Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - Actavis, Inc. | d541119dex993.htm |
| EX-99.1 - EX-99.1 - Actavis, Inc. | d541119dex991.htm |
| 8-K - 8-K - Actavis, Inc. | d541119d8k.htm |
 Creating a Global Specialty Pharmaceutical Leader
The Combination of Actavis and Warner Chilcott
May 20, 2013
Exhibit 99.2 |
 Important Information For Investors And Shareholders
2
This communication does not constitute an offer to sell or the solicitation of an
offer to buy any securities or a solicitation of any vote
or
approval.
New
Actavis
will
file
with
the
Securities
and
Exchange
Commission
(the
“SEC”)
a
registration
statement
on
Form S-4, each of Actavis and Warner Chilcott will file with the SEC a proxy
statement and each of New Actavis, Actavis and Warner Chilcott will file
with the SEC other documents with respect to the proposed transaction. In addition, a definitive proxy
statement/prospectus will be mailed to shareholders of Actavis and Warner
Chilcott. INVESTORS AND SECURITY HOLDERS OF ACTAVIS AND WARNER
CHILCOTT ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT/PROSPECTUS AND
OTHER DOCUMENTS THAT WILL BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY
BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. Investors
and security holders will be able
to
obtain
free
copies
of
the
registration
statement
and
the
proxy
statement/prospectus
(when
available)
and
other
documents filed with the SEC by New Actavis, Actavis and Warner Chilcott through
the website maintained by the SEC at http://www.sec.gov.
on Actavis’
internet
website
at
www.actavis.com
or
by
contacting
Actavis’
Investor
Relations
Department
at
(862)
261-7488.
Copies of the documents filed with the SEC by Warner Chilcott will be available
free of charge on Warner Chilcott’s internet website at www.wcrx.com or
by contacting Warner Chilcott’s Investor Relations Department at (973) 442-3200.
Actavis,
Warner
Chilcott,
their
respective
directors
and
certain
of
their
executive
officers
may
be
considered
participants
in
the
solicitation of proxies in connection with the proposed transaction.
Information about the directors and executive officers of Warner Chilcott is
set forth in its Annual Report on Form 10-K for the year ended December 31, 2012, which was filed with the
SEC on February 22, 2013, its Quarterly Report on Form 10-Q for the quarter
ended March 31, 2013, which was filed with the SEC on May 10, 2013, its proxy
statement for its 2013 annual meeting of stockholders, which was filed with the SEC on April 5,
2013, and certain of its Current Reports on Form 8-K, which were filed with the
SEC on May 2, 2013 and May 8, 2013. Information about the directors and
executive officers of Actavis is set forth in its Annual Report on Form 10-K for the year
ended December 31, 2012, which was filed with the SEC on February 28, 2013, its
Quarterly Report on Form 10-Q for the quarter ended March 31, 2013,
which was filed with the SEC on May 7, 2013, its proxy statement for its 2013 annual meeting of
stockholders, which was filed with the SEC on March 29, 2013, and certain of its
Current Reports on Form 8-K, which were filed with the SEC on January
29, 2013 and May 13, 2013. Other information regarding the participants in the proxy solicitations and
a description of their direct and indirect interests, by security holdings or
otherwise, will be contained in the proxy statement/prospectus and other
relevant materials to be filed with the SEC when they become available.
.
Copies of the documents filed with the SEC by New Actavis and Actavis will be
available free of charge |
 Actavis Cautionary Statement Regarding
Forward-Looking Statements
3
Statements contained in this communication that refer to Actavis’ estimated or anticipated future
results or other non-historical facts are forward-looking statements that reflect
Actavis’ current perspective of existing trends and information as of the date of this communication.
Forward looking statements generally will be accompanied by words such as “anticipate,”
“believe,” “plan,” “could,” “should,” “estimate,”
“expect,” “forecast,” “outlook,” “guidance,”
“intend,” “may,” “might,” “will,” “possible,” “potential,” “predict,” “project,” or other similar words,
phrases or expressions. It is important to note that Actavis’ goals and expectations are not
predictions of actual performance. Actual results may differ materially from Actavis’
current expectations depending upon a number of factors affecting Actavis’ business, Warner Chilcott’s
business and risks associated with acquisition transactions. These factors include, among others, the
inherent uncertainty associated with financial projections; restructuring in connection with,
and successful close of the acquisition of Warner Chilcott; subsequent integration of the
acquisition of Warner Chilcott and the ability to recognize the anticipated synergies and benefits of the acquisition; the receipt of
required regulatory approvals for the acquisition of Warner Chilcott (including the approval of
antitrust authorities necessary to complete the acquisition); the anticipated size of the
markets and continued demand for Actavis’ and Warner Chilcott’s products; the impact of
competitive products and pricing; access to available financing (including financing for the
acquisition of Warner Chilcott) on a timely basis; maintaining a position in the Standard &
Poor’s 500; the risks of fluctuations in foreign currency exchange rates; the risks and uncertainties
normally incident to the pharmaceutical industry, including product liability claims and the
availability of product liability insurance on reasonable terms; the difficulty of predicting
the timing or outcome of pending or future litigation or government investigations; periodic
dependence on a small number of products for a material source of net revenue or income; variability
of trade buying patterns; changes in generally accepted accounting principles; risks that the
carrying values of assets may be negatively impacted by future events and circumstances; the
timing and success of product launches; the difficulty of predicting the timing or outcome of product development efforts
and regulatory agency approvals or actions, if any; market acceptance of and continued demand for
Actavis’ and Warner Chilcott’s products; costs and efforts to defend or enforce
intellectual property rights; difficulties or delays in manufacturing; the availability and pricing
of third party sourced products and materials; successful compliance with governmental regulations
applicable to Actavis’ and Warner Chilcott’s facilities, products and/or businesses;
changes in the laws and regulations affecting, among other things, pricing and reimbursement of
pharmaceutical products; changes in tax laws or interpretations that could increase Actavis’ consolidated tax liabilities;
the loss of key senior management or scientific staff; and such other risks and uncertainties detailed
in Actavis’ periodic public filings with the Securities and Exchange Commission, including
but not limited to Actavis’ Annual Report on form 10-K for the year ended
December 31, 2012 and from time to time in Actavis’ other investor communications. Except as
expressly required by law, Actavis disclaims any intent or obligation to update or revise these
forward-looking statements.
|
 Warner Chilcott Cautionary Statement Regarding
Forward-Looking Statements
4
This communication contains forward-looking statements, including statements concerning the
proposed transaction with Actavis, our industry, our operations, our anticipated financial
performance and financial condition and our business plans, growth strategy and product development efforts. These statements constitute
forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and
Section 21E of the Securities Exchange Act of 1934. The words “may,”
“might,” “will,” “should,” “estimate,” “project,” “plan,” “anticipate,” “expect,” “intend,” “outlook,” “believe” and other similar
expressions are intended to identify forward-looking statements. Readers are cautioned not
to place undue reliance on these forward-looking statements, which speak only as of their dates. These
forward-looking statements are based on estimates and assumptions by our management that, although
we believe to be reasonable, are inherently uncertain and subject to a number of risks and
uncertainties. The following represent some, but not necessarily all, of the factors that could cause actual results to differ from
historical results or those anticipated or predicted by our forward-looking statements: the timing
to consummate the proposed transaction with Actavis; the risk that a condition to closing of
the proposed transaction with Actavis may not be satisfied; the risk that a regulatory approval that may be required for the proposed
transaction with Actavis is delayed, is not obtained or is obtained subject to conditions that are not
anticipated; New Actavis’ ability to achieve the synergies and value creation contemplated
by the proposed acquisition; New Actavis’ ability to promptly and effectively integrate Actavis’ and Warner Chilcott’s businesses; the
diversion of management time on transaction-related issues; our substantial indebtedness,
including increases in the LIBOR rates on our variable-rate indebtedness above the
applicable floor amounts; competitive factors and market conditions in the industry in which we operate, including the approval and
introduction of generic or branded products that compete with our products; our ability to protect our
intellectual property; a delay in qualifying any of our manufacturing facilities that produce
our products, production or regulatory problems with either our own manufacturing facilities or those of third party
manufacturers, packagers or API suppliers upon whom we may rely for some of our products or other
disruptions within our supply chain; pricing pressures from reimbursement policies of private
managed care organizations and other third party payors, government sponsored health systems and regulatory reforms, and
the continued consolidation of the distribution network through which we sell our products; changes in
tax laws or interpretations that could increase our consolidated tax liabilities; government
regulation, including U.S. and foreign health care reform, affecting the development, manufacture, marketing and sale of
pharmaceutical products, including our ability and the ability of companies with whom we do business
to obtain necessary regulatory approvals; adverse outcomes in our outstanding litigation,
regulatory investigations or arbitration matters or an increase in the number of such matters to which we are subject; the loss of key
senior management or scientific staff; our ability to manage the growth of our business by
successfully identifying, developing, acquiring or licensing new products at favorable prices
and marketing such new products; our ability to obtain regulatory approval and customer acceptance of new products, and continued customer
acceptance of our existing products; and the other risks identified in our periodic filings including
our Annual Report on Form 10-K for the year ended December 31, 2012, and from
time-to-time in our other investor communications. We caution you that the foregoing list of important factors is not exclusive. In
addition, in light of these risks and uncertainties, the matters referred to in our
forward-looking statements may not occur. We undertake no obligation to publicly update or
revise any forward-looking statement as a result of new information, future events or otherwise, except as may be required by law.
|
 The
Benefits of Combining Actavis and Warner Chilcott •
Creates Top 20 global pharmaceutical company by sales with
substantial presence in generics and branded products
•
Actavis Specialty Brands Pro Forma 2013 Revenues of ~$3bn
•
Augments Actavis Specialty Brands business in core areas of
Women’s Health and Urology
-
Expands portfolio into Gastroenterology and Dermatology
•
Expect substantial operational synergies coupled with tax savings
from overall tax structure benefits
-
Redomicile as an Irish plc
•
Expected to be immediately accretive to non-GAAP earnings and
strong operating cash flow provides opportunity to de-lever balance
sheet
•
Expected to enhance combined company’s credit profile
5 |
 Proposed Transaction Terms
•
43% premium to the unaffected share price**
•
Pro Forma Warner Chilcott ownership of ~23%
•
Anticipated to close by the end of 2013, subject to customary
conditions including regulatory reviews and shareholder approvals
•
Actavis Management to lead combined company
0.160 shares of “New Actavis”
equity
+ Assumption of $3.4 billion Warner Chilcott’s Net Debt
$8.5 billion Total Consideration*
6
*
Based on the closing share price of ACT of $125.50 on May 17, 2013. **
Under the terms of the Transaction Agreement, at closing Warner Chilcott shareholders will receive 0.160 shares of New Actavis for each Warner Chilcott share
they own, which equates to a value of $20.08 per Warner Chilcott share based on Actavis' closing
share price of $125.50 on May 17, 2013. This represents a 43 percent premium compared to
Warner Chilcott's $14.00 per share volume-weighted average trading price for the 30-day period ending on May 9, 2013 (the day
before Warner Chilcott disclosed it was engaged in preliminary discussions with Actavis) and a 34%
premium to $15.01 being the price on May, 9 2013. Based on the price of Actavis shares
on May, 9 2013 of $106.81 the value per share would represent a premium of 14% to $15.01.
|
 Warner Chilcott Overview
•
Leading specialty pharmaceutical company
-
$2.4 billion LTM 3/31/13 Revenue
-
Spun out of Warner Lambert in 1996
•
Corporate HQ in Dublin, Ireland
-
~2,100 employees; ~1,500 based in the United States, Puerto
Rico and Canada
-
US headquarters: Rockaway, NJ
-
R&D & Manufacturing in Puerto Rico, Northern Ireland, Ireland,
Germany
•
Focused on women’s healthcare, gastroenterology, urology, and
dermatology branded pharmaceutical therapeutic categories
7 |
 Financially Compelling Combination
Enhances
Financial
Potential
Through
Expected
Operational
and Tax
Synergies
•
Strong combined revenues of approximately $11 billion
•
Expected to be more than 30% accretive to Actavis non-GAAP
earnings for 2014, including anticipated synergies
•
Significant combined operating cash flow generation
•
Expect substantial operational synergies and savings coupled
with tax savings
Creates
Platform for
Further
Growth
•
All-stock transaction results in overall de-leveraging
•
Enhances borrowing capacity with substantial cash flow
contribution from anticipated synergies and tax savings
•
Increased specialty branded / Gx mix allows ACT to compete
with larger pharmaceutical companies
8 |
 Commercially Compelling Combination
Enhances
Specialty
Brands Scale
•
Achieves Actavis strategic goal to build multi-billion dollar
Specialty Brands business
•
Creates Top 3 U.S. specialty pharmaceutical company
and Top 20 global pharmaceutical company by sales
Enhanced
Women’s
Health and
Urology
Franchises;
Adds GI,
Dermatology
Presence
•
Premier Women’s Health player in U.S. -
Expands
Portfolio
•
Adds Gastroenterology and Dermatology franchises and
sales infrastructure
•
Expands Urology portfolio
•
Stronger Pipeline –
25 total development projects
-
15 Women’s Health products
-
Several Urology, Gastroenterology, Dermatology
products
Enhances
Branded /
Generics Mix
•
Diversifies ACT’s 2013E Specialty / Generic mix from 7%
standalone to ~ 25% pro forma
9 |
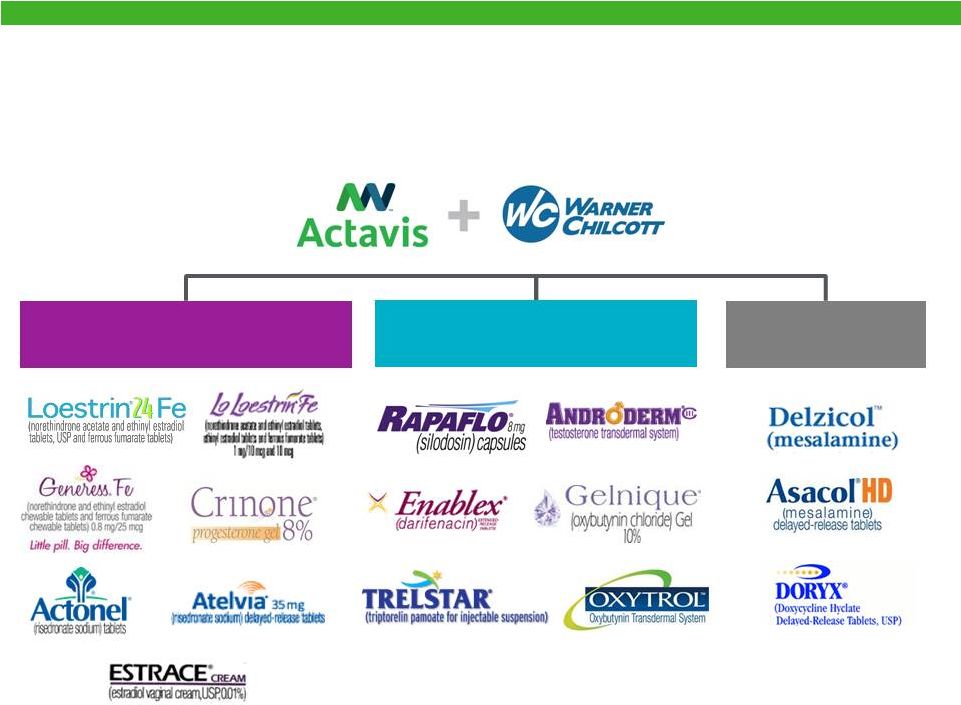
 Actavis Specialty Brands
Urology
10
Women’s Health |
 Well Positioned in Attractive Therapeutic Categories
Dermatology
Urology
Gastroenterology
Women’s Health
Warner Chilcott
11 |
 Stronger Combined Specialty Brands Franchise
Women’s Health
Urology
GI and
Dermatology
12 |
 Combined Women’s Health Pipeline
Phase 1
Phase 2
Phase 3
NDA
Approved
Canada
US
Global
US
PDUFA:
Dec. ‘13
RoW
US
Global
Global
Global
US
PDUFA
July ‘13
US
US
Approved
Apr 2013
Approved
May 2013
Product
Indication
Actavis
Esmya®
Fibroids
Actavis
Progestin Patch
Contraception
Actavis (Uteron)
Diafert®
Infertility
Actavis (Uteron)
Levosert®
IUD
Actavis (Uteron)
Estelle®
Oral Contraceptive
Actavis (Uteron)
Colvir
HPV
Actavis (Uteron)
Vaginate
Vaginal Infections
Actavis
Metronidazole Gel
Bacterial Vaginosis
Warner Chilcott
3042
Oral Contraceptive
Warner Chilcott
Minastrin 24 Fe
Oral Contraceptive
Warner Chilcott
3064
Oral Contraceptive
Warner Chilcott
3065
Oral Contraceptive
Warner Chilcott
3074
Oral Contraceptive
Warner Chilcott
3058
Contraceptive Ring
Warner Chilcott
3011
Hormone Therapy
13
PDUFA:
July ‘13 |
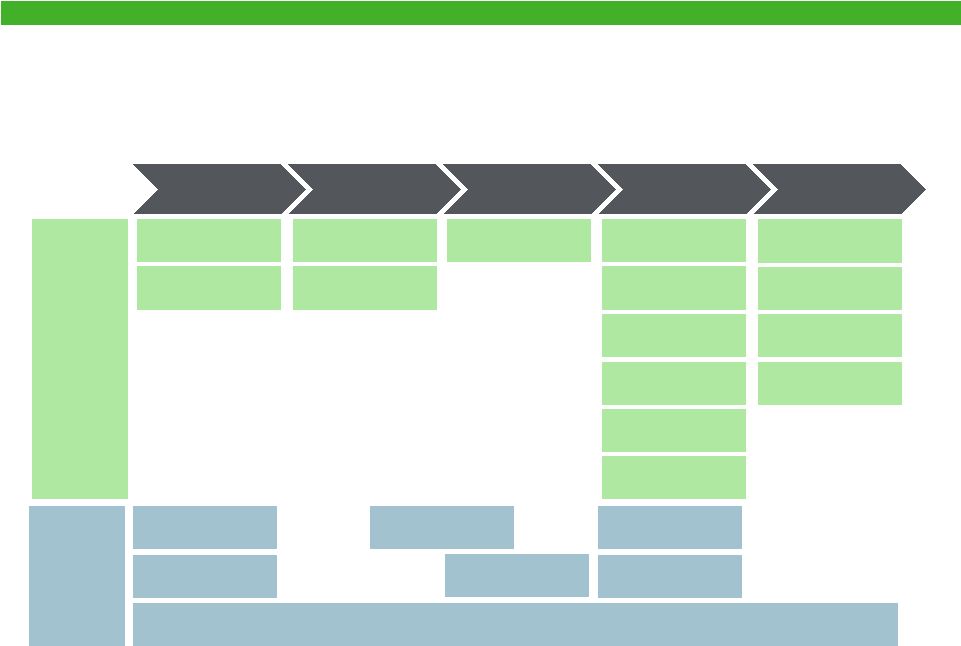 Combined Small Molecule Pipeline Overview
Preclinical
Phase I
Phase II
Phase III
NDA
Alyssa
Novel IUD
(Global)
Vaginate Vaginal
Infections
(Global)
Crinone®
2nd Generation
(U.S.)
Actavis
Warner
Chilcott
Colvir
HPV Lesions
(Global)
Metronidazole Gel
1.35
(US)
Estelle®
Novel OC
(Global)
Progestin Only Patch
Contraceptive
(Global)
Vaginal Ring
Contraceptive
(Global)
Esmya®
Uterine Fibroids
(U.S.)
Levosert®
IUD*
(U.S. + Canada)
Diafert®
Infertility
(U.S.)
Generess®
Fe
Contraceptive
(Canada)
Esmya®
Uterine
Fibroids
(Canada)
Levosert®
IUD*
(Europe)
Diafert®
Infertility
(Europe)
GI Products
Udenafil (BPH)
(Urology)
Sarecycline
(Derm)
Udenafil (ED)
(Urology)
WC2055
(Derm)
Portfolio of Women’s Health Products
Topical (ED)
(Urology)
14 |
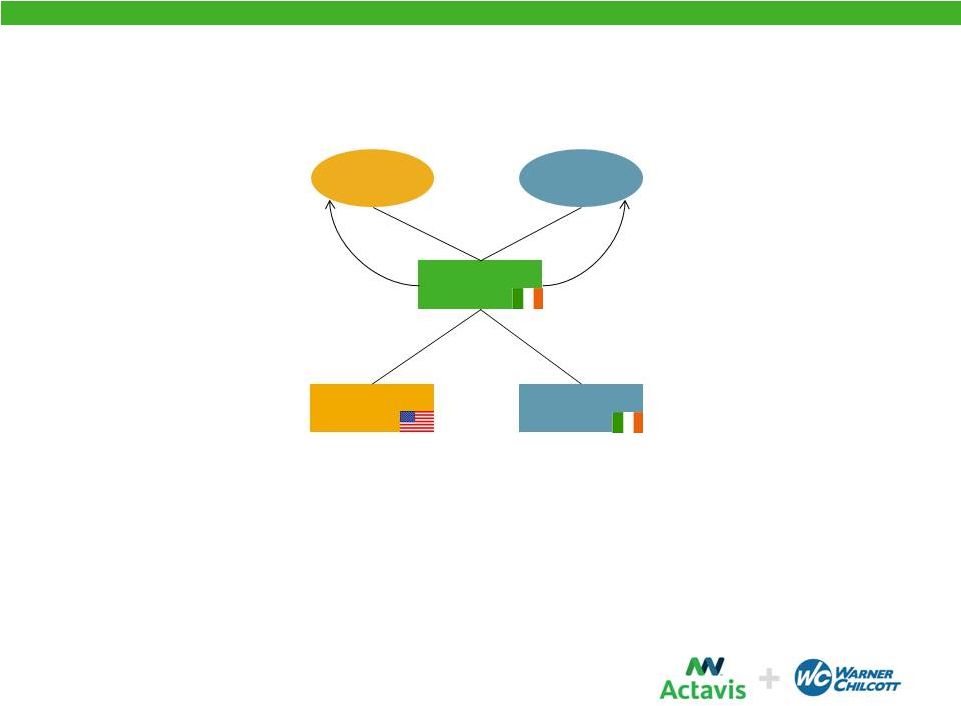 Corporate Redomicile
•
A newly formed Irish plc (“New Actavis”) acquires each of Actavis and
Warner Chilcott in exchange for shares
-
New
Actavis
is
the
publicly
traded
parent
company
(listed
in
the
U.S.
on
NYSE)
-
Actavis shareholders and Warner Chilcott shareholders each receive shares in New
Actavis (Actavis shareholders on a one-for-one basis and Warner
Chilcott shareholders based on an exchange ratio of 0.160)
Actavis
Shareholders
New Actavis
Shares (~23%)
Warner Chilcott
Shareholders
Actavis
New
Actavis
New Actavis
Shares (~77%)
Warner Chilcott
15 |
 Corporate Redomicile: Considerations
•
Receipt of New Actavis shares is taxable to Actavis
shareholders but expected to be tax free for U.S. federal
income tax purposes to Warner Chilcott shareholders
•
Regular U.S. merger process for ACT
-
Proxy/Registration Statement; Majority approval by Actavis
shareholders
-
One-for-one exchange ratio for Actavis shareholders
•
Warner Chilcott acquisition subject to approval of Irish
High Court and shareholder vote (Warner Chilcott
shareholders receive New Actavis shares in exchange
for Warner Chilcott shares)
16 |
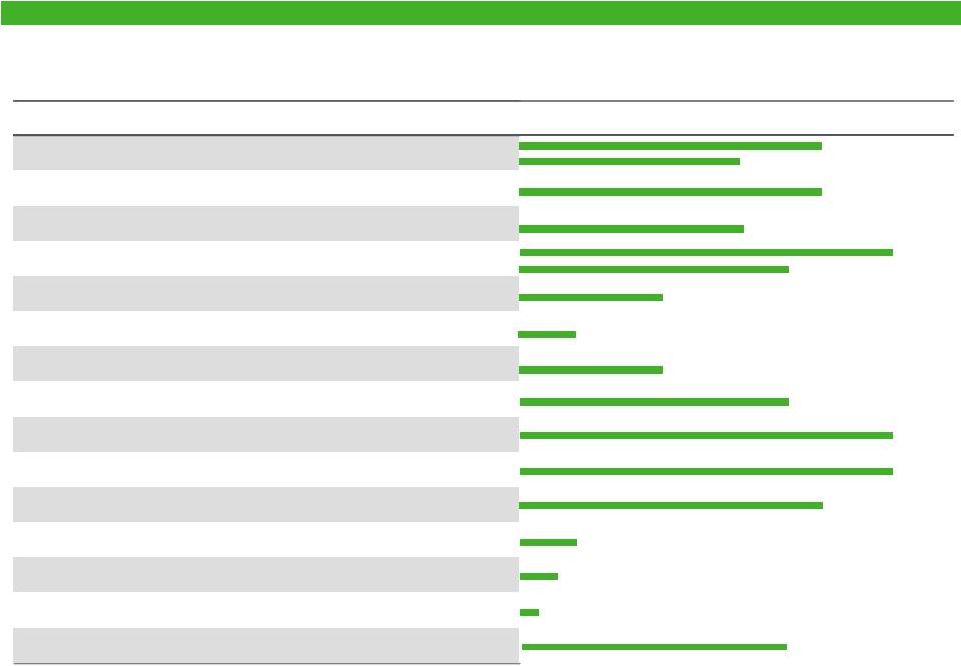
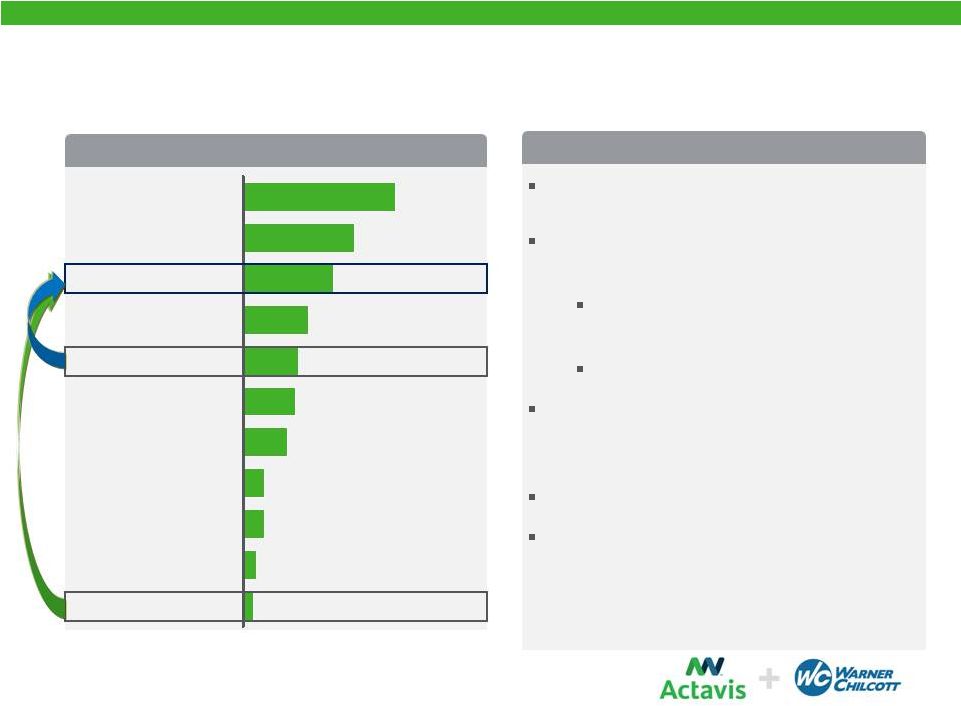 Achieving a Top 3 Position
Actavis
0.5
0.5
0.7
0.8
Jazz
1.7
Salix
Mylan
2.4
Endo
Valeant
2.5
Warner Chilcott
2.0
Allergan
2.9
Forest
Teva
5.9
Combined Co
4.3
2012 US Specialty Sales, $ Billions
~$3 billion Actavis Specialty Brands 2013
Pro Forma Revenues
Augments core areas of Women’s Health
and Urology
Premier Women’s Health player in
US
Larger Portfolio in Urology
Adds Gastroenterology and Dermatology
therapeutic categories and marketing
infrastructure
Enhances product development pipeline
Provides opportunities to bring combined
product portfolio to new and emerging
markets
Strong Growth Potential
17 |
 Proposed Transaction Benefits Summary
Strong combined revenues of approximately $11 billion
Expected to be immediately accretive
More than $400 million in anticipated after-tax operational
synergies and related cost reductions, and tax savings
The majority of these are operational and are expected to be
realized in 2014, with full effect during 2015
These synergies exclude any potential revenue, manufacturing
and interest rate synergies or savings
Strong combined operating cash flow de-levers balance
sheet to below 3.0x debt to adjusted EBITDA immediately at
close
Favorable combined company tax rate of ~17%
Greater than 30% Accretive to 2014 Non-GAAP Earnings Per Share
18 |
 New
Actavis – Growth Profile
Growth Drivers 2014 through 2016
Actavis Pharma
•
North American growth driven by strong PIV
portfolio and complex dosage forms
•
Drive growth in high-value markets
•
Optimize Global Commercial Network
Actavis Specialty Brands
•
Expanded product portfolio, therapeutic categories, pipeline
and geographic footprint
•
Execution on development, launches and next generation
strategies
•
Focused Biosimilar strategy
Actavis Global Operations
•
Supply Chain Optimization
•
Expand Anda distribution services
19 |
 20 |
 Statement Required by the Irish Takeover Rules
21
The directors of Warner Chilcott accept responsibility for the information contained in this
communication relating to Warner Chilcott and its Associates and the directors of Warner
Chilcott and members of their immediate families, related trusts and persons connected with them. To the
best of the knowledge and belief of the directors of Warner Chilcott (who have taken all reasonable
care to ensure such is the case), the information contained in this communication for which
they accept responsibility is in accordance with the facts and does not omit anything likely to
affect the import of such information. The
directors of Actavis accept responsibility for the information contained in this communication other than that relating to Warner Chilcott and its
Associates and the directors of Warner Chilcott and members of their immediate families, related
trusts and persons connected with them. To the best of the knowledge and belief of the
directors of Actavis (who have taken all reasonable care to ensure that such is the case), the information
contained in this communication, for which they accept responsibility, is in accordance with the facts
and does not omit anything likely to affect the import of such information.
Deutsche Bank Securities Inc. is acting exclusively for Warner Chilcott as financial advisor and is
not acting as financial advisor to anyone else in connection with the matters referred to in
this announcement and will not be responsible to anyone other than Warner Chilcott in connection
therewith for providing advice in relation to the matters referred to in this announcement. Deutsche
Bank Securities Inc. has delegated certain of its financial advisory functions and
responsibilities to Deutsche Bank AG, acting through its London branch. Deutsche Bank AG, acting through its
London branch is performing such delegated functions and responsibilities exclusively for Warner
Chilcott and is not acting as a financial adviser for any other person in connection with the
matters referred to in this announcement and will not be responsible to any such other person for
providing advice in relation to the matters referred to in this announcement. Deutsche Bank AG is
authorised under German Banking Law (competent authority: BaFin – Federal Financial
Supervisory Authority) and authorised and subject to limited regulation by the Financial Conduct
Authority. Details about the extent of Deutsche Bank AG’s authorization and regulation by the
Financial Conduct Authority are available on request BofA Merrill Lynch and Greenhill & Co.
are acting exclusively for Actavis and no one else in connection with the matters referred to in this
announcement and will not be responsible to anyone other than Actavis for providing the protections
afforded to clients of BofA Merrill Lynch or Greenhill & Co and for providing advice in
relation to the acquisition of Warner Chilcott, the contents of this announcement or any transaction or
arrangement referred to herein. The
statement that this acquisition is earnings accretive should not be interpreted to mean that the earnings per share in the current or any future
financial period will necessarily match or be greater than those for the relevant preceding period.
|
 Dealing Disclosure Requirements
Under the provisions of Rule 8.3 of the Irish Takeover Panel Act, 1997, Takeover
Rules 2007, as amended (the "Irish Takeover Rules"), if
any
person
is,
or
becomes,
'interested'
(directly
or
indirectly)
in,
1%,
or
more
of
any
class
of
'relevant
securities'
of
Warner
Chilcott
or
Actavis,
all 'dealings' in any 'relevant securities' of Warner Chilcott or Actavis
(including by means of an option in respect of, or a derivative referenced
to, any such 'relevant securities') must be publicly disclosed by not later than
3:30 p.m. (Dublin time) on the business day following the date of
the
relevant
transaction.
This
requirement
will
continue
until
the
date
on
which
the
Scheme
becomes
effective
or
on
which
the
'offer
period'
otherwise ends. If two or more persons co-operate on the basis of any
agreement, either express or tacit, either oral or written, to acquire an
'interest' in 'relevant securities' of Warner Chilcott or Actavis, they will be
deemed to be a single person for the purpose of Rule 8.3 of the Irish
Takeover Rules. Under the provisions of Rule 8.1 of the Irish Takeover
Rules, all 'dealings' in 'relevant securities' of Warner Chilcott by Actavis or ‘relevant
securities’
of Actavis by Warner Chilcott, or by any of their respective 'associates' must
also be disclosed by no later than 12 noon (Dublin time) on the business day
following the date of the relevant transaction. A disclosure table, giving
details of the companies in whose 'relevant securities' 'dealings' should be disclosed can be found on the Panel's
website at www.irishtakeoverpanel.ie.
'Interests in securities' arise, in summary, when a person has long economic
exposure, whether conditional or absolute, to changes in the price of
securities. In particular, a person will be treated as having an 'interest' by virtue of the ownership or control of securities, or by virtue
of any option in respect of, or derivative referenced to, securities.
Terms
in
quotation
marks
are
defined
in
the
Irish
Takeover
Rules,
which
can
also
be
found
on
the
Irish
Takeover
Panel's
website.
If
you
are
in any
doubt
as
to
whether
or
not
you
are
required
to
disclose
a
dealing
under
Rule
8,
please
consult
the
Panel's
website
at
www.irishtakeoverpanel.ie
or contact the Panel on telephone number +353 1 678 9020; fax number +353 1 678
9289. 22 |
