Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a13-12028_18k.htm |
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a13-12028_1ex99d1.htm |
Exhibit 99.2
|
|
At the Forefront of Therapies for Rare and Orphan Diseases™ May 9, 2013 |
|
|
Safe Harbor This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations and financial conditions of Amicus including but not limited to preclinical and clinical development of Amicus’ candidate drug products, the timing and reporting of results from clinical trials evaluating Amicus’ candidate drug products. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus’ forward-looking statements due to numerous known and unknown risks and uncertainties, including the “Risk Factors” described in our Annual Report on Form 10-K for the year ended December 31, 2012. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. Slide 2 |
|
|
Slide 3 Agenda Chaperone-ERT Combination Programs Upcoming Milestones/Concluding Remarks Introduction Migalastat HCl Monotherapy for Fabry Disease FY12 Financial Results and FY13 Guidance Q&A |
|
|
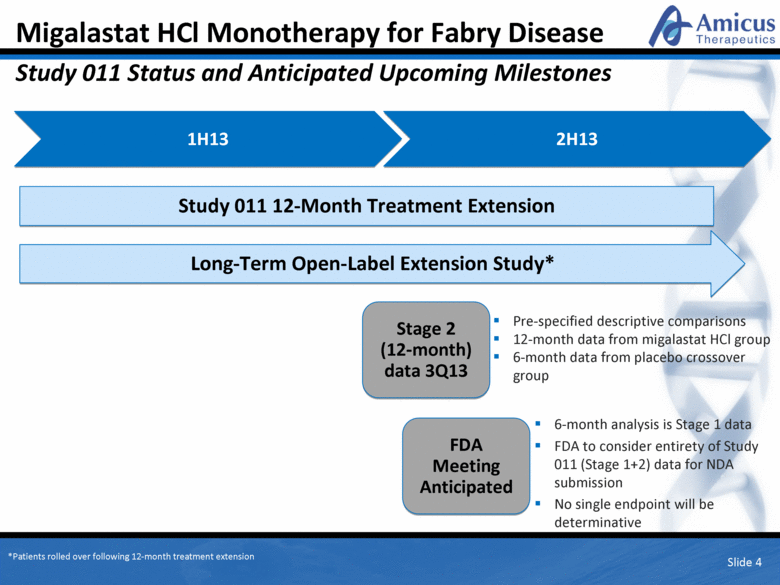
Migalastat HCl Monotherapy for Fabry Disease Study 011 Status and Anticipated Upcoming Milestones Slide 4 1H13 Pre-specified descriptive comparisons 12-month data from migalastat HCl group 6-month data from placebo crossover group 6-month analysis is Stage 1 data FDA to consider entirety of Study 011 (Stage 1+2) data for NDA submission No single endpoint will be determinative 2H13 Stage 2 (12-month) data 3Q13 FDA Meeting Anticipated Study 011 12-Month Treatment Extension Long-Term Open-Label Extension Study* *Patients rolled over following 12-month treatment extension |
|
|
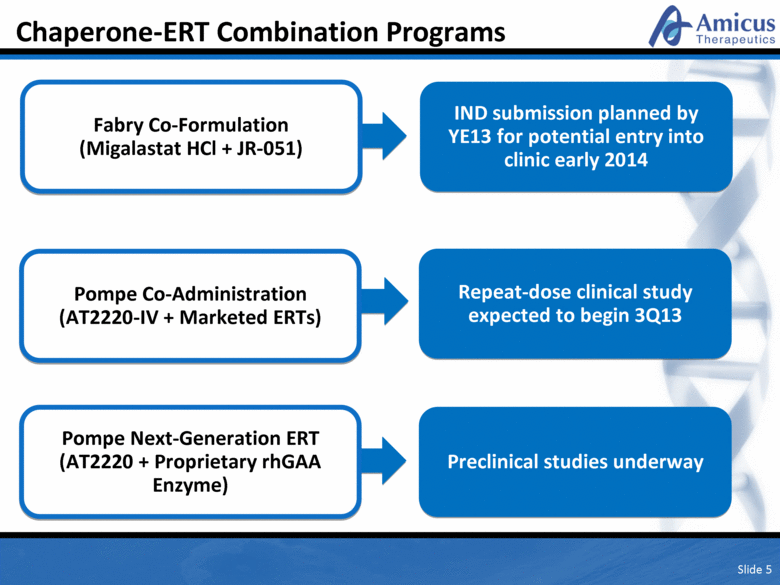
Slide 5 Chaperone-ERT Combination Programs Pompe Co-Administration (AT2220-IV + Marketed ERTs) Repeat-dose clinical study expected to begin 3Q13 Pompe Next-Generation ERT (AT2220 + Proprietary rhGAA Enzyme) Preclinical studies underway Fabry Co-Formulation (Migalastat HCl + JR-051) IND submission planned by YE13 for potential entry into clinic early 2014 |
|
|
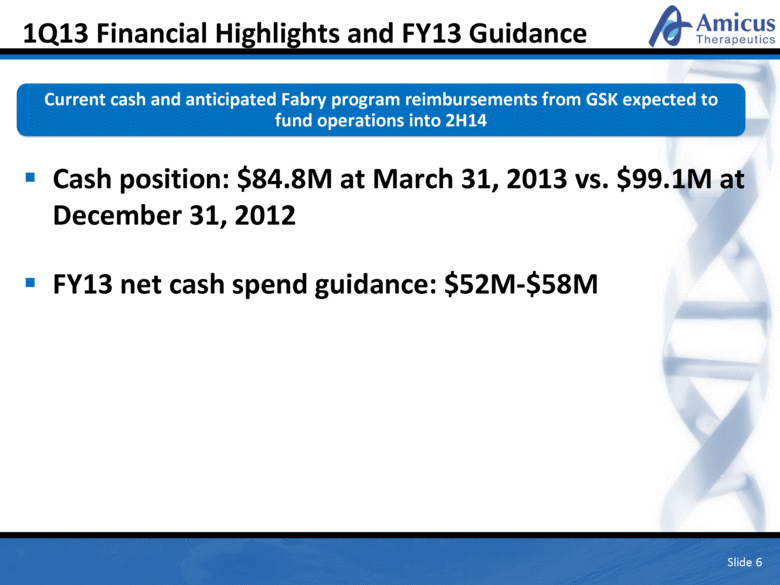
1Q13 Financial Highlights and FY13 Guidance Cash position: $84.8M at March 31, 2013 vs. $99.1M at December 31, 2012 FY13 net cash spend guidance: $52M-$58M Slide 6 Current cash and anticipated Fabry program reimbursements from GSK expected to fund operations into 2H14 |
|
|
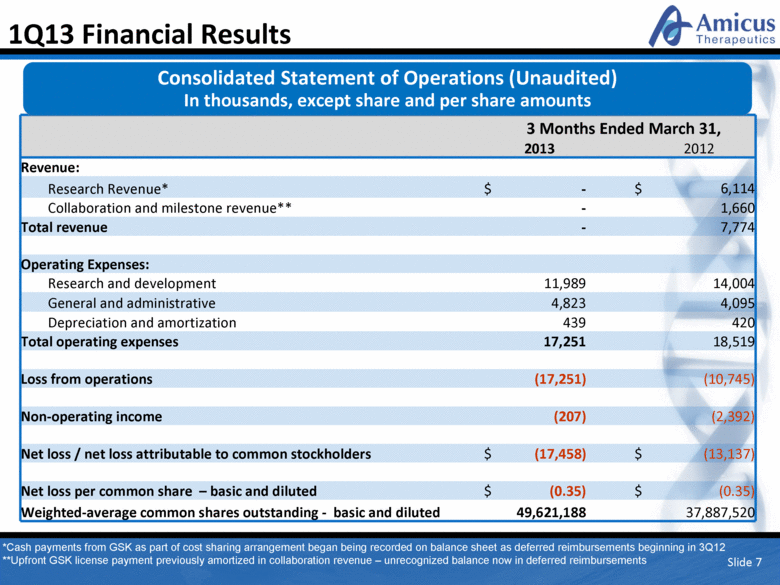
Slide 7 1Q13 Financial Results 3 Months Ended March 31, 2013 2012 Revenue: Research Revenue* $ - $ 6,114 Collaboration and milestone revenue** - 1,660 Total revenue - 7,774 Operating Expenses: Research and development 11,989 14,004 General and administrative 4,823 4,095 Depreciation and amortization 439 420 Total operating expenses 17,251 18,519 Loss from operations (17,251) (10,745) Non-operating income (207) (2,392) Net loss / net loss attributable to common stockholders $ (17,458) $ (13,137) Net loss per common share – basic and diluted $ (0.35) $ (0.35) Weighted-average common shares outstanding - basic and diluted 49,621,188 37,887,520 Consolidated Statement of Operations (Unaudited) In thousands, except share and per share amounts *Cash payments from GSK as part of cost sharing arrangement began being recorded on balance sheet as deferred reimbursements beginning in 3Q12 **Upfront GSK license payment previously amortized in collaboration revenue – unrecognized balance now in deferred reimbursements |
|
|
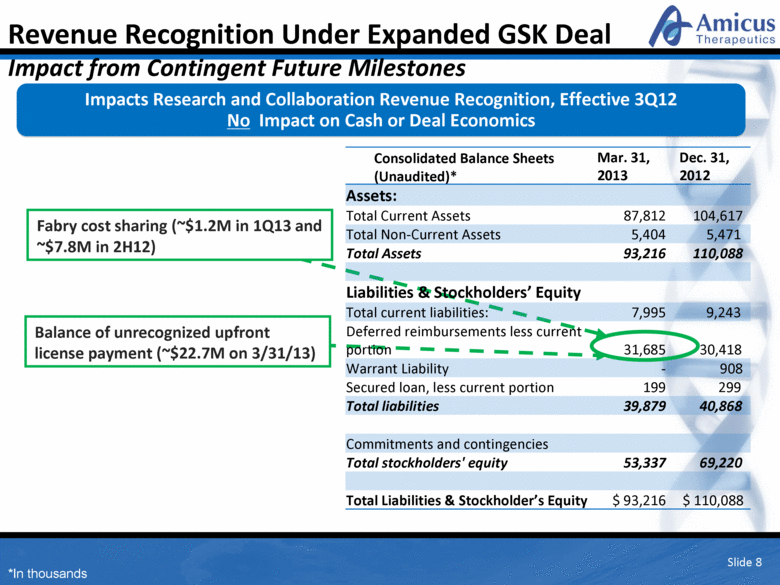
Slide 8 Mar. 31, 2013 Dec. 31, 2012 Assets: Total Current Assets 87,812 104,617 Total Non-Current Assets 5,404 5,471 Total Assets 93,216 110,088 Liabilities & Stockholders’ Equity Total current liabilities: 7,995 9,243 Deferred reimbursements less current portion 31,685 30,418 Warrant Liability - 908 Secured loan, less current portion 199 299 Total liabilities 39,879 40,868 Commitments and contingencies Total stockholders' equity 53,337 69,220 Total Liabilities & Stockholder’s Equity $ 93,216 $ 110,088 Balance of unrecognized upfront license payment (~$22.7M on 3/31/13) Impacts Research and Collaboration Revenue Recognition, Effective 3Q12 No Impact on Cash or Deal Economics Revenue Recognition Under Expanded GSK Deal Impact from Contingent Future Milestones Consolidated Balance Sheets (Unaudited)* *In thousands Fabry cost sharing (~$1.2M in 1Q13 and ~$7.8M in 2H12) |
|
|
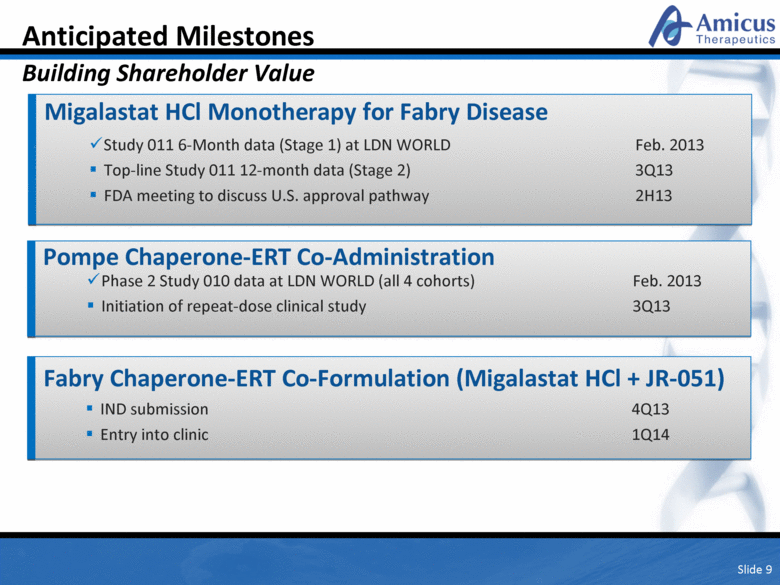
Slide 9 Anticipated Milestones Building Shareholder Value Migalastat HCl Monotherapy for Fabry Disease Study 011 6-Month data (Stage 1) at LDN WORLD Feb. 2013 Top-line Study 011 12-month data (Stage 2) 3Q13 FDA meeting to discuss U.S. approval pathway 2H13 Pompe Chaperone-ERT Co-Administration IND submission 4Q13 Entry into clinic 1Q14 Phase 2 Study 010 data at LDN WORLD (all 4 cohorts) Feb. 2013 Initiation of repeat-dose clinical study 3Q13 Fabry Chaperone-ERT Co-Formulation (Migalastat HCl + JR-051) |
|
|
Q&A Slide 10 John F. Crowley, Chairman & CEO Chip D. Baird, Chief Financial Officer Bradley L. Campbell, Chief Business Officer David J. Lockhart, PhD, Chief Scientific Officer |