Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Inspyr Therapeutics, Inc. | v342111_8k.htm |

Precise targeting of a potent, unique prodrug directly to tumors OTCBB: GNSZ APRIL 2013

SAFE HARBOR STATEMENT GNSZ | APRIL 2013 PAGE 2 Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995: Any statements that are not historical facts are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward - looking statements, which may include, but are not limited to, factors related to GenSpera's anticipated growth strategies, future business development, ability to develop new products, expand to other related industries or markets in other geographical locations, and other information detailed from time to time in the filings and future filings with the United States Securities and Exchange Commission. Readers are advised that this information is intended for the use of investment professionals. Anyone interested in obtaining information on GenSpera should contact GenSpera, as set forth above, to receive GenSpera's most recent financial reports. This presentation was developed by GenSpera and is intended solely for informational purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy the Company's stock. This profile is based upon information available to the public, as well as other information from sources which management believes to be reliable, but is not guaranteed by the Company as being accurate nor does it purport to be complete. Opinions expressed herein are those of management as of the date of presentation and are subject to change without notice.

GENSPERA DEFINED Highly differentiated , targeted therapeutic agents with a unique mechanism of action GNSZ | APRIL 2013 PAGE 3 • Thapsigargin has potential for “complete kill” – active against both slowly and rapidly dividing cells as well as cancer stem cells • Targeting/masking technology directed to tumors and/or related vascular beds • IP acquired from Johns Hopkins University – no milestones or royalties due • Lead drug candidate, G - 202, should be useful against most solid tumors - completed Ph I w positive signal in liver cancer • Ph II enrollment began 1Q13 in patients with hepatocellular carcinoma who have progressed on prior sorafenib therapy; preliminary data expected Q413 • Novel nanoemulsion form with worldwide patent coverage to 2033

PLATFORM TECHNOLOGY GNSZ | APRIL 2013 PAGE 4 12ADT • Derived from T. garganica plant (exclusive supply agreements in place) • Well - characterized, broad spectrum toxin • Kills: ▪ Slow - and fast - growing cancer cells ▪ Cancer stem cells ▪ New and established tumor vasculature • Patent - Protected* • Masking/targeting peptide attached to 12ADT • Renders 12ADT: ▪ Inactive ▪ Soluble ▪ Tumor selective • Intravenous delivery • Phase 1 complete; Phase II enrolling • Patent - Protected* • Prostate - Specific Membrane Antigen (PSMA ) , a cell - surface enzyme on prostate cancer cells & other solid tumor associated vasculature • PSMA cleaves masking/ targeting peptide and liberates 1 2ADT from G - 202 • Localized 12ADT destroys target cells and bystanders • Patent - Protected* PEPTIDE ATTACHED DELIVERED TO TUMOR G - 202 12ADT cleaved peptide PSMA *9 Patents issued, 1 EU & 9 US applications pending incl. nanoemulsion

G - 202 PHASE Ib (ongoing): Results in Patients with HCC GNSZ | APRIL 2013 PAGE 5 Month 1 2 3 4 5 6 7 8 9 10 11 12 Patient A B C D E Median time to progression after failure on sorafenib therapy in patients with advanced HCC is ~2.1 months. Stable disease, remains on study Off study
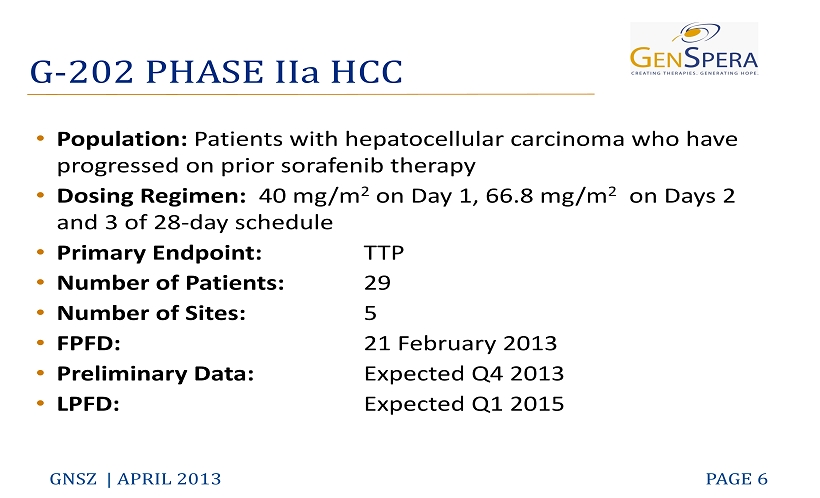
G - 202 PHASE IIa HCC GNSZ | APRIL 2013 PAGE 6 • Population: Patients with hepatocellular ca rcinoma who have progressed on prior sorafenib therapy • Dosing Regimen: 40 mg/m 2 on Day 1, 66.8 mg/m 2 on Days 2 and 3 of 28 - day schedule • Primary Endpoint: TTP • Number of Patients: 29 • Number of Sites: 5 • FPFD: 21 February 2013 • Preliminary Data: Expected Q4 2013 • LPFD: Expected Q1 2015

WORLDWIDE PATENT STRATEGY GNSZ | APRIL 2013 PAGE 7 • Current Infusion Form • Patent status secure in US to 2023 • Data exclusivity outside of US • Future Injectable Nanoemulsion Form • “Product by process” patent application filed October 2012 • Injectable form easier than infusion for patients and treatment centers • Transition to nanoemulsion* after current Phase II program * Worldwide Patent exclusivity expected through 2033

G - 202 CORE DISTINCTIONS GNSZ | APRIL 2013 PAGE 8 • Highly differentiated , novel therapeutic agent with a unique mechanism of action • Preliminary Phase II data expected in Q4 2013 • Out - license or partner subsequent to Phase II • Injectable nanoemulsion form for pivotal studies with worldwide patent coverage to 2033

CONTACT GNSZ | APRIL 2013 PAGE 9 Craig A. Dionne, PhD President & CEO cdionne@genspera.com GenSpera, Inc. 2511 N Loop 1604 W, Suite 204 San Antonio, TX 78258 www.genspera.com Phone: +1 (210) 479 - 8112 Fax: +1 (210) 479 - 8113
