Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - INSMED Inc | a13-15900_1ex99d1.htm |
| 8-K - 8-K - INSMED Inc | a13-15900_18k.htm |
Exhibit 99.2
|
|
Forward Looking Statements This presentation contains forward-looking statements that are made pursuant to provisions of Section 21E of the Securities Exchange Act of 1934. Words, and variations of words, such as "intend," "expect," "will," "anticipate," "believe," "continue," "propose" and similar expressions are intended to identify forward-looking statements. Investors are cautioned that such statements in this presentation, including statements relating to the status, results and timing of clinical trials and clinical data, the anticipated benefits of Insmed's products, the anticipated timing of regulatory submissions, and the ability to obtain required regulatory approvals, bring products to market and successfully commercialize products constitute forward-looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, without limitation, failure or delay of European, Canadian, U.S. Food and Drug Administration and other regulatory reviews and approvals, competitive developments affecting Insmed’s product candidates, delays in product development or clinical trials or other studies, patent disputes and other intellectual property developments relating to Insmed’s product candidates, unexpected regulatory actions, delays or requests, the failure of clinical trials or other studies or results of clinical trials or other studies that do not meet expectations, the fact that subsequent analyses of clinical trial or study data may lead to different (including less favorable) interpretations of trial or study results or may identify important implications of a trial or study that are not reflected in the statements contained in this presentation, and the fact that trial or study results or subsequent analyses may be subject to differing interpretations by regulatory agencies, the inability by Insmed to successfully develop its product candidates or receive necessary regulatory approvals, the inability by Insmed to make product candidates commercially successful, changes in anticipated expenses, changes in Insmed’s financing requirements or ability raise additional capital, and other risks and challenges detailed in Insmed’s filings with the U.S. Securities and Exchange Commission, including its Annual Report on Form 10-K for the year ended December 31, 2012. Investors are cautioned not to place undue reliance on any forward-looking statements that speak only as of the date of this presentation. Insmed undertakes no obligation to update these forward-looking statements to reflect events or circumstances or changes in its expectations. |
|
|
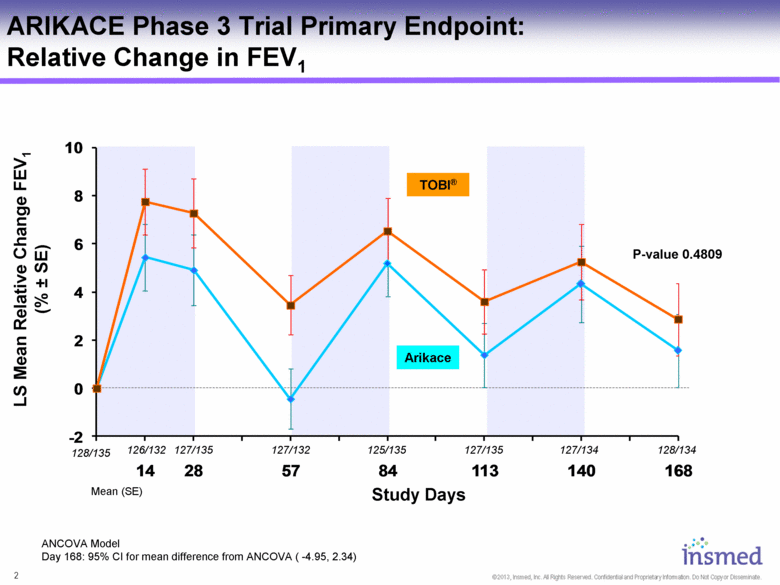
LS Mean Relative Change FEV1 (% ± SE) ARIKACE Phase 3 Trial Primary Endpoint: Relative Change in FEV1 ANCOVA Model Day 168: 95% CI for mean difference from ANCOVA ( -4.95, 2.34) Study Days Mean (SE) 128/135 128/134 127/134 127/135 125/135 127/132 127/135 126/132 TOBI® P-value 0.4809 Arikace |
|
|
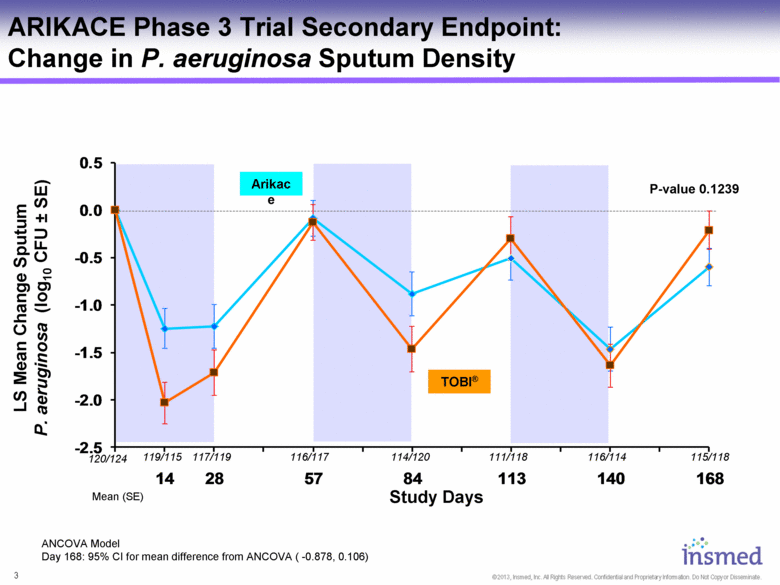
LS Mean Change Sputum P. aeruginosa (log10 CFU ± SE) ANCOVA Model Day 168: 95% CI for mean difference from ANCOVA ( -0.878, 0.106) ARIKACE Phase 3 Trial Secondary Endpoint: Change in P. aeruginosa Sputum Density Study Days Mean (SE) 120/124 115/118 116/114 111/118 114/120 116/117 117/119 119/115 Arikace TOBI® P-value 0.1239 -2.5 -2.0 -1.5 -1.0 -0.5 0.0 0.5 14 28 57 84 113 140 168 |
|
|
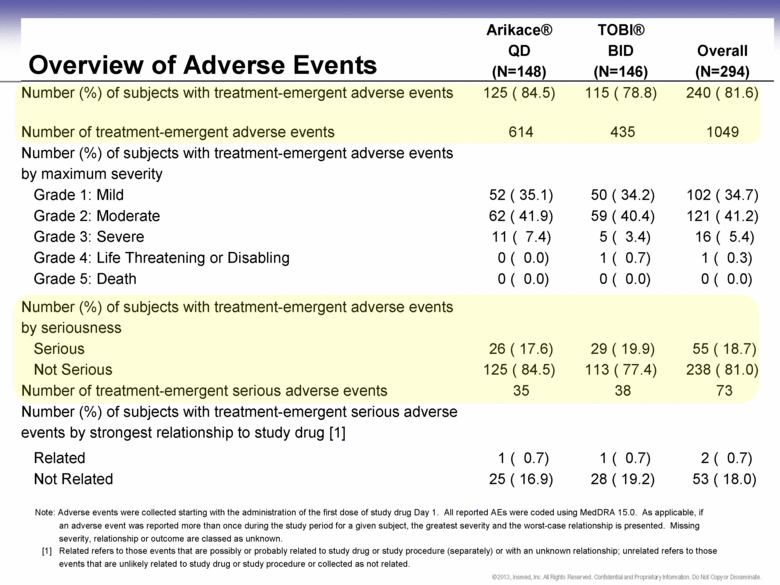
Overview of Adverse Events Arikace® QD (N=148) TOBI® BID (N=146) Overall (N=294) Number (%) of subjects with treatment-emergent adverse events 125 ( 84.5) 115 ( 78.8) 240 ( 81.6) Number of treatment-emergent adverse events 614 435 1049 Number (%) of subjects with treatment-emergent adverse events by maximum severity Grade 1: Mild 52 ( 35.1) 50 ( 34.2) 102 ( 34.7) Grade 2: Moderate 62 ( 41.9) 59 ( 40.4) 121 ( 41.2) Grade 3: Severe 11 ( 7.4) 5 ( 3.4) 16 ( 5.4) Grade 4: Life Threatening or Disabling 0 ( 0.0) 1 ( 0.7) 1 ( 0.3) Grade 5: Death 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Number (%) of subjects with treatment-emergent adverse events by seriousness Serious 26 ( 17.6) 29 ( 19.9) 55 ( 18.7) Not Serious 125 ( 84.5) 113 ( 77.4) 238 ( 81.0) Number of treatment-emergent serious adverse events 35 38 73 Number (%) of subjects with treatment-emergent serious adverse events by strongest relationship to study drug [1] Related 1 ( 0.7) 1 ( 0.7) 2 ( 0.7) Not Related 25 ( 16.9) 28 ( 19.2) 53 ( 18.0) Note: Adverse events were collected starting with the administration of the first dose of study drug Day 1. All reported AEs were coded using MedDRA 15.0. As applicable, if an adverse event was reported more than once during the study period for a given subject, the greatest severity and the worst-case relationship is presented. Missing severity, relationship or outcome are classed as unknown. [1] Related refers to those events that are possibly or probably related to study drug or study procedure (separately) or with an unknown relationship; unrelated refers to those events that are unlikely related to study drug or study procedure or collected as not related. |
|
|
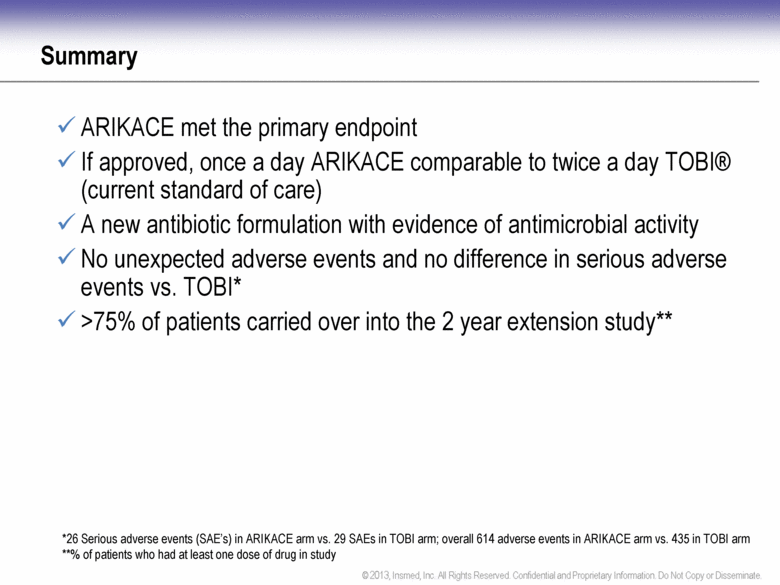
Summary ARIKACE met the primary endpoint If approved, once a day ARIKACE comparable to twice a day TOBI® (current standard of care) A new antibiotic formulation with evidence of antimicrobial activity No unexpected adverse events and no difference in serious adverse events vs. TOBI* >75% of patients carried over into the 2 year extension study** *26 Serious adverse events (SAE’s) in ARIKACE arm vs. 29 SAEs in TOBI arm; overall 614 adverse events in ARIKACE arm vs. 435 in TOBI arm **% of patients who had at least one dose of drug in study |