Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - InspireMD, Inc. | v346499_8k.htm |

May 2013 v50 - 46

Forward - Looking Statements : This presentation contains “forward - looking statements . ” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words . Forward - looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the control of InspireMD , Inc . (the “Company”), and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward - looking statements . Such risks and uncertainties include, without limitation, risks and uncertainties associated with ( i ) market acceptance of the Company’s existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sale of the Company’s products, (iv) intense competition in the medical device industry from much larger, multi - national companies, (v) product liability claims, (vi) the Company’s limited manufacturing capabilities and reliance on subcontractors for assistance, (vii) insufficient or inadequate reimbursement by governmental and other third party payors for the Company’s products, (viii) the Company’s efforts to successfully obtain and maintain intellectual property protection covering its products, which may not be successful, (ix) legislative or regulatory reform of the healthcare system in both the U . S . and foreign jurisdictions, (x) the Company’s reliance on single suppliers for certain product components, (xi) the fact that the Company will need to raise additional capital to meet its business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain and (xii) the fact that the Company conducts business in multiple foreign jurisdictions, exposing it to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction . More detailed information about the Company and the risk factors that may affect the realization of forward - looking statements are set forth in the Company’s filings with the Securities and Exchange Commission, including the Company’s Transition Report on Form 10 - K/T and its quarterly reports on Form 10 - Q . Investors and security holders are urged to read these reports free of charge on the Securities and Exchange Commission’s web site at www . sec . gov . The Company assumes no obligation to publicly update or revise its forward - looking statements as a result of new information, future events or otherwise . 2

3 • InspireMD is a medical device company developing and commercializing its MicroNet technology for interventional cardiology and other vascular procedures • Currently commercializing the MGuard ™ Coronary Embolic Protection Stent (EPS) for the treatment of acute coronary syndromes, namely Acute Myocardial Infarctions (AMI) Company Description • Ticker: NSPR (NYSE MKT) • Corporate Headquarters: Boston, MA • International Headquarters : Tel Aviv, Israel • Fully Diluted Shares Outstanding: 42 million • Fully Diluted Market Cap (as of 5/1/2013) : $115 million Corporate Highlights

* Source: Health Research International, (June 2012) ** JAMA, March 2, 2005 — Vol 293, No. 9 1063 Gregg W. Stone • Inadequate outcomes, as current stents designed primarily for Stable Angina population • Distal embolization occurs in up to 73% of cases** • Majority of AMI market is outside of the U.S . (~60 %) Coronary Stent Market* $4.2B Stable Angina/Other $0.7B US $1.0B e x - US Worldwide AMI Market of $1.7B 4 Current Opportunity Future Opportunity
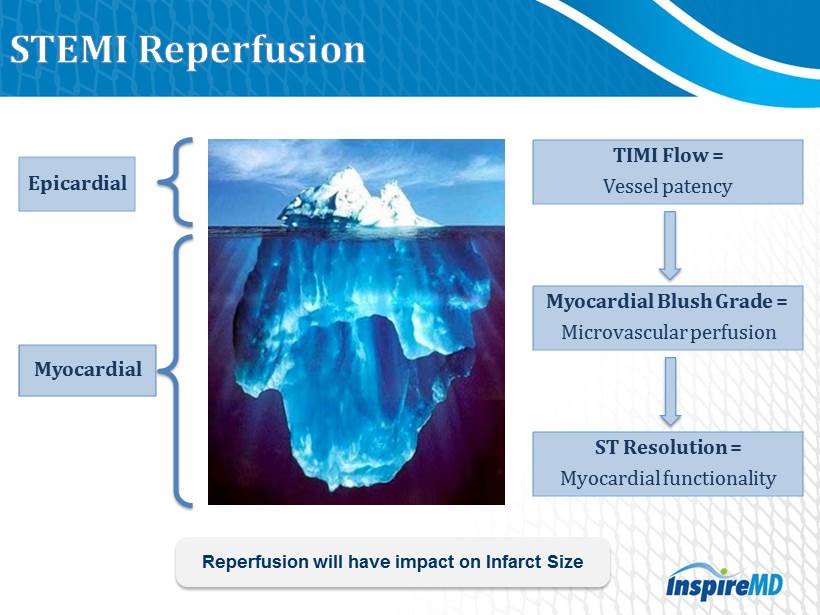
Epicardial Myocardial TIMI Flow = Vessel patency Myocardial Blush Grade = Microvascular perfusion ST Resolution = Myocardial functionality Reperfusion will have impact on Infarct Size

Pre Procedure Embolization Post Procedure Minor heart attack treated with a Bare Metal Stent (BMS) or Drug Eluting Stent (DES) implant Debris to flow down stream, occluding small arteries “ Distal Embolization ” Major Adverse Cardiac Event (MACE) Leading To: Causes: 6

Addressing the AMI Market 7 MGuard ™ Coronary combines InspireMD’ s proprietary MicroNet ™ technology, secured to proximal and distal edges of conventional stent . • Prevent distal embolization • Stabilize ruptured plaque • Improve safety outcome • Maintain standard stenting procedure • No learning curve • First class deliverability MGuard ™ Highlights MicroNet Technology; CoCr Stent | Highly Flexible | Thin Struts | Low Crossing Profile Standard balloon inflated MGuard deployment

8 Repair Perforations Filter Emboli Advantages of technology: • Flexible structure • Minimal foreign body reaction • Does not promote thrombosis Proprietary circular knitted mesh, made of a single fiber from a biocompatible polymer, widely used in medical implantations Potential Applications of Core Technology Seal Aneurysms Drug Delivery Commercial

9 After MGuard ™ Thrombus trapped behind mesh Before Stenting Residual thrombus following aspiration …could lead to embolization when treated with BMS/DES, but with MGuard ™…

STEMI with symptom onset within 12 hours at 432 patients | 50 sites | 9 countries MGuard ™ for Acute ST Elevation Reperfusion PCI with BMS or DES PCI with MGuard Recruitment: 7/2011 – 5/2012 Follow - up: 30 days, 6 months, 1 year Primary endpoint: ST - segment resolution at 60 - 90 minutes Top Line Data: 8/2012 Detailed Data: 10/2012 Substudies Cardiac MRI: 60 patients (30 in each arm) at 3 - 5 days Angio FU: 50 patients in MGuard arm at 13 months
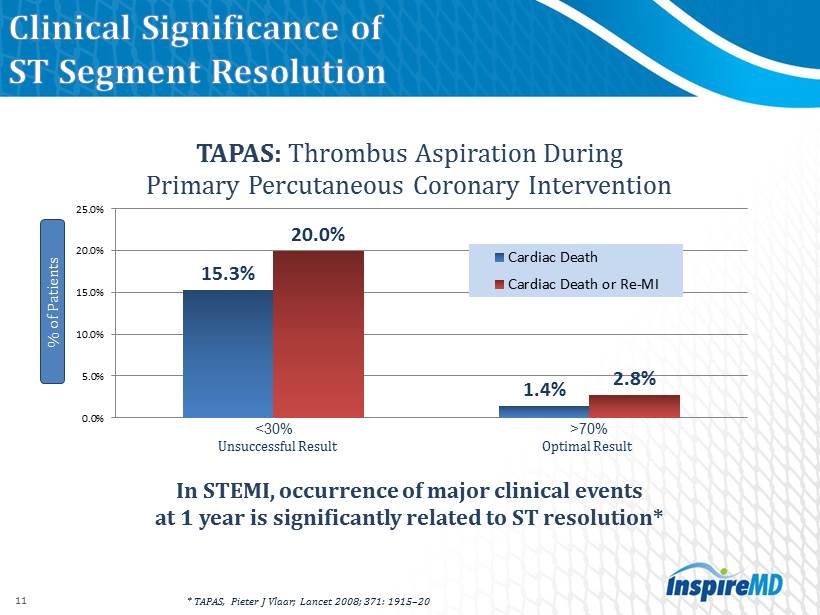
* TAPAS, Pieter J Vlaar ; Lancet 2008 ; 371 : 1915 – 20 15.3% 1.4% 20.0 % 2.8 % 0.0% 5.0% 10.0% 15.0% 20.0% 25.0% Cardiac Death Cardiac Death or Re-MI % of Patients 11 In STEMI, occurrence of major clinical events at 1 year is significantly related to ST resolution * < 30 % > 70 % Unsuccessful Result Optimal Result TAPAS: Thrombus Aspiration During Primary Percutaneous Coronary Intervention
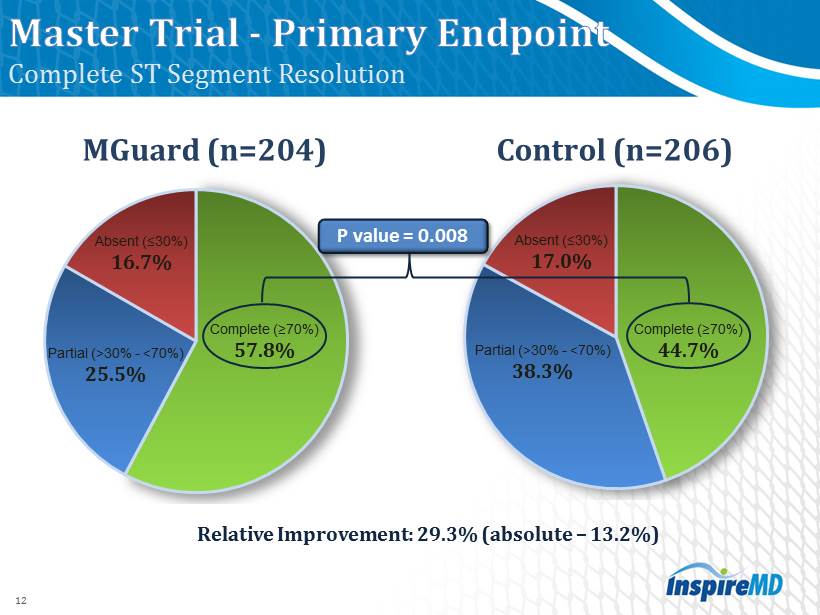
Complete (≥ 70 %) 44.7 % Partial (> 30 % - < 70 %) 38.3 % Absent (≤30%) 17.0 % Complete (≥ 70 %) 57.8 % Partial (> 30 % - < 70 %) 25.5 % Absent (≤ 30 %) 16.7 % Relative I mprovement: 29.3 % (absolute – 13.2 %) 12 Complete ST Segment Resolution MGuard (n=204) Control (n= 206 ) P value = 0.008

AMC Amsterdam (N=2124) ST - Resolution immediately post PCI is a strong predictor of long term mortality N. Verouden – Am J Cardiol 2010 ; 105 ( 12 ): 1692 - 1697 . ST - Resolution after PCI in STEMI
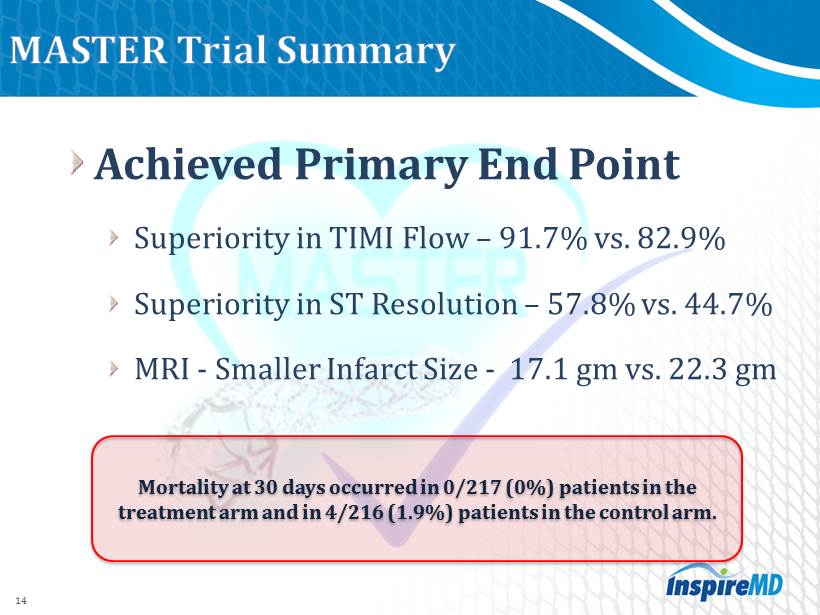
14 Achieved Primary End P oint Superiority in TIMI Flow – 91.7 % vs. 82.9 % Superiority in ST Resolution – 57.8 % vs. 44.7 % MRI - Smaller I nfarct Size - 17.1 gm vs. 22.3 gm Mortality at 30 days occurred in 0 / 217 ( 0 %) patients in the treatment arm and in 4 / 216 ( 1.9 %) patients in the control arm.

Goal: Achieve 90 % TIMI 3 flow within 90 ’ Ndredepa . J Am Coll Cardiol 2008 ; 52 : 512 – 7

8 % 15 % 0% 2% 4% 6% 8% 10% 12% 14% 16% Infarct Size Reflow No Reflow Ndrepepa . J Am Coll Cardiol 2010 ; 55 : 2383 – 9 No Reflow increases infarct size and mortality p < 0.001

• Superior ST Segment resolution • Improved TIMI 3 Flow/ reduced TIMI 0 - 1 occurrence • Trend toward reduced infarct size measured by MRI • Trend towards lower mortality with 0 deaths in treatment group vs. 4 deaths in control arm • What impact over time?

Death - 6 months (%) 0 1 2 3 4 5 Time in Months 0 30 60 90 120 150 180 217 213 213 165 216 209 208 171 Number at risk: MGuard BMS/DES P= 0.056 HR: 0.16 [95% CI: 0.02, 1.36] 0.5% 2.8% MGuard BMS/DES Time in Days 2.8 % 0.5%

MGUARD (N= 217 ) CONTROL BMS / DES (N=216) P Value MACE 11 (5.2%) 7 (3.4%) 0.34 All cause mortality 1 (0.5%) 6 (2.8%) 0.06 Cardiac death 1 (0.5%) 5 (2.3%) 0.10 Reinfarction 3 (1.4%) 2 (0.9%) 0.66 TLR, ischemia - driven 10 (4.8%) 2 (1.0%) 0.02 TVR, ischemia - driven 13 (6.2%) 2 (1.0%) <0.01 Stent Thrombosis Definite or Probable 4 (1.8%) 2 (0.9%) 0.42 Definite 4 (1.8%) 1 (0.5%) 0.18 Stroke 1 (0.5%) 0 (0.0%) 0.32 TIMI Bleeding Major or Minor 5 (2.30%) 5 (2.3%) 0.99 Major 4 (1.8%) 4 (1.3%) 0.71 * Secondary endpoints

• Establish Embolic Protection Stent (EPS) as new stent category • Promote MGuard ™ EPS as the preferred solution for STEMI patients • Utilize positive MASTER Trial results to push adoption and get premium reimbursement in key markets • Develop sales infrastructure to support a focused and phased selling approach in select high - volume markets 20

• Tier 1 - Mix of direct sales agents and distributors, with focus on KOL’s/high - volume AMI centers - 14 - 18 countries, primarily Europe and Brazil • Tier 2 - Country or regional partnerships with high quality local distributors or Strategics - Scaling global reach and frequency • Tier 3 - USA 21

22
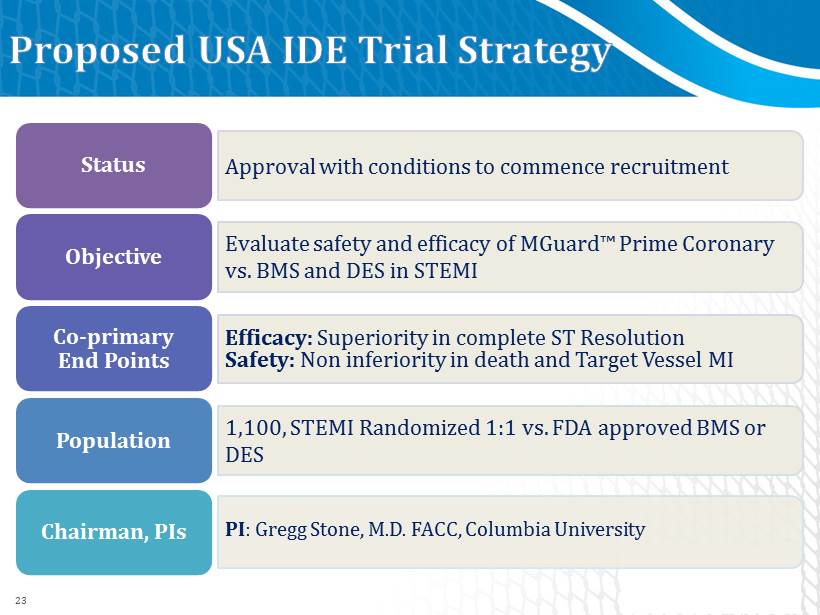
Status Objective Co - primary End Points Population Chairman, PIs Approval with conditions to commence recruitment Evaluate safety and efficacy of MGuard ™ Prime Coronary vs. BMS and DES in STEMI Efficacy: Superiority in complete ST Resolution Safety: Non inferiority in death and T arget V essel MI 1 , 100 , STEMI Randomized 1 : 1 vs. FDA approved BMS or DES PI : Gregg Stone, M.D. FACC, Columbia University 23

Received CE Mark for Coronary Commercial • MGuard Coronary • MGuard Prime Coronary * Planned product 24 MGuard ™ Platform Technology MGuard ™ Coronary EPS MGuard ™ Carotid MGuard™ Peripheral * MGuard™ Drug Eluting * MGuard™ Cerebral * Aneurysm MGuard™ Renal * • MGuard Carotid CE Mark Approved

CE Mark Approved ; Q 1 2013 25 Current Market Solution: MGuard ™ Carotid Solution: Stent + Filter Device + MGuard Embolic Protection Stent During and Post Procedure
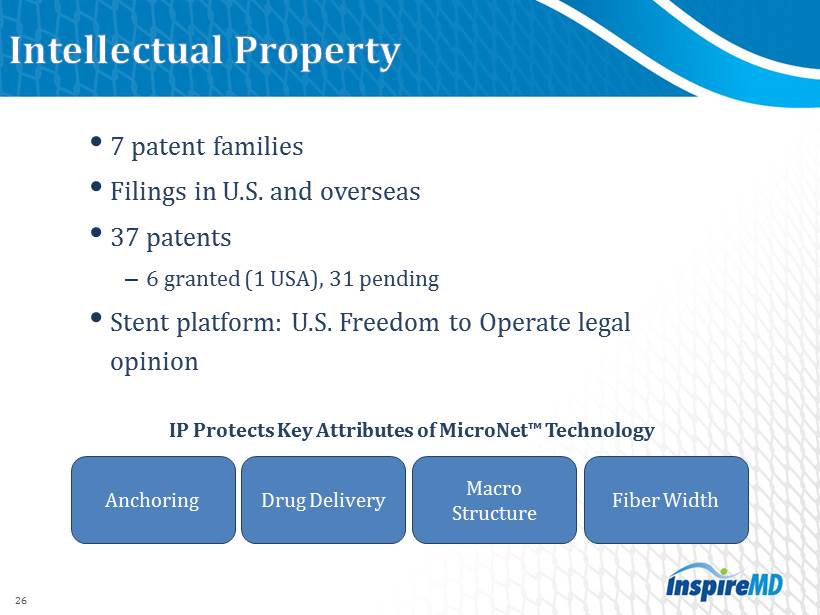
• 7 patent families • Filings in U.S. and overseas • 37 patents – 6 granted ( 1 USA), 31 pending • Stent platform: U.S. Freedom to Operate legal opinion IP Protects Key Attributes of MicroNet ™ Technology 26 Anchoring Macro Structure Drug Delivery Fiber Width

— Use of proceeds from offering to support manufacturing expansion, global launch — Facility has completed requisite EMA inspections — Implementation of Good Manufacturing Practice (GMP) facility in process — Platform technology produced in - house, bare metal stent and catheter manufactured by third party — Final product assembled at InspireMD facility, including attachment of proprietary mesh sleeve to the stent 27

Eli Bar, BSc CTO • Product development • R&D infrastructure • Fully implantable VAD patent Craig Shore, MBA CFO • Pfizer , Bristol Myers Squibb, and Dunn and Bradstreet, General Electric • RIT Technologies (NASDAQ) Chaim Lotan , M.D., F.A.C.C. F.E.S.C Medical Director • Hebrew University • Hadassah Medical Center 28 Sol Barer, PhD Chairman of the Board • Former Chairman and CEO, Celgene, (NASDAQ: CELG) Michael Berman, MBA Director • Pres. Boston Scientific/ Scimed • Aetherworks • Apnex • Benechill • Cardiosonic Robert Ratini, MSc VP Sales & Marketing • Orbusneich Medical • Biosensors Int'l • Abbott Vascular • Boston Scientific • CardiacAssist • Haemonetics James Barry, PhD Director • EVP and COO, Arsenal Medical • VP , Corporate Research and Advanced Technology Development at Boston Scientific • Howmedica Division of Pfizer Alan Milinazzo President & CEO • Orthofix • Medtronic • Boston Scientific Gwen Bame VP Corporate Development • Covidien • Aspect Medical Systems • Boston Scientific

Dr. Yaron Almagor • Director of Cardiac Catheterization and Interventional Cardiology Laboratories at Jesselson Heart Center, Shaare Zedek Medical Center; Jerusalem, Israel Prof. Antonio Colombo • Director of the Cardiac Catheterization • Laboratory at Columbus Hospital • Chief of Invasive Cardiology at San Raffaele Hospital, both in Milan, Italy Prof. Elazer Edelman M.D., Ph.D., F.A.C.C • Professor of Health Sciences and Technology at MIT. Cardiologist at the Brigham and Women ’ s Hospital in Boston • Directs the Harvard - MIT Biomedical Engineering Center (BMEC) Prof. Eberhard Grube • Professor of Medicine, Chief of the Department of Cardiology and Angiology at Siegburg Heart Centre, Germany Dr. Edo Kaluski, MD, FACC, FESC • Director of Cardiac Catheterization Laboratories and Invasive Cardiology at the University Hospital in Newark, New Jersey • Co - founder of InspireMD Prof. Dariusz Dudek • Executive Board of the Working Group on Invasive Cardiology of the Polish Cardiac Society • Associate professor of the Jagiellonian University, Krakow, Poland Prof. Gregg Stone • Professor of Medicine at the Columbia University Medical Center • Director of Cardiovascular Research and Education at the Center for Interventional Vascular Therapies at New York - Presbyterian Hospital, and CRF in New York, NY 29 Dr. Martin B. Leon • Director, Center for Interventional Vascular Therapy Columbia University Medical Center / New York - Presbyterian Hospital, New York.NY • Professor of Medicine Columbia University College of Physicians and Surgeons New York, NY Prof. Alexandre A. Abizaid • Chief of Coronary Interventions at Institute Dante Pazzanese de Cardiologia in São Paulo, Brazil • Associate - director of TCT (Columbia University, U.S .) Prof. Chaim Lotan • Head of The Heart Institute Hadassah University Medical Center. • Chairman of the Ministry of Health Committee for Certification & Licensing of Coronary Stents
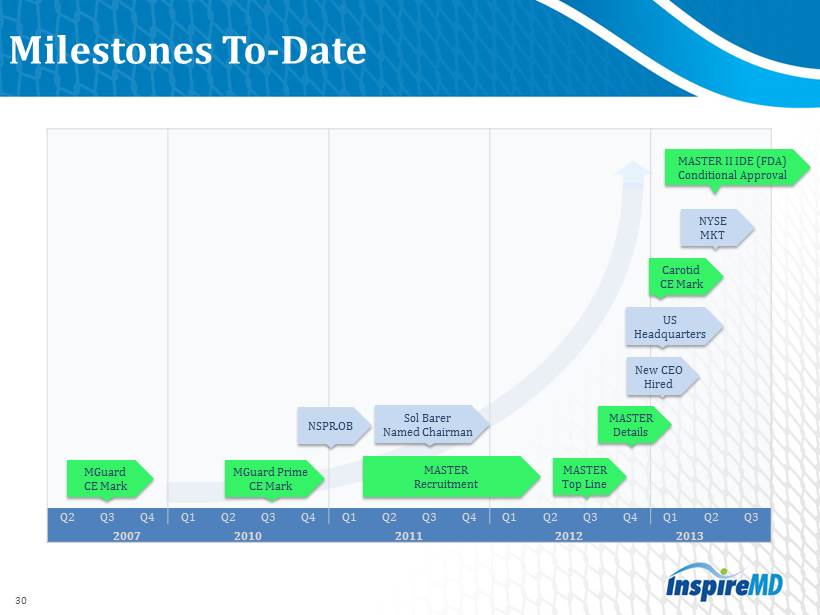
Milestones To - Date Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 2007 2010 2011 2012 2013 MGuard CE Mark NSPR.OB Sol Barer Named Chairman 30 MASTER Recruitment MASTER Top Line MASTER Details New CEO Hired US Headquarters Carotid CE Mark NYSE MKT MGuard Prime CE Mark MASTER II IDE (FDA) Conditional Approval

Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 2013 2014 2015 2016 Anticipated Future Milestones Development Partnership I 31 MASTER Subset Analysis ( EuroPCR ) Manufacturing Expansion MASTER 12 - Month Data (TCT) Distribution Partnership I FDA Approval Peripheral CE Mark Complete MASTER II Enrollment Distribution Partnership II MASTER II Results New Upscaled Manufacturing Facility Development Partnership II

32 NSPR (NYSE MKT) 34.3 million Basic Shares Outstanding $ 2.80 Stock Price (5/1/2013) $ 118 million Fully Diluted Market Capitalization ( 5 / 1 / 2013 ) 7.7 million Warrants, Options and Convertible Loan Shares Q3 – $1.5M | Q2 – $1.4M | Q1 – $0.5M Revenue Growth 42 million Fully Diluted Shares Outstanding

• MGuard ™ Coronary Embolic Protection Stent designed to transform the standard of care for AMI • Initial focus is STEMI patient population • AMI market value $ 1.7 B Unmet Clinical Need • MASTER Trial ( r andomized s tudy) • Primary Endpoint: Statistical significance achieved with superior results • Chairman: Dr . Gregg Stone of the Cardiovascular Research Foundation Strong Clinical Evidence • CE Mark approved, currently active in 24 countries • FDA USA IDE trial recruitment approved with conditions • Lead PI: Dr. Gregg Stone Regulatory Status • 6 patents granted • 31 patents pending • Key aspects of core technology covered Intellectual Property 33

Inspire MD Thank You

35 Appendix
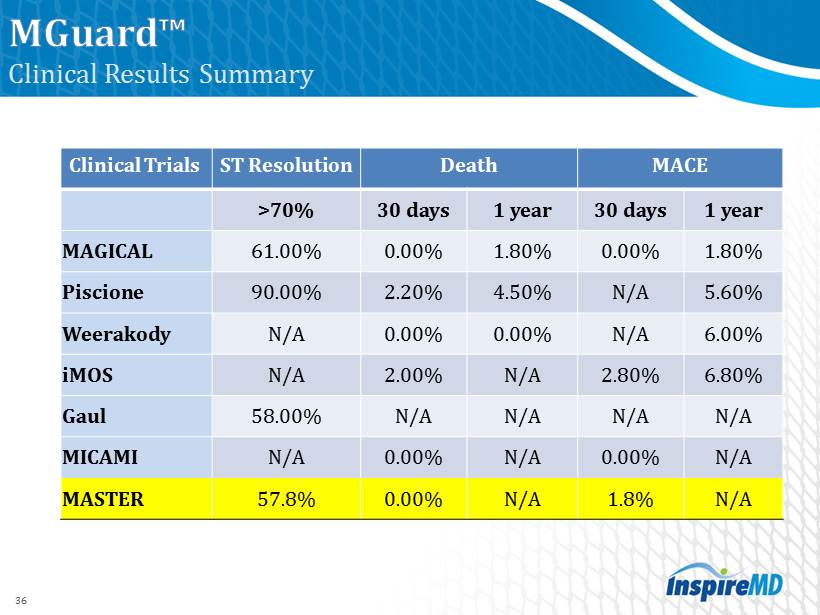
Clinical Results Summary Clinical Trials ST Resolution Death MACE > 70% 30 days 1 year 30 days 1 year MAGICAL 61.00% 0.00% 1.80% 0.00% 1.80% Piscione 90.00% 2.20% 4.50% N/A 5.60% Weerakody N/A 0.00% 0.00% N/A 6.00% iMOS N/A 2.00% N/A 2.80% 6.80% Gaul 58.00% N/A N/A N/A N/A MICAMI N/A 0.00% N/A 0.00% N/A MASTER 57.8% 0.00% N/A 1.8% N/A 36

Detailed Analysis Clinical Events at 30 Days MGuard (n=217) Control (n=216) MACE 4 (1.8%) 5 (2.3%) - Cardiac mortality 0 (0.0%) 4 (1.9%) - Reinfarction 3 (1.4%) 2 (0.9%) - TLR, ischemia - driven 4 (1.8%) 1 (0.5%) 3 - 5 Day MRI Substudy Results MGuard (n=30) Control (n=29) Infarct mass, grams 17.1 [10.0,30.0] 22.3 [15.7,30.1] Infarct mass (% total LV mass) 13.3 [7.9,25.0] 16.6 [10.0,22.6] Total MVO, grams 0.3 [0.0,1.6] 1.0 [0.2, 2.8] MVO (% total LV mass) 0.4 [0.0,1.4] 0.8 [0.2,1.9] LVEF (%) 48.3 [44.5,52.3] 47.3 [42.0,54.5] Measurement of Blood Flow MGuard (n=217 ) Control (n=216) Aspiration Performed 65.9% 67.1% Stent Type - BMS 59.7% - DES 39.8% TIMI Flow = 3 91.7% 82.9% TIMI Flow = 2 6.5% 11.6% TIMI Flow = 0/1 1.8% 5.6% Myocardial Blush = 2/3 83.9% 84.7% (1) ( 2 ) ( 3 ) ( 4 ) ( 3 ) (1) MASTER, although not powered for non primary endpoint measurements , showed statistical significance and positive trends for key measurements . ( 1 ) Data showing a positive trend ( 2 ) MVO = microvascular obstruction ( 3 ) Data is statistically significant ( 4 ) 191 MGuard , 26 MGuard Prime
