Attached files
| file | filename |
|---|---|
| EX-23.2 - EX 23.2 - Acer Therapeutics Inc. | d551477dex232.htm |
Table of Contents
As filed with the Securities and Exchange Commission on June 17, 2013
Registration No. 333-
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT
OF 1933
OPEXA THERAPEUTICS, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Texas | 2834 | 76-0333165 | ||
| (State or Other Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
2635 Technology Forest Blvd.
The Woodlands, Texas 77381
(281) 272-9331
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Neil K. Warma
President and Chief Executive Officer
OPEXA THERAPEUTICS, INC.
2635 Technology Forest Blvd.
The Woodlands, Texas 77381
(281) 272-9331
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent for Service)
Copies to:
| Mike Hird, Esq. Patty M. DeGaetano, Esq. Pillsbury Winthrop Shaw Pittman LLP 12255 El Camino Real, Suite 300 San Diego, CA 92130 (619) 234-5000 |
Christopher S. Auguste, Esq. Kramer Levin Naftalis & Frankel LLP 1177 Avenue of the Americas New York, NY 10036 (212) 715-9100 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ |
Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company x | |||
| (Do not check if a smaller reporting company) | ||||||
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of Each Class of Securities To Be Registered | Proposed Maximum Aggregate Offering Price(1) |
Amount of Registration Fee(2) | ||
| Common Stock, $0.01 par value(3) |
$17,250,000 | $2,352.90 | ||
|
| ||||
|
| ||||
| (1) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended. |
| (2) | Pursuant to Rule 416(a) under the Securities Act of 1933, the registrant is also registering hereunder an indeterminate number of shares that may be issued and resold resulting from stock splits, stock dividends or similar transactions. |
| (3) | Includes shares of common stock which may be issued upon exercise of an option granted to the underwriters to cover over-allotments, if any. |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED JUNE 17, 2013 |
Shares
Common Stock

We are offering shares of our common stock. Our common stock is listed on the NASDAQ Capital Market under the symbol “OPXA.” On June 14, 2013, the last reported sales price for our common stock was $1.70 per share.
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page 14 in this prospectus. You should carefully consider these risk factors, as well as the information contained in this prospectus, before you invest.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| Per share | Total | |||||||
| Public offering price |
$ | $ | ||||||
| Underwriting discount(1) |
$ | $ | ||||||
| Proceeds to us, before expenses |
$ | $ | ||||||
| (1) | The underwriters will receive compensation in addition to the underwriting discount. See “Underwriting” for a description of compensation payable to the underwriters. |
We have granted a 30-day option to the representative of the underwriters to purchase up to additional shares of common stock solely to cover over-allotments, if any.
The underwriters expect to deliver our shares of common to purchasers in this offering on or about , 2013.
Aegis Capital Corp
Table of Contents
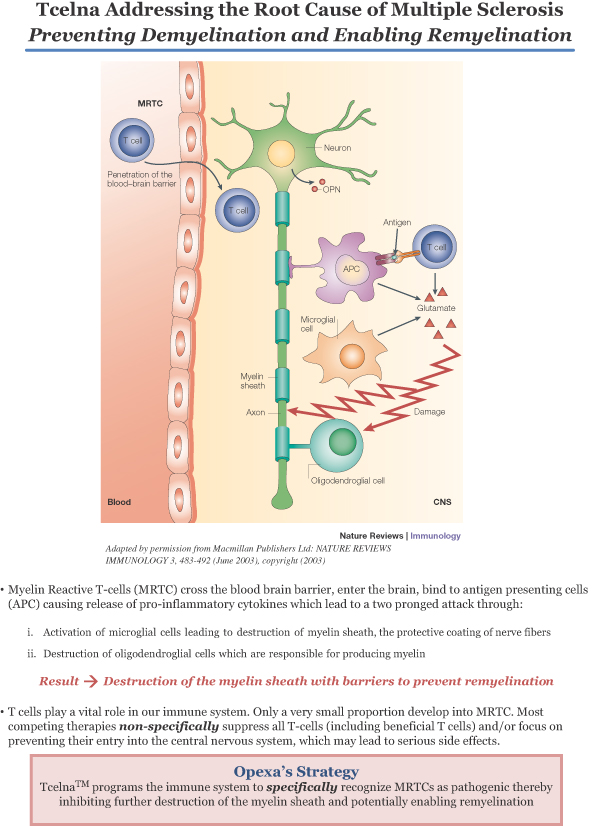
Table of Contents
| Page | ||||
| 1 | ||||
| 1 | ||||
| 10 | ||||
| 14 | ||||
| 37 | ||||
| 39 | ||||
| 40 | ||||
| 42 | ||||
| Market Price of our Common Stock and Related Stockholder Matters |
43 | |||
| 43 | ||||
| 44 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
45 | |||
| 51 | ||||
| 67 | ||||
| 69 | ||||
| Security Ownership of Certain Beneficial Owners and Management |
74 | |||
| 78 | ||||
| 80 | ||||
| 81 | ||||
| 89 | ||||
| 89 | ||||
| 89 | ||||
| 89 | ||||
| F-1 | ||||
ABOUT THIS PROSPECTUS
The registration statement we filed with the Securities and Exchange Commission, or SEC, includes exhibits that provide more detail of the matters discussed in this prospectus. You should read this prospectus and the related exhibits filed with the SEC, together with the additional information described under the heading “Where You Can Find More Information,” before making your investment decision.
You should rely only on the information provided in this prospectus or in a prospectus supplement or amendment thereto. We have not authorized anyone else to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any state where the offer or sale is not permitted. You should assume that the information in this prospectus is accurate only as of the date hereof. Our business, financial condition, results of operations and prospects may have changed since that date.
Unless the context otherwise requires, references in this prospectus to “Opexa,” “the Company,” “we,” “us” and “our” refer to Opexa Therapeutics, Inc. TcelnaTM and ImmPathTM are trademarks of Opexa. All other product and company names are trademarks of their respective owners.
i
Table of Contents
This summary contains basic information about us and this offering. Because it is a summary, it does not contain all of the information that you should consider before investing. Before you decide to invest in our common stock, you should read this entire prospectus carefully, including the section entitled “Risk Factors,” and our consolidated financial statements and the related notes.
Our Business
Opexa is a biopharmaceutical company developing a personalized immunotherapy with the potential to treat major illnesses, including multiple sclerosis (MS). This therapy is based on our proprietary T-cell technology. Our mission is to lead the field of Precision ImmunotherapyTM by aligning the interests of patients, employees and shareholders. Information related to our product candidate, Tcelna™, is preliminary and investigative. Tcelna has not been approved by the U.S. Food and Drug Administration (FDA) or other global regulatory agencies for marketing.
MS is a chronic, often disabling disease that affects the central nervous system (CNS), which is made up of the brain, spinal cord and optic nerves. MS attacks the covering surrounding nerve cells, or myelin sheaths, leading to loss of myelin (demyelination) and nerve damage. Symptoms may be mild, such as numbness in the limbs, or severe, such as paralysis or loss of vision. The progress, severity and specific symptoms of MS are unpredictable and vary from one person to another. We believe that our product candidate, Tcelna, has the potential to fundamentally address the root cause of MS by stopping the demyelination process and in supporting the generation of new myelin sheaths where demyelination has occurred (remyelination).
Tcelna is an autologous T-cell immunotherapy that is currently being developed for the treatment of Secondary Progressive MS (SPMS) and is specifically tailored to each patient’s immune response profile to myelin. Tcelna is designed to reduce the number and/or functional activity of specific subsets of myelin-reactive T-cells (MRTCs) known to attack myelin. This technology was originally licensed from Baylor College of Medicine in 2001.
Tcelna is manufactured using our proprietary method for the production of an autologous T-cell product, which comprises the collection of blood from the MS patient and the expansion of MRTCs from the blood. Upon completion of the manufacturing process, an annual course of therapy consisting of five doses is cryopreserved. At each dosing time point, a single dose of Tcelna is formulated and attenuated by irradiation before returning the final product to the clinical site for subcutaneous administration to the patient.
Tcelna has received Fast Track designation from the FDA in SPMS, and we believe it is positioned as a potential first-to-market personalized T-cell therapy for MS patients. The FDA’s Fast Track program is designed to facilitate the development and expedite the review of drug candidates intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
Opexa was incorporated in Texas in March 1991. Our principal executive offices are located at 2635 Technology Forest Blvd., The Woodlands, Texas 77381, and our telephone number is (281) 775-0600.
T-Cell Therapy and Tcelna
Tcelna is a novel T-cell immunotherapy in Phase IIb clinical development for the treatment of patients with SPMS. It is also positioned to enter Phase III clinical development for the treatment of patients with relapsing remitting MS (RRMS), subject to the availability of sufficient resources. Tcelna is a personalized therapy that is specifically tailored to each patient’s disease profile. Tcelna is manufactured using ImmPathTM, our proprietary
1
Table of Contents
method for the production of a patient-specific T-cell immunotherapy which encompasses the collection of blood from the MS patient, isolation of peripheral blood mononuclear cells, generation of an autologous pool of MRTCs raised against selected peptides from myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) and proteolipid protein (PLP), and the return of these expanded, irradiated T-cells back to the patient. These attenuated T-cells are reintroduced into the patient via subcutaneous injection to trigger a therapeutic immune system response.
Abili-T Trial: Phase IIb Clinical Study in Patients with SPMS
In September 2012, we announced the initiation of a Phase IIb clinical trial of Tcelna in patients with SPMS. The trial is entitled: A Phase II Double-Blind, Placebo Controlled Multi-Center Study to Evaluate the Efficacy and Safety of Tcelna in Subjects with Secondary Progressive Multiple Sclerosis and has been named the “Abili-T” trial. The Abili-T trial is a double-blind, 1:1 randomized, placebo-controlled study in SPMS patients who demonstrate evidence of disease progression with or without associated relapses. The trial is expected to enroll 180 patients who have Expanded Disability Status Scale (EDSS) scores between 3.0 and 6.0 at approximately 30 leading clinical sites in the U.S. and Canada. According to the study protocol, patients will receive two annual courses of Tcelna treatment consisting of five subcutaneous injections per year at weeks 0, 4, 8, 12 and 24.
The primary efficacy endpoint of the trial is the percentage of brain volume change (whole brain atrophy) at 24 months. Study investigators will also measure several important secondary outcomes commonly associated with MS including sustained disease progression as measured by EDSS, changes in EDSS, time to sustained progression, annualized relapse rate (ARR), change in Multiple Sclerosis Functional Composite (MSFC) assessment of disability and change in Symbol Digit Modality Test. Data on certain exploratory endpoints such as quality of life metrics as measured by the Multiple Sclerosis Quality of Life Inventory (MSQLI), magnetic resonance imaging (MRI) measures and immune monitoring metrics are also being collected.
2
Table of Contents
As part of the Abili-T trial, we are undertaking a comprehensive immune monitoring program for all patients enrolled in the study. The goals of this program are to further understand the biology behind the mechanism of action for Tcelna and to possibly identify novel biomarkers that are dominant in the pathophysiology of SPMS patients. The program encompasses an analysis of various pro-inflammatory and anti-inflammatory biomarkers. We believe that the blinded data, which will be analyzed during the course of the trial, may potentially signal responders and non-responders. Directional movement of certain biomarkers, when corroborated with final clinical trial data, may be indicative of responders and disease stabilization or progression. A summary of pro-inflammatory and anti-inflammatory biomarkers to be studied, with their potential outcomes, is set forth in the graphic below.
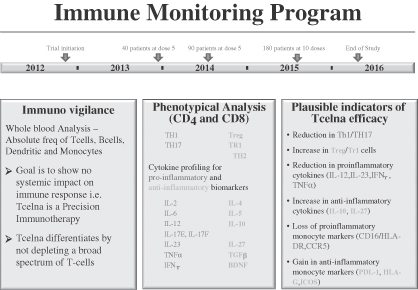
As of June 14, 2013, the Abili-T clinical trial has randomized 53 patients. A scheduled Data Safety Monitoring Board meeting took place during the week of May 20, 2013, and a recommendation was made to continue the study. The Abili-T clinical study in North America of Tcelna is expected to complete enrollment of 180 patients by late 2013 or early 2014, with the resulting top-line data expected to be available in the first half of 2016.
During the first quarter of 2013, the pace of on-boarding clinical sites for the Abili-T clinical study was tempered pending the completion of our negotiations with Ares Trading SA (“Merck”), a wholly owned subsidiary of Merck Serono S.A., for the Option (see “—Option and License Agreement with Merck Serono”) as well as financial considerations. Upon receipt of the upfront payment of $5 million for granting the Option, we were able to refocus on execution of the Abili-T clinical trial, including enrollment. The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of May 31, 2013 to be between $30-$32 million. Our existing resources are not adequate to permit us to complete such study or the majority of it. We anticipate that $ million of the proceeds from the offering will be applied to funding the continuation of the clinical study as well as the ongoing expenses of our operations during such development. We will need to secure significant additional resources to complete the trial and support our operations during the pendency of the trial. There can be no assurance that any such financings or potential opportunities and alternatives can be consummated on acceptable terms, if at all. We believe we have sufficient liquidity to support our clinical trial activities into the fourth quarter of 2013. Given our need for substantial amounts of capital to continue the Abili-T clinical study in
3
Table of Contents
North America of Tcelna in SPMS, we intend to continue to explore potential opportunities and alternatives to obtain the significant additional resources, including one or more additional financings, that will be necessary to complete the Abili-T study and to support our operations during the pendency of such study.
Option and License Agreement with Merck Serono
On February 4, 2013, we entered into an Option and License Agreement with Merck. Pursuant to the agreement, Merck has an option (the “Option”) to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck prior to or upon completion of our ongoing Abili-T trial of Tcelna in patients with SPMS.
Under the terms of the agreement, we received an upfront payment of $5 million for granting the Option. If the Option is exercised, Merck would pay us an upfront license fee of $25 million unless Merck is unable to advance directly into a Phase III clinical trial of Tcelna for SPMS without a further Phase II clinical trial (as determined by Merck), in which event the upfront license fee would be $15 million. After exercising the Option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights to use for other indications outside of MS.
Based upon the achievement of development milestones by Merck for Tcelna in SPMS, we would be eligible to receive one-time milestone payments totaling up to $70 million as follows: (i) milestone payments aggregating $35 million if Tcelna is submitted for regulatory approval and commercialized in the United States; (ii) milestone payments aggregating $30 million if Tcelna is submitted for regulatory approval in Europe and commercialized in at least three major countries in Europe; and (iii) a milestone payment of $5 million if Tcelna is commercialized in certain markets outside of the United States and Europe. If Merck elects to develop and commercialize Tcelna in RRMS, we would be eligible to receive milestone payments aggregating up to $40 million based upon the achievement by Merck of various development, regulatory and first commercial sale milestones.
If Tcelna receives regulatory approval and is commercialized by Merck, we would be eligible to receive royalties pursuant to a tiered structure at rates ranging from 8% to 15% of annual net sales, with step-ups over such range occurring when annual net sales exceed $500 million, $1 billion and $2 billion. Any royalties would be subject to offset or reduction in various situations, including if third party rights are required or if patent protection is not available in an applicable jurisdiction. We would also be responsible for royalty obligations to certain third parties, such as Baylor College of Medicine from which we originally licensed related technology. If we were to exercise an option to co-fund certain of Merck’s development, the royalty rates payable by Merck would be increased to rates ranging from 10% to 18%. In addition to royalty payments, we would be eligible to receive one-time commercial milestones totaling up to $85 million, with $55 million of such milestones achievable at annual net sales targets in excess of $1 billion.
SPMS Overview and Tcelna Mechanism of Action
SPMS is characterized by a steady accrual of irreversible disability, despite, in some cases, relapses followed by remissions or clinical plateaus. Older age at onset of MS diagnosis is the strongest predictor of conversion to SPMS. Males have a shorter time to conversion to SPMS compared with females. Available immunomodulating and immunosuppressive therapies used for RRMS have not been effective in SPMS. In clinical trials, these therapies have demonstrated anti-inflammatory properties as measured by the reduction in number and volume of contrast-enhancing or acutely inflammatory CNS lesions most commonly seen in patients with RRMS. The typical SPMS patient, however, has little or no radiographic evidence of acute inflammation. It is commonly observed that contrast-enhancing CNS lesions are uncommon among these patients, despite a clearly deteriorating neurologic course.
4
Table of Contents
The lack of effect of conventional MS therapeutics in SPMS suggests that the cerebral deterioration characterizing progressive disease may be driven by factors other than acute inflammation. For instance, the immunopathology of SPMS is more consistent with a transition to a chronic T-cell dependent inflammatory type, which may encompass the innate immune response and persistent activation of microglia cells.
Radiographic features that stand out among patients with SPMS include significantly more atrophy of gray matter compared with RRMS patients. Of note, long-term disability in MS in general appears more closely correlated to gray matter atrophy than to white matter inflammation. Such atrophy may be suggestive of progressive clinical disability. Both clinically and radiographically, SPMS represents a disease process with certain features distinct from those of RRMS, and one with extremely limited treatment options.
Tcelna immunotherapy in SPMS may reduce the drivers of this chronic disease by down-regulating anti-myelin immunity through priming regulatory responses that may act in the periphery as well as within the CNS. We believe that our clinical results show therapeutic subcutaneous dosing of 30-45 million cells of Tcelna stimulates host reactivity to the over-represented MRTCs and, as a consequence, a dominant negative regulatory T-cell response is induced leading to down-regulation of similar endogenous disease-causing MRTCs.
We believe that Tcelna has the potential to induce an up-regulation of regulatory cells, such as Foxp3+ Treg cells and IL-10 secreting Tr1 cells, which may effect a reduction in inflammation and provide neuroprotection should they gain entry to the CNS. Data from our TERMS study showed statistically significant changes from baseline (p=0.02) in Foxp3+ Treg cells for the subset of Tcelna patients who had ARR >1. The placebo arm for this subset was not statistically different from its baseline levels. Three SPMS patients from prior clinical studies, whose blood samples were analyzed to measure Tr1 cells prior to treatment and post treatment, showed an increase in the levels of Tr1 cells from non-detectable levels to the range of healthy donor samples. These three patients who had relapses in the preceding 12-24 month period remained relapse free during the 52-week assessment period and also showed a 57% to 67% reduction in MRTCs.
Current Treatment Options for SPMS
Only one product, mitoxantrone, is currently approved for the indication of SPMS. However, as of 2005, this drug carries a black box warning, due to significant risks of decreased systolic function, heart failure, and leukemia. The American Academy of Neurology has issued a report indicating that these risks are even higher than suggested in the original report leading to the black box warning. Hence, a safe and effective treatment for SPMS remains a significant unmet medical need.
Tcelna Clinical Overview in SPMS
In multiple previously conducted clinical trials for the treatment of patients with MS (which have been weighted significantly toward patients with RRMS), Tcelna has demonstrated one of the safest side effect profiles for any marketed or development-stage MS therapy, as well as encouraging efficacy signals. A total of 144 MS patients have received Tcelna in previously conducted Opexa trials for RRMS and SPMS. The therapy has been well-tolerated in all subjects and has demonstrated an excellent overall safety profile. The most common side effect is mild to moderate irritation at the site of injection, which is typically resolved in 24 hours. Tcelna has been administered to a total of 36 subjects with SPMS across three previous clinical studies.
In a pooled assessment of data from 36 SPMS patients treated in Phase I open label studies at the Baylor College of Medicine completed in 1998 and in Opexa sponsored studies completed in 2006 and 2007, approximately 80% of the 35 SPMS patients who completed two years of treatment showed disease stabilization as measured by EDSS following two years of treatment with Tcelna, with the other 20% showing signs of progression. This compares to historical control data which showed a progression rate of 40% in SPMS patients (as reported in ESIMS Study published in Hommes Lancet 2004). The 10 SPMS patients in Opexa sponsored
5
Table of Contents
studies showed a substantial reduction in ARR at two years from 0.5 to an ARR less than 0.1. Only 1 out of the 10 patients experienced one episode of relapse during the two years of assessment. This same cohort showed no worsening of physical or psychological condition (key quality of life indicators as measured by the MS Impact Scale) after two years of treatment with Tcelna. Additionally, there were no reported serious adverse events (SAEs) in any of the patients. Based on preliminary data suggesting stabilized or improved disability among SPMS subjects receiving Tcelna, we believe that further development of this product candidate in SPMS is warranted.
Summary of Phase I Dose Escalation Study in MS
A Phase 1 dose escalation study completed in 2006 was conducted in patients with both RRMS and SPMS who were intolerant or unresponsive to current approved therapies for MS. The open-label, dose escalation study evaluated safety and clinical benefit by administering a primary series of four treatments at one of three dose levels administered at baseline and weeks four, eight, and twelve. Results of the efficacy analyses provide some evidence of the effectiveness of Tcelna in the treatment of MS. The follow-on TERMS Phase IIb clinical study provided further encouraging signs of efficacy in ARR and Multiple Sclerosis Impact Scale (MSIS-29). Data from the Phase I study evaluating the EDSS showed improvements in some subjects in comparison to baseline for weeks 20 and 28.
Subjects showed statistically significant improvement in overall reduction of MRTC counts over baseline at all visits through week 52 for subjects receiving 30-45 million cells per dose, as assessed by total MRTC count percentage changes. These data indicate that Tcelna treatment causes a depletion or immunomodulation of these cells, most obvious at time points closer to the injections. These findings, which demonstrate that administration of Tcelna induces a reduction in MRTCs, were published in Clinical Immunology (2009) 131,202-215.
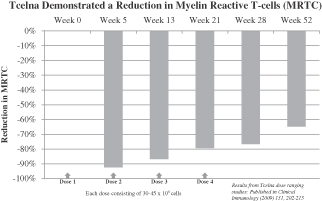
Overall, results of the safety analyses indicate that treatment with Tcelna is well-tolerated. Reported adverse events were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. In conclusion, data from this study suggest that Tcelna is safe for the treatment of MS.
Summary of Phase I/IIA Clinical Trial Data in MS
The second clinical study performed by Opexa was an open-label extension study completed in 2007 to treat patients who were previously treated with T-cell immunotherapy but who saw a rebound in MRTC activity. The purpose of this extension study was to continue evaluating the efficacy, safety and tolerability of Tcelna in patients with RRMS and SPMS with repeated administration of Tcelna. Results of the study provide evidence of the effectiveness of Tcelna in the treatment of MS with repeated dosing. Improvements from baseline at both week 28 and week 52 of the extension study were observed for the frequency of MS exacerbations
6
Table of Contents
(ARR). Evaluation of MSIS-29 component scores suggests a trend for Tcelna therapy in the improvement of physical and psychological parameters assessed by the MSIS-29. The EDSS score analysis revealed an upward trend for the percentage of subjects that reported improvement and sustained improvement over baseline as a result of Tcelna treatment.
Subjects showed statistically significant improvement over baseline in the MRTC counts for each time point through month nine of the extension study. These results indicate that Tcelna treatment results in a statistically significant impact on these cells.
Overall, results of the safety analyses indicate that repeated treatment with Tcelna is well-tolerated. Reported adverse events (AEs) were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. Furthermore, results from this study suggest that repeated dosing of Tcelna has a substantive effect in reduction of ARR in subjects with MS and was well-tolerated.
Summary of TERMS Phase IIb Clinical Trial Data in RRMS
Tovaxin for Early Relapsing Multiple Sclerosis (TERMS) was a Phase IIb clinical study of Tcelna in RRMS patients completed in 2008. Although the study did not show statistical significance in its primary endpoint (the cumulative number of gadolinium-enhanced brain lesions using MRI scans summed at various points in the study), the study showed compelling evidence of efficacy in various clinical and other MRI endpoints.
The TERMS study was a multi-center, randomized, double blind, placebo-controlled trial in 150 patients with RRMS or high risk Clinically Isolated Syndrome. Patients received a total of five subcutaneous injections at weeks 0, 4, 8, 12 and 24. Key results from the TERMS trial included:
| • | In the modified intent to treat patient population consisting of all patients who received at least one dose of study product and had at least one MRI scan at week 28 or later (n=142), the ARR for Tcelna-treated patients was 0.214 as compared to 0.339 for placebo-treated patients, which represented a 37% decrease in ARR for Tcelna as compared to placebo in the general population; |
| • | In a prospective group of patients with more active disease (ARR>1, n=50), Tcelna demonstrated a 55% reduction in ARR as compared to placebo, 88% reduction in whole brain atrophy and a statistically significant improvement in disability (EDSS) compared to placebo (p<0.045) during the 24-week period following the administration of the full course of treatment; and |
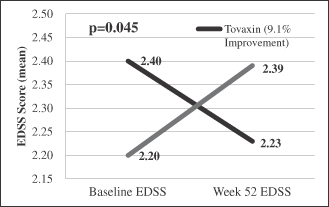
7
Table of Contents
| • | In a retrospective analysis in patients naïve to previous disease modifying treatment, the results showed that patients, when treated with Tcelna, had a 56% to 73% reduction in ARR versus placebo for the various subsets and p values ranged from 0.009 to 0.06. |
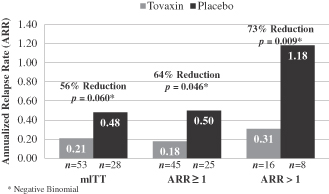
We remain committed to further advancing Tcelna in RRMS at a later date assuming the availability of sufficient resources. For Opexa, however, SPMS is an area which we believe represents a higher unmet medical need. Depending upon the outcome of further feasibility analyses, the T-cell platform may have applications in development treatments for other autoimmune disorders such as rheumatoid arthritis, Type 1 diabetes, and orphan indications such as myasthenia gravis. The primary focus of Opexa remains the development of Tcelna in SPMS.
Other Opportunities
Our proprietary T-cell technology has enabled us to develop intellectual property and a comprehensive sample database that may enable discovery of novel biomarkers associated with MS.
We have developed (and, in part, licensed from the University of Chicago) a proprietary adult stem cell technology to produce monocyte-derived stem cells (MDSC) from blood. These MDSC can be derived from a patient’s monocytes, expanded ex vivo, and then administered to the same patient. Our initial focus for this technology is the further development of this monocyte-derived stem cell technology as a platform for the in vitro generation of highly specialized cells for potential application in autologous cell therapy for patients with diabetes mellitus. The diabetes program is in an early (pre-clinical) development stage.
Risks Associated with our Business
We are a development stage company and have generated minimal revenues to date. Since our inception, we have incurred substantial losses. Our business and our ability to execute our business strategy are subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the “Risk Factors” section of this prospectus, and among these important risks are the following:
| • | We will be required to raise significant additional capital in the near-term, and our ability to obtain funding is uncertain. If sufficient capital is not available, we may not be able to continue our operations as proposed (including any Phase IIb clinical trial initiated or ongoing for Tcelna), which may require us to modify our business plan, curtail various aspects of our operations, cease operations or seek relief under applicable bankruptcy laws. |
| • | We have a history of operating losses and do not expect to be profitable in the foreseeable future. |
8
Table of Contents
| • | Our business is at an early stage of development. We are largely dependent on the success of our product candidate, Tcelna, and we cannot be certain that Tcelna will receive regulatory approval or be successfully commercialized. |
| • | We might be unable to service our debt due to a lack of cash flow or otherwise fail to comply with terms of the convertible secured promissory notes or related agreements and might be subject to default. The convertible secured promissory notes are secured by a pledge of all of our assets. The issuance of securities upon the conversion of such notes and/or the exercise of warrants issued in tandem with such notes will result in significant dilution for our shareholders. |
| • | We have provided Merck with the Option, which provides Merck with the opportunity, if exercised, to control the development and commercialization of Tcelna in MS. |
| • | We will need regulatory approvals for any product candidate, including Tcelna, prior to introduction to the market, which will require successful testing in clinical trials. Clinical trials are subject to extensive regulatory requirements, and are very expensive, time-consuming and difficult to design and implement. Any product candidate, such as Tcelna, may fail to achieve necessary safety and efficacy endpoints during clinical trials in which case we will be unable to generate revenue from the commercialization and sale of our products. |
| • | We will rely on third parties to conduct our clinical trials and perform data collection and analysis, which may result in costs and delays that may hamper our ability to successfully develop and commercialize any product candidate, including Tcelna. |
| • | We are dependent upon our management team and a small number of employees. |
Corporate Information
We were incorporated in Texas in March 1991. Our principal executive offices are located at 2653 Technology Forest Blvd., The Woodlands, Texas 77381, and our telephone number is (281) 775-0600. Our website address is www.opexatherapeutics.com. The information contained on, or that can be accessed through, our website is not part of this prospectus.
9
Table of Contents
| Common stock offered by us |
shares of common stock |
| Common stock outstanding after this offering |
shares of common stock |
| Over-allotment option |
The underwriters have a 30-day option to purchase a maximum of additional shares of common stock. |
| Use of proceeds |
We estimate that the net proceeds from our sale of shares of our common stock in this offering will be approximately $ million, or approximately $ million if the underwriters exercise their over-allotment option in full, after deducting the estimated underwriting discount and estimated offering expenses payable by us. We currently expect to use the net proceeds from this offering as follows: |
| • | approximately $ million to fund further clinical development of Tcelna and the Phase IIb study in patients with SPMS as well as the ongoing expenses of our operations during such development; and |
| • | the balance, if any, for general corporate purposes, including potential repayment of certain outstanding indebtedness. |
| Our existing resources, together with the proceeds from this offering, will not be adequate to permit us to complete such study. We will need to secure significant additional resources to complete the trial and support our operations during the pendency of the trial. See “Use of Proceeds” on page 39 of this prospectus. |
| Risk factors |
See the “Risk Factors” section beginning on page 14 of this prospectus for factors to consider before deciding to purchase our securities. |
| NASDAQ Capital Market Symbol |
OPXA |
Unless we indicate otherwise, all information in this prospectus:
| • | is based on 7,991,559 shares of common stock outstanding as of May 31, 2013: |
| • | excludes 1,111,111 shares of common stock issuable upon the exercise of stock options outstanding at a weighted average exercise price of $4.51 per share; |
| • | excludes 3,069,113 shares of common stock issuable upon the exercise of outstanding warrants at a weighted average exercise price of $4.12 per share; |
| • | excludes 1,020,007 shares of common stock issuable if all of the 12% convertible secured promissory notes outstanding are converted to Series A convertible preferred stock and such stock is then converted into shares of common stock; |
| • | excludes 27,109 shares of common stock available for future grants under our 2010 Stock Incentive Plan; |
10
Table of Contents
| • | excludes any additional shares of common stock that we may issue to Lincoln Park Capital Fund, LLC, or Lincoln Park, pursuant to a $1,500,000 purchase agreement and a $15,000,000 purchase agreement we entered into on November 5, 2012 and November 2, 2012, respectively, which provides that, upon the terms and subject to the conditions and limitation set forth therein, Lincoln Park is committed to purchase up to an aggregate of an additional $15.9 million of shares of our common stock over the term of the purchase agreements, should we elect to sell shares to Lincoln Park; and |
| • | assumes no exercise by the underwriters of the option to purchase up to additional shares of common stock from us to cover over-allotments, if any. |
11
Table of Contents
SUMMARY CONSOLIDATED FINANCIAL DATA
The following summary consolidated statements of operations data for the years ended December 31, 2012 and 2011 have been derived from our audited consolidated financial statements that are included in this prospectus. The summary consolidated statements of operations data for the three-month periods ended March 31, 2013 and 2012 and the consolidated balance sheet data as of March 31, 2013 is derived from our unaudited consolidated financial statements that are included in this prospectus. The historical financial data presented below is not necessarily indicative of our financial results in future periods, and the results for the three-month period ended March 31, 2013 are not necessarily indicative of our operating results to be expected for the full fiscal year ending December 31, 2013 or any other period. You should read the summary consolidated financial data in conjunction with those financial statements and the accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our consolidated financial statements are prepared and presented in accordance with United States generally accepted accounting principles, or U.S. GAAP. Our unaudited consolidated financial statements have been prepared on a basis consistent with our audited financial statements and include all adjustments, consisting of normal and recurring adjustments that we consider necessary for a fair presentation of the financial position and results of operations as of and for such periods.
| For the year ended December 31, | For the three months ended March 31, |
|||||||||||||||
| 2012 | 2011 | 2013 | 2012 | |||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||
| Option revenue |
$ | — | $ | — | $ | 220,100 | $ | — | ||||||||
| Research and development |
6,318,476 | 3,340,038 | 1,621,366 | 1,490,097 | ||||||||||||
| General and administrative |
2,508,541 | 2,406,269 | 1,102,435 | 816,196 | ||||||||||||
| Depreciation and amortization |
303,677 | 210,252 | 78,311 | 67,355 | ||||||||||||
| Loss on disposal of fixed assets |
3,097 | 9,686 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(9,133,791 | ) | (5,966,245 | ) | (2,582,012 | ) | (2,373,648 | ) | ||||||||
| Interest income |
280 | 932 | 1,874 | 136 | ||||||||||||
| Gain on derivative instruments |
552,978 | — | — | — | ||||||||||||
| Other income and expense, net |
— | — | 37,910 | — | ||||||||||||
| Interest expense |
(350,300 | ) | (3,135 | ) | (1,635,254 | ) | (487 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (8,930,833 | ) | $ | (5,968,448 | ) | $ | (4,177,482 | ) | $ | (2,373,999 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted loss per share |
$ | (1.54 | ) | $ | (1.06 | ) | $ | (0.58 | ) | $ | (0.41 | ) | ||||
| Weighted average shares outstanding |
5,785,372 | 5,633,124 | 7,239,102 | 5,762,028 | ||||||||||||
The following table presents consolidated balance sheets data as of March 31, 2013 on:
| • | an actual basis; and |
| • | a pro forma as adjusted basis, giving effect to the sale by us of shares of common stock in this offering at an assumed public offering price of $ per share, after deducting the underwriting discount and estimated offering expenses. |
12
Table of Contents
The pro forma as adjusted information set forth below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this information in conjunction with our consolidated financial statements and notes thereto included in this prospectus.
| As of March 31, 2013 | ||||||||
| Actual | Pro Forma
As Adjusted(1) |
|||||||
| Consolidated Balance Sheet Data: |
||||||||
| Cash and cash equivalents |
$ | 7,834,336 | $ | |||||
| Other current assets |
1,166,430 | |||||||
| Fixed assets, net |
1,189,328 | |||||||
| Restricted cash |
500,000 | |||||||
| Deferred financing costs, net |
158,540 | |||||||
| Other long-term assets |
104,027 | |||||||
| Total assets |
10,952,661 | |||||||
| Total current liabilities |
3,157,125 | |||||||
| Notes payable, net |
376,627 | |||||||
| Deferred revenue |
3,384,552 | |||||||
| Total stockholders’ equity |
4,034,357 | |||||||
| (1) | A $1.00 increase or decrease in the assumed public offering price per share would increase or decrease our cash and cash equivalents, total assets and total stockholders’ equity by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discount and estimated offering expenses payable by us. |
13
Table of Contents
Investing in our common stock involves a high degree of risk. You should consider the following risk factors, as well as other information contained in this prospectus, before deciding to invest in our common stock. The following factors affect our business, our intellectual property, the industry in which we operate and our securities. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known or which we consider immaterial as of the date hereof may also have an adverse effect on our business. If any of the matters discussed in the following risk factors were to occur, our business, financial condition, results of operations, cash flows or prospects could be materially adversely affected, the market price of our common stock could decline and you could lose all or part of your investment in our securities.
Risks Related to Our Business
We will be required to raise significant additional capital in the near-term, and our ability to obtain funding is uncertain. If sufficient capital is not available, we may not be able to continue our operations as proposed (including any Phase IIb clinical trial initiated or ongoing for Tcelna), which may require us to modify our business plan, curtail various aspects of our operations, cease operations or seek relief under applicable bankruptcy laws.
As of March 31, 2013, we had cash and cash equivalents of $7,834,336. During 2012, we closed a private offering in July 2012 consisting of convertible secured notes and warrants to purchase common stock which generated approximately $4.1 million in gross proceeds (of which $500,000 is held in a controlled account, and $500,000 was released to us in January 2013). These convertible secured notes are due July 25, 2014, and to date, notes in the aggregate principal amount of $900,000 have been converted into shares of Series A convertible preferred stock, which in turn, have been converted into an aggregate of 288,229 shares of common stock. From November 2012 through January 2013, we sold an aggregate of 390,000 shares of our common stock to Lincoln Park for gross proceeds of $523,709 pursuant to our $1.5 million purchase agreement with Lincoln Park. We closed a private offering of unsecured convertible promissory notes and warrants to purchase common stock in January 2013 which generated $650,000 in gross proceeds. Upon receipt of the upfront payment from Merck in February 2013, we repaid $550,000 principal amount plus accrued interest of the January 2013 notes and converted the remaining $100,000 principal amount into shares of common stock pursuant to the investor’s election to convert into equity. In February 2013, we sold an aggregate of 167,618 shares of our common stock pursuant to a sales agreement executed on September 6, 2012 with Brinson Patrick Securities Corporation acting as sales agent under an “at-the-market” program, for gross proceeds of $536,417. On February 4, 2013, we entered into an Option and License Agreement with Merck pursuant to which we granted the Option to Merck to acquire an exclusive, worldwide (excluding Japan) license to our Tcelna program for the treatment of MS in consideration for an upfront payment of $5 million. On February 11, 2013, we closed on an offering of 1,083,334 shares of common stock and warrants to purchase 541,668 shares of common stock for gross proceeds of $3,250,002. As part of that offering, which offering Lincoln Park did not participate in, we agreed not to sell shares pursuant to our purchase agreements with Lincoln Park or under our “at-the-market” program for a period of 120 days following that offering (or June 11, 2013).
Our cash burn rate during the three months ended March 31, 2013, inclusive of the cost of our ongoing Abili-T clinical study, was approximately $775,000 per month. During this three-month period, the pace of on-boarding clinical sites for the Abili-T clinical study was tempered pending the completion of our negotiations with Merck for the Option as well as financial considerations. Upon receipt of the upfront payment of $5 million for granting the Option, we were able to refocus on execution of the Abili-T clinical trial, including enrollment. Significant activities in the conduct of the Abili-T clinical trial are expected to result in substantial increases in our monthly cash burn during the balance of 2013. Based on our projected burn rate increasing to an average of $1.2 million per month for the remainder of 2013, we believe we have sufficient liquidity to support our clinical trial activities into the fourth quarter of 2013. However, the Abili-T clinical study in North America of Tcelna is expected to complete enrollment of 180 patients by late 2013 or early 2014, with resulting top-line data expected to be available in the first half of 2016.
14
Table of Contents
The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of May 31, 2013 to be between $30-$32 million. Our existing resources are not adequate to permit us to complete such study or the majority of it. We will need to secure significant additional resources to complete the trial and support our operations during the pendency of the trial.
Given our need for substantial amounts of capital to complete the Phase IIb clinical study for Tcelna in SPMS, we intend to continue to explore potential opportunities and alternatives to obtain the significant additional resources that will be necessary to complete the Phase IIb study and to support our operations during the pendency of such study. These opportunities and alternatives may include one or more additional financing transactions. There can be no assurance that any such financings can be consummated on acceptable terms, if at all. If we are unable to obtain additional funding for operations in the immediate future, we will be forced to suspend or terminate our ongoing clinical trial for Tcelna, which may require us to modify our business plan, curtail various aspects of our operations, cease operations or seek relief under applicable bankruptcy laws.
Assuming we are able to achieve financing which is sufficient to support the Phase IIb study of Tcelna in SPMS and to support our operations during the pendency of such study, we are also exploring a pivotal Phase III clinical study of Tcelna in RRMS. Any such study of Tcelna in RRMS would also depend upon the availability of sufficient resources.
Other than the $1.5 million purchase agreement and the $15 million purchase agreement we entered into with Lincoln Park on November 5, 2012 and November 2, 2012, respectively, each of which is subject to certain limitations and conditions, we have no sources of debt or equity capital committed for funding and we must rely upon best efforts third-party debt or equity funding. We can provide no assurance that we will be successful in any funding effort. The timing and degree of any future capital requirements will depend on many factors, including:
| • | our ability to establish, enforce and maintain strategic arrangements for research, development, clinical testing, manufacturing and marketing; |
| • | the accuracy of the assumptions underlying our estimates for capital needs in 2013 and beyond as well as for the clinical study of Tcelna; |
| • | scientific progress in our research and development programs; |
| • | the magnitude and scope of our research and development programs; |
| • | our progress with preclinical development and clinical trials; |
| • | the time and costs involved in obtaining regulatory approvals; |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; and |
| • | the number and type of product candidates that we pursue. |
If we raise additional funds by issuing equity securities (including pursuant to the $1.5 million purchase agreement and the $15 million purchase agreement with Lincoln Park), shareholders may experience substantial dilution. Debt financing, if available, may involve restrictive covenants that may impede our ability to operate our business. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our shareholders. There is no assurance that our capital raising efforts will be able to attract the capital needed to execute on our business plan and sustain our operations.
If we are unable to obtain additional funding, we may not be able to continue or complete the Phase IIb clinical study of Tcelna in SPMS or otherwise continue our operations as proposed, which may require us to
15
Table of Contents
modify our business plan or curtail various aspects of our operations. If we are unable to maintain an adequate level of capital, it may be necessary to cease operations or seek relief under applicable bankruptcy laws. In such event, our shareholders may lose a portion or even all of their investment.
We may experience delays in our clinical trial enrollment, which could result in increased costs to us.
Once a clinical trial has begun, recruitment and enrollment of subjects may be slower than we anticipate. In addition, clinical trials may take longer than we anticipate if we are required, or believe it is necessary, to enroll additional subjects. Our ongoing Abili-T clinical study in North America of Tcelna is expected to complete enrollment of 180 patients by late 2013 or early 2014, with resulting top-line data expected to be available in the first half of 2016. The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of May 31, 2013 to be between $30-$32 million. These estimated costs are partially a function of the pace of clinical trial enrollment, and should enrollment timelines get delayed beyond our current expectation, the estimated costs are likely to increase due to the additional operational expenses. Similarly, should additional patients be enrolled in the trial, the costs are likely to increase.
We may make changes to discretionary R&D investments that may have an impact on costs.
We are presently complementing the Abili-T clinical trial with an immune monitoring program. Expenses associated with the immune monitoring program are incurred at our discretion and are not required to satisfy any FDA-mandated criteria. Consequently, we may make changes to the parameters that are being analyzed, and these changes may result in either increased or decreased expenses for the study.
We may also incur discretionary expenses related to Phase III development, manufacturing scale-up/automation and technology transfer in the future. There is no assurance that any such future expenses would be recovered by us.
Funding from our purchase agreements with Lincoln Park may be limited or be insufficient to fund our operations or to implement our strategy.
Under our $1.5 million purchase agreement and our $15 million purchase agreement with Lincoln Park, we may direct Lincoln Park to purchase up to $16.5 million of shares of common stock, subject to certain limitations and conditions, over a 30-month period. However, in connection with our February 2013 common stock and warrant offering, we agreed not to sell shares under the purchase agreements with Lincoln Park for a period of 120 days after that offering (or June 11, 2013). From November 2012 through January 2013, we sold an aggregate of 390,000 shares to Lincoln Park pursuant to the $1.5 million purchase agreement, and we issued an aggregate of 56,507 initial commitment shares and 3,585 additional commitment shares in connection therewith. There can be no assurance that we will be able to receive any or all of the additional funds from Lincoln Park because the $1.5 million purchase agreement and the $15 million purchase agreement contain limitations, restrictions, requirements, events of default and other provisions that could limit our ability to cause Lincoln Park to buy common stock from us, including that the closing price of our stock is at least $1.00 and that Lincoln Park own no more than 4.99% of our common stock under the $1.5 million purchase agreement or no more than 9.99% of our common stock under the $15 million purchase agreement. In addition, under the applicable rules of the NASDAQ Capital Market, if we seek to issue shares which may be aggregated with shares sold to Lincoln Park under the $1.5 million purchase agreement and the $15 million purchase agreement in excess of 1,151,829 shares or 19.99% of the total common stock outstanding as of the date of the $15 million purchase agreement, we may be required to seek shareholder approval in order to be in compliance with the NASDAQ Capital Market rules.
The extent to which we rely on Lincoln Park as a source of funding will depend on a number of factors, including the amount of working capital needed, the prevailing market price of our common stock and the extent
16
Table of Contents
to which we are able to secure working capital from other sources. If obtaining sufficient funding from Lincoln Park were to prove unavailable or prohibitively dilutive, we would need to secure another source of funding. Even if we sell all $16.5 million of common stock under the purchase agreements with Lincoln Park, we will still need additional capital to fully implement our business, operating plans and development plans as of the date hereof, including to complete the Phase IIb clinical study of Tcelna in patients with SPMS and to conduct our operations through the expected completion date of such study.
We have a history of operating losses and do not expect to be profitable in the foreseeable future.
We have not generated any profits since our entry into the biotechnology business and we have incurred significant operating losses. We expect to incur additional operating losses for the foreseeable future. We have not received, and we do not expect to receive for at least the next several years, any revenues from the commercialization of any potential products. We do not have any sources of revenues as of the date hereof and may not have any in the foreseeable future.
There is substantial doubt as to our ability to continue as a going concern, which may make it more difficult for us to raise capital.
Our consolidated financial statements as of March 31, 2013 and for the three-month period then ended were prepared assuming that we will continue as a going concern, meaning that we will continue in operation for the foreseeable future and will be able to realize assets and discharge liabilities in the ordinary course of operations. We recognized $220,100 in revenue during the three-month period ended March 31, 2013 related to the $5 million upfront payment from Merck received in February 2013 in connection with the Option and License Agreement. We expect to continue recording revenue related to the $5 million upfront payment from Merck over the exclusive option period based on the expected completion term of the Abili-T clinical trial. However, we do not currently generate any additional revenues resulting in cash receipts, nor do we expect to generate revenues during the remainder of 2013 resulting in cash receipts. Our cash burn rate during the three months ended March 31, 2013, inclusive of the cost of our ongoing Abili-T clinical study, was approximately $775,000 per month. Significant activities in the conduct of the clinical trial are expected to result in substantial increases in our monthly cash burn during the balance of 2013. Based on our projected burn rate increasing to an average of $1.2 million per month for the remainder of 2013, we believe we have sufficient liquidity to support our clinical trial activities into the fourth quarter of 2013. In the absence of significant additional funding, there is substantial doubt about our ability to continue as a going concern. This may make it more difficult for us to raise funds. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures or to generate revenue. If we are unable to obtain additional financing for our operations, we may not be able to continue operations as proposed, requiring us to modify our business plan, curtail various aspects of our operations, cease operations or seek relief under applicable bankruptcy laws. In such event, investors may lose a portion or all of their investment. Our consolidated financial statements contain no adjustment for the outcome of this uncertainty.
Our business is at an early stage of development. We are largely dependent on the success of our product candidate, Tcelna, and we cannot be certain that Tcelna will receive regulatory approval or be successfully commercialized.
Our business is at an early stage of development. We do not have any product candidates that have completed late-stage clinical trials nor do we have any products on the market. We have only one product candidate, Tcelna, which has progressed to the stage of being studied in human clinical trials in the United States. In September 2012, we announced the initiation of a Phase IIb study of Tcelna in patients with SPMS. We are still in the very early stages of identifying and conducting research on any other potential products. Tcelna, and any other potential products, will require regulatory approval prior to marketing in the United States and other countries. Obtaining such approval requires significant research and development and preclinical and clinical testing. We may not be able to develop any products, to obtain regulatory approvals, to continue clinical
17
Table of Contents
development of Tcelna, to enter clinical trials (or any development activities) for any other product candidates or to commercialize any products. Tcelna, and any other potential products, may prove to have undesirable or unintended side effects or other characteristics adversely affecting their safety, efficacy or cost-effectiveness that could prevent or limit their use. Any product using any of our technology may fail to provide the intended therapeutic benefits or to achieve therapeutic benefits equal to or better than the standard of treatment at the time of testing or production.
We might be unable to service our debt due to a lack of cash flow or otherwise fail to comply with terms of the convertible secured promissory notes or related agreements and might be subject to default. The convertible secured promissory notes are secured by a pledge of all of our assets. The issuance of securities upon the conversion of such notes and/or the exercise of warrants issued in tandem with such notes will result in significant dilution for our shareholders.
On July 25, 2012, we closed a private offering consisting of 12% convertible secured notes and warrants to purchase shares of common stock which generated approximately $4.1 million in gross proceeds ($500,000 of which is held in a controlled account). The notes mature on July 25, 2014 and accrue interest at the rate of 12% per annum, compounded annually. Interest is payable semi-annually in either cash or registered shares of common stock at our election. The notes are secured by substantially all of our assets and are convertible into a new class of non-voting Series A convertible preferred stock. The notes can be converted into Series A convertible preferred stock at the option of the investors at a price of $100.00 per share, subject to certain limitations and adjustments. Additionally, we can elect to convert the notes into Series A convertible preferred stock if (i) our common stock closes at or above $10.00 per share for 20 consecutive trading days or (ii) we achieve certain additional funding milestones to continue our clinical trial program. These milestones include (x) executing a strategic agreement with a partner or potential partner by which we will receive a minimum of $5 million to partially fund, or an option to partner with us for, our Phase II clinical trial for Tcelna in patients with SPMS and (y) receiving a minimum of $25 million in additional capital (including the note offering proceeds) from any partner, potential partner or any other source. The Series A convertible preferred stock accrues dividends at the rate of 8% per annum, which are cumulative and payable semi-annually in either cash or registered shares of the common stock at our election. The Series A convertible preferred stock is convertible into shares of our common stock at the option of the holders at a price of $3.1225 per share, subject to certain limitations and adjustments. Additionally, we can elect to convert the Series A convertible preferred stock into common stock if our common stock closes at or above $16.00 per share for 20 consecutive trading days. To date, secured promissory notes in the aggregate principal amount of $900,000 have been converted into shares of Series A convertible preferred stock which, in turn, have been converted into an aggregate of 288,229 shares of common stock. No shares of Series A convertible preferred stock are outstanding as of the date hereof.
As a result of anti-dilution adjustments since the closing of the July 2012 secured promissory note financing, up to 1,020,007 shares of common stock are issuable if all of the 12% convertible secured promissory notes outstanding as of the date hereof are converted to Series A convertible preferred stock and such stock is then converted into shares of our common stock. The noteholders were granted certain registration rights for the shares of common stock underlying the notes and the warrants issued in July 2012.
The warrants have a five-year term and, as a result of anti-dilution adjustments since the closing of the July 2012 secured promissory note financing, (i) have an adjusted exercise price of $2.56 per share and (ii) are exercisable for an aggregate of 1,436,121 shares of common stock. We can redeem the warrants at $0.01 per share if our common stock closes at or above $10.00 per share for 20 consecutive trading days.
As part of the security interest in all of our assets granted to the noteholders, $500,000 of the proceeds is maintained as of the date hereof in a controlled account. This amount was previously $1 million; however, in January 2013 we issued the noteholders five-year warrants to acquire an aggregate of 187,500 shares of our common stock at an exercise price of $1.21 per share in exchange for the reduction of such amount.
18
Table of Contents
If we do not make the required payments when due, either at maturity, or at applicable installment payment dates, or if we breach other terms of the convertible secured notes or related agreements, the noteholders could elect to declare all amounts outstanding, together with accrued and unpaid interest, to be immediately due and payable. Even if we were able to prepay the full amount in cash, any such repayment could leave us with little or no working capital for our business. If we are unable to repay those amounts, the noteholders will have a first claim on our assets pledged under the convertible secured notes. If the noteholders should attempt to foreclose on the collateral, it is unlikely that there would be any assets remaining after repayment in full of such secured indebtedness. Any default under the convertible secured notes and resulting foreclosure would have a material adverse effect on our financial condition and our ability to continue our operations.
We have provided Merck with the Option, which provides Merck with the opportunity, if exercised, to control the development and commercialization of Tcelna in MS.
In February 2013, we granted the Option to Merck. The Option permits Merck to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck prior to or upon completion of our ongoing Phase IIb trial of Tcelna in patients with SPMS. If Merck exercises the Option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights outside of MS. In consideration for the Option, we received an upfront payment of $5 million and may be eligible to receive an option exercise fee as well as milestone and royalty payments based on achievement of development and commercialization milestones. The rights we have relinquished to our product candidate Tcelna, including development and commercialization rights, may harm our ability to generate revenues and achieve or sustain profitability.
If Merck exercises the Option, we would become reliant on Merck’s resources and efforts with respect to Tcelna in MS. In such an event, Merck may fail to develop or effectively commercialize Tcelna for a variety of reasons, including that Merck:
| • | does not have sufficient resources or decides not to devote the necessary resources due to internal constraints such as limited cash or human resources; |
| • | decides to pursue a competitive potential product; |
| • | cannot obtain the necessary regulatory approvals; |
| • | determines that the market opportunity is not attractive; or |
| • | cannot manufacture or obtain the necessary materials in sufficient quantities from multiple sources or at a reasonable cost. |
If Merck does not exercise the Option, we may be unable to enter into a collaboration with any other potential partner on acceptable terms, if at all. We face competition in our search for partners from other organizations worldwide, many of whom are larger and are able to offer more attractive deals in terms of financial commitments, contribution of human resources, or development, manufacturing, regulatory or commercial expertise and support.
If Merck does not exercise the Option, and we are not successful in attracting another partner and entering into collaboration on acceptable terms, we may not be able to complete development of or commercialize any product candidate, including Tcelna. In such event, our ability to generate revenues and achieve or sustain profitability would be significantly hindered and we may not be able to continue operations as proposed, requiring us to modify our business plan, curtail various aspects of our operations or cease operations.
19
Table of Contents
We will need regulatory approvals for any product candidate, including Tcelna, prior to introduction to the market, which will require successful testing in clinical trials. Clinical trials are subject to extensive regulatory requirements, and are very expensive, time-consuming and difficult to design and implement. Any product candidate, such as Tcelna, may fail to achieve necessary safety and efficacy endpoints during clinical trials in which case we will be unable to generate revenue from the commercialization and sale of our products.
Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous FDA requirements, and must otherwise comply with federal, state and local requirements and policies of the medical institutions where they are conducted. The clinical trial process is also time-consuming. We estimate that the Phase IIb clinical trial in North America of our lead product candidate, Tcelna, in SPMS will complete enrollment by late 2013 or early 2014, with the resulting top-line data expected to be available in the first half of 2016. In addition, we anticipate that a pivotal Phase III clinical trial would be necessary before an application could be submitted for approval of Tcelna for SPMS. Failure can occur at any stage of the trials, and problems could be encountered that would cause us or Merck (in the event the Option is exercised) to be unable to initiate a trial, or to abandon or repeat a clinical trial.
The commencement and completion of clinical trials, including the continuation and completion of the Phase IIb clinical trial of Tcelna in SPMS, may be delayed or prevented by several factors, including:
| • | FDA or IRB objection to proposed protocols; |
| • | discussions or disagreement with the FDA over the adequacy of trial design to potentially demonstrate effectiveness, and subsequent design modifications; |
| • | unforeseen safety issues; |
| • | determination of dosing issues and related adjustments; |
| • | lack of effectiveness during clinical trials; |
| • | slower than expected rates of patient recruitment; |
| • | product quality problems (e.g., sterility or purity); |
| • | challenges to patient monitoring and data collection during or after treatment (for example, patients’ failure to return for follow-up visits); and |
| • | failure of medical investigators to follow our clinical protocols. |
In addition, we, Merck (if the Option is exercised) or the FDA (based on its authority over clinical studies) may delay a proposed investigation or suspend clinical trials in progress at any time if it appears that the study may pose significant risks to the study participants or other serious deficiencies are identified. Prior to approval of any product candidate, the FDA must determine that the data demonstrate safety and effectiveness. The large majority of drug candidates that begin human clinical trials fail to demonstrate the desired safety and efficacy characteristics.
Furthermore, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols, or otherwise modify our intended course of clinical development, to reflect these changes. This, too, may impact the costs, timing or successful completion of a clinical trial. In light of widely publicized events concerning the safety risk of certain drug products, regulatory authorities, members of Congress, the U.S. Government Accountability Office, medical professionals and the general public have raised concerns about potential drug safety issues. These events have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products, and establishment of risk management programs that may, for instance, restrict distribution of drug products. The increased attention to drug safety issues may result in a more cautious approach by the FDA to clinical trials. Data from clinical trials may receive greater scrutiny with respect to safety, which may make the FDA or other regulatory authorities more likely to terminate clinical trials before
20
Table of Contents
completion or require longer or additional clinical trials that may result in substantial additional expense and a delay or failure in obtaining approval or approval for a more limited indication than originally sought.
Even if regulatory approval is obtained for any product candidate, such as Tcelna, any such approval may be subject to limitations on the indicated uses for which it may be marketed. Our ability to generate revenues from the commercialization and sale of any potential products, whether directly or through any development arrangement (such as where Merck exercises the Option) will be limited by any failure to obtain or limitation on necessary regulatory approvals.
If Merck exercises the Option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates.
We will rely on third parties to conduct our clinical trials and perform data collection and analysis, which may result in costs and delays that may hamper our ability to successfully develop and commercialize any product candidate, including Tcelna.
Although we have participated in the design and management of our past clinical trials, we do not have the ability to conduct clinical trials directly for any product candidate, including Tcelna. We will need to rely on contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct our clinical trials and to perform data collection and analysis, including the Phase IIb trial of Tcelna in patients with SPMS.
Our clinical trials may be delayed, suspended or terminated if:
| • | any third party upon whom we rely does not successfully carry out its contractual duties or regulatory obligations or meet expected deadlines; |
| • | licenses needed from third parties for manufacturing in order to conduct Phase III trials or to conduct commercial manufacturing, if applicable, are not obtained; |
| • | any such third party needs to be replaced; or |
| • | the quality or accuracy of the data obtained by the third party is compromised due to its failure to adhere to clinical protocols or regulatory requirements or for other reasons. |
Failure to perform by any third party upon whom we rely may increase our development costs, delay our ability to obtain regulatory approval and prevent the commercialization of any product candidate, including Tcelna. While we believe that there are numerous alternative sources to provide these services, we might not be able to enter into replacement arrangements without delays or additional expenditures if we were to seek such alternative sources.
If we fail to identify and license or acquire other product candidates, we will not be able to expand our business over the long term.
We have targeted MS as the first disease to be pursued off our T-cell platform technology. As a platform technology, there exists the potential to address other autoimmune diseases with the technology. Minimal work has been done outside the lead MS indication. Our business over the long term is substantially dependent on our ability to develop, license or acquire product candidates and further develop them for commercialization. The success of this strategy depends upon our ability to expand our existing platform or identify, select and acquire the right product candidates. We have limited experience identifying, negotiating and implementing economically viable product candidate acquisitions or licenses, which is a lengthy and complex process. Also, the market for licensing and acquiring product candidates is intensely competitive, and many of our competitors have greater resources than we do. We may not have the requisite capital resources to consummate product candidate acquisitions or licenses that we identify to fulfill our strategy.
21
Table of Contents
Moreover, any product candidate acquisition that we do complete will involve numerous risks, including:
| • | difficulties in integrating the development program for the acquired product candidate into our existing operations; |
| • | diversion of financial and management resources from existing operations; |
| • | risks of entering new potential markets or technologies; |
| • | inability to generate sufficient funding to offset acquisition costs; and |
| • | delays that may result from our having to perform unanticipated preclinical trials or other tests on the product candidate. |
We are dependent upon our management team and a small number of employees.
Our business strategy is dependent upon the skills and knowledge of our management team. If any critical employee leaves, we may be unable on a timely basis to hire suitable replacements to operate our business effectively. We also operate with a very small number of employees and thus have little or no backup capability for their activities. The loss of the services of any member of our management team or the loss of just a few other employees could have a material adverse effect on our business and results of operations.
If we fail to meet our obligations under our license agreements, we may lose our rights to key technologies on which our business depends.
Our business depends on licenses from third parties. These third party license agreements impose obligations on us, such as payment obligations and obligations diligently to pursue development of commercial products under the licensed patents. We may also need to seek additional licenses as we move into Phase III trials and, if applicable, the commercial stage of operations. These licenses may require increased payments to the licensors. If a licensor believes that we have failed to meet our obligations under a license agreement, the licensor could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation, our ability to carry out the development and commercialization of potential products could be significantly and negatively affected. If our license rights were restricted or ultimately lost, our ability to continue our business based on the affected technology platform could be adversely affected.
Our research and manufacturing facility is not large enough to manufacture product candidates, such as Tcelna, for certain clinical trials or, if such clinical trials are successful, commercial applications.
We conduct our research and development in a 10,200 square foot facility in The Woodlands, Texas, which includes an approximately 1,200 square foot suite of three rooms for the manufacture of T-cell therapies. We believe our facility should have the capacity to support full clinical development of Tcelna in North American trials for SPMS. It is not sufficient, however, to support clinical trials outside North America including Europe and Asia, if required, or the commercial launch of Tcelna. In this case, we would need to expand our manufacturing staff and facility, obtain a new facility, contract with corporate collaborators or other third parties to assist with future drug production and commercialization, or defer to Merck (in the event the Option is exercised) to address manufacturing requirements.
In the event that we decide to establish a commercial-scale manufacturing facility, we will require substantial additional funds and will be required to hire and train significant numbers of employees and comply with applicable regulations, which are extensive. We do not have funds available for building a manufacturing facility, and we may not be able to build a manufacturing facility that both meets regulatory requirements and is sufficient for our commercial-scale manufacturing.
We may arrange with third parties for the manufacture of our future products, if any. However, our third-party sourcing strategy may not result in a cost-effective means for manufacturing our future products. If we
22
Table of Contents
employ third-party manufacturers, we will not control many aspects of the manufacturing process, including compliance by these third parties with current Good Manufacturing Practice (cGMP) and other regulatory requirements. We further may not be able to obtain adequate supplies from third-party manufacturers in a timely fashion for development or commercialization purposes, and commercial quantities of products may not be available from contract manufacturers at acceptable costs.
If any product we may eventually have is not accepted by the market or if users of any such product are unable to obtain adequate coverage of and reimbursement for such product from government and other third-party payors, our revenues and profitability will suffer.
In the instance of Tcelna, if Merck exercises the Option then our ability to achieve revenue will be dependent upon the efforts and success of Merck in developing and commercializing Tcelna. Our ability to successfully commercialize any product we may eventually have, to the extent applicable, and/or our ability to receive any revenue associated with Tcelna in the event Merck exercises the Option, will depend in significant part on the extent to which appropriate coverage of and reimbursement for such product and any related treatments are obtained from governmental authorities, private health insurers and other organizations, such as health maintenance organizations, or HMOs. Third-party payors are increasingly challenging the prices charged for medical products and services. We cannot provide any assurances that third-party payors will consider any product cost-effective or provide coverage of and reimbursement for such product, in whole or in part.
Uncertainty exists as to the coverage and reimbursement status of newly approved medical products and services and newly approved indications for existing products. Third-party payors may conclude that any product is less safe, less clinically effective, or less cost-effective than existing products, and third-party payors may not approve such product for coverage and reimbursement. If adequate coverage of and reimbursement for any product from third-party payors cannot be obtained, physicians may limit how much or under what circumstances they will prescribe or administer them. Such reduction or limitation in the use of any such product would cause sales to suffer. Even if third-party payors make reimbursement available, payment levels may not be sufficient to make the sale of any such product profitable.
In addition, the trend towards managed health care in the United States and the concurrent growth of organizations such as HMOs, which could control or significantly influence the purchase of medical services and products, may result in inadequate coverage of and reimbursement for any product we may eventually have. Many third-party payors, including in particular HMOs, are pursuing various ways to reduce pharmaceutical costs, including, for instance, the use of formularies. The market for any product depends on access to such formularies, which are lists of medications for which third-party payors provide reimbursement. These formularies are increasingly restricted, and pharmaceutical companies face significant competition in their efforts to place their products on formularies of HMOs and other third-party payors. This increased competition has led to a downward pricing pressure in the industry. The cost containment measures that third-party payors are instituting could have a material adverse effect on our ability to operate profitably.
Any product candidate, such as Tcelna, if approved for sale, may not gain acceptance among physicians, patients and the medical community, thereby limiting our potential to generate revenues.
Even if a product candidate, such as Tcelna, is approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any approved product candidate by physicians, healthcare professionals and third-party payors, and our profitability and growth, will depend on a number of factors, including:
| • | demonstration of efficacy; |
| • | relative convenience and ease of administration; |
| • | the prevalence and severity of any adverse side effects; |
23
Table of Contents
| • | availability and cost of alternative treatments, including cheaper generic drugs; |
| • | pricing and cost effectiveness, which may be subject to regulatory control; |
| • | effectiveness of sales and marketing strategies for the product and competition for such product; |
| • | the product labeling or product insert required by the FDA or regulatory authority in other countries; and |
| • | the availability of adequate third-party insurance coverage or reimbursement. |
If any product candidate does not provide a treatment regimen that is as beneficial as the current standard of care or otherwise does not provide patient benefit, that product candidate, if approved for commercial sale by the FDA or other regulatory authorities, likely will not achieve market acceptance and our ability to generate revenues from that product candidate would be substantially reduced.
We have incurred, and expect to continue to incur, increased costs and risks as a result of being a public company.
As a public company, we are required to comply with the Sarbanes-Oxley Act of 2002, or SOX, as well as rules and regulations implemented by the SEC and The NASDAQ Stock Market (NASDAQ). Changes in the laws and regulations affecting public companies, including the provisions of SOX and rules adopted by the SEC and by NASDAQ, have resulted in, and will continue to result in, increased costs to us as we respond to their requirements. Given the risks inherent in the design and operation of internal controls over financial reporting, the effectiveness of our internal controls over financial reporting is uncertain. If our internal controls are not designed or operating effectively, we may not be able to conclude an evaluation of our internal control over financial reporting as required or we or our independent registered public accounting firm may determine that our internal control over financial reporting was not effective. In addition, our registered public accounting firm may either disclaim an opinion as it relates to management’s assessment of the effectiveness of our internal controls or may issue an adverse opinion on the effectiveness of our internal controls over financial reporting. Investors may lose confidence in the reliability of our financial statements, which could cause the market price of our common stock to decline and which could affect our ability to run our business as we otherwise would like to. New rules could also make it more difficult or more costly for us to obtain certain types of insurance, including directors’ and officers’ liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the coverage that is the same or similar to our coverage as of the date hereof. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors, our Board committees and as executive officers. We cannot predict or estimate the total amount of the costs we may incur or the timing of such costs to comply with these rules and regulations.
Under the corporate governance standards of NASDAQ, a majority of our Board of Directors and each member of our Audit Committee must be an independent director. If any vacancies on our Board or our Audit Committee occur that need to be filled by independent directors, we may encounter difficulty in attracting qualified persons to serve on our Board and, in particular, our Audit Committee. If we fail to attract and retain the required number of independent directors, we may be subject to SEC enforcement proceedings and delisting of our common stock from the NASDAQ Capital Market.
Risks Related to Our Intellectual Property
Patents obtained by other persons may result in infringement claims against us that are costly to defend and which may limit our ability to use the disputed technologies and prevent us from pursuing research and development or commercialization of potential products, such as Tcelna.
If third party patents or patent applications contain claims infringed by either our licensed technology or other technology required to make or use our potential products, such as Tcelna, and such claims are ultimately
24
Table of Contents
determined to be valid, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost, if at all, or be able to develop or obtain alternative technology. If we are unable to obtain such licenses at a reasonable cost, we (or, in the event the Option is exercised, Merck with respect to Tcelna) may not be able to develop any affected product candidate commercially. There can be no assurance that we will not be obliged to defend ourselves (or, in the event the Option is exercised, Merck with respect to Tcelna) in court against allegations of infringement of third party patents. Patent litigation is very expensive and could consume substantial resources and create significant uncertainties. An adverse outcome in such a suit could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties, or require us to cease using such technology.
If we are unable to obtain patent protection and other proprietary rights, our operations will be significantly harmed.
Our ability to compete effectively is dependent upon obtaining patent protection relating to our technologies. The patent positions of pharmaceutical and biotechnology companies, including ours, are uncertain and involve complex and evolving legal and factual questions. The coverage sought in a patent application can be denied or significantly reduced before or after the patent is issued. Consequently, we do not know whether pending patent applications for our technology will result in the issuance of patents, or if any issued patents will provide significant protection or commercial advantage or will be circumvented by others. Since patent applications are secret until the applications are published (usually 18 months after the earliest effective filing date), and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that the inventors of our owned or licensed intellectual property rights were the first to make the inventions at issue or that any patent applications at issue were the first to be filed for such inventions. There can be no assurance that patents will issue from pending patent applications or, if issued, that such patents will be of commercial benefit to us, afford us adequate protection from competing products, or not be challenged or declared invalid.
For our licensed intellectual property, we have limited control over the amount or timing of resources that are devoted to the prosecution of such intellectual property. Due to this lack of control and general uncertainties in the patent prosecution process, we cannot be sure that any licensed patents will result from licensed applications or, if they do, that they will be maintained. Issued U.S. patents require the payment of maintenance fees to continue to be in force. We rely on licensors to do this and their failure to do so could result in the forfeiture of patents not timely maintained. Many foreign patent offices also require the payment of periodic annuities to keep patents and patent applications in good standing. As we do not maintain control over the payment of annuities, we cannot assure you that our licensors will timely pay such annuities and that the granted patents and pending patent applications will not become abandoned. In addition, our licensors may have selected a limited amount of foreign patent protection, and therefore applications have not been filed in, and foreign patents may not have been perfected in, all commercially significant countries.
The patent protection of product candidates, such as Tcelna, involves complex legal and factual questions. To the extent that it would be necessary or advantageous for any of our licensors to cooperate or lead in the enforcement of our licensed intellectual property rights, we cannot control the amount or timing of resources such licensors devote on our behalf or the priority they place on enforcing such rights. We may not be able to protect our intellectual property rights against third party infringement, which may be difficult to detect. Additionally, challenges may be made to the ownership of our intellectual property rights, our ability to enforce them, or our underlying licenses.
We cannot be certain that any of the patents issued to us or to our licensors will provide adequate protection from competing products. Our success will depend, in part, on whether we or our licensors can:
| • | obtain and maintain patents to protect our product candidates such as Tcelna; |
| • | obtain and maintain any required or desirable licenses to use certain technologies of third parties, which may be protected by patents; |
25
Table of Contents
| • | protect our trade secrets and know-how; |
| • | operate without infringing the intellectual property and proprietary rights of others; |
| • | enforce the issued patents under which we hold rights; and |
| • | develop additional proprietary technologies that are patentable. |
The degree of future protection for our proprietary rights (owned or licensed) is uncertain. For example:
| • | we or our licensor might not have been the first to make the inventions covered by pending patent applications or issued patents owned by, or licensed to, us; |
| • | we or our licensor might not have been the first to file patent applications for these inventions; |
| • | others may independently develop similar or alternative technologies or duplicate any of the technologies owned by, or licensed to, us; |
| • | it is possible that none of the pending patent applications owned by, or licensed to, us will result in issued patents; |
| • | any patents under which we hold rights may not provide us with a basis for commercially viable products, may not provide us with any competitive advantages or may be challenged by third parties as invalid, or unenforceable under U.S. or foreign laws; or |
| • | any of the issued patents under which we hold rights may not be valid or enforceable or may be circumvented successfully in light of the continuing evolution of domestic and foreign patent laws. |
Confidentiality agreements with employees and others may not adequately prevent disclosure of our trade secrets and other proprietary information and may not adequately protect our intellectual property, which could limit our ability to compete.
We rely in part on trade secret protection in order to protect our proprietary trade secrets and unpatented know-how. However, trade secrets are difficult to protect, and we cannot be certain that others will not develop the same or similar technologies on their own. We have taken steps, including entering into confidentiality agreements with our employees, consultants, outside scientific collaborators and other advisors, to protect our trade secrets and unpatented know-how. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. We also typically obtain agreements from these parties which provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us. Further, we have limited control, if any, over the protection of trade secrets developed by our licensors. Enforcing a claim that a party illegally obtained and is using our trade secrets or know-how is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets or know-how. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
A dispute concerning the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be time consuming and costly, and an unfavorable outcome could harm our business.
A number of pharmaceutical, biotechnology and other companies, universities and research institutions have filed patent applications or have been issued patents relating to cell therapy, T-cells, and other technologies potentially relevant to or required by our product candidate Tcelna. We cannot predict which, if any, of such applications will issue as patents or the claims that might be allowed. We are aware of a number of patent applications and patents claiming use of modified cells to treat disease, disorder or injury.
There is significant litigation in our industry regarding patent and other intellectual property rights. While we are not subject to any pending intellectual property litigation as of the date hereof, and are not aware of any
26
Table of Contents
such threatened litigation, we may be exposed to future litigation by third parties based on claims that our product candidates, such as Tcelna, or their methods of use, manufacturing or other technologies or activities infringe the intellectual property rights of such third parties. If our product candidates, such as Tcelna, or their methods of manufacture are found to infringe any such patents, we may have to pay significant damages or seek licenses under such patents. We have not conducted comprehensive searches of patents issued to third parties relating to Tcelna. Consequently, no assurance can be given that third-party patents containing claims covering Tcelna, its method of use or manufacture do not exist or have not been filed and will not be issued in the future. Because some patent applications in the United States may be maintained in secrecy until the patents are issued, and because patent applications in the United States and many foreign jurisdictions are typically not published until 18 months after filing, we cannot be certain that others have not filed patent applications that will mature into issued patents that relate to our current or future product candidates that could have a material effect in developing and commercializing one or more of our product candidates. A patent holder could prevent us from importing, making, using or selling the patented compounds. We may need to resort to litigation to enforce our intellectual property rights or to determine the scope and validity of third-party proprietary rights. Similarly, we may be subject to claims that we have inappropriately used or disclosed trade secrets or other proprietary information of third parties. If we become involved in litigation, it could consume a substantial portion of our managerial and financial resources, regardless of whether we win or lose. Some of our competitors may be able to sustain the costs of complex intellectual property litigation more effectively than we can because they have substantially greater resources. We may not be able to afford the costs of litigation. Any legal action against us or our collaborators could lead to:
| • | payment of actual damages, royalties, lost profits, potentially treble damages and attorneys’ fees, if we are found to have willfully infringed a third party’s patent rights; |
| • | injunctive or other equitable relief that may effectively block our ability to further develop, commercialize and sell our products; |
| • | we or our collaborators having to enter into license arrangements that may not be available on commercially acceptable terms if at all; or |
| • | significant cost and expense, as well as distraction of our management from our business. |
As a result, we could be prevented from commercializing current or future product candidates.
Risks Related to Our Industry
We are subject to stringent regulation of our product candidates, such as Tcelna, which could delay development and commercialization.
We, our third-party contractors, suppliers and partners (such as Merck, in the event the Option is exercised, with respect to Tcelna), and our product candidates, such as Tcelna, are subject to stringent regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. None of our product candidates can be marketed in the United States until it has been approved by the FDA. No product candidate of ours has been approved, and we may never receive FDA approval for any product candidate. Obtaining FDA approval typically takes many years and requires substantial resources. Even if regulatory approval is obtained, the FDA may impose significant restrictions on the indicated uses, conditions for use and labeling of such products. Additionally, the FDA may require post-approval studies, including additional research and development and clinical trials. These regulatory requirements may limit the size of the market for the product or result in the incurrence of additional costs. Any delay or failure in obtaining required approvals could substantially reduce our ability to generate revenues.
In addition, both before and after regulatory approval, we, our partners and our product candidates, such as Tcelna, are subject to numerous FDA requirements covering, among other things, testing, manufacturing, quality control, labeling, advertising, promotion, distribution and export. The FDA’s requirements may change and additional government regulations may be promulgated that could affect us, our partners and our product
27
Table of Contents
candidates, such as Tcelna. Given the number of recent high profile adverse safety events with certain drug products, the FDA may require, as a condition of approval, costly risk management programs, which may include safety surveillance, restricted distribution and use, patient education, enhanced labeling, special packaging or labeling, expedited reporting of certain adverse events, preapproval of promotional materials and restrictions on direct-to-consumer advertising. Furthermore, heightened Congressional scrutiny on the adequacy of the FDA’s drug approval process and the agency’s efforts to assure the safety of marketed drugs resulted in the enactment of legislation addressing drug safety issues, the FDA Amendments Act of 2007. This legislation provides the FDA with expanded authority over drug products after approval and the FDA’s exercise of this authority could result in delays or increased costs during the period of product development, clinical trials and regulatory review and approval, and increased costs to assure compliance with new post-approval regulatory requirements. We cannot predict the likelihood, nature or extent of government regulation that may arise from this or future legislation or administrative action, either in the United States or abroad.
In order to market any of our products outside of the United States, we and our strategic partners and licensees must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods and the time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks detailed above regarding FDA approval in the United States. Approval by the FDA does not automatically lead to the approval of authorities outside of the United States and, similarly, approval by other regulatory authorities outside the United States will not automatically lead to FDA approval. In addition, regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others. Our product candidates, such as Tcelna, may not be approved for all indications that we request, which would limit uses and adversely impact our potential royalties and product sales. Such approval may be subject to limitations on the indicated uses for which any potential product may be marketed or require costly, post-marketing follow-up studies.
If we fail to comply with applicable regulatory requirements in the United States and other countries, among other things, we may be subject to fines and other civil penalties, delays in approving or failure to approve a product, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, interruption of manufacturing or clinical trials, injunctions and criminal prosecution, any of which would harm our business.
We may need to change our business practices to comply with health care fraud and abuse regulations, and our failure to comply with such laws could adversely affect our business, financial condition and results of operations.
If Merck exercises the Option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. Otherwise, if we are successful in achieving approval to market one or more of our product candidates, our operations will be directly, or indirectly through our customers, subject to various state and federal fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and False Claims Act. These laws may impact, among other things, our proposed sales, marketing, and education programs.
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. The Anti-Kickback Statute is broad
28
Table of Contents
and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized the Department of Health and Human Services, Office of Inspector General, or OIG, to issue a series of regulations, known as the “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG. Penalties for violations of the federal Anti-Kickback Statute include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
The federal False Claims Act prohibits persons from knowingly filing or causing to be filed a false claim to, or the knowing use of false statements to obtain payment from, the federal government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, sometimes known as “relators” or, more commonly, as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The frequency of filing of qui tam actions has increased significantly in recent years, causing greater numbers of healthcare companies to have to defend a False Claims Act action. When an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties. Various states have also enacted laws modeled after the federal False Claims Act.
In addition to the laws described above, the Health Insurance Portability and Accountability Act of 1996 created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines or imprisonment.
If our operations are found to be in violation of any of the laws described above and other applicable state and federal fraud and abuse laws, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from government healthcare programs, and the curtailment or restructuring of our operations.
If our competitors develop and market products that are more effective than our product candidates, they may reduce or eliminate our commercial opportunities.
Competition in the pharmaceutical industry, particularly the market for MS products, is intense, and we expect such competition to continue to increase. We face competition from pharmaceutical and biotechnology companies, as well as numerous academic and research institutions and governmental agencies, in the United States and abroad. Our competitors have products that have been approved or are in advanced development and may succeed in developing drugs that are more effective, safer and more affordable or more easily administered than ours, or that achieve patent protection or commercialization sooner than our products. Our most significant competitors are fully integrated pharmaceutical companies and more established biotechnology companies. These companies have significantly greater capital resources and expertise in research and development, manufacturing, testing, obtaining regulatory approvals, and marketing than we currently do. However, smaller companies also may prove to be significant competitors, particularly through proprietary research discoveries and collaboration arrangements with large pharmaceutical and established biotechnology companies. In addition to
29
Table of Contents
the competitors with existing products that have been approved, many of our competitors are further along in the process of product development and also operate large, company-funded research and development programs. As a result, our competitors may develop more competitive or affordable products, or achieve earlier patent protection or further product commercialization than we are able to achieve. Competitive products may render any products or product candidates that we develop obsolete.
Our competitors may also develop alternative therapies that could further limit the market for any products that we may develop.
Rapid technological change could make our products obsolete.
Biopharmaceutical technologies have undergone rapid and significant change, and we expect that they will continue to do so. As a result, there is significant risk that our product candidates, such as Tcelna, may be rendered obsolete or uneconomical by new discoveries before we recover any expenses incurred in connection with their development. If our product candidates, such as Tcelna, are rendered obsolete by advancements in biopharmaceutical technologies, our future prospects will suffer.
Consumers may sue us for product liability, which could result in substantial liabilities that exceed our available resources and damage our reputation.
Developing and commercializing drug products entails significant product liability risks. Liability claims may arise from our and our collaborators’ use of products in clinical trials and the commercial sale of those products.
In the event that any of our product candidates becomes an approved product and is commercialized, consumers may make product liability claims directly against us and/or our partners (such as Merck, in the event the Option is exercised, with respect to Tcelna), and our partners or others selling these products may seek contribution from us if they incur any loss or expenses related to such claims. We have insurance that covers clinical trial activities. We believe our insurance coverage as of the date hereof is reasonably adequate at this time. However, we will need to increase and expand this coverage as we commence additional clinical trials, as well as larger scale trials, and if any product candidate is approved for commercial sale. This insurance may be prohibitively expensive or may not fully cover our potential liabilities. Our inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the regulatory approval or commercialization of products that we or one of our collaborators develop. Product liability claims could have a material adverse effect on our business and results of operations. Liability from such claims could exceed our total assets if we do not prevail in any lawsuit brought by a third party alleging that an injury was caused by one or more of our products.
Health care reform measures could adversely affect our business.
The business and financial condition of pharmaceutical and biotechnology companies are affected by the efforts of governmental and third-party payors to contain or reduce the costs of health care. In the United States and in foreign jurisdictions, there have been, and we expect that there will continue to be, a number of legislative and regulatory proposals aimed at changing the health care system. For example, in some countries other than the United States, pricing of prescription drugs is subject to government control, and we expect proposals to implement similar controls in the United States to continue. Another example of reform that could affect our business is drug reimportation into the United States (i.e., the reimportation of approved drugs originally manufactured in the United States back into the United States from other countries where the drugs were sold at lower prices). Initiatives in this regard could decrease the price we or any potential collaborators receive for our product candidates if they are ever approved for sale, adversely affecting our future revenue growth and potential profitability. Moreover, the pendency or approval of such proposals could result in a decrease in our stock price or adversely affect our ability to raise capital or to obtain strategic partnerships or licenses.
30
Table of Contents
Risks Related to Our Securities
There is currently a limited market for our securities, and any trading market that exists in our securities may be highly illiquid and may not reflect the underlying value of our net assets or business prospects.
Although our common stock is traded on the NASDAQ Capital Market, there is currently a limited market for our securities and there can be no assurance that an active market will ever develop. Investors are cautioned not to rely on the possibility that an active trading market may develop.
Our stock may be delisted from NASDAQ, which could affect its market price and liquidity.
We are required to meet certain qualitative and financial tests (including a minimum stockholders’ equity requirement of $2.5 million and bid price for our common stock of $1.00 per share) to maintain the listing of our common stock on the NASDAQ Capital Market, and our common stock is in jeopardy of being delisted. On November 26, 2012, we received a staff deficiency letter from NASDAQ notifying us that the stockholders’ equity of $2,339,285 as reported in our Quarterly Report on Form 10-Q for the period ended September 30, 2012 was below the minimum stockholders’ equity of $2.5 million required for continued listing on NASDAQ. We were provided 45 calendar days, or until January 10, 2013, to submit a plan to regain compliance with the minimum stockholders’ equity standard. We submitted such a plan and it was accepted, with NASDAQ thus granting us an extension until May 15, 2013 to evidence compliance with the minimum stockholders’ equity standard. If we had failed to evidence compliance upon filing of the Quarterly Report on Form 10-Q for the period ended March 31, 2013, we could have been subject to delisting. Our stockholders’ equity as of March 31, 2013 was $4,034,357, and we subsequently received notice from NASDAQ that we had regained compliance with the listing standard and the matter was closed in May 2013. While we are exercising diligent efforts to maintain the listing of our common stock on NASDAQ, there can be no assurance that we will be able to maintain compliance with the stockholder’s equity standard.
It is also possible that we could fail to satisfy another NASDAQ requirement for continued listing of our stock, such as the minimum bid price, the market value or number of publicly held shares or number of shareholders, or a corporate governance requirement. For example, during portions of 2008 and 2009, our stockholders’ equity was below the continued listing standard requirement of $2.5 million and the bid price for our common stock was below $1.00 per share for periods of time, and our common stock was in jeopardy of being delisted. Additionally, during 2010 and 2011, the trading price of our common stock was minimally above $1.00 per share for certain periods of time, and our stock closed below $1.00 per share from December 2011 through part of December 2012. In February 2012, we received a staff deficiency letter from NASDAQ indicating that our common stock failed to comply with the minimum bid price requirement because it traded below the $1.00 minimum closing bid price for 30 consecutive trading days, and after an initial and an extended grace period, and implementation of a one-for-four reverse stock split of our common stock on December 14, 2012, we regained compliance with the $1.00 minimum closing bid price listing standard and NASDAQ notified us that the matter was closed in January 2013. However, there can be no assurance that the closing bid price of our common stock will continue to stay above the minimum continued listing standard.
We may receive additional future notices from NASDAQ that we have failed to meet its requirements, and proceedings to delist our stock could be commenced. In such event, NASDAQ rules permit us to appeal any delisting determination to a NASDAQ Hearings Panel. If we are unable to maintain or regain compliance in a timely manner and our common stock is delisted, it could be more difficult to buy or sell our common stock and obtain accurate quotations, and the price of our stock could suffer a material decline. Delisting may also impair our ability to raise capital.
As our share price is volatile, and you may not be able to resell our shares at a profit or at all.
The market prices for securities of biopharmaceutical and biotechnology companies, and early-stage drug discovery and development companies like us in particular, have historically been highly volatile and may
31
Table of Contents
continue to be highly volatile in the future. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:
| • | the development status of any drug candidates, such as Tcelna, including clinical study results and determinations by regulatory authorities with respect thereto; |
| • | the initiation, termination, or reduction in the scope of any collaboration arrangements (such as developments involving Merck and the Option Agreement, including a decision by Merck to exercise or not exercise the Option) or any disputes or developments regarding such collaborations; |
| • | announcements of technological innovations, new commercial products or other material events by our competitors or us; |
| • | disputes or other developments concerning our proprietary rights; |
| • | changes in, or failure to meet, securities analysts’ or investors’ expectations of our financial performance; |
| • | additions or departures of key personnel; |
| • | discussions of our business, products, financial performance, prospects or stock price by the financial and scientific press and online investor communities; |
| • | public concern as to, and legislative action with respect to, the pricing and availability of prescription drugs or the safety of drugs and drug delivery techniques; |
| • | regulatory developments in the United States and in foreign countries; or |
| • | dilutive effects of sales of shares of common stock by us or our shareholders, including Lincoln Park, and sales of common stock acquired upon exercise or conversion by the holders of warrants, options or convertible notes. |
Broad market and industry factors, as well as economic and political factors, also may materially adversely affect the market price of our common stock.
We may be or become the target of securities litigation, which is costly and time-consuming to defend.
In the past, following periods of market volatility in the price of a company’s securities or the reporting of unfavorable news, security holders have often instituted class action litigation. If the market value of our securities experience adverse fluctuations and we become involved in this type of litigation, regardless of the outcome, we could incur substantial legal costs and our management’s attention could be diverted from the operation of our business, causing our business to suffer.
Our “blank check” preferred stock could be issued to prevent a business combination not desired by management or our majority shareholders.
Our articles of incorporation authorize the issuance of “blank check” preferred stock with such designations, rights and preferences as may be determined by our Board of Directors without shareholder approval. Our preferred stock could be utilized as a method of discouraging, delaying, or preventing a change in our control and as a method of preventing shareholders from receiving a premium for their shares in connection with a change of control.
Future sales of our common stock in the public market could lower our stock price.
In July 2012, we closed a private offering consisting of convertible secured notes and warrants to purchase common stock which generated approximately $4.1 million in gross proceeds, of which notes in the aggregate principal amount of $900,000 have been converted into shares of Series A convertible preferred stock which, in
32
Table of Contents
turn, have been converted into an aggregate of 288,229 shares of common stock. From November 2012 through January 2013, we sold an aggregate of 390,000 shares to Lincoln Park pursuant to the $1.5 million purchase agreement and issued an additional 56,507 shares as initial commitment shares and 3,585 shares as additional commitment shares. In January 2013, we issued $650,000 principal amount of unsecured convertible promissory notes of which $100,000 was converted into 77,034 shares of common stock at $1.298125 per share and the remaining $550,000 of principal amount plus accrued interest was repaid. Purchasers of such notes also received five-year warrants to acquire an aggregate of 243,750 shares of our common stock at an exercise price of $1.24 per share. Pursuant to a Sales Agreement executed on September 6, 2012, we have registered for sale up to 1,000,000 shares of common stock through Brinson Patrick Securities Corporation acting as sales agent in an “at-the-market” program. In February 2013, we sold an aggregate of 167,618 shares of our common stock pursuant to such at-the-market program for gross proceeds of $536,417. On February 11, 2013, we closed on an offering of 1,083,334 shares of common stock and warrants to purchase 541,668 shares of common stock, for gross proceeds of $3,250,002. There can be no assurance that our capital raising efforts will be able to attract the capital needed to execute on our business plan and sustain our operations.
Sales of a substantial number of additional shares of our common stock in the public market could cause the market price of our common stock to decline. An aggregate of 7,991,559 shares of common stock were outstanding as of May 31, 2013. As of such date, another (i) 1,111,111 shares of common stock were issuable upon exercise of outstanding options, (ii) 3,069,113 shares of common stock were issuable upon the exercise of outstanding warrants, and (iii) 1,020,007 shares were issuable if all of the outstanding 12% convertible secured promissory notes were converted to Series A convertible preferred stock and such stock was then converted into shares of common stock.
A substantial majority of the outstanding shares of our common stock are freely tradable without restriction or further registration under the Securities Act of 1933. We may sell additional shares of common stock, as well as securities convertible into or exercisable for common stock, in subsequent public or private offerings. We may also issue additional shares of common stock, as well as securities convertible into or exercisable for common stock, to finance future acquisitions. Among other requirements, we will need to raise significant additional capital in order to complete the Phase IIb clinical study of Tcelna in SPMS, and this may require us to issue a substantial amount of securities (including common stock as well as securities convertible into or exercisable for common stock). We cannot predict the size of future issuances of our common stock, as well as securities convertible into or exercisable for common stock, or the effect, if any, that future issuances and sales of our securities will have on the market price of our common stock. Sales of substantial amounts of our common stock, as well as securities convertible into or exercisable for common stock, including shares issued in connection with an acquisition or securing funds to complete our clinical trial plans, or the perception that such sales could occur, may adversely affect prevailing market prices for our common stock.
The sale or issuance of our common stock to Lincoln Park may cause dilution and the sale of the shares of common stock acquired by Lincoln Park, or the perception that such sales may occur, could cause the price of our common stock to fall.
Under the $1.5 million purchase agreement and $15 million purchase agreement with Lincoln Park, we may direct Lincoln Park to purchase up to $16.5 million of shares of common stock, subject to certain limitations and conditions, over a 30-month period. However, in conjunction with our February 2013 offering of common stock and warrants, we agreed not to sell shares under the purchase agreements with Lincoln Park for a period of 120 days after that offering (or June 11, 2013). We have sold an aggregate of 390,000 shares to date under the $1.5 million purchase agreement. Additionally, we issued Lincoln Park 56,507 shares of common stock as initial commitment shares and have issued an aggregate of 3,585 additional commitment shares, and may in the future issue up to an additional 109,428 shares of common stock as additional commitment shares, as a fee for its commitment to purchase the shares under the $1.5 million purchase agreement and the $15 million purchase agreement. The number of shares ultimately offered for sale by Lincoln Park is dependent upon the number of shares purchased by Lincoln Park under the purchase agreements. Depending on market liquidity at the time, sales of shares we issue to Lincoln Park may cause the trading price of our common stock to decline.
33
Table of Contents
Subject to certain conditions, we generally have the right to control the timing and amount of any sales of our shares to Lincoln Park, except that, pursuant to the terms of our agreements with Lincoln Park, we would be unable to sell shares to Lincoln Park if and when the market price of our common stock is below $1.00 per share or if Lincoln Park would own more than 4.99% of our common stock for stock sold to it under the $1.5 million purchase agreement or 9.99% of our common stock for stock sold to it under the $15 million purchase agreement. The purchase price for the shares that we may sell to Lincoln Park will fluctuate based on the price of our common stock and other factors determined by us. As such, Lincoln Park may ultimately purchase all, some or none of the shares of our common stock issuable pursuant to the purchase agreements after the date hereof and, after it has acquired shares, Lincoln Park may sell all, some or none of those shares. Therefore, sales to Lincoln Park by us pursuant to either or both of the purchase agreements could result in substantial dilution to the interests of other holders of our common stock. Additionally, the sale of a substantial number of shares of our common stock to Lincoln Park, or the anticipation of such sales, could cause the trading price of our common stock to decline and could make it more difficult for us to sell equity or equity-related securities in the future.
We presently do not intend to pay cash dividends on our common stock.
We anticipate as of the date hereof that no cash dividends will be paid on the common stock in the foreseeable future. While our dividend policy will be based on the operating results and capital needs of the business, it is anticipated that all earnings, if any, will be retained to finance the future expansion of our business.
Our shareholders may experience substantial dilution in the value of their investment if we issue additional shares of our capital stock.
Our charter allows us to issue up to 100,000,000 shares of our common stock and to issue and designate the rights of, without shareholder approval, up to 10,000,000 shares of preferred stock. In connection with the July 25, 2012 convertible note financing, 80,000 shares of preferred stock were designated as non-voting Series A convertible preferred stock. In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share paid by other investors, and dilution to our shareholders could result. We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by investors, and investors purchasing shares or other securities in the future could have rights superior to existing shareholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by other investors.
We may issue debt and equity securities or securities convertible into equity securities, any of which may be senior to our common stock as to distributions and in liquidation, which could negatively affect the value of our common stock.
In the future, we may attempt to increase our capital resources by entering into debt or debt-like financing that is unsecured or secured by up to all of our assets, or by issuing additional debt or equity securities, which could include issuances of secured or unsecured commercial paper, medium-term notes, senior notes, subordinated notes, guarantees, preferred stock, hybrid securities, or securities convertible into or exchangeable for equity securities. In the event of our liquidation, our lenders and holders of our debt and preferred securities would receive distributions of our available assets before distributions to the holders of our common stock. Because our decision to incur debt and issue securities in future offerings may be influenced by market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of our future offerings or debt financings. Further, market conditions could require us to accept less favorable terms for the issuance of our securities in the future.
For example, on July 25, 2012, we closed a private offering consisting of convertible secured notes and warrants to purchase common stock which generated approximately $4.1 million in gross proceeds. The notes
34
Table of Contents
mature on July 25, 2014 and accrue interest at the rate of 12% per annum, compounded annually, payable in either cash or registered shares of common stock. The notes are secured by substantially all of our tangible and intangible assets, and $500,000 of the proceeds from the note offering is being held in a controlled account as part of the security interest granted to the noteholders. This amount was previously $1 million; however, in January 2013 we issued the noteholders five-year warrants to acquire an aggregate of 187,500 shares of our common stock at an exercise price of $1.21 per share in exchange for the reduction of such amount. The notes are convertible into a new class of non-voting Series A convertible preferred stock at a conversion price of $100.00, subject to certain limitations and adjustments. The Series A convertible preferred stock accrues cumulative dividends at the rate of 8% per annum, payable in either cash or registered shares of common stock, and carries a $100.00 per share liquidation preference. The Series A convertible preferred stock is convertible into common stock at a conversion price of $3.1225, subject to certain limitations and adjustments. As a result of antidilution adjustments since the closing of the July 2012 financing: (i) up to 1,020,007 shares of common stock are issuable if all the outstanding principal balance as of the date hereof of $3,185,000 of 12% convertible secured promissory notes is converted to Series A convertible preferred stock and such stock is then converted into shares of common stock; and (ii) the warrants issued to the purchasers of the convertible secured promissory notes are exercisable at an adjusted exercise price of $2.56 per share for an aggregate of 1,436,121 shares of common stock. As of May 31, 2013, secured promissory notes with an aggregate principal amount of $900,000 have been converted into shares of Series A convertible preferred stock which, in turn, have been converted into an aggregate of 288,229 shares of our common stock.
Our management has significant flexibility in using our current available cash and the proceeds from this offering.
In addition to general corporate purposes (including working capital and operational purposes), we intend to use our current available cash and the proceeds from this offering, as well as the net proceeds from the sale of any common stock that we may sell to Lincoln Park under our purchase agreements from time to time, including the $5 million proceeds received from Merck in February 2013 and the approximately $3.25 million in gross proceeds from the February 2013 registered offering of common stock and warrants, to continue our ongoing Phase IIb clinical study of Tcelna in SPMS.
The Phase IIb clinical study in North America of Tcelna is expected to complete enrollment of 180 patients by late 2013 or early 2014, with the resulting top-line data expected to be available in the first half of 2016. During the first quarter of 2013, the pace of on-boarding clinical sites for the Abili-T clinical study was tempered pending the completion of our negotiations with Merck for the Option as well as financial considerations. Upon receipt of the upfront payment of $5 million for granting the Option, we were able to refocus on execution of the Abili-T clinical trial, including enrollment. The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of May 31, 2013 to be between $30-$32 million. We used the $650,000 gross proceeds from the January 2013 private offering of convertible unsecured promissory notes and warrants as bridge financing to continue our Phase IIb clinical study while continuing discussions with Merck regarding the February 2013 Option and License Agreement. We repaid the principal balance of the January 2013 notes with $550,000 in cash and $100,000 was converted into 77,034 shares of common stock. Our existing resources are not adequate to permit us to complete our ongoing Phase IIb clinical study of Tcelna in SPMS or the majority of it. We will need to secure significant additional resources to complete the trial and support our operations during the pendency of the trial.
Depending on future developments and circumstances, we may use some of our available cash for other purposes. Notwithstanding our intention to use our available cash for further clinical studies of Tcelna, our management will have significant flexibility in using our available cash. The actual amounts and timing of expenditures will vary significantly depending on a number of factors, including the amount and timing of cash used in our operations and our research and development efforts. Management’s failure to use these funds effectively would have an adverse effect on the value of our common stock and could make it more difficult and costly to raise funds in the future.
35
Table of Contents
You may experience immediate dilution in the book value per share of the common stock you purchase.
Because the price per share of our common stock being offered may be substantially higher than the net tangible book value per share of our common stock, you may suffer substantial dilution in the net tangible book value of the common stock you purchase in this offering. If you purchase shares of common stock in this offering at the current market value, you may suffer immediate and substantial dilution in the net tangible book value of the common stock. See “Dilution” in this prospectus for a more detailed discussion of the dilution which may incur in connection with this offering.
36
Table of Contents
When used in this prospectus, the words “expects,” “believes,” “hopes,” “anticipates,” “estimates,” “may,” “could,” “intends,” “exploring,” “evaluating,” “progressing,” “proceeding” and similar expressions are intended to identify forward-looking statements. These forward-looking statements include statements in this prospectus under the headings “Our Company,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” These forward-looking statements do not constitute guarantees of future performance. Investors are cautioned that statements which are not strictly historical statements, including, without limitation, statements regarding current or future financial payments, costs, returns, royalties, performance and position, plans and objectives for future operations, plans and objectives for product development, plans and objectives for present and future clinical trials and results of such trials, plans and objectives for regulatory approval, litigation, intellectual property, product development, manufacturing plans and performance, management’s initiatives and strategies, and the development of the Company’s product candidate, Tcelna (imilecleucel-T), constitute forward-looking statements. Such forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated. These risks and uncertainties include, but are not limited to, those risks discussed in “Risk Factors,” as well as, without limitation, risks associated with:
| • | market conditions; |
| • | our capital position; |
| • | the rights and preferences provided to the Series A convertible preferred stock and investors in the convertible secured notes we issued in July 2012 (including a secured interest in all of our assets); |
| • | our ability to compete with larger, better financed pharmaceutical and biotechnology companies; |
| • | new approaches to the treatment of our targeted diseases; |
| • | our expectation of incurring continued losses; |
| • | our uncertainty of developing a marketable product; |
| • | our ability to raise additional capital to continue our development programs (including to undertake and complete any ongoing or further clinical studies for Tcelna), including in this regard our ability to satisfy various conditions required to access the financing potentially available under the purchase agreements with Lincoln Park (such as the minimum closing price for our common stock, the registration of the underlying shares of common stock under the Securities Act of 1933, as amended, and the requirement for an ongoing trading market for our stock); |
| • | our ability to maintain compliance with NASDAQ listing standards; |
| • | the success of our clinical trials (including the Phase IIb trial for Tcelna in secondary progressive multiple MS which, depending upon results, may determine whether Merck elects to exercise its Option); |
| • | whether Merck exercises its Option and, if so, whether we receive any development or commercialization milestone payments or royalties from Merck pursuant to the Option; |
| • | our dependence (if Merck exercises its Option) on the resources and abilities of Merck for the further development of Tcelna; |
| • | the efficacy of Tcelna for any particular indication, such as for relapsing remitting MS or secondary progressive MS; |
| • | our ability to develop and commercialize products; |
| • | our ability to obtain required regulatory approvals; |
| • | our compliance with all Food and Drug Administration regulations; |
37
Table of Contents
| • | our ability to obtain, maintain and protect intellectual property rights (including for Tcelna); |
| • | the risk of litigation regarding our intellectual property rights or the rights of third parties; |
| • | the success of third party development and commercialization efforts with respect to products covered by intellectual property rights that we may license or transfer; |
| • | our limited manufacturing capabilities; our dependence on third-party manufacturers; |
| • | our ability to hire and retain skilled personnel; |
| • | our volatile stock price; and |
| • | other risks detailed in our filings with the SEC. |
These forward-looking statements speak only as of the date made. We assume no obligation or undertaking to update any forward-looking statements to reflect any changes in expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. You should, however, review additional disclosures we make in our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K filed with the SEC.
38
Table of Contents
We estimate that the net proceeds from the sale of the shares of common stock in the offering at an assumed offering price of $ will be approximately $ million, after deducting the underwriting discount and estimated offering expenses, or $ million if the underwriters exercise their over-allotment option in full.
We currently expect to use the net proceeds from this offering as follows:
| • | approximately $ million to fund further clinical development of Tcelna and the Phase IIb study in patients with SPMS as well as the ongoing expenses of our operations during such development; and |
| • | the balance, if any, for general corporate purposes, including potential repayment of certain outstanding indebtedness. |
With respect to the potential repayment of certain outstanding indebtedness, we currently plan to use up to $3.6 million of our available cash or proceeds from this offering to repay in full our 12% convertible secured promissory notes, subject to the right of the noteholders to convert any unpaid principal amount of the notes into shares of our Series A convertible preferred stock. The notes were originally issued in July 2012 and mature on July 25, 2014.
Our management will retain broad discretion as to the allocation of the net proceeds from this offering. Until we use the net proceeds of this offering, we intend to invest the funds in short-term, interest bearing investments.
39
Table of Contents
The following table presents our cash, cash equivalents and capitalization, as of March 31, 2013:
| • | on an actual basis; and |
| • | on a pro forma as adjusted basis to give effect to the sale by us of shares of common stock in this offering at an assumed public offering price of $ per share, after deducting the underwriting discount and estimated offering expenses. |
The pro forma as adjusted information set forth below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this information in conjunction with our consolidated financial statements and notes thereto included in this prospectus.
| As of March 31, 2013 | ||||||||
| Actual | Pro
Forma As Adjusted(1) |
|||||||
| (Unaudited) | ||||||||
| Cash and cash equivalents |
$ | 7,834,336 | $ | |||||
|
|
|
|
|
|||||
| Long-term liabilities |
$ | 3,761,179 | $ | |||||
| Stockholders’ equity: |
||||||||
| Preferred stock, no par value, 10,000,000 shares authorized; none issued and outstanding, actual or pro forma as adjusted |
— | |||||||
| Common stock, $0.01 par value, 100,000,000 shares authorized; |
79,916 | |||||||
| Additional paid-in capital |
117,743,543 | |||||||
| Deficit accumulated during the development stage |
(113,789,102 | ) | ||||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
$ | 4,034,357 | $ | |||||
|
|
|
|
|
|||||
| (1) | A $1.00 increase or decrease in the assumed public offering price per share would increase or decrease our pro forma as adjusted cash and cash equivalents, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discount and estimated offering expenses payable by us. |
The information above is as of March 31, 2013 and excludes:
| • | 7,991,559 shares of common stock issuable upon the exercise of stock options outstanding at May 31, 2013 with a weighted average exercise price of $4.51 per share; |
| • | 3,069,113 shares of common stock issuable upon the exercise of outstanding warrants at May 31, 2013 with a weighted average exercise price of $4.12 per share; |
| • | 1,020,007 shares of common stock issuable if all of the 12% convertible secured promissory notes outstanding at May 31, 2013 are converted to Series A convertible preferred stock and such stock is then converted into shares of common stock; |
| • | 27,109 shares of common stock available for future grants under our 2010 Stock Incentive Plan at May 31, 2013; |
| • | any additional shares of common stock that we may issue to Lincoln Park Capital Fund, LLC, or Lincoln Park, pursuant to a $1,500,000 purchase agreement and a $15,000,000 purchase agreement we entered into on November 5, 2012 and November 2, 2012, respectively, which provides that, upon the |
40
Table of Contents
| terms and subject to the conditions and limitation set forth therein, Lincoln Park is committed to purchase up to an aggregate of an additional $15.9 million of shares of our common stock over the term of the purchase agreements, should we elect to sell shares to Lincoln Park; and |
| • | any shares issued upon the exercise by the underwriters of the option to purchase up to additional shares of common stock from us to cover over-allotments, if any. |
41
Table of Contents
Our net tangible book value as of March 31, 2013 was approximately $4,034,357 or $0.50 per share, based on 7,991,559 shares of our common stock outstanding on that date. Net tangible book value per share is determined by dividing our total tangible assets (total assets less intangible assets), less total liabilities, by the number of shares of our common stock outstanding.
After giving effect to our assumed sale of all shares of our common stock in this offering at an assumed public offering price of $ per share, our as adjusted net tangible book value as of March 31, 2013 would have been approximately $ , or $ per share of common stock. This represents an immediate increase in net tangible book value of $ per share to existing shareholders and immediate dilution in net tangible book value of $ per share to new investors participating in this offering at the assumed offering price. The following table illustrates this dilution on a per share basis:
| Assumed public offering price per share |
$ | |||||||
| Net tangible book value per share as of March 31, 2013, before this offering |
$ | 0.50 | ||||||
| Increase in pro forma net tangible book value per share attributable to new investors |
$ | |||||||
| Net tangible book value per share as of March 31, 2013, after giving effect to this offering |
$ | |||||||
| Dilution per share to new investors |
$ |
The information above is as of March 31, 2013 and excludes:
| • | 7,991,559 shares of common stock issuable upon the exercise of stock options outstanding at May 31, 2013 with a weighted average exercise price of $4.51 per share; |
| • | 3,069,113 shares of common stock issuable upon the exercise of outstanding warrants at May 31, 2013 with a weighted average exercise price of $4.12 per share; |
| • | 1,020,007 shares of common stock issuable if all of the 12% convertible secured promissory notes outstanding at May 31, 2013 are converted to Series A convertible preferred stock and such stock is then converted into shares of common stock; |
| • | 27,109 shares of common stock available for future grants under our 2010 Stock Incentive Plan at May 31, 2013; and |
| • | any additional shares of common stock that we may issue to Lincoln Park Capital Fund, LLC, or Lincoln Park, pursuant to a $1,500,000 purchase agreement and a $15,000,000 purchase agreement we entered into on November 5, 2012 and November 2, 2012, respectively, which provides that, upon the terms and subject to the conditions and limitation set forth therein, Lincoln Park is committed to purchase up to an aggregate of an additional $15.9 million of shares of our common stock over the term of the purchase agreements, should we elect to sell shares to Lincoln Park. |
If the underwriters’ overallotment option is exercised, our adjusted pro forma net tangible book value following the offering will be $ per share, and the dilution to new investors in the offering will be $ per share.
A $1.00 increase or decrease in the assumed public offering price per share would increase or decrease our pro forma as adjusted net tangible book value per share after this offering by approximately $ million, and dilution per share to new investors by approximately $ for an increase of $1.00, or $ for a decrease of $1.00, after deducting the underwriting discount and estimated offering expenses payable by us.
42
Table of Contents
MARKET PRICE OF OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Our common stock is traded on the NASDAQ Capital Market under the symbol “OPXA.” Our common stock has, from time to time, traded on a limited, sporadic and volatile basis. The table below shows the high and low sales prices for our common stock for the periods indicated, as reported by NASDAQ.
| Price Ranges(1) | ||||||||
| High | Low | |||||||
| Fiscal Quarter Ended March 31, 2013 |
||||||||
| First Quarter |
$ | 3.15 | $ | 1.16 | ||||
| Fiscal Year Ended December 31, 2012 |
||||||||
| First Quarter |
$ | 4.36 | $ | 2.84 | ||||
| Second Quarter |
3.04 | 1.28 | ||||||
| Third Quarter |
3.08 | 1.28 | ||||||
| Fourth Quarter |
3.28 | 1.12 | ||||||
| Fiscal Year Ended December 31, 2011 |
||||||||
| First Quarter |
$ | 11.20 | $ | 6.08 | ||||
| Second Quarter |
8.04 | 6.36 | ||||||
| Third Quarter |
6.56 | 4.44 | ||||||
| Fourth Quarter |
5.60 | 3.60 | ||||||
| (1) | We implemented a 1-for-4 reverse stock split on December 14, 2012. The high and low prices in the table reflect the impact of the reverse stock split for all periods shown in the table. |
The closing price of our common stock on June 14, 2013 was $1.70 per share. We implemented a 1-for-4 reverse stock split of our common stock on December 14, 2012. There are approximately 200 holders of record of our common stock, excluding shareholders for whom shares are held in “nominee” or “street name.”
We have never declared or paid any cash dividends on our common stock and we do not intend to pay cash dividends in the foreseeable future. We expect as of the date hereof to retain any future earnings to fund the operation and expansion of our business.
43
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated statements of operations data for the years ended December 31, 2012 and 2011 have been derived from our audited consolidated financial statements that are included in this prospectus. The summary consolidated statements of operations data for the three-month periods ended March 31, 2013 and 2012 and the consolidated balance sheet data as of March 31, 2013 is derived from our unaudited consolidated financial statements that are included in this prospectus. The historical financial data presented below is not necessarily indicative of our financial results in future periods, and the results for the three-month period ended March 31, 2013 are not necessarily indicative of our operating results to be expected for the full fiscal year ending December 31, 2013 or any other period. You should read the summary consolidated financial data in conjunction with those financial statements and the accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our consolidated financial statements are prepared and presented in accordance with United States generally accepted accounting principles, or U.S. GAAP. Our unaudited consolidated financial statements have been prepared on a basis consistent with our audited financial statements and include all adjustments, consisting of normal and recurring adjustments that we consider necessary for a fair presentation of the financial position and results of operations as of and for such periods.
| For the year
ended December 31, |
For the three months ended March 31, |
|||||||||||||||
| 2012 | 2011 | 2013 | 2012 | |||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||
| Option revenue |
$ | — | $ | — | $ | 220,100 | $ | — | ||||||||
| Research and development |
6,318,476 | 3,340,038 | 1,621,366 | 1,490,097 | ||||||||||||
| General and administrative |
2,508,541 | 2,406,269 | 1,102,435 | 816,196 | ||||||||||||
| Depreciation and amortization |
303,677 | 210,252 | 78,311 | 67,355 | ||||||||||||
| Loss on disposal of fixed assets |
3,097 | 9,686 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(9,133,791 | ) | (5,966,245 | ) | (2,582,012 | ) | (2,373,648 | ) | ||||||||
| Interest income |
280 | 932 | 1,874 | 136 | ||||||||||||
| Gain on derivative instruments |
552,978 | — | — | — | ||||||||||||
| Other income and expense, net |
— | — | 37,910 | — | ||||||||||||
| Interest expense |
(350,300 | ) | (3,135 | ) | (1,635,254 | ) | (487 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (8,930,833 | ) | $ | (5,968,448 | ) | $ | (4,177,482 | ) | $ | (2,373,999 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted loss per share |
$ | (1.54 | ) | $ | (1.06 | ) | $ | (0.58 | ) | $ | (0.41 | ) | ||||
| Weighted average shares outstanding |
5,785,372 | 5,633,124 | 7,239,102 | 5,762,028 | ||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||
| Cash and cash equivalents |
$ | 592,004 | $ | 7,109,215 | $ | 7,834,336 | $ | 4,677,956 | ||||||||
| Other current assets |
1,077,546 | 124,773 | 1,166,430 | 1,005,756 | ||||||||||||
| Fixed assets, net |
1,265,041 | 1,029,236 | 1,189,328 | 1,390,674 | ||||||||||||
| Restricted cash |
1,000,000 | — | 500,000 | — | ||||||||||||
| Deferred financing costs, net |
211,479 | — | 158,540 | — | ||||||||||||
| Other long-term assets |
— | — | 104,027 | — | ||||||||||||
| Total assets |
4,146,070 | 8,263,224 | 10,952,661 | 7,074,386 | ||||||||||||
| Total current liabilities |
885,975 | 1,067,860 | 3,157,126 | 2,047,601 | ||||||||||||
| Notes payable, net |
376,763 | — | 376,627 | — | ||||||||||||
| Deferred revenue |
— | — | 3,384,552 | — | ||||||||||||
| Total stockholders’ equity |
2,883,332 | 7,195,364 | 4,034,357 | 5,026,785 | ||||||||||||
44
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Our results of operations and cash flows should be read in conjunction with our unaudited consolidated financial statements and notes thereto and the audited financial statements and the notes thereto included elsewhere in this prospectus.
Business Overview
Unless otherwise indicated, we use “Opexa,” “the Company,” “we,” “our” and “us” to refer to the businesses of Opexa Therapeutics, Inc.
Opexa is a biopharmaceutical company developing a personalized immunotherapy with the potential to treat major illnesses, including multiple sclerosis (MS). This therapy is based on our proprietary T-cell technology. Our mission is to lead the field of Precision ImmunotherapyTM by aligning the interests of patients, employees and shareholders. Information related to our product candidate, Tcelna™, is preliminary and investigative. Tcelna has not been approved by the U.S. Food and Drug Administration (FDA) or other global regulatory agencies for marketing.
MS is a chronic, often disabling disease that affects the central nervous system (CNS), which is made up of the brain, spinal cord and optic nerves. MS attacks the covering surrounding nerve cells, or myelin sheaths, leading to loss of myelin (demyelination) and nerve damage. Symptoms may be mild, such as numbness in the limbs, or severe, such as paralysis or loss of vision. The progress, severity and specific symptoms of MS are unpredictable and vary from one person to another. We believe that our product candidate, Tcelna, has the potential to fundamentally address the root cause of MS by stopping the demyelination process and in supporting the generation of new myelin sheaths were demyelination has occurred (remyelination).
Tcelna is an autologous T-cell immunotherapy that is currently being developed for the treatment of Secondary Progressive MS (SPMS) and is specifically tailored to each patient’s immune response profile to myelin. Tcelna is designed to reduce the number and/or functional activity of specific subsets of myelin-reactive T-cells (MRTCs) known to attack myelin. This technology was originally licensed from Baylor College of Medicine in 2001.
Tcelna is manufactured using our proprietary method for the production of an autologous T-cell product, which comprises the collection of blood from the MS patient and the expansion of MRTCs from the blood. Upon completion of the manufacturing process, an annual course of therapy consisting of five doses is cryopreserved. At each dosing time point, a single dose of Tcelna is formulated and attenuated by irradiation before returning the final product to the clinical site for subcutaneous administration to the patient.
Tcelna has received Fast Track designation from the FDA in SPMS, and we believe it is positioned as a potential first-to-market personalized T-cell therapy for MS patients. The FDA’s Fast Track program is designed to facilitate the development and expedite the review of drug candidates intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
Critical Accounting Policies
General. Our discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from
45
Table of Contents
other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies affect our most significant judgments and estimates used in preparation of our financial statements.
Revenue Recognition. We adopted the provisions of FASB ASC 605, Revenue Recognition. ASC 605 requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services rendered; (3) consideration is fixed or determinable; and (4) collectability is reasonably assured.
We evaluated the Option and License Agreement (the “Merck Agreement”) with Ares Trading SA (“Merck”), a wholly owned subsidiary of Merck Serono S.A., and determined that the $5 million upfront payment from Merck has “stand-alone value.” Opexa’s continuing performance obligations, in connection with the $5 million payment, include the execution and completion of the Abili-T clinical trial in SPMS using commercially reasonable efforts at our own costs. As a “stand-alone value” term in the Merck Agreement, the $5 million upfront payment is determined to be a single unit of accounting, and is recognized as revenue on a straight-line basis over the exclusive option period based on the expected completion term of the Abili-T clinical trial in SPMS.
Stock-Based Compensation. We adopted the provisions of FASB ASC 718 which establishes accounting for equity instruments exchanged for employee service. We utilize the Black-Scholes option pricing model to estimate the fair value of employee stock based compensation at the date of grant, which requires the input of highly subjective assumptions, including expected volatility and expected life. Changes in these inputs and assumptions can materially affect the measure of estimated fair value of our share-based compensation. These assumptions are subjective and generally require significant analysis and judgment to develop. When estimating fair value, some of the assumptions will be based on, or determined from, external data and other assumptions may be derived from our historical experience with stock-based payment arrangements. The appropriate weight to place on historical experience is a matter of judgment, based on relevant facts and circumstances.
We estimated volatility by considering historical stock volatility. We have opted to use the simplified method for estimating expected term of options as equal to the midpoint between the vesting period and the contractual term.
Research and Development. The costs of materials and equipment or facilities that are acquired or constructed for research and development activities and that have alternative future uses are capitalized as tangible assets when acquired or constructed. The cost of such materials consumed in research and development activities and the depreciation of such equipment or facilities used in those activities are research and development costs. However, the costs of materials, equipment, or facilities acquired or constructed for research and development activities that have no alternative future uses are considered research and development costs and are expensed at the time the costs are incurred.
Results of Operations and Financial Condition
Comparison of the Three Months Ended March 31, 2013 with the Three Months Ended March 31, 2012
Revenue. Revenue of $220,100 related to the $5 million upfront payment from Merck in conjunction with the Option and License Agreement was recognized for the three months ended March 31, 2013. No revenues were recognized during the three months ended March 31, 2012.
Research and Development Expenses. Research and development expenses were $1,621,366 for the three months ended March 31, 2013, compared with $1,490,097 for the three months ended March 31, 2012. The increase in expenses is primarily due to an increase of staff to conduct increased development activities, an increase in the procurement and use of supplies for our product manufacturing and development operations and the engagement of clinical sites for the clinical study of Tcelna in SPMS, and was partially offset by decreases in the use of consultants and contract development costs.
46
Table of Contents
General and Administrative Expenses. General and administrative expenses for the three months ended March 31, 2013 were $1,102,435, compared with $816,196 for the three months ended March 31, 2012. The increase in expense is due to an increase in capital financing expenses and a one-time severance charge, and was partially offset by a decrease in legal expense.
Depreciation and Amortization Expenses. Depreciation and amortization expenses for the three months ended March 31, 2013 were $78,311, compared with $67,355 for the three months ended March 31, 2012. The increase in expense is due to increase in depreciation for laboratory and manufacturing equipment acquired during 2012 to support increased development activities.
Interest Expense. Interest expense was $1,635,254 for the three months ended March 31, 2013, compared to $487 for the three months ended March 31, 2012. The increase in interest expense was primarily related to the amortized debt discount and interest on both the July 25, 2012 convertible secured promissory notes and the January 23, 2013 convertible promissory notes and the amortization of the financing fees over the life of the notes. Interest expense for the three months ended March 31, 2012 related solely to the financing of insurance premiums.
Interest Income. Interest income was $1,874 for the three months ended March 31, 2013, compared to $136 for the three months ended March 31, 2012.
Other Income. Other income of $37,910 for the three months ended March 31, 2013 was related to the extinguishment of membership interests in the mutual insurance company that we participated in for our product liability insurance through January 1, 2013. We recorded no other income for the three months ended March 31, 2012.
Net loss. We had a net loss for the three months ended March 31, 2013 of approximately $4.18 million, or $0.58 per share (basic and diluted), compared with a net loss of approximately $2.37 million or $0.41 per share (basic and diluted) for the three months ended March 31, 2012. The increased net loss is primarily related to increases in compensation and severance costs, capital financing expenses, the procurement and use of supplies for both our laboratory and our product manufacturing operations, the engagement of clinical sites for the clinical study of Tcelna in SPMS, depreciation expense and interest expense, and was partially offset by an increases in revenue and other income and decreases in legal expenses, consulting costs and contract development costs.
Comparison of Year Ended December 31, 2012 with the Year Ended December 31, 2011
Net Sales. We recorded no commercial revenues for the years ended December 31, 2012 and 2011.
Research and Development Expenses. Research and development expenses were $6,318,476 for the year ended December 31, 2012, compared to $3,340,038 for the year ended December 31, 2011. The increase in expenses was primarily due to an increases in staff to conduct increased development activities, the procurement and use of supplies used in both our laboratory and product manufacturing operations, the engagement of consultants and the costs of subject participation in our Phase IIb clinical study, facilities costs and stock compensation expense, and was partially offset by a decrease in legal costs. We have made and expect to continue to make substantial investments in research and development in order to develop and market our technology. We expense research and development costs as incurred. Acquired research and development that has no alternative future use is expensed when acquired. Property, plant and equipment for research and development that has an alternative future use is capitalized and the related depreciation is expensed.
General and Administrative Expenses. Our general and administrative expenses were $2,508,541 for the year ended December 31, 2012, as compared to $2,406,269 for the year ended December 31, 2011. The increase in expense is due to increases in legal expense, capital financing activities, stock compensation expense and facilities costs, and was partially offset by a reduction in professional service fees.
47
Table of Contents
Depreciation and Amortization Expenses. Depreciation and amortization expenses were $303,677 for the year ended December 31, 2012, as compared to $210,252 for the year ended December 31, 2011. The increase in expense is due to an increase in depreciation for facility build-out costs incurred during the first half of 2011, an increase in depreciation for laboratory and manufacturing equipment acquired during 2011 and 2012 to support increased development activities and an increase in depreciation for information technology equipment to replace and upgrade obsolete equipment.
Interest Expense. Interest expense was $350,300 for the year ended December 31, 2012, compared to $3,135 for the year ended December 31, 2011. The increase in interest expense was primarily related to the non-cash amortized debt discount and interest on the July 25, 2012 convertible notes and the amortization of the financing fees over the life of the notes. Interest expense for the year ended December 31, 2011 related solely to the financing costs on insurance policies and the loan payable on an equipment line.
Interest Income. Interest income was $280 for the year ended December 31, 2012, compared to $932 for the year ended December 31, 2011.
Net Loss. We had a net loss for the year ended December 31, 2012 of $8,930,833, or $1.54 per share (basic and diluted), compared with a net loss of $5,968,448, or $1.06 per share (basic and diluted), for the year ended December 31, 2011. The increase in net loss is primarily due to increases in research and development, general and administrative, depreciation and interest expenses.
Liquidity and Capital Resources
Historically, we have financed our operations primarily from the sale of debt and equity securities. As of March 31, 2013, we had cash and cash equivalents of $7,834,336 and $500,000 of restricted cash which is subject to a deposit control agreement in favor of the holders of our July 2012 convertible notes.
During January 2013, we closed a private offering consisting of convertible notes and Series J warrants to purchase common stock which generated $650,000 in gross proceeds, of which we repaid $550,000 and converted $100,000 to 77,034 shares of our common stock during February 2013.
Also during January 2013, we sold 125,000 shares of our common stock to Lincoln Park for gross proceeds of $142,400 under a $1.5 million purchase agreement entered into in November 2012. We also entered into a $15 million purchase agreement and registration rights agreement with Lincoln Park in November 2012. Pursuant to these agreements, we have the right to sell to Lincoln Park an aggregate of up to $16.5 million in shares of our common stock, subject to certain conditions and limitations. Under the terms and subject to the conditions of the purchase agreements, Lincoln Park is obligated to purchase up to an aggregate of $16.5 million in shares of common stock (subject to certain limitations) from time to time over a 30-month period. We may direct Lincoln Park, at our sole discretion and subject to certain conditions, to purchase up to 100,000 shares of common stock in regular purchases, increasing to amounts of up to 300,000 shares depending upon the closing sale price of our common stock. In addition, we may direct Lincoln Park to purchase additional amounts as accelerated purchases. The purchase price of shares of common stock related to the future funding will be based on the prevailing market prices of such shares at the time of sales (or over a period of up to 12 business days leading up to such time), but in no event will shares be sold to Lincoln Park on a day the common stock closing price is less than the adjusted minimum floor price of $1.00. As of May 31, 2013, we have sold 390,000 shares to Lincoln Park under the $1.5 million purchase agreement for gross proceeds of $523,709, and we have a remaining commitment amount of $15,976,291 available to us through Lincoln Park purchase agreements. However, there can be no assurance that we will be able to receive any or all of the additional funds from Lincoln Park because the purchase agreements contain limitations, restrictions, requirements, events of default and other provisions that could limit our ability to cause Lincoln Park to buy common stock from us.
48
Table of Contents
Pursuant to a waiver executed in February 2013 by the holders of in excess of two-thirds (66-2/3%) of the principal amount of the outstanding July 2012 convertible promissory notes and accepted by Opexa, the amount of the cash subject to the deposit control agreement was reduced from $1 million to $500,000.
In February 2013, we entered into the Merck Agreement. Pursuant to this agreement, Merck has an option (the “Option”) to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck prior to or upon our completion of the Phase IIb trial. Under the terms of the Merck Agreement, we received an upfront payment of $5 million for granting the Option. If the Option is exercised, Merck would pay us an upfront license fee of $25 million unless Merck is unable to advance directly into a Phase III clinical trial of Tcelna for SPMS without a further Phase II clinical trial (as determined by Merck), in which event the upfront license fee would be $15 million. After exercising the Option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights outside of MS.
Also during February 2013, we sold an aggregate of 167,618 shares of our common stock, for gross proceeds of $536,417, pursuant to a sales agreement entered into with Brinson Patrick Securities Corporation in September 2012 in connection with the implementation of an “at-the-market” offering program. Pursuant to the sales agreement, we may sell shares of our common stock directly into the open market from time to time depending upon market demand, through our sales agent, in transactions deemed to be an “at-the-market” offering as defined in Rule 415 of the Securities Act of 1933. We have registered up to 1,000,000 shares of our common stock for potential sale under this program. As of May 31, 2013, 167,618 shares had been sold and 832,382 shares remain available for future sale under the sales agreement.
We closed an offering in February 2013 of 1,083,334 shares of common stock and warrants to purchase 541,668 shares of common stock for gross proceeds of $3,250,002. As part of that offering, we agreed not to sell shares pursuant to our purchase agreements with Lincoln Park or under our “at-the-market” program for a period of 120 days following such February 2013 offering (or June 11, 2013).
Our cash burn rate during the three months ended March 31, 2013, inclusive of the cost of our ongoing Abili-T clinical study, was approximately $775,000 per month. During this three-month period, the pace of on-boarding clinical sites for the Abili-T clinical study was tempered pending the completion of our negotiations with Merck for the Option as well as financial considerations. Upon receipt of the upfront payment of $5 million for granting the Option, we were able to refocus on execution of the Abili-T clinical trial, including enrollment. Significant activities in the conduct of the Abili-T clinical trial are expected to result in substantial increases in our monthly cash burn during the balance of 2013. Based on our projected burn rate increasing to an average of $1.2 million per month for the remainder of 2013, we believe we have sufficient liquidity to support our clinical trial activities into the fourth quarter of 2013.
We currently intend to continue to use our available cash to fund general corporate purposes (including working capital and operational purposes) and continue the ongoing Abili-T clinical study of Tcelna in SPMS. The Abili-T clinical study in North America of Tcelna is expected to complete enrollment of 180 patients by late 2013 or early 2014, with resulting top-line data expected to be available in the first half of 2016. The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of May 31, 2013 to be between $30-$32 million. Our existing resources are not adequate to permit us to complete such study or the majority of it. We anticipate that $ million of the proceeds from the offering will be applied to funding the continuation of the clinical study as well as the ongoing expenses of our operations during such development. We will need to secure significant additional resources to continue and complete the trial and support our operations during the pendency of the trial. If we are unable to obtain additional funding for operations, we will be forced to suspend or
49
Table of Contents
terminate our current ongoing clinical trial for Tcelna, which may require us to modify our current business plan and curtail various aspects of our operations, as well as implement significant cost-reduction measures or potentially cease operations.
There can be no assurance that any such financing arrangements can be consummated on acceptable terms, if at all. Given our need for substantial amounts of capital to continue the Abili-T clinical study in North America of Tcelna in SPMS, we intend to continue to explore potential opportunities and alternatives to obtain the additional resources, including one or more financings, that will be necessary to complete the ongoing Phase IIb study and to support ongoing operations during the pendency of such study.
If Merck does not exercise the Option to acquire the exclusive, worldwide (excluding Japan) license of our Tcelna program for MS, or if we are not successful in attracting another partner and entering into a collaboration on acceptable terms, we may not be able to complete development of or commercialize any product candidate, including Tcelna. In particular, we may be unable to undertake, or complete, the planned Phase III clinical study of Tcelna in SPMS. In such event, our ability to generate revenues and achieve or sustain profitability would be significantly hindered and we may not be able to continue operations as proposed, requiring us to modify our business plan, curtail various aspects of our operations or cease operations.
We do not maintain any external lines of credit. Should we need any additional capital in the future beyond the purchase agreements with Lincoln Park and our at-the-market program, management will be reliant upon “best efforts” debt or equity financings. As our prospects for funding, if any, develop during the fiscal year, we will assess our business plan and make adjustments accordingly. Although we have successfully funded our operations to date by attracting additional investors in our equity and debt securities, there is no assurance that our capital raising efforts will be able to attract additional necessary capital for our operations in the future.
Assuming we are able to achieve financing which is sufficient to continue the Abili-T study in North America and to support our operations during the pendency of such study, we are also able to concurrently manage a pivotal Phase III clinical study in RRMS in North America in our present facility. Any such RRMS studies would also depend upon the availability of sufficient resources.
Off-Balance Sheet Arrangements
None.
Inflation
We believe that inflation has not had a material impact on our results of operations for the two years ended December 31, 2012 and 2011, since inflation rates have generally remained at relatively low levels and our operations are not otherwise uniquely affected by inflation concerns.
Recent Accounting Pronouncements
On July 1, 2009, the FASB officially launched the FASB Accounting Standards Codification, which has become the single official source of authoritative, nongovernmental U.S. Generally Accepted Accounting Principles, in addition to guidance issued by the Securities and Exchange Commission. The codification supersedes all prior FASB, AICPA, EITF, and related literature. The codification, which is effective for interim and annual periods ending after September 15, 2009, is organized into approximately 90 accounting topics. The FASB no longer issues new standards in the form of Statements, FASB Staff Positions, or Emerging Issues Task Force Abstracts. Instead, amendments to the codification are made by issuing “Accounting Standards Updates.”
There were various other accounting standards and interpretations issued during 2012 and 2011, none of which are expected to have a material impact on our financial position, operations or cash flows.
For the three months ended March 31, 2013, there were no accounting standards or interpretations issued that are expected to have a material impact on our financial position, operations or cash flows.
50
Table of Contents
Overview
Opexa is a biopharmaceutical company developing a personalized immunotherapy with the potential to treat major illnesses, including multiple sclerosis (MS). This therapy is based on our proprietary T-cell technology. Our mission is to lead the field of Precision ImmunotherapyTM by aligning the interests of patients, employees and shareholders. Information related to our product candidate, Tcelna™, is preliminary and investigative. Tcelna has not been approved by the U.S. Food and Drug Administration (FDA) or other global regulatory agencies for marketing.
MS is an inflammatory autoimmune disease of the central nervous system (CNS), which is made up of the brain, spinal cord and optic nerves, with a clinically heterogeneous and unpredictable course that persists for decades. MS attacks the covering surrounding nerve cells, or myelin sheaths, leading to loss of myelin (demyelination) and nerve damage. In addition to demyelination, the neuropathology of MS is characterized by variable loss of oligodendroglial cells and axonal degeneration and manifests in neurological deficits. Symptoms may be mild, such as numbness in the limbs, or severe, such as paralysis or loss of vision. This inflammatory, demyelinating, autoimmune disease has varied clinical presentations, ranging from relapses and remissions (relapsing remitting MS, or RRMS) to slow accumulation of disability with or without relapses (secondary progressive MS, or SPMS). There are approximately 450,000 MS patients in North America and over 2,000,000 patients worldwide according to estimates from The National MS Society. The SPMS patient population is estimated by various industry sources to be between 30-45% of the total MS patient population.
We believe that our product candidate, Tcelna, has the potential to fundamentally address the root cause of MS by stopping the demyelination process and in supporting the generation of new myelin sheaths where demyelination has occurred (remyelination). Tcelna is an autologous T-cell immunotherapy that is currently being developed for the treatment of SPMS and is specifically tailored to each patient’s immune response profile to myelin. Tcelna is designed to reduce the number and/or functional activity of specific subsets of myelin-reactive T-cells (MRTCs) known to attack myelin. This technology was originally licensed from Baylor College of Medicine in 2001.
Tcelna is manufactured using our proprietary method for the production of an autologous T-cell product, which comprises the collection of blood from the MS patient and the expansion of MRTCs from the blood. Upon completion of the manufacturing process, an annual course of therapy consisting of five doses is cryopreserved. At each dosing time point, a single dose of Tcelna is formulated and attenuated by irradiation before returning the final product to the clinical site for subcutaneous administration to the patient.
Tcelna has received Fast Track designation from the FDA in SPMS, and we believe it is positioned as a potential first-to-market personalized T-cell therapy for MS patients. The FDA’s Fast Track program is designed to facilitate the development and expedite the review of drug candidates intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
Opexa was incorporated in Texas in March 1991. Our principal executive offices are located at 2635 Technology Forest Blvd., The Woodlands, Texas 77381, and our telephone number is (281) 775-0600.
Multiple Sclerosis—Background
MS is a disease that is more common in females than males (2:1) between the ages of 20 and 40, with a peak onset of approximately 25 years of age. MS frequently causes impairment of motor, sensory, coordination and balance, visual, and/or cognitive functions, as well as urinary (bladder) or bowel dysfunction and symptoms of fatigue. The identified autoimmune mechanisms directed at myelin tissue of the CNS may play an important role in the pathogenesis of MS. Epidemiologic studies suggest that a variety of genetic, immunologic, and environmental factors including viral infections may play a role in defining the etiology and in triggering the onset and progression of MS.
51
Table of Contents
At the onset of MS, approximately 85% of MS patients have RRMS. Without disease-modifying medication, one-half of these RRMS patients will develop steadily progressive disease, SPMS, within 10 years, increasing to 90% within 25 years of MS diagnosis. The MS drug market was approximately $13 billion in 2012 and is forecasted to reach as much as $16 billion by 2015.
MS remains a challenging autoimmune disease to treat because the pathophysiologic mechanisms are diverse, and the chronic, unpredictable course of the disease makes it difficult to determine whether the favorable effects of short-term treatment will be sustained. Therapies that are easy to use and can safely prevent or stop the progression of disease represent the greatest unmet need in MS.
In recent years, the understanding of MS pathogenesis has evolved to comprise an initial, T-cell-mediated inflammatory activity followed by selective demyelination (erosion of the myelin coating of the nerve fibers) and then neurodegeneration. The discovery of disease-relevant immune responses has accelerated the development of targeted therapeutic products for the treatment of the early stages of MS. Some subjects, who have the appropriate genetic background, have increased susceptibility for the in vivo activation and expansion of MRTCs. These MRTCs may remain dormant, but at some point they are activated in the periphery, thus enabling them to cross the blood-brain barrier and infiltrate the healthy tissue of the brain and spinal cord. The cascade of pathogenic events leads to demyelination of protrusions from nerve cells called axons, which causes nerve impulse transmissions to diffuse into the tissue resulting in disability to the individual.
52
Table of Contents
Tcelna for MS
We believe that Tcelna works selectively on the MRTCs by harnessing the body’s natural immune defense system and feedback mechanisms to deplete these T-cells and induce favorable immune regulatory responses by rebalancing the immune system. Tcelna is manufactured by isolating the MRTCs from the blood, expanding them to a therapeutic dose ex-vivo, and attenuating them with gamma irradiation to prevent DNA replication and thereby cellular proliferation. These attenuated MRTCs are then injected subcutaneously into the body in therapeutic dosages. The body recognizes specific T-cell receptor molecules of these MRTCs as immunogenic and initiates an immune response reaction against them, resulting in the depletion and/or immunosuppression of circulating, myelin reactive T-cells carrying the peptide-specific T-cell receptor molecules. In addition, we believe that T-cell activation molecules on the surface of the activated MRTCs, that constitute the Tcelna product, promote anti-inflammatory responses. Because the therapy uses an individual’s own cells, the only direct identifiable side effect observed thus far is injection site reactions which typically are minor and generally clear within 24 hours.
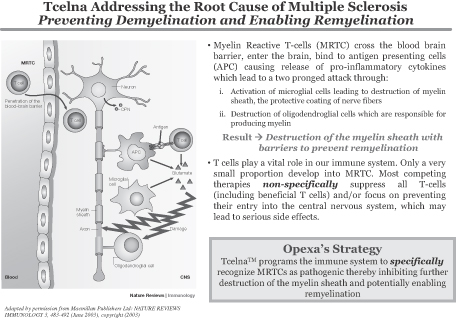
Tcelna Clinical Development Program
Tcelna is a novel T-cell immunotherapy in Phase IIb clinical development for the treatment of patients with SPMS. It is also positioned to enter Phase III clinical development for the treatment of patients with RRMS, subject to the availability of sufficient resources. Tcelna is a personalized immunotherapy that is specifically tailored to each patient’s disease profile. Tcelna is manufactured using ImmPathTM, our proprietary method for the production of a patient-specific T-cell immunotherapy which encompasses the collection of blood from the MS patient, isolation of peripheral blood mononuclear cells, generation of an autologous pool of MRTCs raised against selected peptides from myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) and proteolipid protein (PLP), and the return of these expanded, irradiated T-cells back to the patient. These attenuated T-cells are reintroduced into the patient via subcutaneous injection to trigger a therapeutic immune system response.
53
Table of Contents
The Tcelna clinical development program spans studies conducted at Baylor College of Medicine and Opexa conducted clinical studies. A summary of the various Tcelna clinical studies is shown below:
| Phase |
Completion Dates |
Population |
Total N |
Treatment Duration |
Tcelna | Placebo | ||||||
| Baylor | 1998 | RRMS, PPMS, SPMS (26) |
114 | Up to 24 months |
114 | |||||||
| Phase I dose escalation | 2006 | RRMS (5) SPMS (6) |
16 | 12 | 16 | — | ||||||
|
Phase I/II Open label retreatment |
2007 | RRMS (9) SPMS (4) |
13 | 12 | 13 | — | ||||||
|
Phase IIb TERMS |
2008 | RRMS, CIS | 150 | 12 | 100 | 50 | ||||||
| Phase IIb extension OLTERMS | 2008 | RRMS, CIS | 38 | At least one dose post TERMS |
15 from placebo arm |
|||||||
|
Phase IIb Abili-T |
1st Half 2016* | SPMS | 180* | 24* | 90* | 90* |
| * | Expected upon completion of ongoing SPMS Abili-T trial |
Summary of Phase I Dose Escalation Study in MS
A Phase 1 dose escalation study completed in 2006 was conducted in patients with both RRMS and SPMS who were intolerant or unresponsive to current approved therapies for MS. The open-label, dose escalation study evaluated safety and clinical benefit by administering a primary series of four treatments at one of three dose levels administered at baseline and weeks four, eight and twelve. Results of the efficacy analyses provide some evidence of the effectiveness of Tcelna in the treatment of MS. Data from the Phase I study evaluating the Expanded Disability Status Scale (EDSS) showed improvements in some subjects in comparison to baseline for weeks 20 and 28.
54
Table of Contents
Subjects showed statistically significant improvement in overall reduction of MRTC counts over baseline at all visits through week 52 for subjects receiving 30-45 million cells per dose, as assessed by total MRTC count percentage changes. These data indicate that Tcelna treatment causes a depletion or immunomodulation of these cells, most obvious at time points closer to the injections. These findings, which demonstrate that administration of Tcelna induces a reduction in MRTCs, were published in Clinical Immunology (2009) 131, 202-215.
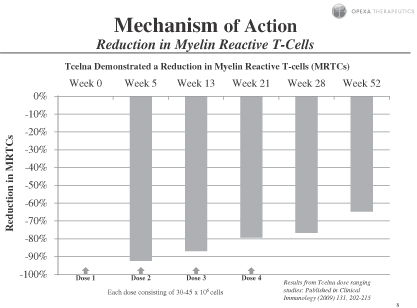
Overall, results of the safety analyses indicate that treatment with Tcelna is well-tolerated. Reported adverse events were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. In conclusion, data from this study suggest that Tcelna is safe for the treatment of MS.
Summary of Phase I/IIA Clinical Trial Data in MS
The second clinical study performed by Opexa was an open-label extension study completed in 2007 to treat patients who were previously treated with T-cell immunotherapy but who saw a rebound in MRTC activity. The purpose of this extension study was to continue evaluating the efficacy, safety and tolerability of Tcelna in patients with RRMS and SPMS with repeated administration of Tcelna. Results of the study provide evidence of the effectiveness of Tcelna in the treatment of MS with repeated dosing. Improvements from baseline at both week 28 and week 52 of the extension study were observed for the frequency of MS exacerbations (ARR). Evaluation of the Multiple Sclerosis Impact Scale (MSIS-29) component scores suggests a trend for Tcelna therapy in the improvement of physical and psychological parameters assessed by the MSIS-29. The EDSS score analysis revealed an upward trend for the percentage of subjects that reported improvement and sustained improvement over baseline as a result of Tcelna treatment.
Subjects showed statistically significant improvement over baseline in the MRTC counts for each time point through month nine of the extension study. These results indicate that Tcelna treatment results in a statistically significant impact on these cells.
Overall, results of the safety analyses indicate that repeated treatment with Tcelna is well-tolerated. Reported adverse events (AEs) were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. Furthermore, results from this study suggest that repeated dosing of Tcelna has a substantive effect in reduction of ARR in subjects with MS and was well-tolerated.
55
Table of Contents
Summary of TERMS Phase IIb Clinical Trial Data in RRMS
Tovaxin for Early Relapsing Multiple Sclerosis (TERMS) was a Phase IIb clinical study of Tcelna in RRMS patients completed in 2008. Although the study did not show statistical significance in its primary endpoint (the cumulative number of gadolinium-enhanced brain lesions using MRI scans summed at various points in the study), the study showed compelling evidence of efficacy in various clinical and other MRI endpoints.
The TERMS study was a multi-center, randomized, double blind, placebo-controlled trial in 150 patients with RRMS or high risk Clinically Isolated Syndrome. The inclusion criteria for TERMS was an EDSS score of 0 to 5.5. Patients received a total of five subcutaneous injections at weeks 0, 4, 8, 12 and 24. Key results from the TERMS trial included:
| • | In the modified intent to treat patient population consisting of all patients who received at least one dose of study product and had at least one MRI scan at week 28 or later (n=142), the ARR for Tcelna-treated patients was 0.214 as compared to 0.339 for placebo-treated patients, which represented a 37% decrease in ARR for Tcelna as compared to placebo in the general population; |
| • | In a prospective group of patients with more active disease (ARR>1, n=50), Tcelna demonstrated a 55% reduction in ARR as compared to placebo, 88% reduction in whole brain atrophy and a statistically significant improvement in disability (EDSS) compared to placebo (p<0.045) at week 52 during the 24-week period following the administration of the full course of treatment; and |

| • | In a retrospective analysis in patients naïve to previous disease modifying treatment, the results showed that patients, when treated with Tcelna, had a 56% to 73% reduction in ARR versus placebo for the various subsets and p values ranged from 0.009 to 0.06. |

56
Table of Contents
We remain committed to further advancing Tcelna in RRMS at a later date assuming the availability of sufficient resources. For Opexa, however, SPMS is an area which we believe represents a higher unmet medical need.
SPMS Overview and Tcelna Mechanism of Action
SPMS is characterized by a steady accrual of irreversible disability, despite, in some cases, relapses followed by remissions or clinical plateaus. Older age at onset of MS diagnosis is the strongest predictor of conversion to SPMS. Males have a shorter time to conversion to SPMS compared with females. Available immunomodulating and immunosuppressive therapies used for RRMS have not been effective in SPMS. In clinical trials, these therapies have demonstrated anti-inflammatory properties as measured by the reduction in number and volume of contrast-enhancing or acutely inflammatory CNS lesions most commonly seen in patients with RRMS. The typical SPMS patient, however, has little or no radiographic evidence of acute inflammation. It is commonly observed that contrast-enhancing CNS lesions are uncommon among these patients, despite a clearly deteriorating neurologic course.
The lack of effect of conventional MS therapeutics in SPMS suggests that the cerebral deterioration characterizing progressive disease may be driven by factors other than acute inflammation. For instance, the immunopathology of SPMS is more consistent with a transition to a chronic T-cell dependent inflammatory type, which may encompass the innate immune response and persistent activation of microglia cells. Meningeal follicles close to cortical gray matter lesions suggests that adaptive immune responses involving antibody and complement contribute to progression in SPMS. Furthermore, chronic MRTCs may be contributing to the development of both innate and adaptive immune responses persisting in the CNS.
Radiographic features that stand out among patients with SPMS include significantly more atrophy of gray matter compared with RRMS patients. Of note, long-term disability in MS in general appears more closely correlated to gray matter atrophy than to white matter inflammation. Such atrophy may be suggestive of progressive clinical disability. Both clinically and radiographically, SPMS represents a disease process with certain features distinct from those of RRMS, and one with extremely limited treatment options.
Tcelna immunotherapy in SPMS may reduce the drivers of this chronic disease by down-regulating anti-myelin immunity through priming regulatory responses that may act in the periphery as well as within the CNS. We believe that our clinical results show therapeutic subcutaneous dosing of 30-45 million cells of Tcelna stimulates host reactivity to the over-represented MRTCs and, as a consequence, a dominant negative regulatory T-cell response is induced leading to down-regulation of similar endogenous disease-causing MRTCs.
We believe that Tcelna has the potential to induce an up-regulation of regulatory cells, such as Foxp3+ Treg cells and IL-10 secreting Tr1 cells, which may effect a reduction in inflammation and provide neuroprotection should they gain entry to the CNS. Data from our TERMS study showed statistically significant changes from baseline (p=0.02) in Foxp3+ Treg cells for the subset of Tcelna patients who had ARR >1. The placebo arm for this subset was not statistically different from its baseline levels. Three SPMS patients from prior clinical studies, whose blood samples were analyzed to measure Tr1 cells prior to treatment and post treatment, showed an increase in the levels of Tr1 cells from non-detectable levels to the range of healthy donor samples. These three patients who had relapses in the preceding 12-24 month period remained relapse free during the 52-week assessment period and also showed a 57% to 67% reduction in MRTCs.
Current Treatment Options for SPMS
Only one product, mitoxantrone, is currently approved for the indication of SPMS in the US. However, since 2005, this drug carries a black box warning, due to significant risks of decreased systolic function, heart failure, and leukemia. The American Academy of Neurology has issued a report indicating that these risks are even higher than suggested in the original report leading to the black box warning. Hence, a safe and effective treatment for SPMS remains a significant unmet medical need.
57
Table of Contents
Tcelna Clinical Overview in SPMS
In multiple previously conducted clinical trials for the treatment of patients with MS (which have been weighted significantly toward patients with RRMS), Tcelna has demonstrated one of the safest side effect profiles for any marketed or development-stage MS therapy, as well as encouraging efficacy signals. A total of 144 MS patients have received Tcelna in previously conducted Opexa trials for RRMS and SPMS. The therapy has been well-tolerated in all subjects and has demonstrated an excellent overall safety profile. The most common side effect is mild to moderate irritation at the site of injection, which is typically resolved in 24 hours. Tcelna has been administered to a total of 36 subjects with SPMS across three previous clinical studies.
In a pooled assessment of data from 36 SPMS patients treated in Phase I open label studies at the Baylor College of Medicine completed in 1998 and in Opexa-sponsored studies completed in 2006 and 2007, approximately 80% of the 35 SPMS patients who completed two years of treatment showed disease stabilization as measured by EDSS following two years of treatment with Tcelna, with the other 20% showing signs of progression. This compares to historical control data which showed a progression rate of 40% in SPMS patients (as reported in ESIMS Study published in Hommes Lancet 2004). The 10 SPMS patients in Opexa sponsored studies showed a substantial reduction in ARR at two years from 0.5 to an ARR less than 0.1. Only 1 out of the 10 patients experienced one episode of relapse during the two years of assessment. This same cohort showed no worsening of physical or psychological condition (key quality of life indicators as measured by the MS Impact Scale) after two years of treatment with Tcelna. Additionally, there were no reported serious adverse events (SAEs) in any of the patients. Based on preliminary data suggesting stabilized or improved disability among SPMS subjects receiving Tcelna, we believe that further development of this product candidate in SPMS is warranted.
Abili-T Trial: Phase IIb Clinical Study in Patients with SPMS
In September 2012, we announced the initiation of a Phase IIb clinical trial of Tcelna in patients with SPMS. The trial is entitled: A Phase II Double-Blind, Placebo Controlled Multi-Center Study to Evaluate the Efficacy and Safety of Tcelna in Subjects with Secondary Progressive Multiple Sclerosis and has been named the “Abili-T” trial. The Abili-T trial is a double-blind, 1:1 randomized, placebo-controlled study in SPMS patients who demonstrate evidence of disease progression with or without associated relapses. The trial is expected to enroll 180 patients who have EDSS scores between 3.0 and 6.0 at approximately 30 leading clinical sites in the U.S. and Canada. According to the study protocol, patients will receive two annual courses of Tcelna treatment consisting of five subcutaneous injections per year at weeks 0, 4, 8, 12 and 24.
58
Table of Contents
The primary efficacy endpoint of the trial is the percentage of brain volume change (whole brain atrophy) at 24 months. Study investigators will also measure several important secondary outcomes commonly associated with MS including sustained disease progression measured by EDSS, changes in EDSS, time to sustained progression, ARR, Change in Multiple Sclerosis Functional Composite (MSFC) assessment of disability and Change in Symbol Digit Modality Test. Data on certain exploratory endpoints such as quality of life metrics as measured by the MS Quality of Life Inventory (MSQLI), magnetic resonance imaging (MRI) measures and immune monitoring metrics are also being collected. The annual treatment and efficacy assessment schedule is shown below:
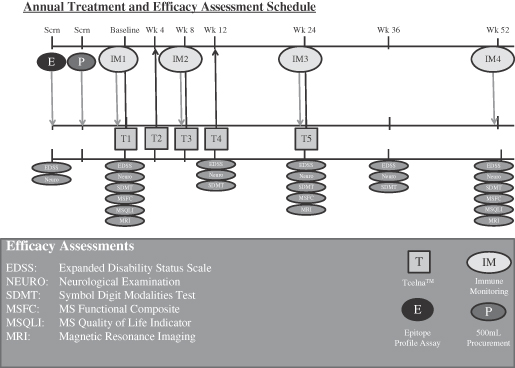
59
Table of Contents
Immune Monitoring Program. As part of the Abili-T trial, we are undertaking a comprehensive immune monitoring program for all patients enrolled in the study. The goals of this program are to further understand the biology behind the mechanism of action for Tcelna and to possibly identify novel biomarkers that are dominant in the pathophysiology of SPMS patients. The program encompasses an analysis of various pro-inflammatory and anti-inflammatory biomarkers. We believe that the blinded data, which will be analyzed during the course of the trial, may signal responders and non-responders. Directional movement of certain biomarkers, when corroborated with final clinical trial data, may be indicative of responders and disease stabilization or progression. It is hypothesized that directional movement higher of the anti-inflammatory biomarkers (listed on the right side in the middle column of the graphic below) may be indicative of clinical efficacy. Similarly, it is hypothesized that a direction movement lower of pro-inflammatory markers (listed on the left side in the middle column of the graphic below) may be indicative of a reduction in inflammation and, consequently, clinical efficacy.

As a prelude to the immune monitoring program, Opexa characterized the status of patients entering the trial at baseline and compared the data sets to those of healthy donors. This baseline data was recently presented at the 2013 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) meeting. The poster showed that SPMS subjects have a reduced frequency of IL-10 secreting TR1 cells and the frequency of nTregs may be reduced in SPMS patients. Although the level of Foxp3 expression in SPMS patients is equivalent to that of healthy donors, the data suggest that SPMS is associated with lower levels of PDL1 and HLA-G expressing monocytes. The impact of Tcelna treatment will be studied over time on a blinded basis as part of the immune monitoring program, and compared to patients receiving placebo post trial completion, to better illustrate the mechanism of action for Tcelna therapy.
As of June 14, 2013, the Abili-T clinical trial has randomized 53 patients. A scheduled Data Safety Monitoring Board meeting took place during the week of May 20, 2013, and a recommendation was made to continue the study. The Abili-T clinical study in North America of Tcelna is expected to complete enrollment of 180 patients by late 2013 or early 2014, with the resulting top-line data expected to be available in the first half of 2016.
During the first quarter of 2013, the pace of on-boarding clinical sites for the Abili-T clinical study was tempered pending the completion of our negotiations with Ares Trading SA (“Merck”), a wholly owned subsidiary of Merck Serono S.A., for the Option (see “—Option and License Agreement with Merck Serono”) as
60
Table of Contents
well as financial considerations. Upon receipt of the upfront payment of $5 million for granting the Option, we were able to refocus on execution of the Abili-T clinical trial, including enrollment. The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of May 31, 2013 to be between $30-$32 million. Our existing resources are not adequate to permit us to complete such study or the majority of it. We anticipate that $ million of the proceeds from the offering will be applied to funding the continuation of the clinical study as well as the ongoing expense of our operations during such development. We will need to secure significant additional resources to complete the trial and support our operations during the pendency of the trial. There can be no assurance that any such financings or potential opportunities and alternatives can be consummated on acceptable terms, if at all. We believe we have sufficient liquidity to support our clinical trial activities into the fourth quarter of 2013. Given our need for substantial amounts of capital to continue the Abili-T clinical study in North America of Tcelna in SPMS, we intend to continue to explore potential opportunities and alternatives to obtain the significant additional resources, including one or more additional financings, that will be necessary to complete the Abili-T study and to support our operations during the pendency of such study.
Option and License Agreement with Merck Serono
On February 4, 2013, we entered into an Option and License Agreement (“Merck Agreement”) with Merck. Pursuant to the Merck Agreement, Merck has an option (the “Option”) to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck prior to or upon completion of our ongoing Abili-T trial of Tcelna in patients with SPMS.
Under the terms of the Merck Agreement, we received an upfront payment of $5 million for granting the Option. If the Option is exercised, Merck would pay us an upfront license fee of $25 million unless Merck is unable to advance directly into a Phase III clinical trial of Tcelna for SPMS without a further Phase II clinical trial (as determined by Merck), in which event the upfront license fee would be $15 million. After exercising the Option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights to use for other indications outside of MS. Based upon the achievement of development milestones by Merck for Tcelna in SPMS, we would be eligible to receive one-time milestone payments totaling up to $70 million as follows: (i) milestone payments aggregating $35 million if Tcelna is submitted for regulatory approval and commercialized in the United States; (ii) milestone payments aggregating $30 million if Tcelna is submitted for regulatory approval in Europe and commercialized in at least three major countries in Europe; and (iii) a milestone payment of $5 million if Tcelna is commercialized in certain markets outside of the United States and Europe. If Merck elects to develop and commercialize Tcelna in RRMS, we would be eligible to receive milestone payments aggregating up to $40 million based upon the achievement by Merck of various development, regulatory and first commercial sale milestones.
If Tcelna receives regulatory approval and is commercialized by Merck, we would be eligible to receive royalties pursuant to a tiered structure at rates ranging from 8% to 15% of annual net sales, with step-ups over such range occurring when annual net sales exceed $500 million, $1 billion and $2 billion. Any royalties would be subject to offset or reduction in various situations, including if third party rights are required or if patent protection is not available in an applicable jurisdiction. We would also be responsible for royalty obligations to certain third parties, such as Baylor College of Medicine from which we originally licensed related technology. If we were to exercise an option to co-fund certain of Merck’s development, the royalty rates payable by Merck would be increased to rates ranging from 10% to 18%. In addition to royalty payments, we would be eligible to receive one-time commercial milestones totaling up to $85 million, with $55 million of such milestones achievable at annual net sales targets in excess of $1 billion.
61
Table of Contents
Other Opportunities
Our proprietary T-cell technology has enabled us to develop intellectual property and a comprehensive sample database that may enable discovery of novel biomarkers associated with MS. Depending upon the outcome of further feasibility analysis, the T-cell platform may have applications in developing treatments for other autoimmune disorders such as rheumatoid arthritis, Type 1 diabetes, and orphan indications such as myasthenia gravis. The primary focus of Opexa remains the development of Tcelna in SPMS.
We have developed (and, in part, licensed from the University of Chicago) a proprietary adult stem cell technology to produce monocyte-derived stem cells (MDSC) from blood. These MDSC can be derived from a patient’s monocytes, expanded ex vivo, and then administered to the same patient. Our initial focus for this technology is the further development of this monocyte-derived stem cell technology as a platform for the in vitro generation of highly specialized cells for potential application in autologous cell therapy for patients with diabetes mellitus. The diabetes program is in an early (pre-clinical) development stage.
Tcelna Manufacturing
We manufacture Tcelna in our own current Good Manufacturing Practice (cGMP) facility. Over 850 Tcelna preparations have been successfully manufactured at our facilities.
Tcelna is a personalized autologous immunotherapy that is not only manufactured for every individual subject but also is tailored to match each subject’s evolving disease profile as defined by T-cell profiling against myelin antigens. In preparing Tcelna, the subject is pre-screened with our proprietary Epitope Profiling Assay (EPA) for immunodominant anti-myelin T-cell responses against specific peptides by assaying peripheral blood mononuclear cell (PBMC) reactivity against 109 peptides tested in pools of six derived from MBP, MOG and PLP. The EPA takes approximately 14 days to conduct and report data.
Using up to six pre-selected peptide pools, the MRTC lines to each pool are expanded to therapeutic levels, mixed and cryopreserved until time for final formulation. The manufacturing and quality control process spans approximately 35 days. Prior to injection, the MRTCs are thawed, formulated and attenuated (by irradiation) to render them unable to replicate but viable for therapy. These attenuated T-cells are administered in a defined schedule of five subcutaneous injections. Patients will be treated with a new, personalized treatment series (five subcutaneous injections) each year based on their altered disease profile or epitope shift and the re-manufacture of a new Tcelna product representing the emerging immunodominant T-cell response to myelin.
If Merck exercises its Option to acquire an exclusive, worldwide license for our Tcelna program for the treatment of MS, we retain certain rights with respect to the manufacture of Tcelna.
Personalized Therapy
The clinical symptoms of MS are the result of an immune attack against the myelin sheaths that insulate nerves in the brain and spinal cord that constitute the CNS. A subset of white cells, called T-cells, is the primary orchestrator of this immunity. Tcelna is an immunotherapy representing an enriched source of the patient’s own MRTCs that are used to invoke a protective response to limit further damage to the myelin sheaths within the patient’s CNS. Immunity to myelin in terms of the specificity of T-cells for myelin proteins varies between individuals. Therefore, Tcelna is further personalized by screening the immune response, and detecting those proteins that are preferentially targeted by T-cells on a per patient basis. This is achieved using protein fragments, called peptides, from the three major myelin proteins (MOG, MBP and PLP) as targets to finely map immunity to myelin. A limited number of peptides are chosen to which immunity appears greatest, and the Tcelna product is manufactured against these peptides. Thus, Tcelna is not only manufactured for each patient, but it is also tailored against each patient’s personalized T-cell immune response to myelin. In preparing Tcelna for a patient, the patient-specific MRTCs are expanded from a unit of whole blood using the selected myelin peptides in the presence of growth factors. Once sufficient numbers of T-cells have been propagated to support
62
Table of Contents
the clinical dosing regimen, they are frozen down as individual Tcelna doses. Prior to clinical use, a frozen Tcelna dose is thawed, formulated, and attenuated (by irradiation) to render the T-cells unable to replicate, but viable for therapy. After quality control and quality assurance, each individual dose is shipped on request overnight to the clinical site for administration over a defined schedule of five subcutaneous injections. Patients will be treated with a new Tcelna series (five subcutaneous injections) each year based on their altered disease profile or epitope shift.
Tcelna Safety and Tolerability
We believe that Tcelna treatment selectively targets, depletes and/or down-regulates the pathogenic T-cell population. It is not a general immune suppressant and, accordingly, it is not associated with the serious side effects seen by those MS treatments that function by systemically suppressing the immune system. In clinical trials conducted to date, there have been no SAEs associated with Tcelna treatment. We believe that this favorable safety profile may be an important advantage as patient compliance represents a significant challenge due to serious side effects associated with many currently available and in development MS treatments.
Licenses, Patents and Proprietary Rights
We believe that proprietary protection of our technologies is critical to the development of our business. We will continue to protect our intellectual property through patents and other appropriate means. We rely upon trade-secret protection for certain confidential and proprietary information and take active measures to control access to that information. We currently have non-disclosure agreements with all of our employees, consultants, vendors, advisory board members and contract research organizations.
The initial T-cell technology on which Tcelna is based was originally discovered by researchers at Baylor College of Medicine in Houston, Texas. Baylor granted Opexa an exclusive, worldwide right and license to commercially exploit such technology, which includes rights to issued patents and pending patent applications owned by Baylor. Opexa has since expanded the development of technology related to Tcelna and T-cell technology and has filed patent applications with respect thereto, from which several patents have issued (including with respect to the specificity and veracity of antigens that have been discovered). There is also substantial proprietary know-how surrounding the Tcelna development and manufacturing processes that remains a trade secret. Consequently, we consider barriers to entry, relative to Tcelna for the treatment of MS, to be high.
Our patent portfolio tracks our scientific development programs in autoimmune disease treatments, with an initial focus on MS. We believe that our scientific platform is adaptable in that any T-cell dependent autoimmune disease with known specific antigens, such as rheumatoid arthritis, may be a candidate for treatment, and we believe that our patent strategy is readily extendable to address these additional indications.
Competition
The development of therapeutic agents for human disease is intensely competitive. Major pharmaceutical companies currently offer a number of pharmaceutical products to treat MS and other diseases for which our technologies may be applicable. Many pharmaceutical and biotechnology companies are investigating new drugs and therapeutic approaches for the same purposes, which may achieve new efficacy profiles, extend the therapeutic window for such products, alter the prognosis of these diseases, or prevent their onset. We believe that our products, when and if successfully developed, will compete with these products principally on the basis of improved and extended efficacy and safety and their overall economic benefit to the health care system. We expect competition to increase. We believe that our most significant competitors will be fully integrated pharmaceutical companies and more established biotechnology companies. Smaller companies may also be significant competitors, particularly through collaborative arrangements with large pharmaceutical or biotechnology companies. Some of our primary competitors in the current treatment of, and in the development of treatments for, MS include Biogen-Idec, Elan, Merck-Serono (which is an affiliate of the entity that holds the Option), Teva, Bayer/Schering AG and Novartis.
63
Table of Contents
Sales and Marketing
If Merck exercises its Option to acquire an exclusive, worldwide license for our Tcelna program for the treatment of MS and pays us an upfront license fee, Merck would be solely responsible for funding future commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights outside of MS. We would consider partnering with large biotech and pharmaceutical companies, if and when applicable, to assist with marketing and sales of an MS T-cell therapy in Japan as well as to assist with marketing and sales in indications beyond MS.
If Merck does not exercise its Option, we may choose to partner with large biotech or other pharmaceutical companies for sales and marketing, if and when applicable, or alternatively develop our own sales force to market our MS cell therapy products in the U.S. Given the concentration of MS treatment among a relatively small number of specialized neurologists in the U.S., we believe that a modest size sales force would be sufficient to market an MS product in the U.S.
Government Regulation
Our research and development activities and the future manufacturing and marketing of our potential products are, and will be, subject to regulation for safety and efficacy by a number of governmental authorities in the U.S. and other countries.
In the U.S., pharmaceuticals, biologicals and medical devices are subject to FDA regulation. The Federal Food, Drug and Cosmetic Act, as amended, and the Public Health Service Act, as amended, the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the testing in human subjects, manufacture, safety, efficacy, labeling, storage, export, record keeping, approval, marketing, advertising and promotion of our potential products. Product development and approval within this regulatory framework take a number of years and involve significant uncertainty combined with the expenditure of substantial resources.
FDA Approval Process
We will need to obtain FDA approval of any therapeutic product we plan to market and sell. The FDA will only grant marketing approval if it determines that a product is both safe and effective. The testing and approval process will require substantial time, effort and expense. The steps required before our products may be marketed in the U.S. include:
Preclinical Laboratory and Animal Tests. Preclinical tests include laboratory evaluation of the product candidate and animal studies in specific disease models to assess the potential safety and efficacy of the product candidate as well as the quality and consistency of the manufacturing process.
Submission to the FDA of an Investigational New Drug Application, or IND, Which Must Become Effective Before U.S. Human Clinical Trials May Commence. The results of the preclinical tests are submitted to the FDA, and the IND becomes effective 30 days following its receipt by the FDA, as long as there are no questions, requests for delay or objections from the FDA. The sponsor of an IND must keep the FDA informed during the duration of clinical studies through required amendments and reports, including adverse event reports.
Adequate and Well-Controlled Human Clinical Trials to Establish the Safety and Efficacy of the Product Candidate. Clinical trials, which test the safety and efficacy of the product candidate in humans, are conducted in accordance with protocols that detail the objectives of the studies, the parameters to be used to monitor safety and the efficacy criteria to be evaluated. Any product candidate administered in a U.S. clinical trial must be manufactured in accordance with cGMP.
The protocol for each clinical study must be approved by an independent Institutional Review Board, or IRB, at the institution at which the study is conducted, and the informed consent of all participants must be
64
Table of Contents
obtained. The IRB will consider, among other things, the existing information on the product candidate, ethical factors, the safety of human subjects, the potential benefits of the therapy and the possible liability of the institution.
Clinical development is traditionally conducted in three sequential phases, which may overlap:
| • | In Phase I, product candidates are typically introduced into healthy human subjects or into selected patient populations (i.e., patients with a serious disease or condition under study, under physician supervision) to test for adverse reactions, dosage tolerance, absorption and distribution, metabolism, excretion and clinical pharmacology. |
| • | Phase II involves studies in a limited population of patients with the disease or condition under study to (i) determine the efficacy of the product candidates for specific targeted indications and populations, (ii) determine optimal dosage and dosage tolerance and (iii) identify possible and common adverse effects and safety risks. (Phase II may divided into Phase IIa and Phase IIb studies to address these issues.) When a dose is chosen and a candidate product is found to have preliminary evidence of effectiveness, and to have an acceptable safety profile in Phase II evaluations, Phase III trials begin. |
| • | Phase III trials are undertaken to develop additional safety and efficacy information from an expanded patient population, generally at multiple study sites. This information obtained is used to develop a better understanding of the risks and benefits of the product candidate and to determine appropriate labeling for use. |
Based on clinical trial progress and results, the FDA may request changes or may require discontinuance of the trials at any time if significant safety issues arise.
Submission to the FDA of Marketing Authorization Applications and FDA Review. The results of the preclinical studies and clinical studies are submitted to the FDA as part of marketing approval authorization applications such as New Drug Applications (NDAs) or Biologics License Applications (BLAs). The FDA will evaluate such applications for the demonstration of safety and effectiveness. A BLA is required for biological products subject to licensure under the Public Health Service Act and must show that the product is safe, pure and potent. In addition to preclinical and clinical data, the BLA must contain other elements such as manufacturing materials, stability data, samples and labeling. FDA approval of a BLA is required prior to commercial sale or shipment of a biologic. A BLA may only be approved once the FDA examines the product and inspects the manufacturing establishment to assure conformity to the BLA and all applicable regulations and standards for biologics.
The time for approval may vary widely depending on the specific product candidate and disease to be treated, and a number of factors, including the risk/benefit profile identified in clinical trials, the availability of alternative treatments, and the severity of the disease. Additional animal studies or clinical trials may be requested during the FDA review period, which might add substantially to the review time.
The FDA’s marketing approval for a product is limited to the treatment of a specific disease or condition in specified populations in certain clinical circumstances, as described on the approved labeling. The approved use is known as the “indication.” After the FDA approves a product for the initial indication, further clinical trials may be required to gain approval for the use of the product for additional indications. The FDA may also require post-marketing testing (Phase IV studies) and surveillance to monitor for adverse effects, which could involve significant expense. The FDA may also elect to grant only conditional approval.
Ongoing Compliance Requirements
Even after product approval, there are a number of ongoing FDA regulatory requirements, including:
| • | Registration and listing; |
65
Table of Contents
| • | Regulatory submissions relating to changes in an NDA or BLA (such as the manufacturing process or labeling) and annual reports; |
| • | Adverse event reporting; |
| • | Compliance with advertising and promotion restrictions that relate to drugs and biologics; and |
| • | Compliance with GMP and biological product standards (subject to FDA inspection of facilities to determine compliance). |
Other Regulations
In addition to safety regulations enforced by the FDA, we are also subject to regulations under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act and other present and potential future foreign, federal, state and local regulations. For instance, product manufacturing establishments located in certain states also may be subject to separate regulatory and licensing requirements.
Outside the U.S., we will be subject to regulations that govern the import of drug products from the U.S. or other manufacturing sites and foreign regulatory requirements governing human clinical trials and marketing approval for products. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursements vary widely from country to country.
Research and Development
Research and development expenses for the year ended December 31, 2012 were approximately $6.3 million, mainly reflecting the costs of preparation, initiation and operation of the Abili-T clinical trial for Tcelna in Multiple Sclerosis. Research and development expenses for the year ended December 31, 2011 were approximately $3.3 million, mainly reflecting the costs of preparation for the Abili-T clinical trial for Tcelna.
Organizational History
We are a development-stage company and have a limited operating history. Our predecessor company for financial reporting purposes was formed on January 22, 2003 to acquire rights to an adult stem cell technology. In November 2004, we acquired Opexa Pharmaceuticals, Inc. and its MS treatment technology. Currently, we remain focused on developing our T-cell technology for MS. To date, we have not generated any commercial revenues from operations. As we continue to execute our business plan, we expect our development and operating expenses to increase.
Employees
As of May 31, 2013, we had 29 full-time employees. We believe that our relations with our employees are good. None of our employees is represented by a union or covered by a collective bargaining agreement.
66
Table of Contents
As of the date of this prospectus, our directors and executive officers are as follows:
| Name |
Age | Position | ||||
| Neil K. Warma |
50 | President, Chief Executive Officer and Director | ||||
| Karthik Radhakrishnan |
42 | Chief Financial Officer | ||||
| Donna R. Rill |
59 | Chief Development Officer | ||||
| David E. Jorden |
50 | Director | ||||
| Gail J. Maderis |
55 | Director | ||||
| Michael S. Richman |
52 | Director | ||||
| Scott B. Seaman |
57 | Director | ||||
Neil K. Warma has served as President and Chief Executive Officer since June 2008 and as a Director since September 2008. He also previously served as our Acting Chief Financial Officer from March 2009 to August 2012. From July 2004 to September 2007, Mr. Warma served as president and chief executive officer of Viron Therapeutics Inc., a privately-held clinical stage biopharmaceutical company. From 2000 to 2003 Mr. Warma was co-founder and president of MedExact USA, Inc., an Internet company providing clinical information and services to physicians and pharmaceutical companies. From 1992 to 2000, Mr. Warma held senior positions of increasing responsibility at Novartis Pharmaceuticals (previously Ciba-Geigy Ltd.) at its corporate headquarters in Basel, Switzerland. While at Novartis, Mr. Warma served as the Head of International Pharma Policy & Advocacy and in senior management within global marketing where he worked on the international launch of a gastrointestinal product. Mr. Warma obtained an honors degree specializing in Neuroscience from the University of Toronto and an International M.B.A. from the Schulich School of Management at York University in Toronto. As our President and Chief Executive Officer, Mr. Warma is directly involved in all aspects of our operations. He has extensive experience in corporate business development within the biopharmaceutical industry, in addition to executive leadership and management experience.
Karthik Radhakrishnan has served as Chief Financial Officer since March 2013. Mr. Radhakrishnan joined Opexa with over 10 years of healthcare capital markets experience and most recently was a Vice President at ING Investment Management in New York. While at ING from 2007 to 2012, he was responsible for healthcare investments in the small & small-mid cap core/growth products that are part of the Fundamental Equity product line. Previously he was the senior analyst at Eagle Asset Management from 2005 to 2007, responsible for large cap growth healthcare. Prior to this, Mr. Radhakrishnan served in various analyst positions including Senior Analyst at The Dow Chemical Company where he worked from 2002 to 2005. Mr. Radhakrishnan served as a member of the Board of Trustees at Cares Foundation, a non-profit organization serving the Congenital Adrenal Hyperplasia community from 2008 to 2011. Mr. Radhakrishnan is a CFA charter holder and has an MBA degree from the University of Michigan, a Masters in Engineering from the State University of New York and a Bachelor’s degree from the Indian Institute of Technology.
Donna R. Rill was appointed as our Chief Development Officer in April 2013 and has served as Senior Vice President of Operations and Quality Systems since January 2009. From November 2004 until January 2009, she served as Vice President of Operations. From April 2003 to November 2004, she was the director of quality systems and process development at Opexa Pharmaceuticals, Inc. From November 1997 to April 2003, she was the director of translational research for the Center for Cell & Gene Therapy at Baylor College of Medicine. Ms. Rill has worked to design and qualify GMP Cell & Gene Therapy Laboratories, GMP Vector Production facilities, and Translational Research Labs at St. Jude Children’s Research Hospital, Texas Children’s Hospital, and Baylor College of Medicine. Ms. Rill received her B.S. in Medical Technology from the University of Tennessee, Memphis.
David E. Jorden has served as a Director since August 2008 and served as our Acting Chief Financial Officer from August 2012 until March 2013. Mr. Jorden has also served as Executive Chairman for Cytomedix, Inc. since February 2012 and previously as an executive board member since October 2008. In June 2013, he was appointed Interim Chief Executive Officer for Nanospectra Biosciences, Inc., a private company focused on
67
Table of Contents
nanoparticle based therapies for tissue ablation. Mr. Jorden previously served as vice president with Morgan Stanley in its Wealth Management group where he was responsible for equity portfolio management for high net worth individuals since 2003. Prior to Morgan Stanley, Mr. Jorden served as vice president and chief financial officer of Genometrix, Inc., a private genomics/life sciences company focused on high-throughput microarray applications from March 2000 to September 2002. Mr. Jorden was a principal with Fayez Sarofim & Co. prior to joining Genometrix. Mr. Jorden earned a MBA from Kellogg School of Management at Northwestern University in 1989 and a BBA from the University of Texas/Austin in 1984. He currently serves as a director of Cytomedix, Inc., PLx Pharma, Inc. and Nanospectra Biosciences, Inc. Mr. Jorden holds Chartered Financial Analyst and Certified Public Accountant designations. He has extensive experience in various aspects of corporate finance and accounting for public companies including capital formation and deployment.
Gail J. Maderis has served as a Director since October 2011. Ms. Maderis has served as President and CEO of BayBio (Bay Area Bioscience Association), an independent, non-profit trade association serving the life sciences industry in Northern California, since October 2009 and joined BayBio’s board in 2004. From July 2003 to June 2009, Ms. Maderis served as President and CEO of FivePrime Therapeutics, Inc., a biotechnology company focused on the discovery and development of innovative protein and antibody drugs, and served as a director until 2010. Prior to that, Ms. Maderis held general management positions at Genzyme Corporation from 1997 to 2003, including founder and president of Genzyme Molecular Oncology, a publicly traded division of Genzyme, and corporate vice president of Genzyme Corporation. Ms. Maderis has served as a director of NovaBay Pharmaceuticals, Inc. since October 2010. Ms. Maderis has been a member of several private company boards, and currently serves on The Mayor’s Biotech Advisory Council of San Francisco, as well as the HBS Healthcare Initiative board. Ms. Maderis received a B.S. degree in business from the University of California at Berkeley and an M.B.A. from Harvard Business School. Ms. Maderis has extensive experience as a senior executive of life sciences companies, giving her valuable operational and industry experience and leadership skills, as well as an extensive network of contacts related to financing, partnering and support services in the biotech industry and visibility into business and policy trends that impact the biopharmaceutical industry.
Michael S. Richman has served as a Director since June 2006. Mr. Richman has served as president and chief executive officer of Amplimmune, Inc. since July 2008. Mr. Richman served as president and chief operating officer of Amplimmune, Inc. from May 2007 to July 2008. From April 2002 to May 2007, Mr. Richman served as executive vice president and chief operating officer of MacroGenics, Inc. Mr. Richman joined MacroGenics, Inc. in 2002 with approximately 20 years’ experience in corporate business development within the biotechnology industry. Mr. Richman served as a director of Cougar Biotechnology from June 2006 to July 2009. Mr. Richman obtained his B.S. in Genetics/Molecular Biology at the University of California at Davis and his MSBA in International Business at San Francisco State University. He has extensive experience in business development and strategic planning for life science companies, as well as executive leadership and management experience.
Scott B. Seaman has served as a Director of since April 2006. Mr. Seaman has served for over five years as (i) the executive director and treasurer of the Albert and Margaret Alkek Foundation of Houston, Texas, a private foundation primarily supporting institutions in the Texas Medical Center in Houston, Texas, (ii) the chief financial officer of Chaswil Ltd., a private family management company, (iii) secretary and treasurer of M & A Properties Inc., a ranching and real estate concern, and (iv) director of Somebody Cares America. In March, 2013, Mr. Seaman was elected a director of Gradalis, Inc., a privately held clinical stage biotechnology company developing cancer-focused immunotherapies. In April 2009, Mr. Seaman became the Managing Member of ICT Development LLC which is the Managing Member of ICT Holdings LLC, an energy services supplier for which he serves as president. From January 2003 to April 2009, Mr. Seaman served as chairman and from July 2004 to April 2009, as president of ICT Management Inc., the general partner of Impact Composite Technology Ltd., a composite industry supplier. From October 2007 to December 2010, Mr. Seaman served on the board of GeneExcel, Inc., a privately held biotechnology company. From May 2004 to December 2010, Mr. Seaman served as a Member of the Investment Committee of Global Hedged Equity Fund LP, a hedge fund. Mr. Seaman received a bachelor’s degree in business administration from Bowling Green State University and is a certified public accountant. Mr. Seaman has extensive experience in overall financial management and corporate development, combined with operational and corporate governance experience.
68
Table of Contents
Executive Officer Compensation
The following table sets forth certain information concerning compensation earned by or paid to certain persons who we refer to as our “Named Executive Officers” for services provided for the fiscal year ended December 31, 2012. Our Named Executive Officers include persons who (i) served as our principal executive officer or acted in a similar capacity during 2012, (ii) were serving at fiscal year-end as our two most highly compensated executive officers, other than the principal executive officer, whose total compensation exceeded $100,000, and (iii) if applicable, up to two additional individuals for whom disclosure would have been provided as a most highly compensated executive officer, but for the fact that the individual was not serving as an executive officer at fiscal year-end.
2012 Summary Compensation Table
| Name and Principal Position |
Year | Salary | Bonus | Options Awards(1) |
All Other Compensation |
Total | ||||||||||||||||||
| Neil K. Warma |
2012 | $ | 396,550 | $ | 50,000 | $ | 688,684 | $ | 100 | $ | 1,135,334 | |||||||||||||
| President, Chief Executive Officer, Acting Chief Financial Officer(2) |
2011 | $ | 385,000 | $ | 50,000 | $ | 115,051 | — | $ | 550,051 | ||||||||||||||
| Donna R. Rill |
2012 | $ | 220,000 | $ | 15,000 | $ | 154,152 | $ | 250 | $ | 389,402 | |||||||||||||
| Senior Vice President of Operations and Quality Systems |
2011 | $ | 200,000 | $ | 15,000 | $ | 38,350 | — | $ | 253,350 | ||||||||||||||
| Jaye L. Thompson, Ph.D.(3) |
2012 | $ | 220,000 | $ | 10,000 | $ | 154,152 | $ | 50 | $ | 384,202 | |||||||||||||
| Senior Vice President of Clinical Development and Regulatory Affairs |
2011 | $ | 200,000 | $ | 15,000 | $ | 38,350 | — | $ | 253,350 | ||||||||||||||
| (1) | Amounts in this column represent the aggregate grant date fair value of awards computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718 (“FASB ASC 718”). Each executive officer was granted two options on January 6, 2012, and the fair value of each was calculated using the Black-Scholes option-pricing model. The first option is based on the achievement of future performance-based, strategic milestone objectives, and the grant date fair value is based upon the probable outcome of the performance conditions. See Note 12 to our financial statements included in this annual report on Form 10-K for assumptions underlying the valuation of equity awards. |
| (2) | Mr. Warma served as Acting Chief Financial officer until August 15, 2012. |
| (3) | Dr. Thompson served as our Senior Vice President of Clinical Development and Regulatory Affairs until March 29, 2013. |
Executive Employment Agreements
Neil K. Warma. We entered into an employment agreement on June 16, 2008 with Neil K. Warma pursuant to which he serves as our President and Chief Executive Officer. Pursuant to the agreement, which automatically renews for 12-month periods, Mr. Warma is currently paid $396,550 per year. In addition, Mr. Warma is entitled to the following: (i) an annual cash bonus of up to 50% of his base salary based upon milestones to be agreed upon; and (ii) a one-time payment of $50,000 cash and 6,250 shares of our common stock to be issued if and when the closing bid price of our common stock equals or exceeds $16.00 for 20 consecutive trading days. In addition, we provide Mr. Warma with our standard benefits and insurance coverage as generally provided to our management, as well as contractual indemnification rights by reason of his service as an officer and employee. If his employment is terminated by the Board without cause, as defined in the agreement, Mr. Warma will be entitled to receive a severance payment equal to 12 months of his base salary plus a payment equal to 30% of base salary in lieu of any potential bonus, in addition any earned but unpaid bonus. In addition, vesting of stock options will accelerate in full. We will also reimburse Mr. Warma for COBRA expenses for a 12-month period, subject to a cap equal to Opexa’s standard contribution to employee health benefits. Upon the effectiveness of a
69
Table of Contents
change in control, as defined in the agreement, Mr. Warma will receive 18 months of salary and COBRA reimbursement and a payment equal to 45% of base salary in lieu of any potential bonus, in addition to any earned but unpaid bonus. In addition, all vesting of options will accelerate in full. Any payment or benefit Mr. Warma might receive upon a change of control which would constitute a “parachute payment” under Section 280G of the Internal Revenue Code will be reduced so as not to trigger excise tax under Section 4999 of such Code. Mr. Warma’s agreement also provides that for a 12-month period following his termination of employment, he will not engage or participate in any competitive business or solicit or recruit any of Opexa’s employees. The severance and change of control benefits are subject to Mr. Warma executing and delivering a general release and waiver of claims in favor of Opexa.
Donna R. Rill. We entered into an amended and restated employment agreement with Donna R. Rill on April 21, 2010 which is effective as of April 1, 2010, pursuant to which Ms. Rill serves as our Senior Vice President of Operations and Quality Systems. This agreement superseded Ms. Rill’s prior agreement. Ms. Rill is currently compensated at the rate of $220,000 per annum and is eligible to receive an annual discretionary bonus of up to 20% of her base salary per 12-month period, based on the achievement of objectives as determined by Opexa’s Board and Chief Executive Officer. In addition, Ms. Rill receives our standard benefits and insurance coverage as generally provided to our management, as well as contractual indemnification rights by reason of her service as an officer and employee. Ms. Rill’s employment may be terminated at any time voluntarily by her or without cause (as defined in the agreement) by the Board. If her employment is terminated by the Board without cause, Ms. Rill will be entitled to receive a severance payment equal to six months of her base salary and vesting for any unvested stock options will accelerate by six additional months. The severance benefits are subject to Ms. Rill having been continuously employed through the termination event, executing and delivering a general release and waiver of claims in favor of Opexa, not being in breach of the employment agreement or Opexa’s proprietary information and inventions agreement, and not engaging in any activity which is competitive with Opexa during the term of the employment agreement or while receiving the severance benefits. The timing of any payments to Ms. Rill under the employment agreement is subject to applicable requirements of Section 409A of the Code and the related Treasury Regulations.
Jaye L. Thompson. Until the termination of her employment on March 29, 2013, Jaye L. Thompson, Ph.D., was employed as our Senior Vice President of Clinical Development and Regulatory Affairs pursuant to an amended and restated employment agreement dated June 27, 2011. This agreement superseded Dr. Thompson’s prior agreement dated November 16, 2009. Dr. Thompson was compensated at the rate of $220,000 per annum and was eligible to receive an annual discretionary bonus of up to 20% of her base salary per 12-month period, based upon the achievement of objectives as determined by Opexa’s Board and Chief Executive Officer. In addition, Dr. Thompson received our standard benefits and insurance coverage as generally provided to our management. The agreement provided that Dr. Thompson’s employment may be terminated at any time voluntarily by her or without cause (as defined in the agreement) by the Board. If her employment was terminated by the Board without cause, Dr. Thompson was entitled to receive a severance payment equal to six months of her base salary. In addition, in the event of a change of control (as defined in the agreement) and Dr. Thompson’s employment is terminated without cause or Dr. Thompson resigned for good reason (as defined in the agreement) within 12 months of such change of control, Dr. Thompson was entitled to receive a severance payment equal to six months of her base salary and all unvested equity awards would immediately vest in full and become exercisable pursuant to their terms. The severance benefits were subject to Dr. Thompson having been continuously employed through the termination event, executing and delivering a general release and waiver of claims in favor of Opexa, not being in material breach of the employment agreement or Opexa’s proprietary information and inventions agreement, and not engaging in any activity which is competitive with Opexa during the term of the employment agreement or while receiving the severance benefits. The timing of any payments to Dr. Thompson under the employment agreement is subject to applicable requirements of Section 409A of the Code and the related Treasury Regulations.
We entered into a termination agreement, waiver, and release with Dr. Thompson as of March 29, 2013, and her employment terminated on such date. On June 7, 2013, we amended and restated the termination agreement,
70
Table of Contents
pursuant to which she received a severance payment in the form of a lump sum payment of $60,000 in satisfaction of any and all severance consideration or other amounts owed to Dr. Thompson through her termination date. In consideration for such severance payment, Dr. Thompson provided us with a general release and waiver of any and all claims. The consulting agreement entered into with Dr. Thompson in connection with her departure was mutually terminated.
2012 Grants of Plan Based Awards
The following table presents information regarding stock options granted during the fiscal year ended December 31, 2012 pursuant to our 2010 Stock Incentive Plan to our Named Executive Officers.
Estimated Future Payouts Under Equity Incentive Plan Awards(1)
| Name |
Grant Date | Threshold | Target | Maximum | All
Other Option Awards: Number of Securities Underlying Options(3) |
Exercise Price of Option Awards |
Grant Date Fair Value of Options |
|||||||||||||||||||||
| Neil K. Warma |
01/06/12 | — | 139,593 | 139,593 | $ | 3.80 | $ | 528,612 | (2) | |||||||||||||||||||
| 01/06/12 | 43,623 | $ | 3.80 | $ | 160,071 | (4) | ||||||||||||||||||||||
| Donna R. Rill |
01/06/12 | — | 31,408 | 31,408 | $ | 3.80 | $ | 118,937 | (2) | |||||||||||||||||||
| 01/06/12 | 9,597 | $ | 3.80 | $ | 35,215 | (4) | ||||||||||||||||||||||
| Jaye L. Thompson |
01/06/12 | — | 31,408 | 31,408 | $ | 3.80 | $ | 118,937 | (2) | |||||||||||||||||||
| 01/06/12 | 9,597 | $ | 3.80 | $ | 35,215 | (4) | ||||||||||||||||||||||
| (1) | The Target and Maximum amounts represent the number of shares of common stock underlying performance-based options that begin vesting, if at all, in two tranches commencing upon achievement of certain key milestone events. So long as the identified key milestones are achieved prior to their respective expiration dates, the applicable portion of the performance options thereupon commence vesting quarterly over a three-year period. Generally, (i) the first tranche of one-third of the performance option shares commences three-year quarterly vesting upon achievement of the first key milestone, which is Opexa initiating a clinical trial for Tcelna in SPMS, and (ii) the second tranche of two-thirds of the performance option shares commences three-year quarterly vesting upon achievement of the second key milestone, which is Opexa entering into a collaboration, partnership or other strategic arrangement involving rights in the United States for Tcelna. The performance options have a term of ten years, but potentially expire in two tranches, with (i) the first tranche to have expired on December 31, 2012 if the first key milestone was not achieved and (ii) the second tranche expiring on June 30, 2013 if the second key milestone is not achieved. On September 12, 2012, the first key milestone objective was met and vesting commenced for the first tranche of one-third of the performance option shares. On February 4, 2013, the second key milestone objective was met and vesting commenced for the second tranche of two-thirds of the performance option shares. |
| (2) | Amount represents the aggregate grant date fair value of the option award computed in accordance with FASB ASC 718. The fair value was calculated using the Black-Scholes option-pricing model, and the grant date fair value is based upon the probable outcome of the performance conditions. See Note 12 to our financial statements included in this annual report on Form 10-K for assumptions underlying the valuation of equity awards. |
| (3) | These options are time-based, have a term of ten years and vest quarterly over a three-year period commencing on the date of grant. |
| (4) | Amount represents the aggregate grant date fair value of the option award computed in accordance with FASB ASC 718. The fair value was calculated using the Black-Scholes option-pricing model. See Note 12 to our financial statements included in this annual report on Form 10-K for assumptions underlying the valuation of equity awards. |
71
Table of Contents
2012 Outstanding Equity Awards at Fiscal Year-End
The following table presents information regarding outstanding equity awards at December 31, 2012 for each of the Named Executive Officers.
| Option Awards | Option Exercise Price |
Option Expiration Date |
||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
||||||||||||||
| Neil K. Warma |
62,500 | — | $ | 4.04 | 06/16/18 | |||||||||||
| 37,500 | — | $ | 0.88 | 01/16/19 | ||||||||||||
| 25,000 | — | $ | 8.20 | 11/30/19 | ||||||||||||
| 10,937 | 7,813 | (1) | $ | 6.24 | 01/04/21 | |||||||||||
| 10,906 | 32,717 | (1) | $ | 3.80 | 01/06/22 | |||||||||||
| 3,635 | 135,958 | (2) | $ | 3.80 | 01/06/22 | |||||||||||
| Donna R. Rill |
1,500 | — | $ | 28.00 | 12/05/15 | |||||||||||
| 5,845 | — | $ | 20.00 | 04/20/16 | ||||||||||||
| 8,000 | — | $ | 21.88 | 06/18/17 | ||||||||||||
| 750 | — | $ | 4.36 | 05/06/18 | ||||||||||||
| 8,250 | — | $ | 4.68 | 06/26/18 | ||||||||||||
| 10,000 | — | $ | 0.88 | 01/16/19 | ||||||||||||
| 2,099 | — | $ | 1.88 | 02/06/19 | ||||||||||||
| 12,500 | — | $ | 8.20 | 11/30/19 | ||||||||||||
| 3,646 | 2,604 | (1) | $ | 6.24 | 01/04/21 | |||||||||||
| 2,399 | 7,198 | (1) | $ | 3.80 | 01/06/22 | |||||||||||
| 872 | 30,536 | (2) | $ | 3.80 | 01/06/22 | |||||||||||
| Jaye L. Thompson |
12,500 | — | $ | 8.20 | 11/30/19 | |||||||||||
| 3,646 | 2,604 | (1) | $ | 6.24 | 01/04/21 | |||||||||||
| 2,399 | 7,198 | (1) | $ | 3.80 | 01/06/22 | |||||||||||
| 872 | 30,536 | (2) | $ | 3.80 | 01/06/22 | |||||||||||
| (1) | The shares vest quarterly over a three-year period from the grant date. |
| (2) | The performance-based options begin vesting, if at all, in two tranches commencing upon achievement of certain key milestone events. So long as the identified key milestones are achieved prior to their respective expiration dates, the applicable portion of the performance options thereupon commence vesting quarterly over a three-year period. Generally, (i) the first tranche of one-third of the performance option shares commences three-year quarterly vesting upon achievement of the first key milestone, which is Opexa initiating a clinical trial for Tcelna in SPMS, and (ii) the second tranche of two-thirds of the performance option shares commences three-year quarterly vesting upon achievement of the second key milestone, which is Opexa entering into a collaboration, partnership or other strategic arrangement involving rights in the United States for Tcelna. The performance options have a term of ten years, but potentially expire in two tranches, with (i) the first tranche to have expired on December 31, 2012 if the first key milestone was not achieved and (ii) the second tranche expiring on June 30, 2013 if the second key milestone is not achieved. On September 12, 2012, the first key milestone objective was met and vesting commenced for the first tranche of one-third of the performance option shares. On February 4, 2013, the second key milestone objective was met and vesting commenced for the second tranche of two-thirds of the performance option shares. |
72
Table of Contents
2012 Director Compensation
The following table presents summary information regarding compensation of the non-employee members of our Board of Directors who served during any part of the fiscal year ended December 31, 2012.
| Name |
Fees Earned or Paid in Cash |
Options Awards(1) |
Total | |||||||||
| David E. Jorden(2) |
$ | 37,500 | (3) | $ | 27,857 | (4)(5) | $ | 65,357 | ||||
| Gail J. Maderis |
— | $ | 27,857 | (4)(5) | $ | 27,857 | ||||||
| Michael S. Richman |
— | $ | 27,857 | (4)(5) | $ | 27,857 | ||||||
| Scott B. Seaman |
— | $ | 27,857 | (4)(5) | $ | 27,857 | ||||||
| (1) | Amounts in this column represent the aggregate grant date fair value of awards computed in accordance with FASB ASC 718. The fair value was calculated using the Black-Scholes option-pricing model. See Note 12 to our financial statements included in this annual report on Form 10-K for assumptions underlying the valuation of equity awards. |
| (2) | Mr. Jorden was appointed as Acting Chief Financial Officer on August 15, 2012. |
| (3) | Compensation for services as chair of the Audit Committee until August 15, 2012. |
| (4) | As compensation for Board services, Messrs. Jorden, Richman and Seaman and Ms. Maderis were issued the following two options on March 1, 2012 to purchase shares of common stock at an exercise price of $3.76 per share, the market value on the date of grant: (i) an option, with a term of ten years, to purchase 2,500 shares, with 50% vesting immediately upon grant and the remaining 50% vesting on March 1, 2013; and (ii) an option, with a term of the earlier of ten years or upon a change of control of Opexa, to purchase 5,150 shares in lieu of cash compensation for services, with 50% vesting immediately upon grant and the remaining 50% vesting on December 31, 2012. |
| (5) | The aggregate number of shares underlying outstanding option awards as of December 31, 2012 was: Mr. Jorden, 46,717 shares; Ms. Maderis, 13,505 shares; Mr. Richman, 43,159 shares; and Mr. Seaman, 45,034 shares. |
Standard Compensation Arrangements
Employee directors do not receive any compensation for services as a member of our Board. We reimburse our directors for travel and lodging expenses in connection with their attendance at Board and committee meetings. As compensation for their services on our Board, in 2012 our non-employee directors were issued options to purchase shares of Opexa common stock in lieu of cash compensation. Each option is granted with an exercise price equal to the fair market value of Opexa’s common stock on the date of grant and is issued either fully vested or with a vesting schedule over a period of time up to one year (or up to two years in the case of an initial grant to a new director). In addition, we paid a quarterly retainer of $15,000 in cash to David E. Jorden while he served as the chair of our Audit Committee until August 15, 2012. As of August 15, 2012, we discontinued payment of a quarterly retainer to the Audit Committee chair.
73
Table of Contents
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of May 31, 2013, the number and percentage of outstanding shares of our common stock beneficially owned by: (a) each person who is known by us to be the beneficial owner of more than 5% of our outstanding shares of common stock; (b) each of our directors; (c) each of our named executive officers; and (d) all current directors and executive officers, as a group. As of May 31, 2013, there were 7,991,559 shares of common stock issued and outstanding. All numbers in the table and the footnotes thereto have been adjusted to reflect the 1-for-4 reverse stock split of our common stock that was implemented December 14, 2012.
Beneficial ownership has been determined in accordance with Rule 13d-3 under the Exchange Act. Under this rule, certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire shares (for example, upon exercise of an option or warrant) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares is deemed to include the amount of shares beneficially owned by such person by reason of such acquisition rights. As a result, the percentage of outstanding shares of any person as shown in the following table does not necessarily reflect the person’s actual voting power at any particular date.
To our knowledge, except as indicated in the footnotes to this table and pursuant to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them.
Beneficial Ownership Table
| Name and Address of Beneficial Owner(1) |
Number of Shares Owned |
Percentage of Class |
||||||
| Beneficial Owners of more than 5%: |
||||||||
| Sabby Management, LLC(2) |
750,000 | (3) | 9.4 | % | ||||
| DLD Family Investments, LLC(4) |
599,985 | (5) | 7.2 | % | ||||
| Alkek & Williams Ventures Ltd.(6) |
589,040 | (7) | 7.1 | % | ||||
| Albert and Margaret Alkek Foundation(8) |
585,543 | (9) | 7.1 | % | ||||
| Charles E. Sheedy(10) |
490,523 | (11) | 6.0 | % | ||||
| Executive Officers and Directors: |
||||||||
| Scott B. Seaman(6) |
656,059 | (12) | 7.8 | % | ||||
| David E. Jorden |
404,919 | (13) | 5.0 | % | ||||
| Neil K. Warma |
200,763 | (14) | 2.5 | % | ||||
| Donna R. Rill |
65,384 | (15) | * | |||||
| Michael S. Richman |
54,231 | (16) | * | |||||
| Jaye L. Thompson(17) |
22,689 | (18) | * | |||||
| Gail J. Maderis |
22,910 | (19) | * | |||||
| All directors and executive officers as a group (7 persons)** |
1,414,683 | (20) | 15.8 | % | ||||
| * | Less than 1% |
| ** | Includes only current directors and officers serving in such capacity as of the date of the table. |
| (1) | Unless otherwise indicated in the footnotes, the mailing address of the beneficial owner is c/o Opexa Therapeutics, Inc., 2635 Technology Forest Boulevard, The Woodlands, Texas 77381. |
| (2) | This information is based on the Schedule 13G filed with the SEC on February 11, 2013, by Sabby Healthcare Volatility Master Fund, Ltd. (“Sabby Healthcare Fund”), Sabby Volatility Warrant Master Fund, Ltd. (“Sabby Warrant Fund”), Sabby Management, LLC (“Sabby Management”) and Hal Mintz. Sabby Management serves as the investment manager of Sabby Healthcare Fund and Sabby Warrant Fund, and |
74
Table of Contents
| Mr. Mintz serves as manager of Sabby Management. Sabby Healthcare Fund has shared voting and investment power over the shares held by it. Sabby Warrant Fund has shared voting and investment power over the shares held by it. Sabby Management and Mr. Mintz have shared voting and investment power of the shares held by Sabby Healthcare Fund and Sabby Warrant Fund. Sabby Management and Mr. Mintz disclaim beneficial ownership over the securities held by Sabby Healthcare Fund and Sabby Warrant Fund except to the extent of their pecuniary interest therein. The mailing address of Sabby Healthcare Fund and Sabby Warrant Fund is c/o Ogier Fiduciary Services (Cayman) Limited, 89 Nexus Way, Camana Bay, Grand Cayman KY1-9007, Cayman Islands. The mailing address of Sabby Management and Mr. Mintz is 10 Mountainview Road, Suite 205, Upper Saddle River, New Jersey 07458. |
| (3) | Consisting of: (i) 500,000 shares of common stock held by Sabby Healthcare Fund; and (ii) 250,000 shares of common stock held by Sabby Warrant Fund. In addition to the shares beneficially owned reported in the table, Sabby Healthcare Fund and Sabby Warrant Fund hold Series L warrants to purchase 250,000 and 125,000 shares, respectively, of common stock that they are contractually prohibited from exercising to the extent the beneficial ownership of Sabby Healthcare Fund or Sabby Warrant Fund, together with their affiliates, would exceed 4.99% of the total number of issued and outstanding shares after such exercise, which beneficial ownership limitation can be increased to 9.99% upon 61 days prior notice to us. |
| (4) | Randa Duncan Williams is the principal of DLD Family and she may be deemed to exercise voting and investment power with respect to such shares. The information in this footnote is primarily based on the Foundation 13D and other information provided to us. The mailing address of the beneficial owner is P.O. Box 4735, Houston, Texas 77210-4735. |
| (5) | Consisting of: (i) 221,126 shares of common stock; (ii) 20,000 shares of common stock underlying Series H warrants; (iii) 160,128 shares of common stock issuable if a 12% convertible secured promissory note is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iv) 175,781 shares of common stock underlying Series I warrants; and (v) 22,950 shares of common stock underlying Series K warrants. |
| (6) | Chaswil, Ltd. is the investment manager of Ventures and holds voting power and investment power with respect to Company securities held by Ventures pursuant to a written agreement. Scott B. Seaman is a principal of Chaswil, Ltd. and has shared voting power and shared investment power over all of the shares of common stock beneficially owned by Ventures. The information in this footnote is primarily based on the Foundation 13D and other information provided to us. The mailing address of the beneficial owner is 1100 Louisiana, Suite 5250, Houston, Texas 77002. |
| (7) | Consisting of: (i) 230,181 shares of common stock; (ii) 160,128 shares of common stock issuable if a 12% convertible secured promissory note is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iii) 175,781 shares of common stock underlying Series I warrants; and (iv) 22,950 shares of common stock underlying Series K warrants. |
| (8) | This information is based on the Schedule 13D/A filed with the SEC on August 23, 2012, by Albert and Margaret Alkek Foundation (“the Foundation”), Alkek & Williams Ventures, Ltd. (“Ventures”), Scott Seaman, DLD Family Investments, LLC (“DLD Family”), and the other reporting persons named therein (“the Foundation 13D”) and other information available to us. The Foundation acts through an investment committee of its board of directors, which includes Mr. Charles Williams, Mr. Daniel Arnold, Mr. Joe Bailey, Mr. Scott Seaman and Ms. Randa Duncan Williams. Mr. Seaman is the executive director of the Foundation and chairman of the investment committee. The investment committee has sole voting and investment power over all of the shares of common stock beneficially owned by the Foundation. However, pursuant to the Foundation 13D, neither the executive director nor any member of the investment committee may act individually to vote or sell shares of common stock held by the Foundation; therefore, the Foundation has concluded that no individual committee member is deemed to beneficially own, within the meaning of Rule 13d-3 of the Exchange Act, any shares of common stock held by the Foundation solely by virtue of the fact that he or she is a member of the investment committee. Additionally, pursuant to the Foundation 13D, the Foundation has concluded that because Mr. Seaman, in his capacity as executive director or chairman of the investment committee, cannot act in such capacity to vote or sell shares of common stock held by the Foundation without the approval of the investment committee, he is not deemed to beneficially own, within the meaning of Rule 13d-3 of the Exchange Act, any shares of common stock |
75
Table of Contents
| held by the Foundation by virtue of his position as executive director or chairman of the investment committee. The mailing address of the beneficial owner is 1100 Louisiana, Suite 5250, Houston, Texas 77002. |
| (9) | Consisting of: (i) 370,228 shares of common stock; (ii) 96,076 shares of common stock issuable if a 12% convertible secured promissory note is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iii) 105,469 shares of common stock underlying Series I warrants; and (iv) 13,770 shares of common stock underlying Series K warrants. Pursuant to the Foundation 13D, the Foundation and other reporting persons named therein may be deemed to constitute a group for purposes of Section 13(d) or Section 13(g) of the Exchange Act. However, the Foundation, Ventures, Chaswil, Ltd. and Mr. Seaman expressly disclaim (i) that, for purposes of Section 13(d) or Section 13(g) of the Exchange Act, they are a member of a group with respect to securities of Opexa held by DLD Family, Mr. Arnold, Mr. Bailey or Ms. Williams and (ii) that they have agreed to act together with DLD Family, Mr. Arnold, Mr. Bailey or Ms. Williams as a group other than as described in the Foundation 13D. Therefore, this does not include the following securities: (i) 221,126 shares of common stock held by DLD Family; (ii) 20,000 shares of common stock underlying Series H warrants held by DLD Family; (iii) 160,128 shares of common stock issuable if a 12% convertible secured promissory note held by DLD Family is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iv) 175,781 shares of common stock underlying Series I warrants held by DLD Family; (v) 22,950 shares of common stock underlying Series K warrants held by DLD Family; (vi) 6,666 shares of common stock held by Mr. Arnold; (vii) 12,500 shares of common stock held by Mr. Bailey; (viii) 230,181 shares of common stock held by Ventures; (ix) 160,128 shares of common stock issuable if a 12% convertible secured promissory note held by Ventures is converted to Series A convertible preferred stock and such stock is then converted into common stock; (x) 175,781 shares of common stock underlying Series I warrants held by Ventures; (xi) 22,950 shares of common stock underlying Series K warrants held by Ventures; (xii) 10,913 shares of common stock held by Mr. Seaman; and (xiii) 56,106 shares of common stock underlying currently exercisable stock options held by Mr. Seaman. |
| (10) | Charles E. Sheedy exercises sole voting and dispositive power over all of the shares of common stock beneficially owned. The information in this footnote is primarily based on information reported on the Schedule 13G/A filed with the SEC on February 14, 2013 by Charles E. Sheedy and other information available to us. The mailing address of the beneficial owner is Two Houston Center, 909 Fannin Street, Suite 2907, Houston, Texas 77010. |
| (11) | Consisting of: (i) 259,594 shares of common stock; (ii) 14,000 shares of common stock underlying Series H warrants; (iii) 80,064 shares of common stock issuable if a 12% convertible secured promissory note is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iv) 87,890 shares of common stock underlying Series I warrants; (v) 37,500 shares of common stock underlying Series J warrants; and (vi) 11,475 shares of common stock underlying Series K warrants. |
| (12) | Consisting of: (i) 230,181 shares of common stock held by Ventures; (ii) 160,128 shares of common stock issuable if a 12% convertible secured promissory note held by Ventures is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iii) 175,781 shares of common stock underlying Series I warrants held by Ventures; (iv) 22,950 shares of common stock underlying Series K warrants held by Ventures; (v) 56,106 shares underlying currently exercisable stock options held by Mr. Seaman; and (vi) 10,913 shares of common stock held by Mr. Seaman. (See footnotes 6 and 7 for additional discussion of the information set forth in clauses (i) through (iv) of the preceding sentence.) Pursuant to the Foundation 13D, this does not include the following shares which Mr. Seaman has determined he does not have beneficial ownership of or has disclaimed beneficial ownership: (i) 370,228 shares of common stock held by the Foundation; (ii) 96,076 shares of common stock issuable if a 12% convertible secured promissory note held by the Foundation is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iii) 105,469 shares of common stock underlying Series I warrants held by the Foundation; and (iv) 13,770 shares of common stock underlying Series K warrants held by the Foundation. (See footnotes 8 and 9 for additional discussion of the information set forth in clauses (i) through (iv) of the preceding sentence.) The mailing address of the beneficial owner is 1100 Louisiana, Suite 5250, Houston, Texas 77002. |
76
Table of Contents
| (13) | Consisting of: (i) 227,094 shares of common stock; (ii) 36,829 shares of common stock issuable if a 12% convertible secured promissory note is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iii) 40,429 shares of common stock underlying Series I warrants; (iv) 37,500 shares of common stock underlying the Series J warrants; (v) 5,278 shares of common stock underlying the Series K warrants; and (vi) 57,789 shares of common stock underlying currently exercisable stock options. |
| (14) | Consisting of: (i) 8,658 shares of common stock; (ii) 4,803 shares of common stock issuable if a 12% convertible secured promissory note is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iii) 5,273 shares of common stock underlying Series I warrants; (iv) 688 shares of common stock underlying Series K warrants; and (v) 181,341 shares of common stock underlying currently exercisable stock options. |
| (15) | Consisting of: (i) 402 shares of common stock and (ii) 64,982 shares of common stock underlying currently exercisable stock options. |
| (16) | Consisting of: 54,231 shares of common stock underlying currently exercisable stock options. |
| (17) | Dr. Thompson’s employment as an executive officer terminated on March 29, 2013. |
| (18) | Consisting of: (i) 1,078 shares of common stock and (ii) 21,611 shares of common stock underlying currently exercisable stock options. |
| (19) | Consisting of: 22,910 shares of common stock underlying currently exercisable stock options. |
| (20) | Consisting of: (a) the following held by Mr. Seaman or for which Mr. Seaman may be deemed to have voting and investment power: (i) 230,181 shares of common stock held by Ventures; (ii) 160,128 shares of common stock issuable if a 12% convertible secured promissory note held by Ventures is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iii) 175,781 shares of common stock underlying Series I warrants held by Ventures; (iv) 22,950 shares of common stock underlying Series K warrants held by Ventures; (v) 56,106 shares underlying currently exercisable stock options held by Mr. Seaman; and (vi) and 10,913 shares of common stock held by Mr. Seaman; (b) the following held by Mr. Jorden: (i) 227,094 shares of common stock; (ii) 36,829 shares of common stock issuable if a 12% convertible secured promissory note is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iii) 40,429 shares of common stock underlying Series I warrants; (iv) 37,500 shares of common stock underlying the Series J warrants; (v) 5,278 shares of common stock underlying the Series K warrants; and (vi) 57,789 shares of common stock underlying currently exercisable stock options; (c) the following held by Mr. Warma: (i) 8,658 shares of common stock; (ii) 4,803 shares of common stock issuable if a 12% convertible secured promissory note is converted to Series A convertible preferred stock and such stock is then converted into common stock; (iii) 5,273 shares of common stock underlying Series I warrants; (iv) 688 shares of common stock underlying Series K warrants; and (v) 181,341 shares of common stock underlying currently exercisable stock options; (d) 402 shares of common stock and 64,982 shares of common stock underlying currently exercisable stock options held by Ms. Rill; (e) 54,231 shares of common stock underlying currently exercisable stock options held by Mr. Richman; (f) 22,910 shares of common stock underlying currently exercisable stock options held by Ms. Maderis; and (g) 10,417 shares of common stock underlying currently exercisable stock options held by Karthik Radhakrishnan, our Chief Financial Officer. |
77
Table of Contents
TRANSACTIONS WITH RELATED PERSONS
Since January 1, 2010, we have engaged in the following reportable transactions with our directors, executive officers, beneficial holders of more than 5% of our voting securities, and affiliates or their immediately family members. We believe that all of these transactions were on terms as favorable as could have been obtained from unrelated third parties.
Entities affiliated with director Scott B. Seaman invested an aggregate of $1.3 million principal amount in our 12% convertible secured promissory notes which were issued in a private offering of notes and warrants to purchase shares of our common stock pursuant to a Note Purchase Agreement dated July 25, 2012. An aggregate of $4.085 million in principal amount of notes was originally issued in the offering, of which $3.185 million is currently outstanding and $900,000 was converted into shares of Series A convertible preferred stock and such shares were then converted into 288,229 shares of common stock. The notes mature on July 25, 2014 and accrue interest at the rate of 12% per annum, compounded annually. Interest is payable semi-annually on June 30 and December 31, commencing December 31, 2012, in either cash or registered shares of our common stock at our election. The notes are secured by substantially all of our tangible and intangible assets, and $500,000 of the proceeds is presently held in a controlled account. Alkek & Williams Ventures Ltd. acts as the collateral agent for the noteholders. The notes are convertible into a new class of non-voting Series A convertible preferred stock, subject to certain limitations and adjustments, which is ultimately convertible into common stock. The warrants have an exercise price of $2.56 per share, a five-year term and are exercisable for 112.5% of the number of shares of common stock into which the notes are ultimately convertible, subject to certain limitations and adjustments. The investors have certain registration rights relating to the shares of common stock underlying the Series A convertible preferred stock and the warrants.
Convertible secured notes and warrants to purchase common stock were issued to investors affiliated with Mr. Seaman in the following amounts:
| Principal Amount of Note |
Number of Shares Subject to Warrant |
|||||||
| Alkek & Williams Ventures Ltd. |
$ | 500,000 | 175,781 | |||||
| Albert and Margaret Alkek Foundation |
$ | 300,000 | 105,469 | |||||
| DLD Family Investments, LLC |
$ | 500,000 | 175,781 | |||||
See footnotes 4, 6 and 8 to the “Beneficial Ownership Table” for a description of the relationship between and among Mr. Seaman and each of these investors, each of whom is also a beneficial owner of more than 5% of our outstanding common stock.
Directors and executive officers David E. Jorden and Neil K. Warma also participated in the note offering and invested $115,000 and $15,000, respectively, and were issued warrants to purchase 40,429 and 5,273 shares, respectively.
While the Audit Committee of our Board of Directors is generally responsible for oversight and review of any related person transactions, an independent special committee of our Board reviewed and negotiated the terms of the convertible secured note offering and recommended that the offering be approved on behalf of Opexa and our Board of Directors.
We issued an aggregate of 163,224 shares of common stock to holders of the July 2012 notes in payment of accrued interest on December 31, 2012, of which entities affiliated with Mr. Seaman were issued an aggregate of 57,418 shares. Mr. Jorden and Mr. Warma were issued 5,080 and 663 shares, respectively.
Mr. Jorden and Charles E. Sheedy, who is the beneficial owner of more than 5% of our outstanding common stock, participated in a private offering on January 23, 2013 with certain other accredited investors who
78
Table of Contents
purchased an aggregate of $650,000 in principal amount of our unsecured convertible promissory notes and warrants to purchase shares of our common stock. Messrs. Jorden and Sheedy each invested $100,000 in the offering and each received a warrant to purchase 37,500 shares of common stock. The notes were originally scheduled to mature on January 23, 2014 and accrued interest at the rate of 12% per annum, compounded annually. Interest was payable quarterly beginning March 31, 2013 in cash. The notes were convertible into common stock at the option of the investors at a price of $1.298125 per share, subject to certain limitations. Fifty percent of the initial principal amount (less any amount of such principal that has otherwise been prepaid or converted) was payable by us five business days following our receipt of an aggregate of at least $5 million in proceeds from the sale of our equity securities and/or as payments from one or more partners or potential partners in return for granting a license, other rights, or an option to license or otherwise acquire rights with respect to Tcelna. The remaining principal was payable five business days following our receipt of an aggregate of at least $7.5 million in proceeds from the sale of our equity securities and/or as payments from one or more partners or potential partners in return for granting a license, other rights, or an option to license or otherwise acquire rights with respect to Tcelna. Upon receipt of the upfront payment of $5 million from Merck in February 2013, we repaid $550,000 principal amount of the notes plus accrued interest and converted the remaining $100,000 principal amount into shares of our common stock pursuant to an investor’s election to convert into equity. The warrants have an exercise price of $1.24 per share, a five-year term and are exercisable for a maximum of an aggregate of 243,750 shares of common stock, subject to certain limitations.
Pursuant to a waiver executed by the holders of in excess of two-thirds of the principal amount of the outstanding July 2012 notes and accepted by Opexa, the amount of the cash subject to the deposit control agreement was reduced to $500,000 on January 29, 2013. In exchange for such waiver, we issued warrants to the holders of the July 2012 notes to purchase an aggregate of 187,500 shares of our common stock. The warrants have an exercise price of $1.21 per share and a five-year term. Entities affiliated with Mr. Seaman were issued warrants to purchase an aggregate of 59,670 shares, and Messrs. Jorden and Warma were issued warrants to purchase 5,278 and 688 shares, respectively.
79
Table of Contents
This section describes the general terms and provisions of the shares of our common stock, $0.01 par value. This description is only a summary and is qualified in its entirety by reference to the description of our common stock included in our restated certificate of formation and our amended and restated bylaws which have been filed as exhibits to the registration statement of which this prospectus is a part. You should read our restated certificate of formation and our amended and restated bylaws for additional information before you buy any of our common stock or other securities. See “Where You Can Find More Information.”
We have 100,000,000 shares of authorized common stock. As of May 31, 2013, there were 7,991,559 shares of common stock issued and outstanding. Each holder of common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of shareholders. We have not provided for cumulative voting for the election of directors in our restated certificate of formation. This means that the holders of a majority of the shares voted can elect all of the directors then standing for election. Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of our common stock are entitled to receive dividends out of assets legally available at the times and in the amounts that our board of directors may determine from time to time. Upon our liquidation, dissolution or winding-up, the holders of common stock are entitled to share ratably in all assets remaining after payment of all liabilities and the liquidation preferences of any outstanding preferred stock. Holders of common stock have no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are fully paid and nonassessable, and the shares of common stock offered, when issued, will be fully paid and nonassessable.
Certain Provisions of our Charter and Bylaws
Certain provisions of our restated certificate of formation and our amended and restated bylaws described below may have the effect of delaying, deferring or discouraging another party from acquiring control of us. Our restated certificate of formation and amended and restated bylaws provided that:
| • | Our board of directors is authorized to issue preferred stock without shareholder approval; and |
| • | We will indemnify officers and directors against losses that they may incur in investigations and legal proceedings resulting from their services to us, which may include services in connection with takeover defense measures. |
Transfer Agent
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company, 17 Battery Place, New York, New York 10004.
80
Table of Contents
Aegis Capital Corp. is acting as the sole manager of the offering and as representative of the underwriters, or the Representative. We have entered into an underwriting agreement dated , 2013 with the Representative. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to each underwriter named below and each underwriter named below has severally agreed to purchase, at the public offering price less the underwriting discount set forth on the cover page of this prospectus, the number of shares of common stock listed next to its name in the following table:
| Name of Underwriter |
Number of Shares | |
| Aegis Capital Corp. |
||
|
| ||
| Total |
||
|
|
The underwriters are committed to purchase all the shares of common stock offered by us other than those covered by the option to purchase additional shares described below, if they purchase any shares. The obligations of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriters of officers’ certificates and legal opinions.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions specified in the underwriting agreement. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
We have granted the underwriters an over-allotment option. This option, which is exercisable for up to 30 days after the date of this prospectus, permits the underwriters to purchase a maximum of additional shares (15% of the shares sold in this offering) from us to cover over-allotments, if any. If the underwriters exercise all or part of this option, they will purchase shares covered by the option at the public offering price per share that appears on the cover page of this prospectus, less the underwriting discount. If this option is exercised in full, the total price to the public will be $ and the total net proceeds, before expenses, to us will be $ .
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect thereof.
Discount
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Share | Total Without Over-Allotment Option |
Total With Over-Allotment Option |
||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discount |
$ | $ | $ | |||||||||
| Proceeds, before expenses, to us |
$ | $ | $ | |||||||||
The underwriters propose to offer the shares offered by us to the public at the public offering price set forth on the cover of this prospectus. In addition, the underwriters may offer some of the shares to other securities dealers at such price less a concession of $ per share. If all of the shares offered by us are not sold at the
81
Table of Contents
public offering price, the Representative may change the offering price and other selling terms by means of a supplement to this prospectus.
We have paid an expense deposit of $10,000 to the Representative, which will be applied against the non-accountable expense allowance of $50,000 that will be paid by us to the Representative in connection with this offering; provided that if less than $10 million in gross proceeds is raised in the offering, the non-accountable expense allowance will be reduced to $20,000.
We have also agreed to pay the Representative’s expenses relating to the offering, including (a) all fees, expenses and disbursements relating to background checks of our officers and directors in an amount not to exceed $5,000 in the aggregate (unless we raise a minimum of $10 million in gross proceeds, and in such event, plus 50% of the additional documented expenses for background checks subject to a maximum addition of $5,000); (b) all fees and disbursements of counsel for the underwriters incurred in clearing this offering with FINRA; (c) all fees, expenses and disbursements relating to the registration, qualification or exemption of securities offered under the securities laws of such states and other jurisdictions designated by the Representative, up to a maximum of $5,000; (d) the cost of all mailing and printing of underwriting documents; (e) upon successfully completing this offering, $21,775 for the underwriters’ use of certain software for this offering; and (f) upon successfully completing this offering, up to $20,000 of the representative’s actual accountable road show expenses for the offering.
We estimate that the total expenses of the offering payable by us, excluding the total underwriting discount, will be approximately $ .
Discretionary Accounts
The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreements
Pursuant to certain “lock-up” agreements, we, our directors and officers and certain holders of 5% or more of our outstanding shares of common stock have agreed, for a period of three months from the date of effectiveness of the offering, not to (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, encumber, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of our securities or any securities convertible into or exercisable or exchangeable for shares of our common stock owned or acquired on or prior to the closing date of this offering (including any shares of common stock acquired after the closing date of this offering upon the conversion, exercise or exchange of such securities); (2) file or caused to be filed any registration statement relating to the offering of any shares of our capital stock; or (3) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock, whether any such transaction described in clause (1), (2) or (3) above is to be settled by delivery of common stock or such other securities, in cash or otherwise, except for certain exceptions and limitations.
The above restrictions do not apply to (i) shares sold in this offering, (ii) the issuance of shares of capital stock upon the exercise of stock options or warrants or the conversion of any outstanding security or in payment of accrued interest on our 12% convertible secured promissory notes issued in July 2012, (iii) the issuance of stock options, restricted stock or other equity-based compensation awards under any employee benefit or equity incentive plan, (iv) the filing of a registration statement on Form S-8, (v) securities issued in connection with a transaction that includes a commercial relationship (including but not limited to joint ventures, marketing or distribution arrangements, option or collaboration agreements or intellectual property license agreements) or any acquisition of assets or not less than a majority or controlling portion of the equity of another entity; provided that the aggregate number of shares or securities issued pursuant to clause (v) does not exceed 10% of the total
82
Table of Contents
number of outstanding shares of common stock immediately following the issuance and sale of the shares in this offering, and (vi) the issuance of shares of common stock under our existing at-the-market and equity line programs prior to the registration statement for this offering being declared effective.
Right of First Refusal
Subject to certain limited exceptions, until six months from the closing date of this offering, the Representative has a right of first refusal to act as sole book-running manager for any public or private equity or public debt offerings in which we or any of our successors or subsidiaries may engage during that period.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representative may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Stabilization
In connection with this offering, the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate-covering transactions, penalty bids and purchases to cover positions created by short sales.
| • | Stabilizing transactions permit bids to purchase securities so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the securities while the offering is in progress. |
| • | Over-allotment transactions involve sales by the underwriters of securities in excess of the number of securities the underwriters are obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of securities over-allotted by the underwriters is not greater than the number of securities that they may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over-allotment option. The underwriters may close out any short position by exercising their over-allotment option and/or purchasing securities in the open market. |
| • | Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of securities to close out the short position, the underwriters will consider, among other things, the price of securities available for purchase in the open market as compared with the price at which they may purchase securities through exercise of the over-allotment option. If the underwriters sell more securities than could be covered by exercise of the over-allotment option and, therefore, have a naked short position, the position can be closed out only by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that after pricing there could be downward pressure on the price of the securities in the open market that could adversely affect investors who purchase in the offering. |
| • | Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the s securities originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions. |
83
Table of Contents
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our securities or preventing or retarding a decline in the market price of our securities. As a result, the price of our securities in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our securities. These transactions may be effected on The NASDAQ Capital Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive market making
In connection with this offering, underwriters and selling group members may engage in passive market making transactions in our common stock on The NASDAQ Capital Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the shares and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
Other Relationships
Certain of the underwriters and their affiliates may in the future provide various investment banking and other financial services for us and our affiliates for which they may in the future receive customary fees.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer for the offeree under this prospectus.
84
Table of Contents
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area—Belgium, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of common stock will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of common stock has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
| (a) | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| (b) | to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statement); |
| (c) | to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)I of the Prospectus Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or |
| (d) | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of common stock shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive. |
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
85
Table of Contents
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority, or the ISA, nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società e la Borsa, “CONSOB”) pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| • | to Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
| • | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
| • | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
| • | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
86
Table of Contents
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissão do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority.
This document is personal to the recipient only and not for general circulation in Switzerland.
87
Table of Contents
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA.
This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to us.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
NASDAQ Capital Market Listing
Our common stock is listed on the NASDAQ Capital Market under the symbol “OPXA.”
88
Table of Contents
The financial statements of Opexa as of December 31, 2012, and for the years ended December 31, 2012 and 2011 included in this prospectus have been so included in reliance on the report of MaloneBailey, LLP, an independent registered public accounting firm, dated March 28, 2013, appearing elsewhere in this prospectus, given upon such firm’s authority as experts in auditing and accounting.
The validity of any securities offered by this prospectus will be passed upon for us by Pillsbury Winthrop Shaw Pittman LLP, San Diego, California. Certain legal matters in connection with this offering will be passed upon for the underwriters by Kramer Levin Naftalis & Frankel LLP, New York, New York.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 with the SEC under the Securities Act of 1933, as amended. This prospectus is part of the registration statement but the registration statement includes additional information and exhibits. We file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy the registration statement and any document we file with the SEC at the public reference room maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a web site that contains reports, proxy and information statements and other information regarding companies, such as ours, that file documents electronically with the SEC. The website address is www.sec.gov. The information on the SEC’s website is not part of this prospectus, and any references to this website or any other website are inactive textual references only.
| AEs |
Adverse events. |
| Antigen presenting cells |
A cell that displays antigenic epitopes within major histocompatibility complexes on their surfaces. T-cells may recognize these complexes using their T-cell receptors. These cells process antigens and present them to T-cells. |
| ARR |
Annualized relapse rate. |
| Attenuation |
Process of irradiation that renders cells replication incompetent but viable for a short period of time. |
| Axon |
The long projection of nerve cells. |
| Biomarker |
Is a molecule that is present (or absent) from a particular cellular type. This facilitates the characterization of a cell type and their identification. A biomarker can be used to identify a cell population, make a diagnostic, measure the progress of disease or the effects of treatment. |
| BLA |
Biologics license application. |
89
Table of Contents
| Blood brain barrier |
Is a separation of circulating blood from the brain extracellular fluid in the central nervous system. |
| cGMP |
Current good manufacturing practice. |
| CNS |
Central nervous system. |
| Cryopreserved |
Storage of cells in a frozen state in liquid nitrogen. |
| Cytokines |
Are small signaling molecules used for cell signaling. Cytokines can be classified as proteins, peptides, or glycoproteins. |
| Demyelination |
Destruction of myelin sheath. |
| Dose |
Number of attenuated MRTCs per vial. |
| EDSS |
Expanded disability status scale. |
| EPA |
An Epitope Profiling Essay is a proprietary assay developed by Opexa to identify the patient specific antigens that might be responsible for triggering the myelin sheath destruction pathway. |
| Epitope |
Also known as antigenic determinant is the part of an antigen that is recognized by the immune system, specifically by antibodies, B-cells, or T-cells. |
| FDA |
U.S. Food and Drug Administration. |
| Immunotherapy |
Is a medical term defined as the “treatment of disease by inducing, enhancing, or suppressing an immune response.” |
| Lymphocytes |
A kind of white blood cell in the vertebrate immune system, specifically, landmark of the adaptive immune system. Lymphocytes can be divided into large lymphocytes and small lymphocytes. Large granular lymphocytes include natural killer cells (NK cells). Small lymphocytes consist of T-cells and B-cells. |
| MBP |
Myelin basic protein is a protein believed to be important in the process of myelination of nerves in the nervous system. MBP maintain the correct structure of myelin, interacting with the lipids in the myelin membrane. |
| MDSC |
Monocyte-derived stem cells. |
| Microglial cells |
Microglia are a type of glial cell that are the resident macrophages of the brain and spinal cord, and thus act as the first and main form of active immune defense in the central nervous system. |
| MS |
Multiple sclerosis is a disease that involves an immune system attack against the central nervous system (brain, spinal cord, and optic nerves). The disease is thought to be triggered in a genetically susceptible individual by a combination of one or more environmental factors. The day-to-day actions of humans are facilitated by neurons as they send electrical impulses at very high speeds to different parts of the body. The ability of the neurons to function properly is determined by the fatty tissue insulation surrounding the axons known |
90
Table of Contents
| as myelin or myelin sheath. In the case of multiple sclerosis, the body’s own immune system attacks the myelin sheath. When this happens the electrical impulses from the brain could be slowed down, distorted or stopped resulting in weakness, lack of coordination, fatigue, vision problems, numbness or paralysis. The diagnosis of multiple sclerosis is usually through an MRI scan which helps identify any scar tissue in the brain or the de-myelinated neurons. |
| MOG |
Myelin oligodendrocyte glycoprotein is a glycoprotein believed to be important in the process of myelinization of nerves in the central nervous system. In humans this protein is encoded by the MOG gene. It is speculated to serve as a necessary “adhesion molecule” to provide structural integrity to the myelin sheath and is known to develop late on the oligodendrocyte. |
| MRTCs |
Myelin-reactive T-cells are a subset of T-cells that are associated with the destruction of the myelin sheath. |
| MSFC |
Multiple sclerosis functional composite. |
| MSIS |
Multiple sclerosis impact scale. |
| MSQLI |
Multiple sclerosis quality of life inventory. |
| Myelin or myelin sheath |
The ability of the neurons to function properly is determined by the fatty tissue insulation surrounding the axons known as myelin or myelin sheath. |
| NDA |
New drug application. |
| nTreg cells |
Natural Treg cells with high-avidity interactions in the thymus whose function is believed to prevent autoimmunity and raise the activation threshold for all immune responses. |
| Oligodendroglial cells |
Are a type of brain cell whose main functions are to provide support and to insulate the axons in the central nervous system. |
| PBMC |
Peripheral blood mononuclear cell. |
| Peptide |
Short chains of amino acids linked by amide bonds. |
| PLP |
In a myelin sheath, as the layers of myelin wraps come together, proteolipid protein will bind itself and tightly hold the cellular membranes together. |
| Precision ImmunotherapyTM |
A trademark owned by Opexa. |
| Primary Progressive MS |
This disease course is characterized by slowly worsening neurologic function from the beginning—with no distinct relapses or remissions. The rate of progression may vary over time, with occasional plateaus and temporary minor improvements. Approximately 10% of people are diagnosed with primary-progressive MS. |
91
Table of Contents
| Regulatory T-Cells |
Treg cells are a sub population of T cells which modulate the immune system. Animal models have suggested that modulation of Tregs can treat autoimmne diseases. |
| Remyelination |
Remyelination is the process propagating oligodendrocyte precursor cells to form oligodendrocytes to create new myelin sheaths on demyelinated axons in the CNS. This is a process naturally regulated in the body and tends to be very efficient in a healthy CNS. |
| RRMS |
Relapsing remitting multiple sclerosis. People with this type of MS experience clearly defined attacks of worsening neurologic function. These attacks—which are called relapses, flare-ups, or exacerbations—are followed by partial or complete recovery periods (remissions), during which no disease progression occurs. Approximately 85% of people are initially diagnosed with relapsing-remitting MS. |
| SAEs |
Serious adverse events. |
| SPMS |
Secondary progressive multiple sclerosis. Following an initial period of relapsing-remitting MS, many people develop a secondary-progressive disease course in which the disease worsens more steadily, with or without occasional flare-ups, minor recoveries (remissions), or plateaus. Before the disease-modifying medications became available, approximately 50% of people with relapsing-remitting MS developed this form of the disease within 10 years. |
| T-cells or T-lymphocytes |
Are a type of lymphocytes (itself a type of white blood cells) that play a central role in cell-mediated immunity. They can be distinguished from other lymphocytes, such as B-cells and natural killer cells (NK-cells), by the presence of a T-cell receptor on the cell surface. They are called T-cells because they mature in the thymus. There are several subsets of T-cells, each with a distinct function. |
| TcelnaTM |
Imilecleucel-T, formerly known as Tovaxin®. |
| TERMS |
Tovaxin for Early Relapsing Multiple Sclerosis was a Phase IIb clinical study of Tcelna in relapsing remitting multiple sclerosis patients completed in 2008. |
| Whole brain atrophy |
Brain atrophy is a condition in which cells in the brain are lost, or the connections between them are damaged. In the brain, losing neurons is highly undesirable, as loss of brain tissue can cause a variety of neurological and cognitive problems. Patients with brain atrophy may develop seizures, dementia, and aphasias. In focal cerebral atrophy, the damage is concentrated on a particular area of the brain, which means that the functions of that area of the brain can become impaired. Generalized brain atrophy involves the whole brain, and may be associated with a range of problems. |
Sources: National MS Society, Wikipedia, Opexa documents
92
Table of Contents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
OPEXA THERAPEUTICS, INC.
| PAGES | ||||
| Audited Financial Statements for years ended December 31, 2012 and 2011 and the period from January 22, 2003 (Inception) through December 31, 2012 |
||||
| F-2 | ||||
| Consolidated Balance Sheets as of December 31, 2012 and 2011 |
F-3 | |||
| F-4 | ||||
| F-5 | ||||
| F-7 | ||||
| F-8 | ||||
| Unaudited Financial Statements as of and for the three months ended March 31, 2013 and 2012 |
||||
| Unaudited Consolidated Balance Sheets as of March 31, 2013 and December 31, 2012 |
F-24 | |||
| F-25 | ||||
| F-26 | ||||
| F-27 | ||||
| F-29 | ||||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
Opexa Therapeutics, Inc.
(a development stage company)
The Woodlands, Texas
We have audited the accompanying consolidated balance sheets of Opexa Therapeutics, Inc., (a development stage company), as of December 31, 2012 and 2011 and the related consolidated statements of expenses, changes in stockholders’ equity and cash flows for each of the years then ended and for the period from January 22, 2003 (Inception) through December 31, 2012. These financial statements are the responsibility of Opexa’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatements. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Opexa as of December 31, 2012 and 2011 and the results of its operations and its cash flows for each of the years then ended and for the period from January 22, 2003 (Inception) through December 31, 2012 in conformity with accounting principles generally accepted in the United States of America.
/s/ MALONEBAILEY, LLP
www.malonebailey.com
Houston, Texas
March 28, 2013
F-2
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
| December 31, 2012 |
December 31, 2011 |
|||||||
| Assets | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 592,004 | $ | 7,109,215 | ||||
| Other current assets |
1,077,546 | 124,773 | ||||||
|
|
|
|
|
|||||
| Total current assets |
1,669,550 | 7,233,988 | ||||||
| Property & equipment, net of accumulated depreciation of $1,494,510 and $1,193,601, respectively |
1,265,041 | 1,029,236 | ||||||
| Restricted cash |
1,000,000 | — | ||||||
| Deferred financing costs, net of amortization of $58,639 and $0, respectively |
211,479 | — | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 4,146,070 | $ | 8,263,224 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 412,096 | $ | 491,315 | ||||
| Accrued expenses |
473,879 | 576,545 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
885,975 | 1,067,860 | ||||||
| Long term liabilities: |
||||||||
| Convertible debt, net of unamortized discount of $3,136,342 and $0, respectively |
318,658 | — | ||||||
| Convertible debt – related parties, net of unamortized discount of $571,895 and $0, respectively |
58,105 | — | ||||||
|
|
|
|
|
|||||
| Total liabilities |
1,262,738 | 1,067,860 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
— | — | ||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, no par value, 10,000,000 shares authorized, none issued and outstanding |
— | — | ||||||
| Common stock, $0.01 par value, 100,000,000 shares authorized, 6,249,369 and 5,762,028 shares issued and outstanding |
62,494 | 57,621 | ||||||
| Additional paid in capital |
112,432,458 | 107,818,530 | ||||||
| Deficit accumulated during the development stage |
(109,611,620 | ) | (100,680,787 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
2,883,332 | 7,195,364 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 4,146,070 | $ | 8,263,224 | ||||
|
|
|
|
|
|||||
See accompanying summary of accounting policies and notes to consolidated financial statements
F-3
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF EXPENSES
Years ended December 31, 2012 and 2011 and the
Period from January 22, 2003 (Inception) to December 31, 2012
| 2012 | 2011 | Inception through 2012 |
||||||||||
| Research and development |
$ | 6,318,476 | $ | 3,340,038 | $ | 76,497,351 | ||||||
| General and administrative |
2,508,541 | 2,406,269 | 30,117,616 | |||||||||
| Depreciation |
303,677 | 210,252 | 1,650,158 | |||||||||
| Loss on disposal of fixed assets |
3,097 | 9,686 | 513,345 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(9,133,791 | ) | (5,966,245 | ) | (108,778,470 | ) | ||||||
| Interest income |
280 | 932 | 1,358,697 | |||||||||
| Other income and expense, net |
— | — | 661,146 | |||||||||
| Gain on extinguishment of debt |
— | — | 1,612,440 | |||||||||
| Gain on derivative instruments |
552,978 | — | 1,941,826 | |||||||||
| Gain on sale of technology |
— | — | 3,000,000 | |||||||||
| Interest expense |
(350,300 | ) | (3,135 | ) | (9,407,259 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (8,930,833 | ) | $ | (5,968,448 | ) | $ | (109,611,620 | ) | |||
|
|
|
|
|
|
|
|||||||
| Basic and diluted loss per share |
$ | (1.54 | ) | $ | (1.06 | ) | ||||||
| Weighted average shares outstanding |
5,785,372 | 5,633,124 | ||||||||||
See accompanying summary of accounting policies and notes to consolidated financial statements
F-4
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
Period from January 22, 2003 (Inception) through December 31, 2012
| Common Stock | Additional Paid in Capital |
Accumulated Deficit |
Total | |||||||||||||||||
| Shares | Par | |||||||||||||||||||
| Shares issued for cash |
131,250 | $ | 65,625 | $ | (64,625 | ) | $ | — | $ | 1,000 | ||||||||||
| Shares repurchased and cancelled |
(42,656 | ) | (21,328 | ) | 21,003 | — | (325 | ) | ||||||||||||
| Discount related to beneficial conversion feature |
— | — | 28,180 | — | 28,180 | |||||||||||||||
| Discount on warrants attached to debt |
— | — | 28,180 | — | 28,180 | |||||||||||||||
| Net loss |
— | — | — | (126,003 | ) | (126,003 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2003 |
88,594 | 44,297 | 12,738 | (126,003 | ) | (68,968 | ) | |||||||||||||
| Shares issued for: |
||||||||||||||||||||
| cash |
562 | 281 | 8,719 | — | 9,000 | |||||||||||||||
| services |
51,625 | 25,813 | 823,187 | — | 849,000 | |||||||||||||||
| license |
6,067 | 3,033 | 424,042 | — | 427,075 | |||||||||||||||
| reverse merger with Sportan |
24,934 | 12,467 | (160,200 | ) | — | (147,733 | ) | |||||||||||||
| acquisition of Opexa |
62,500 | 31,250 | 23,718,750 | — | 23,750,000 | |||||||||||||||
| additional shares attached to convertible debt |
4,025 | 2,012 | 286,354 | — | 288,366 | |||||||||||||||
| conversion of convertible notes |
15,187 | 7,594 | 240,776 | — | 248,370 | |||||||||||||||
| Shares cancelled |
(2,000 | ) | (1,000 | ) | 1,000 | — | — | |||||||||||||
| Discount related to beneficial conversion feature |
— | — | 855,849 | — | 855,849 | |||||||||||||||
| Discount on warrants attached to debt |
— | — | 1,848,502 | — | 1,848,502 | |||||||||||||||
| Option expense |
— | — | 123,333 | — | 123,333 | |||||||||||||||
| Net loss |
— | — | — | (31,411,736 | ) | (31,411,736 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2004 |
251,494 | 125,747 | 28,183,050 | (31,537,739 | ) | (3,228,942 | ) | |||||||||||||
| Shares issued for: |
||||||||||||||||||||
| cash, net of offering costs |
97,362 | 48,681 | 5,297,536 | — | 5,346,217 | |||||||||||||||
| convertible debt |
152,756 | 76,378 | 7,573,068 | — | 7,649,446 | |||||||||||||||
| debt |
575 | 288 | 160,712 | — | 161,000 | |||||||||||||||
| license |
7,298 | 3,649 | 1,864,735 | — | 1,868,384 | |||||||||||||||
| services |
6,000 | 3,000 | 1,009,400 | — | 1,012,400 | |||||||||||||||
| Discount related to beneficial conversion feature |
— | — | 831,944 | — | 831,944 | |||||||||||||||
| Discount on warrants attached to debt |
— | — | 1,433,108 | — | 1,433,108 | |||||||||||||||
| Option expense |
— | — | 2,487,741 | — | 2,487,741 | |||||||||||||||
| Warrant expense |
— | — | 2,373,888 | — | 2,373,888 | |||||||||||||||
| Transition of warrants from equity instruments to liability instruments |
— | — | (10,658,496 | ) | — | (10,658,496 | ) | |||||||||||||
| Net loss |
— | — | — | (14,856,724 | ) | (14,856,724 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2005 |
515,485 | 257,743 | 40,556,686 | (46,394,463 | ) | (5,580,034 | ) | |||||||||||||
| Shares issued for: |
||||||||||||||||||||
| cash, net of offering costs |
1,150,000 | 575,000 | 20,578,519 | — | 21,153,519 | |||||||||||||||
| debt |
8,707 | 4,354 | 175,646 | — | 180,000 | |||||||||||||||
| Option expense |
— | — | 2,749,617 | — | 2,749,617 | |||||||||||||||
| Warrant expense |
— | — | 1,568,966 | — | 1,568,966 | |||||||||||||||
| Net loss |
— | — | — | (12,649,170 | ) | (12,649,170 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2006 |
1,674,192 | 837,097 | 65,629,434 | (59,043,633 | ) | 7,422,898 | ||||||||||||||
F-5
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY—(Continued)
Period from January 22, 2003 (Inception) through December 31, 2012
| Common Stock | Additional Paid in Capital |
Accumulated Deficit |
Total | |||||||||||||||||
| Shares | Par | |||||||||||||||||||
| Cumulative change in derivative liability |
— | — | 10,658,496 | (4,001,820 | ) | 6,656,676 | ||||||||||||||
| Option expense |
— | — | 1,876,103 | — | 1,876,103 | |||||||||||||||
| Warrant expense |
— | — | 845,275 | — | 845,275 | |||||||||||||||
| Net loss |
— | — | — | (14,667,367 | ) | (14,667,367 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2007 |
1,674,192 | 837,097 | 79,009,308 | (77,712,820 | ) | 2,133,585 | ||||||||||||||
| Shares issued for: |
||||||||||||||||||||
| cash, net of offering costs |
1,375,968 | 687,984 | 7,963,595 | — | 8,651,579 | |||||||||||||||
| services |
11,300 | 5,650 | 43,315 | — | 48,965 | |||||||||||||||
| Issuance of warrants for cash |
— | — | 603,850 | — | 603,850 | |||||||||||||||
| Option expense |
— | — | 1,901,570 | — | 1,901,570 | |||||||||||||||
| Net loss |
— | — | — | (11,852,152 | ) | (11,852,152 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2008 |
3,061,460 | 1,530,731 | 89,521,638 | (89,564,972 | ) | 1,487,397 | ||||||||||||||
| Cumulative effect of change in accounting principle |
(1,976,457 | ) | 1,755,622 | (220,835 | ) | |||||||||||||||
| Par value adjustment |
— | (1,500,117 | ) | 1,500,117 | — | — | ||||||||||||||
| Reduction in derivative liability |
— | — | 587,609 | — | 587,609 | |||||||||||||||
| Discount on convertible notes |
— | — | 439,493 | — | 439,493 | |||||||||||||||
| Discount on warrants |
— | — | 37,453 | — | 37,453 | |||||||||||||||
| Shares issued for: |
||||||||||||||||||||
| cash, net of offering costs |
637,500 | 6,375 | 4,682,790 | — | 4,689,165 | |||||||||||||||
| exercise of options |
15,100 | 151 | 63,453 | — | 63,604 | |||||||||||||||
| exercise of warrants |
154,991 | 1,550 | 1,073,385 | — | 1,074,935 | |||||||||||||||
| Option expense |
— | — | 650,249 | — | 650,249 | |||||||||||||||
| Net loss |
— | — | — | (1,433,922 | ) | (1,433,922 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2009 |
3,869,051 | 38,690 | 96,579,730 | (89,243,272 | ) | 7,375,148 | ||||||||||||||
| Conversion of convertible notes |
690,045 | 6,900 | 1,373,191 | — | 1,380,091 | |||||||||||||||
| Shares issued for: |
||||||||||||||||||||
| services |
13,750 | 138 | 64,212 | — | 64,350 | |||||||||||||||
| exercise of options |
35,380 | 354 | 109,287 | — | 109,641 | |||||||||||||||
| exercise of warrants |
8,500 | 85 | (85 | ) | — | — | ||||||||||||||
| Option expense |
— | — | 508,550 | — | 508,550 | |||||||||||||||
| Net loss |
— | — | — | (5,469,067 | ) | (5,469,067 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2010 |
4,616,726 | 46,167 | 98,634,885 | (94,712,339 | ) | 3,968,713 | ||||||||||||||
| Shares issued for: |
||||||||||||||||||||
| cash, net of offering costs |
1,132,726 | 11,328 | 8,606,829 | — | 8,618,157 | |||||||||||||||
| services |
12,576 | 126 | 86,902 | — | 87,028 | |||||||||||||||
| Option expense |
— | — | 489,914 | — | 489,914 | |||||||||||||||
| Net loss |
— | — | — | (5,968,448 | ) | (5,968,448 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2011 |
5,762,028 | 57,621 | 107,818,530 | (100,680,787 | ) | 7,195,364 | ||||||||||||||
| Write off of derivative liability |
— | — | 1,761,657 | — | 1,761,657 | |||||||||||||||
| Discount related to beneficial conversion feature |
— | — | 1,497,634 | — | 1,497,634 | |||||||||||||||
| Shares issued for: |
||||||||||||||||||||
| initial commitment on Lincoln Park $1.5 million share purchase agreement |
56,507 | 565 | 148,566 | — | 149,131 | |||||||||||||||
| cash, net of offering costs |
267,610 | 2,676 | 331,294 | — | 333,970 | |||||||||||||||
| accrued interest |
163,224 | 1,632 | 184,051 | 185,683 | ||||||||||||||||
| Option expense |
— | — | 690,726 | — | 690,726 | |||||||||||||||
| Net loss |
— | — | — | (8,930,833 | ) | (8,930,833 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at December 31, 2012 |
6,249,369 | $ | 62,494 | $ | 112,432,458 | $ | (109,611,620 | ) | $ | 2,883,332 | ||||||||||
See accompanying summary of accounting policies and notes to consolidated financial statements
F-6
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
Years ended December 31, 2012 and 2011 and the
Period from January 22, 2003 (Inception) to December 31, 2012
| 2012 | 2011 | Inception through 2012 |
||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (8,930,833 | ) | $ | (5,968,448 | ) | $ | (109,611,620 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities |
||||||||||||
| Stock payable for acquired research and development |
— | — | 112,440 | |||||||||
| Stock issued for acquired research and development |
— | — | 26,286,589 | |||||||||
| Stock issued for services |
— | 87,028 | 2,061,743 | |||||||||
| Stock issued for debt in excess of principal |
— | — | 109,070 | |||||||||
| Amortization of discount on notes payable due to warrants and beneficial conversion feature |
104,032 | — | 6,856,730 | |||||||||
| Gain on extinguishment of debt |
— | — | (1,612,440 | ) | ||||||||
| Depreciation |
303,677 | 210,252 | 1,650,158 | |||||||||
| Amortization of debt financing costs |
58,639 | — | 583,017 | |||||||||
| Option and warrant expense |
690,726 | 489,914 | 16,265,933 | |||||||||
| Gain on derivative instruments |
(552,978 | ) | — | (1,941,826 | ) | |||||||
| Loss on disposal of fixed assets |
3,097 | 9,686 | 513,345 | |||||||||
| Changes in: |
||||||||||||
| Other current assets |
(611,607 | ) | (39,248 | ) | (1,153,053 | ) | ||||||
| Accounts payable – third parties and related parties |
(71,410 | ) | (13,028 | ) | (160,651 | ) | ||||||
| Accrued expenses |
83,018 | 234,923 | 605,238 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(8,923,639 | ) | (4,988,921 | ) | (59,435,327 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities |
||||||||||||
| Purchase of property & equipment |
(550,389 | ) | (296,949 | ) | (2,222,199 | ) | ||||||
| Restricted cash |
(1,000,000 | ) | — | (1,000,000 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(1,550,389 | ) | (296,949 | ) | (3,222,199 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities |
||||||||||||
| Common stock and warrants sold for cash, net of offering costs |
381,309 | 8,618,157 | 49,453,797 | |||||||||
| Common stock repurchased and canceled |
— | — | (325 | ) | ||||||||
| Proceeds from exercise of warrants and options |
— | — | 1,248,588 | |||||||||
| Proceeds from third party debt |
3,455,000 | — | 12,738,184 | |||||||||
| Proceeds from related party debt |
630,000 | — | 630,000 | |||||||||
| Deferred financing and offering costs |
(509,492 | ) | — | (509,492 | ) | |||||||
| Repayments on loan payable |
— | (35,607 | ) | (311,222 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
3,956,817 | 8,582,550 | 63,249,530 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net change in cash and cash equivalents |
(6,517,211 | ) | 3,296,680 | 592,004 | ||||||||
| Cash and cash equivalents at beginning of period |
7,109,215 | 3,812,535 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of period |
$ | 592,004 | $ | 7,109,215 | $ | 592,004 | ||||||
|
|
|
|
|
|
|
|||||||
| Cash paid for: |
||||||||||||
| Income tax |
$ | — | $ | — | $ | — | ||||||
| Interest |
1,946 | 3,135 | 155,109 | |||||||||
| NON-CASH TRANSACTIONS |
||||||||||||
| Issuance of common stock to Sportan shareholders |
— | — | 147,733 | |||||||||
| Issuance of common stock for accrued interest |
185,683 | — | 789,287 | |||||||||
| Issuance of warrants to placement agent |
— | — | 37,453 | |||||||||
| Conversion of notes payable to common stock |
— | — | 7,709,980 | |||||||||
| Conversion of accrued liabilities to common stock |
— | — | 197,176 | |||||||||
| Conversion of accounts payable to note payable |
— | — | 93,364 | |||||||||
| Discount on convertible notes relating to: |
||||||||||||
| Warrants |
2,314,635 | — | 5,974,372 | |||||||||
| Beneficial conversion feature |
1,497,634 | — | 3,303,153 | |||||||||
| Stock attached to notes |
— | — | 1,287,440 | |||||||||
| Fair value of derivative instrument |
— | — | 4,680,220 | |||||||||
| Derivative reclassified to equity |
1,761,657 | — | 2,349,266 | |||||||||
| Unpaid additions to property and equipment |
7,812 | 136,266 | 144,078 | |||||||||
| Amortization of deferred offering costs to paid-in capital |
47,339 | — | 47,339 | |||||||||
| Shares issued as deferred offering costs |
149,131 | — | 149,131 | |||||||||
See accompanying summary of accounting policies and notes to consolidated financial statements
F-7
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Business Overview and Summary of Accounting Policies
Description of Business. Opexa Therapeutics, Inc. (“Opexa” or “the Company”) was initially incorporated as Sportan United Industries, Inc. (“Sportan”) in Texas in March 1991. In June 2004, PharmaFrontiers Corp. (“PharmaFrontiers”) was acquired by Sportan in a transaction accounted for as a reverse acquisition. PharmaFrontiers’ stockholders were issued Sportan shares in exchange for all of the outstanding common shares of PharmaFrontiers. Concurrent with the transaction, Sportan changed its name to PharmaFrontiers. During its development stage as a biopharmaceutical company, PharmaFrontiers acquired the worldwide exclusive license to a stem cell technology developed at Argonne National Laboratory, a U.S. Department of Energy Laboratory operated by the University of Chicago, in which adult multi-potent stem cells are derived from monocytes obtained from the patient’s own blood (the “Stem Cell License”). A patent application was filed in November 2003 with the United States Patent and Trade Office regarding the technology involved in the Stem Cell License. The initial focus for this technology is the further development of this monocyte-derived stem cell technology as a platform for the in vitro generation of highly specialized cells for potential application in autologous cell therapy for patients with diabetes mellitus (the “Diabetes Program”).
In October 2004, PharmaFrontiers acquired all of the outstanding stock of Opexa Pharmaceuticals, Inc. (“Opexa Pharmaceuticals”), a biopharmaceutical company that previously acquired the exclusive worldwide license from Baylor College of Medicine to an patient specific, autologous T-cell immunotherapy, Tcelna™ (formerly known as Tovaxin®), for the initial treatment of multiple sclerosis (MS). In June 2006, the Company changed its name to Opexa Therapeutics, Inc. from PharmaFrontiers Corp. and, in January 2007, Opexa Therapeutics, Inc., the parent, merged with its wholly owned subsidiary, Opexa Pharmaceuticals with Opexa Therapeutics, Inc. being the surviving company.
In August 2009, Opexa entered into an exclusive agreement with Novartis Institutes for BioMedical Research, Inc. (“Novartis”) whereby Novartis acquired Opexa’s rights to the Stem Cell License and associated technology platform and had full responsibility for funding and carrying out all research, development and commercialization activities. Opexa received an upfront cash payment of $3 million at the time the agreement was entered into and subsequently received $0.5 million as a technology transfer fee milestone. In November 2011, Opexa re-acquired the stem cell assets from Novartis in consideration for releasing Novartis with respect to any further payment obligations owed to Opexa by Novartis. In connection with the re-acquisition of the stem cell assets, a related license agreement with the University of Chicago was re-assigned to Opexa. Opexa and the University of Chicago entered into a Fourth Amended and Restated License Agreement in connection with such assignment to Opexa.
In September 2012, Opexa initiated a Phase IIb clinical trial of Tcelna in patients with secondary progressive MS (“SPMS”). Previously, in September 2008, the Company completed a Phase IIb clinical study of Tcelna in the relapsing-remitting MS (“RRMS”) indication.
Opexa operates in a highly regulated and competitive environment. The manufacturing and marketing of pharmaceutical products require approval from, and are subject to, ongoing oversight by the Food and Drug Administration, or FDA, in the United States, by the European Medicines Agency, or EMA, in the E.U. and by comparable agencies in other countries. Obtaining approval for a new therapeutic product is never certain and may take many years and may involve expenditure of substantial resources. Tcelna is in development stage and Opexa has not applied for a Biologics License Application (BLA) for Tcelna with the FDA nor a similar regulatory licensure in any other country, and thus Tcelna is not approved to be marketed in any country.
F-8
Table of Contents
Development Stage Company. Opexa is considered to be in development stage and has had no commercial revenues to date.
Reverse Stock Split. In June, 2006, Opexa effected a one-for-ten reverse stock split of its common stock.
On December 14, 2012, Opexa effected a one-for-four reverse stock split of its common stock (the “1:4 Reverse Stock Split”) which decreased the number of common shares issued and outstanding from approximately 23.6 million shares to approximately 5.9 million shares as of December 14, 2012. The number of authorized shares of common stock and preferred stock remained the same following the 1:4 Reverse Stock Split.
Unless otherwise noted, impacted amounts included in the consolidated financial statements and notes thereto have been retroactively adjusted for the stock splits as if such stock splits occurred on the first day of the first period presented. Impacted amounts include shares of common stock issued and outstanding, shares underlying convertible promissory notes, warrants and stock options, shares reserved, conversion prices of convertible securities, exercise prices of warrants or options, and loss per share. There was no impact on preferred or common stock authorized resulting from the 1:4 Reverse Stock Split.
Principals of Consolidation. The financial statements include the accounts of Opexa and its former wholly-owned subsidiary, Opexa Pharmaceuticals through December 31, 2006. All intercompany accounts and transactions have been eliminated.
The consolidated financial statements include the accounts of Opexa and its wholly owned subsidiary, Opexa Hong Kong Limited (“Opexa Hong Kong”). Opexa Hong Kong was formed in the Hong Kong Special Administrative Region during 2012 in order to facilitate potential development collaborations in the pan-Asian region. Presently, Opexa Hong Kong has not entered into any agreements and has not recognized any revenues as of December 31, 2012. All intercompany transactions and balances between Opexa and Opexa Hong Kong are eliminated in consolidation.
Use of Estimates in Financial Statement Preparation. The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Certain Risks and Concentrations. Opexa is exposed to risks associated with foreign currency transactions insofar as it has used U.S. dollars to fund Opexa Hong Kong’s bank account denominated in Hong Kong dollars. As the net position of the unhedged Opexa Hong Kong bank account fluctuates, Opexa’s earnings may be negatively affected. In addition, the reported carrying value of the Company’s Hong Kong dollar-denominated assets and liabilities that remain in Opexa Hong Kong will be affected by fluctuations in the value of the U.S. dollar as compared to the Hong Kong dollar. Opexa currently does not utilize forward exchange contracts or any type of hedging instruments to hedge foreign exchange risk as Opexa believes that its overall exposure is relatively limited. As of December 31, 2012, Opexa Hong Kong reported cash and cash equivalents of $3,902 in converted U.S. dollars and does not have any reported liabilities in the consolidated balance sheets.
Cash and Cash Equivalents. For purposes of the statements of cash flows, cash equivalents include all highly liquid investments with original maturities of three months or less. The primary objectives for the fixed income investment portfolio are liquidity and safety of principal. Investments are made with the objective of achieving the highest rate of return consistent with these two objectives. Opexa’s investment policy limits investments to certain types of instruments issued by institutions primarily with investment grade credit ratings and places restrictions on maturities and concentration by type and issuer.
F-9
Table of Contents
Supplies Inventory. Reagents and supplies that will be used to manufacture Tcelna and placebo product in Opexa’s Phase IIb clinical study are recorded as other current assets. The inventory of these reagents and supplies are determined at the lower of cost or market value with cost determined under the first-in first-out (FIFO) method.
Long-lived Assets. Property and equipment are stated on the basis of historical cost less accumulated depreciation. Depreciation is provided using the straight-line method over the estimated useful lives of the assets. Major renewals and improvements are capitalized, while minor replacements, maintenance and repairs are charged to current operations. Impairment losses are recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amount.
Deferred costs. Opexa incurs costs in connection with a debt or equity offering or in connection with the proceeds pursuant to an execution of a strategic agreement. These costs are recorded as deferred offering or deferred financing costs in the consolidated balance sheets. Such costs may consist of legal, accounting, underwriting fees and other related items incurred through the date of the debt or equity offering or the date of the execution of the strategic agreement. Costs in connection with a debt offering are amortized to interest expense over the term of the note instrument. Costs in connection with the execution of a strategic agreement in which an initial upfront payment is received are offset to the gain recognized in the Consolidated Statements of Expenses. Additional paid in capital includes costs recorded as an offset to proceeds in connection with the completion of an equity offering.
Income Taxes. Income tax expense is based on reported earnings before income taxes. Deferred income taxes reflect the impact of temporary differences between assets and liabilities recognized for financial reporting purposes and such amounts recognized for tax purposes, and are measured by applying enacted tax rates in effect in years in which the differences are expected to reverse. A valuation allowance is recorded to reduce the net deferred tax asset to zero because it is more likely than not that the deferred tax asset will not be realized. The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained upon an examination.
Stock-Based Compensation. Opexa accounts for share-based awards issued to employees and non-employees in accordance with FASB ASC 718. Accordingly, employee share-based payment compensation is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the requisite service period (generally the vesting is over a 3-year period). Additionally, share-based awards to non-employees are expensed over the period in which the related services are rendered at their fair value.
Research and Development. Research and development expenses are expensed in the consolidated statements of expenses as incurred in accordance with FASB ASC 730, Research and Development. Research and development expenses include salaries, related employee expenses, clinical trial expenses, research expenses, consulting fees, and laboratory costs. In instances in which the Company enters into agreements with third parties for research and development activities, Opexa may prepay fees for services at the initiation of the contract. Opexa records the prepayment as a prepaid asset in the consolidated balance sheets and amortizes the asset into research and development expense in the consolidated statements of operations over the period of time the contracted research and development services are performed. Other types of arrangements with third parties may be fixed fee or fee for service, and may include monthly payments or payments upon completion of milestones or deliverables. Opexa expenses the costs of licenses of patents and the prosecution of patents until the issuance of such patents and the commercialization of related products is reasonably assured. Research and development expense for the years ended December 31, 2012 and 2011 was $6,318,476 and $3,340,038, respectively.
Foreign Currency Translation and Transaction Gains and Losses. Opexa records foreign currency translation adjustments and transaction gains and losses in accordance with FASB ASC 830, Foreign Currency Matters. For the Company’s operations that have a functional currency other than the U.S. dollar, gains and
F-10
Table of Contents
losses resulting from the translation of the functional currency into U.S. dollars for financial statement presentation are not included in determining net loss, but are accumulated in the cumulative foreign currency translation adjustment account as a separate component of stockholders’ equity, except for intercompany transactions that are of a short-term nature with Opexa Hong Kong that are consolidated, combined or accounted for by the equity method in Opexa’s consolidated financial statements. Opexa Hong Kong has transactions in Hong Kong dollars. Opexa records transaction gains and losses in its consolidated statements of operations related to the recurring measurement and settlement of such transactions. For the year ended December 31, 2012, Opexa did not record any gains and losses resulting from the translation of the functional currency into U.S. dollars and thus did not report any cumulative foreign currency translation adjustments in stockholders’ equity in the consolidated balance sheets.
Net Loss per Share. Basic and diluted net loss per share is calculated based on the net loss attributable to common shareholders divided by the weighted average number of shares outstanding for the period excluding any dilutive effects of options, warrants, unvested share awards and convertible securities.
Note 2. Cash and Cash Equivalents
At December 31, 2012, Opexa invested approximately $24,500 in a money market fund investing exclusively in high-quality, short-term money market instruments consisting of U.S. government obligations and repurchase agreements collateralized by the U.S. Government. While this fund seeks current income while preserving capital and liquidity, the fund is subject to risk, including U.S. government obligations risk, and is not federally insured or guaranteed by or obligations of the Federal Deposit Insurance Corporation or any other agency. For the 12 months ended December 31, 2012, the money market fund recognized an average market yield of 0.01%. Interest income of $280 was recognized for the year ended December 31, 2012 in the statements of expenses.
At December 31, 2011, Opexa invested approximately $7.0 million in a money market account with an average market yield of 0.01%. Interest income of $932 was recognized for the year ended December 31, 2011 in the statements of expenses.
Opexa issued a total of $4,085,000 in principal amount of convertible secured promissory notes to related parties and third parties on July 25, 2012 (see Note 6 and Note 7). As part of the security interest granted by Opexa to the investors, $1.0 million of the proceeds are required to be maintained in an account subject to a deposit account control agreement while the notes are outstanding. As of December 31, 2012, the $1.0 million balance in the controlled account is reported as restricted cash in the consolidated balance sheets. Subsequent to December 31, 2012, the restricted cash was reduced to $500,000 (see Note 14).
Note 3. Other Current Assets
Other current assets consisted of the following at December 31, 2012 and 2011:
| Description |
2012 | 2011 | ||||||
| Supplies inventory |
$ | 604,179 | $ | — | ||||
| Deferred offering costs |
341,166 | — | ||||||
| Prepaid expenses |
132,201 | 124,773 | ||||||
|
|
|
|
|
|||||
| $ | 1,077,546 | $ | 124,773 | |||||
|
|
|
|
|
|||||
Supplies inventory at December 31, 2012 includes reagents and supplies that will be used to manufacture Tcelna and placebo product in Opexa’s Phase IIb clinical study. Opexa expects to amortize these prepaid reagents and supplies to research and development costs in the consolidated statements of expenses over the course of the clinical study.
F-11
Table of Contents
Deferred offering costs at December 31, 2012 include costs incurred from third parties in connection with the implementation of an at-the-market program (“ATM Agreement”) in September 2012 pursuant to which Opexa may sell shares of its common stock from time to time depending upon market demand through a sales agent in transactions deemed to be an “at-the-market” offering as defined in Rule 415 of the Securities Act of 1933. As of December 31, 2012, the costs of $101,972 in connection with the implementation of the ATM Agreement were capitalized and are included in other current assets in the consolidated balance sheets. Upon the sales of any shares of common stock under the ATM Agreement, the capitalized costs will be offset against the proceeds of such sales of shares of common stock.
Deferred offering costs at December 31, 2012 also include costs incurred from third parties in connection with the implementation of a $1.5 million Purchase Agreement and a $15 million Purchase Agreement (collectively, the “Purchase Agreements”) in November 2012 pursuant to which Opexa has the right to sell to Lincoln Park Capital Fund, LLC (“Lincoln Park”) an aggregate of up to $16.5 million in shares of its common stock, subject to certain conditions and limitations. As of December 31, 2012, the remaining costs of $216,198 in connection with the implementation of the Purchase Agreements remained capitalized and are included in other current assets in the consolidated balance sheets. Upon the sales of shares of common stock under the Purchase Agreements, the capitalized costs are offset against the proceeds of such sales of shares of common stock.
Deferred offering costs at December 31, 2012 also include costs incurred from third parties in connection with the Option and License Agreement entered into with Ares Trading SA (“Merck”), a wholly owned subsidiary of Merck Serono S.A., on February 4, 2013. Under the terms of the Agreement, the Company received an upfront payment of $5 million on February 20, 2013. As of December 31, 2012, the remaining costs of $22,996 in connection with the Option and License Agreement remained capitalized and are included in other current assets in the consolidated balance sheets.
Note 4. Property and Equipment
Property and equipment consisted of the following at December 31, 2012 and 2011:
| Description |
Life | 2012 | 2011 | |||||||||
| Computer equipment |
3 years | $ | 121,129 | $ | 99,603 | |||||||
| Office furniture and equipment |
5-7 years | 274,438 | 251,170 | |||||||||
| Software |
3 years | 149,867 | 96,097 | |||||||||
| Laboratory equipment |
7 years | 1,020,158 | 994,994 | |||||||||
| Leasehold improvements |
10 years | 622,772 | 603,445 | |||||||||
| Manufacturing equipment |
7 years | 571,187 | 177,528 | |||||||||
|
|
|
|
|
|||||||||
| Subtotal |
2,759,551 | 2,222,837 | ||||||||||
| Less: accumulated depreciation |
(1,494,510 | ) | (1,193,601 | ) | ||||||||
|
|
|
|
|
|||||||||
| Property and equipment, net |
$ | 1,265,041 | $ | 1,029,236 | ||||||||
|
|
|
|
|
|||||||||
Property and equipment is carried at cost less accumulated depreciation. Depreciation is calculated by the straight-line method over the estimated useful life of three to ten years, depending upon the type of equipment, except for leasehold improvements which are amortized using the straight-line method over the remaining lease term or the life of the asset, whichever is shorter. The cost of repairs and maintenance is charged as an expense as incurred. Depreciation expense totaled $303,677 and $210,252 for the years ended December 31, 2012 and 2011, respectively.
Note 5. Income Taxes
Opexa uses the liability method, where deferred tax assets and liabilities are determined based on the expected future tax consequences of temporary differences between the carrying amounts of assets and liabilities for financial and income tax reporting purposes.
F-12
Table of Contents
At December 31, 2012 and 2011, Opexa had approximately $69 million and approximately $61 million of unused net operating losses, respectively, available for carry forward to future years. The unused net operating losses begin to expire at December 31, 2024. At December 31, 2012 and 2011, Opexa’s deferred tax asset resulting from its cumulative NOLs amounted to $23,678,228 and $20,876,592, respectively which is covered by a full valuation allowance due to uncertainty of Opexa’s ability to generate future taxable income necessary to realize the related deferred tax asset.
Note 6. Convertible Promissory Notes
On July 25, 2012, Opexa issued a total of $4,085,000 in principal amount of secured convertible promissory notes (“Notes”) to third parties and related parties (collectively, the “Noteholders”), of which an aggregate of $630,000 was issued to related parties (See Note 7). The Notes mature on July 25, 2014 and accrue interest at the rate of 12% per annum, compounded annually. Interest is payable semi-annually on June 30 and December 31 in either cash or registered shares of common stock, at Opexa’s election. The Notes are secured by substantially all of Opexa’s assets and are convertible into a new class of non-voting Series A convertible preferred stock. The Notes can be converted into Series A convertible preferred stock at the option of the investors at a price of $100.00 per share, subject to certain limitations and adjustments. Additionally, Opexa can elect to convert the Notes into Series A convertible preferred stock if (i) Opexa’s common stock closes at or above $10.00 per share for 20 consecutive trading days or (ii) Opexa achieves certain additional funding milestones to continue its clinical trial program. These milestones include (x) executing a strategic agreement with a partner or potential partner by which Opexa will receive a minimum of $5 million to partially fund, or an option to partner with Opexa for, its Phase II clinical trial for Tcelna in patients with SPMS and (y) receiving a minimum of $25 million in additional capital (including the Note offering proceeds) from any partner, potential partner or any other source.
The Series A convertible preferred stock accrues dividends at the rate of 8% per annum, which are cumulative and payable semi-annually on June 30 and December 31 in either cash or registered shares of common stock at Opexa’s election. The Series A convertible preferred stock has a liquidation preference of $100.00 per share, entitling holders to payment from the assets of the Company available for distribution to its shareholders before any payment is made to the holders of the common stock. The Series A convertible preferred stock participates in any dividends or other distributions on shares of common stock (other than dividends payable in shares of common stock) along with the common stock. As a result of anti-dilution adjustments following the November 2012 sale of shares of Opexa’s common stock, the Series A convertible preferred stock is convertible into shares of the Company’s common stock at a price of $3.12 per share (the floor price), subject to certain limitations and conditions, and up to 1,308,236 shares of common stock were issuable if all 12% convertible secured promissory notes issued in the July 2012 financing and outstanding at December 31, 2012 were converted to Series A convertible preferred stock and such stock is then converted into common stock. Additionally, Opexa can elect to convert the Series A convertible preferred stock into common stock if the Company’s common stock closes at or above $16.00 per share for 20 consecutive trading days. As of December 31, 2012, no shares of Series A convertible preferred stock were currently outstanding. Subsequent to December 31, 2012, $900,000 in principal amount of the notes were converted into shares of Series A convertible preferred stock and such shares were then converted into common stock (see Note 14).
As part of the security interest in all of the Company’s assets granted to the Noteholders, $1.0 million of the proceeds is maintained in a controlled account (see Note 2). Subsequent to December 31, 2012, the restricted cash was reduced to $500,000 (see Note 14). The Noteholders were granted certain registration rights for the shares of underlying common stock.
If the Company does not make the required payments when due, either at maturity, or at applicable installment payment dates, or if the Company breaches other terms of the convertible secured notes or related agreements, the Noteholders could elect to declare all amounts outstanding, together with accrued and unpaid interest, to be immediately due and payable. Even if the Company was able to prepay the full amount in cash, any
F-13
Table of Contents
such repayment could leave the Company with little or no working capital for its business. If the Company is unable to repay those amounts, the Noteholders will have a first claim on Opexa’s assets pledged under the convertible secured notes. If the Noteholders should attempt to foreclose on the collateral, it is unlikely that there would be any assets remaining after repayment in full of such secured indebtedness. Any default under the convertible secured notes and resulting foreclosure would have a material adverse effect on Opexa’s financial condition and the Company’s ability to continue its operations.
The Notes were analyzed at issuance for a beneficial conversion feature and Opexa concluded that a beneficial conversion feature exists. The beneficial conversion feature was measured using the commitment-date stock price and was determined to be $1,497,634, of which $230,969 was attributable to related parties. This amount was recorded as a debt discount and is amortized to interest expense in the consolidated statements of expenses over the term of the Notes. Opexa also analyzed the Notes for derivative accounting consideration and determined that derivative accounting does not apply.
In connection with the issuance of the Notes, Opexa also issued Series I warrants to the Noteholders to initially purchase an aggregate of 957,422 shares of Opexa’s common stock at $5.00 per share, subject to certain limitations and adjustments. The warrants have a five-year term and are exercisable six months from the date of issuance, or January 25, 2013. As a result of anti-dilution adjustments, the number of warrant shares for which the Series I warrants are exercisable increased to an aggregate increase of 1,436,121 shares of Opexa’s common stock at an adjusted exercise price of $2.56 per share, subject to further certain limitations and adjustments. As a result, Opexa accounted for these reset provisions in accordance with FASB ASC 815-40, which requires Opexa to record the warrants as a derivative liability at the grant date and to record changes in fair value relating to the warrants at each subsequent balance sheet date (see Note 8). Opexa can redeem the warrants at $0.01 per share if its common stock closes at or above $10.00 per share for 20 consecutive trading days.
The initial fair value of the warrant liabilities of $2,314,635, together with the beneficial conversion feature of $1,497,634 were recognized as a debt discount and are amortized to interest expense in the consolidated statements of expenses over the term of the Notes using the effective interest method. The amortized debt discount for the year-ended December 31, 2012 was $104,032 and Opexa recognized $552,978 as a derivative gain in the consolidated statements of expenses due to the change in fair value of the liability. The unamortized discount as of December 31, 2012 amounted to $3,708,237.
The following table provides a summary of the changes in convertible debt – third parties, net of unamortized discount, during 2012:
| Balance at December 31, 2011 |
$ | — | ||
| July 25, 2012 Notes, face value |
3,455,000 | |||
| Discount on beneficial conversion feature of Notes at issuance |
(1,266,665 | ) | ||
| Discount on fair value of Series I warrant liability at issuance |
(1,957,665 | ) | ||
| Amortization of debt discount to interest expense through December 31, 2012 |
87,988 | |||
|
|
|
|||
| Balance at December 31, 2012 |
$ | 318,658 | ||
|
|
|
Note 7. Related Party Transactions
Investors in the July 25, 2012 Note offering included two members of Opexa’s Board of Directors and entities affiliated with a third director. Opexa issued an aggregate of $630,000 in principal amount of Notes to the two directors and an entity for which a third director reports beneficial ownership of Opexa securities. In connection with the issuance of such Notes, Opexa also issued warrants to purchase an aggregate of 221,483
F-14
Table of Contents
shares of common stock. The fair value of the warrants was $356,969. Opexa also determined the Notes contained a beneficial conversation feature with fair value of $230,969. Opexa recorded a total of $587,939 as debt discount associated with the Notes issued to the related parties and amortized $16,044 as interest expense in the consolidated statements of expenses for the year ended December 31, 2012.
On August 15, 2012, Opexa appointed director David E. Jorden as its Acting Chief Financial Officer. As a non-employee officer of Opexa, Mr. Jorden receives cash compensation of $100,000 per annum for his service. For the period of August 15, 2012 through December 31, 2012, cash compensation totaling $37,500 was earned by Mr. Jorden and is reported in general and administrative expense in the consolidated statements of expenses. As of December 31, 2012, cash compensation totaling $8,333 was due to Mr. Jorden and is included in accounts payable in the consolidated balance sheets.
The following table provides a summary of the changes in convertible debt – related parties, net of unamortized discount, during 2012:
| Balance at December 31, 2011 |
$ | — | ||
| July 25, 2012 Notes, face value |
630,000 | |||
| Discount on beneficial conversion feature of Notes at issuance |
(230,970 | ) | ||
| Discount on fair value of Series I warrant liability at issuance |
(356,969 | ) | ||
| Amortization of debt discount to interest expense through December 31, 2012 |
16,044 | |||
|
|
|
|||
| Balance at December 31, 2012 |
$ | 58,105 | ||
|
|
|
Note 8. Fair Value of Derivative Financial Instruments
The carrying value of cash and cash equivalents, receivables, accounts payable and accrued expenses in the consolidated balance sheets approximates their fair values because of the short-term nature of these instruments. The carrying value of the Notes in the consolidated balance sheets approximates fair value since the related rate of interest approximates current market rates. Management believes Opexa is not exposed to significant interest or credit risks arising from these financial instruments.
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. Opexa utilizes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable.
| • | Level 1—Quoted prices in active markets for identical assets or liabilities. These are typically obtained from real-time quotes for transactions in active exchange markets involving identical assets. |
| • | Level 2—Quoted prices for similar assets and liabilities in active markets; quoted prices included for identical or similar assets and liabilities that are not active; and model-derived valuations in which all significant inputs and significant value drivers are observable in active markets. These are typically obtained from readily-available pricing sources for comparable instruments. |
| • | Level 3—Unobservable inputs, where there is little or no market activity for the asset or liability. These inputs reflect the reporting entity’s own beliefs about the assumptions that market participants would use in pricing the asset or liability, based on the best information available in the circumstances. |
FASB ASC 815, “Accounting for Derivatives and Hedging Activities” (“FASB ASC 815”) specifies that a contract that would otherwise meet the definition of a derivative, but is both (a) indexed to its own stock and
F-15
Table of Contents
(b) classified in stockholders’ equity in the statement of financial position would not be considered a derivative financial instrument. FASB ASC 815 provides a new two-step model to be applied in determining whether a financial instrument or an embedded feature is indexed to an issuer’s own stock, including evaluating the instrument’s contingent exercise and settlement provisions, and thus able to qualify for the FASB ASC 815-10 scope exception. It also clarifies the impact of foreign-currency-denominated strike prices and market-based employee stock option valuation instruments on the evaluation. Initially, Opexa evaluated all of its financial instruments and determined that the Series I warrants associated with the July 2012 Note financing (see Note 6) qualified for treatment under FASB ASC 815. Consequently, the Company recorded a derivative liability of $2,314,635 upon issuance of the warrants and a corresponding discount on the convertible debt. On November 8, 2012, it was determined that the floor for resetting the exercise price was met and no further adjustments to the exercise price of the Series I warrants would occur. Therefore, the Series I warrants were considered indexed to the company’s stock and qualified for the scope exception under FASB ASC 815-10 allowing for a transfer from liability classification to equity classification. Consequently, the remaining derivative liability of $1,761,657 at November 8, 2012 was written off to additional paid in capital.
The following table provides a summary of the changes in fair value, including net transfers in and/or out, of the derivative financial instruments, measured at fair value on a recurring basis using significant unobservable inputs (Level 3):
| Balance at December 31, 2011 |
$ | — | ||
| Fair value of warrant derivative liabilities at issuance |
2,314,635 | |||
| Realized derivative gains included in other income (expense) |
(552,978 | ) | ||
| Write off of warrant derivative liability to additional paid in capital |
(1,761,657 | ) | ||
|
|
|
|||
| Balance at December 31, 2012 |
$ | — | ||
|
|
|
The fair value of the derivative liabilities are calculated at the time of issuance using the Lattice option pricing model with Monte Carlo simulation. Opexa records a derivative liability for the calculated value. Changes in the fair value of the derivative liabilities are reported in other income (expense) in the consolidated statements of expenses. The variables used in the Lattice option pricing model for the derivative liabilities during the year ended December 31, 2012 include:
| July 25, 2012 | September 30, 2012 | November 8, 2012 | ||||||||||
| Market value of common stock on measurement date |
$ | 2.56 | $ | 2.70 | $ | 1.96 | ||||||
| Projected exercise price |
$ | 5.00 | $ | 4.52 | $ | 4.56 | ||||||
| Risk free interest rate |
0.56% | 0.56% | 0.72% | |||||||||
| Warrant lives in years |
5 | 4.88 | 4.71 | |||||||||
| Expected volatility |
193% | 193% | 194% | |||||||||
| Expected dividend yield |
0% | 0% | 0% | |||||||||
| Offering price range |
$ | 2.56-$6.56 | $ | 2.72-$6.72 | $ | 2.56-$6.56 | ||||||
Note 9. Commitments and Contingencies
In October 2005, Opexa entered into a ten-year lease for its office and research facilities. The facility including the property is leased for a term of ten years with two options for an additional five years each at the then prevailing market rate. Future minimum lease payments under the non-cancellable operating lease are $157,896 for 2013, $157,896 for 2014 and $118,422 for 2015. Rent expense in the consolidated statements of expenses was approximately $136,000 for each of the years ended December 31, 2012 and 2011.
F-16
Table of Contents
Note 10. Significant Contractual Service and Milestone Agreements
In February 2012, Opexa entered into an agreement with Pharmaceutical Research Associates, Inc. (“PRA”), a contract research organization, in which PRA will provide Opexa with services related to the design, implementation and management of Opexa’s ongoing Phase IIb clinical trial program in SPMS (the “PRA Agreement”). Under the terms of the PRA Agreement, Opexa made upfront cash payments to PRA of $543,766. Future payments by Opexa to PRA under the PRA Agreement are based on the achievement of certain time and performance milestones as presented in the PRA Agreement. In December 2012, Opexa entered into an Amendment #1 to Task Order #1 (the “Amendment”) with PRA in which Opexa agreed to reimburse PRA for additional services and pass-through expenses incurred while performing out-of-scope work. Under the terms of the Amendment, an upfront cash payment of $37,605 is to be made to PRA as payment for certain out-of-scope tasks performed by PRA and future payments by Opexa to PRA under the PRA Agreement on the achievement of certain time milestones in the Amendment. Total payments to PRA during 2012, which were charged to expense, amounted to $1,382,236. Unless terminated by either party without cause on 60 days prior notice or on shorter notice with cause, the initial term of the PRA Agreement is for four years and automatically renews for successive one year terms.
During 2012, Opexa entered into individual Clinical Trial Agreements with 18 clinical institutions (the “Institutions”) across the U.S. and 18 principal investigators (the “Investigators”) acting within their employment or agent positions within their clinical institution. Under the terms of each Clinical Trial Agreement, each of the Investigators will identify and recruit subjects with SPMS meeting certain enrollment requirements and conduct clinical research in conjunction with Opexa’s Phase IIb clinical study, and each of the Institutions will provide appropriate resources and facilities so the Institution’s Investigator can conduct Opexa’s Phase IIb clinical study in a timely and professional manner and according to the terms of the Clinical Trial Agreement. Under the terms of each Clinical Trial Agreement, Opexa paid an upfront cash payment to each Institution for start-up and other costs which were charged directly to expense. Future payments by Opexa to the Institutions during the term of each Clinical Trial Agreement are based on the achievement of certain performance milestones as presented in each Clinical Trial Agreement. Unless terminated by Opexa without cause with 30 days’ notice, or unless terminated by the Institution, Investigator or Opexa for health or safety reasons, the initial term of the Clinical Trial Agreements with each Institution and Investigator is for the duration of their enrolled subjects in the Phase IIb clinical study.
Note 11. Equity
Summary information regarding equity related transactions for the years ended December 31, 2011 and December 31, 2012 is as follows:
During 2011, equity related transactions were as follows:
| • | In January 2011, 96,189 shares of common stock were sold under the Continuous Offering Program Agreement dated May 14, 2010 (the “2010 ATM Agreement”) for net proceeds of $1,066,286. Compensation and fees totaling $10,826 was paid to the placement agent with respect to the shares sold. The 2010 ATM Agreement was subsequently terminated by Opexa on February 7, 2011. |
| • | In February 2011, an aggregate of 1,036,622 units were sold in a public offering, with each unit consisting of one share of common stock and a warrant to purchase four-tenths (0.40) of a share of common stock, at a price to the public of $2.05 per unit, for gross proceeds of $8,500,325. The shares of common stock and warrants were immediately separable and were issued separately such that no units were issued. The warrants were exercisable immediately upon issuance, have a five-year term and an exercise price of $10.44 per share. Net proceeds from this offering were approximately $7,551,891 after deducting underwriting discounts and commissions and other estimated offering expenses. The offering closed on February 11, 2011. |
| • | 12,576 shares of common stock valued at their fair value of $87,028 were issued to a consultant in exchange for services. |
F-17
Table of Contents
During 2012, equity related transactions were as follows:
| • | In November 2012, Opexa entered into two purchase agreements with Lincoln Park pursuant to which the Company has the right to sell to Lincoln Park an aggregate of up to $16.5 million in shares of common stock, subject to certain conditions and limitations. As consideration for its commitment to purchase shares of common stock pursuant to the $1.5 million purchase agreement, Opexa issued to Lincoln Park 56,507 shares of common stock with a fair value of $149,131. |
| • | In November and December 2012, 265,000 shares of common stock were sold and 2,610 additional commitment shares were issued to Lincoln Park for net proceeds of $333,970. |
| • | In December 2012, 163,224 shares of common stock were issued to the Noteholders of the July 2012 Notes as payment of accrued interest. |
Note 12. Options and Warrants
On September 2, 2010, the Board adopted the Opexa Therapeutics, Inc. 2010 Stock Incentive Plan (“the 2010 Plan”) for the granting of equity incentive awards to employees, directors and consultants of Opexa. The 2010 Plan was approved by the Company’s stockholders on October 19, 2010. The 2010 Plan is the successor to and continuation of Opexa’s June 2004 Compensatory Stock Option Plan (the “2004 Plan”). The 2004 Plan reserved a maximum of 575,000 shares of common stock for issuance pursuant to incentive stock options and nonqualified stock options granted to employees, directors and consultants. Awards were made as either incentive stock options or nonqualified stock options, with the Board having discretion to determine the number, term, exercise price and vesting of grants made under the 2004 Plan. All outstanding equity awards granted under the 2004 Plan continue to be subject to the terms and conditions as set forth in the agreements evidencing such stock awards and the terms of the 2004 Plan, but no additional awards will be granted under the 2004 Plan subsequent to approval of the 2010 Plan. Under the 2010 Plan, the total number of shares of common stock reserved for issuance consists of 625,000 shares plus the number of shares subject to stock options outstanding under the 2004 Plan that are forfeited or terminate prior to exercise and would otherwise be returned to the share reserves under the 2004 Plan and any reserved shares not issued or subject to outstanding grants, up to a maximum of 793,204 shares. The 2010 Plan provides for the grant of incentive stock options or nonqualified stock options, as well as restricted stock, stock appreciation rights, restricted stock units and performance awards that may be settled in cash, stock or other property. The Board of Directors or Compensation Committee, as applicable, administers the 2010 Plan and has discretion to determine the recipients, the number and types of stock awards to be granted and the terms and conditions of the stock awards, including the period of their exercisability and vesting. Subject to a limitation on repricing without stockholder approval, the Board or Compensation Committee, as applicable, may also determine the exercise price of options granted under the 2010 Plan.
Employee Options:
During 2011, options to purchase 43,750 shares of common stock were granted by Opexa to its employees at an exercise price of $6.24. These options have a term of ten years and vest over three years. Fair value of $268,451 was recorded using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for options issued during the year ended December 31, 2011 include (1) discount rate of 3.36%, (2) expected term of six years, (3) expected volatility of 192% and (4) zero expected dividends.
During 2011, options to purchase 18,750 shares were forfeited and cancelled.
Opexa recorded $304,024 stock-based compensation expense to management and employees during 2011. Unamortized stock-based compensation expense as of December 31, 2011 amounted to $364,064.
F-18
Table of Contents
During 2012, options to purchase an aggregate of 107,832 shares were granted to employees, at exercise prices ranging from $1.80 to $3.80. These options have terms of ten years and have a vesting schedule of three years. Fair value of $381,020 was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for these options include (1) discount rate range of 1.40% and 1.98%, (2) expected term of 5.25 to 7 years, (3) expected volatility range of 180% and 183% and (4) zero expected dividends.
During 2012, options to purchase an aggregate of 254,756 shares were granted to senior management, based on the achievement of future performance-based, strategic milestone objectives, at an exercise price of $3.80. These options have terms of ten years and have vesting schedules of three years commencing after the two specific milestone objectives have been individually met. Fair value of $964,715 was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for these options include (1) discount rate of 1.98%, (2) expected term of ten years, (3) expected volatility of 183% and (4) zero expected dividends. As of December 31, 2012, one of the two specific milestone objectives had been individually met and an aggregate of 82,009 shares granted to senior management commenced vesting during 2012.
During 2012, options to purchase 4,678 shares were forfeited and cancelled.
Opexa recorded $549,150 stock-based compensation expense to management and employees during 2012, which included the related expense for the options that are expected to vest based on achievement of their related performance conditions. Unamortized stock compensation expense as of December 31, 2012 amounted to $1,142,135.
Non-Employee Options:
During 2011, options to purchase 32,407 shares of common stock were granted by Opexa to its consultants and directors at exercise prices ranging from $3.80 to $7.12. These options have terms of two to ten years, and have vesting dates that vary from either full or partial vesting at date of grant to full vesting within one to two years of the date of grant. Fair value of $196,783 was recorded using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for options issued during the year ended December 31, 2011 include (1) discount rate range of 0.25% to 3.50%, (2) expected term of two to five and one-quarter years, (3) expected volatility of 85%—198% and (4) zero expected dividends.
Opexa recorded $185,890 stock-based compensation expense to consultants and directors during 2011. Unamortized stock-based compensation expense as of December 31, 2011 amounted to $19,658.
During 2012, an option to purchase an aggregate of 18,750 shares was granted to Opexa’s non-employee Acting Chief Financial Officer at an exercise price of $2.04 in connection with his appointment. This option has a term of ten years, with one-third of the shares vesting immediately, one-third of the shares vesting on December 31, 2012 and the remaining one-third of the shares vesting at the earlier of June 30, 2013 or the appointment of a permanent chief financial officer. Fair value of $37,096 was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for this option include (1) discount rate of 1.80%, (2) expected term of 5.25 years, (3) expected volatility of 185% and (4) zero expected dividends.
During 2012, options to purchase an aggregate of 30,600 shares were granted to directors for service on Opexa’s Board at an exercise price of $3.76. Options to purchase an aggregate of 10,000 shares have terms of 10 years, with 50% of the shares vesting immediately and 50% vesting one year from the date of grant. Options to purchase the remaining 20,600 shares will expire on the earlier of 10 years or a change in control of the Company, with 50% of the shares vesting immediately and 50% vesting on December 31, 2012. Fair value of $111,428 was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for these options include (1) discount rate of 2.03%, (2) expected term of 5.25 years, (3) expected volatility of 186% and (4) zero expected dividends.
F-19
Table of Contents
During 2012, options to purchase 25,563 shares were forfeited and cancelled.
Opexa recorded $141,576 of stock-based compensation expense to consultants and directors during 2012. Unamortized stock compensation expense as of December 31, 2012 amounted to $14,770.
Broker and Investor Warrants:
During 2011, warrants to purchase 671,972 shares were forfeited.
In connection with Opexa’s February 2011 public offering, Opexa issued warrants to purchase an aggregate of 414,650 shares of common stock to the investors at an exercise price of $10.44 per share. These warrants have a term of five years and were immediately exercisable.
During 2012, warrants to purchase 464,584 shares were forfeited.
In connection with Opexa’s July 25, 2012 private offering of the Notes (see Note 6), Opexa issued warrants to purchase an aggregate of 1,436,121 shares of common stock at a current adjusted exercise price of $2.56 per share, subject to certain limitations and adjustments. These warrants have a term of five years and are initially exercisable on January 25, 2013.
At December 31, 2011, the aggregate intrinsic value of the outstanding options and warrants was $227,567 and $435,913, respectively. At December 31, 2012, the aggregate intrinsic value of the outstanding options and warrants was $13,846 and $57,891, respectively.
Summary information regarding options and warrants from December 31, 2006 is as follows:
| Options | Weighted Average Exercise Price |
Warrants | Weighted Average Exercise Price |
|||||||||||||
| Outstanding at December 31, 2006 |
190,426 | $ | 45.92 | 917,590 | $ | 78.04 | ||||||||||
| Year ended December 31, 2007: |
||||||||||||||||
| Granted |
73,475 | 21.12 | — | — | ||||||||||||
| Forfeited and canceled |
(4,336 | ) | 30.96 | — | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding at December 31, 2007 |
259,565 | $ | 39.16 | 917,590 | $ | 78.04 | ||||||||||
| Year ended December 31, 2008: |
||||||||||||||||
| Granted |
160,100 | 4.50 | 1,682,209 | 7.84 | ||||||||||||
| Forfeited and canceled |
(31,617 | ) | 24.41 | — | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding at December 31, 2008 |
388,048 | $ | 25.88 | 2,599,799 | $ | 32.60 | ||||||||||
| Year ended December 31, 2009: |
||||||||||||||||
| Granted |
193,583 | 3.83 | 801,143 | 6.68 | ||||||||||||
| Exercised |
(15,193 | ) | 4.21 | (179,691 | ) | 6.64 | ||||||||||
| Forfeited and canceled |
(85,605 | ) | 42.22 | (52,082 | ) | 20.00 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding at December 31, 2009 |
480,833 | $ | 14.80 | 3,169,169 | $ | 27.72 | ||||||||||
| Year ended December 31, 2010: |
||||||||||||||||
| Granted |
38,138 | 8.34 | 1,966 | 8.00 | ||||||||||||
| Exercised |
(36,284 | ) | 3.56 | (17,102 | ) | 8.40 | ||||||||||
| Forfeited and canceled |
(97,171 | ) | 36.62 | (289,160 | ) | 117.60 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-20
Table of Contents
| Options | Weighted Average Exercise Price |
Warrants | Weighted Average Exercise Price |
|||||||||||||
| Outstanding at December 31, 2010 |
385,516 | $ | 8.60 | 2,864,873 | $ | 11.00 | ||||||||||
| Year ended December 31, 2011: |
||||||||||||||||
| Granted |
76,157 | 6.29 | 414,649 | 10.44 | ||||||||||||
| Exercised |
— | — | — | — | ||||||||||||
| Forfeited and canceled |
(18,750 | ) | 20.00 | (671,972 | ) | 23.72 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding at December 31, 2011 |
442,923 | $ | 7.71 | 2,607,550 | $ | 6.66 | ||||||||||
| Year ended December 31, 2012: |
||||||||||||||||
| Granted |
411,938 | 3.68 | 1,436,121 | 2.56 | ||||||||||||
| Exercised |
— | — | — | — | ||||||||||||
| Forfeited and canceled |
(30,241 | ) | 11.80 | (464,584 | ) | 6.12 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding at December 31, 2012 |
824,620 | $ | 5.54 | 3,579,087 | $ | 5.64 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Summary of options outstanding and exercisable as of December 31, 2012 is as follows:
| Range of Exercise Prices |
Weighted Average Remaining Contractual Life (years) |
Number of Options Outstanding |
Number of Options Exercisable |
|||||||||
| $0.88 to $4.99 |
5.84 | 599,706 | 258,337 | |||||||||
| 5.00 to 9.99 |
1.58 | 178,054 | 157,221 | |||||||||
| 10.00 to 39.20 |
0.21 | 46,860 | 46,860 | |||||||||
|
|
|
|
|
|
|
|||||||
| $0.88 to $39.20 |
7.63 | 824,620 | 462,418 | |||||||||
|
|
|
|
|
|
|
|||||||
Summary of warrants outstanding and exercisable as of December 31, 2012 is as follows:
| Range of Exercise |
Weighted Average Remaining Contractual Life (years) |
Number of Warrants Outstanding |
Number of Warrants Exercisable |
|||||||||
| $0.18 to $4.99 |
1.86 | 1,850,102 | 413,981 | |||||||||
| 5.00 to 9.99 |
0.04 | 1,068,905 | 1,068,905 | |||||||||
| 10.00 to 10.44 |
0.53 | 660,080 | 660,080 | |||||||||
|
|
|
|
|
|
|
|||||||
| $0.18 to $10.44 |
2.43 | 3,579,087 | 2,142,966 | |||||||||
|
|
|
|
|
|
|
|||||||
Note 13. Licenses and Gain on Extinguishment of Debt
University of Chicago License Agreement
In 2004, Opexa entered into an agreement with the University of Chicago (“University”) for the worldwide license to technology developed at Argonne National Laboratory, a U.S. Department of Energy Laboratory operated by the University. The license was later amended granting Opexa an exclusive, non-transferable worldwide license to the University’s stem cell technology. In consideration for the license and amendment, Opexa paid the University a total of $232,742 and issued the University 53,462 shares of common stock valued at $2,295,461. Opexa also agreed to pay the University $1.5 million and to issue the University 21,623 shares of Opexa common stock. In April 2007, the $1.5 million cash payment obligation was extended until July 31, 2007 and the obligation to issue shares of Opexa’s common stock was extended until July 31, 2007, with $112,440 accrued as of June 30, 2007.
F-21
Table of Contents
In July 2007, Opexa entered into a second amended and restated license agreement with the University that eliminated the obligations under the prior agreement for the payment of $1.5 million due July 31, 2007 and the obligation to issue 21,623 shares of Opexa common stock. These obligations were recorded as an intangible asset, with the liabilities recorded as a notes payable—current portion of $1.5 million and a stock payable of $112,440. As a result of the amendment and restatement of the license agreement with the University, $1,612,440 was reported as a gain on extinguishment of liability. Opexa applied the accounting guidance related to transfers and servicing of financial assets and extinguishments of liabilities as well as the guidance on debtor’s accounting for a modification or exchange of debt instruments. In August 2009, the University of Chicago license agreement was assigned to Novartis as part of Opexa’s sale of its stem cell technology platform to Novartis, and effective November 2, 2011, the license agreement was re-assigned to Opexa and the license agreement was amended and restated, as further described below.
Stem Cell Technology Agreement
In August 2009, Opexa entered into an exclusive agreement with Novartis for the further development of its stem cell technology. This technology, which has generated preliminary data, was in early preclinical development. Under the terms of the agreement, Novartis acquired the stem cell technology from Opexa and Novartis had full responsibility for funding and carrying out all research, development and commercialization activities. Opexa received an upfront cash payment of $3 million at the time the agreement was entered into and subsequently received $0.5 million as a technology transfer milestone fee.
In November 2011, Opexa re-acquired the stem cell assets from Novartis in consideration for releasing Novartis with respect to any further payment obligations owed to Opexa by Novartis In connection with the re-acquisition of the stem cell assets, a related license agreement with the University of Chicago was re-assigned to Opexa. Opexa and the University of Chicago entered into a Fourth Amended and Restated License Agreement in connection with such assignment to Opexa.
Note 14. Subsequent Events
In January 2013, 125,000 shares of common stock were sold and 975 additional commitment shares were issued to Lincoln Park under the $1.5 million purchase agreement for net proceeds of $142,400.
On January 23, 2013, Opexa closed a private offering consisting of convertible notes (the “January 2013 Notes”) and warrants to purchase shares of common stock for gross proceeds of $650,000 of which $100,000 was from a related party. The January 2013 Notes were scheduled to mature on January 23, 2014 and accrued interest at the rate of 12% per annum, compounded annually. The January 2013 Notes were convertible into common stock at the option of the investors at a price of $1.30 per share, subject to certain limitations. The principal balance plus accrued was payable within five business days of the receipt by Opexa of an aggregate of at least $7.5 million in proceeds from the sale of its equity securities and/or as payments from one or more partners or potential partners in return for granting a license, other rights, or an option to license or otherwise acquire rights with respect to Tcelna. On February 26, 2013, following the receipt of proceeds in excess of $7.5 million, Opexa paid principal and interest totaling $567,368 to holders of the January 2013 Notes and issued 77,034 shares of common stock to one holder of the January 2013 Notes who elected to convert the principal into common stock.
The warrants related to the January 2013 Notes financing have an exercise price of $1.24 per share, a five-year term and are exercisable for a maximum of 243,750 shares of common stock, subject to certain limitations. The Company can redeem the warrants at $0.01 per share if the Company’s common stock closes at or above $10.00 per share for 20 consecutive trading days.
Pursuant to the convertible secured promissory note financing effected by Opexa on July 25, 2012 (the “July 2012 Notes”), $1.0 million of the gross proceeds are maintained in a segregated account and subject to a deposit control agreement while the July 2012 Notes are outstanding. Pursuant to a waiver executed by the holders of in
F-22
Table of Contents
excess of two-thirds (66-2/3%) of the principal amount of the outstanding July 2012 Notes and accepted by Opexa, the amount of the cash subject to the deposit control agreement was reduced to $500,000 on January 29, 2013. In exchange for such waiver, the Company issued warrants to the holders of the July 2012 Notes to purchase an aggregate of 187,500 shares of the Company’s common stock. The warrants have an exercise price of $1.21 per share and a five-year term. The Company can redeem the warrants at $0.01 per underlying share of common stock if the common stock closes at or above $10.00 per share for 20 consecutive trading days.
In February 2013, three of the holders of the July 2012 Notes elected to convert an aggregate of $900,000 of the July 2012 Notes into shares of the Company’s Series A convertible preferred stock with further immediate conversion into shares of the Company’s common stock. Accordingly, the Company issued an aggregate of 288,229 shares of common stock to the holders.
In February 2013, Opexa sold an aggregate of 167,618 shares of common stock under the ATM Agreement dated September 6, 2012 for gross proceeds of $536,417. Under the ATM Agreement, Opexa may sell an aggregate of up to 1,000,000 shares of common stock from time to time through the placement agent with a commission equal to 3% of the gross proceeds. Opexa paid compensation and fees totaling $16,105 to the placement agent with respect to the shares sold.
On February 4, 2013, Opexa entered into an option and license agreement with Merck. Pursuant to the agreement, Merck has an option to acquire an exclusive, worldwide (excluding Japan) license of the Company’s Tcelna program for the treatment of multiple sclerosis. Under the terms of the agreement, the Company received an upfront payment of $5 million on February 20, 2013.
On February 11, 2013, Opexa sold an aggregate of 1,083,334 units in a registered offering, with each unit consisting of one share of common stock and a warrant to purchase half (0.5) a share of common stock, at a price of $3.00 per unit, for gross proceeds of $3,250,002. The shares of common stock and warrants were immediately separable and were issued separately such that no units were issued. The warrants are exercisable immediately upon issuance, have a four-year term and an exercise price of $3.00 per share. A fee of 6.0% of the gross proceeds was paid to the placement agent.
F-23
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
(unaudited)
| March 31, 2013 |
December 31, 2012 |
|||||||
| Assets | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 7,834,336 | $ | 592,004 | ||||
| Other current assets |
1,166,430 | 1,077,546 | ||||||
|
|
|
|
|
|||||
| Total current assets |
9,000,766 | 1,669,550 | ||||||
| Property & equipment, net of accumulated depreciation of $1,572,821 and $1,494,510, respectively |
1,189,328 | 1,265,041 | ||||||
| Restricted cash |
500,000 | 1,000,000 | ||||||
| Deferred financing costs, net of amortization of $111,578 and $58,639, respectively |
158,540 | 211,479 | ||||||
| Other long-term assets |
104,027 | — | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 10,952,661 | $ | 4,146,070 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 830,658 | $ | 412,096 | ||||
| Accrued expenses |
931,119 | 473,879 | ||||||
| Deferred revenue |
1,395,348 | — | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
3,157,125 | 885,975 | ||||||
| Long term liabilities: |
||||||||
| Convertible promissory notes, net of discount of $2,253,590 and $3,136,342, respectively |
301,410 | 318,658 | ||||||
| Convertible promissory notes-R/P, net of discount of $554,783 and $571,895 |
75,217 | 58,105 | ||||||
| Deferred revenue |
3,384,552 | — | ||||||
|
|
|
|
|
|||||
| Total liabilities |
6,918,304 | 1,262,738 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
— | — | ||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, no par value, 10,000,000 shares authorized, none issued and outstanding |
— | — | ||||||
| Common stock, $0.01 par value, 100,000,000 shares authorized, 7,991,559 and 6,249,369 shares issued and outstanding |
79,916 | 62,494 | ||||||
| Additional paid in capital |
117,743,543 | 112,432,458 | ||||||
| Deficit accumulated during the development stage |
(113,789,102 | ) | (109,611,620 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
4,034,357 | 2,883,332 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 10,952,661 | $ | 4,146,070 | ||||
|
|
|
|
|
|||||
See accompanying notes to unaudited consolidated financial statements
F-24
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
| Three Months Ended March 31, 2013 |
Three Months Ended March 31, 2012 |
Inception through March 31, 2013 |
||||||||||
| Revenue: |
||||||||||||
| Option Revenue |
$ | 220,100 | $ | — | $ | 220,100 | ||||||
| Research and development |
1,621,366 | 1,490,097 | 78,118,717 | |||||||||
| General and administrative |
1,102,435 | 816,196 | 31,220,051 | |||||||||
| Depreciation and amortization |
78,311 | 67,355 | 1,728,469 | |||||||||
| Loss on disposal of assets |
— | — | 513,345 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(2,582,012 | ) | (2,373,648 | ) | (111,360,482 | ) | ||||||
| Interest income |
1,874 | 136 | 1,360,571 | |||||||||
| Other income and expense, net |
37,910 | — | 699,056 | |||||||||
| Gain on extinguishment of debt |
— | — | 1,612,440 | |||||||||
| Gain on derivative instruments |
— | — | 1,941,826 | |||||||||
| Gain on sale of technology |
— | — | 3,000,000 | |||||||||
| Interest expense |
(1,635,254 | ) | (487 | ) | (11,042,513 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (4,177,482 | ) | $ | (2,373,999 | ) | $ | (113,789,102 | ) | |||
|
|
|
|
|
|
|
|||||||
| Basic and diluted loss per share |
$ | (0.58 | ) | $ | (0.41 | ) | N/A | |||||
| Weighted avg shares outstanding – Basic and diluted |
7,239,102 | 5,762,028 | N/A | |||||||||
See accompanying notes to unaudited consolidated financial statements
F-25
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(unaudited)
| Common Stock | Additional Paid in Capital |
Accumulated Deficit |
Total | |||||||||||||||||
| Shares | Par | |||||||||||||||||||
| Balances at December 31, 2012 |
6,249,369 | $ | 62,494 | $ | 112,432,458 | $ | (109,611,620 | ) | $ | 2,883,332 | ||||||||||
| Conversion of convertible notes |
365,263 | 3,652 | 996,348 | — | 1,000,000 | |||||||||||||||
| Discount related to beneficial conversion feature |
— | — | 141,829 | — | 141,829 | |||||||||||||||
| Discount on warrants attached to debt |
— | — | 195,969 | — | 195,969 | |||||||||||||||
| Shares issued for: |
||||||||||||||||||||
| cash, net of offering costs |
1,376,927 | 13,770 | 3,565,752 | — | 3,579,522 | |||||||||||||||
| Warrant expense |
— | — | 219,553 | — | 219,553 | |||||||||||||||
| Option expense |
— | — | 191,634 | — | 191,634 | |||||||||||||||
| Net loss |
— | — | — | (4,177,482 | ) | (4,177,482 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balances at March 31, 2013 |
7,991,559 | $ | 79,916 | $ | 117,743,543 | $ | (113,789,102 | ) | $ | 4,034,357 | ||||||||||
See accompanying notes to unaudited consolidated financial statements
F-26
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
| Three Months Ended March 31, |
Inception through March 31, 2013 |
|||||||||||
| 2013 | 2012 | |||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (4,177,482 | ) | $ | (2,373,999 | ) | $ | (113,789,102 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities |
||||||||||||
| Stock payable for acquired research and development |
— | — | 112,440 | |||||||||
| Stock issued for acquired research and development |
— | — | 26,286,589 | |||||||||
| Stock issued for services |
— | — | 2,061,743 | |||||||||
| Stock issued for debt in excess of principal |
— | — | 109,070 | |||||||||
| Amortization of discount on notes payable due to warrants and beneficial conversion feature |
1,237,662 | — | 8,094,392 | |||||||||
| Loss/(Gain) on extinguishment of debt |
— | — | (1,612,440 | ) | ||||||||
| Depreciation |
78,311 | 67,355 | 1,728,469 | |||||||||
| Amortization of debt financing costs |
52,939 | — | 635,956 | |||||||||
| Option expense |
411,187 | 205,420 | 16,677,120 | |||||||||
| Loss/(Gain) on derivative instruments |
— | — | (1,941,826 | ) | ||||||||
| Loss on disposition of fixed assets |
— | — | 513,345 | |||||||||
| Changes in: |
||||||||||||
| Deferred revenue |
4,779,900 | — | 4,779,900 | |||||||||
| Other current assets |
(115,589 | ) | (880,983 | ) | (1,268,642 | ) | ||||||
| Accounts payable - third parties and related parties |
418,562 | 1,022,532 | 257,911 | |||||||||
| Accrued expenses |
457,240 | (225,743 | ) | 1,062,478 | ||||||||
| Other assets |
(104,027 | ) | — | (104,027 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) operating activities |
3,038,703 | (2,185,418 | ) | (56,396,624 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities |
||||||||||||
| Purchase of property & equipment |
(2,598 | ) | (245,841 | ) | (2,224,797 | ) | ||||||
| Restricted cash |
500,000 | — | (500,000 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
497,402 | (245,841 | ) | (2,724,797 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities |
||||||||||||
| Common stock and warrants sold for cash, net of offering costs |
3,623,738 | — | 53,077,535 | |||||||||
| Common stock repurchased and canceled |
— | — | (325 | ) | ||||||||
| Proceeds from exercise of warrants and options |
— | — | 1,248,588 | |||||||||
| Proceeds from third party debt |
550,000 | — | 13,288,184 | |||||||||
| Proceeds from related party debt |
100,000 | — | 730,000 | |||||||||
| Deferred financing and offering costs |
(17,511 | ) | — | (527,003 | ) | |||||||
| Repayment on related party notes payable |
(100,000 | ) | — | (100,000 | ) | |||||||
| Repayments on notes payable |
(450,000 | ) | — | (761,222 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
3,706,227 | — | 66,955,757 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net change in cash and cash equivalents |
7,242,332 | (2,431,259 | ) | 7,834,336 | ||||||||
| Cash and cash equivalents at beginning of period |
592,004 | 7,109,215 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of period |
$ | 7,834,336 | $ | 4,677,956 | $ | 7,834,336 | ||||||
|
|
|
|
|
|
|
|||||||
F-27
Table of Contents
| Three Months Ended March 31, |
Inception through March 31, 2013 |
|||||||||||
| 2013 | 2012 | |||||||||||
| Cash paid for: |
||||||||||||
| Income tax |
$ | — | $ | — | $ | — | ||||||
| Interest |
18,647 | 487 | 173,756 | |||||||||
| NON-CASH TRANSACTIONS |
||||||||||||
| Issuance of common stock to Sportan shareholders |
— | — | 147,733 | |||||||||
| Issuance of common stock for accrued interest |
— | — | 789,287 | |||||||||
| Issuance of warrants to placement agent |
— | — | 37,453 | |||||||||
| Conversion of notes payable to common stock |
1,000,000 | — | 8,709,980 | |||||||||
| Conversion of accrued liabilities to common stock |
— | — | 197,176 | |||||||||
| Conversion of accounts payable to note payable |
— | — | 93,364 | |||||||||
| Discount on convertible notes relating to: |
— | — | ||||||||||
| Warrants |
195,969 | — | 6,170,341 | |||||||||
| Beneficial conversion feature |
141,829 | — | 3,444,982 | |||||||||
| Stock attached to notes |
— | — | 1,287,440 | |||||||||
| Fair value of derivative instrument |
— | — | 4,680,220 | |||||||||
| Derivative reclassified to equity |
— | — | 2,349,266 | |||||||||
| Unpaid additions to property and equipment |
— | 182,952 | — | |||||||||
| Amortization of deferred offering costs to paid-in capital |
45,450 | — | 92,789 | |||||||||
| Shares issued as deferred offering costs |
1,234 | — | 150,365 | |||||||||
See accompanying notes to unaudited consolidated financial statements
F-28
Table of Contents
OPEXA THERAPEUTICS, INC.
(a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(unaudited)
Note 1. Basis of Presentation
The accompanying interim unaudited consolidated financial statements of Opexa Therapeutics, Inc. (“Opexa” or the “Company”), a development stage company, have been prepared in accordance with accounting principles generally accepted in the United States of America and the rules of the Securities and Exchange Commission (“SEC”) and should be read in conjunction with the audited financial statements and notes thereto contained in Opexa’s latest Annual Report filed with the SEC on Form 10-K. In the opinion of management, all adjustments, consisting of normal recurring adjustments, necessary for a fair presentation of financial position and the results of operations for the interim periods presented have been reflected herein. The results of operations for interim periods are not necessarily indicative of the results to be expected for the full year. Notes to the financial statements that would substantially duplicate the disclosure contained in the audited financial statements for the most recent fiscal year as reported in Form 10-K have been omitted.
The accompanying consolidated financial statements include the accounts of Opexa and its wholly owned subsidiary, Opexa Hong Kong Limited (“Opexa Hong Kong”). All intercompany balances and transactions have been eliminated in the consolidation.
Note 2. Significant Accounting Polices
Revenue Recognition. Opexa recognizes revenue in accordance with FASB ASC 605, Revenue Recognition. ASC 605 requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services rendered; (3) consideration is fixed or determinable; and (4) collectability is reasonably assured.
On February 4, 2013, Opexa entered into an Option and License Agreement (the “Merck Agreement”) with Ares Trading SA (“Merck”), a wholly owned subsidiary of Merck Serono S.A. Pursuant to the terms, Merck has an option to acquire an exclusive, worldwide (excluding Japan) license of the Company’s Tcelna™ program for the treatment of multiple sclerosis (“MS”). Tcelna is currently in a Phase IIb clinical trial in patients with Secondary Progressive MS (“SPMS”). The option may be exercised by Merck prior to or upon the Company’s completion of the Phase IIb Trial.
Opexa received an upfront payment of $5 million for granting the option. If the option is exercised, Merck would pay the Company an upfront license fee of $25 million unless Merck is unable to advance directly into a Phase III clinical trial of Tcelna for SPMS without a further Phase II clinical trial (as determined by Merck), in which event the upfront license fee would be $15 million. After exercising the option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although the Company would retain an option to co-fund certain development in exchange for increased royalty rates. The Company would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights outside of MS.
Opexa evaluated the Merck Agreement and determined that the $5 million upfront payment from Merck has “stand-alone value”. Opexa’s continuing performance obligations, in connection with the $5 million payment, include the execution and completion of the Phase IIb clinical trial in SPMS using commercially reasonable efforts at the Company’s own costs. As a “stand-alone value” term in the Merck Agreement, the $5 million upfront payment is determined to be a single unit of accounting, and is recognized as revenue on a straight-line basis over the exclusive option period based on the expected completion term of the Phase IIb clinical trial in SPMS. Opexa includes the unrecognized portion of the $5 million as deferred revenue on the consolidated balance sheets.
F-29
Table of Contents
Cash and Cash Equivalents. Opexa considers all highly liquid investments with an original maturity of three months or less, when purchased, to be cash equivalents. Investments with maturities in excess of three months but less than one year are classified as short-term investments and are stated at fair market value.
Opexa primarily maintains cash balances on deposit in accounts at a U.S.-based financial institution. The aggregate cash balance on deposit in these accounts is insured by the Federal Deposit Insurance Corporation up to $250,000. Opexa’s cash balances on deposit in these accounts may, at times, exceed the federally insured limits. Opexa has not experienced any losses in such accounts.
At March 31, 2013, Opexa invested approximately $7.7 million in a savings account. For the three months ended March 31, 2013, the savings account recognized an average market yield of 0.25%. Interest income of $1,874 was recognized for the three months ended March 31, 2013 in the consolidated statements of expenses.
Note 3. Other Current Assets
Other current assets consisted of the following at March 31, 2013 and December 31, 2012:
| Description |
March 31, 2013 |
December 31, 2012 |
||||||
| Supplies inventory |
$ | 491,377 | $ | 604,179 | ||||
| Deferred offering costs |
309,969 | 341,166 | ||||||
| Prepaid expenses |
365,084 | 132,201 | ||||||
|
|
|
|
|
|||||
| $ | 1,166,430 | $ | 1,077,546 | |||||
|
|
|
|
|
|||||
Supplies inventory at March 31, 2013 and December 31, 2012 includes reagents and supplies that will be used to manufacture Tcelna and placebo product in Opexa’s Phase IIb clinical study. Opexa expects to amortize these prepaid reagents and supplies to research and development costs in the consolidated statements of expenses over the course of the clinical study.
Deferred offering costs at March 31, 2013 and December 31, 2012 include costs incurred from third parties in connection with the implementation of an at-the-market program (“ATM Agreement”) in September 2012 pursuant to which Opexa may sell shares of its common stock from time to time depending upon market demand through a sales agent in transactions deemed to be an “at-the-market” offering as defined in Rule 415 of the Securities Act of 1933. As of March 31, 2013, the remaining costs of $88,446 in connection with the implementation of the ATM Agreement remained capitalized and are included in other current assets in the consolidated balance sheets. Upon the sales of shares of common stock under the ATM Agreement, the remaining capitalized costs are offset against the proceeds of such sales of shares of common stock.
Deferred offering costs at March 31, 2013 also include costs incurred from third parties in connection with the implementation of a $1.5 million purchase agreement and a $15 million purchase agreement (collectively, the “purchase agreements”) in November 2012 pursuant to which Opexa has the right to sell to Lincoln Park Capital Fund, LLC (“Lincoln Park”) an aggregate of up to $16.5 million in shares of its common stock, subject to certain conditions and limitations. As of March 31, 2013, the remaining costs of $221,523 in connection with the implementation of the purchase agreements remained capitalized and are included in other current assets in the consolidated balance sheets. Upon the sales of shares of common stock under the purchase agreements, the remaining capitalized costs are offset against the proceeds of such sales of shares of common stock.
Prepaid expenses at March 31, 2013 include advance payments totaling $220,374 made to vendors and consultants for the conduct of the Phase IIb clinical trial in SPMS.
F-30
Table of Contents
Note 4. Restricted Cash
Pursuant to the July 2012 Note financing, $1.0 million of the gross proceeds were initially required to be maintained in a segregated account and subject to a deposit control agreement while the July 2012 Notes are outstanding. Pursuant to a waiver executed by the holders of in excess of two-thirds (66-2/3%) of the principal amount of the outstanding July 2012 Notes and accepted by Opexa, the amount of the cash subject to the deposit control agreement was reduced to $500,000 on January 29, 2013. In exchange for such waiver, the Company issued warrants to the holders of the July 2012 Notes to purchase an aggregate of 187,500 shares of the Company’s common stock (see Note 11). As of March 31, 2013, the $500,000 balance in the controlled account is reported as restricted cash in the consolidated balance sheets.
Note 5. Deferred Financing Costs
Deferred financing costs at March 31, 2013 consist of costs incurred from third parties in conjunction with the July 2012 Notes. The costs in connection with the debt financing were capitalized and are amortized to interest expense over the term of the related debt. As of March 31, 2013, the unamortized deferred financing costs totaling $158,540 are reported as deferred financing costs in the consolidated balance sheets. During the quarter ended March 31, 2013, Opexa amortized $52,939 of deferred financing costs as interest expense.
Note 6. Other Assets
Other long-term assets at March 31, 2013 consist of legal costs incurred from third parties in conjunction with the Merck Agreement. These costs were capitalized and are amortized to general and administrative expenses on the consolidated statements of operations in conjunction with the recognition of revenue on a straight-line basis over the exclusive option period based on the term of the Phase IIb clinical trial in SPMS. During the quarter ended March 31, 2013, Opexa amortized $6,765 of legal costs to general and administrative expenses on the consolidated statements of operations. Opexa included the current portion of the legal costs of $42,887 in other current assets on the consolidated balance sheets and the long term portion of the legal costs of $104,027 in other long-term assets on the consolidated balance sheets.
Note 7. Convertible Promissory Notes
On January 23, 2013, Opexa closed a private offering consisting of convertible notes (the “January 2013 Notes”) and warrants to purchase shares of common stock for gross proceeds of $650,000 of which $100,000 was from a related party (see Note 8). The January 2013 Notes are scheduled to mature on January 23, 2014 and accrue interest at the rate of 12% per annum, compounded annually. The January 2013 Notes are convertible into common stock at the option of the investors at a price of $1.30 per share, subject to certain limitations. The principal balance plus accrued interest is payable within five business days of the receipt by Opexa of an aggregate of at least $7.5 million in proceeds from the sale of its equity securities and/or as payments from one or more partners or potential partners in return for granting a license, other rights, or an option to license or otherwise acquire rights with respect to Tcelna.
The January 2013 Notes were analyzed at issuance for a beneficial conversion feature and Opexa concluded that a beneficial conversion feature exists. The beneficial conversion feature was measured using the commitment-date stock price and was determined to be $141,829 of which $21,820 was attributable to the related party. Opexa also analyzed the Notes for derivative accounting consideration and determined that derivative accounting does not apply.
In connection with the issuance of the January 2013 Notes, Opexa also issued Series J warrants to purchase an aggregate of 243,750 shares of Opexa’s common stock (see Note 11), subject to certain limitations and adjustments. The relative fair value of the warrant liabilities of $195,969, together with the beneficial conversion feature of $141,829 were recognized as a debt discount and are amortized to interest expense in the consolidated statements of expenses over the term of the January 2013 Notes using the effective interest method.
F-31
Table of Contents
On February 26, 2013, following the receipt of $3.25 million in gross proceeds during February 2013 from the sale of common stock and warrants to purchase shares of common stock, and following the receipt of the upfront payment of $5 million from Merck on February 20, 2013, Opexa paid principal and interest totaling $567,368 to holders of the January 2013 Notes, of which $100,000 was to a related party, and issued 77,034 shares of common stock to one holder of the January 2013 Notes who elected to convert the principal of $100,000.
During the quarter ended March 31, 2013, the debt discount of $337,798 in connection with the January 2013 Notes was fully amortized to interest expense.
In February 2013, three of the third party holders of the July 2012 Notes elected to convert their principal amounts of $900,000 into shares of the Company’s Series A convertible preferred stock with further immediate conversion into 288,229 shares of the Company’s common stock. As of March 31, 2013, an aggregate of $3,185,000 in principal amount of the July 2012 Notes to third parties and related parties is reported as long term liabilities in the consolidated balance sheets, of which $630,000 was issued to related parties (see Note 8).
The following table provides a summary of the changes in convertible debt—third parties, net of unamortized discount, during the quarter ended March 31, 2013:
| Balance at December 31, 2012 |
$ | 318,658 | ||
| January 2013 Notes, face value |
550,000 | |||
| Discount on beneficial conversion feature of January 2013 Notes at issuance |
(120,009 | ) | ||
| Discount on fair value of Series J warrant liability at issuance |
(165,820 | ) | ||
| Repayment of January 23, 2013 Notes |
(450,000 | ) | ||
| Conversion of January 23, 2013 Notes into common stock |
(100,000 | ) | ||
| Conversion of July 25, 2012 Notes into common stock |
(900,000 | ) | ||
| Amortization of debt discount to interest expense through March 31, 2013 |
1,168,581 | |||
|
|
|
|||
| Balance at March 31, 2013 |
$ | 301,410 | ||
|
|
|
Note 8. Related Party Transactions
Investors in the January 2013 Notes offering included one member of Opexa’s Board of Directors who was issued a note with a principal amount of $100,000 (see Note 7).
The following table provides a summary of the changes in convertible debt—related parties, net of unamortized discount, during the quarter ended March 31, 2013:
| Balance at December 31, 2012 |
$ | 58,105 | ||
| January 2013 Notes, face value |
100,000 | |||
| Discount on beneficial conversion feature of January 2013 Notes at issuance |
(21,820 | ) | ||
| Discount on fair value of Series J warrant liability at issuance |
(30,149 | ) | ||
| Repayment of January 23, 2013 Notes |
(100,000 | ) | ||
| Amortization of debt discount to interest expense through March 31, 2013 |
69,081 | |||
|
|
|
|||
| Balance at March 31, 2013 |
$ | 75,217 | ||
|
|
|
F-32
Table of Contents
For the quarter ended March 31, 2013, cash compensation totaling $25,000 earned by Director David E. Jorden for his service as Opexa’s Acting Chief Financial Officer is reported in general and administrative expense in the consolidated statements of expenses. Concurrent with the appointment of Karthik Radhakrishnan as Chief Financial Officer on March 29, 2013, Mr. Jorden resigned as the Company’s Acting Chief Financial Officer but will continue in his role as director on Opexa’s Board. As of March 31, 2013, cash compensation totaling $8,333 was due to Mr. Jorden and is included in accounts payable in the consolidated balance sheets.
Note 9. Equity
For the quarter ended March 31, 2013, equity related transactions were as follows:
| • | In January 2013, 125,000 shares of common stock were sold and 975 additional commitment shares were issued to Lincoln Park under the $1.5 million purchase agreement for net proceeds of $142,400. |
| • | 365,263 shares of common stock were issued in connection with the conversion of the January 2013 and July 2012 Notes (see Note 7). |
| • | In February 2013, Opexa sold an aggregate of 167,618 shares of common stock under the ATM Agreement for gross proceeds of $536,417. |
| • | On February 11, 2013, Opexa sold an aggregate of 1,083,334 units in a registered offering, with each unit consisting of one share of common stock and a warrant to purchase half (0.5) a share of common stock, at a price of $3.00 per unit, for gross proceeds of $3,250,002. The shares of common stock and warrants were immediately separable and were issued separately such that no units were issued. The warrants are exercisable immediately upon issuance, have a four-year term and an exercise price of $3.00 per share. A fee of 6.0% of the gross proceeds was paid to the placement agent. |
For the quarter ended March 31, 2013, $350,530 was netted against additional paid in capital as stock offering costs.
Note 10. Stock-Based Compensation
Stock Options
The 2010 Stock Incentive Plan (the “2010 Plan”) provides for the grant of equity incentive awards to employees, directors and consultants of Opexa in the form of incentive stock options or nonqualified stock options, as well as restricted stock, stock appreciation rights, restricted stock units and performance awards that may be settled in cash, stock or other property. The 2010 Plan is the successor to and continuation of Opexa’s June 2004 Compensatory Stock Option Plan (the “2004 Plan”). A total of 625,000 shares of common stock are authorized to be issued for awards made under the 2010 Plan through September 2020, plus (i) the number of shares subject to stock options outstanding under the 2004 Plan that are forfeited or terminate prior to exercise and would otherwise be returned to the share reserves under the 2004 Plan and (ii) any reserved shares under the 2004 Plan that were not issued or subject to outstanding grants. In addition, shares subject to awards granted under the 2010 Plan that terminate or expire before being exercised or settled will become available for grant under the 2010 Plan. As of March 31, 2013, options to purchase an aggregate of 903,289 shares were issued and outstanding.
Opexa accounts for share-based compensation, including options and nonvested shares, according to the provisions of Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 718, “Share Based Payment.” During the three months ended March 31, 2013, Opexa recognized option expense of $191,634 which includes the related expense for the options that are expected to vest based on achievement of their related performance conditions (see below). Unamortized stock compensation expense as of March 31, 2013 amounted to $1,126,780.
F-33
Table of Contents
Stock Option Activity
A summary of stock option activity for the three months ended March 31, 2013 is presented below:
| Number of Shares |
Wtd. Avg. Exercise Price |
Wtd. Avg. Remaining Contract Term (# years) |
Intrinsic Value |
|||||||||||||
| Outstanding at January 1, 2013 |
824,620 | $ | 5.54 | |||||||||||||
| Granted |
125,000 | 2.34 | ||||||||||||||
| Exercised |
— | — | ||||||||||||||
| Forfeited and canceled |
(46,331 | ) | 4.73 | |||||||||||||
|
|
|
|
|
|||||||||||||
| Outstanding at March 31, 2013 |
903,289 | $ | 5.14 | 7.7 | $ | 104,769 | ||||||||||
| Exercisable at March 31, 2013 |
485,124 | $ | 6.63 | 6.5 | $ | 100,730 | ||||||||||
Option awards are granted with an exercise price equal to the market price of Opexa’s stock at the date of issuance, generally have a ten-year life, and have various vesting dates that range from no vesting or partial vesting upon date of grant to full vesting on a specified date. Opexa estimates the fair value of stock options using the Black-Scholes option-pricing model and records the compensation expense ratably over the service period.
During the three months ended March 31, 2013, an option to purchase an aggregate of 125,000 shares was granted to an employee at an exercise price of $2.34. This option has a term of ten years and has a vesting schedule of three years. Fair value of $285,226 was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for the option issued to an employee during the three months ended March 31, 2013 include (1) discount rate of 1.87%, (2) expected term of 5.25 years, (3) expected volatility of 194% and (4) zero expected dividends.
During the three months ended March 31, 2013, options to purchase 46,331 shares were forfeited and cancelled.
Warrant Activity
A summary of warrant activity for the three months ended March 31, 2013 is presented below:
| Number of Shares |
Wtd. Avg. Exercise Price |
Wtd. Avg. Remaining Contract Term (# years) |
Intrinsic Value |
|||||||||||||
| Outstanding at January 1, 2013 |
3,579,087 | $ | 5.64 | |||||||||||||
| Granted |
972,918 | 2.21 | ||||||||||||||
| Exercised |
— | — | ||||||||||||||
| Forfeited and canceled |
(1,129,359 | ) | 7.65 | |||||||||||||
|
|
|
|
|
|||||||||||||
| Outstanding at March 31, 2013 |
3,422,646 | $ | 4.01 | 3.5 | $ | 480,000 | ||||||||||
| Exercisable at March 31, 2013 |
3,422,646 | $ | 4.01 | 3.5 | $ | 480,000 | ||||||||||
In connection with the January 2013 Notes (see Note 7), investors were issued five-year warrants to purchase up to an aggregate of 243,750 shares of common stock, at an exercise price of $1.24 per share. The estimated relative fair value of the investor warrants was $195,969 and was calculated using the Black-Scholes valuation model. The following assumptions were used: (1) no expected dividends, (2) risk free interest rate of 0.76%, (3) expected volatility of 191% and (4) expected life of five years. Opexa can redeem the warrants at $0.01 per share if the Company’s common stock closes at or above $10.00 per share for 20 consecutive trading days.
F-34
Table of Contents
Pursuant to a waiver executed by the holders of in excess of two-thirds (66-2/3%) of the principal amount of the outstanding July 2012 Notes and accepted by Opexa, the amount of the cash subject to the deposit control agreement was reduced to $500,000 on January 29, 2013. In exchange for such waiver, the Company issued warrants to the holders of the July 2012 Notes to purchase an aggregate of 187,500 shares of the Company’s common stock. The warrants have an exercise price of $1.21 per share and a five-year term. The estimated fair value of the warrants was $219,553 and was calculated using the Black-Scholes valuation model. The following assumptions were used: (1) no expected dividends, (2) risk free interest rate of 0.90%, (3) expected volatility of 191% and (4) expected life of five years. Opexa can redeem the warrants at $0.01 per underlying share of common stock if the common stock closes at or above $10.00 per share for 20 consecutive trading days.
In connection with the February 2013 registered offering (See Note 10), Opexa issued warrants to the investors on February 11, 2013 to purchase an aggregate of 541,668 shares of common stock at an exercise price of $3.00 per share. These warrants have a term of four years and were immediately exercisable.
Note 11. Subsequent Events
Subsequent to March 31, 2013, Opexa granted its directors, officers and employees options to purchase an aggregate of 207,822 shares of common stock with a fair value of $356,335 at an exercise price of $1.75.
F-35
Table of Contents
Shares
Common Stock

PROSPECTUS
Aegis Capital Corp
Through and including , 2013 (the 25th day after the date of this offering), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Table of Contents
PART II
Information Not Required In Prospectus
Item 13. Other Expenses of Issuance and Distribution.
The following is a statement of estimated expenses in connection with the issuance and distribution of the securities being registered, other than the underwriting discount. All expenses incurred with respect to the registration of the common stock will be borne by us. All amounts are estimates except the SEC registration fee and the FINRA filing fee.
| Amount to be Paid |
||||
| SEC Registration Fee |
$ | 2,353 | ||
| FINRA Filing Fee |
2,588 | |||
| NASDAQ Fee |
65,000 | |||
| Printing Expenses |
75,000 | |||
| Legal Fees and Expenses |
225,000 | |||
| Accounting Fees and Expenses |
15,000 | |||
| Blue Sky Fees and Expenses |
5,000 | |||
| Transfer Agent and Registrar Fees and Expenses |
5,000 | |||
| Miscellaneous Expenses |
150,059 | |||
|
|
|
|||
| $ | 545,000 | |||
|
|
|
|||
Item 14. Indemnification of Directors and Officers.
Section 8.101 of the Texas Corporation Law (or the TCA) authorizes the Registrant to indemnify certain persons, including any person who was, is or is threatened to be made a named defendant or respondent in a threatened, pending or completed action or other proceeding, because the person is or was a director or officer, against judgments and reasonable expenses actually incurred by the person in connection with the threatened, pending or completed action or other proceeding. The Registrant is required by Section 8.051 of the TCA to indemnify a director or officer against reasonable expenses actually incurred by him or her in connection with a threatened, pending, or completed action or other proceeding in which he or she is a named defendant or respondent because he or she is or was a director or officer if he or she has been wholly successful, on the merits or otherwise, in the defense of the action or proceeding.
The Registrant’s restated certificate of formation provides that none of its directors shall be personally liable to the Registrant or its shareholders for monetary damages for an act or omission in such director’s capacity as a director; provided, however, that the liability of such director is not limited to the extent that such director is found liable for (i) a breach of the director’s duty of loyalty to the Registrant or its shareholders, (ii) an act or omission not in good faith that constitutes a breach of duty of the director to the Registrant or an act or omission that involves intentional misconduct or a knowing violation of the law, (iii) a transaction from which the director received an improper benefit, whether or not the benefit resulted from an action taken within the scope of the director’s office, or (iv) an act or omission for which the liability of the director is expressly provided by an applicable statute.
The Registrant’s restated certificate of formation and amended and restated bylaws provide that the Registrant shall indemnify its officers, directors, agents and any other persons to the fullest extent permitted by applicable law. The Registrant’s directors and officers are covered by insurance indemnifying them against certain liabilities which might be incurred by them in their capacities as such. Pursuant to terms of their employment contracts, certain of the Registrant’s officers are entitled to indemnification in their capacity as such and to the fullest extent permitted by applicable law.
At the present time, there is no pending litigation or proceeding involving a director, officer, employee or other agent of the Registrant in which indemnification would be required or permitted. The Registrant is not aware of any threatened litigation or proceeding which may result in a claim for such indemnification.
II-1
Table of Contents
Item 15. Recent Sales of Unregistered Securities.
The following is a summary of all securities that we have sold within the past three years without registration under the Securities Act of 1933, as amended. The transaction amount and the price of securities listed below have been adjusted to reflect the 1-for-4 reverse split which was implemented on December 14, 2012.
| • | On August 18, 2010, we issued 13,750 restricted shares of common stock to a consultant for professional services. The common stock was issued in reliance on the exemption from registration contained in Section 4(2) of the Securities Act and the rules and regulations promulgated thereunder. |
| • | On June, 23, 2010, we issued an aggregate of 665,045 shares of common stock to the holders of our 10% convertible promissory notes, consisting of (i) 626,000 shares pursuant to the conversion of all currently outstanding notes with an aggregate outstanding principal balance of $1,252,000 and (ii) 39,045 shares in payment of one-half of the accrued and unpaid interest on said notes of $78,091. On May 6, 2010, we issued 25,000 shares of common stock to a holder of a note pursuant to the conversion of such note with a principal balance of $50,000. The common stock was issued in reliance on the exemptions from registration contained in Sections 3(a)(9) and 4(2) of the Securities Act and the rules and regulations promulgated thereunder. |
| • | On April 18, 2011, we issued 12,576 restricted shares of common stock to a consultant for professional services. The common stock was issued in reliance on the exemption from registration contained in Section 4(2) of the Securities Act and the rules and regulations promulgated thereunder. |
| • | On July 25, 2012, we completed a private offering of $4,085,000 in principal amount of convertible secured promissory notes and Series I warrants to purchase an aggregate of 1,436,121 shares of common stock, subject to certain adjustments, with an exercise price of $2.56 per share. The offers and sales were made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based upon representations made by the investors, each of whom was an accredited investor. In February 2013, we issued an aggregate of 288,229 shares of common stock to three noteholders who elected to convert such notes into shares of Series A convertible preferred stock with further immediate conversion into common stock. |
| • | On November 2, 2012, we completed a private placement to Lincoln Park Capital Fund, LLC pursuant to which we have the right to sell to Lincoln Park up to $15,000,000 in shares of common stock, subject to certain limitations, from time to time over the 30-month period commencing on the date that a registration statement covering the resale of the shares is declared effective by the SEC. The issuance and sale was made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based on the offering of such securities to one investor, the lack of any general solicitation or advertising in connection with such issuance, the representation of such investor that it was an accredited investor and that it was purchasing the shares for its own account and without a view to distribute them. |
| • | On January 23, 2013, we completed a private offering of $650,000 in principal amount of unsecured convertible promissory notes and Series J warrants to purchase an aggregate of 243,750 shares of common stock, subject to certain adjustments, with an exercise price of $1.24 per share. The offers and sales were made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based upon representations made by the investors, each of whom was an accredited investor. On February 26, 2013, we issued 77,034 shares of common stock to a noteholder who elected to convert such note into common stock. |
| • | On January 30, 2013, we issued Series K warrants to purchase an aggregate of 187,500 shares of common stock, at an exercise price of $1.21 per share, to the holders of our July 2012 convertible secured promissory notes in exchange for a waiver and reduction in the cash held in a controlled account as security for repayment of the notes. The issuance was made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based upon representations made by the investors, each of whom was an accredited investor. |
II-2
Table of Contents
Item 16. Exhibits and Financial Statement Schedules.
| (a) | Exhibits |
The exhibits to the registration statement are listed in the Exhibit Index to this registration statement and are incorporated herein by reference.
| (b) | Financial statement schedules |
All schedules have been omitted because either they are not required, are not applicable or the information is otherwise set forth in the financial statements and related notes thereto.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(A) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B) Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
II-3
Table of Contents
(5) That for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to any charter provision, by law or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(7) The undersigned registrant hereby undertakes that:
(i) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(I) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and
(ii) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of The Woodlands, State of Texas, on June 17, 2013.
| OPEXA THERAPEUTICS, INC. | ||
| By: | /s/ Neil K. Warma | |
| Neil K. Warma | ||
| President and Chief Executive Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Neil K. Warma and Karthik Radhakrishnan, and each of them, his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments, including post-effective amendments, to this Registration Statement, and any registration statement relating to the offering covered by this Registration Statement and filed pursuant to Rule 462(b) under the Securities Act of 1933, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully for all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that each of said attorneys in fact and agents or their substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Name |
Title |
Date | ||
| /s/ Neil K. Warma Neil K. Warma |
President, Chief Executive Officer and Director (Principal Executive Officer) |
June 17, 2013 | ||
| /s/ Karthik Radhakrishnan Karthik Radhakrishnan |
Chief Financial Officer (Principal Financial and Accounting Officer) |
June 17, 2013 | ||
| /s/ David E. Jorden David E. Jorden |
Director |
June 17, 2013 | ||
| /s/ Gail J. Maderis Gail J. Maderis |
Director |
June 17, 2013 | ||
| /s/ Michael S. Richman Michael S. Richman |
Director |
June 17, 2013 | ||
| /s/ Scott B. Seaman Scott B. Seaman |
Director |
June 17, 2013 | ||
II-5
Table of Contents
EXHIBIT INDEX
| Exhibit |
Description | |
| 1.1@ |
Form of underwriting agreement. | |
| 2.1 |
Stock Purchase Agreement by and among Sportan United Industries, Inc., Jason G. Otteson, PharmaFrontiers Corp., Warren C. Lau and other PharmaFrontiers stockholders, dated May 5, 2004 (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed June 4, 2004, File No. 000-25513). | |
| 2.2 |
Agreement and Plan of Reorganization by and among PharmaFrontiers Corp., Pharma Acquisition Corp and Opexa Pharmaceuticals, Inc. dated October 7, 2004 (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on 8-K filed October 8, 2004, File No. 000-25513). | |
| 3.1 |
Restated Certificate of Formation of Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 3.2 |
Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock of Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 3.3 |
Certificate of Amendment of the Restated Certificate of Formation of Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on December 14, 2012). | |
| 3.4 |
Amended and Restated By-laws, as amended (incorporated by reference to Exhibit 3.3 to the Company’s Annual Report on Form 10-K filed on March 8, 2011). | |
| 4.1 |
Form of Common Stock Certificate (incorporated by reference to Exhibit 4.7 to the Company’s Registration Statement on Form S-3 filed on November 13, 2009, File No. 333-163108). | |
| 4.2 |
Unit Purchase Agreement dated August 8, 2008 by and among Opexa Therapeutics, Inc. and the Investors named therein in connection with Unit offering of common stock and Series F Warrants (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed August 12, 2008). | |
| 4.3 |
Form of Series F Warrant issued in connection with August 8, 2008 financing (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed August 12, 2008). | |
| 4.4 |
Registration Rights Agreement dated August 8, 2008 between Opexa Therapeutics, Inc. and the Investors named therein in connection with common stock and Series F Warrants (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed August 12, 2008). | |
| 4.5 |
Unit Purchase Agreement dated April 14, 2009 by and among Opexa Therapeutics, Inc. and the Investors party thereto for the 10% Convertible Notes and Series G Warrants (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed April 16, 2009). | |
| 4.6 |
Form of Series G Warrant issued on April 14, 2009 (incorporated by reference to Exhibit 10.4 of the Company’s Current Report on Form 8-K filed April 16, 2009). | |
| 4.7 |
Form of Securities Purchase Agreement dated as of December 9, 2009 by and between Opexa Therapeutics, Inc. and each investor signatory thereto for Unit offering of Common Stock and Series A and Series B Warrants (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed December 10, 2009). | |
| 4.8 |
Form of Common Stock Purchase Warrant for Series A and Series B Warrants (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed December 10, 2009). | |
| 4.9 |
Form of Series H Warrant issued on February 11, 2011 (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed February 8, 2011). | |
Table of Contents
| Exhibit |
Description | |
| 4.10 |
Form of Series I Warrant issued on July 25, 2012 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 4.11 |
Form of Series J Warrant issued on January 23, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on January 23, 2013). | |
| 4.12 |
Form of Series K Warrant issued on January 30, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on January 30, 2013). | |
| 4.13 |
Form of Series L Warrant issued on February 11, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on February 7, 2013). | |
| 4.14 |
Form of Securities Purchase Agreement, dated as of February 7, 2013, by and between Opexa Therapeutics, Inc. and each investor signatory thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 7, 2013). | |
| 4.15 |
Placement Agent Agreement, dated February 7, 2013, by and between Opexa Therapeutics, Inc. and Dawson James Securities, Inc. (incorporated by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed on February 7, 2013). | |
| 5.1@ |
Opinion of Pillsbury Winthrop Shaw Pittman LLP. | |
| 10.1+ |
Opexa Therapeutics, Inc. June 2004 Compensatory Stock Option Plan (incorporated by reference to Exhibit B to the Company’s Definitive Information Statement on Schedule 14C filed on June 29, 2004, File No. 000-25513). | |
| 10.2+ |
Certificate of Amendments to the Opexa Therapeutics, Inc. June 2004 Compensatory Stock Option Plan (incorporated by reference to Exhibit 10.15 of the Company’s Annual Report on Form 10-K filed March 5, 2010). | |
| 10.3+ |
Employment Agreement dated June 16, 2008 by and between Opexa Therapeutics, Inc. and Neil K. Warma (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 18, 2008). | |
| 10.4+ |
Amended and Restated Employment Agreement entered into on April 21, 2010 by and between Opexa Therapeutics, Inc. and Donna R. Rill (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed April 27, 2010). | |
| 10.6 |
License Agreement dated September 5, 2001 by and between Opexa Therapeutics, Inc. and Baylor College of Medicine (incorporated by reference to Exhibit 10.14 to the Company’s Annual Report on Form 10-KSB filed April 15, 2005, File No. 000-25513). | |
| 10.7 |
Lease dated August 19, 2005 by and between Opexa Therapeutics, Inc. and Dirk D. Laukien (incorporated by reference to Exhibit 10.13 to the Company’s Annual Report on Form 10-KSB filed March 31, 2006, File No. 000-25513). | |
| 10.8 |
License Agreement dated January 13, 2006 by and between Opexa Therapeutics, Inc. and Shanghai Institute for Biological Services (incorporated by reference to Exhibit 10.23 to the Company’s Registration Statement on Form SB-2 (Amendment No. 1) filed February 9, 2006, File No. 333-126687). | |
| 10.9 |
Fourth Amended and Restated License Agreement, dated November 2, 2011, by and between Opexa Therapeutics, Inc. and the University of Chicago (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed November 4, 2011). | |
| 10.10+ |
Opexa Therapeutics, Inc. 2010 Stock Incentive Plan (incorporated by reference to Appendix A to the Company’s Schedule 14A definitive proxy statement filed September 14, 2010). | |
| 10.11+ |
Form of award agreement for awards to be made under the Opexa Therapeutics, Inc. 2010 Stock Incentive Plan (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed October 22, 2010). | |
Table of Contents
| Exhibit No. |
Description | |
| 10.12 |
Form of Note Purchase Agreement, dated July 25, 2012, by and among Opexa Therapeutics, Inc. and the investors signatory thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 10.13 |
Form of 12% Convertible Secured Promissory Note issued to investors on July 25, 2012 (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 10.14 |
Form of Security Agreement, dated July 25, 2012, by and among Opexa Therapeutics, Inc., the investors signatory thereto, and Alkek & Williams Ventures, Ltd. as collateral agent for the investors (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 10.15 |
Deposit Account Control Agreement, dated July 25, 2012, by and among Opexa Therapeutics, Inc., Alkek & Williams Ventures, Ltd. as collateral agent for the investors, and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 10.16 |
Form of Registration Rights Agreement, dated July 25, 2012, by and among Opexa Therapeutics, Inc. and the investors signatory thereto (incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| 10.17 |
Sales Agreement, dated September 6, 2012, by and between Opexa Therapeutics, Inc. and Brinson Patrick Securities Corporation (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 7, 2012). | |
| 10.18 |
$15,000,000 Purchase Agreement, dated as of November 2, 2012, by and between Opexa Therapeutics, Inc. and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 5, 2012). | |
| 10.19 |
$1,500,000 Purchase Agreement, dated as of November 5, 2012, by and between Opexa Therapeutics, Inc. and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on November 5, 2012). | |
| 10.20 |
Registration Rights Agreement, dated as of November 2, 2012, by and between Opexa Therapeutics, Inc. and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed November 5, 2012). | |
| 10.21 |
Form of unsecured 12% Convertible Promissory Note issued to investors on January 23, 2013 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on January 23, 2013). | |
| 10.22 |
Form of Waiver and Omnibus Amendment, dated January 30, 2013, by and between Opexa Therapeutics, Inc. and certain investors (incorporated by reference to Exhibit 10.22 to the Company’s Annual Report on Form 10-K filed on March 29, 2013). | |
| 10.23## |
Option and License Agreement, dated February 4, 2013, by and between Ares Trading SA, a wholly owned subsidiary of Merck Serono S.A., and Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 5, 2013). | |
| 10.24+ |
Offer Letter, effective March 29, 2013, by and between Opexa Therapeutics, Inc. and Karthik Radhakrishnan (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 1, 2013). | |
| 21.1 |
List of Subsidiaries (incorporated by reference to Exhibit 21.1 to the Company’s Annual Report on Form 10-K filed on March 29, 2013). | |
| 23.1@ |
Consent of Pillsbury Winthrop Shaw Pittman LLP (included in Exhibit 5.1). | |
Table of Contents
| Exhibit No. |
Description | |
| 23.2* |
Consent of MaloneBailey, LLP. | |
| 24.1* |
Power of Attorney (included on signature page). | |
| 101.INS@ |
XBRL Instance Document. | |
| 101.SCH@ |
Taxonomy Extension Schema Document. | |
| 101.CAL@ |
Taxonomy Extension Calculation Linkbase Document. | |
| 101.DEF@ |
Taxonomy Extension Definition Linkbase Document. | |
| 101.LAB@ |
Taxonomy Extension Labels Linkbase Document. | |
| 101.PRE@ |
Taxonomy Extension Presentation Linkbase Document. | |
| * | Filed herewith |
| + | Management contract or compensatory plan or arrangement |
| ## | Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
| @ | To be filed by amendment. In accordance with Rule 406T of Regulation S-T, the XBRL-related information in Exhibit 101 to this registration statement is deemed not filed or part of this registration statement or prospectus for purposes of Section 11 or 12 of the Securities Act, is deemed not filed for purposes of Section 18 of the Exchange Act, and otherwise is not subject to liability under these sections. |
