Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Cellular Biomedicine Group, Inc. | ebig_8k.htm |

Is Cell Therapy in China an
Opportunity?
Opportunity?
1
William Cao, PhD, BM
Member, Expert Committee of Regenerative Medicine and Cell Therapy Clinical Application,
Chinese Medical Doctor Association (CMDA)
President, COO, Cellular Biomedicine Group Inc. (CBMG)
2/19/13
The 8th Annual Stem Cell Summit
New York
February 19, 2013
1

2/19/13
2
William Cao, PhD, BM
President, COO, Cellular Biomedicine Group Inc.
(CBMG)
(CBMG)
Member, Expert Committee of Regenerative
Medicine and Cell Therapy Clinical Application,
Medicine and Cell Therapy Clinical Application,
Chinese Medical Doctor Association (CMDA)
Chinese Medical Doctor Association (CMDA) Invitation letter electing Dr Cao as member of CMDA
2

3
1. China’s tremendous medical market
2. Government is very supportive
3. Regulatory maturation and outlook
4. What should we do?
To Discuss
2/19/13
3

China’s Tremendous
Medical Market
Medical Market
2/19/13
4
4
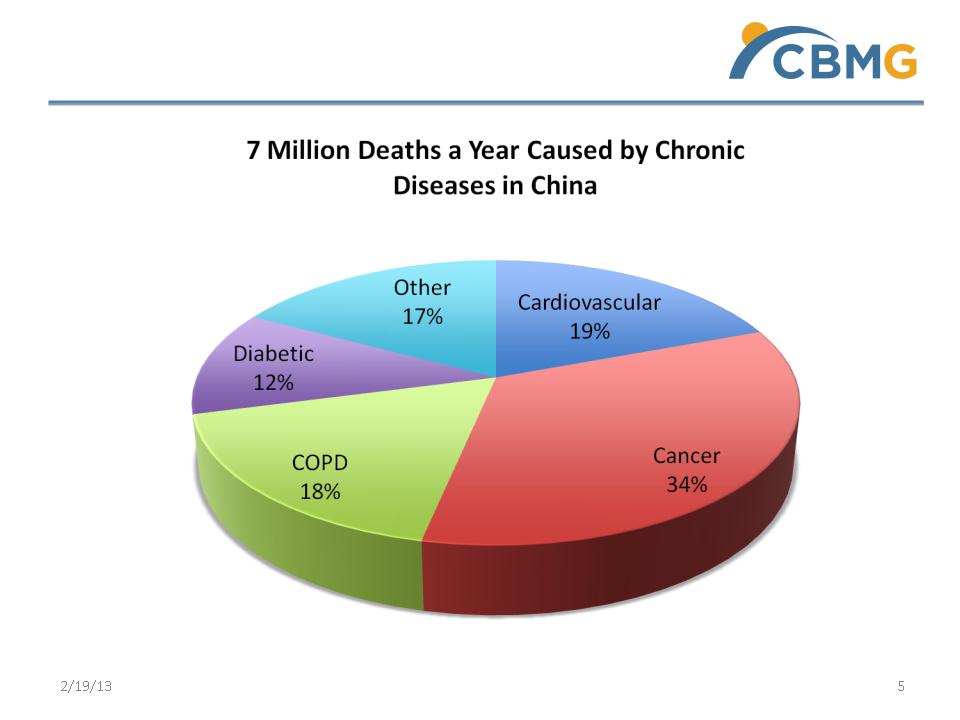
Demand for new therapies
5
2/19/13
6
|
REGULATION
|
Autologous cell therapies are regulated as medical technology
|
|
DUPLICATION
|
Technology that has already passed Phase II Clinical Trials with
US FDA has the potential to be granted the same status in China with relative ease |
|
FASTER PATIENT
RECRUITMENT
|
China has much larger patient base
|
|
BETTER PATIENT
COMPLIANCE
|
Chinese patients typically more compliant with trials. Clinical
Trial SOPs equivalent to USA
|
|
SHORTER CLINICAL
PATHS |
Shortened clinical pathways exist in China for autologous cell-
based therapies, thus greatly reducing the risk of time-to- money. |
|
HIGH CAPITAL
EFFICIENCY |
Demonstrated very high capital efficiency in operations - a
fraction of the cost and 3 times translation speed in comparison to similar trials in US, enabling multiple trials with limited capital. |
The China Advantage
6

Stem Cell Technology is
One of the Key Tasks of
China’s National Science & Technology
Development Long Term Plan
One of the Key Tasks of
China’s National Science & Technology
Development Long Term Plan
2/19/13
7
7

2009-2-19 - Health Reform Speech by Chen Zhu, China’s Health Minister,
2009 National Medical Affairs work conference
2009 National Medical Affairs work conference
http://www.gov.cn/gzdt/2009-05/19/content_1319064.htm
Government Support
“Need to implement new
medical technology
classifications for….stem cell
transplants…”
medical technology
classifications for….stem cell
transplants…”
2/19/13
8
8
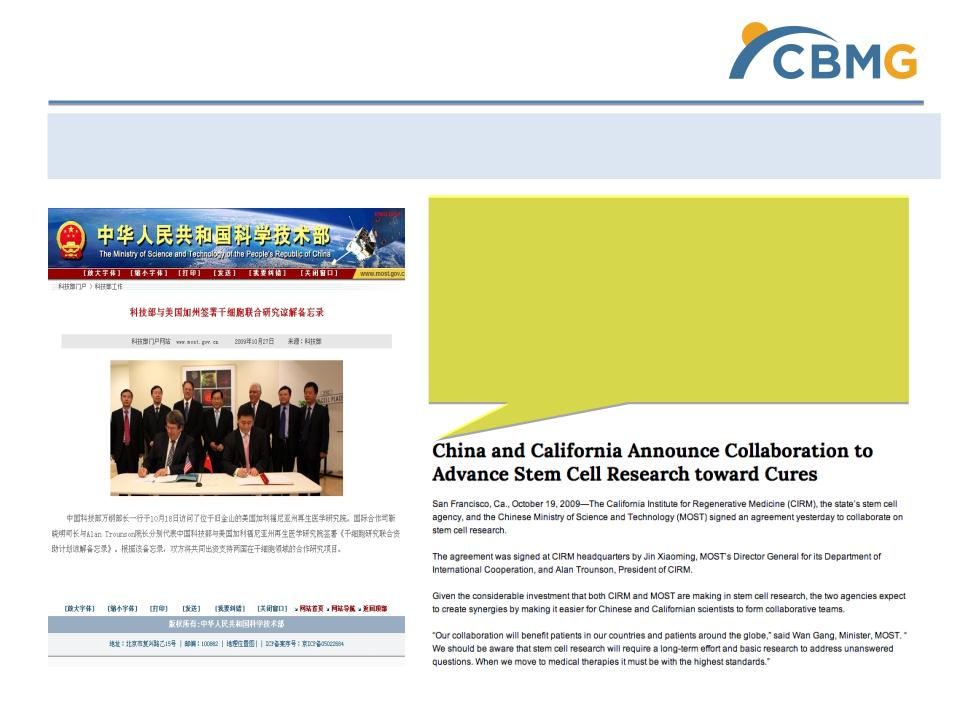
2009-10-18 - China’s Ministry of Science and Technology signs MOU with
CIRM for joint research of stem cells
CIRM for joint research of stem cells
Government Support
http://www.cirm.ca.gov/about-cirm/newsroom/press-releases/10192009/china-and-california-announce-collaboration-advance-stem
“China has made a major commitment to
biomedical research, and stem cell
research in particular…China will now join
California in accelerating critical stem cell
research to relieve the suffering of patients
and families throughout the world.”
biomedical research, and stem cell
research in particular…China will now join
California in accelerating critical stem cell
research to relieve the suffering of patients
and families throughout the world.”
2/19/13
9
9

2009-11-17 - Sino-US
Joint Statement
Government Support
Sino-US joint research in
healthcare area, including stem
cells …
healthcare area, including stem
cells …
2/19/13
10
10

As one of the national strategic objectives, Stem Cell Technology was
included in the “National Long-Term Scientific and Technological
Development Plan”(2006-2020)
included in the “National Long-Term Scientific and Technological
Development Plan”(2006-2020)
Government Support
In the next 15 years
Science and Technology
Development Plan
Outline, tissue engineering
based on stem cell
technologies is listed as
frontier technology
Science and Technology
Development Plan
Outline, tissue engineering
based on stem cell
technologies is listed as
frontier technology
2/19/13
11
11

Government Regulation
2009-06-11 - MOH announces the
directory of third-class medical
technology allowed in clinical
application
directory of third-class medical
technology allowed in clinical
application
2/19/13
12
Stem cell therapy is
listed as the 3rd
Medical Technology
listed as the 3rd
Medical Technology
12

2009-10-22 - The meeting of the
third-class medical technology
clinical application
third-class medical technology
clinical application
Government Regulation
2010-07-08 - The meeting of the
third-class medical technology
clinical application
third-class medical technology
clinical application
2/19/13
13
CMDA is the 1st agency
to approve the 3rd
Medical Technologies
to approve the 3rd
Medical Technologies
13
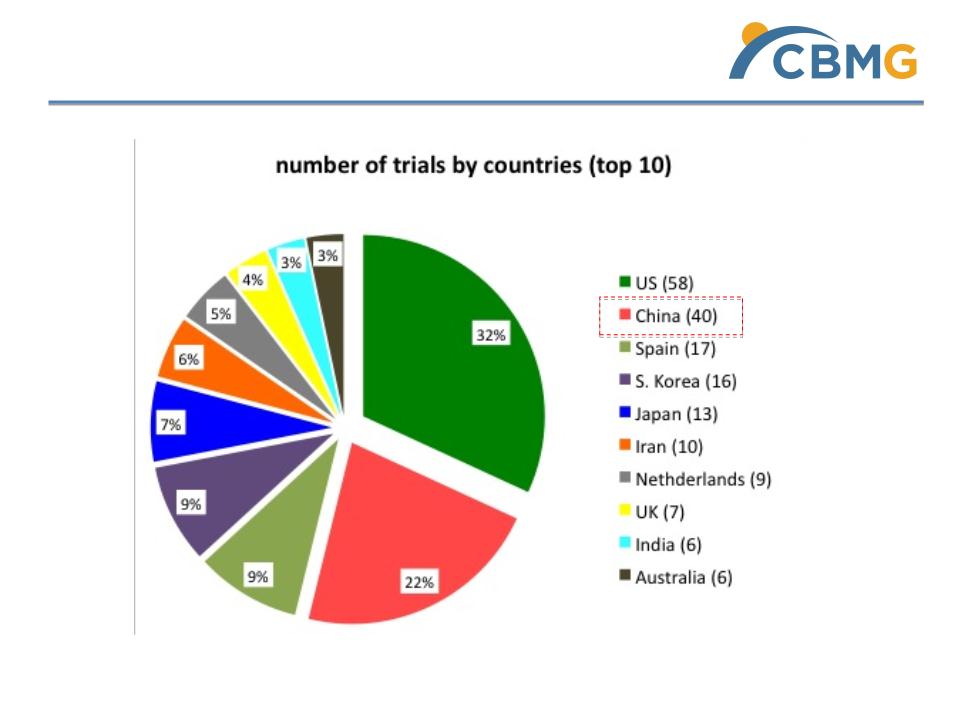
Countries distribution of tracked trials
*Unofficial data collected for Cell therapy clinical trials
2012 report by Alexey Bersenev
2012 report by Alexey Bersenev
2/19/13
14
14

New Regulation Outlook
- My Interpretation
- My Interpretation
2/19/13
15
15

Government Regulation
Good News! New Regulation on Stem Cell Therapies
2/19/13
16
16

2/19/13
17
Cell therapies are classified and regulated as
following:
following:
• Somatic cell therapies, autologous stem cell
therapies are classified as the 3rd Medical
Technology: IRB review, plus two phases trials,
safety and efficacy. Applied for somatic cell
therapies, autologous stem cell therapies.
therapies are classified as the 3rd Medical
Technology: IRB review, plus two phases trials,
safety and efficacy. Applied for somatic cell
therapies, autologous stem cell therapies.
• Allogeneic stem cells are classified as drugs:
much more stringent clinical trials, including
preclinical study, more stringent IRB review, 3
phases clinical trials.
much more stringent clinical trials, including
preclinical study, more stringent IRB review, 3
phases clinical trials.
New Regulation Outlook
17

New Regulation Outlook
Cell therapy technologies are likely to be
regulated according to 3 categories:
regulated according to 3 categories:
2/19/13
18
Regular cells
Autologous
stem cells
stem cells
Allogeneic
stem cells
stem cells
CMDA etc
MOH
SFDA
Med Tech
Med Tech
Drug
18

What Should We Do?
2/19/13
19
19

CBMG Business Model as Example
|
CBMG has built the first stage of a comprehensive platform that can support multiple
cell lines with multiple partners. |
|
Therapies developed fit China’s patient profile
|
|
We in-house develop, in-license technologies.
We establish JVs with partners.
We own trials and clinical protocols.
|
|
Multiple inflection points exist for each platform as therapies enter into clinical use
|
|
Autologous cell therapies are regulated as medical technology
|
|
Build satelite GMP laboratories in large strategic cities
|
|
Actively contribute in regulatory guidelines
|
20

21
CBMG Business Model:
Translational Platform in China
• Principal Investigators
• Expert Group
• Ethic Committee
• Hospital Authorities
• MOH, SFDA
• Regulators
• Pricing Bureau
• Insurance Co
• CROs
• External Labs
Clinical Trials
• Quality Standard
• Regional GMP labs
• Safety
• Cost
• Suppliers
• Auditors
R & D
Lab
Lab
Partner
A
A
JV A
Cell Lab
Cell Lab
Partner
C
C
Licensed
Cell Line
Partner
B
B
JV B
Cell Lab
Cell Lab
Partner
D
D
Licensed
Cell Line
Cell Products
& Therapies
Regional
Hospitals &
Patients
Hospitals &
Patients
21

22
THANK YOU
2/19/13
William.cao@cellbiomedgroup.com
22
