for false or misleading information, particularly in cases where the hallmarks of pump and dump activities may exist, such as rapid share price increases or decreases. We and our shareholders may be subjected to enhanced regulatory scrutiny due to the small number of holders who own the registered shares of our common stock publicly available for resale, and the limited trading markets in which such shares are offered or sold which have often been associated with improper activities concerning penny stocks, such as the OTC BB or the OTC Markets (OTCQB or OTCPink). Until such time as the restricted shares of the Company are registered or available for resale under Rule 144, there will continue to be a small percentage of shares held by a small number of investors, many of whom acquired such shares in privately negotiated purchase and sale transactions that will constitute the entire available trading market. The Supreme Court has stated that manipulative action is a term of art connoting intentional or willful conduct designed to deceive or defraud investors by controlling or artificially affecting the price of securities. Often times, manipulation is associated by regulators with forces that upset the
14
Table of Contents
supply and demand factors that would normally determine trading prices. Securities regulators have often cited thinly traded markets, small numbers of holders, and awareness campaigns as components of their claims of price manipulation and other violations of law when combined with manipulative trading, such as wash sales, matched orders or other manipulative trading timed to coincide with false or touting press releases. There can be no assurance that our or third-parties’ activities, or the small number of potential sellers or small percentage of stock in the float, or determinations by purchasers or holders as to when or under what circumstances or at what prices they may be willing to buy or sell stock, will not artificially impact (or would be claimed by regulators to have affected) the normal supply and demand factors that determine the price of stock.
Our stock price may be volatile.
The stock market in general, and the stock prices of technology-based and biotechnology / biopharmaceutical stocks in particular, have experienced volatility that often has been unrelated to the operating performance of any specific public company. The market price of our common stock is likely to be highly volatile and could fluctuate widely in price in response to various factors, many of which are beyond our control, including the following:
| • | changes in our industry; competitive pricing pressures; our ability to obtain working capital financing; additions or departures of key personnel; |
| • | limited public float in the hands of a small number of persons whose sales or lack of sales could result in positive or negative pricing pressure on the market price for our common stock; |
| • | sales of our common stock; |
| • | our ability to execute our business plan; |
| • | operating results that fall below expectations; |
| • | loss of any strategic relationship; |
| • | regulatory developments; |
| • | changes in federal or state healthcare-related laws; |
| • | economic and other external factors; |
| • | period-to-period fluctuations in our financial results; |
| • | challenges and/or invalidation of key patents; and |
| • | the inability to develop or acquire new or needed technology. |
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
If our shareholders sell substantial amounts of our common stock in the public market, including shares issued in past private offerings, upon the effectiveness of the registration statement required to be filed, or upon the expiration of any statutory holding period, under Rule 144, or upon expiration of lock-up periods applicable to outstanding shares, or issued upon the exercise of outstanding options or warrants, it could create a circumstance commonly referred to as an overhang and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are
15
Table of Contents
occurring, also could make more difficult our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate. Certain shares of common stock held by current and former officers and directors, founders, and institutional shareholders of Q are subject to a lock-up agreement prohibiting sales of such shares for a period of 12 months from July 17, 2012. Following such date, all of those shares will become freely tradable, subject to securities laws and SEC regulations regarding sales by insiders.
Our undesignated preferred stock may inhibit potential acquisition bids; this may adversely affect the market price for our common stock and the voting rights of holders of our common stock.
Our Certificate of Incorporation provides our Board of Directors with the authority to issue up 10,000,000 shares of undesignated preferred stock and to determine or alter the rights, preferences, privileges and restrictions granted to or imported upon these shares without further vote or action by our shareholders. As of the date of this filing, no shares of preferred stock have been designated and the Board of Directors still has authority to designate and issue up to 10,000,000 shares of preferred stock in one or more classes or series. The issuance of shares of preferred stock may delay or prevent a change in control transaction without further action by our shareholders. As a result, the market price of our common stock may be adversely affected. In addition, if we issue preferred stock in the future that has preference over our common stock with respect to the payment of dividends or upon our liquidation, dissolution or winding up, or if we issue preferred stock with voting rights that dilute the voting power of our common stock, the rights of holders of our common stock or the market price of our common stock could be adversely affected.
We have not paid dividends in the past and do not expect to pay dividends in the future. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate doing so in the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as our Board of Directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
Because our directors and executive officers are among our largest shareholders, they can exert significant control over our business and affairs and have actual or potential interests that may depart from those of subscribers in our previous private offerings.
Our directors and executive officers own or control a significant percentage of the common stock. Additionally, the holdings of our directors and executive officers may increase in the future upon vesting or other maturation of exercise rights under any of the options or warrants they may hold or in the future be granted or if they otherwise acquire additional shares of our common stock. The interests of such persons may differ from the interests of our other stockholders. As a result, in addition to their board seats and offices, such persons have significant influence over and control all corporate actions requiring stockholder approval, irrespective of how the Company’s other stockholders, may vote, including the following actions: to elect or defeat the election of our directors; to amend or prevent amendment of our Certificate of Incorporation or By-Laws; to effect or prevent a merger, sale of assets or other corporate transaction; and to control the outcome of any other matter submitted to our stockholders for vote. Such person’s stock ownership may discourage a potential acquirer from making a tender offer or otherwise attempting to obtain control of the Company, which in turn could reduce our stock price or prevent our stockholders from realizing a premium over our stock price.
16
Table of Contents
RISKS RELATED TO CLINICAL AND COMMERCIALIZATION ACTIVITIES
Delays in the commencement of clinical testing of our current and potential product candidates could result in increased costs to us and delay our ability to generate revenues.
The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
| • | obtaining adequate financing; |
| • | demonstrating sufficient safety and efficacy to obtain regulatory clearance to commence a clinical trial; |
| • | manufacturing sufficient quantities or producing products meeting our quality standards of a product candidate; |
| • | obtaining an injection device or methodology meeting our quality standards; |
| • | obtaining approval of an Investigational New Drug (IND) application or proposed trial design from the FDA; |
| • | reaching agreement on acceptable terms with our collaborators on all aspects of the clinical trial, including the contract research organizations (CROs) and the trial sites; and |
| • | obtaining Institutional Review Board (IRB) approval to conduct a clinical trial at a prospective site. |
In addition, clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size and nature of the patient population, the nature of the protocol, the proximity of patients to clinical sites, other competing clinical trials in the same disease indication, the availability of effective treatments for the relevant disease, and the eligibility criteria for the clinical trial. Delays in commencing clinical testing of our potential product candidates could prevent or delay us from obtaining approval for our potential product candidates.
We do not have experience as a company in conducting clinical trials, or in other areas required for the successful commercialization and marketing of our potential product candidates.
We have no experience as an entity in conducting either early stage or large-scale, late stage clinical trials. We cannot be certain that planned clinical trials will begin or be completed on time, if at all. Large-scale trials would require either additional financial and management resources, or reliance on third-party clinical investigators, pharmaceutical partners, CROs and/or consultants. Relying on third-party clinical investigators, pharmaceutical partners or CROs may force us to encounter delays that are outside of our control. Any such delays could have a material adverse effect on our business.
We also do not currently have marketing and distribution capabilities for our potential product candidates. Developing an internal sales and distribution capability would be an expensive and time-consuming process. We may enter into agreements with third parties that would be responsible for marketing and distribution. However, these third parties may not be capable of successfully selling any of our potential product candidates. The inability to commercialize and market our potential product candidates could materially adversely affect our business.
Obtaining regulatory approvals to market our potential product candidates in the United States and other countries is a costly and lengthy process and we cannot predict whether or when we will be permitted to commercialize our potential product candidates.
Federal, state and local governments in the United States and governments in other countries have significant regulations in place that govern many of our activities and may prevent us from creating commercially viable products from our discoveries. The regulatory process, particularly for biopharmaceutical potential product candidates like ours, is uncertain, can take many years and requires the expenditure of substantial resources.
17
Table of Contents
Our potential product candidates will require extensive pre-clinical and clinical testing prior to submission of any regulatory application to commence commercial sales. In particular, human pharmaceutical therapeutic potential product candidates are subject to rigorous requirements of the FDA in the United States and similar health authorities in other countries in order to demonstrate safety and efficacy. Data obtained from pre-clinical and clinical activities is susceptible to varying interpretations that could delay, limit or prevent regulatory agency approvals. In addition, delays or rejections may be encountered as a result of changes in regulatory agency policy during the period of product development and/or the period of review of any application for regulatory agency approval for a product candidate.
Any product candidate that we or our collaborators develop must receive all relevant regulatory agency approvals/clearances before it may be marketed in the United States or other countries. Obtaining regulatory approvals/clearances is a lengthy, expensive and uncertain process. Because certain of our potential product candidates involve the application of new technologies or are based upon a new therapeutic approach, they may be subject to substantial additional review by various government regulatory authorities, and, as a result, the process of obtaining regulatory approvals/clearances for them may proceed more slowly than for potential product candidates based upon more conventional technologies.
Delays in obtaining regulatory agency approvals/clearances could:
| • | significantly delay and harm the marketing of any product that we or our collaborators develop; |
| • | impose costly procedures upon our activities or the activities of our collaborators; |
| • | diminish any competitive advantages that we or our collaborators may attain; or |
| • | adversely affect our ability to receive royalties and generate revenues and profits. |
Even if we commit the necessary time and resources, the required regulatory agency approvals/clearances may not be obtained for any potential product candidates developed by us or in collaboration with us. If we obtain regulatory agency approvals/clearances for a new product, these approvals/clearances may entail limitations on the indicated uses for which it can be marketed that could limit the potential commercial use of the product.
Failure to achieve continued compliance with government regulation over approved products could delay or halt commercialization of our products.
Approved products and their manufacturers are subject to continual review, and discovery of previously unknown problems with a product or its manufacturer may result in restrictions on the product or manufacturer, including withdrawal of the product from the market. The future sale by us or our collaborators of any commercially viable product will be subject to government regulation from several standpoints, including the processes of:
| • | manufacturing; |
| • | adverse-event reporting; |
| • | advertising and promoting; |
| • | selling and marketing; |
| • | labeling; and |
| • | distributing. |
18
Table of Contents
If, and to the extent that, we are unable to comply with these regulations, our ability to earn revenues will be materially and negatively impacted.
Failure to comply with regulatory requirements can result in severe civil and criminal penalties, including but not limited to:
| • | recall or seizure of products; |
| • | injunction against the manufacturing, distributing, selling and marketing of products, and |
| • | criminal prosecution. |
The imposition of any of these penalties or other commercial limitations could significantly impair our business, financial condition and results of operations.
RISKS RELATED TO PROTECTING OUR INTELLECTUAL PROPERTY
Impairment of our intellectual property rights may adversely affect the value of our technologies and potential product candidates and limit our ability to pursue their development.
Protection of our proprietary technology is critically important to our business. Our success will depend in part on our ability to obtain and enforce our patents and maintain trade secrets, both in the United States and in other countries. Further, our patents may be challenged, invalidated or circumvented, and our patent rights may not provide proprietary protection or competitive advantages to us. In the event that we are unsuccessful in obtaining and enforcing patents, we may not be able to further develop or commercialize our potential product candidates and our business would be negatively impacted.
The patent positions of pharmaceutical and biopharmaceutical companies, including ours, are highly uncertain and involve complex legal and technical questions. In particular, legal principles for biotechnology and pharmaceutical patents in the United States and in other countries are evolving, and the extent to which we will be able to obtain patent coverage to protect our technology, or enforce issued patents, is uncertain. In the United States, recent court decisions in patent cases as well as proposed legislative changes to the patent system only exacerbate this uncertainty. Furthermore, significant amendments to the regulations governing the process of obtaining patents were proposed in a new rule package by the United States Patent and Trademark Office (the Patent Office) in 2007. The proposed new rules were widely regarded as detrimental to the interests of biotechnology and pharmaceutical companies. The implementation of the rule package was blocked by a court injunction requested by a pharmaceutical company. The Patent Office challenged the court decision through an appeal to the U.S. Court of Appeals for the Federal Circuit (CAFC), but the appeal was dismissed in November 2009, after the Patent Office changed course and rescinded the proposed new rules. At this point we do not know whether the Patent Office will attempt to introduce new rules to replace those that were recently withdrawn or whether any such new rules would also be challenged.
We are uncertain of what patent coverage we will obtain for our products in Europe or Asia. At this time, we do not know whether or to what extent we will be able to obtain future patent protection for our technologies and products in Europe or Asia. If we are unable to protect our inventions related to cell therapy products in Europe or Asia, our future business opportunities could be negatively impacted.
Challenges to our patent rights can result in costly and time-consuming legal proceedings that may prevent or limit development of our potential product candidates.
Publication of discoveries in scientific or patent literature tends to lag behind actual discoveries by at least several months and sometimes several years. Therefore, the persons or entities that we or our licensors name as inventors in our patents and patent applications may not have been the first to invent the inventions
19
Table of Contents
disclosed in the patent applications or patents, or the first to file patent applications for these inventions. As a result, we may not be able to obtain patents for discoveries that we otherwise would consider patentable and that we consider to be extremely significant to our future success.
Where more than one party seeks U.S. patent protection for the same technology, the Patent Office may declare an interference proceeding in order to ascertain the party to which the patent should be issued. Patent interferences are typically complex, highly contested legal proceedings, subject to appeal. They are usually expensive and prolonged, and can cause significant delay in the issuance of patents. Moreover, parties that receive an adverse decision in an interference can lose important patent rights. Our pending patent applications, or our issued patents, may be drawn into interference proceedings which may delay or prevent the issuance of patents, or result in the loss of issued patent rights.
Outside of the United States, certain jurisdictions, such as Europe, New Zealand and Australia, permit oppositions to be filed against the granting of patents. Because our intent is to commercialize products internationally, securing both proprietary protection and freedom to operate outside of the United States is important to our business. We may be, in the future, involved in both opposing the grant of patents to others through such opposition proceedings and in defending our patent applications against oppositions filed by others.
European opposition and appeal proceedings can take several years to reach final decision. The potential oppositions discussed above reflect the complexity of the patent landscape in which we operate, and illustrate the risks and uncertainties.
Patent opposition proceedings are not currently available in the U.S. patent system, but legislation has been proposed to introduce them. However, issued U.S. patents can be re-examined by the Patent Office at the request of a third party. Patents owned or licensed by Q may therefore be subject to re-examination. As in any legal proceeding, the outcome of patent re-examinations is uncertain, and a decision adverse to our interests could result in the loss of valuable patent rights.
As more groups become engaged in scientific research and product development in the area of cell therapy, the risk of our patents being challenged through patent interferences, oppositions, re-examinations, litigation or other means will likely increase. Challenges to our patents through these procedures can be extremely expensive and time-consuming, even if the outcome is favorable to us. An adverse outcome in a patent dispute could severely harm our business by:
| • | causing us to lose patent rights in the relevant jurisdictions(s); |
| • | subjecting us to litigation, or otherwise preventing us from commercializing potential products in the relevant jurisdiction(s); |
| • | requiring us to obtain licenses to the disputed patents; |
| • | forcing us to cease using the disputed technology; or |
| • | requiring us to develop or obtain alternative technologies. |
Furthermore, if such challenges to our patent rights are not resolved promptly in our favor, our existing and future business relationships may be jeopardized and we could be delayed or prevented from entering into new collaborations or from commercializing certain products, which could materially harm our business.
20
Table of Contents
If we fail to meet our obligations under license agreements, we may lose our rights to key technologies on which our business depends.
Our business depends on several critical technologies that are based in part on patents licensed from third parties. Those third-party license agreements impose obligations on us, such as payment obligations and obligations to diligently pursue development of commercial products under the licensed patents. If a licensor believes that we have failed to meet our obligations under a license agreement, the licensor could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation our ability to carry out the development and commercialization of potential products could be significantly and negatively affected. If our license rights were restricted or ultimately lost, our ability to continue our business based on the affected technology would be severely adversely affected.
We may be subject to litigation that will be costly to defend or pursue and uncertain in its outcome.
Our business may bring us into conflict with our licensees, licensors, others with whom we have contractual or other business relationships, or with our competitors or others whose interests differ from ours. If we are unable to resolve those conflicts on terms that are satisfactory to all parties, we may become involved in litigation brought by or against us. That litigation is likely to be expensive and may require a significant amount of management’s time and attention at the expense of other aspects of our business. The outcome of litigation is always uncertain, and in some cases could include judgments against us that require us to pay damages, enjoin us from certain activities, or otherwise affect our legal or contractual rights, which could have a significant adverse effect on our business.
The development, manufacturing and commercialization of cell-based therapeutic products expose us to product liability claims, which could lead to substantial liability.
By developing and, ultimately, commercializing therapeutic products, we are exposed to the risk of product liability claims. Product liability claims against us could result in substantial litigation costs and damage awards against us. We intend to obtain liability insurance that covers our clinical trials, and we will need to increase our insurance coverage if and when we begin commercializing products. We may not be able to obtain insurance on acceptable terms, if at all, and the policy limits on our insurance policies may be insufficient to cover our liability.
We may be subject to infringement claims that are costly to defend, and which may limit our ability to use disputed technologies and may prevent us from pursuing research and development or commercialization of potential products.
Our commercial success depends significantly on our ability to operate without infringing patents and the proprietary rights of others. Our technologies may infringe the patents or proprietary rights of others. In addition, we may become aware of discoveries and technology controlled by third parties that are advantageous to our programs. In the event our technologies infringe the rights of others or we require the use of discoveries and technology controlled by third parties, we may be prevented from pursuing research, development or commercialization of potential products; may be required to obtain licenses to those patents or other proprietary rights or develop or obtain alternative technologies. We have obtained or are negotiating licenses from universities and companies for technologies that we anticipate incorporating into our potential products, and we initiate negotiation for licenses to other technologies as the need or opportunity arises. We may not be able to obtain a license to patented technology on commercially favorable terms, or at all. If we do not obtain a necessary license, we may need to redesign our technologies or obtain rights to alternate technologies, the research and adoption of which could cause delays in product development. In cases where we are unable to license necessary technologies, we could be prevented from developing certain potential products. Our failure to obtain alternative technologies or a license to any technology that we may require to research, develop or commercialize our potential product candidates would significantly and negatively affect our business.
21
Table of Contents
Much of the information and know-how that is critical to our business is not patentable and we may not be able to prevent others from obtaining this information and establishing competitive enterprises.
We sometimes rely on trade secrets to protect our proprietary technology, especially in circumstances in which we believe patent protection is not appropriate or available. We attempt to protect our proprietary technology in part by confidentiality agreements with our employees, consultants, collaborators and contractors. We cannot assure you that these agreements will not be breached, that we would have adequate remedies for any breach, or that our trade secrets will not otherwise become known or be independently discovered by competitors, any of which would harm our business significantly.
RISKS RELATED TO OUR RELATIONSHIPS WITH THIRD PARTIES
We depend on other parties to help us develop, manufacture and test our potential product candidates, and our ability to develop and commercialize potential products may be impaired or delayed if collaborations are unsuccessful.
Our strategy for the development, clinical testing and commercialization of our potential product candidates requires that we enter into collaborations with corporate partners, licensors, licensees and others. We are dependent upon the subsequent success of these other parties in performing their respective responsibilities and the continued cooperation of our partners. By way of example, we have key agreements with Goodwin Biosciences, Inc. and the University of Utah related to manufacture of our products, and are working with Johns Hopkins University and MPI Research, Inc. on pre-clinical studies for our first product. We have agreements with Life Technologies, Inc. concerning commercialization of certain of our products for the research market. Our collaborators, contractors and licensees may not cooperate with us or perform their obligations under our agreements with them. We cannot control the amount and timing of our collaborators’ resources that will be devoted to activities related to our collaborative agreements with them. Our collaborators may choose to pursue existing or alternative technologies in preference to those being developed in collaboration with us.
Under agreements with other parties, we may rely significantly on them to, among other activities:
| • | conduct research and development activities and pre-clinical safety studies in conjunction with or for us; |
| • | manufacture product(s) or reagents used for such product manufacture for use by us, our contractors and our partners; |
| • | design and conduct advanced clinical trials in the event that we reach clinical trials; |
| • | fund research and development activities with us; |
| • | manage and license certain patent rights; |
| • | pay us fees upon the achievement of milestones; and |
| • | market with us any commercial products that result from our collaborations. |
The development and commercialization of potential products will be delayed if collaborators or other partners fail to conduct these activities in a timely manner or at all. In addition, our collaborators could terminate their agreements with us and we may not receive any development or milestone payments. If we do not achieve milestones set forth in the agreements, or if our collaborators breach or terminate their collaborative agreements with us, our business may be materially harmed.
We also rely on other companies for certain reagent development, manufacturing or other technical scientific work, with respect to our cell products. We have contracts with these companies that specify the
22
Table of Contents
work to be done and results to be achieved, but we do not have direct control over their personnel or operations. If these companies do not perform the work that they were assigned, our ability to develop or manufacture our potential product candidates could be significantly harmed.
Our reliance on the activities of our consultants, research institutions, and scientific contractors, whose activities are not wholly within our control, may lead to delays in development of our potential product candidates.
We rely extensively upon and have relationships with scientific consultants at academic and other institutions, some of whom conduct research at our request, and other consultants who assist us in formulating our research and development and clinical strategy or other matters. These consultants are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. We have limited control over the activities of these consultants and, except as otherwise required by our collaboration and consulting agreements, can expect only limited amounts of their time to be dedicated to our activities.
In addition, we have formed research collaborations with academic and other research institutions in the U.S. These research facilities may have commitments to other commercial and noncommercial entities. We have limited control over the operations of these laboratories and can expect only limited amounts of their time to be dedicated to our research goals. If any of these third parties are unable or refuse to contribute to projects on which we need their help, our ability to generate advances in our technologies and develop our potential product candidates could be significantly harmed.
RISKS RELATED TO COMPETITIVE FACTORS
The loss of key personnel could slow our ability to conduct research and develop potential product candidates.
Our future success depends to a significant extent on the skills, experience and efforts of our executive officers and key members of our scientific staff. We face intense competition for qualified individuals from numerous pharmaceutical, biopharmaceutical and biotechnology companies, as well as academic and other research institutions. We may be unable to retain our current personnel or attract or assimilate other highly qualified management and scientific personnel in the future on acceptable terms. The loss of any or all of these individuals could harm our business and might significantly delay or prevent the achievement of research, development or business objectives.
Our potential product candidates are likely to be expensive to manufacture, and they may not be profitable if we are unable to achieve a selling price in excess of the costs of manufacturing.
Our Q-Cells and other cell-based products could be more expensive to manufacture than other treatments currently on the market today or that will be on the market should we even be in a position to market any of our potential product candidates. Our present manufacturing processes are conducted at a modest scale and we may be able to reduce manufacturing costs through process improvements, as well as through scale increases. If we are not able to do so, however, and, depending on the pricing of the potential product, the profit margin on our product may be significantly less than that of most drugs on the market today. If we are unable to scale our production of Q-Cells at a commercially acceptable manufacturing cost, it would impact the affordability of the therapy for patients and reduce our potential profitability.
The cell-based therapies we are developing may require large quantities of cells. We continue to develop processes to scale up production and increase yields of the cells in a cost-effective way. We may not be able to charge a high enough price for any cell therapy product we develop, even if it is safe and effective, to make a profit. If we are unable to realize significant profits from our potential product candidates, our business would be materially harmed.
23
Table of Contents
Some of our competitors may develop technologies that are superior to or more cost-effective than ours, which may impact the commercial viability of our technologies and which may significantly damage our ability to sustain operations.
The pharmaceutical and biotechnology industries are intensely competitive. Other pharmaceutical and biotechnology companies and research organizations currently engage in or have in the past engaged in efforts related to the biological mechanisms that are the focus of our programs in glial cell therapies, including the study of neural stem cells as precursors for glial cells and use of embryonic stem cells to derive glial cells. In addition, other products and therapies that could compete directly with the potential product candidates that we are seeking to develop and market currently exist or are being developed by pharmaceutical and biopharmaceutical companies and by academic and other research organizations.
Other companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Academic institutions, government agencies and other public and private research organizations may also conduct research, seek patent protection and establish collaborative arrangements for research, clinical development and marketing of products similar to ours. These companies and institutions compete with us in recruiting and retaining qualified scientific and management personnel as well as in acquiring technologies complementary to our programs.
In addition to the above factors, we expect to face competition in the following areas:
| • | product efficacy and safety; |
| • | the timing and scope of regulatory consents; |
| • | availability of resources; |
| • | reimbursement coverage; |
| • | price; and |
| • | patent position, including potentially dominant patent positions of others. |
As a result of the foregoing, our competitors may develop more effective or more affordable products, or achieve earlier patent protection or product commercialization than we do. Most significantly, competitive products may render any potential product candidates that we develop obsolete, which would negatively impact our business and ability to sustain operations. Although we believe that our approach holds the promise of achieving the desired safety and efficacy profiles for treatment of many neurodegenerative diseases, competitor companies may indeed demonstrate safety and efficacy of their competing products, as well as achieve a marketed product before we do.
To be successful, our potential product candidates must be accepted by the health care community, which can be very slow to adopt or unreceptive to new technologies and products.
Our potential product candidates and those developed by our collaborators, if approved for marketing, may not achieve market acceptance since hospitals, physicians, patients or the medical community in general may decide not to accept and utilize these products. The potential product candidates that we are attempting to develop represent substantial departures from established treatment methods and will compete with a number of conventional drugs and therapies manufactured and marketed by major pharmaceutical companies. The degree of market acceptance of any of our developed potential products will depend on a number of factors, including:
| • | our establishment and demonstration to the medical community of the clinical efficacy and safety of our potential product candidates; |
| • | our ability to create products that are superior to the alternatives currently on the market; |
24
Table of Contents
| • | our ability to establish in the medical community the potential advantage of our treatments over alternative treatment methods; and |
| • | reimbursement policies of government and third-party payers. |
If the health care community does not accept our potential products for any of the foregoing reasons, or for any other reason, our business would be materially harmed.
Because of the uncertainty of pharmaceutical pricing, reimbursement and healthcare reform measures, we or our licensees may be unable to sell our products profitably.
The availability of reimbursement by governmental and other third-party payers affects the market for any pharmaceutical product. These third-party payers continually attempt to contain or reduce the costs of healthcare. There have been a number of significant legislative and regulatory changes to the healthcare system enacted in recent years and further proposals and changes are likely. Medicare’s policies may decrease the market for our products. Significant uncertainty exists with respect to the reimbursement status of newly approved healthcare products.
In addition, third-party payers are increasingly challenging the price and cost-effectiveness of medical products and services. We might not be able to sell our products profitably or recoup the value of our investment in product development if reimbursement is unavailable or limited in scope, particularly for potential product candidates addressing small patient populations.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. We expect that there will continue to be a number of U.S. federal and state proposals to implement governmental pricing controls. While we cannot predict whether such legislative or regulatory proposals will be adopted, the adoption of such proposals could have a material adverse effect on our business, financial condition and liquidity.
On July 15, 2008, the Medicare Improvements for Patients and Providers Act of 2008 became law with a number of Medicare and Medicaid reforms to establish a bundled Medicare payment rate that includes services and drug/labs that are currently separately billed. The implementation of this program is currently not expected to begin until at least 2016. Bundling initiatives that have been implemented in other healthcare settings have occasionally resulted in lower utilization of services that had not previously been a part of the bundled payment.
Changes in government regulations or private third-party payers’ reimbursement policies may reduce reimbursement for our products and adversely affect our future results. In addition, when a new medical product is approved, the availability of government and private reimbursement for that product is uncertain, as is the amount for which that product will be reimbursed. We cannot predict the availability or amount of reimbursement for our potential product candidates.
The Patient Protection and Affordable Care Act (PPACA) substantially changes the way healthcare is financed by government and private insurers, encourages improvements in healthcare quality, and impacts the medical device industry. The PPACA includes an excise tax on entities that manufacture or import medical devices offered for sale in the United States; a new Patient-Centered Outcomes Research Institute to conduct comparative effectiveness research; and payment system reforms.
The PPACA also imposes new reporting and disclosure requirements on device and drug manufacturers for any payment or transfer of value made or distributed to physicians or teaching hospitals. Under these
25
Table of Contents
provisions, known as the Physician Payment Sunshine Act, affected device and drug manufacturers need to begin data collection on August 1, 2013, with the first reports due in 2014. These provisions require, among other things, extensive tracking and maintenance of databases regarding the disclosure of relationships and payments to physicians and teaching hospitals. In addition, certain states have passed or are considering legislation restricting our interactions with health care providers and/or requiring disclosure of many payments to them. Failure to comply with these tracking and reporting laws could subject us to significant civil monetary penalties.
The Health Insurance Portability and Accountability Act of 1996 (HIPAA) created new federal statutes to prevent healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs such as the Medicare and Medicaid programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines or imprisonment or exclusion from government sponsored programs. HIPAA also established uniform standards governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of individually identifiable health information maintained or transmitted by healthcare providers, health plans and healthcare clearinghouses.
Both federal and state government agencies are continuing heightened and coordinated civil and criminal enforcement efforts. As part of announced enforcement agency work plans, the federal government will continue to scrutinize, among other things, the billing practices of hospitals and other providers of healthcare services. The federal government also has increased funding to fight healthcare fraud, and it is coordinating its enforcement efforts among various agencies, such as the U.S. Department of Justice, the Office of Inspector General and state Medicaid fraud control units. We believe that the healthcare industry will continue to be subject to increased government scrutiny and investigations.
In addition, the Budget Control Act of 2011 mandates, among other things, reductions in Medicare payment rates if a sufficient deficit reduction plan is not approved and a reduction in funding for Medicare, Medicaid or similar government programs may adversely affect our future results. Economic pressure on state budgets may result in states increasingly seeking to achieve budget savings through mechanisms that limit coverage or payment for our drugs. In recent years, some states have considered legislation that would control the prices of drugs. State Medicaid programs are increasingly requesting manufacturers to pay supplemental rebates and requiring prior authorization by the state program for use of any drug for which supplemental rebates are not being paid. Managed care organizations and Accountable Care Organizations (ACOs) continue to seek price discounts and, in some cases, to impose restrictions on the coverage of particular drugs. Government efforts to reduce Medicaid expenses may lead to increased use of managed care organizations by Medicaid programs. This may result in managed care organizations and ACOs influencing prescription decisions for a larger segment of the population and a corresponding constraint on prices and reimbursement for our products.
In the European Union and some other international markets, the government provides health care at low cost to consumers and regulates pharmaceutical prices, patient eligibility or reimbursement levels to control costs for the government-sponsored health care system. Many countries are reducing their public expenditures and we expect to see strong efforts to reduce healthcare costs in international markets, including patient access restrictions, suspensions on price increases, prospective and possibly retroactive price reductions and other recoupments and increased mandatory discounts or rebates, recoveries of past
26
Table of Contents
price increases, and greater importation of drugs from lower-cost countries to higher-cost countries. These cost control measures could reduce our revenues. In addition, certain countries set prices by reference to the prices in other countries where our products are marketed. Thus, our inability to secure adequate prices in a particular country may not only limit the marketing of our products within that country, but may also adversely affect our ability to obtain acceptable prices in other markets. This may create the opportunity for third party cross border trade or influence our decision to sell or not to sell a product, thus adversely affecting our geographic expansion plans and revenues.
Our competition has significantly greater experience and financial resources.
The Biotechnology industry is characterized by intense competition. We compete against numerous companies, and many have substantially greater resources. Several of these companies have initiated cell therapy research programs and/or efforts to treat the same diseases that we target. Companies such as Neuralstem, Inc., StemCells, Inc., BioTime, Inc., and Genzyme Corporation, as well as others, may have substantially greater resources and experience in our fields that put us at a competitive disadvantage.
This is a best efforts offering, with no minimum, and there can be no assurance that the offering will result in any proceeds, or enough proceeds to continue to operate our business operations. We intend to use the proceeds from the sale of the securities, if any, to complete the pre-clinical studies required by the FDA in support of our anticipated Phase 1/2a clinical study and to conduct a Phase 1/2a clinical trial of our Q-Cells product in patients with Amyotrophic Lateral Sclerosis (ALS or Lou Gehrig’s disease). We plan to conduct the trial at Johns Hopkins University and Emory University and may include additional clinical trial sites. The goal of this trial is to generate clinical data read-outs beginning in 2014. Additionally, we intend to use some proceeds from this offering for working capital needs, capital expenditures, and other general corporate purposes to support the development of Q-Cells. Pending any ultimate use of any portion of the proceeds from this offering, we intend to invest the proceeds in a variety of capital preservation investments, including short-term, interest-bearing instruments, such as United States government securities and municipal bonds.
DETERMINATION OF OFFERING PRICE
The $ per unit consisting of [ ] share of common stock and a warrant to purchase up to [ ] shares of common stock was determined based on our internal assessment of what the market would support. There is no relationship whatsoever between this price and our assets, results of operations, book value or any other objective criteria of value.
A FINRA registered broker-dealer unaffiliated with the underwriter has applied to have our common stock quoted on the OTC Bulletin Board. We intend to file a registration statement under the Exchange Act concurrently with the effectiveness of the registration statement of which this prospectus is a part. If our common stock becomes quoted on the OTC Bulletin Board and a market for the stock develops, the actual price of stock will be determined by prevailing market prices at the time of sale or by private transactions. The offering price would thus be determined by market factors.
Our net tangible book value as of March 31, 2013 was approximately a negative $435,688 or a negative $0.018 per share, based on 24,786,833 shares of our common stock outstanding on that date. Net tangible book value per share is determined by dividing our total tangible assets (total assets less intangible assets), less total liabilities, by the number of shares of our common stock outstanding.
After giving effect to our assumed sale of all shares of our common stock in this offering at an assumed public offering price of $[ ] per share, our as adjusted net tangible book value as of March 31, 2013 would have been approximately $[ ], or $[ ] per share of common stock. This represents an immediate increase in net tangible book value of $[ ] per share to existing shareholders and immediate dilution in net tangible book value of $[ ] per share to new investors participating in this offering at the assumed offering price. The following table illustrates this dilution on a per share basis:
| Assumed public offering price per share |
$ | |||
| Net tangible book value per share as of March 31, 2013, before this offering |
($ | 0.018 | ) | |
| Increase in pro forma net tangible book value per share attributable to new investors |
$ | |||
| Net tangible book value per share as of March 31, 2013, after giving effect to this offering |
$ | |||
| Dilution per share to new investors |
$ |
The information above is as of March 31, 2013 and excludes:
| • | 3,865,440 shares of common stock issuable upon the exercise of stock options outstanding at July 5, 2013 with a weighted average exercise price of $0.20 per share; |
| • | 12,104,518 shares of common stock issuable upon the exercise of outstanding warrants at July 5, 2013 with a weighted average exercise price of $1.40 per share; and |
| • | 3,987,529 shares of common stock available for future grants under our 2012 Equity Incentive Plan at July 5, 2013. |
Under the terms and subject to the conditions contained in an underwriting agreement, dated the date of this prospectus, between us and MLV & Co., we have agreed to issue and sell to the public through the underwriter, and the underwriter has agreed to offer and sell up to [ ] units, each unit consisting of one share of our common stock, and one warrant exercisable for a quarter (.25) of a share of our common stock, on a best-efforts basis.
27
Table of Contents
The underwriting agreement provides that the obligation of the underwriter to offer and sell the units, on a best-efforts basis, is subject to certain conditions precedent, including but not limited to delivery of legal opinions. The underwriter is under no obligation to purchase any units for its own accounts. As a “best-efforts” offering, there can be no assurance that the offering contemplated hereby will ultimately be consummated. The underwriter may, but is not obligated to, retain other selected dealers that are qualified to offer and sell the units and that are members of the Financial Industry Regulatory Authority.
The underwriter proposes to offer the units to investors at the public offering prices less the underwriting discounts and commissions set forth on the cover of this prospectus. There is no arrangement for funds to be received in escrow, trust or similar arrangement.
The following table summarizes the compensation and estimated expenses we will pay:
| Per unit |
Total | |||||||
| Offering price |
$ | [ ] | $ | [ ] | ||||
| Underwriting commissions |
$ | [ ] | $ | [ ] | ||||
| Proceeds, before expenses, to us |
$ | [ ] | $ | [ ] | ||||
In addition, we estimate that our share of the total expenses of this offering, excluding underwriting commissions referred to above and payment of the underwriter’s expenses referred to below, will be approximately $[ ].
We have also agreed to pay the underwriter’s reasonable out-of-pocket expenses (including fees and expense of the underwriter’s counsel) incurred by the underwriter in connection with this offering up to [$ ].
As additional compensation to the underwriter, upon consummation of this offering, we will issue to the underwriter or its designees warrants to purchase an aggregate number of shares of our common stock equal to 1.0% percent of the number of shares of common stock covered by warrants issued in this offering, at an exercise price per share equal to $[ ] (the “Underwriter Warrants”). The Underwriter Warrants and the underlying shares of common stock will not be exercised, sold, transferred, assigned, or hypothecated or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the Underwriter Warrants by any person for a period of 180 days from the date of the underwriting agreement in accordance with FINRA Rule 5110. The Underwriter Warrants will be exercisable at an initial exercise price of $[ ] per share and expire on the [ ] anniversary of the date of issuance.
We have agreed to indemnify the underwriter against some specified types of liabilities, including liabilities under the Securities Act of 1933, as amended, or the Securities Act, and to contribute to payments the underwriter may be required to make in respect of any of these liabilities.
Each of our executive officers and directors, have agreed, subject to certain exceptions, not to dispose of or hedge any of our shares of common stock or securities convertible into or exercisable or exchangeable for common stock for 90 days after the date of this prospectus without first obtaining the written consent of MLV & Co. The 90-day “lock-up” period during which our executive officers and directors are restricted from engaging in transactions in our common stock or securities convertible into or exercisable or exchangeable for common stock is subject to extension such that, in the event that either (i) during the last 17 days of the “lock-up” period, we issue an earnings or financial results release or material news or a material event relating to us occurs, or (ii) prior to the expiration of the “lock-up” period, we announce that we will release earnings or financial results during the 16-day period beginning on the last day of the “lock-up” period, then in either case the expiration of the “lock-up” period will be extended until the expiration of the 18-day period beginning on the issuance of the earnings or financial results release or the occurrence of the material news or material event, as applicable, unless MLV & Co waives, in writing, such an extension.
28
Table of Contents
This is a brief summary of the material provisions of the underwriting agreement and does not purport to be a complete statement of its terms and conditions. A copy of the underwriting agreement will be filed with the SEC and incorporated by reference into the registration statement of which this prospectus forms a part. See “Where You Can Find More Information” on page 78 of this prospectus.
The transfer agent for our common stock to be issued in this offering is Continental Stock Transfer & Trust.
We intend to apply to list our common stock on the OTC Bulletin Board under the symbol “QCEL.”
The underwriter and its affiliates may provide from time to time in the future certain financial advisory, investment banking and other services for us in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, the underwriter and its affiliates may effect transactions for their own accounts or the accounts of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
The following description of certain matters relating to our common stock does not purport to be complete and is subject in all respects to the Delaware General Business Corporation Law and to the provisions of our Certificate of Incorporation (“Certificate of Incorporation”) and By-Laws (the “By-Laws”).
Common Stock
Our certificate of incorporation, as amended, authorizes the issuance of up to 100,000,000 shares of common stock, par value $0.0001 per share, and up to 10,000,000 shares designated as preferred stock, par value $0.0001 per share.
Common Stock Outstanding. As of July 5, 2013 there were 24,811,833 shares of our common stock issued and outstanding. All shares of our common stock currently outstanding are fully paid and non-assessable.
Voting Rights. Each share of stock shall entitle the holder thereof to one vote. Directors shall be elected by a plurality of the votes of the shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors. Any other action shall be authorized by a majority of the votes cast except where the General Corporation Law prescribes a different percentage of votes and/or a different exercise of voting power, and except as may be otherwise prescribed by the provisions of the certificate of incorporation and the Bylaws. In the election of directors, and for any other action, voting need not be by ballot. The Bylaws may be adopted, amended or repealed at any time by the unanimous written consent of the Board of Directors. Each of our directors will be elected annually by our stockholders voting as a single class.
Dividend Rights. Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of our common stock are entitled to receive dividends out of assets legally available at the time if, as and when declared by our board of directors.
29
Table of Contents
Preemptive Rights. Holders of our common stock are not entitled to preemptive rights and our common stock is not subject to redemption or conversion. There are no redemption or sinking fund provisions applicable to our common stock.
Rights upon Liquidation. Upon the liquidation, dissolution or winding-up of the Company, the holders of our common stock are entitled to share pro-rata in all assets remaining after payment of all our debts and other liabilities and the liquidation preferences of any outstanding preferred stock.
Listing. We intend to apply to list our common stock on the OTC Bulletin Board under the symbol “QCEL”.
Warrants
The following summary of certain terms and provisions of the warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by the provisions of the warrant, the form of which has been filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions of the form of the warrant for a complete description of the terms and conditions of the warrants.
Duration and Exercise Price . The warrants offered hereby will entitle the holders thereof to purchase up to an aggregate of 1,250,000 shares of our common stock at an initial exercise price of $[ ] per share of common stock. The warrants will be immediately exercisable and will expire on the fourth anniversary of the date of issuance. The warrants will be issued separately from the common stock included in the units, and may be transferred separately immediately thereafter. The warrants will be issued in certificated form only.
Exercisability . The warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise. A holder (together with its affiliates) may not exercise any portion of the warrant to the extent that the holder would own more than 4.9% of the outstanding common stock after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s warrants up to 9.9% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants.
Capital Adjustments. In the event of any capital adjustment, as described in the warrants and generally including any recapitalization of the company or reclassification of our common stock or merger or consolidation with or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, then upon any subsequent exercise of a warrant, the holder will have the right to receive as alternative consideration, for each share of our common stock that would have been issuable upon such exercise immediately prior to the occurrence of such transaction, the number of shares of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of our common stock for which the warrant is exercisable immediately prior to such event.
Transferability. Subject to applicable laws and the restriction on transfer set forth in the warrant, the warrants may be transferred at the option of the holder upon surrender of the warrant to us together with the appropriate instruments of transfer.
Listing. We do not intend to list the warrants on any securities exchange or other trading market.
Right as a Stockholder. Holders of the warrants will not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their warrants.
Call Right. The warrant may be redeemed prior to its expiration date, at the option of the Company, at a price of $0.001 per share, upon not less than 10 days prior written notice to the Holder notifying holder of the Company’s intent to exercise such right; provided, that no redemption may occur unless (i) the Company’s common stock has had a per share closing sales price of at least 150% of the warrant exercise price for ten (10) consecutive trading days and (ii) there is an effective registration statement covering the sale of the shares of common stock issuable upon exercise of the warrant. The warrant may be exercised by the holder, for cash, at any time after notice of redemption has been given by the company and prior to the time and date fixed for redemption.
Preferred stock
Our Certificate of Incorporation provides our Board of Directors with the authority to issue up 10,000,000 shares of undesignated Preferred stock, par value $0.0001 per share, and to determine or alter the rights, preferences, privileges and restrictions granted to or imported upon these shares without further vote or action by our shareholders. As of the date of this prospectus, no shares of Preferred stock have been designated and the Board of Directors still has authority to designate and issue up to 10,000,000 shares of Preferred stock in one or more classes or series.
Anti-Takeover Provisions of Delaware Law and Charter Provisions
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a publicly held Delaware corporation from engaging in a “business combination,” except under certain circumstances, with an “interested stockholder” for a period of three years following the date such person became an “interested stockholder” unless:
| • | before such person became an interested stockholder, the board of directors of the corporation approved either the business combination or the transaction that resulted in the interested stockholder becoming an interested stockholder; |
| • | upon the consummation of the transaction that resulted in the interested stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding shares held by directors who also are officers of the corporation and shares held by employee stock plans; or |
| • | at or following the time such person became an interested stockholder, the business combination is approved by the board of directors of the corporation and authorized at a meeting of stockholders by the affirmative vote of the holders of 66 2/3% of the outstanding voting stock of the corporation which is not owned by the interested stockholder. |
The term “interested stockholder” generally is defined as a person who, together with affiliates and associates, owns, or, within the three years prior to the determination of interested stockholder status, owned, 15% or more of a corporation’s outstanding voting stock. The term “business combination” includes mergers, asset or stock sales and other similar transactions resulting in a financial benefit to an interested stockholder. Section 203 makes it more difficult for an “interested stockholder” to effect various business combinations with a corporation for a three-year period. The existence of this provision would be expected
30
Table of Contents
to have an antitakeover effect with respect to transactions not approved in advance by our board of directors, including discouraging attempts that might result in a premium over the market price for the shares of common stock held by stockholders.
The ability of the board of directors to issue shares of preferred stock and to set the voting rights, preferences and other terms thereof, without further stockholder action, may be deemed to have an anti-takeover effect and may discourage takeover attempts not first approved by the board of directors, including takeovers which stockholders may deem to be in their best interests. If takeover attempts are discouraged, temporary fluctuations in the market price of our common stock, which may result from actual or rumored takeover attempts, may be inhibited. These provisions, together with the ability of our board of directors to issue preferred stock without further stockholder action could also delay or frustrate the removal of incumbent directors or the assumption of control by stockholders, even if the removal or assumption would be beneficial to our stockholders. These provisions could also discourage or inhibit a merger, tender offer or proxy contests, even if favorable to the interests of stockholders, and could depress the market price of our common stock. In addition, our bylaws may be amended by action of the Board of Directors.
31
Table of Contents
We undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this prospectus. Readers are urged to carefully review and consider the various disclosures made throughout the entirety of this prospectus, which attempt to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
Overview
Q Therapeutics, Inc. (hereinafter Q Therapeutics, Q, we, us, our and similar expressions) conducts its business and operations through its wholly owned subsidiaries, Q Therapeutic Products, Inc. and NeuroQ Research, Inc. Q Therapeutics is a Salt Lake City, Utah-based biopharmaceutical company that is developing human cell-based therapies intended to treat degenerative diseases of the brain and spinal cord, the primary components of the central nervous system (CNS). Incorporated in the state of Delaware on March 28, 2002, Q merged with Q Acquisition, Inc., a Delaware corporation and a wholly owned subsidiary of Grace 2, Inc., on October 13, 2011. On November 2, 2011, Grace 2 changed its name to Q Holdings, Inc., and on December 10, 2012, it changed its name to Q Therapeutics, Inc.
The technology upon which these potential therapies are based was developed by Q’s’ co-founder Mahendra Rao, M.D., Ph.D., a leader in glial stem cell biology, during his tenure at the University of Utah and as Head of the Stem Cell Section at the National Institutes of Health (NIH). Dr. Rao was one of the first scientists to identify and seek patent coverage on stem cells and their progeny cells found in the CNS. After licensing Dr. Rao’s technology from the University of Utah and NIH, Q commenced operations in the spring of 2004 to develop cell-based therapeutic products that can be sold as “off-the-shelf” pharmaceuticals. Q is managed by an experienced team of biotechnology executives with demonstrated start-up success and is advised by leaders in the neurology and stem cell therapeutics fields.
The proceeds from this offering, if any, will be used to fund completion of the pre-clinical studies required by FDA in support of our anticipated Phase 1/2a clinic study and to conduct a Phase 1/2a clinical study with Q’s proprietary Q-Cells® product in patients with Amyotrophic Lateral Sclerosis. We intend to conduct the trial at Johns Hopkins University and Emory University and it is intended to demonstrate the safety of Q’s product and measures of biological activity which can lead to surrogate measures of efficacy for the subsequent clinical trials.
Objectives of Q Therapeutics
Every year, hundreds of thousands of people suffer from debilitating and often fatal diseases of the brain and spinal cord. Q’s primary business objective is to develop and commercialize novel therapeutic products to treat these diseases as they represent areas of significant clinical need and commercial opportunity. Q is advancing its initial product, trademarked “Q-Cells®” to the market to treat Amyotrophic Lateral Sclerosis (Lou Gehrig’s Disease) and eventually other indications, potentially including Multiple Sclerosis, Transverse Myelitis, Spinal Cord Injury, Stroke, Parkinson’s disease and Alzheimer’s disease.
Initially, Q is targeting orphan diseases, where the Food and Drug Administration (FDA) allows applying for fast-track approvals and market exclusivity, and for which smaller, less-expensive clinical trials may be warranted. This approach can result in accelerated commercialization efforts while maintaining a financing approach focused on capital efficiency.
32
Table of Contents
The Problem of Neurodegenerative Diseases
Neurodegenerative diseases are by nature complex. For most such diseases, there is not just one ‘cause’ nor just one ‘disease’; there can be multiple different causes with many factors and pathways contributing to the many disease symptoms. Despite much effort by the pharmaceutical/biotechnology industry, current approaches have not been effective. Traditional drugs have focused on single disease pathways. Due to the multi-factorial nature of neurodegenerative diseases, these drugs tend to fail to treat the nerve damage caused by these diseases as they do not fully address all of the disease factors or pathways. As a result, patients suffering from these diseases can, in many cases, only hope to slow the inexorable disease progression and the associated disabilities. We believe that a worldwide market measured in the tens of billions of dollars awaits those who are successful in addressing this substantial medical need.
Q’s Approach to the Problem
A new approach is needed for treating the multimodal nature of neurodegenerative disease. Q has identified and patented a new treatment modality that provides a multi-factorial approach, bypassing the need to address individual pathways. Specifically, Q is developing a cell-based therapeutic that employs a natural cell type found in the healthy CNS: human glial restricted progenitors and their progeny cells. We call them, Q-Cells®. Q-Cells produce astrocytes and oligodendrocytes, the support cells that enable the normal function of neurons. We believe that Q-Cells may provide multiple and complementary mechanisms of action in the treatment of many neurodegenerative diseases. An advantage of this approach is that one need not fully understand the mechanistic aspects of these diseases, as we are tapping into the natural cellular machinery that enables healthy systems to function. We believe this is a more realistic approach than seeking a drug that affects a single disease pathway, although the addition of Q-Cell therapy may in fact enhance benefits seen with such single drugs. Based on data in animal models, we believe that repairing damaged neurons is a faster, more realistic and easier approach than trying to replace them. In addition to using its proprietary cellular technology in a therapeutic mode, Q might also evaluate novel ways to use Q-Cells to screen for new drugs, such as small molecule compounds, that could also provide treatments for neurological diseases.
Q’s Initial Proprietary Product - Q-Cells; Summary of Pre-clinical Studies
Most cells in the brain and spinal cord are glial cells, which protect the health and function of neurons. Glial cells form an insulating “myelin sheath” around neurons, provide the necessary growth factors needed to maintain a healthy nervous system and remove toxic compounds. Disease arises when glial cells are damaged or destroyed, causing neurons to malfunction and eventually die. Q-Cells technology aims to treat neurodegenerative diseases by providing new, healthy glial cells to help maintain and/or restore neuron function to a more robust state.
Q-Cells’ actions are multimodal in that they:
| • | Produce needed growth factors and nutrients as well as remove toxic compounds; and |
| • | Form the insulating myelin layer around the axons of neurons, enabling their normal function for signal transmission, |
Q-Cells accomplish these functions in a process that is unique to stem and progenitor cells. They are administered into and act locally in brain or spinal cord, where they differentiate into the two mature glial cell subtypes found in the brain and spinal cord:
| • | Oligodendrocytes, which provide the myelin “insulation” around the axons of neurons required for proper signal transmission by the conducting neurons as well as provide nutritional support, |
| • | Astrocytes, which provide support to neurons through production of essential growth factors and other trophic support mechanisms as well as removal of destructive toxins (Figure 1). |
33
Table of Contents
This technology aims to restore health and function to neurons prior to their death by using the natural support mechanisms in the CNS, rather than by replacing the neurons. No current treatment can achieve this outcome.
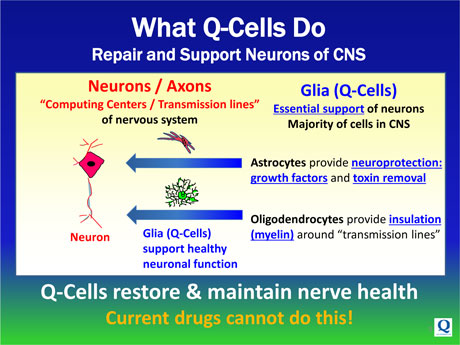
Figure 1. Most cells in the brain and spinal cord are glial cells that provide support function for the neurons. Q-Cells support neurons, which Q believes is a more straightforward task than replacing neurons.
Benefits and features of Q-Cells
Advantages of Q-Cells, as demonstrated in animal models, include:
| • | Q-Cells can provide important growth factors and other trophic functions that support neuronal health, which may provide treatment options for diseases directly involving neuronal degeneration such as ALS and Parkinson’s disease. |
| • | Q-Cells can provide myelin repair functions for diseases or injuries involving demyelination such as MS, Transverse Myelitis, Cerebral Palsy and Spinal Cord Injury. |
| • | When placed in the CNS, Q-Cells predictably replicate, migrate, and terminally differentiate into physiologically relevant glia cells: oligodendrocytes and astrocytes. |
| • | Q-Cells don’t give rise to appreciable numbers of neurons, reducing potential for unwanted effects due to aberrant neuronal connections. |
| • | The isolation process is readily scalable to good manufacturing practices (GMP). |
| • | Tissue-sourced Q-Cells spend little time in culture prior to freezing of the cells to produce the product for shipment to treatment sites, providing advantages in production. |
Many normal CNS cells remain viable throughout a person’s life. Our clinical trials are intended to ascertain whether transplanted Q-Cells function for significant periods of time, or if periodic (e.g., annual) treatments will be more appropriate. This replacement therapy will build on the model of successful bone marrow transplants, wherein stem cells from donor bone marrow replace diseased blood cells. However, unlike bone
34
Table of Contents
marrow transplants, Q-Cells will be banked in vials containing the cells and shipped frozen to hospitals and clinics. Thus, Q plans to sell products following the model of a traditional specialty pharmaceutical company in that Q-Cells will be prepared and inventoried in advance, and will not be generated uniquely for each patient.
The CNS has a degree of immune privilege, and Q plans to initially use marketed immunosuppressants for a transient period, to mitigate potential for immune rejection associated with transplants.
Q-Cells are late-stage, lineage-restricted human progenitor cells. In contrast, earlier-stage CNS stem cells (neural stem cells or neuroepithelial precursor cells, see Figure 6) can produce neurons as well as glial cells. This less-restricted differentiation potential can produce neurons inappropriately, possibly forming aberrant synaptic connections that lead to neurological complications. Q has confirmed that terminal differentiation of Q-Cells in animal models is naturally restricted to astrocytes and oligodendrocytes.
All of the factors cited above lead us to believe that Q-Cells represent a desirable cell therapy approach to neurodegenerative disease.
The Science behind Q-Cells, Pre-clinical Results and Their Application to Disease
During normal development, glia are formed by a progenitor cell called the glial-restricted precursor (GRP). Q has trademarked the human GRPs, together with their progeny, under the name Q-Cells®. Q-Cells have been shown to naturally replicate, migrate and differentiate into mature glial cells in their native CNS environment. The resulting differentiated cells (astrocytes and oligodendrocytes, shown in Figure 2) then perform the essential support functions described above. Q Therapeutics is taking advantage of Q-Cells’ natural abilities to follow the local molecular cues present in the brain and spinal cord, and intends to transplant Q-Cells into patients to treat diseases where glia are defective.
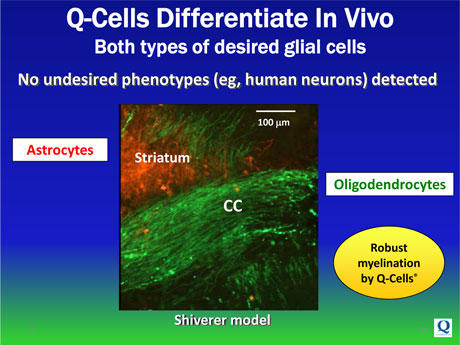
Figure 2. Q-Cells, when implanted into the brains of newborn “shiverer” mice, differentiate into both types of glial cells: oligodendrocytes (green stain for myelin basic protein [MBP]) and astrocytes (red stain for human glial fibrillary acidic protein [GFAP]). Shiverer mice are genetically engineered to be born with defective myelin.
35
Table of Contents
A. Diseases Involving Myelination
In demyelinating diseases such as Multiple Sclerosis and Transverse Myelitis, an inflammatory attack damages the oligodendrocytes, resulting in the loss of the insulating myelin sheath (i.e. demyelination); this is the primary pathology that causes loss of proper neuronal function and can lead to neuron death. Q-Cells follow natural cues to replace the missing myelin on damaged neurons, providing restorative therapy for demyelinating diseases. Other drugs have been unable to achieve this result.
Windrem et al (Cell Stem Cell, published 2008) and Walczak with his colleagues at Johns Hopkins University (2012, unpublished data) demonstrated widespread migration of implanted human GRPs/Q-Cells and robust remyelination in the brains of “shiverer” mice (Figure 3), which are genetically engineered to be born with defective myelin. This treatment led to a substantially prolonged life span in a subset of treated mice, with many mice ‘cured’ of the widespread dysmyelination in their brains.
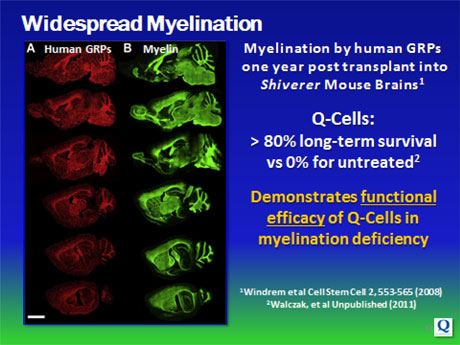
Figure 3. Transplanted human GRPs differentiate into myelinating oligodendrocytes in the shiverer mouse model. Human GRPs were implanted into brains of newborn shiv/rag2-/- mice and analyzed 12 months post-transplantation. Panel A shows the presence of human cells (stained in red) throughout the brain in treated mice, and Panel B shows the presence of myelin (green) formed by the resulting human oligodendrocytes. Shiverer mice lack the ability to form functional myelin, and untreated mice were all dead within five months. The human GRPs or Q-Cells resulted in functional benefits and mice that survived long-term were essentially cured.
Sandrock et al (Regenerative Medicine, 2010) showed engraftment, migration, integration and differentiation of Q-Cells into myelin-forming oligodendrocytes and astrocytes as well as safety (no tumor formation) when implanted into newborn shiverer and shiverer/rag2-/- mice. Walczak et al (Glia, 2011) showed safety (no tumors), extensive migration, expansion into spinal cord lesions, and adoptation of a mature glial phenotype (in shiv/rag2-/- mice), and preservation of electrophysiological conduction across the spinal cord in rats with focal (inflammatory) spinal cord lesions. Jin et al (Journal of Neurotrauma, 2011) (Figure 4) working in rat models of traumatic spinal cord injury, showed that Q-Cells reduced cyst and scar formation at the injury site (cysts and scars can prevent healing and repair), and had beneficial effects on bladder function and did not increase sensitivity to pain (safety).
36
Table of Contents
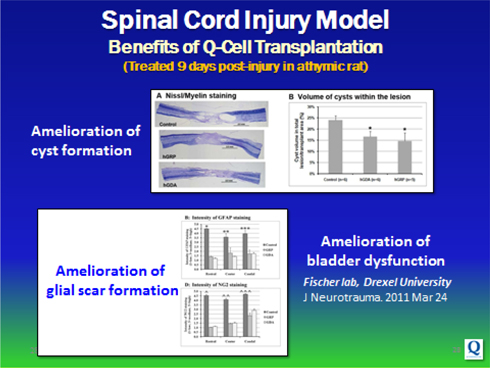
Figure 4. Benefits of Q-Cell transplantation in models of traumatic spinal cord injury. Q-Cells were transplanted into the thoracic spinal cords of athymic rats 9 days after a contusion spinal cord injury. Top graph, Q-Cells resulted in amelioration of cyst formation at the injury site. Bottom graph, Q-Cells reduced formation of glial scars at the injury site. Q-Cells also reduced bladder dysfunction (see figure in the publication.)
B. Neurodegeneration and Amyotrophic Lateral Sclerosis (ALS, also known as Lou Gehrig’s Disease)
Neurodegenerative diseases are characterized by the death of neurons, generally due to the lack of supporting functions that healthy glial cells provide. These diseases include Parkinson’s disease, Alzheimer’s disease and ALS. ALS is a neurodegenerative disease characterized by the progressive deterioration and loss of motor neurons resulting in muscle atrophy, progressive weakness, paralysis and ultimately death. Most ALS cases have no apparent hereditary basis; of the approximately 10% of cases that are inherited, more than a dozen different genetic mutations have been implicated. The precise mechanism(s) underlying the disease is not understood, although hypotheses include glutamate excitotoxicity, mitochondrial dysfunction, protein aggregation, aberrant RNA metabolism and protein trafficking defects. This list and the varied presentation of disease have led to the idea that multiple pathways are involved in ALS, and that therapies focusing on a single target or pathway are likely to be of limited utility in disease amelioration. In contrast, a cellular therapeutic with multimodal activities targeting the underlying support of motor neuron health on several levels may provide neuroprotection and slow or halt disease progression, and potentially even improvement.
Studies in mouse models of ALS suggest that abnormalities in astrocytes and microglia, in addition to motor neurons, play a role in disease onset as well as progression. Healthy astrocytes play a prominent role in CNS health, including regulation of extracellular ionic environment, providing critical amino acids and metabolites to neurons (lactate, glutamine), and regulating glutamate neurotransmission. Loss of the glutamate transporter in astrocytes is common to both genetic and sporadic ALS in humans. In both ALS patients and in animal
37
Table of Contents
models, there is abundant evidence that astrocyte abnormalities and physiological dysfunction can be seen many months before motor neuron degeneration and precede clinical disease [literature references include Clement et al, Science 302, 113-117 (2003); Rothstein et al Neuron 18, 327-338 (1997); Lepore et al, Nature Neuroscience 11, 1294-1301 (2008); Yamanaka et al, Nature Neuroscience 11, 251-253 (2008); Wang et al, Human Molecular Genetics 20, 286-293 (2011)]. Further, the Maragakis lab recently demonstrated that transplanting rat astrocyte precursors carrying the SOD mutation, which gives rise to ALS in people who have this mutation, into healthy rats results in degeneration of motor neurons in the previously healthy rats [Proc Natl Acad Sci on line edition Oct 2011 10.1073 (2011)]. Together, these data are consistent with the notion that diseased astrocytes in ALS (regardless of the cause of ALS) have a role in motor neuron pathology and death, while healthy astrocytes can be protective of diseased motor neurons. This altered physiology of CNS astrocytes contributes to disease progression by resulting in further susceptibility to motor neuron loss. Supplementing diseased astrocytes with healthy cells is a promising approach for slowing and/or halting the course of ALS.
While the role of astrocyte dysfunction in ALS has been established, the role of oligodendrocytes has only begun to be appreciated. The lactate transporter (SLC16A1, expressed in oligodendrocytes) has been shown to be reduced in spinal cord of ALS patients and in mouse models of ALS. Given that oligodendrocytes appear to be the main supplier of energy-rich lactate to nerve cells, treatments that boost numbers of oligodendrocytes which can deliver this critical energy-rich metabolite may slow the progression of the disease (Lee et al., Nature, July 2012). Further, selective removal of mutant SOD1 from oligodendrocytes delayed disease onset and prolonged survival in SOD1 mice and demyelination is seen in gray matter in ALS mice and men (Kang et al., Nature Neuroscience, March 2013). Together, these findings are consistent with the notion that oligodendrocyte dysfunction also can contribute to ALS.
The involvement of glial dysfunction in motor neuron loss in ALS forms the basis for the therapeutic approach of introducing healthy glia via transplantation of glial restricted progenitors (Q-Cells) into ALS patients to slow and/or halt disease course. Q-Cells may be able to functionally replace diseased host glia (both astrocytes and oligodendrocytes), thereby reducing motor neuron death and slowing or halting disease progression. The glutamate transport capacity of Q-Cells may not be the only mechanism for neuroprotection; production of growth factors; lactate transport and trophic factor delivery may also play a protective role. Glial supplementation may positively affect multiple pathways of natural motor neuron support and could therefore represent an effective cell therapy approach to ALS.
Dr. Maragakis and his colleagues have demonstrated the validity of this hypothesis, showing therapeutic benefits following transplantation of healthy rat GRPs into the SOD1 rat model (Lepore et al, Nature Neuroscience 11, 1294-1301 (2008)). Administration of rat GRPs (the rat cells homologous to human Q-Cells) into the cervical spinal cord after onset of disease enhanced survival and motor function.
38
Table of Contents
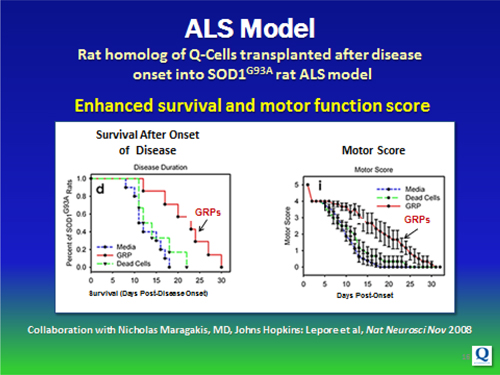
Figure 5. Beneficial effects of GRPs in rat model of ALS. Healthy rat GRPs were transplanted into the cervical spinal cords of the rat SOD1 model of ALS after symptoms of disease onset. Left graph: Survival after disease onset approximately doubled in the group treated with rat GRPs. Right graph: Motor score in the forelimbs increased significantly in rats treated with rat GRPs.
The above study in the rat ALS model (Figure 5), which targeted cervical motor neurons, documented that following GRP transplantation in this model of ALS, sparing of the motor neurons that innervate the diaphragm (the phrenic nerve) and hence control respiratory function occurred. Further, GRP transplantation resulted in lifespan extension and improvement in grip strength. Importantly, these pre-clinical animal experiments have demonstrated clinically relevant improvements in diaphragmatic electrophysiology, resulting in the maintenance of diaphragmatic function. Using the same transplantation paradigm, robust survival and wide spread distribution of Q-Cells has been demonstrated following transplantation into the cervical spinal cord of SOD1 mice. In these studies, Q-Cells differentiated into mature glial cells but not unwanted neurons, one demonstration of safety (Lepore et al. 2011).
Initial Clinical Trial Plans in ALS patients
In January 2012, we held a productive pre-IND meeting with the FDA to discuss Q-Cells for the treatment of ALS during which we discussed pre-clinical requirements, cell manufacturing and clinical trial design and protocol. The FDA is supportive of our approach and required that we complete definitive GLP safety studies in preparation for our first in man clinical trial. In December 2012, we initiated these studies to demonstrate the local biodistribution of Q-Cells within the CNS and the lack of tumor formation. Final data from these studies is expected during Q4 2013. To date, we have not seen any safety issues associated with Q-Cells. We plan to complete all requisite GLP pre-clinical safety and device studies during the second half of 2013 with the intention of submitting our IND to FDA shortly thereafter.
The initial clinical sites for our planned Phase 1/2a study include Emory University School of Medicine and Johns Hopkins University School of Medicine, both institutions with a leadership position in ALS clinical research.
We anticipate commencing enrollment in our Phase 1/2a clinical study in 12-15 ALS patients during the first half of 2014. It is anticipated that the two site trial will be completed in 18-24 months pursuant to the initiation of enrollment, pending a favorable and timely review by the FDA. The end points of the Phase 1/2a clinical trial are safety of Q-Cell administration as a primary measure, with surrogate measures of biological activity and efficacy as secondary endpoints. The funding we receive from this financing will be used to complete the studies required by the FDA for IND submission and to conduct a Phase 1/2a.
Upon completion of the initial Phase 1/2a portion of the trial, we plan to meet with the FDA to discuss an extended Phase 2a trial consisting of 20 additional patients. We have ongoing discussions with investigators at the University of California at San Francisco and the University of California at San Diego who have demonstrated interest in participating in an expanded trial.
We have designed this trial in close consultation with the FDA. It is based upon a proposed Mechanism of Action (MOA) of Q-Cells. Both oligodendrocytes and astrocytes may be expected to contribute to the MOA which could be local (i.e. cell-cell mediated; graft-host) and/or could be global (release of soluble factors, “conditioning, filtering, & cleansing” of CSF). Focal treatment in the region of the cervical spinal cord will target the muscles of the arm and the diaphragm, and we will monitor measures of the biological effect of these muscle groups (e.g., local muscle integrity, (electrical impedance myography), muscle strength (hand held dynamometry), and respiratory function (forced vital capacity), as well as overall physical condition and function (ALSFRS-R). However, we plan to also obtain measures of muscle integrity in distal muscle groups not innervated by the neurons in the locally treated area in order to begin to understand if and how the allograft is functioning, e.g. are growth factors released in the cerebral spinal fluid that may provide benefits to motor neurons originating in other regions of the spinal cord. Additionally, we intend to investigate imaging modalities to look for biological changes such as neuronal integrity, myelination and transplant integration.
39
Table of Contents
Subsequent Clinical Trials
We believe our proprietary Q-Cells will have broad applicability across myriad neurodegenerative diseases and injuries of the CNS. Potential therapeutic indications for our Q-Cells include Amyotrophic Lateral Sclerosis (ALS or Lou Gehrig’s disease), Transverse Myelitis, Multiple Sclerosis, Spinal Cord Injury, Stroke, Parkinson’s and Alzheimer’s disease.
Following the initiation of our clinical trial with Q Cells in ALS, we plan to pursue additional orphan indications for Q-Cells that demonstrate their inherent broad capability and multiple mechanisms of action. These indications include transverse myelitis and spinal cord injury. This approach can result in accelerated commercialization efforts while maintaining a financing approach focused on capital efficiency. We believe that following this strategy will lead to partnerships with larger biotechnology, pharmaceutical, or cell therapy companies for larger indications including multiple sclerosis, stroke, Parkinson’s and Alzheimer’s disease where larger clinical trials and more substantial sales and marketing programs are necessary to access the markets. In addition, we intend to pursue improvements in manufacturing processes that lead to larger production runs as well as derivation of our product from multiple tissue sources. These efforts should, in turn, lead to more efficient and diversified manufacturing capabilities for Q Therapeutics and allow for deeper penetration of both existing and new markets.
Q-Cells - Benefits of Localized Therapy
The ability of animal results to predict human results, and accordingly the drug development risk profile, depends on many factors including:
(1) The pharmacokinetic and metabolic profiles of a drug in animals vs. humans, which can greatly influence both efficacy and safety;
(2) How the drug affects the target diseased and disease-causing tissues; and
(3) What the drug does to non-target cells.
Many drug candidates fail in development because they affect not only their intended target but have toxic off-target effects in other organ systems, as well. For example, a drug intended to treat headache pain may fail to become a commercial product due to unacceptable toxic systemic effects in other organs, e.g., heart or kidneys. Q-Cells are a localized treatment in the CNS. Studies conducted by Windrem et.al. (Cell Stem Cell 2, 553-565(2008)) indicate that human GRPs stop at the boundary where the CNS meets the peripheral nervous system. Q anticipates that this localized nature of the planned Q-Cell therapy may reduce risk from systemic toxicity, which Q believes may reduce the risk of drug failure in early clinical trials due to systemic toxicities. Additional animal safety studies are being conducted to establish safety both locally in the spinal cord and brain as well as systemically before initiating clinical trials.
Validation of Q’s technology and approach
Q’s technology, approach and progress have been validated by several outside groups. In 2008, Invitrogen, Inc. (now Life Technologies, Inc.) conducted diligence on Q prior to making an investment of $2.6 million. In 2009, Q received confirmation of notice of award of a $4 million NIH grant, which involved review by top scientists in the neurology regenerative medicine and ALS areas (at Harvard University, Columbia University, University of Wisconsin). To obtain each subsequent year of grant funding, NIH reviewed and confirmed the progress on developmental milestones. In 2011, Cephalon (now Teva), did extensive diligence prior to making an investment of $3.7 million into Q, following their 2010 investment in Mesoblast, another cell therapy company. Cephalon/Teva also entered into an agreement with the Company for a one-time limited right of first negotiation to one indication for Q-Cells. Once triggered, if a definitive agreement is not reached within certain time periods, this right is terminated. The quality of our academic collaborations also attests to what we have been able to accomplish in our development of Q-Cells. Finally, the granting of multiple patents on our intellectual property validates the inventiveness and provides further value to the company.
The funding received from these sources has enabled Q to characterize and develop manufacturing protocols for Q-Cells, transferring them to a GLP/GMP manufacturing facility, obtain animal data in multiple models of neurodegenerative disease (diseases of myelination- multiple sclerosis, transverse myelitis and general dysmyelination; spinal cord injury, ALS), generate much of the pre-clinical data package that will support IND filing, and obtain numerous patents.
40
Table of Contents
Q Therapeutics’ Collaborative Business Model
Q Therapeutics uses a collaborative business model to develop its products. Q believes this type of business model to be capital efficient while allowing it to augment its own expertise and capabilities with those of its development partners at the time that outside resources are needed. Q’s collaborative business model includes utilizing third parties for certain functions and outsourced services. For example, Q utilizes outside collaborators and contractors to:
| • | Leverage its research and development resources by forming collaborations with outside scientists specialized in our areas of interest; |
| • | Contract Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) manufacturing to facilities specialized in such production; |
| • | Utilize experienced regulatory consultants to work with the FDA; |
| • | Contract safety/toxicology studies to qualified GLP labs; |
| • | Conduct the clinical trials with physicians and institutions with relevant experience; and |
| • | Enter into pharmaceutical company collaborations to maximize product sales. |
Collaborations and Grant Funding
Q Therapeutics pursues opportunities to obtain grant funding from government and private organizations when such grants are aimed at an area that augments Q’s internal development efforts. Generally, we seek opportunities that have the potential to provide funding over several years and that can advance programs into clinical development. We normally seek qualified academic collaborators to work with us on these programs to complement our internal developmental expertise. Grant funding is very competitive to obtain, and it is normal to apply for many grants for each one funded. As applying for grants requires expenditure of internal resources, Q is selective as to which grant opportunities it will pursue that are aligned with our corporate goals. To date, Q has been the beneficiary of:
1) Two SBIR Phase 1 grants (~$120,000 each);
2) A 4 year, $4 million (in direct costs) grant from National Institutes of Health (NIH) to help fund the pre-clinical studies of Q-Cells to result in submission of an IND to treat ALS patients;
3) An $850,000 grant from the Maryland Stem Cell Fund, to help fund pre-clinical studies with Johns Hopkins on Q-Cells for treatment of ALS; and
4) A $250,000 grant from the Neilsen Foundation to fund studies of Q-Cells in traumatic spinal cord injury.
Q Therapeutics works in conjunction and maintains collaborations with the highly respected laboratories of Dr. Nicholas Maragakis, Dr. Piotr Walczak and Dr. Itzhak Fischer. The collaborations with these laboratories do not involve any milestone or royalty payments or any form of agreement for compensation of any kind. Material transfer agreements cover the supply and use of cells from Q to the collaborators. To date, most of their research has been funded through grant funding provided by the NIH, Maryland Stem Cell Fund, and the Neilsen Foundation.
The NIH grant that has funded the collaboration with Dr. Maragakis’ laboratory is in its 3rd year, with renewal for the 4th pending achievement of certain milestones. Approximately $800,000 to $900,000 in direct costs was funded in each of year one and two, with a year three award of $1.0 million for direct costs. Year three funding will be primarily devoted to Q-Cell production at the University of Utah, antibody production at Goodwin Biotechnology, Inc., and GLP safety studies at MPI Research to support the Company’s IND submission for ALS.
41
Table of Contents
Material Terms of the NIH Grant
The NIH grant is intended to fund work leading to an IND submission for use of Q-Cells in the treatment of ALS. The grant was issued directly to our collaborators and covers costs that would otherwise be borne by Q, including our collaboration with Dr. Maragakis’ laboratory, the University of Utah, MPI Research and Goodwin Biotechnology. In December 2012, the Company received a sub-award of $631,383 under the grant for the 2012-2013 plan year. As of March 31, 2013, $483,303 has been invoiced of which $481,469 has been paid.
Q Therapeutics has the proprietary rights to the cells that are the subject of all of these grants.
Agreement Terms of Maragakis Collaboration
With respect to the Maragakis collaboration, Q provides technical expertise as well as product for testing and actively assists with experimental design and analysis. The Maragakis Laboratory provides its technical expertise and carries out experiments as mutually agreed upon with Q Therapeutics. Q maintains all rights to the Q-Cells product. Upon the development of any new intellectual property in which technologies outside of Q-Cells are involved, Q Therapeutics has the right of first negotiation to obtain an exclusive license on such innovation with Johns Hopkins University.
Future Collaborations with Third Parties
We are in discussions with third parties about collaborating in the development of our Q-Cell® product and may, under the right terms, enter into one or more partnership(s) to advance the program.
Manufacturing
Q Therapeutics has developed manufacturing protocols to isolate Q-Cells directly from somatic or fetal cadaver tissue, without the need for additional differentiation in vitro. Q has developed proprietary methods to expand the cells at this stage to enable treatment of many patients from each preparation. Q’s strategy is to start with the most straightforward path: isolate and characterize unmodified cells that are already, naturally, at the desired stage of differentiation, to achieve proof of activity in clinical trials. Q believes that this strategy enables development of Q-Cells and other cellular therapeutics in the most cost and time-effective manner.
Q Therapeutics will use a centralized laboratory (contract GMP cell production manufacturer) for cell isolation and expansion and to provide the necessary quality control. The clinical transplant sites will receive frozen cells, which they will subsequently thaw, wash and inject into the target site of the patient. Use of allogeneic cells (i.e., cells derived from tissue and not obtained from the patient), rather than autologous cells (cells derived from the patient’s own tissues) both enables use of what Q believes may be the most effective healthy cells as well as permits cell therapy to be an off-the-shelf treatment, promoting more widespread use. The manufacturing of the Q-Cells is done via a proprietary process developed by Q. This process can be shared with Q’s contract manufacturer of choice, or with several manufacturers to mitigate the risk of loss of any one manufacturing subcontractor.
Q Therapeutics is currently working with the GLP/GMP Cell Therapy & Regenerative Medicine Facility (CTRM) operated by the University of Utah. Q Therapeutics may seek other manufacturers as its development programs progress as well as once Q-Cells have been approved for commercial sale.
Manufacturing Agreement with the University of Utah
In December 2011, Q Therapeutics entered into a service and supply agreement with the University of Utah for an initial period of five years, with an option to renew the agreement on an annual basis. The basic terms of the agreement stipulate the pricing for the manufacturing and processing of batches of cells and the index
42
Table of Contents
to be used for any price modifications, the granting of non-exclusive rights to the CTRM work product, and a non-exclusive, non-transferable and royalty-free license to the University of Utah to manufacture Q-Cells for use by Q and by collaborators specified by Q, with payment of manufacturing costs to the University of Utah. Either party can terminate the agreement upon appropriate notice.
Manufacturing Agreement with Goodwin Biotechnology
Q Therapeutics has an agreement with Goodwin Biotechnology (GBI) that specifies the contract research to achieve GMP production and conjugation of the antibody used in cell purification for manufacture of Q-Cells. Q maintains all rights to the products. The Agreement with GBI, which sets out obligations of GBI to provide process development and GMP manufacture and stability studies of the antibody originally provided by Q, is close to completion with stability studies ongoing. Q Therapeutics can terminate the project at any time, subject to a cancellation penalty.
Q-Cells Can Be Obtained from a Variety of Sources
Q-Cells can be obtained from a variety of sources (Figure 6), including: (i) from somatic tissue (termed adult stem cells, isolated from fetal or adult tissue), (ii) differentiated from CNS stem cells [neuroepithelial stem cells (NEP) or neural stem cells (NSC)] which can be isolated from somatic tissue or derived from pluripotent cells), or (iii) differentiated from pluripotent cells (e.g., embryonic stem cells (ESC) or other pluripotent cells such as induced pluripotent stem cells (iPSC)). Q Therapeutics has intellectual property (IP) covering multiple sources, giving it a broad IP position. Because of this broad IP position, Q has the ability to follow a development path that enables cost and time-efficient development of its cellular therapeutics. For these reasons, Q has chosen to develop its initial Q-Cells product by isolating the cells directly from somatic (fetal brain) tissue, followed by expansion in the production facility, as this provides cells that are at the desired cell type without the need for additional in vitro differentiation.
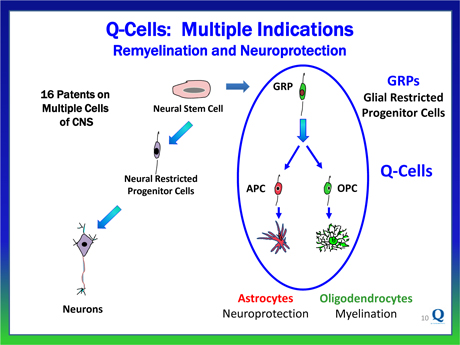
Figure 6. Stem and progenitor cells of the brain and spinal cord. Stem cells can be derived from pluripotent stem cells (e.g., ESCs or iPSCs) or isolated from somatic tissue sources (called Adult Stem Cells).
43
Table of Contents
Q Therapeutics’ strategy is to start with the more straightforward path: isolate and use unmodified cells that are already, naturally, at the desired cell type. Q believes that the restricted fate of Q-Cells lowers the chance of unwanted side effects, such as inappropriate production of neurons. NRP = neuronal restricted precursor, GRP = glial restricted precursor, APC = astrocyte precursor cell, OPC = oligodendrocyte precursor cell.
Q Therapeutics’ Intellectual Property
Q Therapeutics has exclusive worldwide rights to its Q-Cells product, either through an agreement with the University of Utah Research Foundation (UURF or the Foundation) or through owned, internally developed intellectual property. The Q patent portfolio encompasses five families of neural lineage progenitor or stem cell technologies. Currently, our patent portfolio includes 16 issued patents plus U.S. and international patents pending encompassing composition of matter, methods of production and methods of use of multiple cell types of the CNS (including Q-Cells) as well as some of the peripheral nervous system, giving us a strong intellectual property position for Q-Cells as well as other neural lineage cells. Some of our patents are the subject of a license agreement with the University of Utah and the NIH. This license provides for royalties on Q Therapeutics’ and sublicensees’ sales and contains due diligence obligations and related provisions; a portion of the consideration to the University of Utah was equity in Q. In addition, Q has exclusive rights to internally developed patent applications on its glial cell products, as well a non-exclusive license to two patents on the delivery device.
Terms of License Agreement with the University of Utah Research Foundation
The License Agreement (the “Agreement”) with the Foundation obligates Q Therapeutics to diligently proceed with development, manufacture, sale and use of licensed products through the term of the Agreement.
Pursuant to the Agreement, Q Therapeutics has financial obligations to pay milestones to the University of Utah. More specifically, approximately $1.3 million in total milestones will be due to the Foundation for each product that receives market approval, with most of the payment weighted towards approval. Additionally, the Foundation is entitled to royalties resulting from the sale of products designated for research or human therapeutic use.
To date, less than $1,000,000 has been paid in aggregate pursuant to these contractual payment obligations. The term of the Agreement spans the life of the patents. The Foundation can terminate the Agreement for uncured default after a 30-day notice. Q Therapeutics may terminate the Agreement at any time without cause upon 90 days’ notice.
Trademarks
Q also holds a U.S. Trademark to its first product name, Q-Cells (serial number: 78869175; registration number: 3,385,490), and U.S. and International Trademarks/Service marks to: Q Therapeutics (U.S. serial number: 78-415,125; U.S. registration number: 3,280,432; international registration number: 867,474).
Patents and Patent Applications
Q’s issued/granted patents and patent applications that are licensed or owned by Q as of December 31, 2012, include the following:
Common Neural Progenitor for the CNS and PNS
Mahendra S. Rao, Tahmina Mujtaba
ISSUED U.S. Patent 6,830,927
44
Table of Contents
Generalization, Characterization, and Isolation of Neuroepithelial Stem Cells and Lineage Intermediate Precursor
Mahendra S. Rao, Margot Mayer-Proschel, Tahmina Mujtaba
PCT/US98/ 09630, Filed 5/7/98, published PCT application
Isolation of Lineage-Restricted Neuronal Precursors
Mahendra S. Rao, Margot Mayer-Proschel
ISSUED U.S. Patent 6,734,015
Lineage-Restricted Neuronal Precursors
Mahendra S. Rao, Margot Mayer-Proschel and Anjali J. Kalyani
ISSUED U.S. Patent 6,787,353
Lineage-Restricted Neuronal Precursors
Mahendra S. Rao, Margot Mayer-Proschel, Anjali J. Kalyani
PCT/US98/ 13875, filed, 7/3/98, published PCT application
Lineage Restricted Glial Precursors from the Central Nervous System
Mahendra S. Rao, Mark Noble, Margot Mayer-Proschel
ISSUED U.S. Patent 6,235,527
Lineage Restricted Glial Precursors from the Central Nervous System
Mahendra S. Rao, Mark Noble, Margot Mayer-Proschel
PCT/US98/ 24456, filed, 11/17/98, published PCT application
Generation, Characterization, and Isolation of Neuroepithelial Stem Cells and Lineage Restricted Intermediate Precursor
Mahendra S. Rao, Margot Mayer-Proschel, Tahmina Mujtaba
2,289,021, filed 5/7/98, published Canadian patent application
Generation, Characterization, and Isolation of Neuroepithelial Stem Cells and Lineage Restricted Intermediate Precursor
Mahendra S. Rao, Margot Mayer-Proschel, Tahmina Mujtaba
GRANTED Israeli Patent, 132584
Lineage-Restricted Neuronal Precursors
Mahendra S. Rao, Margot Mayer-Proschel, Anjali J. Kalyani
2,294,737, filed 7/3/98, published Canadian patent application
Lineage-Restricted Neuronal Precursors
Mahendra S. Rao, Margot Mayer-Proschel, Anjali J. Kalyani
GRANTED Israeli Patent, 133799
Lineage-Restricted Neuronal Precursors
Mahendra S. Rao, Margot Mayer-Proschel, Anjali J. Kalyani
ISSUED Japanese Patent 4,371,179
Lineage-Restricted Precursor Cells Isolated From Mouse Neural Tube and Mouse Embryonic Stem Cells
Tahmina Mujtaba and Mahendra S. Rao
PCT/US00/ 12446, filed 5/5/00, published PCT application
Lineage Restricted Glial Precursors from the Central Nervous System
Mahendra S. Rao, Mark Noble and Margot Mayer-Proschel
ISSUED U.S. Patent 6,900,054
45
Table of Contents
Method of Isolating Human Neuroepithelial Precursor Cells from Human Fetal Tissue
Margot Mayer-Proschel, Mahendra S. Rao, Patrick A. Tresco and Darin J. Messina
ISSUED U.S. Patent 6,852,532
Isolation of Mammalian CNS Glial Restricted Precursor Cells
Mahendra S. Rao and Margot Mayer-Proschel
ISSUED U.S. Patent 7,037,720
Isolation of Mammalian CNS Glial-Restricted Precursor Cells
Mahendra S. Rao and Margot Mayer-Proschel
ISSUED U.S. Patent 7,595,194
Generation, Characterization and Isolation of Neuroepithelial Stem Cells and Lineage Restricted Intermediate Precursor
Mahendra S. Rao and Margot Mayer-Proschel
12/568,419, filed 9/28/09, published U.S. patent application
Methods Using Lineage Restricted Glial Precursors from the Central Nervous System
Mahendra S. Rao, Mark Noble and Margot Mayer-Proschel
ISSUED U.S. Patent 7,214,372
Lineage Restricted Glial Precursors
Mahendra S. Rao and Tahmina Mujtaba
ISSUED U.S. Patent 7,795,021
Lineage Restricted Glial Precursors
Mahendra S. Rao and Tahmina Mujtaba
12/209,559, filed 9/12/08, published U.S. patent application
Pure Populations of Astrocyte Restricted Precursor Cells
Mahendra S. Rao, Tahmina Mujtaba and YuanYuan Wu
PCT/US2003 /002356, filed 1/23/03, published PCT application
Lineage-Restricted Neuronal Precursors
Mahendra S. Rao, Margot Mayer-Proschel and Anjali J. Kalyani
12/233,857, filed 9/19/08, published U.S. patent application
Pure Populations of Astrocyte Restricted Precursor Cells
Mahendra S. Rao, Tahmina Mujtaba, YuanYuan Wu and Ying Liu
2,473,749, filed 1/23/03, published Canadian patent application
Pure Populations of Astrocyte Restricted Precursor Cells
Mahendra S. Rao, Tahmina Mujtaba, YuanYuan Wu and Ying Liu
03732101.5, filed 1/23/03, published European patent application
Pure Populations of Astrocyte Restricted Precursor Cells
Mahendra S. Rao, Tahmina Mujtaba, YuanYuan Wu and Ying Liu
2008-139534, filed 1/23/03, published Japanese patent application
Pure Populations of Astrocyte Restricted Precursor Cells
Mahendra S. Rao, Tahmina Mujtaba, YuanYuan Wu and Ying Liu
2004-7011381, filed 1/23/03, published South Korean patent application
46
Table of Contents
Pure Populations of Astrocyte Restricted Precursor Cells
Mahendra S. Rao, Tahmina Mujtaba, YuanYuan Wu and Ying Liu
12/433,060, filed 4/30/09, published U.S. patent application
Method of Isolating Human Neuroepithelial Precursor Cells from Human Fetal Tissue
Margot Mayer-Proschel, Mahendra S. Rao, Patrick A. Tresco and Darin J. Messina
ISSUED U.S. Patent 7,517,521
Method of Isolating Human Neuroepithelial Precursor Cells from Human Fetal Tissue
Margot Mayer-Proschel, Mahendra S. Rao, Patrick A. Tresco and Darin J. Messina
ISSUED U.S. Patent 8,168,174
Methods and Compositions for Expanding, Identifying, Characterizing and Enhancing Potency of Mammalian-
Derived Glial Restricted Progenitor Cells. Robert Sandrock, James Campanelli and Deborah A. Eppstein, PCT/US2010/055956, filed November 2010, published PCT application
In September 2012, Q Therapeutics entered into two non-exclusive sub-licenses with Neuralstem, Inc. for the following patents related to the clinical delivery device that Q intends to use:
| • | U.S. Patent 8,092,495 relating to the development of a spinal platform and |
| • | U.S. Patent 7,833,217 relating to the development of a cannula. |
The agreements with Neuralstem require an annual maintenance fee of $5,000 per year to maintain its perpetual irrevocable licenses of Neuralstem’s technology. Q can terminate the agreements at any time upon written notice to Neuralstem at least 90 days prior to the end of the calendar year.
In addition, Q has entered into out-license agreements with Life Technologies, Inc. (LIFE), Molecular Transfer, Inc. (MTI), and XCell Science LLC (XCell), under which the licensees may develop, manufacture and sell certain cell products covered by Q’s IP for research and drug discovery use only, for which Q (or its subsidiary NeuroQ) is anticipated to receive royalty payments, and in the case of XCell, Q may provide certain payments for research activities.
CNS Therapeutic Market Overview
The global market for CNS Therapeutics is projected to reach US$133 billion by the year 2018, primarily driven by the increase in disease prevalence rates due to aging population, introduction of new class of drugs, and increased expenditure on healthcare. (“CNS Therapeutics: A Global Strategic Business Report,” Global Industry Analysts Inc., February 2013). In addition, the reported incidence of CNS disorders is increasing due to better diagnostic techniques. The global CNS therapeutics market is comprised of three main segments:
(1) Neurology (e.g. Parkinson’s and Alzheimer’s diseases, MS, Spinal Cord Injury, ALS);
(2) Psychiatry (e.g. Depression, ADHD, Schizophrenia); and
(3) Pain (e.g. Migraine).
While the Psychiatry market represents approximately half of all dollars spent on CNS drug therapies, the neurology segment of the market is the fastest growing. The United States represents approximately half of this global market and, until recently, was realizing the fastest growth rates. This is the result of three factors: higher prices charged for the drugs themselves compared to other markets; the larger volume of patients seeking treatment; and the higher rates of pharmacotherapy compared to other countries. The Asia/Pacific market for CNS drug therapy is now the most rapidly growing as these factors begin to make an impact on the worldwide market.
47
Table of Contents
The Unmet Need in the CNS Therapeutic Market
The CNS Therapeutic market is based primarily on traditional drug therapies (small molecules and biologics). While this growing market is large and represents nearly 25% of all dollars spent on prescription pharmaceuticals worldwide, there remains a high unmet need in the treatment of many neurological diseases where current therapies are inadequate or yet to be developed. Two examples are MS and ALS.
The annual worldwide pharmacotherapy market for MS is approximately $10 billion. MS is estimated to affect 2.5 million people worldwide. The vast majority of all approved MS therapies seek to slow disease progression by “knocking down” the autoimmune component of the disease, which causes the damage and destruction of the myelin sheath that surrounds the neurons. However there is no approved product that repairs this damage once it has occurred. Thus, while current therapies may slow or even halt the progression of the disease, once the damage is done, there is currently no drug that can repair that damage. This represents a substantial unmet need in the MS disease space.
Similarly, there is only one approved drug for the treatment of ALS. This drug, Rilutek®, prolongs the life expectancy of ALS patients by approximately 60-90 days. ALS is always fatal, and there are no approved drugs that substantially slow or halt the progression of the disease, much less reverse its devastating effects. This represents another unmet need in the CNS Therapeutic market. Given the estimated prevalence of ALS in the U.S. is approximately 30,000 patients; it is classified as an orphan disease.
Considering these and many other shortcomings in the approved pharmacopeia for injuries and diseases of the CNS, there is an opportunity to substantially expand the CNS Therapeutic market with new technologies that address the unmet needs. Cell-based therapy holds the promise of significantly expanding the CNS market and satisfying the unmet needs that exist.
The Potential of Cell-Based Therapy
Cell-based therapy is seen by many as the next great advance in the treatment of disease and injury. It holds the promise of better therapy for disorders which are currently not well treated and new therapies to meet currently unmet needs. Should this promise hold true, the currently available therapies could be displaced and the Pharmacotherapeutic market substantially expanded as new stem and progenitor cell therapies are approved for human use.
The immense promise of stem cell biology has prompted the formation of new companies seeking to exploit the therapeutic potential of stem cells. Approaches to creating stem cell therapies fall into two broad categories:
| (1.) | Isolating (or generating) and purifying new populations of stem or progenitor cells from various tissues, expanding them as necessary and transplanting the cells into various target organs. One challenge with this approach is ensuring that the exact desired population can be reproducibly purified and expanded. This affects safety and efficacy as well as the ease of scaled-up manufacturing. |
| (2.) | Stimulating existing stem cells to “wake up,” expand and differentiate inside the body at an accelerated rate. Challenges associated with this approach include manipulating complex interactions among multiple growth factors and endogenous signals to generate the desired outcomes, as well as complications due to malfunctioning or mutant endogenous cells in certain diseases. |
Q believes that the fastest route to a safe and effective product is through the first route, using therapy with well characterized, unmodified cells that perform their tasks as nature intended; functioning as “mini-factories” and playing roles appropriate to the specific disease state.
48
Table of Contents
Several types of cells are under evaluation for use in cell therapy by several companies, including embryonic stem cells, human cord blood and placental stem cells, and fetal or adult tissue from various organ sites. Purified cell populations can be injected intravenously or transplanted directly into target organs such as brain, heart, pancreas, blood and bone. For most neurodegenerative diseases, Q believes that CNS cells, not hematopoietic or mesenchymal lineage cells, are needed for long-term benefits.
Market Opportunity for Q Therapeutics
Q Therapeutics aims to change the way medicine is practiced in the treatment of many debilitating and often fatal diseases of the brain and spinal cord by bringing to market a patented cell-based therapeutic that addresses substantial unmet needs in the CNS Therapeutic market today. Q Therapeutics believes that its initial Q-Cells product could meet a variety of these needs across a number of CNS diseases. By demonstrating that Q-Cells are safe and effective first in smaller orphan indications and later in larger target markets, Q Therapeutics believes it can both augment and/or displace current therapeutic approaches as well as expand the therapeutic market in currently untreatable CNS conditions. Should this prove true, Q-Cells would address a multi-billion dollar market opportunity in treatment of neurodegenerative diseases for which there are no effective treatments.
Q intends to bring Q-Cells to the market first to treat ALS to demonstrate the safety and efficacy of its cell-based therapeutic. Q also intends that Q-Cells will be brought to other indications potentially including TM, MS, Spinal Cord Injury, Stroke, Parkinson’s and Alzheimer’s diseases. Q estimates that the annual market opportunity for its initial orphan disease targets exceeds $1 billion worldwide. Application of its Q-Cells product to other larger market diseases such as MS would substantially expand the market available to Q.
Orphan Disease Strategy for Initial Commercialization
Q’s first commercial targets are orphan diseases with billion-dollar market potential (Figure 7). The Orphan Drug Act of 1983 (in this paragraph, the Act) defined an “orphan drug” as a therapy intended to treat rare “orphan” diseases, those affecting fewer than 200,000 Americans. The benefits provided by the Act may include more rapid regulatory timelines, tax benefits and seven-year market exclusivity for the first product approved for an indication. Despite the smaller numbers of affected patients, orphan diseases often have highly motivated patient advocacy groups that are eager to assist companies with patient recruitment and therapeutic development. Moreover, substantial annual per-patient treatment prices effectively offset the relatively small patient populations. Due to the focused nature of marketing permitted by targeting orphan indications, Q might be able to capture a significant portion of the U.S. market for certain orphan diseases without the need of a major marketing partner, provided that it has adequate financial resources. This ability to target patients suffering from orphan diseases also provides an incentive to potential Q development partners with interest in funding development costs and providing developmental expertise in exchange for marketing rights.
Q intends to submit an application to the FDA seeking designation of Q-Cells for the treatment of ALS as an Orphan Drug.
49
Table of Contents
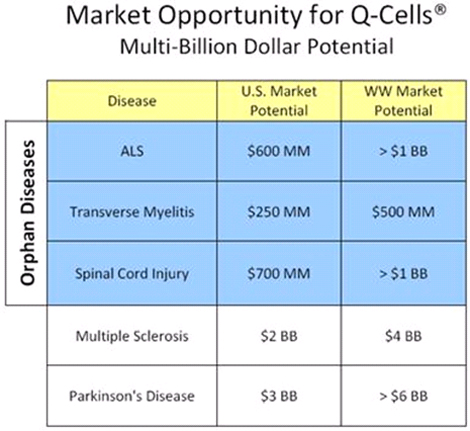
Figure 7. Multi-billion dollar market potential for Q-Cells. The initial orphan diseases may allow Q or its partner(s) to market the product with a focused specialty sales force. Market opportunity size was calculated using a lifetime treatment cost projection of $200,000, assuming 50%, 30% and 35% patient population penetration for the orphan diseases of ALS, Transverse Myelitis and Spinal Cord Injury, respectively; and 2% and 10% patient penetration for MS and Parkinson’s disease, respectively. Further market opportunities exist for additional neurodegenerative disease targets.
Q Market Development Strategy – Orphan Indications First, Larger Markets Follow
By targeting orphan diseases first (ALS, TM, and SCI), Q can continue its strategy of increasing the value of the Company in a capital efficient manner by taking advantage of the benefits of the Act. Evaluation of Q-Cells in these various neurodegenerative diseases provides multiple opportunities for success utilizing this single therapeutic product. We believe that such success will be very attractive to larger pharmaceutical and biotechnology companies who will want to partner with us in the application of Q-Cells to larger markets where the development and sales capacity of those partners can be advantageous.
Amyotrophic Lateral Sclerosis (ALS), also called Lou Gehrig’s disease
ALS is diagnosed in 5,000-6,000 new individuals per year in the U.S., comparable in annual incidence to Multiple Sclerosis. Patient death generally occurs two-five years after diagnosis, resulting in an estimated U.S. prevalence of 30,000 with 450,000 people living with ALS worldwide (ALS Therapy Development Institute). ALS is a neurodegenerative disease causing progressive deterioration and loss of motor neurons, affecting both upper and lower motor neurons. The loss of nerve stimulus to specific muscles results in atrophy and progressive weakness that leads to paralysis. Death usually results from respiratory failure.
There is no curative treatment for ALS. The only drug approved for treatment for the disease - riluzole (Rilutek®®)—is believed to reduce (but not reverse) damage to motor neurons by decreasing the release of glutamate, and prolongs survival by only 60-90 days. Costs for treating individuals with advanced ALS can average $200,000 a year or more.
50
Table of Contents
The rationale for a clinical trial using Q-Cells to treat ALS patients has been described above.
Multiple Sclerosis and Transverse Myelitis
Q is also evaluating Q-Cells in animal studies for MS, a chronic autoimmune-triggered demyelinating neurodegenerative disease, and Transverse Myelitis (TM), a related but acute, localized inflammatory disease of the spinal cord. Multiple clinical and pathological studies suggest that there are many common features of the inflammation and neural injury between TM and MS, with a shared primary pathology of demyelination due to immune attack destroying the oligodendrocytes.
Multiple Sclerosis
The annual worldwide pharmacotherapy market for MS is approximately $10 billion. Diagnosis of MS typically occurs between 20 and 40 years of age with over 2.5 million people worldwide estimated to have been diagnosed (National MS Society). While MS is rarely fatal in the short term, individuals diagnosed with MS are usually required to implement both temporary and/or permanent modifications to their lifestyle as they experience the varying range of MS symptoms. Currently, the vast majority of all approved MS therapies seek to slow disease progression by “knocking down” the autoimmune component of the disease, which causes the damage and destruction of the myelin sheath that surrounds the neurons. However, there is no approved product that repairs this damage once it has occurred. Thus, while current therapies may slow or even halt the progression of the disease, once the damage is done, there is currently no drug to effect repairing that damage.
Traverse Myelitis
It is estimated that approximately 1,400 new cases of TM are diagnosed each year in the U.S. with approximately 33,000 individuals currently suffering the effects of the disease (National Institute of Neurological Disorders and Stroke). Lack of treatment or incorrect treatment can leave the individual with a disability such as partial or complete paralysis resulting from the disorder. Researchers are currently uncertain of the primary causes and there are currently no effective cures for TM. Current treatment is targeted towards minimizing inflammation and pain. Estimated costs for diagnosing and treating TM are approximately $600,000 per patient in the U.S.
Due to the similarity of the lesion pathology in TM and MS, demonstration of efficacy of remyelination in the orphan disease TM may be an indicator for efficacy in the more prevalent disease of MS. The rationale for using Q-Cells in these indications is based on data obtained using both animal and human GRPs in models of disease.
Demonstration of remyelination in lesions in TM and MS patients may also be relevant for developing treatments of other diseases in which demyelination is a significant factor such as cerebral palsy, white-matter stroke and certain traumatic spinal cord injuries. Shiverer is an established myelin-deficiency model in which the entire CNS is defective in normal myelination. Q and its collaborators at Johns Hopkins have demonstrated that Q-Cells engrafted into shiverer (a) effectively compete with host (defective) oligodendrocytes, (b) myelinate host axons resulting in normal myelin and (c) demonstrate benefits in a focal inflammatory spinal cord lesion model (Figure 8, Q data; Walczak et al, GLIA 59, 499-501 (2011)). Others have shown that human GRPs transplanted into shiverer/rag2 mice extended survival of a portion of the mice (Windrem et al, Cell Stem Cell 2, 553-565 (2008)). They also documented that the implanted human cells interacted effectively with the host proteins and did not leave the CNS. The efficacy of a localized treatment that does not produce toxicities in other organs is a significant finding. This is supported further by an extensive body of literature demonstrating remyelination by rodent glial precursors and related cells in
51
Table of Contents
multiple animal models [including publications from Blakemore (1999), Ben Hur (2006), Cummings (2005), Duncan (2004, 2005), Goldman (2008), Keirstead (2004, 2005), Whittemore (2010), Rao (1997-2010), Sandrock (2010), and Walczak (2011).] This myelination has occurred in both the presence and the absence of an ongoing inflammatory process.
Figure 8. Myelination by implanted Q-Cells that produced oligodendrocytes.
Shiverer is a mouse with a mutation that produces defective myelin basic protein (MBP) and hence defective myelin. Q-Cells implanted in shiverer/rag2 brain mature into oligodendrocytes that produced normal MBP (green). All MBP seen here is produced by Q-Cells that matured into oligodendrocytes (human cell-specific red nuclei stain).
Traumatic Spinal Cord Injury
Approximately 12,000 people are paralyzed each year in the U.S. due to a traumatic spinal Cord Injury (SCI) and it is estimated that over 270,000 people are living with Spinal Cord Injury in the U.S. (National Spinal Cord Injury Statistical Center, Birmingham, Alabama). The average age of occurrence for SCI is 41. This has tremendous costs both in terms of patient care and lost productivity as well as quality of life. Average medical costs are estimated at between $15,000 to $30,000 a year with an estimated lifetime cost up to $3 million depending on the severity of the injury and the age at which the injury occurred. It has been projected that the U.S. could save up to $400 billion in future healthcare costs if there were effective therapies to treat and prevent spinal cord injuries (Christopher and Dana Reeve Foundation, Centers for Disease Control, University of Alabama National Spinal Cord Injury Statistical Center).
Q has collaborated with Itzhak Fischer, PhD, at Drexel University to test Q-Cells in animal models of traumatic Spinal Cord Injury. Recently published studies by Q and its collaborators at Drexel document the safety and statistically significant, reproducible, disease modifying activity of Q-Cells transplanted into the injury site in an athymic rat model of thoracic contusion SCI (Jin et al., J Neurotrauma; 28(4):579-94; 2011).
52
Table of Contents
Parkinson’s disease
Parkinson’s disease (PD) affects more than one million patients in the U.S., with approximately 60,000 new cases diagnosed each year (Parkinson’s Disease Foundation). Due to the increase in the aging population, it is anticipated that the number of individuals diagnosed by PD will continue to increase. PD involves the breakdown and death of vital nerve cells, or neurons, found in the substantia nigra of the brain. These neurons normally secrete dopamine, and lack of dopamine leads to symptoms such as shaking, rigidity, difficulty and slowness in movement, and postural instability. While the cause of PD is currently unknown and there is no cure, there are therapies available to manage its symptoms, but they become less effective as the disease progresses. Combined direct and indirect costs of treating PD in the U.S. alone are estimated to be $25 billion a year with medication costs averaging $2,500 a year. If surgery is needed, the costs can approach up to a $100,000 per patient.
Although patients initially respond to treatment with L-dopa or dopaminergic agonists, over time this response decreases. Several early trials have found that administration of single growth factors failed to provide meaningful clinical benefits in PD patients. Studies in animal PD models have suggested non-cell autonomous killing of neurons, with diseased astrocytes playing a critical initiating role. Astrocytes in culture exert a protective effect on neuronal cells in a setting where both cell-types are co-cultured. Studies in a primate model of PD showed functional benefits after implantation of neural cells. Upon autopsy, a large number of progeny astrocytes were found juxtaposed with the host nigrostriatal circuitry suggesting that the “homeostatic adjustments” to the microenvironment results in preservation of the remaining host nigrostriatal pathway (Redmond et al, Proceedings of National Academy of Sciences USA 104, 12175–12180 (2007)). As Q-Cells produce healthy astrocytes, this provides potential for protective effects including those via production of multiple growth factors and other trophic support, rather than relying on a single factor. Q anticipates that it will pursue studies of Q-Cells in Parkinson’s disease models upon availability of appropriate additional funding.
Other Disease Targets
Q-Cells provide a platform technology that may be useful to treat not only demyelinating diseases, but other neurodegenerative diseases that can benefit from the neuronal support provided by growth factors and other trophic support by Q-Cells. The patient populations and markets for Q’s follow-on therapeutic targets may be substantial, in addition to the opportunity in ALS, MS, TM, SCI and PD discussed above.
Alzheimer’s disease
Alzheimer’s disease (AD) is a progressive, neurodegenerative disease characterized by abnormal clumps (amyloid plaques) and tangled bundles of fibers (neurofibrillary tangles) in the brain. Symptoms include memory loss, language deterioration and confusion and eventually lead to loss of cognition and personality. Currently, there is not a way to prevent, cure or even delay its progression. It is the sixth leading cause of death in the U.S. and is estimated to affect about 5.4 million people in the U.S., with annual healthcare costs in excess of $200 billion. The number of patients is projected to reach 16 million by 2050 (Alzheimer’s Association, 2012).
Transplantation of neural cells that produce glial cells (both astrocytes and oligodendrocytes) as well as transplantation of astrocyte precursors, provided benefits in AD models, rescuing neurons and improving memory (Hampton et al, Journal of Neuroscience 30(3), 9973-9983 (2010); Blurton-Jones et al, PNAS 106(32), 13594-13599 (2009)). Since Q-Cells mature into glial cells after transplantation, Q believes there
53
Table of Contents
may be an opportunity for use of Q-Cells potentially to both slow decline and/or restore function for AD patients. Q will explore collaboration to further investigate the efficacy of Q-Cells in animal models once appropriate financing has been obtained.
Traumatic Brain Injury
It is estimated that 1.7 million people in the U.S. sustain a Traumatic Brain Injury (TBI) each year with approximately 5.3 million Americans living with a TBI-related injury (Centers for Disease Control and Prevention). In 2010, it was estimated that direct and indirect medical costs for those with TBI were approximately $76.5 billion. Other than acute phase treatments to prevent further injury (e.g., surgery to relieve pressure build-up), treatment of headaches, seizures and physical rehabilitation, there is no treatment for and nothing to reverse TBI. The multiple support functions of Q-Cells may be beneficial in achieving recovery from TBI.
Cerebral Palsy
Cerebral Palsy (CP) has a U.S. patient population of about 764,000 children and adults (United Cerebral Palsy) and refers to any of a number of neurological disorders that permanently affect muscle coordination and mobility. No cure currently exists, but the earlier the disease is diagnosed in an individual’s life, the higher likelihood there is of being able to improve muscle function and coordination. An initial target may be for spastic diplegia, with involves injury to the cerebral cortex and represents approximately 70% of CP. As Q-Cells can provide myelinating oligodendrocytes, they may provide benefits to CP patients.
Stroke
Stroke afflicts almost 800,000 people and is the third leading cause of death in the U.S, with total annual costs over $40 billion. Market projections are significant even if only a small portion of these patients were treated. Q-Cells may be beneficial in treating stroke both for restoring myelination, as well as the support provided by astrocytes to restoring neuron function.
Leukodystrophies and CNS storage diseases
These are several inherited pediatric diseases for which the molecular cause is known to involve a single gene defect. There are no treatment options for most of these diseases, often resulting in death at a young age. Although a few companies including Genzyme/Sanofi and Biomarin have developed enzyme replacement therapies to successfully treat systemic manifestations of a few of these diseases, the systemically administered enzyme pharmaceuticals do not cross the blood-brain barrier to treat CNS targets. Such CNS diseases may provide future opportunity to Q as there are many storage disorders with severe CNS manifestations that involve destruction of oligodendrocytes with concomitant demyelination. Q-Cells, as they are transplanted directly into the CNS, may be able to provide both the missing enzyme in the CNS, as well as replace the destroyed oligodendrocytes and thus provide remyelination.
Peripheral Neuropathies
Peripheral neuropathies have no effective treatments. In addition to causing severe pain, they are a leading cause of amputations in the developed world. Eight million diabetes patients have neuropathy in the U.S. alone. Q has patents on cells that support the peripheral nervous system.
Competition
The competitive landscape includes companies working on a range of alternative treatments for diseases of the CNS including traditional drug products, protein therapeutics, gene therapy and different types of cell therapy. A handful of companies are exploring the possibility of harnessing the power of stem cells to treat
54
Table of Contents
neurodegenerative disease, though no clear leader has emerged. Q believes that Q-Cells are different from other cell therapy products both in their novel mechanism of action/capabilities as well as the cost-effective methods of production. Q believes that the cell therapy approach has many advantages over single drug therapeutics for many neurodegenerative diseases. Thus, the closest direct competition is other cell therapy companies working on the same disease targets.
Direct Competition
A limited number of companies are working on the therapeutic neurological application of stem and progenitor cells for CNS treatment, including the public companies: StemCells Inc. (NASDAQ: STEM); ReNeuron (LSE: RENE.L); Brainstorm Cell Therapeutics (OTCBB: BCLI); and Neuralstem (NYSE MKT: CUR); and the private company California Stem Cells, Inc.
StemCells, Inc. uses human fetal-derived neural stem cells and has initiated a Phase 1/2 clinical trial and recently reported positive interim date for SCI patients. Stem Cells Inc. is also conducting a Phase 1 clinical trial for treatment of Pelizaeus-Merzbacher disease, a rare myelination disorder. ReNeuron uses human fetal-derived neural stem cells to treat patients disabled by stroke. ReNeuron has submitted an application to the UK regulatory body to commence a multi-site Phase 2 clinical trial for its cell based treatment ReN001. Brainstorm is conducting Phase 2a clinical trials in ALS patients in Israel using modified mesenchymal stem cells (MSCs) derived from the patient’s own (autologous) bone marrow. Neuralstem has completed its Phase 1 safety study for ALS and has been awarded orphan status by the FDA. Neuralstem has also announced approval by the FDA to commence a Phase 2 trial in ALS, and has commenced a Phase 1 safety trial for SCI. California Stem Cells has submitted an IND to treat spinal muscular atrophy with its embryonic stem cell-derived motor neuron therapy that has been placed on Clinical Hold by the FDA. They have stated an intent to submit a second IND using its technology to treat ALS.
Other Potential Competitors
Another approach is the use of cells or virus-based vectors implanted in the brain as delivery vehicles for gene therapy, with the goal of achieving local production of desired proteins and growth factors. NsGene is studying the use of encapsulated, genetically modified cells to secrete biological growth factors in the treatment of Alzheimer’s and Parkinson’s diseases. Ceregene is conducting clinical trials with virus-based vectors for gene therapy for Parkinson’s and Alzheimer’s diseases, with other disease targets in earlier research. Cedars Sinai is developing neural progenitors engineered to secrete glial cell-derived neurotrophic factor (GDNF) for treatment of ALS. Q’s approach differs significantly in that it does not rely on a single growth factor for success, and in that it does not use genetically-modified cells or viruses; rather, unmodified Q-Cells produce the range of growth factors and trophic support that is typical of healthy glial cells.
Other companies working in the CNS space have focused on stimulating endogenous differentiation of nascent adult stem cells with administration of molecules to trigger their expansion and differentiation in vivo (e.g., Braincells). Still other pharmaceutical and biotechnology companies (including, among others, Genzyme/Sanofi-Aventis, Biogen-Idec, Acorda, Merck-Serono, GlaxoSmithKline and Roche) have programs to identify therapeutics to treat neurodegenerative diseases such as MS, Parkinson’s disease and Alzheimer’ disease. Some of these programs might yield effective therapeutics that could compete with Q’s products, and which might prove less costly and easier to administer.
Why Q Therapeutics’ approach may prove superior
A noteworthy distinction between Q and other CNS cell therapy companies involves the specific properties of Q-Cells. These cells naturally produce mature glial cells (astrocytes and oligodendrocytes) that can both achieve myelination of neuronal axons as well as otherwise promote neuron health, while minimizing unwanted and potentially deleterious neuron formation. Q-Cells are a more mature cell type - at a more
55
Table of Contents
advanced stage of differentiation - than are the cells used by StemCells Inc., ReNeuron, and Neuralstem, who all use the less-differentiated CNS stem cells that produce neurons as well as glial cells (see Figure 3). Unlike ReNeuron, Q does not use genetically immortalized cells, which Q believes could potentially raise safety issues, e.g., concerns about deleterious effects such as tumor formation. Unlike Brainstorm, which uses non-CNS autologous MSCs that it modifies in vitro to make what they call “glial-like” cells, Q uses non-autologous natural CNS glial cells that are not modified in vitro and that are intended to be an “off-the shelf” product.
Pluripotent stem cells could also be a starting source. This approach requires the identification of proper signaling factors and establishing the sequence of use to correctly and safely induce differentiation into the desired CNS cell type and to ensure sufficient elimination of the undifferentiated ESCs which can form tumors, prior to transplantation. Our current Q-Cells are not derived from undifferentiated pluripotent cells, such as embryonic stem cells. Rather, Q-Cells are isolated already at the desired final cell type, and require no in vitro differentiation protocols. Q believes that the progenitor cell therapies it is developing as its first products, generated from pre-formed cells that naturally occur, offer safety and efficacy benefits as well as a faster path to market.
Government Regulation
Any products we may develop and our research and development activities are subject to stringent government regulation in the United States by the FDA and, in many instances, by corresponding foreign and state regulatory agencies. The European Union, or EU, has vested centralized authority in the European Medicines Agency and Committee on Proprietary Medicinal Products to standardize review and approval across EU member nations.
These regulatory agencies enforce comprehensive statutes, regulations and guidelines governing the drug development process. This process involves several steps. Initially, a company must generate pre-clinical data to show safety before human testing may be initiated. In the United States, a drug company must submit an IND to the FDA prior to securing authorization for human testing. The IND must contain adequate data on product candidate chemistry, toxicology and metabolism and, where appropriate, animal research testing to support initial safety.
A Clinical Trial Agreement, or CTA, is the European equivalent of the IND. CTA requirements are issued by each competent authority within the European Union and are enacted by local laws and directives.
Any of our potential product candidates will require regulatory approval and compliance with regulations made by United States and foreign government agencies prior to commercialization in such countries. The process of obtaining FDA or foreign regulatory agency approval has historically been extremely costly and time consuming. The FDA regulates, among other things, the development, testing, manufacture, safety, efficacy, record keeping, labeling, storage, approval, advertising, promotion, sale, and distribution of biologics and new drugs.
The standard process required by the FDA before a pharmaceutical agent may be marketed in the United States includes:
| • | pre-clinical tests in animals that demonstrate a reasonable likelihood of safety and effectiveness in human patients; |
| • | submission to the FDA of an IND, which must become effective before clinical trials in humans can commence. If Phase 1 clinical trials are to be conducted initially outside the United States, a different regulatory filing is required, depending on the location of the trial; |
| • | adequate and well controlled human clinical trials to establish the safety and efficacy of the drug or biologic in the intended disease indication; |
56
Table of Contents
| • | for drugs, submission of a New Drug Application, or NDA, or a Biologic License Application, or BLA, with the FDA; and |
| • | FDA approval of the NDA or BLA before any commercial sale or shipment of the drug. |
Pre-clinical studies can take several years to complete, and there is no guarantee that an IND based on those studies will become effective to permit clinical trials to begin. The clinical development phase generally takes five to seven years, or longer, to complete (i.e., from the initiation of Phase 1 through completion of Phase 3 studies). After successful completion of clinical trials for a new drug or biologic product, FDA approval of the NDA or BLA must be obtained. This process requires substantial time and effort and there is no assurance that the FDA will accept the NDA or BLA for filing and, even if filed, that the FDA will grant approval. In the past, the FDA’s approval of an NDA or BLA has taken, on average, one to two years, but in some instances may take substantially longer. If questions regarding safety or efficacy arise, additional studies may be required and then followed by a resubmission of the NDA or BLA. Review and approval of an NDA or BLA can take several years.
In addition to obtaining FDA approval for each product, each drug manufacturing facility must be inspected and approved by the FDA. All manufacturing establishments are subject to inspections by the FDA and by other federal, state, and local agencies, and must comply with good manufacturing practices, or GMP, requirements. We do not currently have any GMP manufacturing capabilities, and will rely on contract manufacturers to produce material for any clinical trials that we may conduct.
We must also obtain regulatory approval in other countries in which we intend to market any drug. The requirements governing conduct of clinical trials, product licensing, pricing, and reimbursement vary widely from country to country. FDA approval does not ensure regulatory approval in other countries. The current approval process varies from country to country, and the time spent in gaining approval varies from that required for FDA approval. In some countries, the sale price of the drug must also be approved. The pricing review period often begins after market approval is granted. Even if a foreign regulatory authority approves a drug product, it may not approve satisfactory prices for the product.
In addition to regulations enforced by the FDA, we are also subject to regulation under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act, and other present and potential future federal, state, or local regulations. Our research and development involves the controlled use of hazardous materials, chemicals, biological materials, and various radioactive compounds. Although we believe that our safety procedures for handling and disposing of such materials currently comply in all material respects with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result and any such liability could exceed our available resources.
Research and Development
Our research and development expenses for the years ended December 31, 2012 and 2011 were $2,089,321 and $444,834, respectively. Since our inception, we have incurred total research and development expenses of $10,972,892. Our research and development expenses to date have been primarily the result of our research and the research of our collaborative partners relating to the development of Q-Cells.
57
Table of Contents
Our Employees
As of July 5, 2013, we had five full-time employees and five part-time employees. All of our employees are located in Salt Lake City, Utah. Approximately 60% of our employees are involved with research, development and clinical affairs and 40% are involved with finance, human resources and administration.
We currently maintain compensation, benefits, equity participation and work environment policies intended to assist in attracting and retaining qualified personnel. We believe that the success of our business will depend to a significant extent on our ability to attract and retain such personnel.
Properties
Our facility in Salt Lake City, Utah is comprised of approximately 5,300 square feet of research and office space located at 615 Arapeen Drive, Suite 102, Salt Lake City, Utah, 84108. We own the equipment summarized in the financial statements. The total lease expense including common area maintenance charges for the year ended December 31, 2012 was $150,528. Our current lease term expired March 31, 2013, but is expected to be renewed. We are currently paying rent on a month-to-month basis. We cannot be assured that we will be able to agree on the terms with the landlord for continuing in the space and that we will remain in the same space after our lease expires. If we need to move to a new facility, this will require additional expense to add the improvements needed for our operations as well as delay our development activities until we are fully operational in a new space.
We are not aware of any environmental issues that may affect the use of our property. We currently have no investments in real estate, real estate mortgages or real estate securities, and do not anticipate making any such investments.
We are not currently a party to any legal proceedings nor do we have knowledge of any pending or threatened legal claims.
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS
Our common stock is not currently trading on any stock exchange and it is not quoted on any quotation system or traded in any other manner in the public markets. We are not aware of any market activity in our stock since inception through the date of this filing.
A FINRA-registered broker-dealer unaffiliated with the underwriter has submitted a Form 211 Application to FINRA, in an effort to have the Company’s common stock quoted on the OTC Bulletin Board. We cannot guarantee that such a listing will be obtained. There is currently no trading activity in our securities, and there can be no assurance that a regular trading market for our common stock will ever be developed.
As of July 5, 2013, there are approximately 168 record holders of 24,811,833 shares of our common stock.
We have never paid any dividends to stockholders and presently do not intend to pay cash dividends on our common stock in the foreseeable future. We intend to retain any future earnings to fund the development and growth of our business.
58
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
Company Overview
Q Therapeutics, Inc. conducts its business and operations through its wholly owned subsidiaries, Q Therapeutic Products, Inc. (hereinafter Q Products) and NeuroQ Research, Inc. Q Therapeutics is a Salt Lake City, Utah-based biopharmaceutical company that is developing human cell-based therapies intended to treat degenerative diseases of the brain and spinal cord, the primary components of the central nervous system. Q was incorporated in the state of Delaware in March 2002.
The technology upon which these therapies are based was developed by Q’s co-founder Mahendra Rao, M.D., Ph.D., a global leader in glial stem cell biology, during his tenure as a Professor at the University of Utah and as Head of the Stem Cell Section at the National Institutes of Health. Q is managed by an experienced team of biotechnology executives with demonstrated start-up success and advised by leaders in the neurology and stem cell therapeutics fields.
Every year, hundreds of thousands of people suffer with debilitating neurodegenerative diseases such as Amyotrophic Lateral Sclerosis (ALS, or Lou Gehrig’s disease), Multiple Sclerosis (MS), Transverse Myelitis (TM) and Spinal Cord Injury (SCI). Traditional drugs tend to fail to treat the nerve damage caused by these diseases due to the multifactorial nature of these diseases and the inability of most drugs to address all of these factors. Q Therapeutics is developing a new and nontraditional approach targeted to improve the health of people suffering from neurodegenerative diseases: a human cell-based product called Q-Cells.
Q-Cells are healthy human glial cells. The role of glial cells in the brain and spine is to support and protect neurons, the signal transmission lines of the nervous system. Glial cells perform many functions including forming an insulating “myelin sheath” around neurons, providing the necessary growth factors needed to maintain a healthy nervous system, and removing compounds that are toxic to neurons. Many neurodegenerative diseases arise when glial cells are damaged or destroyed, causing neurons to malfunction and eventually die. Q-Cells technology aims to treat neurodegenerative diseases by supplementing the damaged or missing glia in the CNS with new, healthy cells that can help maintain and/or restore neuron function to a more robust state.
The diseases targeted by Q Therapeutics’ products are not well treated with current drug therapies. At best, patients suffering from these diseases can, in some cases, only hope to slow their inexorable progression and the associated disabilities. A handful of companies are exploring the possibility of harnessing the power of stem and progenitor cells to treat these conditions, though no clear leader has emerged. In addition to utilizing its proprietary cellular products as therapeutic products, Q may evaluate novel ways to utilize these cells to screen for new drugs (such as small molecule compounds) that could also provide treatments for neurological diseases.
Q Therapeutics believes that a worldwide market exists for those companies whose cell-based treatments become commercial products. Q Therapeutics’ patent protected technology represents an opportunity to build on the recent advancements in the cell therapy field and bring to market a therapeutic approach that will change the way medicine is practiced in treating many disabling and fatal conditions of the central nervous system.
Results of Operations for the Three Months Ended March 31, 2013 compared to the Three Months Ended March 31, 2012:
For the period from March 28, 2002 (date of inception) through March 31, 2013, we have not generated significant revenues and have been focused on developing our products for commercial sale. Q Therapeutics is considered to be a development stage company.
59
Table of Contents
Since entering the development stage, we have not generated revenues in excess of expenses and have been dependent on government grants and debt and equity raised from individual investors to sustain our operations. Our products have not yet been approved by the FDA for commercial sale and as a result we have not yet generated revenues from therapeutic product sales. We have incurred losses and used cash in operating activities since inception. As of March 31, 2013, the Company had an accumulated deficit of $20,991,557 and negative working capital of $474,759.
Revenues
Historically, the Company has generated minimal revenues through (1) research grants from government agencies such as the National Institutes of Health, (2) granting rights to the Company’s technology to other entities, and (3) sales of its product for research purposes.
Grant revenues for the three months ended March 31, 2013 and 2012 were $5,501 and $0, respectively. The increase was primarily due to being the recipient of a sub-award of an NIH grant issued to The Johns Hopkins University. The sub-award was approved in December 2012 for a total of $631,383 for the 2012-2013 grant budget year. As of March 31, 2013, approximately $483,000 has been billed to NIH. We anticipate grant revenues to increase as we identify new opportunities for grants that are available and specific to our research of cell-based therapies.
Research and Development Expenses
Q Therapeutics anticipates that development activities and costs will remain at similar levels to the first quarter of 2013 as we advance the work necessary to complete our future Investigational New Drug (IND) submission. This includes Good Laboratory Practices animal safety studies, injection device studies, manufacturing activities and working with clinical and regulatory consultants. Should additional financing be obtained, we may also increase research and development activities to evaluate use of our proprietary products in other disease indications, including working with outside collaborators.
Research and development expenses for the three months ended March 31, 2013, were $131,109, a decrease of $93,098, or 41.5%, from $224,207 for the three months ended March 31, 2012. The decrease is primarily due to the one-time costs associated with the initiation of our pilot studies that occurred in 2012 that did not recur in 2013. We anticipate our research and development expenses will remain at similar levels to those of the first quarter of 2013 as we continue our safety studies, until additional financing can be obtained.
General and Administrative Expenses
The following table details the general and administrative expenses incurred by the Company:
| For the Three Months Ended March 31, |
||||||||||||||||
| 2013 | 2012 | Change | % | |||||||||||||
| Salaries and benefits |
$ | 166,946 | $ | 146,835 | $ | 20,111 | 13.7 | % | ||||||||
| Legal and professional fees |
119,186 | 147,971 | (28,785 | ) | -19.5 | % | ||||||||||
| Facility and office related |
52,125 | 47,330 | 4,795 | 10.1 | % | |||||||||||
| Stock-based compensation |
25,438 | 29,308 | (3,870 | ) | -13.2 | % | ||||||||||
| Travel and entertainment |
10,557 | 13,845 | (3,288 | ) | -23.7 | % | ||||||||||
| Depreciation |
467 | 445 | 22 | 5.0 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total general and administrative expenses |
$ | 374,719 | $ | 385,734 | $ | (11,015 | ) | -2.9 | % | |||||||
|
|
|
|
|
|
|
|||||||||||
60
Table of Contents
The net decrease in general and administrative expenses is due to a reduction in legal and professional fees and associated travel and entertainment expenses resulting from the costs of the private placement of the Company’s securities and the evolution to a public reporting entity in 2012 that did not recur in 2013, a decrease in stock-based compensation and a reduction in annual fees and licenses; offset primarily by a non-qualified contribution to the Company’s retirement plan. We anticipate general and administrative expenses to remain at approximately the same levels, or decrease as necessary, due to our cash constraints until further financing becomes available.
Liquidity and Capital Resources
For the three months ended March 31, 2013, net cash used in operating activities totaled $120,304 compared to $549,108 for the three months ended March 31, 2012. Cash expenditures decreased in 2013 primarily due to the initiation costs related to our pilot study in 2012 that did not recur in 2013, payment for professional services with Company securities in lieu of cash, and deferral of the payment of certain executive salaries.
For the three months ended March 31, 2013, net cash used in investing activities related to the purchase of equipment totaled $19,851 compared to $1,323 for the three months ended March 31, 2012.
For the three months ended March 31, 2013, net cash provided by financing activities was $0 compared to $175,000 for the three months ended March 31, 2012. The primary reason for the change was that the Company raised $190,000 from the private placement of its securities offset by the repayment of a $15,000 note payable in the first quarter of 2012, which did not recur in the first quarter of 2013.
As of March 31, 2013, the Company had negative working capital of $474,759. Approximately $950,000 of the amount included in accounts payable is owed to a vendor and is not payable until the current project is completed, which is expected to be in the fourth quarter of 2013. Upon completion of the project, the Company will have the option to convert the balance owed to this vendor into equity and a five-year note payable.
Results of Operations for the Year Ended December 31, 2012 compared to the Year Ended December 31, 2011:
For the period from March 28, 2002 (date of inception) through December 31, 2012, we have not generated significant revenues and have been focused on developing our products for commercial sale. Q Therapeutics is considered to be a development stage company.
Since entering the development stage, we have not generated revenues in excess of expenses and have been dependent on government grants and debt and equity raised from individual investors to sustain our operations. Our products have not yet been approved by the FDA for commercial sale and as a result we have not yet generated revenues from product sales. We have incurred losses and used cash in operating activities since inception. As of December 31, 2012, the Company had an accumulated deficit of $20,492,278 and negative working capital.
Between October 13, 2011 and December 31, 2011, the Company raised $3,828,047 in connection with a private placement offering to accredited investors. Between January 1, 2012 and May 31, 2012, as part of a subsequent tranche of the private placement an additional $190,000 was raised. Management believes that the Company currently has sufficient liquidity and capital resources to sustain operations through at least December 31, 2013 as long as it manages and, in some cases reduces expenses until additional financing can be obtained.
61
Table of Contents
| For the Years Ended December 31, |
||||||||||||||||
| 2012 | 2011 | Change | % | |||||||||||||
| Revenues: |
||||||||||||||||
| Grant revenues |
$ | 485,031 | $ | 10,173 | $ | 474,858 | 4667.8 | % | ||||||||
| License fees and other revenues |
— | 164,400 | (164,400 | ) | -100.0 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total revenues |
485,031 | 174,573 | 310,458 | 177.8 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
2,089,321 | 444,834 | 1,644,487 | 369.7 | % | |||||||||||
| General and administrative |
1,458,088 | 1,567,012 | (108,924 | ) | -7.0 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
3,547,409 | 2,011,846 | 1,535,563 | 76.3 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(3,062,378 | ) | (1,837,273 | ) | (1,225,105 | ) | 66.7 | % | ||||||||
| Other income (expense), net |
4,538 | (762,065 | ) | 766,603 | -100.6 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Loss before provision (benefit) for income taxes |
(3,057,840 | ) | (2,599,338 | ) | (458,502 | ) | 17.6 | % | ||||||||
| Provision (benefit) for income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (3,057,840 | ) | $ | (2,599,338 | ) | $ | (458,502 | ) | 17.6 | % | |||||
|
|
|
|
|
|
|
|||||||||||
Grant Revenues
Grant revenues for the years ended December 31, 2012 and 2011 were $485,031 and $10,173, respectively, an increase of $474,858, or 4,668%. The increase was primarily due to being the recipient of a sub-award of an NIH grant issued to Johns Hopkins University. The sub-award was approved in December 2012 for a total of $631,383 for the 2012-2013 budget year. We anticipate grant revenues to increase as we identify new opportunities for grants that are available and specific to our research of cell-based therapies.
License Fees and Other Revenues
License fees and other revenues for the year ended December 31, 2012 were $0, a decrease of $164,400, or 100%, from $164,400 for the year ended December 31, 2011. The decrease was primarily attributable to non-recurring revenue of $150,000 recognized in 2011 relating to the right of first negotiation to a related party. As we do not currently have any agreements which require annual license fees, we do not expect to generate license fee revenue during 2013.
Research and Development Expenses
Q Therapeutics anticipates that development activities and costs will remain at levels similar to the three months ended December 31, 2012, as we advance the work necessary to complete our future IND submission. This includes GLP animal safety studies, injection device studies, manufacturing activities and working with clinical and regulatory consultants. Should additional financing be obtained, we may also increase research and development activities to evaluate use of our proprietary products in other disease indications, including working with outside collaborators. The following table details the research and development expenses we have incurred:
| For the Years Ended December 31, |
||||||||||||||||
| 2012 | 2011 | Change | % | |||||||||||||
| Lab materials and supplies |
$ | 1,369,780 | $ | 37,595 | $ | 1,332,185 | 3543.5 | % | ||||||||
| Salaries and benefits |
318,724 | 295,987 | 22,737 | 7.7 | % | |||||||||||
| License fees |
195,083 | — | 195,083 | 100.0 | % | |||||||||||
| Consulting |
100,540 | 3,413 | 97,127 | 2845.8 | % | |||||||||||
| Facility-related expenses |
88,798 | 81,045 | 7,753 | 9.6 | % | |||||||||||
| Travel and entertainment |
— | 1,288 | (1,288 | ) | -100.0 | % | ||||||||||
| Depreciation |
16,396 | 25,506 | (9,110 | ) | -35.7 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total research and development expenses |
$ | 2,089,321 | $ | 444,834 | $ | 1,644,487 | 369.7 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
62
Table of Contents
Research and development expenses for the year ended December 31, 2012 were $2,089,321, an increase of $1,644,487, or 369.7%, from $444,834 for the year ended December 31, 2011. The increase in expense is primarily the result of the associated costs relating to conducting our pilot and GLP animal safety studies, the initial cost and maintenance of licensing agreements which will support our clinical studies and an increase in consulting fees. We anticipate our research and development expenses will remain at similar levels for 2013 as we continue our safety studies, until additional financing can be obtained.
General and Administrative Expenses
The following table details the general and administrative expenses incurred by the Company:
| For the Years Ended December 31, |
||||||||||||||||
| 2012 | 2011 | Change | % | |||||||||||||
| Salaries and benefits |
$ | 594,343 | $ | 621,863 | $ | (27,520 | ) | -4.4 | % | |||||||
| Legal and professional fees |
456,334 | 361,337 | 94,997 | 26.3 | % | |||||||||||
| Stock-based compensation |
160,237 | 42,378 | 117,859 | 278.1 | % | |||||||||||
| General office and administrative |
102,625 | 88,283 | 14,342 | 16.2 | % | |||||||||||
| Facility-related expenses |
81,527 | 84,048 | (2,521 | ) | -3.0 | % | ||||||||||
| Travel and entertainment |
34,246 | 63,829 | (29,583 | ) | -46.3 | % | ||||||||||
| Bad debt expense |
26,518 | 2,628 | 23,890 | 909.1 | % | |||||||||||
| Depreciation |
1,846 | 2,222 | (376 | ) | -16.9 | % | ||||||||||
| Royalties |
412 | 300,424 | (300,012 | ) | -99.9 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total general and administrative expenses |
$ | 1,458,088 | $ | 1,567,012 | $ | (108,924 | ) | -7.0 | % | |||||||
|
|
|
|
|
|
|
|||||||||||
The net decrease in general and administrative expenses is primarily due to a royalty expense payment of $300,000 made in October 2011 that did not occur in 2012, offset, in part, by an increase in stock-based compensation related to option grants in 2012 and an increase in legal and professional fees resulting from the Company becoming a public reporting company. We anticipate general and administrative expenses to remain at approximately the same levels, or, if necessary, decrease due to our cash constraints until further financing becomes available.
Liquidity and Capital Resources
For the year ended December 31, 2012, net cash used in operating activities totaled $2,121,749 compared to $1,665,370 for the year ended December 31, 2011. Cash expenditures increased in 2012 primarily due to
63
Table of Contents
costs associated with conducting our pilot and GLP animal safety studies, the acquisition and maintenance costs associated with licensing technology, and legal and professional services resulting from becoming a public reporting company.
For the year ended December 31, 2012, net cash used in investing activities was $2,363 compared to $5,627 for the year ended December 31, 2011.
For the year ended December 31, 2012, net cash provided by financing activities was $176,800 compared to $3,990,318 for the year ended December 31, 2011. The primary reason for the change was that the Company raised $190,000 in 2012 compared to $3,828,047 in 2011 from the private placement of its securities.
We believe that our current levels of cash, when combined with (1) our expected cash flows from grant revenues, and (2) reductions to our current operating expenses, will be sufficient to meet our liquidity needs through at least the end of 2013. However, we will require additional cash resources in the future, as we anticipate changed business conditions and other developments in the Company’s progress. We will need additional cash resources in the future if we pursue opportunities for investment, acquisition, strategic cooperation or other similar actions. To satisfy future cash requirements, we expect to seek funding through the issuance of debt or equity securities or through a credit facility lender. Any future issuance of equity securities would cause dilution for our shareholders. Any incurrence of indebtedness will increase our debt service obligations and may cause us to be subject to restrictive operating and financial covenants. It is possible that financing may be available to us in amounts or on terms that are not favorable to the Company or not available at all.
Recent Accounting Pronouncements
As of the date of this report, there are no accounting pronouncements that had not yet been adopted by the Company that we believe would have a material impact on our financial statements. Additionally, the Company has reviewed all recently issued, but not yet effective, accounting pronouncements and does not believe the future adoption of any such pronouncements will have a material impact on the Company’s financial position, results of operations or liquidity.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, financings, or other relationships with unconsolidated entities or other persons, also known as special purpose entities.
Significant Accounting Policies
Our discussion and analysis of financial condition and results of operations is based upon the consolidated financial statements included herein, which have been prepared in accordance with U.S. generally accepted accounting principles and the requirements and regulations of the Securities and Exchange Commission (SEC). The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the dates of the financial statements and the reported amounts of sales and expenses during the reporting periods. Significant accounting policies and areas where substantial judgments are made by management include:
Stock-Based Compensation – We calculate the estimated fair value of our stock options and warrants on the grant date using the Black-Scholes option-pricing model and recognize the estimated fair value as compensation expense on a straight-line basis over the vesting period. We recognize stock compensation expense in the period in which the employee is required to provide service, which is generally over the
64
Table of Contents
vesting period of the individual equity instruments. Stock options issued in lieu of cash to non-employees for services performed are recorded at the fair value of the options at the time they are issued and are expensed as service is provided.
The volatility assumption used in the Black-Scholes option-pricing model is based on the volatility of publicly traded companies in our industry. The expected term of the options and warrants granted represent the periods of time that the options granted are expected to be outstanding. The risk free rate for periods within the contractual lives of the options and warrants is based on the U.S. treasury securities constant maturity rate that corresponds to the expected term in effect at the time of grant.
Revenue Recognition and Grants Receivable – We apply for research grants generally as a sub-recipient to grants funded by government agencies through research institutions. Grants receivable are recorded in accordance with the provisions defined in the sub-award or grant agreement. Grants receivable are considered past due when payment has not been received within 30 days of the invoice date though certain institutions customarily pay within 60 days of the invoice date. The amounts of the specific reserves are estimated by management based on various assumptions including the age of the individual grant receivable, as well as changes in payment schedules and histories. Grants receivable balances are charged off against the allowance for doubtful accounts when management determines the potential for recovery is remote. Recoveries of receivables previously charged off are recorded when payment is received. We did not incur any losses relating to bad debts during the last two years.
Income Taxes – We calculate federal and state taxes using the asset and liability method. Under the asset and liability method, deferred income tax assets or liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred income tax assets or liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates is recognized in net income (loss) in the period that includes the enactment date.
Net Loss Per common Share – Basic earnings (loss) per share (EPS) is calculated by dividing income (loss) available to common stockholders by the weighted-average number of common shares outstanding during the period.
Diluted EPS is similar to Basic EPS except that the weighted-average number of common shares outstanding is increased using the treasury stock method to include the number of additional common shares that would have been outstanding if the dilutive potential common shares had been issued. Such potentially dilutive common shares include stock options and warrants. Shares having an antidilutive effect on periods presented are not included in the computation of Diluted EPS.
65
Table of Contents
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors, Executive Officers, Promoters and Control Persons and Corporate Governance; Compliance with Section 16(a) of the Exchange Act.
The following table lists the names, age and positions of the individuals who serve on the Board of Directors of the Company:
| Name |
Age |
Directors and Officers | ||
| Deborah A. Eppstein, Ph.D. |
64 | President, Chief Executive Officer and Director | ||
| (Principal Executive Officer) | ||||
| Steven J. Borst, M.B.A. |
56 | Chief Financial Officer and Vice President Corporate Development | ||
| (Principal Financial and Accounting Officer) | ||||
| Dinesh C. Patel, Ph.D. |
62 | Chairman of the Board of Directors | ||
| Peter Barton Hutt |
78 | Director | ||
| Linda F. Powers |
57 | Director | ||
| Peter Grebow, Ph.D. |
66 | Director | ||
| Diane Jorkasky, M.D. |
61 | Director |
Business Experience of Directors
Deborah A. Eppstein, Ph.D. is President, CEO and a Member of the Board of Directors. Dr. Eppstein has been employed since February 2006 by Q Therapeutics, initially as President, then as President and CEO commencing September 2006. Dr. Eppstein’s business experience with Q encompasses strategic planning, scientific direction and oversight, capital formation, and managing and running the Company.
Dr. Eppstein has more than 30 years of experience in the pharmaceutical and biotech industries, with the latter 20 on the entrepreneurial side. She was the founding CEO of Altea Therapeutics. Previously, she was Vice President of Corporate Development at TheraTech, where she was involved with the IPO, partnering, achieving profitability and subsequent sale of the company. Earlier she was Director of Corporate Development and Department Head of biochemistry, virology and tumor biology at Syntex (now Roche). Dr. Eppstein received a B.A. from Grinnell College, a Ph.D. in biochemistry from the University of Arkansas, and she conducted research in virology and cell biology as an NIH postdoctoral fellow at the University of California, Santa Barbara. Dr. Eppstein has received the Women’s Technology Leadership and Businesswoman of the Year awards in Salt Lake City.
Steven J. Borst, M.B.A., is Chief Financial Officer and Vice President of Corporate Development. Mr. Borst has been employed at Q Therapeutics since 2002. He has served the Company as Vice President, Finance and Corporate Development, and as of October 2011, Mr. Borst has served as CFO and Vice President Corporate Development. Mr. Borst’s business experience with the Company over the last nine years encompasses seeking investors and concluding financings, working with corporate partners and managing facilities and operations.
Mr. Borst also serves concurrently as one of three Managing Directors of UpStart Ventures Management, which manages a seed stage life science-focused venture capital fund, and is considered an early-stage healthcare fund in Salt Lake City. He possesses both an operational and venture capital background in healthcare and the life sciences. He has also co-founded five Salt Lake biotech companies. Mr. Borst was previously associated with two Chicago-based venture funds and served in senior management positions with two venture-backed healthcare portfolio companies. Mr. Borst holds a B.S. degree in Industrial Engineering from the University of Michigan and an M.B.A. from the J.L. Kellogg Graduate School of Management at Northwestern University.
66
Table of Contents
Board of Directors
In addition to Dr. Eppstein, the Q Board of Directors is composed of:
Dinesh C. Patel, Ph.D., serves as Chairman of the Company’s Board of Directors. Dr. Patel is a Managing Director and Founding Partner of vSpring Capital (which is now SignalPeak), an early-stage venture capital fund with over $400 million under management (2000 to present). From 1985-1999, Dr. Patel served as co-founder, Chairman, President and CEO of TheraTech, Inc., a Salt Lake City, Utah-based company that has been a pioneer in the development and manufacturing of innovative drug delivery products. TheraTech went public in 1992, became profitable in 1997, and in 1999 was acquired for approximately $350 million by Watson Pharmaceuticals.
Dr. Patel has been the recipient of numerous awards including; Honorary Doctor of Business Degree, University of Utah (2008), the 2006 Utah Technology Council Hall of Fame, 2006 Pathfinder Award-Edison Showcase University of Utah, 2006 Ellis Island Medal of Honor, Utah Asian Chamber of Commerce Outstanding Business Award, U.S. Small Business Administration’s Business Achiever Award, Scientific and Technology Award (State of Utah), Entrepreneur of the Year Award (Mountain West Capital Network) and Scientific and Technology Development Pioneer of Progress Award. Dr. Patel served as co-chair of Governor Huntsman’s transition team and is currently on the board of the Utah Policy Partnership (UPP), the board of the Utah Symphony & Opera, the Chairman of the USTAR Governing Authority board and sits on the Utah Technology Council executive committee.
Born and raised in Zambia, Africa, Dr. Patel received his undergraduate degree in India and his doctorate degree from University of Michigan. He and his wife, Kalpana reside in Salt Lake City, Utah. Dr. Patel is active in the Indian and local community serving on several boards and is an active donor for various charitable causes.
The Company believes that the demonstrated leadership, excellent academic credentials, numerous awards and proven track record with both TheraTech and vSpring Capital, particularly his vast experience as it specifically relates to development and manufacturing of innovative drug delivery products, renders Dr. Patel an asset to our Company and an exemplary member and a qualified Chairperson of our Board of Directors.
Peter Barton Hutt, Director, has been a partner of the Washington, D.C.-based law firm of Covington & Burling, specializing in food and drug law, since 1968, except for the period from 1971 to 1975 when he served as Chief Counsel of the FDA. He received a B.A., magna cum laude, from Yale University, a L.L.B. from Harvard Law School and a L.L.M. from New York University. Mr. Hutt has served on the boards of several publicly traded biotechnology companies and is a member of the Institute of Medicine, National Academy of Sciences. Mr. Hutt has received numerous honors, including being named by the National Law Journal as one of the 40 best health care lawyers in the United States.
The Company believes that Mr. Hutt’s 40-plus year stellar legal and professional record, excellent academic credentials, numerous prior board of director memberships and experience for publicly traded biotech companies, and particularly his vast experience as it specifically relates to the biotech and medical fields in both a legal and business capacity, render Mr. Hutt an asset to our Company and a qualified member of our Board of Directors.
Linda F. Powers, Director, is a Managing Director and co-founder of Toucan Capital LLC, a Bethesda, MD venture fund that invested in Q’s Series A-2 round. From 2001 until 2011, Ms. Powers’ principal employment was as Managing Director of Toucan Capital, a biotech investment fund. Presently, her principal employment is CEO of Northwest Biotherapeutics (nwbio.com), a portfolio company of Toucan Capital. Northwest Biotherapeutics business involves developing immune therapies for cancer. Ms. Powers continues to serve as a Managing Director of Toucan Capital.
67
Table of Contents
Ms. Powers has more than 10 years of experience in seed and early-stage venture investing, and more than 18 years of experience in corporate finance and restructuring, mergers and acquisitions, joint ventures and intellectual property licensing. Ms. Powers holds an A.B. degree in economics from Princeton University, magna cum laude and Phi Beta Kappa, as well as a J.D. degree, magna cum laude, from Harvard Law School.
The Company believes that the combination of Ms. Powers’ distinguished academic credentials, substantial biotech venture fund management experience, and particularly her vast knowledge and business dealings as they specifically relate to the biotherapeutics and research and development fields in both a legal and business capacity, render Ms. Powers an asset to our Company and a qualified member of our Board of Directors.
Peter Grebow Ph.D., Director, has served as a director since December of 2011. Dr. Grebow held several key senior management positions at Cephalon Inc., a biopharmaceutical company (Cephalon) before retiring. Dr. Grebow joined Cephalon in January 1991, where he has served in several positions including Senior Vice President, Worldwide Business Development Senior Vice President, Drug Development, Executive Vice President of Technical Operations, and most recently Executive Vice President of Cephalon Ventures. Prior to joining Cephalon, Dr. Grebow served as the Vice President, Drug Development for Rorer Central Research, a division of Rhone-Poulenc Rorer Pharmaceuticals Inc., from 1988 to 1990. In addition, Dr. Grebow also serves as a director for Optimer Pharmaceuticals, Inc. since February 2009 and GenSpera, Inc. since May 2012.
Dr. Grebow received his undergraduate degree from Cornell University, a Master’s of Science in Chemistry from Rutgers University and a Ph.D. in Physical Biochemistry from the University of California, Santa Barbara. Dr. Grebow’s demonstrated leadership in his field, his knowledge of scientific matters affecting our business and his understanding of our industry render Dr. Grebow an asset to our Company and a qualified member of our Board of Directors.
Diane Jorkasky M.D., Director, joined our Board of Directors on March 8, 2013. She is a 25-year veteran of the pharmaceutical industry with experience in Phases 1-4 clinical trials, including protocol development, conduct and reporting; site placement strategy; and regulatory interaction. During nine years at Pfizer, Dr. Jorkasky held a number of positions of increasing responsibility advancing to Vice President of Development and Head of Worldwide Clinical Research Operations as well as the leader of the Operational Excellence Board for Development. She was responsible for ensuring that all exploratory development, clinical pharmacology, translational medicine and clinical technology studies were conducted and reported. In this role, she led the execution of over 160 clinical studies per year. At SmithKline Beecham, Dr. Jorkasky served as Vice President and Director of Clinical Pharmacology for North America. Currently, Dr. Jorkasky serves on the Board of Tengion, Inc., a regenerative medicine company focused on the repair and replacement of tissues and organs. She is a member of the Scientific Advisory Board of BioMotive and is the Chief Scientific Officer for Complexa, Inc., a biopharmaceutical company focused on discovering and developing innovative therapies for the treatment of inflammatory and metabolic diseases. Dr. Jorkasky is board certified in internal medicine, nephrology and clinical pharmacology. She is on the medical school faculties of Yale University, University of Pennsylvania, Uniformed Service of Health Sciences and is a Professor of Bioengineering and Therapeutic Sciences at the University of California, San Francisco. She has published over 100 peer review articles and is a Woodrow Wilson Visiting Fellow.
68
Table of Contents
Scientific Advisors and Consultants
Ian D. Duncan, BVMS, Ph.D., is a Professor in the Department of Medical Sciences at the University of Wisconsin. He is a leading research authority on myelin repair by cell transplantation using stem cells, with Multiple Sclerosis as a main interest. Dr. Duncan has also done extensive studies of leukodystrophies with stem cells. He received degrees in Veterinary Medicine and a Ph.D. from Glasgow University in Scotland. Dr. Duncan collaborates with Q in running animal studies in inflammatory demyelinating diseases such as MS.
Itzhak Fischer, Ph.D. is Professor and Chair, Neurobiology and Anatomy at Drexel University. Dr. Fischer is an expert in study of traumatic spinal cord injury and the role of transplanted progenitor cells of the central nervous system in providing therapeutic benefits in animal models. Dr. Fischer collaborates with the Company in evaluating activity of glial restricted progenitor cells in treatment of spinal cord injury in rodent models.
Douglas Kerr, M.D., Ph.D. is Medical Director, Neurology Research and Development at Biogen-Idec where he oversees late stage neurodegenerative programs, focusing on motor neuron disorders ALS and SMA. Dr. Kerr is an expert in autoimmune neurodegenerative diseases such as Multiple Sclerosis and Transverse Myelitis. Dr. Kerr previously established the world-leading Transverse Myelitis center at Johns Hopkins Medical School.
Nicholas J. Maragakis, M.D. is Associate Professor of Neurology at Johns Hopkins University. Dr. Maragakis treats patients with a variety of neuromuscular disorders, with an emphasis on patients with motor neuron diseases, such as Amyotrophic Lateral Sclerosis. This expertise is coordinated with the ALS clinic at Johns Hopkins, a multidisciplinary clinic. In addition to his clinical practice, Dr. Maragakis’ laboratory studies the role of astrocytes in neurological diseases, such as ALS. Q is conducting animal studies with Dr. Maragakis in models of ALS. Dr. Maragakis will be the PI for the Phase 1/2a clinical trial in ALS.
Thomas N. Parks, Ph.D. is Vice President of Research at the University of Utah School of Medicine, as well as Professor and Executive Director of the Brain Institute. He was a co-founder of NPS Pharmaceuticals, Inc. Dr. Parks received a B.S. in Biology from University of California, Irvine, and a Ph.D. in Psychobiology from Yale University.
Regulatory Consultants
Q Therapeutics has engaged experienced FDA consultants to advise on pre-clinical studies and manufacturing process development, and to assist in preparation of an IND application. They include:
Joy A. Cavagnaro, Ph.D. of Access Bio was previously Senior Pharmacologist at the CBER division of the FDA and was responsible for pre-clinical development and safety assessment of biological p Dr. Cavagnaro advises on pre-clinical studies.
Andra Miller, Ph.D., Senior Consultant with Biologics Consulting Group, provides advice on Chemistry, Manufacturing and Control (CMC) aspects of manufacturing Q-Cells.
Involvement in Certain Legal Proceedings
None of our executive officers, directors or named consultants has, during the past five years:
| a) | Had any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
69
Table of Contents
| b) | Been convicted in a proceeding or subject to a pending criminal proceeding; |
| c) | Been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities, futures, commodities or banking activities; |
| d) | Been found by a court of competent jurisdiction (in a civil action), the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated. |
Family Relationships
None.
Section 16(A) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our executive officers and directors, and persons who beneficially own more than 10% of a registered class of our equity securities to file with the Securities and Exchange Commission initial statements of beneficial ownership, reports of changes in ownership and annual reports concerning their ownership of our common shares and other equity securities, on Forms 3, 4 and 5, respectively. Executive officers, directors and greater than 10% shareholders are required by the Securities and Exchange Commission regulations to furnish us with copies of all Section 16(a) reports they file. Based on our review of the copies of such forms received by us, and to the best of our knowledge, all executive officers, directors and greater than 10% shareholders filed the required reports in a timely manner, except for the following: Deborah A. Eppstein, Steven J. Borst, Peter Grebow, Peter Barton Hutt had not filed their respective Form 4-s for their stock options granted on March 28, 2012 timely.
CORPORATE GOVERNANCE
During the year ended December 31, 2012, our Board of Directors held eight meetings. During the year ended December 31, 2012, all but one director, Joydeep Goswami, attended at least 75% of the total meetings of our Board of Directors which were held during the period the director was a director. Effective March 8, 2013, Joydeep Goswami resigned from his position as a director of the Company. The independent directors met in executive sessions at the end of scheduled Board meetings.
Compensation, Audit, and Nominating Committees
The Board of Directors has established a Compensation Committee, but does not yet have a charter. The Compensation Committee’s primary objective is to review, approve and recommend to the Board annual goals, objectives and compensation of the Chief Executive Officer and Chief Financial Officer and to evaluate their performance against those goals, to oversee executive performance and to approve and recommend their compensation, to oversee the administration of equity-based compensation and to recommend to the Board granting of stock option grants and awards, and to manage the overall compensation policies relating to employees. In 2012, a peer review of compensation for executives in similar biotech companies in our industry was conducted and compared to the current compensation of executives. The Compensation Committee currently consists of director Peter Grebow.
The Board has not yet formally established separate audit, or nominating committees though the Board performs many of the functions that would otherwise be delegated to such committees. Currently, the Board of Directors believes that the cost of establishing such committees, including the costs necessary to recruit and retain qualified independent directors to serve on the Board of Directors and such committees and the
70
Table of Contents
legal costs to properly form and document the authority, policies and procedures of such committees are not justified under the Company’s current circumstances. However, it is anticipated that our Board of Directors will seek qualified independent directors to serve on the Board and ultimately form standing nominating and compensation committees and nominate other directors to serve on its audit committee.
Communications with the Board
Stockholders and other interested parties may communicate with one or more directors or the non-management directors as a group in writing by regular mail. The following address may be used by those who wish to send such communications by regular mail:
Board of Directors or Name of Individual Director(s)
c/o Corporate Secretary
Q Therapeutics, Inc.
615 Arapeen Drive, Suite 102
Salt Lake City, UT 84108
Code of Ethics and Conduct
In September 2012, the Board of Directors adopted a written Code of Ethics and Conduct for our directors, officers, employees and consultants. The Code of Ethics and Conduct is available on the Company’s website at www.qthera.com or upon written request to Q Therapeutics, Inc. 615 Arapeen Dr. Suite 102, Salt Lake City, Utah 84108, Attention: Investor Relations. All officers, directors and employees are subject to the Code of Ethics and Conduct. We have implemented a whistleblower policy which provides a means by which any conduct that is not compliant with the Code of Ethics and Conduct can be reported anonymously and confidentially.
Our Board of Directors has authorized the compensation of its officers with the following annual cash salaries for 2013:
| Deborah A. Eppstein, President and Chief Executive Officer |
$ | 257,500 | ||
| Steven J. Borst, Chief Financial Officer and Vice President of Corporate Development |
$ | 206,000 | ||
In March 2013, certain of the Company’s executives made the decision to defer cash payment of 60% of their salaries until additional funding has been obtained.
The following table shows all compensation awarded to, earned by, or paid to Deborah A. Eppstein, our President and Chief Executive Officer, and Steven J. Borst, our Chief Financial Officer and Vice President of Corporate Development, for the years ended December 31, 2012 and 2011.
SUMMARY COMPENSATION TABLE
| Name |
Title |
Fiscal Year |
Salary ($) |
Bonus ($) |
Option Awards (1) ($) |
Other Comp. (2) ($) |
Total ($) |
|||||||||||||||||||
| Deborah A. Eppstein, Ph.D. |
President and CEO | 2012 | 250,000 | 50,000 | 153,850 | 225 | 454,075 | |||||||||||||||||||
| 2011 | 121,076 | 134,000 | — | 213 | 255,289 | |||||||||||||||||||||
| Steven J. Borst |
CFO and Vice President of Corporate |
2012 | 200,000 | 50,000 | 153,850 | 215 | 404,065 | |||||||||||||||||||
| 2011 | 90,000 | 106,000 | — | 213 | 196,213 | |||||||||||||||||||||
71
Table of Contents
| (1) | Represents the grant date fair value of options computed in accordance with FASB ASC Topic 718. Options vest over a four-year period which includes cliff vesting for the first three months and monthly vesting thereafter. These amounts represent the fair value of the awards on the grant date and do not correspond to the actual income that will be recognized by the officers. |
| (2) | Represents Group Term Life insurance premiums paid. |
NARRATIVE DISCLOSURES TO SUMMARY COMPENSATION TABLE
It is our policy to award stock options at the fair market value of our common stock on the business day prior to the date of the grant in conjunction with the terms of the 2011 Q Holdings Stock Incentive Plan. The grant date is deemed to be the date on which the Board of Directors approves of the stock option grant.
Q Therapeutic Products, Inc. entered into an Employment and Proprietary Rights Agreement (original agreement) with Deborah A. Eppstein on February 24, 2006. The terms of the original agreement specified salary, vacation, bonus, employee stock option grants, and change of control conditions. On October 13, 2011, in conjunction with the reverse merger, Q Therapeutics, Inc. entered into an Employment and Proprietary Rights Agreement, which defaulted to the original agreement should any inconsistencies or omissions arise. On March 28, 2012, Ms. Eppstein received an option to purchase 250,000 shares of common stock at $1.00 per share, with 15,625 shares immediately vested upon the initial grant and the remaining shares to be vested in an equal amount over the next 45 months.
Q Therapeutic Products, Inc. entered into an Employment and Proprietary Rights Agreement (original agreement) with Steven J. Borst. The terms of the original agreement specified salary, vacation, bonus, employee stock option grants, and change of control conditions. On October 13, 2011, in conjunction with the reverse merger, Q Therapeutics, Inc. entered into an Employment and Proprietary Rights Agreement, which defaulted to the original agreement should any inconsistencies or omissions arise. On March 28, 2012, Mr. Borst received an option to purchase 250,000 shares of common stock at $1.00 per share, with 15,625 shares immediately vested upon the initial grant and the remaining shares to be vested in an equal amount over the next 45 months.
On December 18, 2012, our Board of Directors approved and subsequently amended the terms of our employment agreements with our executives. The amendment provided clarity in regards to the definition of compensation as including salary, cash bonus, equity incentives and benefits as determined by the Company from time to time, at the Board of Director’s discretion, and the liability associated with the employee’s separation from the Company. Should the employee be terminated for cause or voluntarily terminate without good reason, he or she is entitled to receive accrued compensation through the date of termination. Termination for cause can result from the employee being convicted of a felony, performing an act of fraud, theft or embezzlement involving his or her employment with the Company, or refusing to carry out the duties and responsibilities of his or her employment in a manner reasonably satisfactory to a majority of the members of the Board, and such refusal is not cured within 30 days after receipt of written notification. Should the employee be terminated without cause or voluntarily terminate for good reason, he or she is entitled to receive 18 month’s salary for Ms. Eppstein or 12 month’s salary for Mr. Borst (plus benefits, accrued vacation earned and any bonus granted) to be paid within ten days of such termination.
72
Table of Contents
Additionally, the Board of Directors approved the 2012 Compensation Plan (the 2012 Plan) for our executives and employees. The 2012 Plan provided for salary increases, cash bonus and stock/option awards. However, the achievement of the bonus portion is tied to successfully raising at least $2.5 million in financing. To date, no such financing has been completed.
OUTSTANDING EQUITY AWARDS AS OF DECEMBER 31, 2012
EQUITY INCENTIVE PLAN: STOCK OPTION AWARDS
EQUITY INCENTIVE PLAN: STOCK OPTION AWARDS
OUTSTANDING EQUITY AWARDS AS OF DECEMBER 31, 2012
| Name (a) |
Number of securities underlying unexercised options (#) exercisable (b) |
Number of securities underlying unexercised options (#) unexerciseable (c) |
Option Exercise Price ($) (e) |
Option Expiration Date (f) |
||||||||||||
| Deborah Eppstein |
141,377 | — | 0.1525 | (1) | 2/24/2017 | |||||||||||
| Deborah Eppstein |
28,327 | — | 0.1525 | (2) | 2/9/2019 | |||||||||||
| Deborah Eppstein |
346,141 | — | 0.1525 | (3) | 3/19/2019 | |||||||||||
| Deborah Eppstein |
448,956 | — | 0.0786 | (4) | 10/13/2019 | |||||||||||
| Deborah Eppstein |
216,338 | — | 0.1941 | (5) | 12/22/2020 | |||||||||||
| Deborah Eppstein |
216,338 | 24,037 | 0.1941 | (6) | 12/22/2020 | |||||||||||
| Deborah Eppstein |
250,000 | 187,500 | 1.0000 | (7) | 3/28/2022 | |||||||||||
| Steven Borst |
18,930 | — | 0.1525 | (2) | 2/9/2019 | |||||||||||
| Steven Borst |
173,071 | — | 0.1525 | (3) | 3/19/2019 | |||||||||||
| Steven Borst |
209,995 | — | 0.0786 | (4) | 10/13/2019 | |||||||||||
| Steven Borst |
216,338 | — | 0.1941 | (5) | 12/22/2020 | |||||||||||
| Steven Borst |
216,338 | 24,037 | 0.1941 | (6) | 12/22/2020 | |||||||||||
| Steven Borst |
250,000 | 187,500 | 1.0000 | (7) | 3/28/2022 | |||||||||||
| (1) | 25% of the option shares vested immediately on February 24, 2007 with the remaining option shares vesting monthly over the following 36 months. |
| (2) | 16.67% of the option shares vested monthly for six months. |
| (3) | 25% of the option shares vested immediately on March 19, 2009 with the remaining option shares vesting monthly over the following 36 months. |
| (4) | 58.34% of the option shares vested immediately on November 1, 2009, with the remaining option shares vesting monthly over the following five months. |
| (5) | 50% of the option shares vested immediately on December 22, 2010 with the remaining option shares vesting monthly over the following 18 months. |
| (6) | 50% of the option shares vested immediately upon a qualifying event dated October 13, 2011 with the remaining option shares vesting monthly over the following 18 months following the qualifying event. |
| (7) | 6.25% of the option shares vested on March 28, 2012 with the remaining option shares vesting monthly over the following 45 months. |
73
Table of Contents
Plan:
2002 stock Option Plan
In April 2002, the Q Therapeutics’ Board of Directors approved the Q Therapeutics 2002 stock Incentive Plan (the 2002 Plan) and in February 2003, the holders of a majority of the outstanding voting capital stock of Q Therapeutics approved of the 2002 Plan. The 2002 Plan permits the grant of Incentive stock Options, Non-Qualified stock Options and Restricted stock. Subject to the provisions of the Plan, a designated committee (Committee) of the Board of Directors (or if none, the Board) may from time to time, in its sole discretion select from among eligible employees, non-employee directors, consultants and advisors, those to whom awards shall be granted under the 2002 Plan, and shall determine in its discretion the nature, terms, conditions, and amount of each award, subject to the terms of the 2002 Plan. The term of the 2002 Plan commenced on April 10, 2002 (the Effective Date) and remained in effect, subject to the right of the Committee or the Board to amend or terminate the Plan at any time pursuant to the 2002 Plan, until the earlier of (i) the tenth anniversary date of the Effective Date, or (ii) all shares subject to the 2002 Plan have been purchased or acquired according to the 2002 Plan’s provisions.
The 2002 Plan initially provided for a reservation pool of up to 1,100,000 shares of common stock reserved for issuance pursuant to the 2002 Plan. The total number of shares reserved for issuance under the 2002 Plan has since been increased to a total of 2,120,000 shares. Upon the consummation of the Merger, each option to purchase shares of common stock of Q Therapeutics Products, Inc. was exchanged for 2.1633835 options to purchase shares of common stock of the Company resulting in a total of 4,586,373 shares of common stock reserved for issuance under the 2002 Plan. All shares available for award under the 2002 Plan have been granted except for potential awards to acquire 228,472 shares of common stock. By Board authorization on December 6, 2011, the Board added the remaining 228,472 shares available but unissued pursuant to the 2002 Option Plan to the authorized/reserved option pool for the 2011 Plan (discussed below), and thus effectively terminated the 2002 Plan.
2011 Equity Incentive Compensation Plan
In connection with the Merger, on October 13, 2011 the Board of Directors and stockholders approved the Q Holdings 2011 Equity Incentive Compensation Plan (the 2011 Plan). Subject to the provisions of the 2011 Plan, a designated committee (Committee) of the Board of Directors (or if none, the Board) may, from time to time, in its sole discretion select from among eligible employees, non-employee directors and consultants those to whom awards shall be granted under the 2011 Plan, and shall determine in its discretion the nature, terms, conditions and amount of each award, subject to the terms of the 2011 Plan. The term of the 2011 Plan commenced on October 13, 2011 (the Effective Date) and remains in effect, subject to the right of the Committee or the Board to amend or terminate the 2011 Plan at any time pursuant to the 2011 Plan, until the earlier of (i) the tenth anniversary of the Effective Date, or (ii) all shares subject to the 2011 Plan have been purchased or acquired according to the 2011 Plan’s provisions.
The 2011 Plan initially provided for a reservation pool of up to 1,500,000 shares of common stock reserved for issuance pursuant to the 2011 Plan. By Board authorization on December 6, 2011, the Board voted to add the remaining 228,472 shares available but unissued pursuant to the 2002 Plan to the authorized/reserved option pool for the 2011 Plan, bringing the 2011 Plan pool to 1,728,472 shares of common stock reserved for issuance pursuant to the 2011 Plan. In 2012, the Company issued options to acquire 890,000 shares of common stock pursuant to the 2011 Plan. Additionally, the Board also resolved to roll over all forfeited or expired awards made under the 2002 Plan into the 2011 Plan.
On December 18, 2012, the Board of Directors approved, and in May 2013 68.9% of the stockholders approved, the addition of 3,000,000 shares to the 2011 Plan. As of July 5, 2013, there were 3,987,529 options available to be issued under the 2011 Plan.
74
Table of Contents
Director Compensation:
SUMMARY COMPENSATION TABLE
| Name |
Title | Fiscal Year |
Option Awards (1)($) |
|||||
| Peter Grebow |
Director | 2012(2) | 13,948 | |||||
| Peter Barton Hutt |
Director | 2012(3) | 13,948 | |||||
| (1) | Represents the grant date fair value of options computed in accordance with FASB ASC Topic 718. Options vest over twelve months. These amounts represent the fair value of the awards on the grant date and do not correspond to the actual income that will be recognized by the officers. |
| (2) | Mr. Grebow had 25,000 options outstanding as of December 31, 2012. |
| (3) | Mr. Hutt had 122,352 options outstanding as of December 31, 2012. |
For 2012, the Board of Directors approved compensation for Peter Grebow and Peter Barton Hutt, the two outside board members who are not also officers or investors of the Company with an annual stock option grant to purchase 25,000 shares of common stock for each year of service. In December 2012, the annual stock option grant to the directors increased to 50,000 shares of common stock for each year of service thereafter. Such options vest monthly over the course of a 12-month period and are considered fully vested at year end. The Company has not yet issued these options as the option price is not yet determinable. Once a fair market value can be determined, the options will be issued accordingly. As of December 31, 2012, the 25,000 options granted to each individual for 2012 were fully vested. No such grants were made to directors in 2011. Aside from these two outside director compensation arrangements, our Directors receive no compensation for their service. Directors may be reimbursed for expenses incurred in attending meetings of the Board of Directors.
75
Table of Contents
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
| Name of Beneficial Owner |
Number of Shares of Voting Stock Beneficially Owned (1) |
Percentage of Class (1) |
||||||
| Deborah A. Eppstein, Ph.D., President, CEO and Director (2) |
2,202,707 | 8.4 | % | |||||
| Steven J. Borst, CFO and Vice President of Corporate Development (3) |
1,465,765 | 5.7 | % | |||||
| Dinesh C. Patel, Ph.D., Chairman (4) |
7,243,123 | 28.4 | % | |||||
| Linda F. Powers, Director (5) |
1,497,496 | 6.0 | % | |||||
| Peter Barton Hutt, Director (6) |
154,803 | 0.6 | % | |||||
| Peter Grebow Ph.D., Director (7) |
25,000 | 0.1 | % | |||||
| Cephalon, Inc., |
11,056,641 | 34.4 | % | |||||
| UpStart Life Sciences Capital, L.P. |
2,884,576 | 10.8 | % | |||||
| Life Technologies Corporation, |
2,445,564 | 9.9 | % | |||||
| All Directors and Executive Officers as a Group (6 persons) (11) |
12,588,894 | 49.6 | % | |||||
| (1) | Percentage ownership is based on 24,811,833 shares of common stock outstanding as of July 5, 2013 and any shares of common stock that the beneficial owner has the right to acquire within 60 days. Any securities not outstanding which are subject to such options, warrants, rights or conversion privileges shall be deemed to be outstanding for the purpose of computing the percentage of outstanding securities of the class owned by such person but shall not be deemed to be outstanding for the purpose of computing the percentage of the class by any other person. Except as otherwise indicated, each of the persons and entities named in the table has sole voting and dispositive power with respect to all shares of common shares owned by them. |
| (2) | Includes 701,062 shares of common stock and 1,501,645 options exercisable for shares of common within 60 days of July 5, 2013. |
| (3) | Includes 495,871 shares of common stock and 938,839 options exercisable for shares of common stock within 60 days of July 5, 2013 owned directly by Mr. Borst. Includes indirect ownership of 5,435 shares of common stock, 10,185 Series A warrants to purchase common stock, 10,185 Series B warrants to purchase common stock and 5,250 Bridge warrants to purchase common stock owned by Mr. Borst’s spouse. |
| (4) | Shares are owned by vSpring, Mr. Patel’s employer and includes 5,246,849 shares of common stock and warrants to purchase 535,154 shares of common stock each held directly vSpring SBIC, L.P. Includes 1,099,410 shares of common stock and warrants to purchase 156,949 shares of common stock held directly by vSpring, L.P. Includes 141,319 shares of common stock and warrants to purchase 20,174 shares of common stock held directly by vSpring Partners, L.P. Includes 43,268 shares of Common Stock owned by Mr. Patel personally, for which he also has sole voting and dispositive power. |
| (5) | Includes 1,400,916 of common stock owned by Toucan Capital Fund III, L.P. and warrants to purchase 96,580 of common stock owned by Toucan Capital Corp. |
| (6) | Includes 32,451 shares of common stock and 122,352 options exercisable to purchase common stock within 60 days of July 5, 2013. |
| (7) | Includes 25,000 options exercisable to purchase common stock within 60 days of July 5, 2013. |
| (8) | Includes 3,685,547 shares of common stock and warrants to purchase 7,371,094 shares of common stock. |
| (9) | Includes 962,384 shares of common stock and warrants to purchase 1,922,192 shares of common stock. Upstart Ventures Management, L.L.C. as the general partner of UpStart Life Sciences Capital, LP has voting and dispositive control over the shares held by UpStart Life Sciences Capital, LP. UpStart Ventures Management, LLC is managed collectively by Dennis B. Farrar, Theodore H. Stanley, and Steven J. Borst. |
| (10) | Includes 2,445,564 shares of common stock. |
| (11) | Includes options for an aggregate of 2,587,836 shares of common stock held by executive officers and directors as a group that are exercisable within 60 days of July 5, 2013. |
76
Table of Contents
Related Party and Certain Transactions
None.
Director Independence
Our determination of independence of our directors is made using the definition of “independent director” contained under NASDAQ Marketplace Rule 4200(a)(15), even though such definitions do not currently apply to us because we are not listed on NASDAQ. With the exception of Ms. Eppstein, who currently serves in the capacity of Officer and Director, we believe our Board of Directors is comprised of members who qualify as independent pursuant to this Rule.
Indemnification
Pursuant to the Certificate of Incorporation and By-Laws of the Company, we may indemnify an officer or director who is made a party to any proceeding, including a law suit, because of his position, if he acted in good faith and in a manner he reasonably believed to be in our best interest. In certain cases, we may advance expenses incurred in defending any such proceeding. To the extent that the officer or director is successful on the merits in any such proceeding as to which such person is to be indemnified, we must indemnify him against all expenses incurred, including attorney’s fees. With respect to a derivative action, indemnity may be made only for expenses actually and reasonably incurred in defending the proceeding, and if the officer or director is judged liable, only by a court order. The indemnification is intended to be to the fullest extent permitted by the laws of the State of Delaware.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the provisions above, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities, other than the payment by us of expenses incurred or paid by one of our directors, officers, or controlling persons in the successful defense of any action, suit or proceeding, is asserted by one of our directors, officers, or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification is against public policy as expressed in the Securities Act, and we will be governed by the final adjudication of such issue.
INTERESTS OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis, or had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in our company or any of its parents or subsidiaries. Nor was any such person connected with our company or any of its parents or subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
77
Table of Contents
Validity of the securities offered by this prospectus will be passed upon for us by Life Science Law, PC. The underwriter is being represented by LeClairRyan, A Professional Corporation.
The financial statements included in this prospectus have been audited by Tanner LLC, an independent registered public accounting firm, as indicated in their report with respect thereto, and are included herein in reliance upon the authority of said firm as experts in auditing and accounting in giving said report.
DISCLOSURE OF COMMISSION POSITION OF
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our Certificate of Incorporation and By-Laws provide no director shall be liable to the Company or any of its stockholders for monetary damages for breach of fiduciary duty as a director, except with respect to (1) a breach of the director’s duty of loyalty to the Company or its stockholders, (2) acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) liability which may be specifically defined by law or (4) a transaction from which the director derived an improper personal benefit, it being the intention of the foregoing provision to eliminate the liability of the Company’s directors to the Company or its stockholders to the fullest extent permitted by law. The Company shall indemnify to the fullest extent permitted by law each person that such law grants the Company the power to indemnify.
We have been advised that, in the opinion of the SEC, indemnification for liabilities arising under the Securities Act is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, unless directed otherwise by the opinion of our legal counsel, we will submit the question of whether such indemnification is against public policy to a court of appropriate jurisdiction.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 under the Securities Act with the Securities and Exchange Commission with respect to the shares of our common stock and warrants to purchase common stock offered through this prospectus. This prospectus is filed as a part of that registration statement, but does not contain all of the information contained in the registration statement and exhibits. Statements made in the registration statement are summaries of the material terms of the referenced contracts, agreements or documents of our company. We refer you to our registration statement and each exhibit attached to it for a more detailed description of matters involving our company and the statements we have made in this prospectus are qualified in their entirety by reference to these additional materials. You may inspect the registration statement, exhibits and schedules filed with the Securities and Exchange Commission at the SEC’s principal office in Washington, D.C. Copies of all or any part of the registration statement may be obtained from the Public Reference Section of the SEC, Room 1580, 100 F Street NE, Washington D.C. 20549. Please call the Securities and Exchange Commission at 1-800-SEC-0330 for further information on the operation of the public reference rooms. The Securities and Exchange Commission also maintains a
78
Table of Contents
website at http://www.sec.gov that contains reports, proxy statements and information regarding registrants that file electronically with the SEC. Our registration statement and the referenced exhibits can also be found on this site. You can also request copies of these documents, via our website at www.qthera.com. In addition, we will provide electronic or paper copies of our filings free of charge upon request.
79
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
| Item: |
Page: | |
| Condensed Consolidated Financial Statements as of March 31, 2013 and December 31, 2012 and for the Quarters Ended March 31, 2013 and 2012 (Unaudited) |
||
| F-1 | ||
| F-2 | ||
| F-3 | ||
| F-5 | ||
| Consolidated Financial Statements as of and for the Years Ended December 31, 2012 and 2011 (Audited) |
||
| F-10 | ||
| F-11 | ||
| F-12 | ||
| F-13 | ||
| F-15 | ||
| F-17 | ||
80
Table of Contents
(A Development Stage Company)
Condensed Consolidated Balance Sheets (Unaudited)
As of March 31, 2013 and December 31, 2012
| March 31, 2013 |
December 31, 2012 |
|||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash |
$ | 654,052 | $ | 794,207 | ||||
| Receivables, net of allowance of $28,800 as of each of March 31, 2013 and December 31, 2012 |
1,834 | 477,802 | ||||||
| Prepaid expenses and other |
9,657 | 10,366 | ||||||
|
|
|
|
|
|||||
| Total current assets |
665,543 | 1,282,375 | ||||||
| Property and equipment, net |
31,558 | 16,044 | ||||||
| Other assets |
7,513 | 7,513 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 704,614 | $ | 1,305,932 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity (Deficit) |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 997,363 | $ | 1,203,365 | ||||
| Accrued liabilities |
28,124 | 9,685 | ||||||
| Accrued compensation |
114,815 | 87,892 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
1,140,302 | 1,300,942 | ||||||
|
|
|
|
|
|||||
| Stockholders’ equity (deficit): |
||||||||
| Common stock, $0.0001 par value: 100,000,000 shares authorized; 24,786,833 and 24,761,832 shares outstanding as of March 31, 2013 and December 31, 2012, respectively |
2,479 | 2,476 | ||||||
| Additional paid-in capital |
20,553,390 | 20,494,792 | ||||||
| Accumulated deficit |
(20,991,557 | ) | (20,492,278 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity (deficit) |
(435,688 | ) | 4,990 | |||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity (deficit) |
$ | 704,614 | $ | 1,305,932 | ||||
|
|
|
|
|
|||||
See accompanying notes to condensed consolidated financial statements.
F-1
Table of Contents
(A Development Stage Company)
Condensed Consolidated Statements of Operations (Unaudited)
For the Three Months Ended March 31, 2013 and 2012 and
for the Period from March 28, 2002 (Date of Inception) to March 31, 2013
| For the Three Months Ended March 31, |
Cumulative From |
|||||||||||
| 2013 | 2012 | Inception | ||||||||||
| Grant revenues |
$ | 5,501 | $ | — | $ | 1,095,760 | ||||||
| License fees and other revenues |
— | — | 282,900 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating revenues |
5,501 | — | 1,378,660 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Research and development |
131,109 | 224,207 | 11,104,001 | |||||||||
| General and administrative |
374,719 | 385,734 | 9,493,954 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
505,828 | 609,941 | 20,597,955 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(500,327 | ) | (609,941 | ) | (19,219,295 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other income (expense): |
||||||||||||
| Interest income |
— | 441 | 187,616 | |||||||||
| Interest expense |
(325 | ) | (2,020 | ) | (2,111,739 | ) | ||||||
| Other income (expense) |
1,373 | 606 | 151,861 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total other expense, net |
1,048 | (973 | ) | (1,772,262 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before provision for income taxes |
(499,279 | ) | (610,914 | ) | (20,991,557 | ) | ||||||
| Provision for income taxes |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (499,279 | ) | $ | (610,914 | ) | $ | (20,991,557 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted average number of common shares outstanding - basic and diluted |
24,778,221 | 24,636,955 | ||||||||||
| Net loss per common share - basic and diluted |
$ | (0.02 | ) | $ | (0.02 | ) | ||||||
See accompanying notes to condensed consolidated financial statements.
F-2
Table of Contents
(A Development Stage Company)
Condensed Consolidated Statements of Cash Flows (Unaudited)
For the Three Months Ended March 31, 2013 and 2012 and
for the Period from March 28, 2002 (Date of Inception) to March 31, 2013
| For the Three Months Ended March 31, |
Cumulative From |
|||||||||||
| 2013 | 2012 | Inception | ||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (499,279 | ) | $ | (610,914 | ) | $ | (20,991,557 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
4,337 | 4,968 | 395,984 | |||||||||
| Original debt discount |
— | — | 450,000 | |||||||||
| Accretion of debt costs and beneficial conversion feature |
— | — | 1,237,263 | |||||||||
| Stock-based compensation |
25,438 | 29,308 | 457,239 | |||||||||
| Debt issued for services |
— | — | 90,000 | |||||||||
| Common stock issued for services |
25,001 | — | 181,751 | |||||||||
| Preferred stock issued for services |
— | — | 44,750 | |||||||||
| Warrants issued for services |
8,162 | — | 21,248 | |||||||||
| Provision for losses on receivables |
— | — | (43,677 | ) | ||||||||
| Decrease (increase) in: |
— | |||||||||||
| Receivables |
475,968 | 22,328 | 41,843 | |||||||||
| Prepaid expenses and other assets |
709 | — | (17,170 | ) | ||||||||
| Increase (decrease) in: |
— | |||||||||||
| Accounts payable and accrued liabilities |
(187,563 | ) | (24,739 | ) | 1,424,606 | |||||||
| Accrued compensation |
26,923 | 29,941 | 114,815 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(120,304 | ) | (549,108 | ) | (16,592,905 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities: |
||||||||||||
| Purchase of property and equipment |
(19,851 | ) | (1,323 | ) | (427,322 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from issuance of notes payable |
— | — | 5,257,562 | |||||||||
| Payments on short-term note payable |
— | (15,000 | ) | (90,000 | ) | |||||||
| Issuance of preferred stock for cash |
— | — | 8,671,747 | |||||||||
| Issuance of common stock for cash |
— | 190,000 | 3,821,137 | |||||||||
| Proceeds from exercise of common stock options |
— | — | 11,600 | |||||||||
| Proceeds from exercise of preferred stock warrants |
— | — | 2,233 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
— | 175,000 | 17,674,279 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net change in cash |
(140,155 | ) | (375,431 | ) | 654,052 | |||||||
| Cash as of the beginning of the period |
794,207 | 2,741,519 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash as the end of the period |
$ | 654,052 | $ | 2,366,088 | $ | 654,052 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information: |
||||||||||||
| Cash paid for interest |
$ | 325 | $ | 2,020 | $ | 7,996 | ||||||
See accompanying notes to condensed consolidated financial statements.
F-3
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Condensed Consolidated Statements of Cash Flows (Unaudited)
(Continued)
For the Three Months Ended March 31, 2013 and 2012 and
for the Period from March 28, 2002 (Date of Inception) to March 31, 2013
Supplemental disclosure of noncash investing and financing activities for the period from March 28, 2002 (date of inception) to March 31, 2013:
| • | The Company issued 219,658 shares of common stock in exchange for technology valued at $220. |
| • | The Company converted $1,050,000 of notes payable and $29,691 of accrued interest to 482,008 shares of Series A2 preferred stock. |
| • | The Company converted $3,740,000 of notes payable and $370,346 of accrued interest to 1,787,104 shares of Series B preferred stock. |
| • | The Company recorded a debt discount of $1,237,263 related to preferred stock warrants issued with debt and the beneficial conversion feature. |
| • | The Company converted $900,000 of bridge notes payable and $16,644 of accrued interest to 916,644 shares of common stock. |
| • | The Company converted 250,000 shares of Series A1 preferred stock, 2,022,190 shares of Series A2 preferred stock, and 4,102,654 shares of Series B preferred stock to 13,791,231 shares of common stock. |
| • | Two stockholders forfeited, and the Company retired, 200,000 shares of common stock with a net impact on equity of $19 as a result of untimely payments on their notes. |
See accompanying notes to condensed consolidated financial statements.
F-4
Table of Contents
(A Development Stage Company)
Notes to Condensed Consolidated Financial Statements
1. Organization
Q Therapeutics, Inc. (formerly Q Holdings, Inc.) is a development stage biopharmaceutical company headquartered in Salt Lake City, Utah. The Company is engaged in developing adult progenitor cell therapies to treat debilitating diseases of the central nervous system. Q Therapeutics, Inc. was formed October 27, 2005 as a Delaware corporation. The Company’s operating subsidiary, Q Therapeutic Products, Inc., was formed on March 28, 2002 as a Delaware corporation. The Company’s first product candidate, Q-Cells®, is a cell-based therapeutic intended to restore or preserve normal activity of neurons by providing essential support functions that occur in healthy central nervous system tissues. The Company believes that Q-Cells may be applicable to a wide range of central nervous system diseases, including demyelinating conditions such as multiple sclerosis, transverse myelitis, cerebral palsy and stroke, as well as other neurodegenerative diseases and injuries, such as ALS (Lou Gehrig’s disease), spinal cord injury, Parkinson’s disease and Alzheimer’s disease.
2. Significant Accounting Policies
The following significant accounting policies are followed by the Company in preparing its consolidated financial statements:
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements of Q Therapeutics, Inc. and subsidiary do not include all information and notes necessary for a complete presentation of financial position, results of operations, and cash flows in conformity with U.S. generally accepted accounting principles (US GAAP).
The accompanying unaudited condensed consolidated balance sheets, statements of operations, and statements of cash flows reflect all normal recurring adjustments that are, in the opinion of management, necessary for a fair presentation of the Company’s financial position, results of operations and cash flows. The results of operations for the three-month period ended March 31, 2013 are not necessarily indicative of the results to be expected for the full year ending December 31, 2013.
Basis of Consolidation
The accompanying unaudited condensed consolidated financial statements include the accounts of Q Therapeutics, Inc. and its wholly owned subsidiary, Q Therapeutic Products, Inc., and Q Therapeutic Products, Inc.’s wholly owned subsidiary, NeuroQ Research Inc. (collectively, the Company). The Company is a development stage company engaged in the discovery, research, development and eventual commercialization of products as potential treatments for debilitating and fatal diseases of the central nervous system. All significant intercompany amounts have been eliminated.
Development Stage and Liquidity
For the period from March 28, 2002 (date of inception) through March 31, 2013, the Company has not generated significant revenues and has been developing its products. Therefore, the Company is considered to be in the development stage in accordance with the provisions of Accounting Standards Codification (ASC) Topic 915, Development Stage Entities. Cumulative amounts have been presented for the period from March 28, 2002 (date of inception) through March 31, 2013. Historically, the Company has been dependent on government grants and debt and equity raised from individual investors to sustain its operations. The Company’s continued operations will depend on its ability to raise funds through various sources such as government grants and equity and debt financing. The Company expects to continue to fund operations through similar sources of capital previously described. There can be no assurance that such capital will be available on favorable terms or at all. If it is unable to raise additional capital, the Company will likely be forced to curtail desired development activities, which will delay the development of its potential product candidates. The Company’s products have not been approved by the U.S. Food and Drug Administration (FDA) for commercial sale; therefore, the Company has not generated revenues from commercial therapeutic product sales. The Company has incurred losses and used cash for operating activities since inception. As of March 31, 2013, the Company had an accumulated deficit of $20,991,557.
F-5
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Condensed Consolidated Financial Statements Continued
Given the current pace of pre-clinical and clinical development of its potential product candidates, management believes that the Company has sufficient resources to fund the Company’s operations through at least December 31, 2013. However, the Company will require additional cash resources beyond 2013 and will need to identify sources for capital infusion. The Company is working to raise capital through the sale of common stock. The Company has not raised any capital through the sale of common stock through the date of this Form S-1 filing.
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosures of contingent assets and liabilities as of the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Accordingly, actual results could differ from those estimates. Key estimates include allowances for doubtful accounts receivable, useful lives for property and equipment, valuation allowances for net deferred income tax assets, and valuations for stock-based compensation awards. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances.
Revenue Recognition and Grants Receivable
The Company periodically applies for research grants, generally as a sub-recipient to grants funded by government agencies through research universities. Grant revenues are recognized as corresponding expenses are incurred and are billed in conjunction with the terms of the grants. The Company records its grants receivable in accordance with the provisions of the grant agreements. The Company’s grants receivable are considered past due when payment has not been received within 30 days of the invoice date, although certain institutions customarily do not pay within 30 days of the invoice date. The amounts of the specific reserves are estimated by management based on various assumptions including the age of the individual receivable, as well as changes in payment schedules and histories. Grants receivable balances are charged off against the allowance for doubtful accounts when management determines the potential for recovery is remote. Recoveries of receivables previously charged off are recorded when payment is received. For the three months ended March 31, 2013, all revenue was derived from a single customer.
In December 2012, the Company was notified of a sub-award as part of grant funding awarded to The Johns Hopkins University from the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health. The sub-award for the 2012-2013 grant plan year is $631,383. As of March 31, 2013, $483,303 has been invoiced under the sub-award and $1,834 is included in the grants receivable balance.
Stock-Based Compensation
The Company calculates the estimated fair value of its stock options and warrants on the grant date using the Black-Scholes option-pricing model. The Company recognizes stock-based compensation expense as services are provided, which is generally over the vesting period of the individual equity instruments. Expense related to stock options issued in lieu of cash to non-employees for services performed are measured at the fair value of the options on the date they are earned.
The volatility assumption used in the Black-Scholes option-pricing model is based on the volatility of publicly traded companies in the same industry segment as the Company. The expected lives of the options and warrants granted represent the periods of time that the options granted are expected to be outstanding. The risk free rates for periods within the contractual lives of the options and warrants are based on the U.S. treasury securities constant maturity rate that corresponds to the expected terms in effect at the time of grant. Stock compensation expense recorded by the Company was $25,438 and $29,308 for the three months ended March 31, 2013 and 2012, respectively, and is included in general and administrative expense in the statements of operations.
F-6
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Condensed Consolidated Financial Statements Continued
Net Loss Per Common Share
Basic net income or loss per common share (Basic EPS) is computed by dividing net income or loss by the weighted average number of common shares outstanding. Diluted net income or loss per common share (Diluted EPS) is computed by dividing net income or loss by the sum of the weighted average number of common shares outstanding and the dilutive potential common share equivalents then outstanding. Potential dilutive common share equivalents consist of shares issuable upon the exercise of outstanding stock options and warrants to acquire common stock.
Due to the fact that for all periods presented the Company has incurred net losses, potential dilutive common share equivalents as of March 31, 2013 and 2012, totaling 15,907,458 and 15,707,202, respectively, are not included in the calculation of Diluted EPS because they are anti-dilutive. Therefore, basic loss per common share is the same as diluted loss per common share for the three months ended March 31, 2013 and 2012.
Recent Accounting Pronouncements
The Company has reviewed accounting pronouncements that are effective during 2013 and does not believe any of those pronouncements will modify its financial reporting. Additionally, the Company has reviewed all recently issued, but not yet effective, accounting pronouncements and does not believe the future adoption of those pronouncements will have a material impact on the Company’s financial position, results of operations or liquidity.
3. Accrued Compensation
Accrued compensation consists of the following:
| March 31, 2013 | December 31, 2012 | |||||||
| Accrued wages |
$ | 36,053 | $ | 17,046 | ||||
| Accrued vacation |
78,762 | 70,846 | ||||||
|
|
|
|
|
|||||
| Total accrued compensation |
$ | 114,815 | $ | 87,892 | ||||
|
|
|
|
|
|||||
In March 2013, certain of the Company’s executives made the decision to defer cash payment of 60% of their salaries until additional funding has been obtained.
4. Stockholders’ Equity
Common Stock
During the quarter ended March 31, 2013, the Company issued 25,001 shares of its Common Stock to an investor and public relations firm in lieu of cash for services rendered during that period.
F-7
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Condensed Consolidated Financial Statements Continued
Stock Options
The following summarizes the outstanding common stock options and related activity for the three months ended March 31, 2013:
| Number of Options |
Weighted Average Exercise Price Per Share |
|||||||
| Outstanding as of January 1, 2013 |
3,865,440 | $ | 0.34 | |||||
| Granted |
— | — | ||||||
| Exercised |
— | — | ||||||
| Forfeited |
— | — | ||||||
|
|
|
|||||||
| Outstanding as of March 31, 2013 |
3,865,440 | 0.34 | ||||||
|
|
|
|||||||
| Exercisable as of March 31, 2013 |
3,263,420 | 0.22 | ||||||
|
|
|
|||||||
As of March 31, 2013, options to purchase 987,529 shares of common stock under the Company’s stock option plan were authorized and reserved for future grant. On December 18, 2012, the Board of Directors approved, subject to stockholder approval, the addition of 3,000,000 shares to the 2011 Plan. The Company is in process of obtaining stockholder approval to increase the authorized shares under the 2011 Plan by this amount.
The following summarizes information about stock options outstanding as of March 31, 2013:
| Exercise Price |
Numbers of Options Outstanding |
Weighted Average Remaining Contractual Life (Years) |
Weighted Average Price Exercise |
Number of Options Exercisable |
Weighted Average Price Exercise |
|||||||||||||||
| $0.06 - $0.08 | 902,600 | 5.78 | $ | 0.08 | 902,600 | $ | 0.08 | |||||||||||||
| $0.15 - $0.19 | 2,072,840 | 5.83 | 0.17 | 2,060,820 | $ | 0.17 | ||||||||||||||
| $1.00 | 890,000 | 8.60 | 1.00 | 300,000 | $ | 1.00 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 3,865,440 | 6.46 | 0.34 | 3,263,420 | 0.22 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
As of March 31, 2013, the aggregate intrinsic value of outstanding stock options was $2,549,625 and the aggregate intrinsic value of outstanding exercisable stock options was $2,539,939.
Stock-based compensation for the three months ended March 31, 2013 and 2012 was $25,438 and $29,308, respectively. As of March 31, 2013, the Company had $270,102 of unrecognized stock-based compensation expense related to non-vested awards that will be recognized over a weighted average period of 2.77 years.
F-8
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Condensed Consolidated Financial Statements Continued
Warrants
In October 2012, the Company entered into a service agreement with an investor relations and public advisory firm. As part of the agreement, the service provider was entitled to receive up to 250,000 warrants to purchase common stock at prices varying from $1.25 to $2.75 per share with a term of two years. The warrants are immediately exercisable upon issuance. On January 1, 2013, the first tranche of 62,500 warrants was issued with an exercise price of $1.25.
As of March 31, 2013, 12,042,018 warrants to purchase common stock had been issued with exercise prices ranging from $.046 to $2.00 per share and terms ranging from two to seven years. The weighted average warrant exercise price is $1.40 and the weighted average remaining life is 5.2 years.
5. Commitments and Contingencies
Supplier Agreements
In March 2010, the Company entered into a service agreement with an outside research firm to support the Company’s pre-clinical Good Laboratory Practice (GLP) safety studies. The Company was provided financing terms by the supplier which included partial payments of invoices, with remaining balances rolled into a convertible note payable accruing interest at 8% for the first 36 months and increasing to 10% for the 24 months thereafter. A provision in the note provided the supplier the right and option at any time prior to payment of the note to convert all or any portion of the outstanding balance into shares of the Company’s Series B preferred stock. As a result of the merger in October 2011, the Company eliminated its Series B preferred instruments and converted all outstanding shares into the Company’s common stock.
In November 2012, the Company and the supplier amended the terms of their original agreement via a Letter of Understanding (LOU) and increased the total purchase commitment with the supplier to approximately $2,600,000 with an expected completion by year-end 2013. As the Company no longer has Series B preferred stock, the amendment provided the Company with the option of paying part of its commitment with cash or with shares of common stock. Upon conclusion of the supplier’s final project report, any outstanding balance will be converted into a note payable accruing interest at 8% until July 31, 2015. Any outstanding amounts thereafter shall accumulate interest at 10% through March 31, 2016. Should the Company be successful in obtaining additional financing, the amendment calls for a percentage of the funds raised to immediately pay down the outstanding balance. As of March 31, 2013, the amount owed under this agreement totaled $949,818 and is included in accounts payable in the accompanying consolidated balance sheet.
Additionally, the supplier was granted, upon successful completion of the clinical study, a right of first negotiation on any future pre-clinical GLP safety to toxicology studies.
Advisory Agreements
In February 2013, the Company entered into an agreement to engage a financial advisory firm for a minimum term of twelve months. The financial advisory firm is entitled to 7% of the proceeds from its financing efforts payable in cash and if warrants are issued as part of the placement of securities, an issuance of warrants equivalent to 7% of the aggregate number of warrants purchased in the offering. To date, no placement of securities has occurred.
F-9
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Q Therapeutics, Inc.
We have audited the accompanying consolidated balance sheets of Q Therapeutics, Inc. and subsidiary (collectively, the Company) as of December 31, 2012 and 2011, and the related consolidated statements of operations, stockholders’ equity, and cash flows for the years then ended and for the period from inception of the development stage (March 28, 2002) through December 31, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Q Therapeutics, Inc. and subsidiary as of December 31, 2012 and 2011, and the results of their operations and their cash flows for the years then ended and for the period from inception of the development stage (March 28, 2002) through December 31, 2012 in conformity with U.S. generally accepted accounting principles.
| /s/ Tanner LLC |
| Salt Lake City, Utah |
| March 28, 2013 |
F-10
Table of Contents
(A Development Stage Company)
Consolidated Balance Sheets
As of December 31, 2012 and 2011
| December 31, 2012 |
December 31, 2011 |
|||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash |
$ | 794,207 | $ | 2,741,519 | ||||
| Receivables, net of allowance of $28,800 and $2,282 as of December 31, 2012 and 2011, respectively |
477,802 | 53,796 | ||||||
| Prepaid expenses and other |
10,366 | — | ||||||
|
|
|
|
|
|||||
| Total current assets |
1,282,375 | 2,795,315 | ||||||
| Property and equipment, net |
16,044 | 31,923 | ||||||
| Other assets |
7,513 | 7,513 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 1,305,932 | $ | 2,834,751 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 1,203,365 | $ | 77,181 | ||||
| Accrued liabilities |
9,685 | 24,212 | ||||||
| Accrued compensation |
87,892 | 177,401 | ||||||
| Note payable |
— | 15,000 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
1,300,942 | 293,794 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Common stock, $0.0001 par value: 100,000,000 shares authorized; 24,761,832 and 24,582,632 shares outstanding as of December 31, 2012 and 2011, respectively |
2,476 | 2,458 | ||||||
| Additional paid-in capital |
20,494,792 | 19,972,937 | ||||||
| Accumulated deficit |
(20,492,278 | ) | (17,434,438 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
4,990 | 2,540,957 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 1,305,932 | $ | 2,834,751 | ||||
|
|
|
|
|
|||||
See accompanying notes to consolidated financial statements.
F-11
Table of Contents
(A Development Stage Company)
Consolidated Statements of Operations
For the Years Ended December 31, 2012 and 2011 and
for the Period from March 28, 2002 (Date of Inception) to December 31, 2012
| For the
Years Ended December 31, |
Cumulative From |
|||||||||||
| 2012 | 2011 | Inception | ||||||||||
| Revenues: |
||||||||||||
| Grant revenues |
$ | 485,031 | $ | 10,173 | $ | 1,090,259 | ||||||
| License fees and other revenues |
— | 164,400 | 282,900 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
485,031 | 174,573 | 1,373,159 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Research and development |
2,089,321 | 444,834 | 10,972,892 | |||||||||
| General and administrative |
1,458,088 | 1,567,012 | 9,119,235 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
3,547,409 | 2,011,846 | 20,092,127 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(3,062,378 | ) | (1,837,273 | ) | (18,718,968 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other income (expense): |
||||||||||||
| Interest income |
4,274 | 547 | 187,616 | |||||||||
| Interest expense |
(2,237 | ) | (763,088 | ) | (2,111,414 | ) | ||||||
| Other income, net |
2,501 | 476 | 150,488 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense), net |
4,538 | (762,065 | ) | (1,773,310 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before provision (benefit) for income taxes |
(3,057,840 | ) | (2,599,338 | ) | (20,492,278 | ) | ||||||
| Provision (benefit) for income taxes |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (3,057,840 | ) | $ | (2,599,338 | ) | $ | (20,492,278 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted average number of common shares outstanding - basic and diluted |
24,683,787 | 8,656,708 | ||||||||||
|
|
|
|
|
|||||||||
| Net loss per common share - basic and diluted |
$ | (0.12 | ) | $ | (0.30 | ) | ||||||
|
|
|
|
|
|||||||||
See accompanying notes to consolidated financial statements.
F-12
Table of Contents
(A Development Stage Company)
Consolidated Statements of Stockholders’ Equity
For the Years Ended December 31, 2012 and 2011 and
for the Period from March 28, 2002 (Date of Inception) to December 31, 2012
| Series
A1 Preferred Stock |
Series A2 Preferred Stock |
Series B Preferred Stock |
Common Stock | Additional Paid-in Capital |
Accumulated Deficit |
Notes Receivable |
Total Stockholders’ Equity (Deficit) |
|||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||
| Balance March 28, 2002 |
— | $ | — | — | $ | — | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||||||||||||
| Recapitalization due to reverse merger |
— | — | — | — | — | — | 2,600,000 | 260 | (260 | ) | — | — | — | |||||||||||||||||||||||||||||||||||
| Common stock issued at inception, $0.001 per share |
— | — | — | — | — | — | 818,500 | 82 | 737 | — | — | 819 | ||||||||||||||||||||||||||||||||||||
| Common stock issued for technology |
— | — | — | — | — | — | 219,658 | 22 | 198 | — | — | 220 | ||||||||||||||||||||||||||||||||||||
| Series A1 preferred stock issued for cash, $1.20 per share |
250,000 | 25 | — | — | — | — | — | — | 299,975 | — | — | 300,000 | ||||||||||||||||||||||||||||||||||||
| Series A2 preferred stock issued for: |
||||||||||||||||||||||||||||||||||||||||||||||||
| Cash, $2.24 per share (net of issuance cost of $178,827) |
— | — | 1,517,859 | 152 | — | — | — | — | 3,221,026 | — | — | 3,221,178 | ||||||||||||||||||||||||||||||||||||
| Conversion of notes payable, including accrued interest of $29,692; $2.24 per share |
— | — | 482,008 | 48 | — | — | — | — | 1,079,643 | — | — | 1,079,691 | ||||||||||||||||||||||||||||||||||||
| Series B preferred stock issued for: |
||||||||||||||||||||||||||||||||||||||||||||||||
| Cash, $2.30 per share (net of issuance cost of $130,441) |
— | — | — | — | 1,750,002 | 175 | — | — | 3,894,386 | — | — | 3,894,561 | ||||||||||||||||||||||||||||||||||||
| Conversion of notes payable, including accrued interest of $369,427; $2.30 per share |
— | — | — | — | 1,780,183 | 178 | — | — | 4,094,250 | — | — | 4,094,428 | ||||||||||||||||||||||||||||||||||||
| Exercise of Series A2 preferred stock warrants for cash |
— | — | 13,952 | 1 | — | — | — | — | 1,395 | — | — | 1,396 | ||||||||||||||||||||||||||||||||||||
| Exercise of options for cash |
— | — | — | — | — | — | 63,901 | 6 | 9,794 | — | — | 9,800 | ||||||||||||||||||||||||||||||||||||
| Proceeds from debt allocated to preferred stock warrants and beneficial conversion feature |
— | — | — | — | — | — | — | — | 940,819 | — | — | 940,819 | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | — | — | 103,112 | — | — | 103,112 | ||||||||||||||||||||||||||||||||||||
| Exercise of options for notes receivable |
491,144 | 49 | 135,253 | — | (135,302 | ) | — | |||||||||||||||||||||||||||||||||||||||||
| Net loss (cumulative from March 28, 2002 through December 31, 2008) |
— | — | — | — | — | — | — | — | — | (13,214,519 | ) | — | (13,214,519 | ) | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Balance December 31, 2008 |
250,000 | 25 | 2,013,819 | 201 | 3,530,185 | 353 | 4,193,203 | 419 | $ | 13,780,328 | (13,214,519 | ) | (135,302 | ) | 431,505 | |||||||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | — | — | 42,776 | — | — | 42,776 | ||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (755,731 | ) | — | (755,731 | ) | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Balance December 31, 2009 |
250,000 | $ | 25 | 2,013,819 | $ | 201 | 3,530,185 | $ | 353 | 4,193,203 | $ | 419 | $ | 13,823,104 | $ | (13,970,250 | ) | $ | (135,302 | ) | $ | (281,450 | ) | |||||||||||||||||||||||||
See accompanying notes to consolidated financial statements.
F-13
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Consolidated Statements of Stockholders’ Equity Continued
| Series
A1 Preferred Stock |
Series A2 Preferred Stock |
Series B Preferred Stock |
Common Stock | Additional Paid-in Capital |
Accumulated Deficit |
Notes Receivable |
Total Stockholders’ Equity (Deficit) |
|||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||
| Series B preferred stock issued for: |
||||||||||||||||||||||||||||||||||||||||||||||||
| Cash, $2.30 per share |
— | — | — | — | 541,743 | 54 | — | — | 1,245,954 | — | — | 1,246,008 | ||||||||||||||||||||||||||||||||||||
| Conversion of notes payable, including accrued interest of $918; $2.30 per share |
— | — | — | — | 6,921 | 1 | — | — | 15,917 | — | — | 15,918 | ||||||||||||||||||||||||||||||||||||
| Subscription receivable $2.30 per share |
— | — | — | — | 4,348 | — | — | — | 10,000 | — | — | 10,000 | ||||||||||||||||||||||||||||||||||||
| Exercise of Series A2 preferred stock warrants for cash |
— | — | 8,371 | 1 | — | — | — | — | 836 | — | — | 837 | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | — | — | 83,298 | — | — | 83,298 | ||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (864,850 | ) | — | (864,850 | ) | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Balance December 31, 2010 |
250,000 | 25 | 2,022,190 | 202 | 4,083,197 | 408 | 4,193,203 | 419 | 15,179,109 | (14,835,100 | ) | (135,302 | ) | 209,761 | ||||||||||||||||||||||||||||||||||
| Series B preferred stock issued for services |
— | — | — | — | 19,457 | 2 | — | — | 44,748 | — | — | 44,750 | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | — | — | 42,378 | — | — | 42,378 | ||||||||||||||||||||||||||||||||||||
| Value of warrants issued with bridge financing |
— | — | — | — | — | — | — | — | 296,444 | — | — | 296,444 | ||||||||||||||||||||||||||||||||||||
| Conversion of preferred stock to common in conjunction with reverse merger |
(250,000 | ) | (25 | ) | (2,022,190 | ) | (202 | ) | (4,102,654 | ) | (410 | ) | 13,791,231 | 1,379 | (742 | ) | — | — | — | |||||||||||||||||||||||||||||
| Conversion of bridge notes to common stock in conjunction with reverse merger |
— | — | — | — | — | — | 916,644 | 92 | 916,552 | — | — | 916,644 | ||||||||||||||||||||||||||||||||||||
| Exchange of Q Therapeutics common stock for Q Holdings in conjunction with reverse merger |
— | — | — | — | — | — | 1,853,507 | 185 | (135,487 | ) | — | 135,302 | — | |||||||||||||||||||||||||||||||||||
| Common stock issued for cash, $1.00 per share (net of issuance costs of $197,729) |
— | — | — | — | — | — | 3,828,047 | 383 | 3,629,935 | — | — | 3,630,318 | ||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (2,599,338 | ) | — | (2,599,338 | ) | |||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Balance December 31, 2011 |
— | — | — | — | — | — | 24,582,632 | 2,458 | 19,972,937 | (17,434,438 | ) | — | 2,540,957 | |||||||||||||||||||||||||||||||||||
| Common stock forfeited for loan default |
— | — | — | — | — | — | (200,000 | ) | (19 | ) | 19 | — | — | — | ||||||||||||||||||||||||||||||||||
| Common stock issued for: |
||||||||||||||||||||||||||||||||||||||||||||||||
| Cash, $1.00 per share |
— | — | — | — | — | — | 190,000 | 19 | 189,981 | — | — | 190,000 | ||||||||||||||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | — | 32,451 | 3 | 1,797 | — | — | 1,800 | ||||||||||||||||||||||||||||||||||||
| Services |
— | — | — | — | — | — | 156,749 | 15 | 156,735 | — | — | 156,750 | ||||||||||||||||||||||||||||||||||||
| Warrants issued for services |
— | — | — | — | — | — | — | — | 13,086 | — | — | 13,086 | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | — | — | 160,237 | — | — | 160,237 | ||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (3,057,840 | ) | — | (3,057,840 | ) | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Balance December 31, 2012 |
— | $ | — | — | $ | — | — | $ | — | 24,761,832 | $ | 2,476 | $ | 20,494,792 | $ | (20,492,278 | ) | $ | — | $ | 4,990 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
See accompanying notes to consolidated financial statements.
F-14
Table of Contents
(A Development Stage Company)
Consolidated Statements of Cash Flows
For the Years Ended December 31, 2012 and 2011 and
for the Period from March 28, 2002 (Date of Inception) to December 31, 2012
| 2012 | 2011 | Cumulative From Inception |
||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (3,057,840 | ) | $ | (2,599,338 | ) | $ | (20,492,278 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
18,242 | 27,729 | 391,647 | |||||||||
| Original debt discount |
— | 450,000 | 450,000 | |||||||||
| Accretion of debt costs and beneficial conversion feature |
— | 296,444 | 1,237,263 | |||||||||
| Stock-based compensation |
160,237 | 42,378 | 431,801 | |||||||||
| Debt issued for services |
— | 90,000 | 90,000 | |||||||||
| Common stock issued for services |
156,750 | — | 156,750 | |||||||||
| Preferred stock issued for services |
— | 44,750 | 44,750 | |||||||||
| Warrants issued for services |
13,086 | — | 13,086 | |||||||||
| Provision for losses on receivables |
(26,518 | ) | 2,282 | (43,677 | ) | |||||||
| Decrease (increase) in: |
||||||||||||
| Receivables |
(397,488 | ) | 26,872 | (434,125 | ) | |||||||
| Prepaid expenses and other assets |
(10,366 | ) | — | (17,879 | ) | |||||||
| Increase (decrease) in: |
||||||||||||
| Accounts payable and accrued liabilities |
1,111,657 | (183,009 | ) | 1,612,169 | ||||||||
| Accrued compensation |
(89,509 | ) | 136,522 | 87,892 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(2,121,749 | ) | (1,665,370 | ) | (16,472,601 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities: |
||||||||||||
| Purchase of property and equipment |
(2,363 | ) | (5,627 | ) | (407,471 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from issuance of notes payable |
— | 360,000 | 5,257,562 | |||||||||
| Payments on short-term note payable |
(15,000 | ) | — | (90,000 | ) | |||||||
| Issuance of preferred stock for cash |
— | — | 8,671,747 | |||||||||
| Issuance of common stock for cash |
190,000 | 3,630,318 | 3,821,137 | |||||||||
| Proceeds from exercise of common stock options |
1,800 | — | 11,600 | |||||||||
| Proceeds from exercise of preferred stock warrants |
— | — | 2,233 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
176,800 | 3,990,318 | 17,674,279 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net (decrease) increase in cash |
(1,947,312 | ) | 2,319,321 | 794,207 | ||||||||
| Cash as of beginning of the period |
2,741,519 | 422,198 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash as of end of the period |
$ | 794,207 | $ | 2,741,519 | $ | 794,207 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information: |
||||||||||||
| Cash paid for interest |
$ | 2,237 | $ | 201 | $ | 7,671 | ||||||
See accompanying notes to consolidated financial statements.
F-15
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Consolidated Statements of Cash Flows Continued
For the Period from March 28, 2002 (Date of Inception) to December 31, 2012
Supplemental Disclosure of noncash investing and financing activities:
| • | The Company issued 219,658 shares of common stock in exchange for technology valued at $220. |
| • | The Company converted $1,050,000 of notes payable and $29,691 of accrued interest to 482,008 shares of Series A2 preferred stock. |
| • | The Company converted $3,740,000 of notes payable and $370,346 of accrued interest to 1,787,104 shares of Series B preferred stock. |
| • | The Company received proceeds of $1,237,263 from debt related to preferred stock warrants and their beneficial conversion feature. |
| • | The Company converted $900,000 of bridge notes payable and $16,644 of accrued interest to 916,644 shares of common stock. |
| • | The Company converted 250,000 shares of Series A1 preferred stock, 2,022,190 shares of Series A2 preferred stock, and 4,102,654 shares of Series B preferred stock to 13,791,231 shares of common stock. |
| • | Two stockholders forfeited and the Company retired 200,000 shares of common stock with a net impact on equity of $19 as a result of untimely payments on their notes. |
See accompanying notes to consolidated financial statements.
F-16
Table of Contents
(A Development Stage Company)
Notes to Consolidated Financial Statements
1. Organization
Q Therapeutics, Inc. (formerly Q Holdings, Inc.) is a development stage biopharmaceutical company headquartered in Salt Lake City, Utah. The Company is engaged in developing adult stem cell therapies to treat debilitating diseases of the central nervous system. Q Therapeutics, Inc. was formed October 27, 2005 as a Delaware corporation. The Company’s operating subsidiary, Q Therapeutic Products, Inc. was formed on March 29, 2002 as a Delaware corporation. The Company’s first product candidate, Q-Cells ®, is a cell-based therapeutic intended to restore or preserve normal activity of neurons by providing essential support functions that occur in healthy central nervous system tissues. The Company believes that Q-Cells may be applicable to a wide range of central nervous system diseases, including demyelinating conditions such as multiple sclerosis, transverse myelitis, cerebral palsy and stroke; as well as other neurodegenerative diseases and injuries, such as ALS (Lou Gehrig’s disease), spinal cord injury, Parkinson’s disease and Alzheimer’s disease.
2. Significant Accounting Policies
The following significant accounting policies are followed by the Company in preparing its consolidated financial statements:
Basis of Consolidation
The accompanying consolidated financial statements include the accounts of Q Therapeutics, Inc. and its wholly owned subsidiary, Q Therapeutic Products, Inc., and Q Therapeutic Products, Inc.’s wholly owned subsidiary, NeuroQ Research Inc. (collectively, the Company). The Company is a development stage company engaged in the discovery, research, development and eventual commercialization of products as potential treatments for debilitating and fatal diseases of the central nervous system. All significant intercompany amounts have been eliminated.
Merger with Public Company
On October 13, 2011, Q Therapeutics, Inc. entered into an agreement and plan of merger (the Merger Agreement) with Grace 2, Inc., a public shell corporation, and its wholly owned subsidiary, Q Acquisition, Inc. (Merger Sub). On October 18, 2011, Q Therapeutics, Inc. completed a reverse merger transaction in which Merger Sub merged with and into Q Therapeutics, Inc. and Q Therapeutics, Inc. became a wholly owned subsidiary of Grace 2 (the Reverse Merger). Upon the consummation of the Reverse Merger, Grace 2 acquired 100% of Q Therapeutics, Inc. by issuing 2.1633835 shares of common stock for each outstanding equity instrument of Q Therapeutics, Inc. After the effective date of the Reverse Merger, the former Q Therapeutics, Inc. stockholders owned 89.7% of the issued and outstanding common shares of Grace 2, Inc. Also, at the time of the Reverse Merger, Grace 2, Inc. was renamed Q Holdings, Inc. The transaction has been accounted for as a reverse acquisition with Q Therapeutics, Inc. as the accounting acquirer.
Since completing the Reverse Merger, Q Therapeutics, Inc., the wholly owned subsidiary of Q Holdings, Inc. has changed its name to Q Therapeutic Products, Inc. and the parent company, Q Holdings, Inc. has changed its name to Q Therapeutics, Inc.
The consolidated financial statements include the activity of Q Therapeutic Products, Inc. from inception (March 28, 2002) through December 31, 2012), and the activity of Q Therapeutic Products, Inc. and Q Therapeutics, Inc. from the date of acquisition (October 13, 2011) forward.
Development Stage and Liquidity
For the period from March 28, 2002 (date of inception) through December 31, 2012, the Company has not generated significant revenues and has been developing its products. Therefore, the Company is considered to be in the development stage in accordance with the provisions of Accounting Standards Codification (ASC) Topic 915, Development Stage Entities. Cumulative amounts have been presented for the period from March 28, 2002 (date of inception) through December 31, 2012. Historically, the Company has been dependent on government grants and debt and equity raised from individual investors to sustain its operations. The Company’s continued operations will depend on its ability to raise funds through various sources such as government grants and equity and debt financing. The Company expects to continue to fund operations through similar sources of capital previously described. There can be no assurance that such capital will be available on favorable terms or at all. If it is unable to
F-17
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
raise additional capital, the Company will likely be forced to curtail desired development activities, which will delay the development of its potential product candidates. The Company’s products have not been approved by the U.S. Food and Drug Administration (FDA) for commercial sale; therefore, the Company has not generated revenues from commercial product sales. The Company has incurred losses and used cash for operating activities since inception. As of December 31, 2012, the Company had an accumulated deficit of $20,492,278.
From October 13, 2011 through the date the financing closed (May 31, 2012), the Company raised $4,018,047 through a private placement of its common stock. Although there can be no assurance, given the current pace of pre-clinical and clinical development of its potential product candidates, management believes that the Company has sufficient resources to fund the Company’s operations through at least December 31, 2013.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements and the reported amounts of the revenues and expenses during the reporting periods. Accordingly, actual results could differ from those estimates. Key estimates include allowances for doubtful accounts, useful lives for property and equipment, valuation allowances for net deferred income tax assets, and valuations for stock-based compensation awards. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances.
Concentrations of Credit Risk
The Company maintains its cash in bank deposit accounts which, at times, exceeds federally insured limits. To date, the Company has not experienced a loss or lack of access to its invested cash; however, there can be no assurance that access to the Company’s invested cash will not be impacted by adverse conditions in the financial markets.
Cash Equivalents
The Company considers all highly liquid investments with original maturities to the Company of three months or less to be cash equivalents. As of December 31, 2012 and 2011, the Company did not have any cash equivalents.
Revenue Recognition and Grants Receivable
The Company periodically applies for research grants, generally as a sub-recipient to grants funded by government agencies through research universities. Grant revenues are recognized as corresponding expenses are incurred and are billed in conjunction with the terms of the grants. The Company records its grants receivable in accordance with the provisions of the grant agreements. The Company’s grants receivable are considered past due when payment has not been received within 30 days of the invoice date, although certain institutions customarily pay within 60 days of the invoice date. The amounts of the specific reserves are estimated by management based on various assumptions including the age of the individual grants receivable, as well as changes in payment schedules and histories. Grants receivable balances are charged off against the allowance for doubtful accounts when management determines the potential for recovery is remote. Recoveries of receivables previously charged off are recorded when payment is received. A majority of, if not all revenue, is derived from a single customer. The Company did not incur any losses relating to bad debts associated with grant revenues during the last two years.
In December 2012, the Company was awarded a sub-award as part of grant funding awarded to The Johns Hopkins University from the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) to help fund the manufacturing and pre-clinical safety studies for Q-Cells ®, the Company’s product. The sub-award for the 2012-2013 grant plan year is $631,383. As of December 31, 2012, $477,802 has been invoiced and is included in the grants receivable balance. As of December 31, 2011, grants receivable were $0 as revenue relating to the sub-award for the 2011-2012 grant plan year had been invoiced and paid in the first half of 2011.
F-18
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
Concentration of Suppliers
The Company has entered into agreements with outside research facilities to assist in the clinical research, monitoring, and reporting of its pilot and clinical studies. In some instances, the Company is dependent upon a single supplier. The loss of key suppliers could have a material adverse effect upon the Company’s operations by interrupting or delaying the progress or completion of the Company’s clinical trials.
For the year ended December 31, 2012, one supplier accounted for 68% of the Company’s research and development purchases.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization are calculated using the straight-line method over the estimated economic useful lives of the assets as follows:
| Lab equipment | 5 years | |
| Computers and software | 3 years | |
| Leasehold improvements | 7 years | |
| Office equipment and furniture | 3 years |
Expenditures that materially increase values or capacities or extend useful lives of property and equipment are capitalized. Routine maintenance and repairs are expensed as incurred. Upon sale or other retirement of depreciable property, the cost and accumulated depreciation are removed from the related accounts and any gain or loss is reflected in the statements of operations.
Impairment of Long-Lived Assets
The Company reviews its property and equipment, and other long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amounts of the assets may be impaired. Management does not consider any of the Company’s assets to be impaired as of December 31, 2012 and 2011.
Leases
The Company leases its office and research facility under a cancelable operating lease. If rent escalations are material, the Company records the total rent payable during the lease term on a straight-line basis over the term of the lease. The Company records any difference between the rent paid and the straight-line rent as a deferred rent liability in the accompanying consolidated balance sheets.
Stock-Based Compensation
The Company calculates the estimated fair value of its stock options and warrants on the grant date using the Black-Scholes option-pricing model. The Company recognizes stock-based compensation expense as services are provided, which is generally over the vesting period of the individual equity instruments. Stock options issued in lieu of cash to non-employees for services performed are measured at the fair value of the options on the date they are earned and the related expense is recognized as services are provided.
The volatility assumption used in the Black-Scholes option-pricing model is based on the volatility of publicly traded companies in the same industry segment as the Company. The expected lives of the options and warrants granted represent the periods of time that the options granted are expected to be outstanding. The risk free rates for periods within the contractual lives of the options and warrants are based on the U.S. treasury securities constant maturity rate that corresponds to the expected terms in effect at the time of grant. Stock compensation expense recorded by the Company was $160,237 and $42,378 for the years ended December 31, 2012 and 2011, respectively, and is included in general and administrative expense in the statements of operations.
F-19
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
Income Taxes
The Company is a C corporation and federal and state income taxes are computed using the asset and liability method. Under the asset and liability method, deferred income tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates is recognized in net income (loss) in the period that includes the enactment date.
Uncertain Tax Positions
The Company recognizes the financial statement amount of an uncertain tax position only after considering the probability that a tax authority would sustain the position in an examination. For tax positions meeting a “more likely-than-not” threshold, the amount recognized in the financial statements is the amount expected to be realized upon settlement with the tax authority. For tax positions not meeting the threshold, no financial statement benefit is recognized. The Company recognizes interest and penalties, if any, related to uncertain tax positions as general and administrative expenses. The Company currently has no federal or state examinations in progress. The Company’s tax years subject to federal and state tax examination are 2009, 2010, 2011 and 2012.
Research and Development Costs
Research and development (R&D) costs, including research performed under contract by third parties, are expensed as incurred. Major components of R&D expenses consist of personnel costs including salaries and benefits, outside research services, consulting fees, lab supplies and materials, license fees, and facility-related expenses. R&D expenses recorded by the Company were $2,089,321 and $444,834 for the years ended December 31, 2012 and 2011, respectively. Since its inception, the Company has incurred total R&D expenses of $10,972,892.
Net Loss Per Common Share
Basic net income or loss per common share (Basic EPS) is computed by dividing net income or loss by the weighted average number of common shares outstanding. Diluted net income or loss per common share (Diluted EPS) is computed by dividing net income or loss by the sum of the weighted average number of common shares outstanding and the dilutive potential common share equivalents then outstanding. Potential dilutive common share equivalents consist of shares issuable upon the exercise of outstanding stock options and warrants to acquire common stock.
Due to the fact that for all periods presented, the Company has incurred net losses, potential dilutive common share equivalents as of December 31, 2012 and 2011, totaling 15,844,958 and 14,717,466, respectively, are not included in the calculation of Diluted EPS because they are anti-dilutive. Therefore, basic loss per common share is the same as diluted loss per common share for the years ended December 31, 2012 and 2011.
Recent Accounting Pronouncements
The Company has reviewed all accounting pronouncements that were effective during 2012 and does not believe any of those pronouncements modified its financial reporting. Additionally, the Company has reviewed all recently issued, but not yet effective, accounting pronouncements and does not believe the future adoption of those pronouncements will have a material impact on the Company’s financial position, results of operations or liquidity.
Subsequent Events
The Company has evaluated all subsequent events through the issuance date of the financial statements.
F-20
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
3. Property and Equipment
Property and equipment consists of the following as of December 31:
| As of December 31, | ||||||||
| 2012 | 2011 | |||||||
| Lab equipment |
$ | 299,876 | $ | 298,836 | ||||
| Computers and software |
65,234 | 63,911 | ||||||
| Leasehold improvements |
38,934 | 38,934 | ||||||
| Office equipment and furniture |
3,647 | 3,647 | ||||||
|
|
|
|
|
|||||
| 407,691 | 405,328 | |||||||
| Less accumulated depreciation and amortization |
(391,647 | ) | (373,405 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 16,044 | $ | 31,923 | ||||
|
|
|
|
|
|||||
Depreciation and amortization expense for the years ended December 31, 2012 and 2011 was $18,242 and $27,729, respectively.
4. Accrued Compensation
Accrued compensation consists of the following as of December 31:
| As of December 31, | ||||||||
| 2012 | 2011 | |||||||
| Accrued wages |
$ | 17,046 | $ | 126,050 | ||||
| Accrued vacation |
70,846 | 51,351 | ||||||
|
|
|
|
|
|||||
| Total accrued compensation |
$ | 87,892 | $ | 177,401 | ||||
|
|
|
|
|
|||||
As of December 31, 2011, $108,650 of the total accrued wages was for compensation due to executives upon the one-year anniversary of the reverse merger. The bonuses and respective taxes were paid in full in October 2012. No such liability existed as of December 31, 2012.
5. Note Payable
As of December 31, 2011, the Company had an unsecured note payable to a stockholder totaling $15,000 with an annual interest rate of 6%. The note and related interest of $2,020 were repaid in March 2012.
6. Income Taxes
The benefit for income taxes differs from the amount computed at federal statutory rates as follows for the years ended December 31:
| 2012 | 2011 | |||||||
| Federal income tax at statutory rates |
$ | (1,039,665 | ) | $ | (883,776 | ) | ||
| State income tax at statutory rates |
(97,234 | ) | (85,273 | ) | ||||
| Research and development credits |
(67,011 | ) | (278,818 | ) | ||||
| Change in valuation allowance |
1,165,081 | 1,242,665 | ||||||
| Other |
38,829 | 5,202 | ||||||
|
|
|
|
|
|||||
| $ | — | $ | — | |||||
|
|
|
|
|
|||||
F-21
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
Significant components of the Company’s deferred income tax assets (liabilities) are as follows as of December 31:
| 2012 | 2011 | |||||||
| Current: |
||||||||
| Accruals and reserves |
$ | 37,168 | $ | 62,366 | ||||
| Non-qualified stock options and other |
— | 56,015 | ||||||
| Valuation allowance |
(37,168 | ) | (118,381 | ) | ||||
|
|
|
|
|
|||||
| $ | — | $ | — | |||||
|
|
|
|
|
|||||
| Long-term: |
||||||||
| Net operating loss carryforwards |
$ | 6,952,741 | $ | 5,794,922 | ||||
| Depreciation and amortization |
271 | (1,438 | ) | |||||
| Non-qualified stock options and other |
69,072 | — | ||||||
| Research and development credits |
621,497 | 603,803 | ||||||
| Valuation allowance |
(7,643,581 | ) | (6,397,287 | ) | ||||
|
|
|
|
|
|||||
| $ | — | $ | — | |||||
|
|
|
|
|
|||||
As of December 31, 2012, the Company had net operating loss (NOL) carryforwards available to offset future taxable income, if any, of approximately $18,826,000, which will begin to expire in 2022.
The Company has research and development credits totaling approximately $621,000 available for offset against future federal income tax, if any. The credits begin to expire in 2022.
The utilization of the NOL carryforwards is subject to annual limitations under Section 382 of the Internal Revenue Code. Section 382 imposes limitations on a corporation’s ability to utilize its NOL carryforwards if it experiences an “ownership change.” In general terms, an ownership change results from transactions increasing the ownership of certain stockholders in the stock of a corporation by more than 50% over a three-year period.
The Company has concluded that there are no significant uncertain tax positions requiring disclosure.
7. Stockholders’ Equity
Common Stock
As of December 31, 2012, the Company is authorized to issue 100,000,000 shares of common stock, of which 24,761,832 shares were outstanding. In addition, a sufficient number of shares of common stock have been reserved for issuance pursuant to the Company’s 2011 Stock Incentive Plan, as amended, as well as to permit the exercise in full of all outstanding warrants.
Holders of shares of common stock are entitled to cast one vote for each share held at all stockholders’ meetings for all purposes, including the election of directors. The common stock does not have cumulative voting rights.
Between October 13, 2011 and May 31, 2012, the Company conducted the 2011 Private Placement (the Offering) of its securities solely to accredited investors. The Offering was conducted on a best efforts basis, whereby the Company offered for sale a minimum of 3,000,000 and a maximum of 6,000,000 Units, each Unit consisting of one share of common stock, one 7-year common stock purchase warrant exercisable at $1.00 per share, and one 7-year common stock purchase warrant exercisable at $2.00 per share. Units sold totaled 4,018,047 through May 31, 2012, when the Offering closed. The Company raised an aggregate of $3,820,318 after paying issuance costs of $197,729.
Preferred Stock
As of December 31, 2012, the Company was authorized to issue 10,000,000 shares of preferred stock; however, no shares of preferred stock were outstanding.
F-22
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
Conversion of Preferred Stock to Common
On October 13, 2011, the Company completed a reverse merger transaction with a public shell company at which time all Series A-1, A-2, and B preferred shares of the Company were converted into common stock of Q Therapeutics, Inc. Each share of preferred stock converted into 2.1633835 common shares of Q Therapeutics, Inc. Along with this conversion to common stock of Q Therapeutics, Inc., the holders of Q Therapeutic Products, Inc. preferred stock forfeited all rights and preferences.
Stock Options
2002 Stock Option Plan
In April 2002, the Q Therapeutics’ Board of Directors approved the Q Therapeutics 2002 Stock Incentive Plan (the 2002 Plan) and in February 2003, the holders of a majority of the outstanding voting capital stock of Q Therapeutics approved of the 2002 Plan. The 2002 Plan permits the grant of Incentive Stock Options, Non-Qualified Stock Options and Restricted Stock. Subject to the provisions of the Plan, a designated committee (Committee) of the Board of Directors (or if none, the Board) may from time to time, in its sole discretion select from among eligible employees, non-employee directors, consultants and advisors, those to whom awards shall be granted under the 2002 Plan, and shall determine in its discretion the nature, terms, conditions, and amount of each award, subject to the terms of the 2002 Plan. The term of the 2002 Plan commenced on April 10, 2002 (the Effective Date) and remained in effect, subject to the right of the Committee or the Board to amend or terminate the Plan at any time pursuant to the 2002 Plan, until the earlier of (i) the tenth anniversary date of the Effective Date, or (ii) all shares subject to the 2002 Plan have been purchased or acquired according to the 2002 Plan’s provisions.
The 2002 Plan initially provided for a reservation pool of up to 1,100,000 shares of common stock reserved for issuance pursuant to the 2002 Plan. The total number of shares reserved for issuance under the 2002 Plan has since been increased to a total of 2,120,000 shares. Upon the consummation of the Merger, each option to purchase shares of common stock of Q Therapeutics Products, Inc. was exchanged for 2.1633835 options to purchase shares of common stock of the Company resulting in a total of 4,586,373 shares of common stock reserved for issuance under the 2002 Plan. All shares available for award under the 2002 Plan have been granted except for potential awards to acquire 228,472 shares of common stock. By Board authorization on December 6, 2011, the Board added the remaining 228,472 shares available but unissued pursuant to the 2002 Option Plan to the authorized/reserved option pool for the 2011 Plan (discussed below), and thus effectively terminated the 2002 Plan.
2011 Equity Incentive Compensation Plan
In connection with the Merger, on October 13, 2011 the Board of Directors and stockholders approved the Q Holdings 2011 Equity Incentive Compensation Plan (the 2011 Plan). Subject to the provisions of the 2011 Plan, a designated committee (Committee) of the Board of Directors (or if none, the Board) may, from time to time, in its sole discretion select from among eligible employees, non-employee directors and consultants those to whom awards shall be granted under the 2011 Plan, and shall determine in its discretion the nature, terms, conditions and amount of each award, subject to the terms of the 2011 Plan. The term of the 2011 Plan commenced on October 13, 2011 (the Effective Date) and remains in effect, subject to the right of the Committee or the Board to amend or terminate the 2011 Plan at any time pursuant to the 2011 Plan, until the earlier of (i) the tenth anniversary of the Effective Date, or (ii) all shares subject to the 2011 Plan have been purchased or acquired according to the 2011 Plan’s provisions.
The 2011 Plan initially provided for a reservation pool of up to 1,500,000 shares of common stock reserved for issuance pursuant to the 2011 Plan. By Board authorization on December 6, 2011, the Board voted to add the remaining 228,472 shares available but unissued pursuant to the 2002 Plan to the authorized/reserved option pool for the 2011 Plan, bringing the 2011 Plan pool to 1,728,472 shares of common stock reserved for issuance pursuant to the 2011 Plan. In 2012, the Company issued options to acquire 890,000 shares of common stock pursuant to the 2011 Plan. Additionally, the Board also resolved to roll over all forfeited or expired awards made under the 2002 Plan into the 2011 Plan. As of December 31, 2012, there were 987,529 options available to be issued under the 2011 Plan.
F-23
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
Stock-based compensation for the years ended December 31, 2012 and 2011 was $160,237 and $42,378, respectively. As of December 31, 2012, the Company had $299,263 of unrecognized stock-based compensation expense related to non-vested awards that will be recognized over a weighted average period of 2.96 years. On December 18, 2012, the Board of Directors approved, subject to stockholder approval, the addition of 3,000,000 shares to the 2011 Plan.
The following sets forth the outstanding common stock options and related activity for the years ended December 31, 2012 and 2011:
| Number of Options |
Weighted Average Exercise Price Per Share |
|||||||
| Outstanding as of January 1, 2011 |
3,065,005 | $ | 0.14 | |||||
| Granted |
135,211 | 0.15 | ||||||
| Exercised |
— | — | ||||||
| Forfeited |
(43,268 | ) | 0.11 | |||||
|
|
|
|||||||
| Outstanding as of December 31, 2011 |
3,156,948 | 0.14 | ||||||
| Granted |
890,000 | 1.00 | ||||||
| Exercised |
(32,451 | ) | 0.06 | |||||
| Forfeited |
(149,057 | ) | 0.08 | |||||
|
|
|
|||||||
| Outstanding as of December 31, 2012 |
3,865,440 | 0.34 | ||||||
|
|
|
|||||||
| Exercisable as of December 31, 2012 |
3,175,803 | 0.21 | ||||||
|
|
|
|||||||
The Company determines the expected term of its stock option awards by using the simplified method, which assumes each vesting tranche of the award has a term equal to the midpoint between when the award vests and when the award expires. Expected volatility is calculated by weighting the stock price of similar industry public companies equivalent to the expected term of each grant. The risk-free interest rate for the expected term of each option granted is based on the U.S. Treasury securities rate in effect at the time of the grant with the period that approximates the expected term of the option.
For the year ended December 31, 2012, the Company calculated the fair value of each stock-based compensation award on the date of grant using the Black-Scholes option-pricing model with the following weighted average assumptions:
| Risk-free interest rate |
1.39 | % | ||
| Expected stock price volatility |
63.73 | % | ||
| Expected dividend yield |
0 | % | ||
| Expected life of options |
6.92 years |
The weighted average grant date fair value per share of options granted during the year ended December 31, 2012 was $.62. The aggregate intrinsic value of outstanding stock options and the aggregate intrinsic value of outstanding exercisable stock options as of December 31, 2012 was $2,549,625 and $2,510,883, respectively. The aggregate intrinsic value of stock options exercised as of December 31, 2012 was $30,651.
F-24
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
The following summarizes information about stock options outstanding as of December 31, 2012:
| Exercise Price | Numbers of Options Outstanding |
Weighted Average Remaining Contractual Life (Years) |
Weighted Average Exercise Price for Options Outstanding |
Number of Options Exercisable |
Weighted Average Exercise Price for Options Exercisable |
|||||||||||||||
| $0.06 - $ 0.08 | 902,600 | 6.26 | $ | 0.08 | 902,600 | $ | 0.08 | |||||||||||||
| $0.15 - $ 0.19 | 2,072,840 | 6.31 | 0.17 | 2,024,765 | 0.17 | |||||||||||||||
| $1.00 | 890,000 | 9.08 | 1.00 | 248,438 | 1.00 | |||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 3,865,440 | 6.94 | 0.34 | 3,175,803 | 0.21 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
Warrants
In conjunction with the reverse merger, the warrants to purchase preferred stock in Q Therapeutic Products, Inc. were exchanged for warrants to purchase common stock in Q Therapeutics, Inc. Each warrant in Q Therapeutic Products, Inc. was exchanged for 2.1633835 warrants in Q Therapeutics, Inc., the exercise price was adjusted down by the same factor, and all other terms remained unchanged. Additional warrants were issued in 2012 as a result of the completion of the 2011 Offering and in lieu of cash for the transfer of technology and services.
F-25
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
In connection with certain stock offerings and debt issuances, the Company has issued warrants to purchase stock. The following summarizes information about stock warrants as of December 31, 2012, all of which are exercisable:
| Warrants to Purchase |
Year of Expiration |
Number of Shares |
Exercise Price |
|||||||
| Common stock warrants issued for previously issued Series A2 warrants |
2015 | 132,797 | $ | 0.046 | ||||||
| Common stock warrants issued for previously issued Series B warrants |
2015 | 823,347 | 1.035 | |||||||
| Common stock warrants issued for previously issued Series B warrants |
2017 | 192,242 | 0.532 | |||||||
| Warrants issued in conjunction with bridge notes (August 31, 2011) |
2018 | 1,816,644 | 1.00 | |||||||
| Warrants issued in conjunction with bridge notes (August 31, 2011) |
2018 | 916,644 | 2.00 | |||||||
| Warrants issued in conjunction with private placement (October 13 and December 30, 2011) |
2018 | 3,828,047 | 1.00 | |||||||
| Warrants issued in conjunction with private placement (October 13 and December 30, 2011) |
2018 | 3,828,047 | 2.00 | |||||||
| Warrants issued for fees in conjunction with private placement (October 13 and December 30, 2011) |
2016 | 22,750 | 1.20 | |||||||
| Warrants issued in conjunction with private placement (February 2 and March 30, 2012) |
2019 | 190,000 | 1.00 | |||||||
| Warrants issued in conjunction with private placement (February 2 and March 30, 2012) |
2019 | 190,000 | 2.00 | |||||||
| Warrants issued for fees in conjunction with private placement (February 2 and March 30, 2012) |
2017 | 19,000 | 1.20 | |||||||
| Warrants issued in conjunction with asset purchase agreement (April 9, 2012) |
2019 | 10,000 | 1.00 | |||||||
| Warrants issued in conjunction with asset purchase agreement (April 9, 2012) |
2019 | 10,000 | 2.00 | |||||||
|
|
|
|||||||||
| 11,979,518 | ||||||||||
|
|
|
|||||||||
F-26
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
Each warrant with an exercise price of $1.00 or $2.00 per share may be redeemed by the Company at a price of $0.001 per share in the event (i) the closing sales price of the Company’s common stock is at least $1.50 for the $1.00 warrants or $3.00 for the $2.00 warrants for ten consecutive trading days and (ii) there is an effective public registration statement covering the resale of the common stock issuable upon the exercise of the warrants. The average warrant exercise strike price is $1.40 with an average remaining life of 5.5 years.
8. Commitments and Contingencies
Litigation
From time to time, the Company may become involved in lawsuits and legal proceedings, which arise in the ordinary course of business. Litigation is subject to inherent uncertainties and an unfavorable outcome in various matters may arise that could have a material adverse effect on the Company. The Company is not aware of any current or pending litigation that is expected to have a significant impact on the Company’s financial position or results of operations.
Employee Agreements and the 2012 Incentive Plan
The Company has entered into employment and proprietary rights agreements with all of its employees. These agreements stipulate that employment is on an at-will basis and outline salary, benefits, non-disclosure of confidential information, and assignment of intellectual property to the Company.
The Company has employment agreements with its CEO and CFO that specify compensation as salary, benefits and vacation, as well as addresses severance for termination of employment or the provisions should a change of control occur. In December 2012, the Board of Directors approved the 2012 Incentive Plan (2012 Plan) which provides bonus compensation of up to approximately $173,000 for all eligible employees upon the achievement of certain performance goals during 2013.
License and Royalty Agreements
The Company has entered into an exclusive license agreement with a university, where the Company has obtained certain intellectual property from the university to commercialize, produce, manufacture, use and sell the patent rights. The Company is required to pay the university a contractual dollar amount for each new investigational drug application filed with the Food and Drug Administration (FDA) using the licensed intellectual property. Should the Company receive cash payments from licensing revenue during human trial research, the Company is required to pay 10% of all net licensing revenue (but not including payments for product development activities or equity purchases) to the university up to a certain maximum amount. The Company is also required to pay an amount upon New Drug Application (NDA) approval. The NDA approval occurs when the FDA has approved all prior drug testing and allows a new drug to go to market. Once the Company has an NDA, the Company must pay the university a royalty of 2% of net sales up to a certain dollar amount and 2.5% of net sales in excess of that amount of human therapeutics and must pay the university a royalty of 5% of net sales on any services. If the Company sublicenses the intellectual property, then the Company must pay the university in accordance with the provisions of the agreement.
The Company has a product development agreement with an entity, whereby the Company has granted the entity a non-exclusive right to certain intellectual property owned by the Company and the entity has granted to the Company a non-exclusive right to use certain intellectual property owned by the entity in order to develop new products (Products) to be sold primarily by the Company or its designees to the research market. The Company has agreed to pay the entity certain amounts for product development in the form of the Company’s common stock. In addition, the Company has agreed to pay the entity royalty payments of 10% of net sales of the Company to third parties for Products developed using the entity’s intellectual property. If the Company agrees that the entity can also sell Products to third parties and if the entity has sales to a third party of Products using the Company’s intellectual property, then the entity will pay the Company a royalty payment of 10% of net sales. However, if the Products sold by the entity are covered solely by the Company’s patents and know-how, then a 50-50 financial sharing arrangement between the entity and the Company will apply to such Products.
F-27
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
In September 2012, the Company entered into two separate license agreements with one company (the licensor) whereby the Company has been granted two non-exclusive sub-licenses to make, have made and use the licensor’s intellectual technology. Each license agreement required an initial payment of $85,000, as well as an annual license fee of $5,000 per year. Additionally, the agreement allows the Company to purchase product on a per unit basis. No purchase commitments have been entered into at this time. The term of the license agreements is consistent with the term of the life of the patents.
Collaborative Arrangements
From time to time, the Company enters into collaborative arrangements for research and development, manufacture and/or commercialization of product and potential product candidates. These collaborations can provide for non-refundable, upfront license fees, R&D and commercial performance milestones, cost sharing, and royalty payments. The Company’s collaboration agreements with outside parties are performed on a “best efforts” basis with no guarantees of success.
On March 26, 2012, the Company executed an agreement to acquire certain intellectual property and technology based on the achievement of pre-determined milestones. Upon the achievement of certain milestones, the Company was to issue up to 400,000 shares of common stock, 400,000 common stock warrants with an exercise prices of $1.00 per share, and 400,000 common stock warrants with an exercise price of $2.00 per share to be issued as intellectual technology and property were delivered. In April 2012, the Company incurred $10,000 of licensing expense relating to the initial transfer of intellectual property and issued 10,000 shares of common stock, 10,000 common stock warrants with an exercise price of $1.00 per share, and 10,000 common stock warrants with an exercise price of $2.00 per share as initial payment. As of December 31, 2012, no subsequent issuances occurred as the predefined milestones were not met.
Right of First Negotiation
Pursuant to an agreement (Agreement) the Company has irrevocably granted a stockholder with a right of first negotiation to license certain technology of the Company related to Q-Cells ®. The term of the Agreement is for a period of the later of (i) 3 years from the effective date of the Agreement (October 6, 2011) or (ii) 6 months after the date of completion of certain dosing of the technology.
Supplier Agreements
In March 2010, the Company entered into a service agreement with an outside research firm to support the Company’s submission of an investigational new drug application (IND) to the FDA. As part of this purchase commitment, the Company was provided financing terms by the supplier which included partial payments of invoices, with remaining balances rolled into a convertible note payable accruing interest at 8% for the first 36 months and increasing to 10% for the 24 months thereafter. A provision in the note provided the supplier the right and option at any time prior to payment of the note to convert all or any portion of the outstanding balance into shares of the Company’s Series B preferred stock. As a result of the merger in October 2011, the Company eliminated its Series B preferred instruments and converted all outstanding shares to the Company’s common stock.
In November 2012, the Company and the supplier amended the terms of their original agreement via a Letter of Understanding (LOU) and increased the total purchase commitment with the supplier to approximately $2,600,000 with an expected completion by year end 2013. As the Company no longer has Series B preferred stock, the amendment provided the Company with the option of paying part of its commitment with cash or with shares of common stock. Upon conclusion of the supplier’s final project report, any outstanding balance will be converted into a note payable accruing interest at 8% until July 31, 2015. Any outstanding amounts thereafter shall accumulate interest at 10% through March 31, 2016. Should the Company be successful in obtaining additional financing, the amendment calls for a percentage of the funds raised to immediately pay down the outstanding balance. As of December 31, 2012, the amount owed under this agreement totaled $1,177,840 and is included in accounts payable in the accompanying balance sheet.
F-28
Table of Contents
Q Therapeutics, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements Continued
Additionally, the supplier was granted, upon successful completion of the clinical study, a right of first negotiation on any future pre-clinical GLP safety to toxicology studies.
Advisory Agreements
On October 1, 2012, the Company entered into an agreement with an investor relations firm for a minimum term of six months. In lieu of cash, the firm will receive 50,000 shares of restricted stock for services rendered through March 2013. Upon expiration of the initial term, a monthly cash retainer of up to $10,000 will be paid. Additionally, 250,000 warrants will be granted between January 1, 2013 through October 1, 2013 based on achievement of time-based objectives, with exercise prices varying between $1.25 and $2.75. During 2011, 24,999 shares of common stock were issued under the terms of the agreement.
On October 16, 2012, the Company entered into a services agreement with a public relations and investor relations firm.
Under the agreement, the financial advisor will receive a commission of 7% on offering proceeds and warrants to purchase shares of common stock equal to 10% of the number of common shares sold. The warrants shall have a life of five years with a strike price equal to 120% of the price of the common stock sold in the offering. Additionally, the Company agreed to reimburse reasonable expenses up to a maximum of $15,000. The agreement is valid for six months, but can be terminated by either party with at least 10 days written notice.
Operating Leases
The Company leases its research and office facility under an operating lease. The current lease was renewed effective April 1, 2011 and expires in March 2013. The Company expects the lease will be renewed. The minimum future lease payments under this operating lease as of December 31, 2012 total $27,721.
Lease expense under operating leases was $150,528 and $149,770 for the years ended December 31, 2012 and 2011, respectively.
9. Benefit Plans
The Company sponsors a defined contribution 401(k) retirement plan (the Plan). Employees who are 18 years of age or older are eligible to participate in the Plan. Employees may elect to contribute to the Plan up to 100% of their annual compensation, up to the maximum amount allowed by the Internal Revenue Service.
The Company may elect to match part of the employee’s contribution, make a profit sharing contribution, or a non-qualified contribution at its discretion. The Company elected to make a contribution of $9,482 to the Plan during the year ended December 31, 2012. No contribution was made to the Plan during the year ended December 31, 2011.
10. Related-Party Transactions
On August 30, 2011, the Company entered into notes payable totaling $610,000 with an officer and a company in which the officer is a general partner. The notes bore interest at 15% and were converted into common stock on October 13, 2011.
On October 13, 2011, the Company entered into promissory notes receivable from two minority stockholders that totaled $31,968. The terms of the agreements allowed for the assessment of interest at 8% per year, with the notes maturing and payable on December 31, 2011. In the event that the notes were not paid upon maturity, the interest rate was to immediately increase to 15% per year and the stockholders would forfeit 16,667 shares of their common stock per month, up to 200,000 shares, until the notes were paid in full. During the year, the Company received total payments of $7,988 of which $5,941 was applied towards principal and $2,047 to interest. As of December 31, 2012, the amount owed was $28,800 of which $2,215 related to interest and all 200,000 shares of common stock had been forfeited by the owners and returned to the Company. As no resolution of this debt has been identified, an allowance has been provided against the full amount of the notes receivable.
F-29
Table of Contents
Q THERAPEUTICS, INC.
5,000,000 SHARES OF COMMON STOCK
AND
WARRANTS TO PURCHASE UP TO 1,250,000 SHARES OF COMMON STOCK
Until ninety days after the date this registration statement is declared effective, all dealers that effect transactions in these securities whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Sole Book-running Manager

, 2013
Table of Contents
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution. |
The estimated costs of this offering are as follows:
| Expenses(1) |
Amount US ($) |
|||
| SEC Registration Fee |
$ | 682.00 | ||
| Transfer Agent Fees |
* | |||
| Accounting Fees and Expenses |
* | |||
| Legal Fees and Expenses |
* | |||
| Printers |
* | |||
|
|
|
|||
| Total |
$ | |||
|
|
|
|||
| * | To be completed by amendment |
We are paying all expenses of the offering listed above.
| Item 14. | Indemnification of Directors and Officers |
Indemnification
Pursuant to the Certificate of Incorporation and By-Laws of the Company, we may indemnify an officer or director who is made a party to any proceeding, including a law suit, because of his position, if he acted in good faith and in a manner he reasonably believed to be in our best interest. In certain cases, we may advance expenses incurred in defending any such proceeding. To the extent that the officer or director is successful on the merits in any such proceeding as to which such person is to be indemnified, we must indemnify him against all expenses incurred, including attorney’s fees. With respect to a derivative action, indemnity may be made only for expenses actually and reasonably incurred in defending the proceeding, and if the officer or director is judged liable, only by a court order. The indemnification is intended to be to the fullest extent permitted by the laws of the State of Delaware.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the provisions above, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities, other than the payment by us of expenses incurred or paid by one of our directors, officers, or controlling persons in the successful defense of any action, suit or proceeding, is asserted by one of our directors, officers, or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification is against public policy as expressed in the Securities Act, and we will be governed by the final adjudication of such issue.
| Item 15. | Recent Sales of Unregistered Securities |
Recent Sales of Unregistered Securities
On October 1, 2012, the Company issued restricted stock in lieu of cash as part of an agreement with an investor relations firm for a minimum term of six months. In lieu of cash, the investor relations firm received 50,000 shares of restricted stock for services rendered from October 1, 2012 through March 31, 2013 as described below:
| • | 8,333 shares of common stock issued on the first day of October, November, December of 2012 and January of 2013, and |
| • | 8,334 shares of common stock issued on the first day of February and March of 2013. |
The issuance of the securities was made to one party in a private offering exempt from registration on reliance of Section 4(2) of the Securities Act of 1933, as amended.
Additionally, the Company agreed to issue up to 250,000 warrants between January 1, 2013 and October 1, 2013 based on achievement of time-based objectives, with exercise prices ranging between $1.25 and $2.75. Warrants to purchase 62,500 shares were issued and immediately vested on January 1, 2013 with an exercise price of $1.25. Warrants to purchase 62,500 shares were issued and immediately vested on April 1, 2013. The warrants have a two-year life. The issuance of the securities was made in a private offering exempt from registration on reliance of Section 4(2) of the Securities Act of 1933, as amended.
II-1
Table of Contents
In connection with the Merger Agreement, on October 13, 2011, we issued an aggregate of 22,671,923 shares of common stock (including shares underlying warrants and options), or 89.7% of the outstanding shares prior to the offering, to Q Therapeutics, Inc. stockholders in exchange for 100% of the outstanding shares of Q Therapeutics, Inc., which merger resulted in Q Therapeutics, Inc. becoming our wholly owned subsidiary. These securities qualified for exemption under Section 4(2) of the Securities Act since the issuing of securities by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of securities offered. We did not undertake an offering in which we sold a high number of securities to a high number of investors. In addition, these stockholders had the necessary investment intent as required by Section 4(2) since they agreed to and received share certificates bearing a legend stating that such securities are restricted pursuant to Rule 144 of the Securities Act. This restriction ensures that these securities would not be immediately redistributed into the market and therefore not be part of a “public offering.” Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
On August 30, 2011, Q Therapeutics, Inc. received $450,000 in bridge financing from investors, (including $90,000 in credit towards the advancement of consulting fees and out-of-pocket expenses) evidenced by secured convertible promissory notes (Notes) and one Seven-Year common stock Purchase Warrant to purchase one share of the Company’s common stock with an exercise price equal to the lesser of (a) the price per share of the Company’s Shares issued in a financing or (b) the Fair Market Value (FMV) of the common stock of Q Therapeutics, Inc. The Notes were issued at 50% of face value, bore interest at the rate of 15% per annum, and contained an automatic conversion feature whereby upon the attainment of the Company’s Private Placement Offering’s minimum raise amount of $3,000,000, each Note’s outstanding principal and interest balance was automatically converted into a unit, consisting of an aggregate of: (a) one share of the Company’s common stock, (b) one Seven-Year common stock Purchase Warrant to purchase one share of the Company’s common stock exercisable at $1.00 per share of the Company’s common stock (Series A warrants), (c) one Seven-Year common stock Purchase Warrant to purchase one share of the Company’s common stock exercisable at $2.00 per share of the Company’s common stock (Series B warrants) at the same purchase price and upon the same terms as investors in the Company’s Private Placement Offering.
On October 13, 2011, in connection with the Merger, the Notes were automatically converted into 916,644 units consisting of: (a) 916,644 shares of common stock, (b) 916,644 Series A warrants, and (c) 916,644 Series B warrants. These securities qualified for exemption under Section 4(2) of the Securities Act since the issuance securities by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of securities offered. Q Therapeutics, Inc. did not undertake an offering in which we sold a high number of securities to a high number of investors. In addition, these stockholders had the necessary investment intent as required by Section 4(2) since they agreed to and received share certificates bearing a legend stating that such securities are restricted pursuant to Rule 144 of the Securities Act. This restriction ensures that these securities would not be immediately redistributed into the market and therefore not be part of a “public offering.” Based on an analysis of the above factors, Q Therapeutics, Inc. has met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
On October 13, 2011, immediately following the Merger, pursuant to the Purchase Agreement, we consummated the Private Placement for the issuance and sale of 3,803,047 Units for $1.00 per Unit, each Unit consisting of an aggregate of (a) one share of common stock, (b) one Seven-Year common stock Purchase Warrant to purchase one share of the Company’s common stock exercisable at $1.00 per share (Series A Warrant), and (c) one Seven-Year common stock Purchase Warrant to purchase one share of the Company’s common stock exercisable at $2.00 per share (Series B Warrant), for aggregate gross proceeds to
II-2
Table of Contents
date of $3,803,047 from seven investors. Between October 13, 2011 and April 9, 2012, four additional tranches consisting of 11 accredited investors occurred for an additional 215,000 Units generating proceeds of $215,000, resulting in aggregate proceeds of $4,028,047. Such securities were not registered under the Securities Act. The issuance of these securities was exempt from registration under Regulation S and Section 4(2) of the Securities Act. We made this determination based on the representations of Investors, which included, in pertinent part, that such stockholders were either (a) “accredited investors” within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, or (b) not a “U.S. person” as that term is defined in Rule 902(k) of Regulation S under the Act, and that such stockholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the stockholders understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
As noted above, upon consummation of the Merger, all securities of Q Therapeutics issued and outstanding were converted into securities of the Company on a pro rata basis.
| Item 16. | Exhibits |
Index to Exhibits
| 1.1 |
Form of Underwriting Agreement.** | |
| 2.1 |
Agreement and Plan of Merger by and between Grace 2, Inc., Q Merger Sub., and Q Therapeutics, Inc., dated October 13, 2011 (incorporated by reference from the Company’s Current Report on Form 8-K filed on October 18, 2011). | |
| 3.1 |
Certificate of Incorporation of Grace 2, Inc. (incorporated by reference from the Company’s Registration Statement on Form 10SB filed on June 19, 2006). | |
| 3.2 |
Certificate of Amendment to Certificate of Incorporation of Grace 2, Inc. dated October 18, 2011 (incorporated by reference from the Company’s Registration Statement on Form S-1 filed on June 29, 2012). | |
| 3.3 |
By Laws of the Company (incorporated by reference from the Company’s Registration Statement on Form 10SB filed on June 19, 2006). | |
| 3.4 |
Certificate of Amendment to the Certificate of Incorporation of Q Therapeutics, Inc. dated October 9, 2012 (incorporated by reference from the Company’s Form 10-Q filed November 13, 2012). | |
| 3.5 |
Certificate of Amendment to the Certificate of Incorporation of Q Holdings, Inc. dated October 30, 2012 (incorporated by reference from the Company’s Form 10-Q filed November 13, 2012). | |
| 4.1 |
Form of Series A Warrant (incorporated by reference from the Company’s Form 10-Q filed on August 10, 2012.) | |
| 4.2 |
Form of Series B Warrant (incorporated by reference from the Company’s Registration Statement on Form S-1 filed on June 29, 2012). | |
| 4.3 |
Form of Bridge Warrant (incorporated by reference from the Company’s Registration Statement on Form S-1/A filed on August 16, 2012). | |
| 4.4 |
Form of Amendment to Bridge Warrant (incorporated by reference from the Company’s Registration Statement on Form S-1/A filed on August 16, 2012). | |
II-3
Table of Contents
|
4.5 |
Q Holdings stock Purchase Agreement (incorporated by reference from the Company’s Current Report on Form 8-K/A filed on December 9, 2011.) | |
| 4.6 |
Q Holdings Security Rights Agreement (incorporated by reference from the Company’s Current Report on Form 8-K/A filed on December 9, 2011.) | |
| 4.7 |
Amended and Restated Q Therapeutics 2002 stock Incentive Plan (incorporated by reference from the Company’s Registration Statement on Form S-1 filed on June 29, 2012). | |
| 4.8* |
Q Holdings 2011 Equity Incentive Compensation Plan (incorporated by reference from the Company’s Registration Statement on Form S-1 filed on June 29, 2012.) | |
| 4.9 |
Form of Warrant(1) | |
| 5.1 |
Legal Opinion of Life Science Law, PC.** | |
| 10.1 |
Exclusive License Agreement between Q Therapeutics, Inc. and the University of Utah Research Foundation dated October 5,2005, as amended, re-filed pursuant to SEC recommendations (incorporated by reference from the Company’s Current Report on From 8-K filed on March 13, 2012. Certain provisions have been omitted pursuant to Confidential Treatment Requests and have been filed separately with the SEC.) | |
| 10.2* |
Employment Agreement between Q Holdings, Inc. and Deborah A. Eppstein Ph.D. dated October 13, 2011 (incorporated by reference from the Company’s Current Report on Form 8-K filed on October 18, 2011). | |
| 10.2.1* |
Amendment to Employment & Proprietary Rights Agreement between Q Therapeutics, Inc. and Deborah A. Eppstein Ph.D. dated December 18, 2012. (Incorporated by reference from the Company’s Annual Report on Form 10-K filed on March 28, 2013) | |
| 10.3* |
Employment Agreement between Q Holdings, Inc. and Steven J. Borst, dated October 13, 2011 (incorporated by reference from the Company’s Current Report on From 8-K filed on October 18, 2011). | |
| 10.3.1* |
Amendment to Employment & Proprietary Rights Agreement between Q Therapeutics, Inc. and Steven J. Borst, dated December 18, 2012. (Incorporated by reference from the Company’s Annual Report on Form 10-K filed on March 28, 2013) | |
| 10.4 |
Lease Agreement dated January 30, 2004 between Q Therapeutics, Inc. and Paradigm Resources, LLC (incorporated by reference from the Company’s Current Report on From 8-K/A filed on December 9, 2011). | |
| 10.5 |
Form of Bridge Note for $450,000 Bridge Financing on August 30, 2011 (incorporated by reference from the Company’s Registration Statement on Form S-1 filed on June 29, 2012). | |
| 10.6* |
Compensation Plan for Employees of Q Therapeutics, Inc. as approved by the Board of Directors on December 18, 2012. (Incorporated by reference from the Company’s Annual Report on Form 10-K filed on March 28, 2013) | |
| 21.1 |
List of Subsidiaries.(3) | |
| 23.1 |
Consent of Independent Registered Public Accounting Firm.(1) | |
| 23.2 |
Legal Consent of Life Science Law, PC.** | |
II-4
Table of Contents
|
24.1 |
Power of Attorney (3) | |
| 101.INS(2)(3) |
XBRL Instance Document | |
| 101.SCH(2)(3) |
XBRL Taxonomy Extension Schema Document | |
| 101.CAL(2)(3) |
XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF(2)(3) |
XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB(2)(3) |
XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE(2)(3) |
XBRL Taxonomy Extension Presentation Linkbase Document | |
| (1) | Filed herewith. |
| (2) | Pursuant to Rule 406T of Regulation S-T, these interactive data files are deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934 and otherwise are not subject to liability under those sections. |
| (3) | Previously filed. |
| * | Management contract or compensatory plan or arrangement. |
| ** | To be filed by amendment. |
| Item 17. | Undertakings |
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424 (b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
Table of Contents
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(i) If the registrant is relying on Rule 430B:
(A) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B) Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date; or
(ii) If the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
II-6
Table of Contents
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6) The undersigned registrant hereby undertakes to supplement the prospectus, after the expiration of the subscription period, to set forth the results of the subscription offer, the transactions by the underwriters during the subscription period, the amount of unsubscribed securities to be purchased by the underwriters, and the terms of any subsequent reoffering thereof. If any public offering by the underwriters is to be made on terms differing from those set forth on the cover page of the prospectus, a post-effective amendment will be filed to set forth the terms of such offering.
(7) The undersigned registrant hereby undertakes to deliver or cause to be delivered with the prospectus, to each person to whom the prospectus is sent or given, the latest annual report to security holders that is incorporated by reference in the prospectus and furnished pursuant to and meeting the requirements of Rule 14a–3 or Rule 14c–3 under the Securities Exchange Act of 1934; and, where interim financial information required to be presented by Article 3 of Regulation S-X are not set forth in the prospectus, to deliver, or cause to be delivered to each person to whom the prospectus is sent or given, the latest quarterly report that is specifically incorporated by reference in the prospectus to provide such interim financial information.
(8) The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-7
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in Salt Lake City, State of Utah, on July 5, 2013.
| Q THERAPEUTICS, INC. | ||
| By: | /s/ Deborah A. Eppstein | |
| Deborah A. Eppstein, Ph.D. | ||
| President and Chief Executive Officer, Director | ||
| (Principal Executive Officer) | ||
| By: | /s/ Steven J. Borst | |
| Steven J. Borst, M.B.A. | ||
| Chief Financial Officer and Vice President of Corporate Development | ||
| (Principal Financial Officer, Principal Accounting Officer) | ||
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Deborah A. Eppstein |
President and Chief Executive Officer, Director (Principal Executive Officer) |
July 5, 2013 | ||
| Deborah A. Eppstein, Ph.D. | ||||
| /s/ Steven J. Borst |
Chief Financial Officer and Vice President of Corporate Development (Principal Financial Officer and Principal Accounting Officer) |
July 5, 2013 | ||
| Steven J. Borst | ||||
| * |
Chairman of the Board of Directors | July 5, 2013 | ||
| Dinesh C. Patel, Ph.D. | ||||
| * |
Director | July 5, 2013 | ||
| Peter Barton Hutt | ||||
1
Table of Contents
| * |
Director | July 5, 2013 | ||
| Linda F. Powers | ||||
| * |
Director | July 5, 2013 | ||
| Peter Grebow, Ph.D. | ||||
| * |
Director | July 5, 2013 | ||
| Diane Jorkasky, M.D. | ||||
| * By: | /s/ Stephen J. Borst | |
| Stephen J. Borst Attorney-in-fact |
2
