Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
(Mark One)
| x QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the quarterly period ended March 31, 2013 |
or
| o TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the transition period from ______ to _____ |
| Commission File Number 000-54882 |
|
BIOLOGIX HAIR INC.
|
||||||||||||||||||||
|
(Exact name of registrant as specified in its charter)
|
|
Nevada
|
27-4588540
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(IRS Employer Identification No.)
|
|
82 Avenue Road, Toronto, Ontario, Canada
|
M5R 2H2
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
(647) 344-5900
|
||||||||||||||||||||
|
(Registrant’s telephone number, including area code)
|
||||||||||||||||||||
|
N/A
|
||||||||||||||||||||
|
(Former name, former address and former fiscal year, if changed since last report)
|
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
|
||||||||||||||||||||
|
x
|
YES
|
o
|
NO
|
|||||||||||||||||
|
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
|
||||||||||||||||||||
|
o
|
YES
|
x
|
NO
|
|||||||||||||||||
|
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a small reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
|||
|
Large accelerated filer
|
o
|
Accelerated filer o | |
|
Non-accelerated filer
|
o
|
(Do not check if a smaller reporting company)
|
Smaller reporting company x
|
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act
|
|||
|
o
|
YES
|
x
|
NO
|
|
APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY
PROCEEDINGS DURING THE PRECEDING FIVE YEARS
Check whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Exchange Act after the distribution of securities under a plan confirmed by a court.
|
|
o
|
YES
|
o
|
NO
|
|
APPLICABLE ONLY TO CORPORATE ISSUERS
|
||||
|
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date.
|
||||
|
56,630,000 common shares issued and outstanding as of May 20, 2013.
|
TABLE OF CONTENTS
|
PART I - FINANCIAL INFORMATION
|
3
|
|
|
Item 1.
|
Financial Statements
|
3
|
|
Item 2.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
4
|
|
Item 3.
|
Quantitative and Qualitative Disclosures About Market Risk
|
16
|
|
Item 4.
|
Controls and Procedures
|
16
|
|
PART II – OTHER INFORMATION
|
16
|
|
|
Item 1.
|
Legal Proceedings
|
16
|
|
Item 1A.
|
Risk Factors
|
16
|
|
Item 2.
|
Unregistered Sales of Equity Securities and Use of Proceeds
|
16
|
|
Item 3.
|
Defaults Upon Senior Securities
|
17
|
|
Item 4.
|
Mine Safety Disclosures
|
17
|
|
Item 5.
|
Other Information
|
17
|
|
Item 6.
|
Exhibits
|
19
|
|
SIGNATURES
|
21
|
|
PART I - FINANCIAL INFORMATION
Item 1. Financial Statements
These consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial information and the SEC instructions to Form 10-Q. In the opinion of management, all adjustments considered necessary for a fair presentation have been included. Operating results for the interim period ended March 31, 2013 are not necessarily indicative of the results that can be expected for the full year.
3
|
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
INTERIM CONSOLIDATED FINANCIAL STATEMENTS
THREE MONTHS ENDED MARCH 31, 2013
(As expressed in US dollars)
(Unaudited – Prepared by Management)
|
F-1
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – Prepared by Management)
INTERIM CONSOLIDATED BALANCE SHEETS
|
As at
|
March 31
2013
|
December 31
2012
|
||||||
| $ | $ | |||||||
|
ASSETS
|
||||||||
|
Current
|
||||||||
|
Cash and cash equivalents
|
67,136 | 63,755 | ||||||
|
Taxes receivable
|
- | 69,633 | ||||||
|
Prepaid expenses and deposit (Note 4)
|
175,729 | 250,682 | ||||||
|
Total current assets
|
242,865 | 384,070 | ||||||
|
Prepaid expense – long term (Note 4)
|
- | 31,270 | ||||||
|
Property and equipment (Note 5)
|
133,439 | 154,079 | ||||||
|
Website development cost (Note 6)
|
97,296 | 94,272 | ||||||
|
Total assets
|
473,600 | 663,691 | ||||||
|
LIABILITIES
|
||||||||
|
Current
|
||||||||
|
Accounts payable and accrued liabilities
|
494,735 | 423,876 | ||||||
|
Due to related parties (Note 8)
|
5,673 | 189,489 | ||||||
|
Current portion of promissory and convertible promissory notes (Note 7)
|
11,504,439 | 7,604,595 | ||||||
| 12,004,847 | 8,217,960 | |||||||
|
Non-current portion of promissory and convertible promissory notes (Note 7)
|
3,302,873 | 6,663,675 | ||||||
|
Total liabilities
|
15,307,720 | 14,881,635 | ||||||
|
STOCKHOLDERS’ EQUITY
|
||||||||
|
Common stock (Notes 1 and 9)
|
||||||||
|
Authorized: 900,000,000 common shares, $0.001 par value
|
||||||||
|
Issued and outstanding: 56,630,000 (December 31, 2012: 26,430,000 at $0.001 par value) common shares
|
56,630 | 26,430 | ||||||
|
Additional paid-in capital
|
13,161,857 | 11,248,754 | ||||||
|
Share subscriptions received in advance (Note 9)
|
650,000 | - | ||||||
|
Accumulated other comprehensive income
|
5,156 | 6,087 | ||||||
|
Deficit
|
(28,707,763 | ) | (25,499,215 | ) | ||||
|
Total stockholders’ equity (deficiency)
|
(14,834,120 | ) | (14,217,944 | ) | ||||
|
Total liabilities and stockholders’ equity
|
473,600 | 663,691 | ||||||
See accompanying notes to the interim consolidated financial statements
F-2
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – Prepared by Management)
INTERIM CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
|
Three months
ended
March 31,
2013
|
Three months
ended
March 31,
2012
|
Cumulative
from October 4,
2011 to
March 31,
2013
|
||||||||||
| $ | $ | $ | ||||||||||
|
OPERATING EXPENSES
|
||||||||||||
|
Amortization of website development costs (Note 6)
|
11,608 | 7,306 | 46,881 | |||||||||
|
Bank charges
|
1,454 | 2,782 | 14,749 | |||||||||
|
Clinical research and reformulation
|
83,104 | - | 1,103,087 | |||||||||
|
Consulting and management fees (Note 8)
|
116,994 | 158,871 | 973,060 | |||||||||
|
Depreciation (Note 5)
|
20,640 | 18,264 | 103,189 | |||||||||
|
Foreign exchange (gain) or loss
|
(2,059 | ) | 67 | (185 | ) | |||||||
|
Insurance
|
3,785 | 2,677 | 7,110 | |||||||||
|
Interest
|
814,942 | 5,425 | 2,965,898 | |||||||||
|
IPR&D expense (Note 2b)
|
- | - | 18,679,530 | |||||||||
|
Licences and permits
|
312 | - | 6,702 | |||||||||
|
Loss on disposition of assets (Note 5)
|
- | - | 6,362 | |||||||||
|
Marketing and sales promotion
|
55,348 | - | 426,278 | |||||||||
|
Office and administrative
|
29,196 | 3,495 | 103,148 | |||||||||
|
Professional fees
|
35,883 | 22,536 | 320,494 | |||||||||
|
Relocation fees
|
8,236 | 57,435 | 79,985 | |||||||||
|
Rent
|
123,617 | 46,980 | 395,639 | |||||||||
|
Royalty
|
119,870 | - | 388,847 | |||||||||
|
Stock based compensation
|
1,615,956 | - | 2,578,484 | |||||||||
|
Telephone
|
6,000 | 1,242 | 27,998 | |||||||||
|
Transfer agent fees
|
1,651 | 1,000 | 4,857 | |||||||||
|
Travel and promotion
|
53,963 | 144,603 | 445,083 | |||||||||
|
Wages and benefits
|
52,758 | - | 113,735 | |||||||||
|
Warehousing and distribution (Note 8)
|
- | - | 10,000 | |||||||||
|
Website maintenance
|
55,290 | - | 141,220 | |||||||||
|
NET LOSS BEFORE INCOME TAXES FOR THE PERIOD
|
(3,208,548 | ) | (472,683 | ) | (28,942,151 | ) | ||||||
|
Deferred income tax recovery
|
- | - | 234,388 | |||||||||
|
NET LOSS FOR THE PERIOD
|
(3,208,548 | ) | (472,683 | ) | (28,707,763 | ) | ||||||
|
OTHER COMPREHENSIVE INCOME
|
||||||||||||
|
Currency translation adjustments
|
(931 | ) | 18,901 | 5,156 | ||||||||
|
COMPREHENSIVE LOSS FOR THE PERIOD
|
(3,209,478 | ) | (453,782 | ) | (28,702,607 | ) | ||||||
|
|
||||||||||||
|
LOSS PER SHARE – BASIC AND DILUTED
|
(0.06 | ) | (47.27 | ) | ||||||||
|
WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING - BASIC AND DILUTED
|
53,610,000 | 10,000 | ||||||||||
See accompanying notes to the interim consolidated financial statements
F-3
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
INTERIM CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
From inception October 4, 2011 to March 31, 2013
|
Common
Shares
|
Stock
Amount
|
Additional
Paid-in
Capital
|
Accumulated
Other
Comprehensive
Income
|
Share
Subscriptions Received
In Advance
|
Deficit Accumulated in Development
Stage
|
Total
Stockholders’
Equity
(Deficiency)
|
||||||||||||||||||||||
| $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||
|
Balance, October 4, 2011
|
- | - | - | - | - | - | - | |||||||||||||||||||||
|
Issuance of common shares for cash
|
||||||||||||||||||||||||||||
|
- $0.01 per share
|
10,000 | 10 | 90 | - | - | - | 100 | |||||||||||||||||||||
|
Share subscriptions received in advance, net (Note 9)
|
- | - | - | - | 527,500 | - | 527,500 | |||||||||||||||||||||
|
Net loss for the period
|
- | - | - | - | - | (131,371 | ) | (131,371 | ) | |||||||||||||||||||
|
Balance, December 31, 2011
|
10,000 | 10 | 90 | - | 527,500 | (131,371 | ) | 396,229 | ||||||||||||||||||||
|
Common shares issued for cash (Note 9)
|
||||||||||||||||||||||||||||
|
- $0.05 per share, on April 18, 2012
|
9,450,000 | 9,450 | 463,050 | - | (437,500 | ) | - | 35,000 | ||||||||||||||||||||
|
- $0.25 per share, on April 18, 2012
|
6,220,000 | 6,220 | 1,548,780 | - | (90,000 | ) | - | 1,465,000 | ||||||||||||||||||||
|
- $0.80 per share, on May 2, 2012
|
2,500,000 | 2,500 | 1,997,500 | - | - | - | 2,000,000 | |||||||||||||||||||||
|
- $1.00 per share, on June 22, 2012
|
1,500,000 | 1,500 | 1,498,500 | - | - | - | 1,500,000 | |||||||||||||||||||||
|
- $1.00 per share, on September 24, 2012
|
250,000 | 250 | 249,750 | - | - | - | 250,000 | |||||||||||||||||||||
|
Share issuance costs
|
- | - | (210,000 | ) | - | - | - | (210,000 | ) | |||||||||||||||||||
|
Common shares issued for settlement of debt
|
||||||||||||||||||||||||||||
|
- $0.25 per share (Note 2 and Note 9), on April 28, 2012
|
2,000,000 | 2,000 | 498,000 | - | - | - | 500,000 | |||||||||||||||||||||
|
- $1.00 per share (Note 7 and Note 9), on June 6, 2012
|
500,000 | 500 | 499,500 | - | - | - | 500,000 | |||||||||||||||||||||
|
$0.80 per share, acquisition of BHSL (Note 2), on May 18, 2012
|
4,000,000 | 4,000 | 3,196,000 | - | - | - | 3,200,000 | |||||||||||||||||||||
|
Stock based compensation
|
- | - | 962,528 | - | - | - | 962,528 | |||||||||||||||||||||
|
Equity component of conversion beneficiary features, net of tax impact (Note 7)
|
- | - | 278,776 | - | - | - | 278,776 | |||||||||||||||||||||
|
Allocated value of warrants, net of tax impact (Note 7)
|
- | - | 266,280 | - | - | - | 266,280 | |||||||||||||||||||||
|
Net loss for the year
|
- | - | - | - | - | (25,367,844 | ) | (25,367,844 | ) | |||||||||||||||||||
|
Currency translation adjustments
|
- | - | - | 6,087 | - | - | 6,087 | |||||||||||||||||||||
|
Balance, December 31, 2012
|
26,430,000 | 26,430 | 11,248,754 | 6,087 | - | (25,499,215 | ) | (14,217,944 | ) | |||||||||||||||||||
See accompanying notes to the interim consolidated financial statements
F-4
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
INTERIM CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
From inception October 4, 2011 to March 31, 2013
|
Common
Shares
|
Stock
Amount
|
Additional
Paid-in
Capital
|
Accumulated
Other
Comprehensive
Income
|
Share Subscriptions Received In Advance
|
Deficit Accumulated in Development
Stage
|
Total
Stockholders’
Equity
(Deficiency)
|
||||||||||||||||||||||
| $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||
|
Balance, December 31, 2012
|
26,430,000 | 26,430 | 11,248,754 | 6,087 | - | (25,499,215 | ) | (14,217,944 | ) | |||||||||||||||||||
|
Recapitalization – Biologix Nevada (Note 2(c) and 9)
|
30,200,000 | 30,200 | 297,147 | - | - | - | 327,347 | |||||||||||||||||||||
|
Share subscriptions received in advance, net (Note 9)
|
- | - | - | - | 650,000 | - | 650,000 | |||||||||||||||||||||
|
Stock based compensation
|
- | - | 1,615,956 | - | - | - | 1,615,956 | |||||||||||||||||||||
|
Net loss for the period
|
- | - | - | - | - | (3,208,548 | ) | (3,208,548 | ) | |||||||||||||||||||
|
Currency translation adjustments
|
- | - | - | (931 | ) | - | - | (931 | ) | |||||||||||||||||||
|
Balance, March 31, 2013
|
56,630,000 | 56,630 | 13,161,857 | 5,156 | 650,000 | (28,707,763 | ) | (14,834,120 | ) | |||||||||||||||||||
See accompanying notes to the interim consolidated financial statements
F-5
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
INTERIM CONSOLIDATED STATEMENTS OF CASH FLOWS
|
Three
months ended
March 31,
2013
|
Three
months ended
March 31,
2012
|
Cumulative
from
October 4,
2011 to
March 31,
2013
|
||||||||||
| $ | $ | $ | ||||||||||
|
OPERATING ACTIVITIES:
|
||||||||||||
|
Net loss from operations
|
(3,208,548 | ) | (472,683 | ) | (28,707,763 | ) | ||||||
|
Items not affecting cash and cash equivalents
|
||||||||||||
|
- Amortization of website development costs (Note 6)
|
11,608 | 7,306 | 46,881 | |||||||||
|
- Depreciation (Note 5)
|
20,640 | 18,264 | 103,189 | |||||||||
|
- Accrued interest
|
814,042 | 5,425 | 2,963,941 | |||||||||
|
- IPR&D expense (Note 2)
|
- | - | 18,679,530 | |||||||||
|
- Stock based compensation
|
1,615,956 | - | 2,578,484 | |||||||||
|
- Loss on disposition of assets
|
- | - | 6,362 | |||||||||
|
- Deferred income tax recovery
|
- | - | (234,388 | ) | ||||||||
|
Changes in non-cash working capital items
|
||||||||||||
|
- Taxes receivable
|
69,633 | (16,940 | ) | - | ||||||||
|
- Prepaid expenses and deposit
|
74,953 | (41,841 | ) | (207,000 | ) | |||||||
|
- Prepaid expenses – long term
|
31,270 | - | 31,270 | |||||||||
|
- Accounts payable and accrued liabilities
|
65,691 | 69,232 | (160,278 | ) | ||||||||
|
- Due to related parties
|
(183,816 | ) | (9,347 | ) | 5,673 | |||||||
|
Net cash provided by (used in) operating activities
|
(688,571 | ) | (356,902 | ) | (4,894,099 | ) | ||||||
|
FINANCING ACTIVITIES:
|
||||||||||||
|
Proceeds from issuance of common stock
|
- | 1,065,000 | 4,640,100 | |||||||||
|
Share subscriptions received in advance
|
650,000 | - | 1,177,500 | |||||||||
|
Note payable
|
50,000 | - | 1,700,000 | |||||||||
|
Net cash provided by (used in) financing activities
|
700,000 | 1,065,000 | 7,517,600 | |||||||||
|
INVESTING ACTIVITIES:
|
||||||||||||
|
Net cash paid on acquisition of BHSL (Note 2)
|
- | - | (2,192,569 | ) | ||||||||
|
Net cash received on reverse acquisition (Note 2)
|
7,515 | 7,515 | ||||||||||
|
Intellectual property licence
|
- | (500,000 | ) | - | ||||||||
|
Purchase of equipment
|
- | (144,331 | ) | (245,718 | ) | |||||||
|
Proceeds from disposition of assets
|
- | - | 2,728 | |||||||||
|
Website development costs
|
(14,632 | ) | (50,815 | ) | (133,477 | ) | ||||||
|
Net cash provided by (used in) investing activities
|
(7,117 | ) | (695,146 | ) | (2,561,521 | ) | ||||||
|
Currency translation adjustments
|
(931 | ) | 226 | 5,156 | ||||||||
|
INCREASE IN CASH AND CASH EQUIVALENTS
|
3,381 | 13,178 | 67,136 | |||||||||
|
CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD
|
63,755 | 218,416 | - | |||||||||
|
CASH AND CASH EQUIVALENTS, END OF PERIOD
|
67,136 | 231,594 | 67,136 | |||||||||
|
Supplemental disclosure of cash flow information:
|
||||||||||||
|
Income tax paid
|
- | - | - | |||||||||
|
Interest paid
|
- | - | - | |||||||||
See accompanying notes to the interim consolidated financial statements
F-6
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 1 – INCORPORATION, NATURE AND CONTINUANCE OF OPERATIONS
Biologix Hair Inc. (formerly T&G Apothecary, Inc.) (the “Company” or “Biologix Nevada”) was incorporated under the laws of the State of Nevada, U.S.A. on January 18, 2011. The Company was in the business of developing and marketing a 100% USDA Certified Organic personal care product line for women. However, as described below, the Company has recently changed its primary business focus to the development and commercialization of a hair therapy system. The Company has limited operations and in accordance with ASC 915, is considered a development stage company that had no revenues from inception to date. The Company has a December 31 year-end.
Effective January 9, 2013, the Company acquired 100% of the issued and outstanding shares of Biologix Hair Inc. (“Biologix Florida”), a company incorporated on October 4, 2011 in the state of Florida, U.S.A. in exchange for 26,430,000 post-split shares of the Company’s common stock and resulting in a reverse acquisition. On December 13, 2012, the Company changed its name from T&G Apothecary, Inc. to Biologix Hair Inc. to better reflect its new business. Upon the completion of the acquisition, the former shareholders of Biologix Florida held 47% of the Company’s issued and outstanding common stock and the composition of the board of directors has been changed by the majority of existing board members of Biologix Florida. As a result, the transaction was accounted for as a reverse acquisition for accounting purpose, as Biologix Florida was deemed to be the acquirer, and the interim consolidated financial statements are a continuation of the financial statements of Biologix Florida. As a result of these transactions, the business combination resulted in the combined Company having 56,630,000 issued and outstanding common shares. The Company and its subsidiaries resulted from the reverse acquisition are collectively referred to as the “Company”. See Note 2 (c).
The Company has incurred accumulated deficit of $28,707,763 to March 31, 2013 and has no source of revenue. The continuity of the Company’s future operations is dependent upon its ability to obtain financing and profitable operations from its establishment of Hair Therapy Centers. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The Company intends to continue relying upon the issuance of equity securities to finance its operations. However there can be no assurance it will be successful in raising the funds necessary to maintain operations, or that a self-supporting level of operations will ever be achieved. The likely outcome of these future events is indeterminable. The financial statements do not include any adjustment to reflect the possible future effect on the recoverability and classification of the assets or the amounts and classification of liabilities that may result should the Company cease to continue as a going concern.
F-7
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 2 – ACQUISITIONS
(a) Cranium Technologies Ltd.
Effective November 1, 2011, Biologix Florida acquired 100% of the outstanding common shares of Cranium Technologies Ltd. for total cash consideration in the amount of $5,000. As Cranium does not have any viable operation at the time of acquisition, the transaction does not constitute a business combination.
As the acquisition does not qualify as a business combination, with no assets and liabilities as at the date of acquisition, the consideration paid has been considered to be a transaction cost which has been expensed.
(b) Intellectual Property License (BHSL)
Biologix Florida entered into an Intellectual Property License Agreement (the “License Agreement”) on December 9, 2011 with BHSL, whereby Biologix Florida was granted an exclusive, perpetual license to market, sell and distribute the Invention (“Hair Growth Process”), being all formulae, products, processes, and technical know-how for the stimulation of hair growth and treatment of hair loss developed, owned or controlled by Hair Research and Science Est, (“HRSE”) and Licensed Intellectual Property relating to the Invention, within North America, Central America and the Caribbean, including all sovereign and non-sovereign areas therein. In full consideration of all rights granted, Biologix Florida agreed to pay to BHSL $250,000 within 30 days of signing of the License Agreement and $750,000 by January 30, 2012, and subject to a full due diligence review of the Invention and the Licensed Intellectual Property. As at December 31, 2011, Biologix Florida had not yet completed the related full due diligence review of the above noted Invention and recorded the related consideration commitment as prepaid expense and accounts payable and accrued liabilities of $1,000,000, respectively.
During the fiscal year 2012 and on March 7, 2012, Biologix Florida completed its related due diligence review of the above noted Invention and signed an amendment to the License Agreement whereby the payment of the consideration was extended to March 31, 2012. Biologix Florida made cash payment of $500,000 with the remaining balance of $500,000 settled by the common shares of Biologix Florida at a value of $0.25 per share for 2,000,000 common shares on April 18, 2012. (Also see Note 9). Upon the completion of the payments, Biologix Florida charged the considerations paid to in-process research and development (“IPR&D”) expenditures.
On April 11, 2012, BHSL entered into an amended Intellectual Property Purchase and Sale Agreement with HRSE whereby BHSL acquired certain Intellectual Property, including processes and formulae for the stimulation of hair growth and treatment of hair loss, for consideration in the amount of $10,100,000. $100,000 was paid upon execution of the agreement and $10,000,000 in the form of a promissory note bears interest rate of 3% per annum with following maturity date:
|
●
|
$2,000,000 on or before July 31st, 2012;
|
|
●
|
$3,000,000 on or before December 31, 2012; and
|
|
●
|
$5,000,000 on or before July 31, 2013.
|
F-8
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 2 – ACQUISITION (cont’d...)
(b) Intellectual Property License (BHSL) (cont’d...)
On April 19, 2012, Biologix Florida, through a Share Purchase Agreement entered into with the shareholders of the BHSL, acquired 100% of the issued and outstanding shares of BHSL for total consideration of $9,200,000. Pursuant to the Share Purchase Agreement, the consideration payment schedule was as follows:
|
1.
|
$2,100,000 cash payment within 30 days following the execution of the Share Purchase Agreement (paid);
|
|
2.
|
$3,900,000 payment in the form of a promissory note payable by April 19, 2014 (See Note 7); and
|
|
3.
|
An aggregate of 4,000,000 shares of Biologix Florida’s common stock with a fair value based on Biologix Florida’s recently announced financing of $0.80 per share (issued).
|
Upon the completion of the Share Purchase Agreement and in accordance with FASB ASC 805 Business Combinations, Biologix Florida determined that the above noted Share Purchase Agreement transaction does not constitute a business combination, and accordingly, has accounted for it as an asset acquisition. The operations of BHSL have been included in the consolidated financial statements from the date of acquisition. The total purchase consideration has been measured at fair value of $8,450,713:
|
Cash consideration
|
$ | 2,100,000 | ||
|
Promissory note payable
|
3,150,713 | |||
|
Shares consideration
|
3,200,000 | |||
|
Fair value of total purchase considerations
|
$ | 8,450,713 |
$3,900,000 promissory note has been measured at fair value of $3,150,713 on the date of acquisition, using discounted cash flow method. The fair market interest rate is 12.5%, which is the interest rate that was payable on comparable notes.
The following table summarizes BHSL’s assets and liabilities acquired on the acquisition date:
|
Cash
|
$ | 7,431 | ||
|
Receivable
|
75,100 | |||
|
IPR&D expense - Intellectual Property
|
17,679,530 | |||
|
Taxes payable
|
(8,610 | ) | ||
|
Due to Hair & Research Science Est.
|
(9,272,102 | ) | ||
|
Accounts payable and accrued liabilities
|
(30,636 | ) | ||
|
Total considerations
|
$ | 8,450,713 |
The Company intended to further engage an arm’s length party to conduct R&D of the Hair Growth Process in order to unify the various components of the current formula into a single formulation that has been tested for consistency at various temperatures, thus creating a predictable and reliable matrix for industrial manufacture, long term storage and transport (see Note 10). The engaged arm’s length party will also conduct extensive controlled testing in accordance with the requirements of the FDA, Health Canada and the European Medicines Agency in preparation for the anticipated pre-clinical trials. Hence, the Licensed Intellectual Property acquired in this asset acquisition is deemed for use in R&D activities as recognized as an in-process research and development (“IPR&D”) project.
F-9
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 2 – ACQUISITION (cont’d...)
(b) Intellectual Property License (BHSL) (cont’d...)
Pursuant to ASC 730-10, such assets are capitalized only if they have alternative future uses; otherwise, such assets are expensed. For an asset acquired in an asset acquisition for use in R&D activities to have an alternative future use, the following two criteria need to be met: (a) it is reasonably expected that the Company will use the asset acquired in the alternative manner and anticipates economic benefit from that alternative use, and (b) the Company’s use of the asset acquired is not contingent on further development of the asset subsequent to the acquisition date (that is, the asset can be used in the alternative manner in the condition in which it existed at the acquisition date). As the Company’s use of the Intellectual Property is contingent on further development and approval for the anticipated pre-clinical trials, it is deemed to have no alternative future use. Therefore, the costs allocated to acquire IPR&D in the above noted asset acquisition of $17,679,530 and the aforementioned $1,000,000 paid to BHSL were expensed as IPR&D expense accordingly.
In connection with the above noted asset acquisition, the Company made significant estimates and assumptions regarding the fair values of the elements of an asset acquisition as of the date of acquisition, including the fair values of due to Hair & Research Science Est. The Company also refined these estimates over a measurement period not to exceed one year to reflect new information obtained about facts and circumstances that existed as of the acquisition date that, if unknown, would have affected the measurement of the amounts recognized as of that date. If the Company is required to retroactively adjust provisional amounts that the Company has recorded for the fair values of assets and liabilities in connection with acquisitions, these adjustments could have a material impact on our financial condition and results of operations.
(c) Reverse Acquisition - Biologix Nevada
In connection with the reverse acquisition described in Note 1 and prior to the acquisition, Biologix Nevada had no business and did not meet the definition of a business under ASC 805, “Accounting for Business Combinations”. Accordingly, the reverse acquisition of Biologix Nevada by Biologix Florida has been accounted for as a capital transaction, in respect of which the net assets of Biologix Nevada on January 9, 2013 were accounted for as a recapitalization of Biologix Florida. A breakdown of Biologix Nevada’s net assets as at January 9, 2013 is as follows:
|
January 9, 2013
|
||||
|
Cash
|
$ | 7,515 | ||
|
Promissory note receivable – Biologix Florida
|
325,000 | |||
|
Accounts payable
|
(5,168 | ) | ||
|
Net assets acquired
|
$ | 327,347 | ||
The obligation to repay the $325,000 promissory note formerly payable from Biologix Florida to the Company was terminated promptly upon execution of the Share Exchange Agreement.
F-10
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation and Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries, Biologix Florida, Biologix Canada and BHSL, and BHSL’s wholly owned subsidiaries Biologix Hair, Panama S.A. (“Biologix Panama”), Biologix Hair South America S.A. (“Biologix South America”) and Biologix Hair (Hong Kong) Limited (“Biologix HK”).
These consolidated financial statements have been prepared in accordance with United States generally accepted accounting principles (“US GAAP”). All significant inter-company transactions and balances have been eliminated upon consolidation.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with a maturity of three months or less at the time of issuance to be cash equivalents. As at March 31, 2013, the Company has cash and cash equivalents in the amount of $56,409 (December 31, 2012 - $44,577) which are over the federally insured limit. As at March 31, 2013 and December 31, 2012, the Company has $Nil of cash equivalents.
Deferred cost
The consideration commitment on the Intellectual Property License with BHSL are deferred until the completion of the due diligence at which time the cost will be charged to operation as IPR&D expense.
Website Development Costs
In accordance with ASC 350-50, “Website Development Costs”, the Company capitalizes qualifying website development costs. Costs incurred during the application development stage as well as upgrades and enhancements that result in additional functionality are capitalized. Accordingly, direct costs incurred during the application stage of development are capitalized and amortized over the estimated useful life of three years, the expected period of benefit. Fees incurred for website hosting are expensed over the period of the benefit. Costs of operating a website are expensed as incurred.
Property and Equipment
Property and equipment consists of computer equipment, furniture and equipment and leasehold improvements which are carried at cost, net of accumulated depreciation and impairment loss. Amortization is recorded on the straight line method at the following rates:
|
Furniture and equipment
|
3 years
|
|
Leasehold improvements
|
2 years
|
|
Computer equipment
|
2 years
|
Amortization will commence once the property and equipment is put in use. The property and equipment is written down to its net realizable value if it is determined that its carrying value exceeds estimated future benefits to the Company.
F-11
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont’d…)
Acquired In-Process Research and Development
In accordance with ASC 730-10, the initial costs of rights to acquired IPR&D projects acquired in an asset acquisition are expensed as IPR&D unless the project has an alternative future use. These costs include initial payments incurred prior to regulatory approval in connection with research and development collaboration agreements that provide rights to develop, manufacture, market and/or sell pharmaceutical products. The fair value of IPR&D projects acquired in a business are capitalized and accounted for as indefinite-lived intangible assets until the underlying project receives regulatory approval, at which point the intangible asset will be accounted for as definite-lived intangible assets, or discontinuation, at which point the intangible asset will be written off. Development costs incurred after the acquisition are expensed as incurred. Indefinite- and definite-lived assets are subject to impairment reviews.
Stock-Based Compensation
The Company adopted ASC Topic 718-10, Compensation - Stock Compensation - Overall, to account for its stock options and similar equity instruments issued. Accordingly, compensation costs attributable to stock options or similar equity instruments granted are measured at the fair value at the grant date, and expensed over the expected vesting period. ASC Topic 718-10 requires excess tax benefits be reported as a financing cash inflow rather than as a reduction of taxes paid.
Warrant Issuance and Note Conversion Feature
In accordance with ASC 470-20, Debt with conversions and other options, the proceeds from the notes were allocated based on the relative fair values of the notes without the warrants issued in conjunction with the notes and of the warrants themselves at the time of issuance. The Company recorded the allocated value of the warrants at the time of issuance as additional paid-in capital and as a debt discount to the notes. The Company amortize the debt discount as interest expense over the life of the note. Additionally, as a result of issuing the warrants with the convertible notes, a beneficial conversion option is recorded as a debt discount reflecting the incremental conversion option intrinsic value of the conversion option provided to the holders of the notes. The Company also amortize this debt discount as interest expense over the life of the notes. The intrinsic value of each conversion option was calculated as the difference between the effective conversion price and the fair value of the common stock, multiplied by the number of shares into which the note is convertible.
Foreign Currency Translation
The Company’s functional currency and presentation currency is the U.S. dollars. Biologix Canada’s functional currency is Canadian dollars. Biologix Florida, BHSL, Biologix HK, Biologix Panama and Biologix South America’s functional currency are all U.S. dollars.
Transactions in a currency other than the functional currency (“foreign currency”) are measured in the respective functional currencies of the Company and its subsidiaries and are recorded on initial recognition in the functional currencies at exchange rates approximating those ruling at the transaction dates. Exchange gains and losses are recorded in the statements of income and comprehensive income.
F-12
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont’d…)
Foreign Currency Translation (cont’d…)
Assets and liabilities of the Company and its subsidiaries are translated into the U.S. dollars at exchange rates at the balance sheet date, equity accounts are translated at historical exchange rate and revenues and expenses are translated by using the average exchange rates. Translation adjustments are reported as cumulative translation adjustments and are shown as a separate component of other comprehensive income in the statements of stockholders’ equity.
Basic Net Income (Loss) per Share
Basic earnings per share is calculated using the weighted average number of shares outstanding during the year. The Company has adopted ASC Topic 260-10, Earnings per Share - Overall, and uses the treasury stock method to compute the dilutive effect of options, warrants and similar instruments. Under this method, the dilutive effect on earnings per share is recognized on the use of the proceeds that could be obtained upon exercise of options, warrants and similar instruments. It assumes that the proceeds would be used to purchase common shares at the average market price during the period. Diluted loss per share is equal to basic loss per share as any possible dilutive instruments are anti-dilutive.
Income Taxes
The Company accounts for income taxes under the provisions of ASC Topic 740-10, Income Taxes, which requires the Company to recognize deferred tax liabilities and assets for the expected future tax consequences of events that have been recognized in the Company’s financial statements or tax returns using the liability method. Under this method, deferred tax liabilities and assets are determined based on the temporary differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse. The effect on deferred income tax assets and liabilities of a change in income tax rates is included in the period that includes the enactment date. Valuation allowances are established when necessary to reduce deferred income tax assets to the amount expected to be realized.
Long-lived Assets Impairment
Long-term assets of the Company are reviewed for impairment whenever events or circumstances indicate that the carrying amount of assets may not be recoverable, pursuant to guidance established in ASC 360-05, Impairment or Disposal of Long-Lived Assets.
Management considers assets to be impaired if the carrying value exceeds the future projected cash flows from related operations (undiscounted and without interest charges). If impairment is deemed to exist, the assets will be written down to fair value. Fair value is generally determined using a discounted cash flow analysis.
F-13
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont’d…)
Use of Estimates
The preparation of the Company's consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Significant items subject to such estimates and assumptions include fair values of the elements of an asset acquisition as of the date of acquisition; asset impairments; useful lives for depreciation, depletion, and amortization; current and deferred income taxes; and fair value of financial instruments. Estimates are based on facts and circumstances believed to be reasonable at the time; however, actual results could differ from those estimates.
Fair Value of Financial Instruments
The estimated fair values for financial instruments under ASC Topic 825-10, Financial Instruments, are determined at discrete points in time based on relevant market information. These estimates involve uncertainties and cannot be determined with precision. The estimated fair value of the Company’s financial instruments includes cash and cash equivalents, accounts payable and accrued liabilities, amounts due to related parties, promissory and convertible promissory notes. The carrying value of these financial instruments approximates their fair value based on their liquidity or their short-term nature. Unless otherwise noted, it is management’s opinion that the Company is not exposed to significant interest, currency or credit risks arising from these financial instruments due to their short term nature.
The Company adopted ASC Topic 820-10, Fair Value Measurements and Disclosures, which defines fair value, establishes a framework for measuring fair value in US GAAP, and expands disclosures about fair value measurements.ASC Topic 820-10 does not require any new fair value measurements, but provides guidance on how to measure fair value by providing a fair value hierarchy used to classify the source of the information. The fair value hierarchy distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
|
Level one – Quoted market prices in active markets for identical assets or liabilities;
|
|
Level two – Inputs other than level one inputs that are either directly or indirectly observable; and
|
Level three – Unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use.
As at March 31, 2013 and December 31, 2012, the fair value of cash and cash equivalents was measured using Level one inputs.
F-14
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont’d…)
Recently Adopted Accounting Standards
In May 2011, the FASB issued ASU 2011-04, “Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs”, which is effective for annual reporting periods beginning after December 15, 2011. This guidance amends certain accounting and disclosure requirements related to fair value measurements. Additional disclosure requirements in the update include: (1) for Level 3 fair value measurements, quantitative information about unobservable inputs used, a description of the valuation processes used by the entity, and a qualitative discussion about the sensitivity of the measurements to changes in the unobservable inputs; (2) for an entity’s use of a nonfinancial asset that is different from the asset’s highest and best use, the reason for the difference; (3) for financial instruments not measured at fair value but for which disclosure of fair value is required, the fair value hierarchy level in which the fair value measurements were determined; and (4) the disclosure of all transfers between Level 1 and Level 2 of the fair value hierarchy. The Company adopted ASU 2011-04 on January 1, 2012 and the adoption did not have a material effect on the Company’s consolidated financial position or results of operations.
In June 2011, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2011-05, Comprehensive Income (Topic 220): Presentation of Comprehensive Income, which is effective for annual reporting periods beginning after December 15, 2011. ASU 2011-05 will become effective for the Company on January 1, 2012. This guidance eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity. In addition, items of other comprehensive income that are reclassified to profit or loss are required to be presented separately on the face of the financial statements. This guidance is intended to increase the prominence of other comprehensive income in financial statements by requiring that such amounts be presented either in a single continuous statement of income and comprehensive income or separately in consecutive statements of income and comprehensive income.
The Company adopted ASU 2011-05 on January 1, 2012 and the adoption did not have a material impact on the Company’s financial position or results of operations.
In September 2011, the Financial Accounting Standards Board (“FASB”) issued ASU 2011-08, “Intangibles – Goodwill and Other (Topic 350), Testing Goodwill for Impairment”. ASU 2011-08 amends the required annual impairment testing of goodwill by providing an entity an option to first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If, after assessing the totality of events and circumstances, an entity determines it is not more likely than not that the fair value of a reporting unit is less than its carrying amount, then performing the two-step impairment test under Topic 350-24 and Topic 350-20-35-9 is unnecessary. However, if an entity concludes otherwise, then it is required to perform the impairment testing under Topic 350-24 by calculating the fair value of the reporting unit and comparing the results with the carrying amount. If the fair value exceeds the carrying amount, then the entity must perform the second step test of measuring the amount of the impairment test under Topic 350-20-35-9. An entity has the option to bypass the qualitative assessment and proceed directly to the two step goodwill impairment test. Additionally, the entity has the option to resume with the qualitative testing in any subsequent period. The Company adopted ASU 2011-08 on January 1, 2012 and the adoption did not have a material effect on the Company’s consolidated financial position or results of operations.
F-15
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont’d…)
Recent Accounting Pronouncements
In December 2011, the FASB issued ASU 2011-11, “Balance Sheet (Topic 210), Disclosures about Offsetting Assets and Liabilities”. The guidance in this update requires the Company to disclose information about offsetting and related arrangements to enable users of its financial statements to understand the effect of those arrangements on its financial position. The pronouncement is effective for fiscal years and interim periods beginning on or after January 1, 2013 with retrospective application for all comparative periods presented. The Company’s adoption of the new standard is not expected to have a material effect on the Company’s consolidated financial position or results of operations.
In July, 2012, the FASB issued ASU 2012-02, “Intangibles – Goodwill and Other (Topic). ASU 2012-02 amends the required annual impairment testing of indefinite-lived intangible assets by providing an entity an option to first assess qualitative factors to determine whether it is more likely than not that the fair value of the indefinite-lived asset is less than its carrying amount. If, after assessing the totality of events and circumstances, an entity determines it is not more likely than not that the fair value of the indefinite-lived asset is less than its carrying amount, then performing the two-step impairment test under Topic 350-30 is unnecessary. However, if an entity concludes otherwise, then it is required to perform the impairment testing under Topic 350-30-35-18F by calculating the fair value of the reporting unit and comparing the results with the carrying amount. If the fair value exceeds the carrying amount, then the entity must perform the second step test of measuring the amount of the impairment test under Topic 350-30-35-19. An entity has the option to bypass the qualitative assessment and proceed directly to the two step goodwill impairment test. Additionally, the entity has the option to resume with the qualitative testing in any subsequent period. The pronouncement is effective for annual and interim impairment tests performed for fiscal years beginning after September 15, 2012 and early adoption is permitted. The Company’s adoption of the new standard is not expected to have a material effect on the Company’s consolidated financial position or results of operations.
In February 2013, the FASB issued ASU 2013-02, "Comprehensive Income (Topic 220), Reporting of Amounts Reclassified Out of Accumulated Other Comprehensive Income". The amendments do not change the current requirements for reporting net income or other comprehensive income in financial statements. However, the amendments require an entity to provide information about the amounts reclassified out of accumulated other comprehensive income by component. In addition, an entity is required to present, either on the face of the statement where net income is presented or in the notes, significant amounts reclassified out of accumulated other comprehensive income by the respective line items of net income but only if the amount reclassified is required under U.S. GAAP to be reclassified to net income in its entirety in the same reporting period. For other amounts that are not required under U.S.GAAP to be reclassified in their entirety to net income, an entity is required to cross-reference to other disclosures required under U.S. GAAP that provide additional detail about those amounts. The pronouncement is effective for fiscal years and interim periods ending after December 15, 2012. The adoption of this pronouncement is not expected to have a material effect on the Company's consolidated financial position or results of operations.
F-16
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 4 – PREPAID EXPENSES AND DEPOSIT
|
March 31,
2012
|
December 31,
2012
|
|||||||
|
Current
|
||||||||
|
Deposits and advances
|
1,000 | 1,000 | ||||||
|
Prepaid rent
|
174,729 | 224,025 | ||||||
|
Employee advances
|
- | 25,657 | ||||||
|
Prepaid expenses and deposit – current
|
175,729 | 250,682 | ||||||
|
Non-current
|
||||||||
|
Prepaid rent
|
- | 31,270 | ||||||
|
Prepaid expenses and deposit – non-current
|
- | 31,270 | ||||||
NOTE 5 – PROPERTY AND EQUIPMENT
Property and equipment at March 31, 2013 and December 31, 2012 were summarized as follows:
|
March 31, 2013
|
Cost
$
|
Accumulated
Amortization
$
|
Net book
Value
$
|
||||||||||
|
Computer equipment
|
17,124 | 5,351 | 11,773 | ||||||||||
|
Furniture and equipment
|
93,536 | 33,219 | 60,317 | ||||||||||
|
Leasehold improvements
|
125,968 | 64,619 | 61,349 | ||||||||||
| 236,628 | 103,189 | 133,439 | |||||||||||
|
December 31, 2012
|
Cost
$
|
Accumulated
Amortization
$
|
Net book
Value
$
|
||||||||||
|
Computer equipment
|
17,124 | 4,281 | 12,843 | ||||||||||
|
Furniture and equipment
|
93,536 | 26,574 | 66,962 | ||||||||||
|
Leasehold improvements
|
125,968 | 51,694 | 74,274 | ||||||||||
| 236,628 | 82,549 | 154,079 | |||||||||||
During the three months ended March 31, 2013, $20,640 (2012: $18,264) amortization expense was charged to operations. During the year ended December 31, 2012, $82,549 amortization expense was charged to operations. During the year ended December 31, 2012 the Company recognized loss on disposition of the asset of $6,362.
F-17
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 6 – WEBSITE DEVELOPMENT COSTS
|
March 31, 2013
|
Cost
$
|
Accumulated
Amortization
$
|
Net book
Value
$
|
|||||||||
|
Website development
|
144,177 | 46,881 | 97,296 | |||||||||
|
December 31, 2012
|
Cost
$
|
Accumulated
Amortization
$
|
Net book
Value
$
|
|||||||||
|
Website development
|
129,545 | 35,273 | 94,272 | |||||||||
During the three months ended March 31, 2013, $11,608 (2012: $7,306) amortization expense was charged to operations. During the year ended December 31, 2012, $35,273 amortization expense was charged to operations.
NOTE 7 – PROMISSORY AND CONVERTIBLE PROMISSORY NOTES
|
March 31,
|
December 31,
|
|||||||
|
2013
|
2012
|
|||||||
| $ | $ | |||||||
|
Promissory and convertible promissory notes - face value
|
||||||||
|
(a) Promissory note
|
||||||||
|
(b) Convertible promissory note (Unionashton)*
|
1,155,829 | 1,155,829 | ||||||
| 200,000 | 200,000 | |||||||
| 50,000 | 50,000 | |||||||
| 50,000 | - | |||||||
| 100,000 | - | |||||||
|
(c) Convertible promissory note (HRSE)*
|
9,540,000 | 9,540,000 | ||||||
|
(d) Promissory note (BHSL acquisition)
|
3,900,000 | 3,900,000 | ||||||
|
(e) Promissory note (Biologix Nevada)
|
- | 325,000 | ||||||
| 14,995,829 | 15,170,829 | |||||||
|
Effective interest rate - 12.5%
|
(1,538,712 | ) | (1,538,712 | ) | ||||
|
Net present value
|
13,457,117 | 13,632,117 | ||||||
|
Payment
|
(200,000 | ) | (100,000 | ) | ||||
|
Interest accretion
|
1,550,195 | 736,153 | ||||||
| 14,807,312 | 14,268,270 | |||||||
|
Current portion
|
11,504,439 | 7,604,595 | ||||||
|
Non-current portion
|
3,302,873 | 6,663,675 | ||||||
| 14,807,312 | 14,268,270 | |||||||
F-18
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 7 – PROMISORRY AND CONVERTIBLE PROMISSORY NOTES (cont’d ...)
The total amount of principal plus accrued interest are all convertible into common shares (“Common Shares”) of the Company. Each Common Share so issued will for these purposes be valued based on a conversion price (the “Conversion Price”) equal to: (i) if the Company is a privately held company upon the Notice of Conversion, the greater of (a) $1 per Common Share and (b) the price per Common Share of most recent private placement of securities completed by the Company as at the applicable maturity date less 20%; or (ii) if upon the Notice of Conversion the Company is a public company whose securities are listed on a stock exchange or quoted on an over-the-counter quotation system (whether directly or resulting from a Capital Reorganization), the greater of (a) $1 per Common Share and (b) a price per Common Share equal to the average daily closing price of the Common Shares during the 10 day period beginning on the date of the Notice of Conversion, less 20%.
a) Promissory note
On December 23, 2011, the Company signed a promissory note for $400,000. The loan bears interest at 5% per annum commencing on the day the principal sum is advanced to the Company and is due on or before December 31, 2012. On March 12, 2012 the $400,000 was applied to the subscription of 1,600,000 common of the Company at a value of $0.25 per share. Interest in the amount of $4,384 was accrued as at March 12, 2012.
b) Convertible Promissory notes – Honeywagon & Unionashton
On June 5, 2012 the Company signed a promissory note with Honeywagon Holdings Ltd. (“Honeywagon”) for $400,000 due and payable on or before August 6, 2012. The loan bears interest at 10% for the period ending August 6, 2012, payable on maturity. Any interest and principal due under the promissory note is convertible, at the lenders option, into the Company’s common shares at the Conversion Price per share. Interest in the amount of $36,721 was accrued as at August 1, 2012.
The fair value of the convertible promissory note is $391,837 on the date of issuance, with $5,388 representing the value net of tax impact ascribed to the creditors’ option to convert the principal amount into common shares of the Company and classified as additional paid-in capital and with the equal amoun$8,163 t charged to profit and loss and interest expense on August 1, 2012 (see below).
On June 26, 2012 the Company signed a promissory note with Honeywagon for an additional $200,000 due and payable on or before August 27, 2012. The loan bears interest at 10% for the period ending August 27, 2012, payable on maturity. Any interest and principal due under the promissory note is convertible, at the lenders option, into the Company’s common shares at the Conversion Price per share. Interest in the amount of $11,613 was accrued as at August 1, 2012. The fair value of the convertible promissory note is $195,918 on the date of issuance, with $2,694 representing the value net of tax impact ascribed to the creditors’ option to convert the principal amount into common shares of the Company and classified as additional paid-in capital and with $4,082 charged to profit and loss and interest expense on August 1, 2012 (see below).
On July 17, 2012 the Company signed a promissory note with Honeywagon for an additional $100,000 due and payable on or before September 17, 2012. The loan bears interest at 10% for the period ending August 27, 2012, payable on maturity. Any interest and principal due under the promissory note is convertible, at the lenders option, into the Company’s common shares at the Conversion Price per share.
F-19
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 7 – PROMISORRY AND CONVERTIBLE PROMISSORY NOTES (cont’d ...)
b) Convertible Promissory notes – Honeywagon & Unionashton (cont’d ...)
Interest in the amount of $2,420 was accrued as at August 1, 2012. The fair value of the convertible promissory note is $97,959 on the date of issuance, with $1,347 representing the value net of tax impact ascribed to the creditors’ option to convert the principal amount into common shares of the Company and classified as additional paid-in capital and with $2,041 charged to profit and loss and interest expense on August 1, 2012 (see below).
On August 1, 2012, the Company received an additional $300,000 from Honeywagon and replaced the aforementioned Promissory Notes including in the aggregate the principal sum of $700,000 and accrued interest in the amount of $50,754, with one for the principal sum of $1,050,754, due and payable on or before October 1, 2012. The loan bears interest at the rate of 10% for the period and shall be payable upon maturity. Interest in the amount of $105,075 was recorded for the period from August 1, 2012 to October 1, 2012. The fair value of the convertible promissory note is $1,029,310 on the date of issuance, with $14,153 representing the value net of tax impact ascribed to the creditors’ option to convert the principal amount into common shares of the Company and classified as additional paid-in capital and with $21,444 charged to profit and loss and interest expense at the date of assignment on October 1, 2012 (see below).
Pursuant to a convertible promissory note assignment agreement dated October 1, 2012 between Honeywagon and Unionashton Managment Ltd. (“Unionashton”), the promissory note in the amount of $1,050,754 plus accrued interest to October 1, 2012 of $105,075 for a total amount of $1,155,829 was assigned from Honeywagon to Unionashton and the Company replaced and signed a new convertible promissory note in the amount of $1,155,829, due and payable on demand. The loan bears interest at the rate of 5% per annum and shall be payable upon maturity. As additional consideration of the loans, the Company have granted Unionashton warrants to purchase an aggregate of 1,155,829 shares of the Company’s common share exercisable for a 5 year period at the Conversion Price per share. As a result, the aggregate principal value was allocated to the individual components on a relative fair value basis and the Company recorded $266,280 to additional paid-in capital as the fair value of the warrants, net of tax impact and $403,455 was charged to operations immediately as the convertible promissory note is due on demand (see Note 9 – warrants). Interest in the amount of $14,250 was accrued during the three months ended March 31, 2013 and cumulatively $28,817 was accrued as at March 31, 2013.
On November 14, 2012 the Company signed a promissory note for an additional $200,000 with Unionashton, due and payable on demand. The loan bears interest at 5%. Any interest and principal due under the promissory note is convertible, at the lenders option, into the Company’s common shares at the Conversion Price per share. Interest in the amount of $2,466 was accrued during the three months ended March 31, 2013 and cumulatively $3,781 was accrued as at March 31, 2013.
On December 14, 2012 the Company signed a promissory note for an additional $50,000 due and payable on demand. The loan bears interest at 5%. Any interest and principal due under the promissory note is convertible, at the lenders option, into the Company’s common shares at the Conversion Price per share. Interest in the amount of $617 was accrued during the three months ended March 31, 2013 and cumulatively $740 was accrued as at March 31, 2013.
F-20
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 7 – PROMISORRY AND CONVERTIBLE PROMISSORY NOTES (cont’d ...)
b) Convertible Promissory notes – Honeywagon & Unionashton (cont’d ...)
On January 4, 2013 the Company signed a promissory note for an additional $50,000 with Unionashton, due and payable on demand. The loan bears interest at 5%. Any interest and principal due under the promissory note is convertible, at the lenders option, into the Company’s common shares at the Conversion Price per share. Interest in the amount of $596 was accrued as at March 31, 2013.
On January 16, 2013 the Company signed a promissory note for an additional $100,000 with Unionashton, due and payable on demand. The loan bears interest at 5%. Any interest and principal due under the promissory note is convertible, at the lenders option, into the Company’s common shares at the Conversion Price per share. Interest in the amount of $1,027 was accrued as at March 31, 2013.
c) Convertible promissory note – HRSE
On April 11, 2012 BHSL entered into an Intellectual Property Purchase and Sale Agreement with Hair Research and Science Est, (“HRSE”) whereby it acquired certain Intellectual Property, including processes and formulae for the stimulation of hair growth and treatment of hair loss, for consideration in the amount of $10,100,000 of Convertible Promissory Note. (See Note 2b).
On August 1, 2012, as the Company was unable to make the first term payment in full at due date, the Convertible Promissory Note with unpaid accrued interests of $9,090,833 became in default and due on demand. On August 1, 2012 (further amended on November 30, 2012), the Company entered into an Amending Agreement with HRSE to amend the Convertible Promissory Note, repayable as follow:
|
●
|
$1,040,000 on or before February 28, 2013;
|
|
●
|
$2,000,000 on or before June 30, 2013;
|
|
●
|
$3,000,000 on or before October 31, 2013; and
|
|
●
|
$3,500,000 on or before January 31, 2014.
|
The Convertible Promissory Note shall bear interest at the rate of 5% per annum and is payable by January 31, 2014. In the event that the Company become in default of the agreement, the Convertible Promissory Note shall bear interest at 12% per annum, which interest will be payable on demand. The Convertible Promissory Note and any accrued interest are convertible at any time into the Company’s common shares at the Conversion Price per share.
The fair value of the amended Convertible Promissory Note is $9,090,833 on the date of amendment (August 1, 2012), with $255,194 representing the value net of tax impact ascribed to the creditors’ option to convert the principal amount into common shares of the Company and classified as additional paid-in capital and with $8,750,574 classified as liability portion of the amended Convertible Promissory Note, calculated as the net present value using an interest rate of 12.5%, which is the market interest rate that was payable on comparable notes without the conversion feature. On February 28, 2013, as the Company was unable to make the first term payment in full at due date, the Convertible Promissory Note with unpaid accrued interests of $9,913,650 are in default and are now due on demand. An imputed interest of $701,905 was accrued during the three months ended March 31, 2013 and cumulatively $1,163,075 was accrued as at March 31, 2013. The Company is currently negotiating a new term with HRSE.
F-21
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 7 – PROMISORRY AND CONVERTIBLE PROMISSORY NOTES (cont’d ...)
d) Promissory note – BHSL acquisition
Pursuant to the acquisition of 100% of the shares of BHSL, the Company made a payment of $ 3,900,000 in the form of a non-interest bearing promissory note payable by April 19, 2014 (see Note 2b). The fair value of the promissory note payable is $3,150,713 at inception, calculated as the net present value using an interest rate of 12.5%, which is the interest rate that was payable on comparable notes. An imputed interest of $93,180 was accrued during the three months ended March 31, 2013 and cumulatively $352,160 was deemed to have been accrued as at March 31, 2013. As at March 31, 2013 the Company has made principal payments in the amount of $200,000 against the $3,900,000 promissory notes. See also Note 12.
e) Promissory note – Biologix Nevada
On October 15, 2012, Biologix Florida signed a promissory note for $300,000 due and payable to Biologix Nevada on or before January 1, 2013. On December 20, 2012, Biologix Nevada further advanced a promissory note of $25,000. The loans bear interest at 10% per annum, payable on maturity. The obligation to repay the promissory note plus interest terminated promptly upon execution of the Share Exchange Agreement on January 9, 2013. See also Note 1 and Note 2 (c).
NOTE 8 – RELATED PARTY TRANSACTIONS
Related party transactions not disclosed elsewhere in these consolidated financial statements are as follows:
|
Related party transactions with directors/executives or formal directors/executives and companies controlled by directors/executives or formal directors/executives:
|
|
Three
months
|
Three
months
|
|||||||
|
ended
|
ended
|
|||||||
|
March 31,
|
March 31,
|
|||||||
|
2013
|
2012
|
|||||||
| $ | $ | |||||||
|
Clinical research and reformulatio
|
79,118 | - | ||||||
|
Consulting and management fees
|
85,813 | 72,788 | ||||||
|
Professional fees
|
15,000 | 16,032 | ||||||
|
Wages and benefits
|
39,887 | - | ||||||
|
Website development and maintenance
|
55,290 | 87,668 | ||||||
| 275,108 | 176,488 | |||||||
F-22
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 8 – RELATED PARTY TRANSACTIONS (cont’d ...)
|
Amounts due to related parties:
|
As at
|
As at
|
||||||
|
March 31,
|
December 31,
|
|||||||
|
2013
|
2012
|
|||||||
| $ | $ | |||||||
|
Ron Holland, CEO and a director
|
- | 45,683 | ||||||
|
Christian Gomez, former CFO and director
|
- | 3,000 | ||||||
|
Daniel Hunter, former President
|
- | 42,337 | ||||||
|
Donna Lieder, VP Clinician Licensing
|
660 | 32,210 | ||||||
|
H. H Research Partners Corp, a company controlled by VP Media Relations
|
5,013 | - | ||||||
|
Jolee Consulting Corp., a company controlled by a former VP of Shareholder Communications
|
- | 9,875 | ||||||
|
Richardo Faria, former CTO
|
- | 24,150 | ||||||
|
Dr. Diego Castresana, VP R&D of BHSL, former director
|
- | 10,000 | ||||||
|
MacDonald Tuskey, a principal is a former director of the Company
|
- | 22,234 | ||||||
| 5,673 | 189,489 | |||||||
These transactions were in the normal course of operations and were measured at the exchange amount, which is the amount of consideration established and agreed to by the related parties.
NOTE 9 – STOCKHOLDERS’ EQUITY
Common stock
During the period ended December 31, 2011, the Company issued 10,000 common shares for $100 to a director of the Company at $0.01 per share.
On April 18, 2012 the Company issued 9,450,000 common shares at $0.05 per share pursuant to a private placement with total proceeds of $472,500; issued 6,220,000 common shares at $0.25 per share pursuant to a private placement with gross proceeds of $1,555,000, of which $400,000 was paid for by redemption of the promissory note on March 12, 2012, and commission expense of $10,000; and issued 2,000,000 common shares as settlement of debt at a value of $500,000, using a deemed value of $0.25 per share. See Note 2b.
On May 2, 2012, the Company issued 2,500,000 common shares at $0.8 per share pursuant to a private placement, with total proceeds of $2,000,000 and share issuance cost of $200,000.
On May 18, 2012, the Company issued 4,000,000 common shares at a value of $0.80 per share as partial consideration for the acquisition of 100% of the issued and outstanding shares of BHSL. See Note 2(b).
F-23
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 9 – STOCKHOLDERS’ EQUITY (cont’d ...)
Common stock (cont’d ...)
On June 6, 2012, the Company issued 500,000 common shares for settlement of debt at a value of $1 per share pursuant to a purchase and sale agreement entered into on April 11, 2012. See Note 2.
On June 22, 2012, the Company issued 1,500,000 common shares at $1 per share pursuant to a private placement, with total proceeds of $1,500,000.
On September 24, 2012, the Company issued 250,000 common shares at $1 per share pursuant to a private placement, with total proceeds of $250,000.
Upon the reverse acquisition on January 9, 2013, 30,200,000 common shares issued by the Company prior to the acquisition were considered as a recapitalization to Biologix Florida.
Share subscriptions received in advance
As at March 31, 2013, share subscriptions received in advance consists of $650,000 (December 31, 2012 - $Nil) for 650,000 common shares at a value of $1.00 per share that have not yet been issued as at March 31, 2013. See Note 12.
Stock options
A summary of stock option activities is as follows:
|
Options outstanding
|
||||||||||||
|
Weighted average
|
Weighted average
|
|||||||||||
|
Number of
|
exercise
|
remaining
|
||||||||||
|
shares
|
price
|
contractual life (years)
|
||||||||||
|
Outstanding and exercisable at December 31, 2011
|
- | - | - | |||||||||
|
Granted
|
1,620,000 | 0.25 | 5.00 | |||||||||
|
Cancelled
|
(1,620,000 | ) | 0.25 | 5.00 | ||||||||
|
Outstanding and exercisable at December 31, 2012
|
- | - | - | |||||||||
|
Granted
|
1,475,000 | 1.00 4.83 | ||||||||||
|
Outstanding and exercisable at March 31, 2013
|
1,475,000 | 1.00 4.83 | ||||||||||
F-24
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 9 – STOCKHOLDERS’ EQUITY (cont’d ...)
Stock options (cont’d ...)
During the three months ended March 31, 2013, the Company granted 1,475,000 stock options to employees, directors and consultants of the Company, entitling the holders to purchase common shares of the Company for proceeds of $1 per common share expiring January 28, 2018. These options were vested immediately upon granting. The fair value of the options, estimated using Black-Scholes Option Pricing Model, was $1,615,956. The amount has been expensed as stock based compensation during the period ended March 31, 2013.
During the fiscal year ended December 31, 2012, the Company granted 1,620,000 stock options to employees, directors and consultants of the Company, entitling the holders to purchase common shares of the Company for proceeds of $0.25 per common share expiring June 1, 2017. These options were vested immediately upon granting. The fair value of the options, estimated using Black-Scholes Option Pricing Model, was $962,528. This amount has been expensed as stock based compensation during the fiscal year ended December 31, 2012. All 1,620,000 stock options were cancelled prior to December 31, 2012.
|
March 31, 2013
|
December 31, 2012
|
|||||||
|
Risk free interest rate
|
0.29 | % | 0.62 | % | ||||
|
Expected life
|
2 years
|
1 year
|
||||||
|
Annualized volatility
|
126 | % | 121 | % | ||||
|
Expected dividends
|
- | - | ||||||
The fair value of each option was estimated using Black-Scholes Option Pricing Model. The assumptions about stock-price volatility have been based exclusively on the implied volatilities of publicly traded options to buy the Company’s stock with contractual terms closest to the expected life of options granted to employees, directors or consultants.
Warrants
The Company has the following warrants outstanding at March 31, 2013 and December 31, 2012:
|
|
Number
|
Weighted
|
Weighted
average
|
|||||||||
|
of
|
average
|
remaining
|
||||||||||
|
warrants
|
exercise
|
contractual life
|
||||||||||
|
price*
|
(in years)
|
|||||||||||
|
Balance outstanding, December 31, 2012
|
1,155,829 | $ | 1.00 | 4.75 | ||||||||
|
Balance outstanding, March 31, 2013
|
1,155,829 | $ | 1.00 | 4.50 | ||||||||
F-25
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 9 – STOCKHOLDERS’ EQUITY (cont’d ...)
Warrants (cont’d ...)
Pursuant to the convertible promissory note assignment agreement with Unionashton dated October 1, 2012, the Company has granted Unionashton warrants to purchase an aggregate of 1,155,829 shares of the Company’s common share exercisable for a 5 year period equal to the exercise price noted below (see Note 7b). The warrants were fair valued at $403,455 based on the Black-Scholes pricing model which utilizes the following assumptions: expected dividend yield of nil, expected stock price volatility of 111.84%, risk free interest rate of 0.62 and an average expected life of 1 year. The fair value of the convertible promissory note without the warrants attachment is $1,155,829 as it is due on demand. As a result, the Company allocated $403,455 to additional paid-in capital and an equal amount to operations as interest expense based on the relative fair values of the notes without the warrants issued in conjunction with the convertible promissory note and of the warrants themselves at the time of assignment.
The Company did not issue any warrants during the year ended December 31, 2011 and did not have any outstanding warrants as at December 31, 2011.
* The exercise value is equal to: (i) if the Company is a privately held company upon exercise, the greater of (a) $1 per Common Share and (b) the price per Common Share of most recent private placement of securities completed by the Company as at the applicable maturity date less 20%; or (ii) if the Company is a public company upon exercise and whose securities are listed on a stock exchange or quoted on an over-the-counter quotation system (whether directly or resulting from a Capital Reorganization), the greater of (a) $1 per Common Share and (b) a price per Common Share equal to the average daily closing price of the Common Shares during the 10 day period beginning on the date of the Notice of Conversion, less 20%.
NOTE 10 – COMMITMENTS
BHSL has entered into a research and development partnership agreement with Beijing BIT & GY Pharmaceutical R&D Co. Ltd. (“BIT”). The primary focus of the $1,050,000 R&D project is for BIT to refine and potentially enhance the formulation of Biologix Revive – the core of the Biologix Hair Therapy System™ – as well as to conduct extensive controlled testing in accord with the stringent requirements of regulatory agencies such as the Food and Drug Administration (FDA), Health Canada and the European Medicines Agency (EMA). During the year ended December 31, 2012, $787,500 of the total contract amount was charged to operations with $262,500 payable to BIT as at December 31, 2012 and March 31, 2013.
F-26
BIOLOGIX HAIR INC.
(formerly T & G Apothecary, Inc.)
(A development stage company)
(As expressed in US dollars)
(Unaudited – prepared by management)
NOTES TO THE INTERIM CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2013
NOTE 10 – COMMITMENTS (cont’d ...)
On September 1, 2012, BHSL entered into a warehousing and distribution agreement with KD Consultoria & Servicios S.A.S. (“KDCS”), an affiliated company of a director of the Company incorporated under the laws of Colombia, pursuant to which BHSL has engaged KDCS to provide certain pharmaceutical warehousing, inventory, and distribution services via KDCS’s facility located in Baranquilla, Colombia or Medellin, Colombia. Either party may terminate the agreement with 30 days written notice. The term of the agreement ends August 31, 2014 unless earlier terminated. The facility will serve as a distribution center by the sale of Biologix Revive in Colombia, and other eligible South America jurisdictions subject to requisite regulatory approvals. The Company has agreed to pay to KDCS $15,000 for each 6 months period during the term of the agreement, and royalty equal to 4% of the wholesale distribution price of each unit of Biologix Revive distributed through KDCS’s facility. The agreement was terminated at the end of the first 6 month period.
NOTE 11 – SEGMENTED INFORMATION
For the three months ended March 31, 2013, the Company operated solely for the Biologix therapy business. The Company is located and operated in Canada and Barbados. The Company’s total assets and net loss by geographic locations for the three months ended March 31, 2013 is as follows:
|
Loss
|
|
March 31,
2013
|
||||||||||
| $ | ||||||||||||
|
Canada
|
(2,428,228 | ) | ||||||||||
|
Barbados
|
(780,320 | ) | ||||||||||
| (3,208,548 | ) | |||||||||||
|
Assets
|
Canada
|
Barbados
|
Total
|
|||||||||
|
|
$ | $ | $ | |||||||||
|
|
||||||||||||
|
Cash
|
65,414 | 1,722 | 67,136 | |||||||||
|
Other assets
|
406,464 | - | 406,464 | |||||||||
| 471,878 | 1,722 | 473,600 | ||||||||||
NOTE 12 – SUBSEQUENT EVENTS
In accordance with ASC 855, Subsequent Events, the Company evaluated subsequent events through the date the financial statements are issued and as a result, is reporting the following:
|
a.
|
Subsequent to March 31, 2013, the Company signed promissory notes in the amount of $250,000 on May 1, 2013, due and payable on demand. The loan bears interest at the rate of 12% per annum and is payable on demand.
|
|
b.
|
Pursuant to the acquisition of 100% of the shares of BHSL, the Company made a payment of $ 3,900,000 in the form of a non-interest bearing promissory note payable by April 19, 2014 (see Note 7 (d)). On April 30, 2013, the Company entered agreements to convent debt for equity with two of the selling shareholders, Pendolino Investments Ltd. (“Pendolino”) and Gruppen Investments Ltd. (“Gruppen”). Effective immediately, Pendolino agreed to convert USD$1,887,000 of debt into 943,500 common shares of our company and Gruppen agreed to convert USD$906,500 of debt into 453,250 common shares of the company. Pursuant to these two agreements, the Company converted an aggregate of $2,793,500 of debt, payable pursuant to the promissory notes, into 1,396,750 common shares of the Company at a conversion price of USD$2.00 per common share.
|
|
c.
|
See also Note 7 (c).
|
F-27
Forward-Looking Statements
This quarterly report contains forward-looking statements. These statements relate to future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as "may", "should", "expects", "plans", "anticipates", "believes", "estimates", "predicts", "potential" or "continue" or the negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors that may cause our or our industry's actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
Our unaudited financial statements are stated in United States Dollars (US$) and are prepared in accordance with United States Generally Accepted Accounting Principles. The following discussion should be read in conjunction with our financial statements and the related notes that appear elsewhere in this quarterly report. The following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed below and elsewhere in this quarterly report.
In this quarterly report, unless otherwise specified, all dollar amounts are expressed in United States dollars. All references to "US$" refer to United States dollars and all references to "common stock" refer to the common shares in our capital stock.
As used in this quarterly report, the terms "we", "us", "our" and "our company" mean Biologix Hair Inc., and our wholly owned subsidiary, Biologix Hair Inc., a Florida company, unless otherwise indicated.
Corporate History and Background
We were incorporated on January 18, 2011, as T & G Apothecary, Inc. Our initial business plan was to develop and market a 100% USDA Certified Organic personal care product line for women, including bath additives and exfoliating facial cleansers. Owing to our inability to raise sufficient financing to execute our business plan, our management began considering alternative strategies, such as business combinations or acquisitions to create value for our shareholders.
On August 21, 2012, we received resignations from Carolyne S. Johnson and Scott A. Stupprich. Ms. Johnson resigned as president, chief executive officer, chief financial officer and as a director of our company. Mr. Stupprich resigned as secretary of our company. Their resignations were not the result of any disagreements with our company regarding its operations, policies, practices or otherwise. Concurrently with Ms. Johnson’s and Mr. Stupprich’s resignations, we appointed Lilia Roberts as president, chief executive officer, chief financial officer, secretary, treasurer and as a member to our board of directors, effective August 21, 2012.
Also on August 21, 2012, Ms. Roberts acquired a total of 5,000,000 shares of our common stock from Ms. Johnson, our former director and officer, for total consideration of $50,000. The funds used for this share purchase were Ms. Roberts’ personal funds. Ms. Roberts’ 5,000,000 shares amount to approximately 8.8% of our currently issued and outstanding common stock. In conjunction with the sale of her shares Ms. Johnson provided us with a release from any debt owed to her by our company.
4
On November 23, 2012, we entered into a share exchange agreement with Biologix Hair Inc., a Florida corporation, and its shareholders. Pursuant to the terms of the share exchange agreement, we agreed to acquire all of the issued and outstanding shares of Biologix Florida’s common stock in exchange for the issuance by our company of 26,430,000 post-split shares of our common stock to the shareholders of Biologix Florida.
On December 13, 2012, we effected a forward split of our issued and outstanding shares on a 7 new for 1 old basis, increased our authorized capital to 900,000,000 shares, with $0.001 par value, and changed our name from T & G Apothecary, Inc., to Biologix Hair Inc.
On January 9, 2013, we closed the share exchange by issuing the required 26,430,000 post-split common shares to the Biologix Florida shareholders. Concurrently our former director and officer, Lilia Roberts, cancelled 30,700,000 and transferred 3,300,000 of her 35,000,000 post-split shares of our stock. As a result of these transactions, we had 56,630,000 issued and outstanding common shares upon the closing of the share exchange with Biologix Florida.
Overview of Biologix Hair Inc.
As a result of the closing of the share exchange agreement with Biologix Florida, Biologix Florida has become our wholly owned subsidiary and we now carry on business as a development stage pharmaceutical and cosmeceutical company. We have only recently begun operations and we rely upon the sale of our securities to fund those operations. We are engaged in the research, development and commercialization of therapeutic pharmaceutical and cosmeceutical products and therapeutic methods for the treatment of human hair loss, including products for hair regeneration, hair loss prevention, and the treatment of alopecia aereata. Currently, our business is focused on the research, development and commercialization of our proprietary drug formula, known as the “Biologix Revive Hair FormulaTM.” The Biologix Revive Hair FormulaTM and the method for its application are marketed as the Biologix Hair Therapy SystemTM. We also intend to develop and market additional complimentary aftercare products under the umbrella of the Biologix Hair Therapy SystemTM, including health supplements, cosmeceutical shampoos, conditioners, creams, and related hair care products.
We were incorporated on January 18, 2011, as T & G Apothecary, Inc. We have one direct subsidiary, Biologix Hair Inc. (“Biologix Florida”), a company incorporated under the laws of the State of Florida, U.S.A. as Cranium Technologies (USA) Inc. on October 4, 2011. Biologix Florida changed its name to Biologix Hair Inc. (“Biologix”) on December 5, 2011. Biologix Florida has two direct subsidiaries, Biologix Hair (Canada) Inc., a company incorporated under the Canada Business Corporations Act, and Biologix Hair Science Ltd., a Barbados corporation. Biologix Barbados has three subsidiaries, Biologix Hair, Panama S.A., a Panama corporation, Biologix Hair South America S.A., a Panama corporation, and Biologix Hair (Hong Kong) Limited, a Hong Kong corporation.
All of our subsidiaries are wholly owned. Our current corporate structure is as follows:
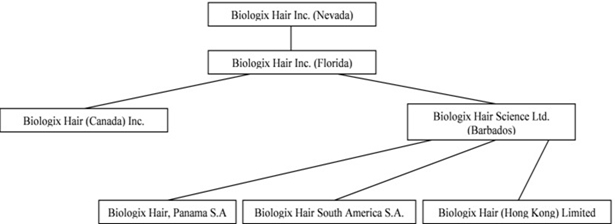
5
Our principal executive offices are located at 82 Avenue Road, Toronto, Ontario, Canada, M5R 2H2. The telephone number at our principal executive office is 647-344-5900. Our fiscal year end is December 31.
Business Strategy and Development
Our business model currently includes the following activities:
|
●
|
researching and developing our proprietary Biologix Revive Hair FormulaTM to achieve an optimal formulation for efficient mass production, storage and shipping;
|
|
|
●
|
expanding our intellectual property rights in the Biologix Revive Hair FormulaTM by prosecuting patents in strategic jurisdictions, including North America and Europe, among others;
|
|
|
●
|
conducting research and development in preparation of eventual pre-clinical and clinical trials of the Biologix Revive Hair FormulaTM to achieve regulatory approval of the formula in strategic jurisdictions, including North America and Europe, among others;
|
|
|
●
|
establishing a worldwide network of major regional licensees for our current and future products to market and wholesale distribute our products;
|
|
|
●
|
in cooperation with our wholesale distributors in jurisdictions where regulatory approval has been obtained or is not required, identifying, training and certifying a network of health professionals to act as retailers of the Biologix Revive Hair FormulaTM and the Biologix Hair Therapy SystemTM; and
|
|
|
●
|
conducting research and development of cosmeceutical hair care products based upon and complimentary to the Biologix Revive Hair FormulaTM, which will comprise part of the Biologix Hair Therapy SystemTM.
|
Development of the Biologix Revive Hair FormulaTM and the Biologix Hair Therapy SystemTM was initiated by Dr. Guillermo Duran in 1992. Between mid-2004 and late 2011, more than 30,000 treatments of Biologix ReviveTM were administered to 5,000-plus patients by a select private consortium of medical clinicians located in South American jurisdictions where regulatory approval was not required. The participating treatment clinicians subjectively observed and reported positive results for retention of healthy hair and for hair regeneration, including among alopecia areata patients. No side effects were reported. Based on this experience, we intend to optimize the formulation for mass production, storage and shipping and to conduct pre-clinical and clinical research to demonstrate to the relevant regulatory authorities, including the United States FDA, that the product is safe and effective for these uses.
In spite the prior successful clinical application of the Biologix Revive Hair FormulaTM in South America, our business will face a wide range of common pharmaceutical industry challenges. For example, the Biologix Revive Hair FormulaTM and any future products that we may develop will be required to undergo a time-consuming, costly and burdensome pre-market approval process in several major markets, including North America and Europe, and we may be unable to obtain regulatory approval for them.
We may not commence clinical testing of any products that we may develop and the commercial value of any clinical study that we conduct will depend significantly upon our choice of indication and our patient population selection. If we are unable to commence clinical testing or if we make a poor choice in terms of clinical strategy, we may never achieve revenues. Our clinical trials may also fail to adequately demonstrate the safety or efficacy of our product candidates for our chosen indications, which may force us to abandon our business plan. Even if we are able to ultimately obtain regulatory approval for any products that we may develop, we may never become profitable.
6
We will require substantial additional funds to complete our research and development activities, and if such funds are not available we may need to significantly curtail or cease our operations. We began our research and development activities in June, 2012 and we require financing to complete our research and development activities. Our financing needs may also change substantially because of a number of factors which are difficult to predict or which may be outside of our control; these include increased competition, the costs of protecting rights to our intellectual property, the resources required to complete pre-clinical and clinical studies, and the length and results of the regulatory approval process.
To date, we have outsourced all other research and development work in relation to the Biologix Revive Hair FormulaTM to Beijing BIT&GY Pharmaceutical R&D Co. Ltd. (BIT&GY), a third party laboratory affiliated with the Beijing Institute of Technology (BIT). We anticipate that we will continue to rely on third parties to satisfy our research and development requirements, including pre-clinical and clinical trial planning, laboratory services, data management, statistical services and report writing, until such time as it becomes necessary or cost effective to hire employees to satisfy those requirements.
Material Corporate Developments of Biologix Hair Inc. (Biologix Florida) and Biologix Hair Science Ltd. (Biologix Barbados).
On December 9, 2011, Biologix Florida entered into an intellectual property license agreement with Biologix Barbados pursuant to which Biologix Florida acquired a 99 year license to distribute Biologix ReviveTM and the Biologix Hair Therapy SystemTM in the territories of North America, the Caribbean, and Central America. In consideration of the distribution rights, we paid to Biologix Barbados $1,000,000. The distribution rights are subject to a royalty of $50 per injectable vial of Biologix ReviveTM sold in the territory, a 20% gross royalty on any Biologix Hair Therapy SystemTM aftercare products sold in the territory, and a minimum quarterly royalty guarantee of $100,000 for each quarter during the term of the agreement beginning with the quarter ended March 31, 2012.
On April 11, 2012, Biologix Barbados entered into an intellectual property purchase and sale agreement and convertible grid promissory note with Hair Research and Science Est., a company incorporated under the laws of the Principality of Liechtenstein. The agreement was subsequently amended by amending agreements dated August 1, 2012 and November 30, 2012, respectively (collectively the “purchase agreement”). Pursuant to the purchase agreement Biologix Barbados acquired 100% of all right, title and interest throughout the world in and to the Biologix Revive Hair FormulaTM and the Biologix Hair Therapy SystemTM, including all related formulae, products, processes, and technical know-how. The rights acquired pursuant to the agreement were subject only to the reservation of certain rights by the inventor of the formula, which are limited to the exclusive, non-assignable, royalty free right to provide, solely within the city of Barranquilla, Colombia, individual therapeutic treatments and services that employ the formula in relation to inventor’s private clinical practice located in the city of Barranquilla, Colombia.
7
In full consideration of all rights, title and interest acquired by Biologix Barbados in the purchase agreement, Biologix Barbados agreed to pay to Hair Research and Science Est. aggregate consideration of $10,640,000 payable as follows:
|
●
|
US $100,000 upon execution of the purchase agreement by the parties (which amount has been paid);
|
||
|
●
|
500,000 common shares of Biologix Hair Inc. valued at US$1 per common share (which shares have been issued);
|
||
|
●
|
US $10,040,000 in the form of a promissory note granted to Hair Research and Science Est., for which the following payment schedule has been agreed:
|
||
|
●
|
US $500,000 on or before June 30, 2012 (which amount has been paid);
|
||
|
●
|
US $1,040,000 on or before February 28, 2013 (as at the date of this report this amount has not been paid and our Company is currently re-negotiating the terms);
|
||
|
●
|
US $2,000,000 on or before June 30, 2013;
|
||
|
●
|
US $3,000,000 on or before October 31, 2013; and
|
||
|
●
|
US $3,500,000 on or before January 31, 2014.
|
||
The promissory note shall bear interest at the rate of 5% per annum and is payable by July 31, 2014. In the event that we become in default of the agreement, the note shall bear interest at 12% per annum, which interest will be payable on demand. The note and any accrued interest are convertible at any time into shares of our company’s common stock at a price per share equal to the lower of $1.00 per shares or the 10 day moving average price of our common shares as quoted on the Over The Counter Bulletin Board discounted by 20%.
As additional consideration Biologix Barbados has agreed to pay to Hair Research and Science Est. royalties as follows:
|
●
|
a perpetual, per treatment royalty equal to USD$20 for each vial of “Revive” injectable hair growth treatment manufactured and shipped by Biologix Barbados (or its sublicensee or assignee). The treatment royalty payable will be reviewed upon completion of each calendar year and adjusted if necessary for future years to account for inflation or deflation according to the United States CPI index certified by the Bureau of Labor Statistics to a maximum of 2.5% of the then current royalty rate.
|
|
●
|
a royalty equal to 10% of gross sales received by Biologix Barbados in respect of the sale of any after-treatment products, such as hair gels, shampoos, conditioners or similar after-treatment products based upon the intellectual property.
|
|
●
|
a royalty equal to 6% of gross sales actually received by Biologix Barbados in respect of sales of the Biologix ReviveTM in South America;
|
|
●
|
a minimum quarterly guarantee of US$50,000 payable upon completion of each of the first four fiscal quarters beginning with the quarter ending on March 31, 2012;
|
|
●
|
a quarterly guarantee of USD$100,000 payable upon completion of the fiscal quarter ending on March 31, 2013 and for each fiscal quarter completed thereafter;
|
|
●
|
all quarterly guarantees paid shall accrue and be deductible from all treatment or product royalties payable in future periods.
|
Subsequent to the period ended December 31, 2012, the payment of $1,040,000 payable by February 28, 2013 of the Convertible Promissory Note with Hair Research and Science Est. is in default and our company is currently negotiating new terms with Hair Research and Science Est.
8
On April 19, 2012, Biologix Florida entered into a share purchase agreement with Biologix Barbados and the selling shareholders of Biologix Barbados to acquire 100% of the outstanding securities of Biologix Barbados. In consideration of all outstanding common shares of Biologix Barbados, we promised to pay to the selling shareholders 4,000,000 shares in the common stock of Biologix Florida (which have been issued with a deemed value of $0.80 per share), $2,100,000 payable within 30 days of the agreement (which has been paid), and $3,900,000 in the form of a promissory note payable by April 19, 2014 deliverable on a pro-rata basis to the shareholders of Biologix Barbados.
On July 3, 2012, Biologix Barbados entered into a research and development agreement with BIT&GY, a laboratory affiliated with the Beijing Institute of Technology. Pursuant to the agreement, we have engaged BIT&GY to conduct research and development of Biologix ReviveTM in order to unify the various components of the current formula into a single formulation that has been tested for consistency at various temperatures, thus creating a predictable and reliable matrix for industrial manufacture, long term storage, and transport. BIT&GY will also conduct extensive controlled testing in accord with the requirements of the Food and Drug Administration (FDA), Health Canada and the European Medicines Agency (EMA) in preparation for our anticipated pre-clinical and clinical trials. The consideration payable to BIT&GY’s for its services is $1,100,000. To date, Biologix Barbados has paid $787,500 to BIT&GY in accordance with the agreement. The balance is payable in installments upon the completion of certain project milestones. Biologix Barbados may terminate the agreement upon 30 days written notice.
On September 1, 2012, Biologix Barbados entered into a warehousing and distribution agreement with KD Consultoria & Servicios S.A.S (KDCS), a company incorporated under the laws of Colombia, pursuant to which Biologix Barbados has engaged KDCS to provide certain pharmaceutical warehousing, inventory, and distribution services via KDCS’s facility located in Baranquilla, Colombia or Medellin, Colombia. Either party may terminate the agreement with 30 days written notice. The term of the agreement ends August 31, 2014 unless earlier terminated. The facility will serve as a distribution center for the sale of Biologix ReviveTM in Colombia, and other eligible South American jurisdictions subject to requisite regulatory approvals. We have agreed to pay to KDCS $15,000 for each 6 month period during the term of the agreement, and a royalty equal to 4% of the wholesale distribution price of each unit of Biologix ReviveTM distributed through KDCS’s facility. KDCS is an affiliated company of our former director, Dr. Diego Castresana.
On October 1, 2012, Biologix Florida entered into a letter of agreement with Unionashton Management Ltd. pursuant to which we received a loan of $1,155,828.92, accruing interest at 5% per annum and payable upon demand. The loan and accrued interest are convertible into shares of the common stock of our common stock at a price per share equal to the lower of $1.00 per share or the 10 day moving average price of our common shares as quoted on the Over The Counter Bulletin Board discounted by 20%. On November 14, 2012, December 14, 2012, and January 4, 2013, respectively, we entered into additional letters of agreement with Unionashton pursuant to which we received loans of $200,000, $50,000, and $50,000 upon terms identical to our October 1, 2012 agreement. As additional consideration of the loans, we have granted to Unionashton warrants to purchase an aggregate of 1,455,828 shares of our common exercisable for a five year period at a price per share equal to the lower of $1.00 per or the 10 day moving average price of our common shares as quoted on the Over The Counter Bulletin Board discounted by 20%.
On April 19, 2012, Biologix Florida entered into a share purchase agreement with Biologix Barbados and the shareholders of Biologix Barbados. Pursuant to the agreement, Biologix Florida acquired 100% of the outstanding securities of Biologix Barbados. As consideration for all of the outstanding securities of Biologix Barbados, we issued the selling shareholders 4,000,000 shares of common stock of Biologix Florida, and $2,100,000 in cash and $3,900,000 in promissory notes, payable by April 19, 2014, on a pro-rata basis to the selling shareholders.
On April 30, 2013, we entered agreements to convert debt for equity with two of the selling shareholders, Pendolino Investments Ltd. and Gruppen Investments Ltd. Effective April 30, 2013, Pendolino agreed to convert $1,887,000 of debt into 943,500 common shares of our company and Gruppen agreed to convert $906,500 of debt into 453,250 common shares of our company. Pursuant to these two agreements, we converted an aggregate of $2,793,500 of debt, payable pursuant to the promissory notes, into 1,396,750 common shares of our company at a conversion price of $2.00 per common share.
9
Plan of Operation
A brief summary of the major stages of our business plan that we are seeking to complete over the next 12 months (beginning April, 2013) and the cost estimates to complete each step are as follows:
|
Tasks
|
Status
|
Estimated Completion
|
Costs
|
|
Research & Development:
Reformulation of Revive Hair Formula:
● Active Pharmaceutical
Ingredient Inspection:
● Formula Composition Tests
● Development of Preparative
Technology and Quality Controls:
● Accelerated Stability Study
● Long Term Stability Study
|
Research & Development was initiated on our behalf by BIT&GY. in July, 2012.
|
Winter 2013
|
$1,100,000
($787,500 paid to date)
|
|
Fulfill Contractual Obligations Pursuant to Intellectual Property Purchase and Sale Agreement and Convertible Grid Promissory Note with Hair Research and Science Est.
|
Ongoing
|
Perpetual
|
Minimum of
$6,640,000 payable
during 2013.
|
We will also be required to complete additional steps in order to market and sell any of our products to the public in certain major jurisdictions, including North America and Europe. The following table sets out the various steps we anticipate we must complete in order to carry out our business plan for the Biologix Revive Hair FormulaTM. Estimated costs and completion times have been indicated where estimable, as has any progress made to date.
|
Anticipated Steps
|
Status
|
Estimated Completion
|
|
Secure Patent Protection of Revive Hair FormulaTM
|
Patent Application Submitted on May 12, 2012
|
Spring 2015
|
|
Secure Trademark Protection for Various Biologix Brands
|
Trademark Applications submitted on January 13 ,2012
|
Fall 2013
|
|
Pre-Clinical Testing
|
Not in Progress
|
2014
|
|
Secure Investigational New Drug Approval or Equivalent
|
Not in Progress
|
2014
|
|
Phase I Clinical Trials
|
Not in Progress
|
2015
|
|
Phase II Clinical Trials
|
Not in Progress
|
2017
|
|
Phase III Clinical Trials
|
Not in Progress
|
2020
|
|
Submit New Drug Application or Equivalent and Obtain Marketing Approval
|
Not in Progress
|
2021
|
|
Finance Marketing and Manufacturing of Approved Drug or Secure Marketing and Manufacturing Partner
|
Not in Progress
|
2021
|
Results of Operations for the Three Months Ended March 31, 2013 and 2012 and Period from January 18, 2011 (inception) to March 31, 2013
The following discussion of our results of operations should be read in conjunction with our unaudited financial statements for the three month periods ended March 31, 2013 and 2012 which are included herein.
10
Our operating results for the three month periods ended March 31, 2013 and 2012 and the period from January 18, 2011 (inception) to March 31, 2013 are summarized as follows:
|
Three Months Ended
|
From January 18, 2011 (Inception) To
|
|||||||||||
|
March 31,
|
March 31,
|
|||||||||||
|
2013
|
2012
|
2013
|
||||||||||
|
Revenue
|
$
|
Nil
|
$
|
Nil
|
$
|
Nil
|
||||||
|
Operating expenses
|
(3,208,548
|
)
|
(472,683
|
)
|
(28,942,151
|
)
|
||||||
|
Other comprehensive income
|
(931
|
)
|
18,901
|
5,156
|
||||||||
|
Net Loss
|
$
|
(3,209,478
|
)
|
$
|
(453,782
|
)
|
$
|
(28,702,607
|
)
|
|||
We generated no revenue for the period from January 18, 2011 (inception) to March 31, 2013. Our operating expenses during three months ended March 31, 2013 were $3,208,548 compared to $472,683 during the same period ended 2012. We incurred operating expenses of $28,942,151 for the period from January 18, 2011 (inception) to March 31, 2013. We recorded a net loss of $3,209,478 for the three months ended March 31, 2013, as compared with $453,782 for the same period ended March 31, 2012 and $28,702,607 for the period from January 18, 2011 (inception) until March 31, 2013.
We anticipate our operating expenses will increase as we implement our business plan. The increase will be attributable to expenses to implement our business plan, and the professional fees to be incurred in connection with our ongoing operating expenses as a reporting company under the Securities Exchange Act of 1934.
Liquidity and Capital Resources
Working Capital
|
As of
|
As of
|
|||||||
|
March 31,
2012
|
December 31, 2012
|
|||||||
|
Current Assets
|
$
|
242,865
|
$
|
384,070
|
||||
|
Current Liabilities
|
$
|
12,004,847
|
$
|
8,217,960
|
||||
|
Working Capita (Deficit)l
|
$
|
(11,761,982
|
)
|
$
|
(7,833,890
|
)
|
||
Cash Flows
|
Three Month
|
Three Month
|
|||||||
|
Period Ended
|
Period Ended
|
|||||||
|
March 31,
2013
|
March 31,
2012
|
|||||||
|
Cash used in Operating Activities
|
$
|
(688,571
|
)
|
$
|
(356,902
|
)
|
||
|
Cash provided by Financing Activities
|
700,000
|
1,065,000
|
||||||
|
Cash provided by (used in) Investing Activities
|
(7,117
|
)
|
695,146
|
)
|
||||
|
Net Increase (Decrease) in Cash and Cash Equivalents
|
$
|
67,136
|
$
|
231,594
|
||||
As of March 31, 2013, we had total current assets of $242,865 and current liabilities of $384,070. We have a working capital deficit of $11,761,982 as of March 31, 2013.
We used $688,571 in cash for operating activities for the three month period ended March 31, 2013 compared with $356,902 for the same period in 2012.
11
Cash provided by financing activities for the three month period ended March 31, 2013 was $700,000 compared to $1,065,000 for the same period in 2012.
Cash used in investing activities for the three month period ended March 31, 2013 was $7,117 compared to $695,146 for the same period in 2012.
As of March 31, 2013, we have insufficient cash to operate our business at the current level for the next twelve months and insufficient cash to achieve our business goals. The success of our business plan beyond the next 12 months is contingent upon us obtaining additional financing. We intend to fund operations through debt and/or equity financing arrangements, which may be insufficient to fund our capital expenditures, working capital, or other cash requirements. We do not have any formal commitments or arrangements for the sales of stock or the advancement or loan of funds at this time. There can be no assurance that such additional financing will be available to us on acceptable terms, or at all.
Off Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to stockholders.
Going Concern
Our financial statements are prepared using generally accepted accounting principles in the United States of America applicable to a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business. We have not yet established an ongoing source of revenues sufficient to cover our operating costs and allow us to continue as a going concern. Our ability to continue as a going concern is dependent on our obtaining adequate capital to fund operating losses until we become profitable. If we are unable to obtain adequate capital, we could be forced to cease operations.
In order to continue as a going concern, we will need, among other things, additional capital resources. Management's plan is to obtain such resources for us by obtaining capital from management and significant shareholders sufficient to meet our minimal operating expenses and seeking equity and/or debt financing. However management cannot provide any assurances that we will be successful in accomplishing any of our plans.
Our ability to continue as a going concern is dependent upon our ability to successfully accomplish the plans described in the preceding paragraph and eventually secure other sources of financing and attain profitable operations. The accompanying financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
Critical Accounting Policies
Basis of Presentation and Consolidation
The consolidated financial statements include the accounts of our company and its wholly owned subsidiaries, Biologix Florida, Biologix Canada and BHSL, and BHSL’s wholly owned subsidiaries Biologix Hair, Panama S.A. (“Biologix Panama”), Biologix Hair South America S.A. (“Biologix South America”) and Biologix Hair (Hong Kong) Limited (“Biologix HK”).
These consolidated financial statements have been prepared in accordance with United States generally accepted accounting principles (“US GAAP”). All significant inter-company transactions and balances have been eliminated upon consolidation.
12
Cash and Cash Equivalents
Our company considers all highly liquid instruments with a maturity of three months or less at the time of issuance to be cash equivalents. As at March 31, 2013, our company has cash and cash equivalents in the amount of $56,409 (December 31, 2012 - $44,577) which are over the federally insured limit. As at March 31, 2013 and December 31, 2012, our company has $Nil of cash equivalents.
Deferred cost
The consideration commitment on the Intellectual Property License with BHSL are deferred until the completion of the due diligence at which time the cost will be charged to operation as IPR&D expense.
Website Development Costs
In accordance with ASC 350-50, “Website Development Costs”, our company capitalizes qualifying website development costs. Costs incurred during the application development stage as well as upgrades and enhancements that result in additional functionality are capitalized. Accordingly, direct costs incurred during the application stage of development are capitalized and amortized over the estimated useful life of three years, the expected period of benefit. Fees incurred for website hosting are expensed over the period of the benefit. Costs of operating a website are expensed as incurred.
Property and Equipment
Property and equipment consists of computer equipment, furniture and equipment and leasehold improvements which are carried at cost, net of accumulated depreciation and impairment loss. Amortization is recorded on the straight line method at the following rates:
|
Furniture and equipment
|
3 years
|
|
Leasehold improvements
|
2 years
|
|
Computer equipment
|
2 years
|
Amortization will commence once the property and equipment is put in use. The property and equipment is written down to its net realizable value if it is determined that its carrying value exceeds estimated future benefits to our company.
Acquired In-Process Research and Development
In accordance with ASC 730-10, the initial costs of rights to acquired IPR&D projects acquired in an asset acquisition are expensed as IPR&D unless the project has an alternative future use. These costs include initial payments incurred prior to regulatory approval in connection with research and development collaboration agreements that provide rights to develop, manufacture, market and/or sell pharmaceutical products. The fair value of IPR&D projects acquired in a business are capitalized and accounted for as indefinite-lived intangible assets until the underlying project receives regulatory approval, at which point the intangible asset will be accounted for as definite-lived intangible assets, or discontinuation, at which point the intangible asset will be written off. Development costs incurred after the acquisition are expensed as incurred. Indefinite- and definite-lived assets are subject to impairment reviews.
Stock-Based Compensation
Our company adopted ASC Topic 718-10, Compensation - Stock Compensation - Overall, to account for its stock options and similar equity instruments issued. Accordingly, compensation costs attributable to stock options or similar equity instruments granted are measured at the fair value at the grant date, and expensed over the expected vesting period. ASC Topic 718-10 requires excess tax benefits be reported as a financing cash inflow rather than as a reduction of taxes paid.
13
Warrant Issuance and Note Conversion Feature
In accordance with ASC 470-20, Debt with conversions and other options, the proceeds from the notes were allocated based on the relative fair values of the notes without the warrants issued in conjunction with the notes and of the warrants themselves at the time of issuance. Our company recorded the allocated value of the warrants at the time of issuance as additional paid-in capital and as a debt discount to the notes. Our company amortize the debt discount as interest expense over the life of the note. Additionally, as a result of issuing the warrants with the convertible notes, a beneficial conversion option is recorded as a debt discount reflecting the incremental conversion option intrinsic value of the conversion option provided to the holders of the notes. Our company also amortize this debt discount as interest expense over the life of the notes. The intrinsic value of each conversion option was calculated as the difference between the effective conversion price and the fair value of the common stock, multiplied by the number of shares into which the note is convertible.
Foreign Currency Translation
Our company’s functional currency and presentation currency is the U.S. dollars. Biologix Canada’s functional currency is Canadian dollars. Biologix Florida, BHSL, Biologix HK, Biologix Panama and Biologix South America’s functional currency are all U.S. dollars.
Transactions in a currency other than the functional currency (“foreign currency”) are measured in the respective functional currencies of our company and its subsidiaries and are recorded on initial recognition in the functional currencies at exchange rates approximating those ruling at the transaction dates. Exchange gains and losses are recorded in the statements of income and comprehensive income.
Assets and liabilities of our company and its subsidiaries are translated into the U.S. dollars at exchange rates at the balance sheet date, equity accounts are translated at historical exchange rate and revenues and expenses are translated by using the average exchange rates. Translation adjustments are reported as cumulative translation adjustments and are shown as a separate component of other comprehensive income in the statements of stockholders’ equity.
Basic Net Income (Loss) per Share
Basic earnings per share is calculated using the weighted average number of shares outstanding during the year. Our company has adopted ASC Topic 260-10, Earnings per Share - Overall, and uses the treasury stock method to compute the dilutive effect of options, warrants and similar instruments. Under this method, the dilutive effect on earnings per share is recognized on the use of the proceeds that could be obtained upon exercise of options, warrants and similar instruments. It assumes that the proceeds would be used to purchase common shares at the average market price during the period. Diluted loss per share is equal to basic loss per share as any possible dilutive instruments are anti-dilutive.
Income Taxes
Our company accounts for income taxes under the provisions of ASC Topic 740-10, Income Taxes, which requires our company to recognize deferred tax liabilities and assets for the expected future tax consequences of events that have been recognized in our company’s financial statements or tax returns using the liability method. Under this method, deferred tax liabilities and assets are determined based on the temporary differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse. The effect on deferred income tax assets and liabilities of a change in income tax rates is included in the period that includes the enactment date. Valuation allowances are established when necessary to reduce deferred income tax assets to the amount expected to be realized.
14
Long-lived Assets Impairment
Long-term assets of our company are reviewed for impairment whenever events or circumstances indicate that the carrying amount of assets may not be recoverable, pursuant to guidance established in ASC 360-05, Impairment or Disposal of Long-Lived Assets.
Management considers assets to be impaired if the carrying value exceeds the future projected cash flows from related operations (undiscounted and without interest charges). If impairment is deemed to exist, the assets will be written down to fair value. Fair value is generally determined using a discounted cash flow analysis.
Use of Estimates
The preparation of our company's consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Significant items subject to such estimates and assumptions include fair values of the elements of an asset acquisition as of the date of acquisition; asset impairments; useful lives for depreciation, depletion, and amortization; current and deferred income taxes; and fair value of financial instruments. Estimates are based on facts and circumstances believed to be reasonable at the time; however, actual results could differ from those estimates.
Fair Value of Financial Instruments
The estimated fair values for financial instruments under ASC Topic 825-10, Financial Instruments, are determined at discrete points in time based on relevant market information. These estimates involve uncertainties and cannot be determined with precision. The estimated fair value of our company’s financial instruments includes cash and cash equivalents, accounts payable and accrued liabilities, amounts due to related parties, promissory and convertible promissory notes. The carrying value of these financial instruments approximates their fair value based on their liquidity or their short-term nature. Unless otherwise noted, it is management’s opinion that our company is not exposed to significant interest, currency or credit risks arising from these financial instruments due to their short term nature.
Our company adopted ASC Topic 820-10, Fair Value Measurements and Disclosures, which defines fair value, establishes a framework for measuring fair value in US GAAP, and expands disclosures about fair value measurements.ASC Topic 820-10 does not require any new fair value measurements, but provides guidance on how to measure fair value by providing a fair value hierarchy used to classify the source of the information. The fair value hierarchy distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
|
Level one – Quoted market prices in active markets for identical assets or liabilities;
|
|
Level two – Inputs other than level one inputs that are either directly or indirectly observable; and
|
Level three – Unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use.
As at March 31, 2013 and December 31, 2012, the fair value of cash and cash equivalents was measured using Level one inputs.
15
Item 3. Quantitative and Qualitative Disclosures About Market Risk
A smaller reporting company is not required to provide the information required by this Item.
Item 4. Controls and Procedures
Management’s Report on Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to our management, including our president and chief financial officer (our principal executive officer, principal financial officer and principal accounting officer) to allow for timely decisions regarding required disclosure.
As the end of the quarter covered by this report, we carried out an evaluation, under the supervision and with the participation of our president (our principal executive officer) and our and chief financial officer (principal financial officer and principal accounting officer), of the effectiveness of the design and operation of our disclosure controls and procedures. Based on the foregoing, our president (our principal executive officer) and our and chief financial officer (principal financial officer and principal accounting officer)concluded that our disclosure controls and procedures were not effective in providing reasonable assurance in the reliability of our reports as of the end of the period covered by this quarterly report.
Changes in Internal Control over Financial Reporting
During the period covered by this report there were no changes in our internal control over financial reporting that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II – OTHER INFORMATION
Item 1. Legal Proceedings
We know of no material, active or pending legal proceedings against our company, nor are we involved as a plaintiff in any material proceeding or pending litigation. There are no proceedings in which any of our directors, officers or affiliates, or any registered or beneficial shareholder, is an adverse party or has a material interest adverse to our interest.
Item 1A. Risk Factors
A smaller reporting company is not required to provide the information required by this Item.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
Effective January 28, 2013, we granted an aggregate of 1,475,000 stock options to certain employees, officers, directors and consultants of our company pursuant to our 2013 Stock Plan. The stock options are exercisable for five years from the date of grant at an exercise price of $1.00 per share.
Also effective January 28, 2013, we entered into stock option agreements with certain employees, officers, directors and consultants in conjunction with granting of the stock options.
The stock options were issued an aggregate of 400,000 stock options to two (2) US persons, relying on Rule 506 under Regulation D and/or Section 4(2) of the Securities Act of 1933 and an aggregate of 1,075,000 stock options to eight (8) non-US person (as that term is defined in Regulation S of the Securities Act of 1933) in an offshore transaction relying on Regulation S of the Securities Act of 1933.
16
On April 30, 2013, we issued an aggregate of 1,396,750 common shares of our company to two accredited investors (as that term is defined in Section 4(2) of the Securities Act of 1933) pursuant to the exemption from registration provided for under Rule 506 of Regulation D, promulgated under the United States Securities Act of 1933, as amended.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
Effective April 30, 2013, Lilia Roberts resigned as a director and Tom McDermott resigned as a director and as chairman of our board of directors. Neither Ms. Roberts’ resignation nor Mr. McDermott’s resignation was the result of any disagreements with our company regarding our operations, policies, practices or otherwise.
Concurrently with Mr. McDemott’s resignation, we appointed Ronald Holland as chairman of our board of directors.
On May 1, 2013, we appointed David Csumrik as a director of our company.
Effective May 5, 2013, Diego Castresana resigned as a director of our company. Mr. Castresana’s resignation was not the result of any disagreements with our company regarding our operations, policies, practices or otherwise. He remains as the vice president of research and development of Biologix Hair Science Ltd., our wholly-owned Barbados subsidiary, which holds the intellectual property rights to Biologix Revive.
On May 6, 2013, we appointed Dr. Pankaj Modi as a director and as vice president of research and development, of our company.
On May 7, 2013, we appointed Wilhelm Keller as a director of our company.
On May 13, 2013, we appointed Ken Zadoorian as a director of our company.
On May 14, 2013, we appointed Michael Stocker as a director and chief executive officer and appointed Greg Wilson as chief financial officer of our company. Subsequently, on May 14, 2013, Ronald Holland resigned as our chief executive officer.
Ken Zadoorian – Director
Ken Zadoorian has built a long track record as a successful executive with an extensive background in large organizations and smaller start-ups as well as experience managing engineering, manufacturing, information technology, community relations and sales. Mr. Zadoorian has proven success in building and developing organizations as well as mergers and acquisitions and has offered business consulting through Axeo Consulting and Scottsdale Consulting.
During his 25-plus years at Eli Lilly & Company, Mr. Zadoorian held several management positions including director of human resources, Worldwide Manufacturing and Engineering and director of human resources, Corporate Staff Components, each position having responsibility for several thousand employees.
17
Additionally, Mr. Zadoorian has served as vice president of operations for American Optical Services/Exela Hearing Services; vice president, operations and human resources for Zounds, Inc.; vice president of human resources for Cox Communications; senior vice president of operations, facilities and human resources for PCS Health Systems; and vice president of administration for Advanced Cardiovascular Systems.
Ken Zadoorian’s formal education has included a B.S. in industrial management from Purdue University and the executives MBA Consortium at Indiana University. He has served as chairman of the board for Boys and Girls Clubs of Scottsdale, board director for Maricopa Community Colleges Foundation and board director for the Cal Ripken Sr. Foundation.
Dr. Michael Stocker – Director and CEO
Michael Stocker, PhD, has held executive positions in several companies, mainly in Latin America. He was part of the FUNDES International management team as well as Regional Manager Latin America for PROPEL Colombia. He also worked for the Boston Consulting Group, in its offices in Mexico, Spain and Switzerland, in connection with the development of business strategies and management structure for several worldwide active corporations and companies, among them Fortune 500 companies.
The consulting and technology branch of his own company, the Stocker Group, has offices in five countries and offers risk and knowledge management to international clients in several countries, including the United Nations and several governments in Latin America. In the mining industry, Dr. Stocker worked as a consultant and advisor for several companies and served as chief executive officer of a US public company.
Dr. Stocker has initiated and invested in several ventures, among them a company he founded in 2010 (Medtechtrade.com) in the medical equipment sector, which is currently offering second-hand medical equipment from hospitals in Europe to hospitals on all continents in need of such equipment.
Dr. Stocker has previously been retained as an adviser and consultant by the Inter-American Development Bank, the United Nations Industrial Development Organization, the Swiss State Secretariat for Economic Affairs, the World Bank's International Finance Corporation and the Organization of American States as well as several private companies.
Dr. Stocker holds a PhD in Economics from the University of St. Gallen, Switzerland and an MBA from the Community of European Management Schools. He has lived and worked in Latin America, chiefly in Chile, Colombia and Costa Rica, since 1993.
J. Gregory Wilson – Chief Financial Officer
J. Gregory Wilson is an entrepreneur and corporate finance strategist with more than 20 years experience advising and structuring capital market financings for start-up enterprises and assisting in developing and implementing successful business plans.
Upon graduation with a Bachelor’s degree in Economics from Queen’s University in Kingston, Ontario, Canada in 1985, Mr. Wilson began his career as a marketing representative with IBM. In 1988, he started and operated a business in the wellness industry that ultimately employed a staff of over 200 and generated annual revenues in excess of $13 million. Mr. Wilson sat on the National Advisory Board for this company in 1989 and 1990.
In 1992, Greg Wilson began a career in finance working with top Canadian investment firm Scotia McLeod. Over the ensuing 5 years, he went on to work with the Bank of Montreal’s investment arm, Nesbitt Burns, before finishing his mainstream financial career with Merrill Lynch in 1997. In late 1997, Mr. Wilson started EMT Capital Corp. and over the past 15 years has worked on a number of successful capital market transactions, including mergers and acquisitions, initial public offerings and private equity financings.
18
In 2005, Greg Wilson was part of the group that founded Paramount Gold & Silver Corp., a precious metals exploration company that currently trades on the NYSE and TSX. In early 2009, he co-founded and led the financing of a renewable energy company with a portfolio of over 600 megawatts of wind projects in Canada, the United States and Germany. He advised a client in late 2009 on the purchase of Consumer Choice Awards, a 23-year-old private company with operations across Canada. Mr. Wilson negotiated and structured the acquisition with the seller and raised the equity component of the purchase price. Mr. Wilson continues to sit on the Board of Directors.
Greg has completed the Canadian Investment Management program, and has been awarded the Fellow of the Canadian Securities Institute designation.
Biographies of the other directors and officers appointed above are incorporated by reference to our Form 8-K filed with the SEC on May 13, 2013.
Item 6. Exhibits
|
Exhibit No.
|
Description
|
|||
|
(2)
|
Plan of Acquisition, Reorganization, Arrangement of Liquidation or Succession
|
|||
|
2.1
|
Share Exchange Agreement with Biologix Hair Inc. (Florida), and the Shareholders of Biologix Hair Inc. (Florida) dated November 23 2012 (incorporated by reference to our Current Report on Form 8-K filed on November 23, 2012).
|
|||
|
(3)
|
Articles of Incorporation; Bylaws
|
|||
|
3.1
|
Articles of Incorporation (incorporated by reference to our Registration Statement on Form S-1 filed on April 7, 2011).
|
|||
|
3.2
|
Certificate of Amendment filed on December 5, 2012 (incorporated by reference to our Current Report on Form 8-K filed on December 5, 2012.).
|
|||
|
3.4
|
Bylaws (incorporated by reference to our Registration Statement on Form S-1 filed on April 7, 2011).
|
|||
|
(4)
|
Instrument Defining the Rights of Security Holders, Including Indentures
|
|||
|
4.1
|
Instrument Defining the Right of Holders – Form of Share Certificate (incorporated by reference to our Registration Statement on Form S-1 filed on April 7, 2011).
|
|||
|
(10)
|
Material Contracts
|
|||
|
10.1
|
Intellectual Property License Agreement dated December 9, 2011 between Cranium Technologies Ltd. (now Biologix Florida) and Biologix Barbados (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.2
|
Lease Agreement between Cranium Technologies Ltd. and Tom David dated December 15, 2011 (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.3
|
Lease Agreement between Cranium Technologies Ltd. and PSS Investments I Inc., et al, dated July 1, 2012 (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.4
|
Intellectual Property Purchase and Sale Agreement and Convertible Grid Promissory Note dated April 11, 2012 between Biologix Barbados Hair Research and Science Est. (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.5
|
Amending Agreement dated August 1, 2012 between Biologix Barbados Hair Research and Science Est. (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013)
|
|||
|
10.6
|
Amending Agreement dated November 30, 2012 between Biologix Barbados Hair Research and Science Est. (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.7
|
Share Purchase Agreement among Biologix Hair Inc. (Biologix Florida) and Biologix Hair Science Ltd. (Biologix Barbados) and the selling shareholders of Biologix Barbados dated April 19, 2012 (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
19
|
Exhibit No.
|
Description
|
|||
|
10.8
|
Letter of Agreement dated October 1, 2012 between Biologix Florida and Unionashton Management Ltd. (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.9
|
Letter of Agreement dated November 14, 2012 between Biologix Florida and Unionashton Management Ltd. (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.10
|
Letter of Agreement dated December 14, 2012 between Biologix Florida and Unionashton Management Ltd. (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.11
|
Letter of Agreement dated January 4, 2012 between Biologix Florida and Unionashton Management Ltd. (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.12
|
Research and Development Agreement dated July 3, 2012 between Biologix Barbados and Beijing BIT&GY Pharmaceutical R&D Co. Ltd. (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.13
|
Warehousing & Distribution Agreement dated September 1, 2012 between Biologix Barbados and KD Consultoria & Servicios S.A.S (incorporated by reference to our Current Report on Form 8-K filed on January 14, 2013).
|
|||
|
10.14
|
2013 Stock Option Plan (incorporated by reference to our Current Report on Form 8-K filed on January 28, 2013).
|
|||
|
10.15
|
Form of Stock Option Agreement (incorporated by reference to our Current Report on Form 8-K filed on January 28, 2013).
|
|||
|
10.16
|
Form of Agreement to Convert Debt to Equity (incorporated by reference to our Current Report on Form 8-K filed on May 6, 2013).
|
|||
|
(21)
|
Subsidiaries of the Registrant
|
|||
|
21.1
|
The following is a list of subsidiaries of our subsidiaries. Our company owns, directly or indirectly, 100% of the voting securities of each of the listed subsidiaries.
|
|||
|
Biologix Hair Inc.
|
Florida
|
|||
|
Biologix Hair (Canada) Inc.
|
Canada
|
|||
|
Biologix Hair Science Ltd.
|
Barbados
|
|||
|
Biologix Hair, Panama S.A
|
Panama
|
|||
|
Biologix Hair South America S.A.
|
Panama
|
|||
|
Biologix Hair (Hong Kong) Limited
|
Hong Kong
|
|||
|
(31)
|
Rule 13a-14(a)/15d-14(a) Certifications
|
|||
|
31.1*
|
Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 of the Principal Executive Officer
|
|||
|
31.2*
|
Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 of the Principal Financial Officer and Principal Accounting Officer
|
|||
|
(32)
|
Section 1350 Certifications
|
|||
|
32.1*
|
Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 of the Principal Executive Officer
|
|||
|
32.2*
|
Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 of the Principal Financial Officer and Principal Accounting Officer
|
|||
|
101**
|
Interactive Data Files
|
|||
|
101.INS
|
XBRL Instance Document
|
|||
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|||
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|||
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|||
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
|||
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|||
|
*
|
Filed herewith.
|
|
**
|
Furnished herewith. Pursuant to Rule 406T of Regulation S-T, the Interactive Data Files on Exhibit 101 hereto are deemed not filed or part of any registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, are deemed not filed for purposes of Section 18 of the Securities and Exchange Act of 1934, and otherwise are not subject to liability under those sections.
|
20
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
BIOLOGIX HAIR INC.
|
|
|
Date: May 20, 2013
|
/s/ Michael Stocker
|
|
Michael Stocker
|
|
|
Chief Executive Officer
|
|
|
(Principal Executive Officer)
|
|
|
Date: May 20, 2013
|
/s/ Greg Wilson
|
|
Greg Wilson
|
|
|
Chief Financial Officer
|
|
|
(Principal Financial Officer and Principal Accounting Officer)
|
21
