Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Trius Therapeutics Inc | d508916d8k.htm |
| EX-99.1 - EX-99.1 - Trius Therapeutics Inc | d508916dex991.htm |
 The
Future of Anti-Infectives ESTABLISH 2 Top Line Data Release
March 25, 2013
Exhibit 99.2 |
 2
Forward Looking Statements
Statements
contained
in
this
data
release
regarding
matters
that
are
not
historical
facts
are
“forward-looking
statements”
within
the
meaning
of
the Private Securities Litigation Reform Act of 1995. Because such
statements are subject to risks and uncertainties, actual results may
differ materially from those expressed or implied by such forward-looking
statements.
Risks
that
contribute
to
the
uncertain
nature
of
the
forward-
looking
statements
include:
the
accuracy
of
Trius’
estimates
regarding
expenses, future revenues and capital requirements; the success and
timing of Trius’
preclinical studies and clinical trials; regulatory
developments
in
the
United
States
and
foreign
countries;
changes
in
Trius’
plans to develop and commercialize its product candidates; Trius’
ability
to
obtain
additional
financing;
Trius’
ability
to
obtain
and
maintain
intellectual
property
protection
for
its
product
candidates;
and
the
loss
of
key
scientific
or
management
personnel.
These
and
other
risks
and
uncertainties
are
described
more
fully
in
Trius’
most
recently
filed
SEC
documents, including its Form 10-K, Forms 10-Q and other documents
filed with the United States Securities and Exchange Commission,
including
those
factors
discussed
under
the
caption
“Risk
Factors”
in
such filings. All forward-looking statements contained in this press
release speak only as of the date on which they were made. Trius
undertakes no obligation to update such statements to reflect events that
occur or circumstances that exist after the date on which they were
made. |
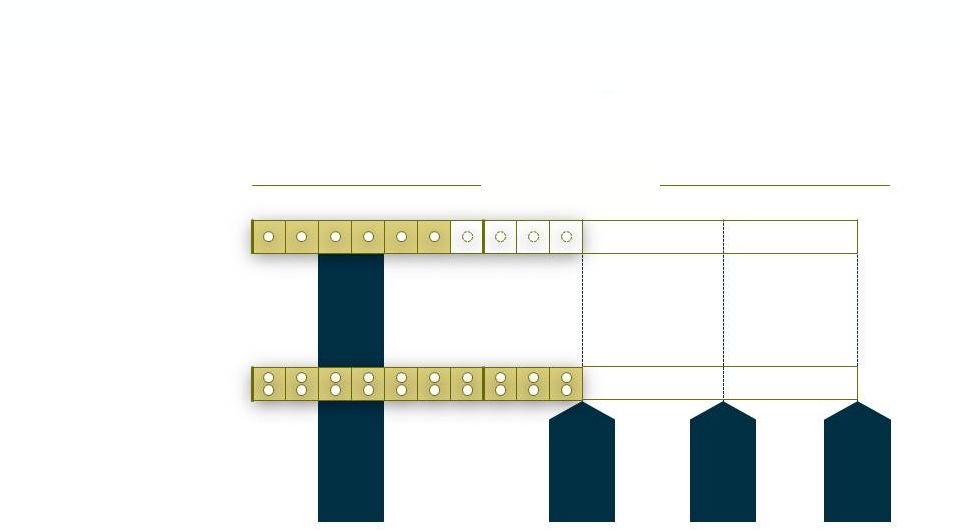 3
ESTABLISH 2 (IV/PO) Phase 3 Trial Design
2
o
EMA
1
o
EMA
Tedizolid
Linezolid
1x 200mg
2x 600mg
n=666
1
o
FDA
2
o
FDA
Placebo
Post-Treatment Evaluations
END-POINTS FOR GLOBAL REGISTRATION
Safety Analysis
Non-inferiority trial design vs. Linezolid |
 4
ESTABLISH 2 Primary & Secondary Endpoints
Primary Endpoint:
•
20% or greater reduction in lesion area at 48-72 hours after first dose of
drug Secondary Endpoints:
•
Programmatic clinical response at end of therapy (EOT)
•
Investigator’s
assessment of clinical response at post treatment evaluation (PTE)
|
 5
ESTABLISH 2 Demographics and Baseline Characteristics
Tedizolid, %
n = 332
Linezolid, %
n = 334
Male, %
67.8
64.1
Age, mean
45.6
45.6
Geographic Region, %
North America (US)
47.0
47.3
Ex-U.S.
53.0
52.7
Clinical Syndrome, %
Cellulitis/erysipelas
50.0
50.3
Major abscess
20.5
20.4
Wound infection
29.5
29.3
MRSA (MITT), %
27.2
26.9
MSSA (MITT), %
52.8
53.0
Lymphadenopathy, %
70.8
70.4
WBC < 4,000 or > 10,000, %
53.0
45.2
Immature neutrophils, %
16.2
12.2
Fever, %
31.0
29.0 |
 6
ESTABLISH 2 Efficacy
ITT Analysis Set
Endpoint
Tedizolid
6 days
treatment, %
n = 332
Linezolid
10 days
treatment, %
n = 334
Treatment
Difference
(95% CI), %
Primary Endpoint
>20% decrease from
baseline in lesion
area at 48-72 hours
85.2
82.6
2.6 (-3.0 to 8.2)
Key Secondary
Endpoints
Sustained clinical
response at end of
therapy
87.0
88.0
-1.0 (-6.1 to 4.1)
Investigators
assessment of
clinical response at
7-14 days after end
of therapy
88.0
87.7
0.3 (-4.8 to 5.3)
Sensitivity
analysis
(2010 FDA
Guidance)
Cessation of lesion
spread and absence
of fever at 48-72
hours
85.8
81.4
4.4 (-1.2 to 10.1) |
 7
ESTABLISH 2 Efficacy by Infection Type
ITT Analysis Set
Tedizolid
6 days
treatment, %
n = 332
Linezolid
10 days
treatment, %
n = 334
Treatment
Difference
(95% CI), %
Cellulitis/erysipelas
80.7
n = 166
80.4
n = 168
0.37 (-8.2 to 8.9)
Major cutaneous
abscess
86.8
n = 68
89.7
n = 68
-2.9 (-14.5 to 8.4)
Wound infection
91.8
n = 98
81.6
n = 98
10.2 (0.71 to 20.1) |
 8
ESTABLISH 2 Safety: Tedizolid was Safe and Well
Tolerated with a Favorable AE Profile vs. Linezolid
Safety Analysis Set
Tedizolid
6 days treatment, %
Linezolid
10 days treatment, %
Any Treatment Emergent
Adverse Event (TEAE)
45.3
43.7
Any Drug Related TEAE
20.5
24.8
Gastrointestinal Disorders
16.0
20.5
No Safety Signals |
 9
ESTABLISH 2 Incidence of TEAEs >2%
System Organ Class
Tedizolid, (%)
n = 331
Linezolid, (%)
n =327
Gastrointestinal disorders
53 (16.0)
67 (20.5)
General disorders and
administration site conditions
23 (6.9)
24 (7.3)
Infections and infestations
40 (12.1)
40 (12.2)
Metabolism and nutrition
disorders
9 (2.7)
7 (2.1)
Musculoskeletal and connective
tissue disorders
9 (2.7)
9 (2.8)
Nervous system disorders
29 (8.8)
36 (11.0)
Psychiatric disorders
10 (3.0)
4 (1.2)
Respiratory, thoracic and
mediastinal disorders
6 (1.8)
13 (4.0)
Skin and subcutaneous tissue
disorders
21 (6.3)
24 (7.3)
Vascular disorders
7 (2.1)
4 (1.2) |
 10
ESTABLISH 2 Post-Baseline Substantially Abnormal
Hematology Values
Tedizolid, (%)
n = 331
Linezolid, (%)
n =327
Hemoglobin
292
287
Below LLN
147 (50.3)
148 (51.6)
Substantially abnormal
4 (1.4)
2 (0.7)
White blood cells
272
258
Below LLN
16 (5.9)
20 (7.8)
Substantially abnormal
3 (1.1)
5 (1.9)
Platelets
275
269
Below LLN
37 (13.5)
35 (13.0)
Substantially abnormal
9 (3.3)
5 (1.9) |
 11
Differences Between ESTABLISH 1 and 2
ESTABLISH 2
ESTABLISH 1
Dosing
IV to oral
(minimum
1
st
day’s
dosing
IV)
Oral
Demographics
US 47%, x-US 53%
US 80%, x-US 20%
MRSA, %
27.1
42.6
Primary
Endpoint*
>
20% reduction in lesion
area @ 48 to 72 hours
Cessation of lesion spread,
absence of fever @ 48 to 72 hours
*ESTABLISH 2 SPA amended under agreement with FDA to reflect expected new primary
endpoint. Both endpoints prospectively captured as sensitivity analyses in
respective studies. Integrated summary of efficacy will reflect the ESTABLISH
2 primary endpoint under the amended SPAs |
 12
ESTABLISH Program Comparison
Endpoint
Study
Tedizolid
6 days
treatment, %
Linezolid
10 days
treatment, %
Treatment
Difference
(95% CI), %
>20% decrease from
baseline in lesion area at
48-72 hours
ESTABLISH 2
ESTABLISH 1
85.2
78.0
82.6
76.1
2.6 (-3.0 to 8.2)
1.9 (-4.5 to 8.3)
Sustained clinical
response at end of
therapy
ESTABLISH 2
ESTABLISH 1
87.0
87.0
88.0
87.8
-1.0 (-6.1 to 4.1)
-0.8 (-5.8 to 4.4)
Investigators assessment
of clinical response at 7-
14 days after end of
therapy
ESTABLISH 2
ESTABLISH 1
88.0
85.5
87.7
86.0
0.3 (-4.8 to 5.3)
-0.5 (-5.8 to 4.9)
Cessation of lesion
spread and absence of
fever at 48-72 hours
ESTABLISH 2
ESTABLISH 1
85.8
79.5
81.4
79.4
4.4 (-1.2 to 10.1)
0.1 (-6.1 to 6.2) |
 13
ESTABLISH Program Safety Comparison
Safety Analysis Set
Study
Tedizolid %
Linezolid %
Any Treatment
Emergent Adverse
Event (TEAE)
ESTABLISH 2
45.3
43.7
ESTABLISH 1
40.8
43.3
Any Drug Related
TEAE
ESTABLISH 2
20.5
24.8
ESTABLISH 1
24.2
31.0
Gastrointestinal
Disorders
ESTABLISH 2
16.0
20.5
ESTABLISH 1
16.3
25.4 |
| 14
Summary
•
All primary and secondary efficacy endpoints were met for FDA and EMA
•
Safety profile indicated that tedizolid was safe and well tolerated
•
Results are consistent with those from ESTABLISH 1 and support filings for
global regulatory approval
•
Combined results of all clinical studies support tedizolid differentiation:
–
Safe, well tolerated, fast acting drug for resistant gram positive infections
–
Convenient once daily IV or oral administration over short course of therapy
–
Fewer drug-drug interactions
–
Active against key linezolid resistant strains |
 15
*Efficacy performance ratings among those aware of brand
Tedizolid
ATU
Study,
Wave
1
–
USA
Report,
conducted
by
CMI
on
behalf
of
Trius,
November
2012,
N=505
Strong Response to Tedizolid Efficacy, Dosing and
Administration
*
Tedizolid, 65%
Tedizolid, 52%
Zyvox, 62%
Zyvox, 42%
Cubicin, 54%
Cubicin, 37%
Vancomycin, 25%
Vancomycin, 27%
Tedizolid, 45%
Tedizolid, 42%
Zyvox, 45%
Zyvox, 38%
Cubicin, 42%
Cubicin, 37%
Vancomycin, 30%
Vancomycin, 30%
Offers simple dosing and
administration
Has a lower potential for
resistance development
Has superior efficacy vs.
other products
Is effective for all patient
types |
 16
Strong Response to Tedizolid Safety & Tolerability
Has a low frequency of
DDI (drug-drug
interaction)
Does not cause renal
and hepatic impairment
Has a low frequency of
safety/adverse events
*Efficacy performance ratings among those aware of brand
Tedizolid
ATU
Study,
Wave
1
–
USA
Report,
conducted
by
CMI
on
behalf
of
Trius,
November
2012,
N=505
Tedizolid, 48%
Tedizolid, 53%
Tedizolid, 59%
Zyvox, 27%
Zyvox, 45%
Zyvox, 31%
Cubicin, 48%
Cubicin, 42%
Cubicin, 44%
Vancomycin, 39%
Vanco
., 14%
Vancomycin, 25% |
 17
Tedizolid
ATU
Study,
Wave
1
–
USA
Report,
conducted
by
CMI
on
behalf
of
Trius,
November
2012,
N=505
Base: Total respondents
Q8.1
How
interesting
is
tedizolid
to
you
for
suspected
or
confirmed
MRSA
ABSSSI
patients
overall?
Use
a
1
to
10
scale
where
“1”
means
“Not
at
all
Interesting”
and
“10”
means
“Very
Interesting,”
to
indicate
your
level
of
interest.
Interest in Tedizolid, by Specialty
(0 = Not Interested, 10 = Very Interested)
Physician Interest in Tedizolid Strong Across Specialties
Total
(N = 505)
Critical Care,
Pulmonologist
(n = 120)
Internist
(n = 137)
General
Surgeon
(n = 86)
Hospitalist
(n = 175)
Infectious
Disease
Specialist
(n = 101)
Infusion Clinic
Specialist
(n = 75)
80%
69%
74%
72%
76%
92%
83%
19%
31%
25%
27%
23%
7%
17%
High Interest (8-10)
Neutral (4-7)
Low Interest (1-3) |
| 18
Upcoming Milestones
•
Late breaker presentations at ECCMID conference (submitted for
April)
•
Detailed data presentations at ICAAC conference (September)
•
Advance EU partnership discussions
•
NDA Filing (H2 2013)
•
Implement pre-commercial activities for potential U.S. launch (H2 2013)
•
Initiation of Phase 3 ventilated nosocomial pneumonia study (H2 2013)
•
EMA Filing (H1 2014)
•
Potential PDUFA date for ABSSSI (mid 2014) |
