Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Advaxis, Inc. | v337386_8k.htm |

2013 Business Outlook Conference Call and Webcast Thomas A. Moore, CEO Advaxis, Inc. (OTC BB: ADXS) Exhibit 99.1

Forward Looking Statements This presentation contains forward - looking statements, including, but not limited to: statements as to the anticipated timing of clinical studies and other business developments, statements as to the development of new constructs, expectations as to the adequacy of our cash balances to support our operations for specified periods of time and as to the nature and level of cash expenditures, expectations as to market opportunities, our ability to take advantage of those opportunities, and the risk factors set forth from time to time in Advaxis' SEC filings, including but not limited to its report on Form 10 - K for the fiscal year ended October 31, 2012, which is available at http://www.sec.gov . The Company undertakes no obligation to publicly release the result of any revision to these forward - looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, except as required by law. You are cautioned not to place undue reliance on any forward - looking statements. 2

Thomas A. Moore Chairman and CEO 3 3

Thomas A. Moore, Chairman / CEO 25 years experience in healthcare, executive management and business development. As CEO of Nelson Communications, engineering the sale of the company to Publicis Group for $246 million. President of Procter & Gamble ’ s (NYSE: PG) worldwide healthcare products business. Mark J. Rosenblum, CFO, Secretary, Senior VP 25 years experience in accounting and financial leadership. VP, Chief Accounting Officer of Wellman, Inc., a $1.2Bn chemical company; CFO and Secretary, HemoBioTech, Inc. Robert Petit, Ph.D., VP Clinical Operations & Medical Affairs 25+ years experience in oncology drug development. US medical strategy lead for Yervoy® (ipilimumab) program at Bristol Myers Squibb (NYSE: BMY) as the Director of Medical Strategy for new oncology products and Director of Global Clinical Research. VP of Clinical Development at MGI Pharma and Aesgen, Inc. Daniel J. O ’ Connor, Esq. , Senior VP, Chief Legal and Business Development Officer 15 years of executive, legal, and regulatory experience in the biopharmaceutical industry with ImClone Systems, PharmaNet and Bracco Diagnostics. As SVP/ GC at ImClone, played a key role in extensive licensing negotiations, paving the way for company to be sold to Eli Lilly in 2008. Chris French, Executive Director of Medical Affairs 20 years of science research and pharmaceutical experience in drug development in start - up, midsize and large pharmaceutical companies. She has held management positions in medical affairs, regulatory affairs, business development and scientific communications, most recently as US Director of Oncology Scientific Communications at Bristol Myers Squibb. Advaxis Management Team 4 4

Advaxis Overview • Advaxis is headquartered in Princeton, NJ • Dr. Yvonne Paterson developed technology at the University of Pennsylvania in the early 1990s and licensed exclusively to Advaxis in 2002 • Advaxis proprietary platform: 2 key elements: – Live attenuated Listeria monocytogenes ( Lm ): • Attenuation is 10,000 to 100,000 times to enhance safety • Stimulates a balanced and comprehensive cellular immune response to Lm • PAMP driven response drives a more complex immune reaction • Lm is further engineered to manufacture and secrete antigen/t - LLO fusions – Antigen proteins fused to truncated LLO (tLLO) and manufactured/secreted by Lm • tLLO drives its own PAMP response • Overcomes tumor protection mechanisms inside the tumor - NOT systemically • Current constructs target HPV - associated cancers and dysplasia, prostate cancer, HER2 over expressing cancers, with several other antigens in R&D 5

5 Ongoing Studies with ADXS - HPV – Phase 1 refractory cervical cancer (15 patients)* – Phase 2 refractory cervical cancer (110 patients)** – Phase 2 refractory cervical cancer (67 patients) – Phase 2 cervical neoplastic dysplasia (CIN) 2/3 (120 patients)** – Phase 1/2 head and neck cancer (27 patients) – Phase 1/2 anal cancer (25 patients) *results published, ** preliminary data reported 6

Advaxis Clinical Pipeline Clinical Pipeline Construct Indication Pre Phase 1 Phase 2 ADXS - HPV Cervical Cancer, India ADXS - HPV Cervical Cancer, US, GOG ADXS - HPV CIN 2/3, US ADXS - HPV Head & Neck Cancer, CRUK ADXS - HPV Anal Cancer, BrUOG ADXS - PSA Prostate Cancer ADXS - cHER2 Canine Osteosarcoma, Penn 7
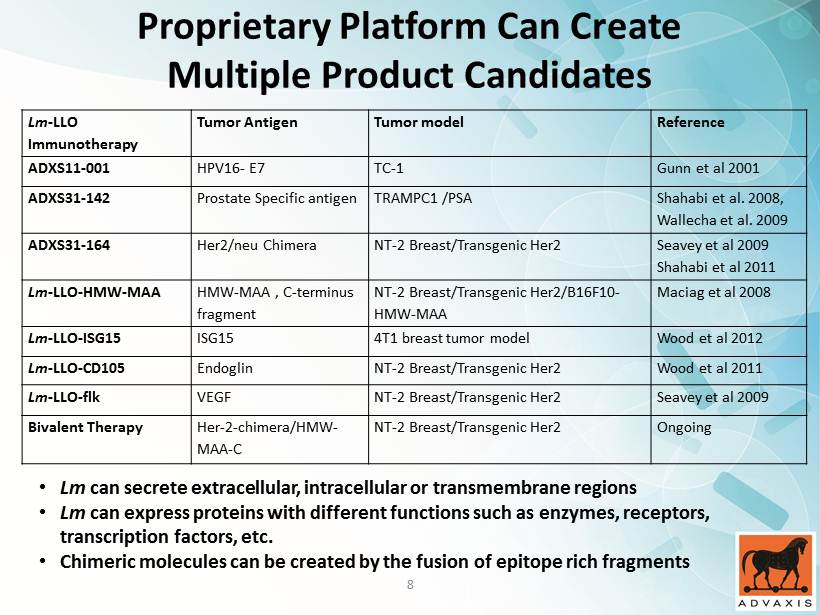
Proprietary Platform Can Create Multiple Product Candidates Lm - LLO Immunotherapy Tumor Antigen Tumor model Reference ADXS11 - 001 HPV16 - E7 TC - 1 Gunn et al 2001 ADXS31 - 142 Prostate Specific antigen TRAMPC1 /PSA Shahabi et al. 2008, Wallecha et al. 2009 ADXS31 - 164 Her2/neu Chimera NT - 2 Breast/Transgenic Her2 Seavey et al 2009 Shahabi et al 2011 Lm - LLO - HMW - MAA HMW - MAA , C - terminus fragment NT - 2 Breast/Transgenic Her2/B16F10 - HMW - MAA Maciag et al 2008 Lm - LLO - ISG15 ISG15 4T1 breast tumor model Wood et al 2012 Lm - LLO - CD105 Endoglin NT - 2 Breast/Transgenic Her2 Wood et al 2011 Lm - LLO - flk VEGF NT - 2 Breast/Transgenic Her2 Seavey et al 2009 Bivalent Therapy Her - 2 - chimera/HMW - MAA - C NT - 2 Breast/Transgenic Her2 Ongoing • Lm can secrete extracellular, intracellular or transmembrane regions • Lm can express proteins with different functions such as enzymes, receptors, transcription factors, etc. • Chimeric molecules can be created by the fusion of epitope rich fragments 8

9

2012 Achievements • Secured access to $10M committed equity line • Eliminated ~ 80% of debt • Achieved proof of concept for ADXS - HPV • Bolstered existing management team 10 10

Overarching 2013 Objectives 1. Continue to advance lead ADXS - HPV toward registration development program 2. Continue to significantly strengthen financial position 11 11

Daniel J. O ’ Connor Senior Vice President Chief Business Development and Legal Officer 12 12

13 Phrma.org 2012 Report Medicines in Development for Cancer Source http://www.phrma.org/sites/default/files/1000/phrmamedicinesindevelopmentcancer2012.pdf 13
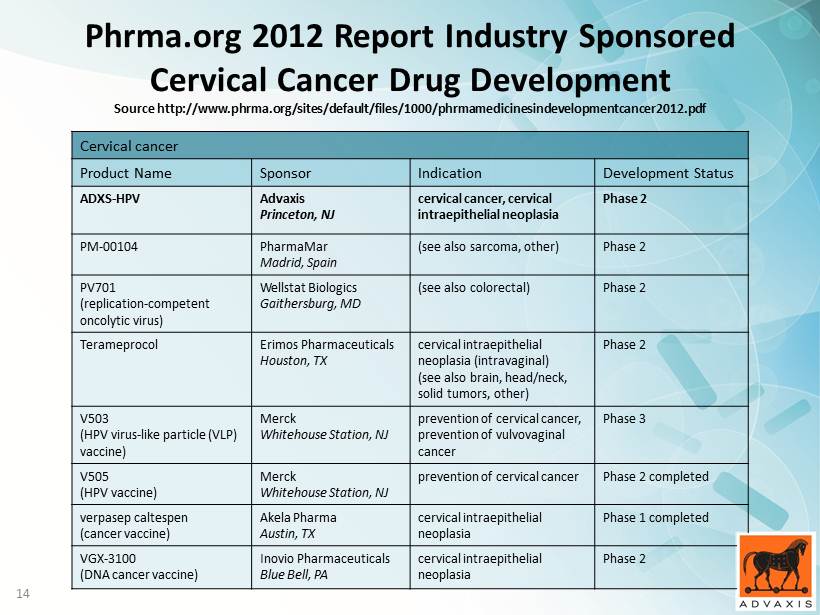
14 Cervical cancer Product Name Sponsor Indication Development Status ADXS - HPV Advaxis Princeton, NJ cervical cancer, cervical intraepithelial neoplasia Phase 2 PM - 00104 PharmaMar Madrid, Spain (see also sarcoma, other) Phase 2 PV701 (replication - competent oncolytic virus) Wellstat Biologics Gaithersburg, MD (see also colorectal) Phase 2 Terameprocol Erimos Pharmaceuticals Houston, TX cervical intraepithelial neoplasia ( intravaginal ) (see also brain, head/neck, solid tumors, other) Phase 2 V503 (HPV virus - like particle (VLP) vaccine) Merck Whitehouse Station, NJ prevention of cervical cancer, prevention of vulvovaginal cancer Phase 3 V505 (HPV vaccine) Merck Whitehouse Station, NJ prevention of cervical cancer Phase 2 completed verpasep caltespen (cancer vaccine) Akela Pharma Austin, TX cervical intraepithelial neoplasia Phase 1 completed VGX - 3100 (DNA cancer vaccine) Inovio Pharmaceuticals Blue Bell, PA cervical intraepithelial neoplasia Phase 2 Phrma.org 2012 Report Industry Sponsored Cervical Cancer Drug Development Source http://www.phrma.org/sites/default/files/1000/phrmamedicinesindevelopmentcancer2012.pdf

Growth in HPV mediated cancers… drives even wider potential for ADXS - HPV ~527,000 Incidence per year Worldwide (12,000 US) 15
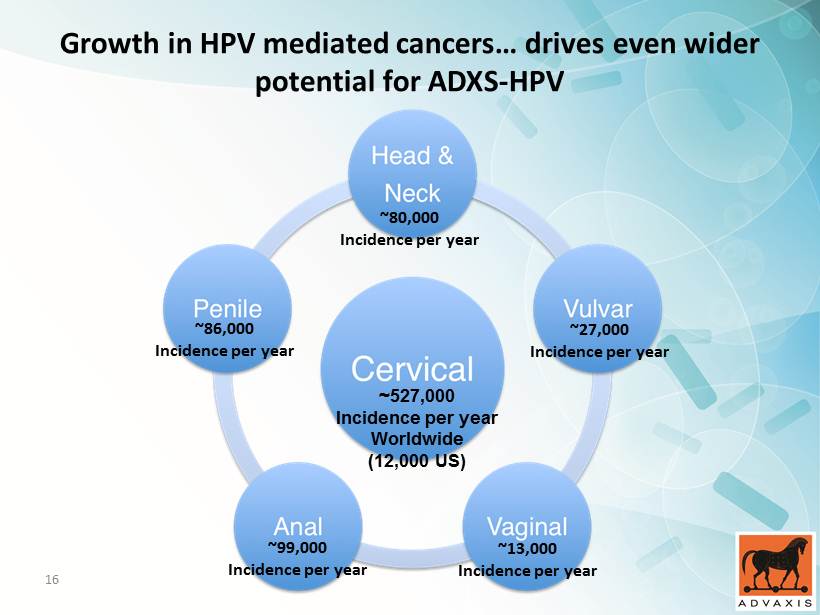
Growth in HPV mediated cancers… drives even wider potential for ADXS - HPV ~80,000 Incidence per year ~86,000 Incidence per year ~27,000 Incidence per year ~13,000 Incidence per year ~99,000 Incidence per year ~527,000 Incidence per year Worldwide (12,000 US) 16

Robert Petit, Ph.D. VP Clinical Operations & Medical Affairs 17 17

Disease burden is concentrated: ~50% of cervical cancer burden exists in India, China & South America 18

Cervical Cancer is a Worldwide Problem • According to WHO, ~ 630 million people are infected with one of the over 100 strains of HPV • Global prevalence of clinically pre - malignant HPV infections to be between 28 to 40 million women • Approximately 500,000 newly diagnosed cases of cervical cancer • Cervical cancer ranks as the 2 nd leading cause of cancer death of women in the world • A bout 11.4% of women in the general population are estimated to harbor cervical HPV infection 19

ADXS - HPV Lead Indication: Phase 2 Cervical Cancer Study +/ - Cisplatin Study Design Recurrent/ Refractory Cervical (N = 110) Arm A N = 55 ADXS Only Arm B N = 55 ADXS+Cisplatin Treatment Follow - up Phase Scans ADXS ADXS ADXS ADXS C C C C ADXS ADXS C ADXS 3m 6m 9m 12m R 3m 6m 9m 12m 18m 18m // // * * Low dose cisplatin: 40mg/m 2 20

6 mo. 9 mo. 12 mo. 18 mo. # Alive/N 71/109 39/88 23/70 6/36 % Survival 65% 44% 33% 17% Preliminary Phase 2 Data Show Encouraging Survival Compared to Historical Controls ADXS - HPV Preliminary Landmark Survival Data (as of October 22, 2012) *NCCN Guidelines: Plaxe SC, et. al., 2002, Cancer Chemother Pharmacol; 50: 151 - 4. Garcia AA, et. al., 2007, Am J Clin Oncol; 30: 428 - 431. Published Phase 2 single agent trials report 12 months survival of 0 - 22% * 21

Proof of Concept Achieved • ADXS - HPV is emerging as an active agent in recurrent/refractory cervical cancer with a predictable safety profile • Apparent prolonged survival, durable complete and partial tumor reductions, as well as stabilization of disease have been observed with ADXS - HPV treatment • ADXS - HPV can be safely combined with chemotherapy 22 22

2013 Clinical and Regulatory Objectives • Analyze Phase 2 data and work to optimize dose and schedule • Proceed with clinical plan with the advice and collaboration of global thought leaders in cervical cancer • Finalize clinical plan and consult with regulatory authorities to advance ADXS - HPV to registrational trials 23 23

Mark J. Rosenblum Senior Vice President Chief Financial Officer 24 24

Financial Overview Exchange / Ticker OTC BB: ADXS Cash Raised Since Inception - approximately $40M Committed Equity Financing Facility $10M Monthly Cash Spend (including clinical) $600K Debt non - affiliate at Y/E 2012 Down from $9M Year Ago <$2.0M Common Stock Outstanding 515.1M Warrants 100M Options 44.8M Market Capitalization – March 5, 2013 $55M Avg. Daily Volume (3 months) 5.1M Public Float 495M 25

Early Q2 2013 Q2 2013 H2 2013 H1 2014 • Announce CIN 2/3 mid - dose Cohort 2 data • Announce 12 - month survival data from India study • Announce final Phase 2 cervical cancer results • Initiate the Phase 2 High - dose Cohort 3 CIN 2/3 • File IND with the FDA for ADXS - PSA 26 By end of 2013 • Complete the safety portion of the ADXS - cHER2 Phase 1/2 canine study • Report preliminary data from canine study Clinical Milestones 2013

Thank You Advaxis Inc. (OTC BB: ADXS) 305 College Road East Princeton, NJ 08540 609.452.9813 ir@advaxis.com www.advaxis.com
