Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Bausch Health Companies Inc. | d479902d8k.htm |
 Valeant Pharmaceuticals International, Inc.
Lenders’
Presentation
February 8, 2013
Exhibit 99.1 |
 Forward-looking Statements
Forward-looking Statements
Certain statements made in this presentation may constitute forward-looking
statements, including, but not limited to, statements regarding preliminary
results and guidance with respect to expected revenues, non-GAAP cash earnings per share, adjusted cash flows
from operations, organic product sales growth, integration-related activities and
benefits, synergies, launches and approvals of products, and assumptions with
respect to 2013 guidance of Valeant Pharmaceuticals International, Inc. (the “Company”). Forward-
looking
statements
may
be
identified
by
the
use
of
the
words
“anticipates,”
“expects,”
“intends,”
“plans,”
“could,”
“should,”
“would,”
“may,”
“will,”
“believes,”
“estimates,”
“potential,”
or “continue”
and variations or similar expressions. These statements are based upon
the current expectations and beliefs of management and are subject to certain risks
and uncertainties that could cause actual results to differ materially from
those described in the forward-looking statements. These risks and uncertainties include, but are not limited to,
risks and uncertainties discussed in the company's most recent annual or quarterly
report filed with the Securities and Exchange Commission ("SEC") and
other risks and uncertainties detailed from time to time in the Company's filings with the SEC and the
Canadian Securities Administrators ("CSA"), which factors are incorporated
herein by reference. Readers are cautioned not to place undue reliance on any
of these forward-looking statements. The Company undertakes no obligation to update any of these forward-
looking statements to reflect events or circumstances after the date of this
presentation or to reflect actual outcomes. Non-GAAP Information
To
supplement
the
financial
measures
prepared
in
accordance
with
generally
accepted
accounting
principles
(GAAP),
the
Company
uses
non-GAAP financial measures that exclude certain items. Management uses
non-GAAP financial measures internally for strategic decision making,
forecasting future results and evaluating current performance. By disclosing non-GAAP financial measures,
management intends to provide investors with a meaningful, consistent comparison of
the Company’s core operating results and trends for the periods
presented. Non-GAAP financial measures are not prepared in accordance with GAAP; therefore, the information is not
necessarily comparable to other companies and should be considered as a supplement
to, not a substitute for, or superior to, the corresponding measures calculated
in accordance with GAAP. The Company has provided preliminary results and guidance with
respect to cash earnings per share, adjusted cash flows from operations and organic
product growth rates, which are non-GAAP financial measures. The Company
has not provided a reconciliation of these preliminary and forward-looking non-GAAP financial
measures due to the difficulty in forecasting and quantifying the exact amount of the
items excluded from the non-GAAP financial measures that will be included
in the comparable GAAP financial measures. Reconciliations of historical non-GAAP financials can be
found at www.valeant.com.
2
Note 1: The guidance in this presentation is only effective as of the date given,
February
7,
2013,
and
will
not
be
updated
or
affirmed
unless
and
until
the
Company
publicly announces updated or affirmed guidance. |
 Agenda
Executive Summary & Transaction Overview
Amendment Overview & Timeline
Business Update
2012 Achievements
Medicis Integration Update
2012 Year End Performance
Financial Guidance for 2013
Questions and Answers
3 |
 Transaction Overview |
 5
Executive Summary
1
Pro Forma revenue adjusted for full year impact of Medicis acquisition.
2
PF
LTM
12/31/12
EBITDA
reflects
Medicis
acquisition
addbacks
in
accordance
with
Amendment
No.
3
to
the
Credit
Agreement.
3
Calculated assuming the mid-point of FY 2012 EBITDA guidance and net of $250
million cash. Valeant
Pharmaceuticals
International,
Inc.
(“Valeant”
or
the
“Company”)
is
a
multinational specialty pharmaceutical company with a diverse global product
portfolio
2012 on-track to be another successful year for Valeant
2012
Full
Year
Revenue
reaffirmed
at
$3.3
-
$3.5
billion
(excluding
one-time
items)
2012
Full
Year
Adjusted
Cash
Flow
from
Operations
reaffirmed
at
$1.1
-
$1.2
billion
2012
Pro
Forma
Revenue
reaffirmed
at
~$4.3
billion
1
Valeant has continued to demonstrate strong financial performance
For the Fiscal Year ended December 31, 2012, pro forma for all announced
acquisitions,
the
Company’s
guidance
for
adjusted
EBITDA
2
is
in
the
range
of
$2,445 -
$2,495 million
Based on the mid-point of EBITDA guidance Valeant expects net secured
leverage
3
of
1.7x
and
net
total
leverage
3
of
4.3x,
including
synergies |
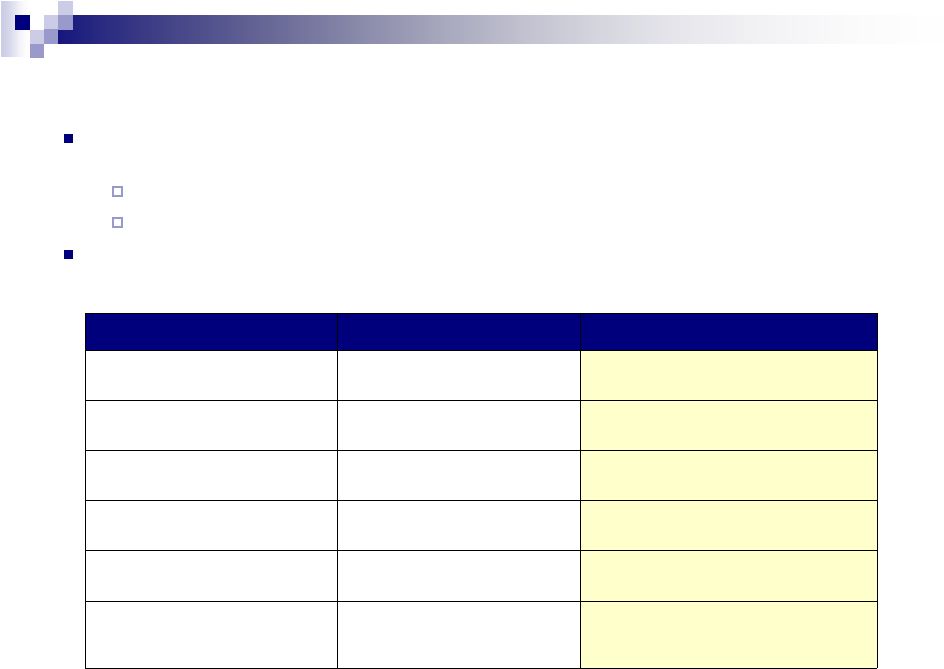 Amendment Overview
Existing
Proposed
Corporate Ratings
Ba3 / BB
Ba3 / BB
Credit Facility Ratings
Ba1 / BBB-
Ba1 / BBB-
Pricing (bps)
L + 325
L + 275
LIBOR floor
1.00%
0.75%
Soft call
101 through Oct. 2, 2013
101 for six months
Cash & equivalents deduction
for leverage covenants
Up to $150 million
Up to $250 million
6
The
Company
is
planning
to
take
advantage
of
the
attractive
debt
markets
to
execute an
amendment
to
reprice
the
Term
Loan
B
–
Series
C
and
Series
D
tranches
Valeant is seeking to reduce pricing to L+275 bps
The Company is also seeking to reduce the LIBOR floor to 0.75%
In conjunction with the repricing, Valeant will be seeking an amendment to
increase the amount of cash that can be netted for the purposes of
calculating the leverage covenant |
 7
Capitalization
Pro forma for Amendment and Medicis Acquisition Close
($ in millions)
12/31/2012
Pro Forma
x LTM 12/31/12
Net debt
Pro Forma
Amount
Amount
PF EBITDA
x PF EBITDA
Coupon
2
LIBOR
Floor
Maturity
Cash
$ 921
$ 894
-
-
-
Revolver ($450 million)
-
-
L + 225
-
4/20/2016
Term Loan A
2,114
2,114
L + 225
-
4/20/2016
Term Loan B -
Series D
3
1,300
1,300
L + 275
0.75%
2/13/2019
Term Loan B -
Series C
4
1,000
1,000
L + 275
0.75%
12/11/2019
Total Secured Debt
$ 4,414
$ 4,414
1.8x
1.7x
6.500% Senior
Unsecured Notes
916
916
2016
6.750% Senior Unsecured Notes
500
500
2017
6.875% Senior Unsecured Notes
945
945
2018
7.000% Senior Unsecured Notes
690
690
2020
6.375% Senior Unsecured Notes
1,750
1,750
2020
6.375% Senior Unsecured Notes
500
500
2020
6.750% Senior Unsecured Notes
650
650
2021
7.250% Senior Unsecured Notes
550
550
2022
Total Debt
5
$ 10,915
$ 10,915
4.4x
4.3x
PF LTM 12/31/2012 EBITDA
1
$2,445
-
$2,495
1
PF LTM 12/31/12 EBITDA reflects Medicis acquisition addbacks in accordance with Amendment No. 3 to the
Credit Agreement. Leverage net of $250 million cash; Leverage multiple based on
mid-point of EBITDA guidance. 2
3
4
5
¹
Revolver and Term Loan A coupon at L+225 pro forma for the January 2013 re-pricing and based on
expected leverage as of 12/31/12.
Term Loan B – Series D issued on 10/02/12 in connection with the repricing of the then existing
Term Loan B series.
Term Loan B – Series C issued on 12/11/12 in connection with the Medicis acquisition close. Total debt does not
include Medicis convertible debt acquired at acquisition close.
1 |
 Summary Terms & Conditions
Senior Secured Term Loan B –
Series C/D
Terms
Senior Secured Term Loan B Facilities
Borrower
Valeant Pharmaceuticals International, Inc. (the “Borrower”)
Guarantors
Material direct and indirect wholly-owned subsidiaries of the Borrower subject
to customary exclusions (consistent with existing Senior Secured Credit
Facilities) Ranking
Senior secured (consistent with existing Senior Secured Credit Facilities)
Security
1st priority security interests in substantially all assets of the Borrowers and
the Guarantors as well as 100% of the capital stock of each material
foreign and domestic subsidiary of Borrower, limited to 65% of the capital stock of each first tier foreign subsidiary of
VPI or of any Guarantor that is a subsidiary of VPI, and all intercompany debt
subject to customary exceptions consistent with existing Senior Secured
Credit Facilities Maturity
Term Loan B –
Series D: February 13, 2019
Term Loan B –
Series C: December 11, 2019
Amount
Term Loan B –
Series D: $1,300 million
Term Loan B –
Series C: $1,000 million
Pricing
L + 275 bps
0.75% LIBOR floor
100.0 OID
Amortization Schedule
1% per annum with bullet at maturity
Call Protection
101 call protection on repricing for six months
Financial Covenants
Interest Coverage test of 3.0x (consistent with existing)
Incremental Facility
Negative Covenants
Mandatory Prepayments
Consistent with existing
8
1
Net of unrestricted and unencumbered cash not in excess of $250 million
1
1
1
Senior
Secured
Leverage
test
of
2.5x
(consistent
with
existing)
Senior
Secured
Leverage
test
of
2.5x
and
Total
Leverage
test
of
5.25x;
50bps
MFN
(consistent
with
existing)
Consistent with existing, including limitations on indebtedness, restricted payments, liens,
transactions with affiliates, asset sales, mergers and consolidations and other
fundamental changes and subsidiary dividends |
 Business Update |
 2012
Achievements Operations:
Revenue growth vs. 2011 of >$1B
1
and >40%
1
Overcame ~$200 M decline related to genericization of Cesamet, Cardizem CD, Ultram
ER, Wellbutrin XL
Cash EPS growth vs. 2011 of >50%
1
Organic growth of ~8% on a same store basis, ~10% Pro Forma
Business Development:
Completed over 25 acquisitions
Probiotica, Pedinol, OraPharma, Medicis, Gerot Lannach
Majority between 2-3 X sales
Established new growth platforms
Oral Health, Podiatry, Aesthetics , Russia, South East Asia/South Africa
Medicis Acquisition Closed in Mid-December
1
Excludes impact of one time items
Strengthened Senior Management Team
Marcelo
Noll
Barboza
–
President,
Valeant
Brazil
Jacques
Dessureault
-
President,
Valeant
Canada
Jason
Hanson
–
Company
Group
Chairman
Andrew
Howden
–
CEO
iNova
Vince
Ippolito
–
SVP,
GM
Aesthetics
Laizer
Kornwasser
–
Company
Group
Chairman
Pavel
Mirovsky
–
President,
Valeant
Europe
Steve
Sembler
–
SVP,
President
OraPharma
Justin
Smith
–
SVP,
GM
U.S.
Rx
Derm
10 |
 2012
Achievements (continued) R&D Productivity and
Product Launches: Filed multiple New Drug Applications
Efinaconazole -
Onychomycosis (Valeant)
Luliconazole -
Tinea Pedis (Medicis)
Xerese (in Canada) –
Herpes Labialis (Valeant)
Launched more than 300 branded generic products in Emerging Markets
Launched multiple patented/OTC products
Regederm in Brazil
Zyclara Pump (Medicis) and Potiga in U.S.
Sublinox and Lodalis in Canada
>25 OTC line extensions in U.S. and Canada (CeraVe, Bedoyecta)
Achieved several regulatory approvals
Dysport (Medicis) in Canada
Restylane-L (Medicis) in U.S.
Balance Sheet Management:
Repurchased 5.3 million common shares at average cost of ~$53 per share
Raised $4.55 billion in high yield notes and loans
11 |
 12
Medicis Integration Update
Valeant
closed
the
acquisition
on
December
11
th
with
the
concurrent
closing
and
funding
of
the
$1.0bn
Term
Loan
B
–
Series
C
Leadership team in place for nearly one month
Nearly all personnel decisions have been made and communicated
Sales incentive programs in place to ensure Q1 success
Approximately 350 reps (Dermatology, Aesthetics, Podiatry) in the field
Upsides from Medicis R&D Pipeline
2 scheduled product launches: Zyclara Pump launched Q3 2012, Dysport Canada to be
launched Q1 2013
2
late
stage
products:
Lulicanazole
filed
Q4
2012,
MetroGel
1.3%
Hydrogel
-
Bacterial
Vaginosis
(Medicis
–
to
be
filed
1H
2013)
Life cycle management opportunities
Synergies
We now expect synergies to exceed $275M on a run rate basis by end of 2013
Significant amount of synergies will not occur until back half of 2013 (ie.
R&D and Legal) Restructuring costs expected to be less than full year
run rate synergies with the majority incurred in Q4 2012
|
 2012
Full Year Performance Note : The
guidance
in
this
presentation
is
only
effective
as
of
the
date
given,
February
7,
2013,
and
will
not
be
updated
or
affirmed
unless and until the Company publicly announces updated or affirmed guidance.
2011
As
Reported
2011 w/o
One-time
Items
2012
Guidance
2012
Guidance w/o
One-time Items
Growth
$2.46 billion
$2.39 billion
$3.4 -
$3.6 billion
$3.3 -
$3.5 billion
~40% -
45%
$2.93
$2.63
$4.48
-
$4.53
$4.11 -
$4.16
~55% -
60%
Adjusted
Cash Flow
from
Operations
$925 million
$849 million
$1.2 -
$1.3 billion
$1.1
-
$1.2
billion
~30% -
40%
13
Cash EPS
Revenue |
 Previous Q4 Guidance Unchanged
Fourth Quarter 2012 Guidance
Revenue expected to be >$900 million
Cash EPS expected to be between $1.18 -
$1.23
Adjusted cash flows from operations expected to be between $330 -
$430
million
Medicis impact expected to be immaterial
14
Note : The
guidance
in
this
presentation
is
only
effective
as
of
the
date
given,
February
7,
2013,
and
will
not
be
updated
or
affirmed
unless and until the Company publicly announces updated or affirmed guidance.
|
 Financial Guidance for 2013*
2013
% over 2012
Revenue
$4.4 -
$4.8 billion
~35%
Cash EPS
$5.45 -
$5.75
~35%
Cash EPS Including
Royalty to Meda
$5.35 -
$5.65
~33%
Adjusted Cash Flow from
Operations
$1.5 -
$1.75 billion
~40%
* Excluding all potential acquisitions
15
Note
:
The
guidance
in
this
presentation
is
only
effective
as
of
the
date
given,
February
7,
2013,
and
will
not
be
updated
or
affirmed
unless and until the Company publicly announces updated or affirmed guidance.
|
 2013
Guidance Assumptions Exchange rates are based on current spot rates
Impact from generics to be >$100M in revenues vs. 2012
Retin-A Micro, BenzaClin, and Cesamet
No generic assumption included for Zovirax
~$40-$50M in revenue declines as a result of planned divestitures
Solodyn revenues of ~$250M -
$275M
Excluding all potential acquisitions
Efinaconazole launch to be breakeven in 2013
Cash EPS expected to be 45%/55% 1H vs. 2H
Q2 expected to be lowest quarter
Q4 expected to be highest quarter
Cash tax rate expected <5%
Leverage reduced to <4x Pro Forma EBITDA by the end of Q3
16 |
 Appendix |
 18
Pro Forma EBITDA Reconciliation
December 31, 2012 LTM EBITDA ($ millions)
Estimated Range
Consolidated Net Income
(a)
(140)
$
(190)
$
+ Consolidated Interest Expense, net
470
485
+ Provision (Benefit) for Income Taxes
(220)
$
(260)
$
+ Depreciation and Amortization
975
995
+ Restructuring and Integration Costs
(b)
325
350
+ Acquisition Related Costs
(c)
60
70
+ Acquisition In-Process Research and Development
190
200
+ Stock-based Compensation
50
65
+ Legal Settlements
50
60
+ Loss on Extinguishment of Debt
10
20
+ Write down of deferred financing costs
5
10
+ Other Non-Cash Charges
(d)
130
140
LTM 31-December-2012 Consolidated Adjusted EBITDA (per Credit Agreement)
1,905
$
1,945
$
+ EBITDA and Synergy Adjustments for Announced and Completed Acquisitions
540
550
LTM 31-December 30-2012 Pro Forma Consolidated Adjusted EBITDA (per Credit
Agreement) 2,445
$
2,495
$
(a) Consolidated Net Income as defined by the Third Amended and Restated Credit Guaranty
Agreement, reflects the adjustment to (i) exclude gains and losses on Asset sales and
(ii) net income or loss derived from Joint Ventures. (b) Medicis related restructuring
and integrations costs amount to $186M. This amount reflects $24M of Medicis costs recorded within Acquisition
costs on the GAAP income statement related to the Galderma settlement agreement.
In addition, restructuring costs as defined by the Third Amended and Restated Credit
Guaranty Agreement, shall not exceed $100M during a twelve month period, except for
costs related to Biovail-Valeant merger, Sanitas, Dermik and Ortho acquisitions. As a result of the cap, $37M is
excluded for the LTM period.
(c) Acquisition costs reflects a reclass of $24M from the GAAP income statement
to restructuring costs related to the Galderma settlement agreement ($24M).
(d) Includes impairment charges, contingent consideration fair value
adjustments, amortization of inventory step up , amortization of alliance product
assets & pp&e step up and other one-time items. |
