UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C.20549
FORM 10-K
(X) ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
For the Fiscal Year Ended September 30, 2008
OR
( ) TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
For the transition period from N/A to N/A
Commission File Number: 000-28745
Cloud Medical Doctor Software Corporation
(Formerly, National Scientific Corporation)
(Name of small business issuer as specified in its charter)
(Formerly National Scientific Corporation)
| Texas | 86-0837077 |
| State of Incorporation | IRS Employer Identification No. |
736 East Braeburn Drive
Phoenix, AZ 85022
(Address of principal executive offices)
(Former Address 8361 East Evans Road, Suite 106, Scottsdale, AZ 85260-3617)
(702) 818-9011
(Issuer’s telephone number)
(former telephone number (480) 948-8324)
Securities registered under Section 12(b) of the Exchange Act:
None
Securities registered under Section 12(g) of the Exchange Act:
Common Stock, $0.001 par value per share
(Title of Class)
Common Stock, $.001 Par Value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. [ ] Yes [x] No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. [x] Yes [ ] No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [ ] No [x]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [ ] No [x]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,”“accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | [ ] | Accelerated filer | [ ] |
| Non–Accelerated filer | [ ] | Small reporting company | [x] |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b–2 of the Exchange Act). Yes [ ] No [x]
Aggregate market value of the voting stock held by non-affiliates: $6,661,688 as based on the closing price of the stock on December 2, 2012. The voting stock held by non-affiliates on that date consisted of 166,542,212 shares of common stock.
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date. As of December 2, 2012, there were 211,891,212 shares of common stock, par value $0.01, issued and outstanding 4,000,000 shares of preferred stock, par value $0.10.
Documents Incorporated by Reference: None
THE COMPANY PREVIOUSLY HAD INSUFFICIENT WORKING CAPITAL TO PAY FOR THE PROFESSIONAL SERVICES REQUIRED TO PREPARE, AUDIT, AND FILE THE QUARTERLY AND ANNUAL REPORTS REQUIRED BY THE SECURITIES ACT OF 1934. AS A RESULT, THE AUDITORS WHO AUDITED THE SEPTEMBER 30, 2007 FINANCIAL STATEMENTS AND REVIEWED THE QUARTERLY REPORTS THROUGH JUNE 30, 2008 RESIGNED EFFECTIVE DECEMBER 13, 2011.
IN FEBRUARY 2010 THE BOARD OF DIRECTORS VOTED TO DISCONTINUE THE MOBILE DVR AND LOCATIONPRODUCTS LINE OF BUSINESS REPORTED IN THESE FINANCIAL STATEMENTS DUE TO THE CUMULATIVE EFFECTS OF SEVERELY DELCLINING REVENUES RESULTING FROM THE 2008 RECESSION. SINCE THIS DECISION WAS MADE SUBSEQUENT TO THE YEARS ENDED SEPTEMBER 30, 2008 AND 2009, THE QUARTERLY AND YEAR END STATEMENTS FOR THOSE YEARS ARE BEING REPORTED ON A GOING CONCERN BASIS RATHER THAN AS DISCONTINUED OPERATIONS. THEY WILL, HOWEVER, BE REPORTED AS DISCONTINUED OPERATIONS COMMENCING WITH THE FISCAL 2010 FILINGS.
NEW MANAGEMENT HAS SUBSEQUENTLY INFUSED SUFFICIENT WORKING CAPITAL TO BRING THE 1934 ACT FILINGS CURRENT. ADDITIONALLY, A NEW LINE OF BUSINESS HAS ALSO COMMENCED WHICH WILL BE REPORTED ON IN THE FISCAL 2011 AND 2012 FILINGS.
ACCORDINGLY, THIS FILING IS BEING SUBMITTED ONLY TO COMPLY WITH SEC RULES AND REGULATIONS AND SHOULD NOT BE RELIED UPON FOR YOUR INVESTMENT DECISIONS. THE OPERATIONS REPORTED ON IN THIS DOCUMENT HAVE BEEN DISCONTINUED AS OF FEBRUARY 4, 2010. NEW MANAGEMENT ENCOURAGES THE READERS OF THIS DOCUMENT TO SUSPEND ANY INVESTMENT DECISIONS PERTAINING TO THIS STOCK UNTIL THE FILINGS ARE BROUGHT CURRENT.
Cloud Medical Doctor Software Corporation
FORM 10-K ANNUAL REPORT
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2008 and 2007
| PART I | ||
| ITEM 1. | BUSINESS | 5 |
| ITEM 1A. | RISK FACTORS | 12 |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS | 17 |
| ITEM 2. | PROPERTIES | 17 |
| ITEM 3. | LEGAL PROCEEDINGS | 17 |
| ITEM 4. | REMOVED AND RESERVED | 17 |
| PART II | ||
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | 18 |
| ITEM 6. | SELECTED FINANCIAL DATA | 20 |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 20 |
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 23 |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | 23 |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 49 |
| ITEM 9A. | CONTROLS AND PROCEDURES | 50 |
| ITEM 9B. | OTHER INFORMATION | 51 |
| PART III | ||
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE | 52 |
| ITEM 11. | EXECUTIVE COMPENSATION | 54 |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | 56 |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 59 |
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES | 59 |
| PART IV | ||
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES | 60 |
| SIGNATURES | 61 | |
| Certifications | ||
| Exhibit 31 | Management Certification | 62 |
| Exhibit 32 | Sarbanes-Oxley Act | 63 |
Special Note Regarding Forward-Looking Statements
Some of our statements under "Business," "Properties," "Legal Proceedings," "Management's Discussion and Analysis of Financial Condition and Results of Operations,"" the Notes to Financial Statements and elsewhere in this report constitute "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These statements are subject to certain events, risks and uncertainties that may be outside our control. Some of these forward-looking statements include statements of:
| • | management's plans, objectives and budgets for its future operations and future economic performance; |
| • | capital budget and future capital requirements; |
| • | meeting future capital needs; |
| • | realization of any deferred tax assets; |
| • | the level of future expenditures; |
| • | impact of recent accounting pronouncements; |
| • | the outcome of regulatory and litigation matters; |
| • | the assumptions described in this report underlying such forward-looking statements; and |
| • | Actual results and developments may materially differ from those expressed in or implied by such statements due to a number of factors, including: |
| o | those described in the context of such forward-looking statements; | |
| o | future service costs; | |
| o | changes in our incentive plans; | |
| o | the markets of our domestic operations; | |
| o | the impact of competitive products and pricing; | |
| o | the political, social and economic climate in which we conduct operations; and | |
| o | the risk factors described in other documents and reports filed with the Securities and Exchange Commission. |
In some cases, forward-looking statements are identified by terminology such as "may," "will," "should," "could," "would," "expects," "plans," "intends," "anticipates," "believes," "estimates," "approximates," "predicts," "potential" or "continue" or the negative of such terms and other comparable terminology.
Although we believe that the expectations reflected in these forward-looking statements are reasonable, it cannot guarantee future results, levels of activity, performance or achievements. Moreover, neither we nor anyone else assumes responsibility for the accuracy and completeness of such statements and is under no duty to update any of the forward-looking statements after the date of this report.
General
The financial statements presented in this report are those of Cloud Medical Doctor Software Corporation, a Texas corporation. When the terms “Cloud, the Company,” “we,” “us” or “our” are used in this document, those terms refer to Cloud Medical Doctor Software Corporation.
Our Company
The Company was incorporated in Texas on June 22, 1953 as American Mortgage Company. On May 16, 1996, the Company changed its name to National Scientific Corporation. On April 3, 2012, the Company changed its name to Cloud Medical Doctor Software Corporation. During 1996, the Company acquired the operations of Eden Systems, Inc. (Eden) as a wholly owned subsidiary. Eden was engaged in water treatment and the retailing of cleaning products. Eden’s operations were sold on October 1, 1997. From September 30, 1997 through the year ended September 30, 2001, we aimed our efforts in the research and development of semiconductor proprietary technology and processes and in raising capital to fund its operations and research. Beginning in calendar 2002, we focused our efforts on the development, acquisition, enhancement and marketing of location device technologies. Our revenue is derived from sales of electronic devices, recognized as the product is delivered.
In February 4, 2010 the prior Board members Mr. Michael Grollman and Mr. Greg Szabo, decided to discontinue and wind down the operations of the Company's Mobile DVR and Location Products business and operate these remaining Company assets in a Limited Liability Company named NSC Labs, LLC controlled and owned by Mr. Grollman. Mr. Grollman and NSC Labs, LLC was to pay the Company 2% of revenues and $100,000. However, Mr. Grollman nor NSC Labs, LLC never paid the consideration for this transaction.
In November 19, 2010, the prior management was terminated and changed management and began working on operations related to the Company’s medical billing software.
Our Current Business of the Company – Cloud Doctor Medical Software
Cloud-MD Software Solutions introduces the Cloud-MD Office, a “Cloud Based”, 5010 and ICD-10 compliant, fully integrated and interoperable suite of medical software and services, designed by experienced healthcare analysts and programmers for healthcare providers, that produces “Actionable Information” to help Independent Physician Practices, New Care Delivery Models (ACO), Healthcare Systems and Billing Services optimize a wide range of business processes resulting in Increased Profits, Higher Quality, Greater Efficiency, Noticeable Cost Reductions and Better Patient Care. Current software product offerings include Practice Management, Electronic Medical Records, Revenue Management, Patient Financial Solutions, Medical and Pharmaceutical Supply Management, Claims Management and PHI Exchange.
Cloud-MD Billing Services provides: Management of Medical Claims from posting physician charges into our medical billing software to posting of payments; Continuous Insurance Claim Follow Up to track and research all rejected or denied medical claims; Comprehensive Reporting including monthly financial Statements sent to our clients so they can see how their practice is doing and a variety of detailed reports give our clients the important information and tools used to assist in the increased production which leads to more profit; Patient Account Inquiries & Support to assist patients with their billing and insurance questions.
Cloud-MD Medical and Pharmaceutical Supply Management Software Services provides: a ground breaking cloud-based Medical Supply Inventory Management System, specifically designed for small and medium Medical Practices, DME’s, Home Health, Long-term Care, and Surgery Centers that offers:
Centralized management control over your medical supply and drug inventory
Real time utilization and financial inventory summary
Low price notifications
Par level/reorder tracking
RX expiration tracking
Auto supply re-ordering
Based on a barcode scanning system, designed to reduce workloads by automating inventory control processes, the Cloud-MD® Inventory Management System requires no installation, on-site software or special hardware. The Cloud-MD® application suite is fully delivered via the Internet and requires no special computing environment at the end-user facility.
Cloud-MD Healthcare Systems Consulting Services specializes in the implementation of sustainable, comprehensive solutions that increase financial and operational performance in healthcare businesses. By partnering with clients, we deliver solutions across the healthcare enterprise that improve quality, increase revenue, reduce operational expenses and attract physicians, patients and employees. We bring resources, in-depth knowledge, and significantly deeper experience than most in the field.
Cloud-MD Acquisition Services provides medical supply acquisition services that are fully integrated with Cloud-MD Office’s Medical and Pharmaceutical Inventory Management software and offers a full range of medical, surgical, and laboratory supply products and equipment for medical offices and surgery centers. Cloud-MD Acquisition Services easy-to-use and seamless process makes supply purchasing quicker and more cost efficient.
Item 1. Description of Business
Discontinued Operations of the Business up through February 4, 2010
Mobile DVR and Location Products
Most of our customers require monitoring and or tracking of a person or object, and reporting this information back to a central location.
Our current MDVR technology has not been awarded any patents as of the date of this report and has been awarded trademark protection on one other. We use a combination of confidentiality agreements, copyright and other trade secret management techniques to protect our trade secrets.
Location Products
Overview of This Technology
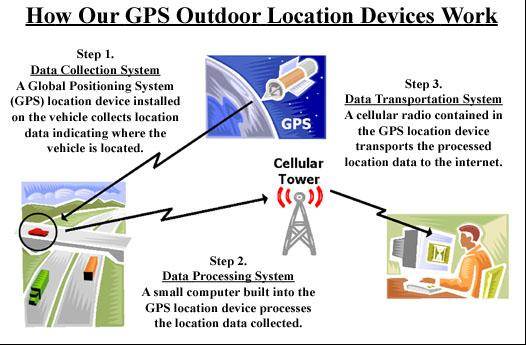
We have developed a group of mobile products with the capability to record high quality digital video, determine location and identify individuals and concatenate this information into a homogeneous information stream that can be easily displayed on a remote computer. We use a technology called Global Positioning System or GPS to determine location. These products report the location information as well as other desired information back to the user through a radio network. The products typically contain a small computer to provide the overall control and data processing capacity for the device. The products can be thought of as having three distinct pieces or systems. These are the data collection system, the data control & processing system, and the data transport system. This design concept is the basis for our Mobile DVR products. The diagram below shows in a general way how the system works with our products:
Data Collection System
This system can be thought of as the “eyes and ears” of the product. It is comprised of two systems. One determines location of the product, while the other system records specific events that the customer may be interested in, such as a door opening on a school bus or the speed of the bus at a certain location. The location system determines the position of the product using a technology developed by the U.S. Government called the Global Positioning System, or GPS, as it is commonly known. The U.S. Government developed the system which consists of approximately 24 satellites that orbit the earth rapidly. It is a worldwide navigation support system that allows users of GPS receivers to determine their precise geographic locations to within a few meters in most cases. The network of satellites and their ground control and monitoring stations are maintained and operated by the United States Department of Defense, which maintains an ongoing satellite replenishment program to ensure continuous global system coverage. Access to the system for all users is currently provided free of charge by the U.S. Government.
GPS works by ranging and triangulating the product’s position from a group of satellites. Of the 24 GPS satellites in orbit, a minimum of four is needed to reliably determine the product’s three-dimensional position. A GPS receiver measures distance by calculating the amount of time it takes a navigation and time reference radio signal from the satellite to make a one-way trip to the GPS receiver.
GPS receivers typically are very compact; and it is not necessary to have a large dish antenna to receive GPS signals. Typical information that can be obtained from these GPS signals are latitude, longitude, elevation, speed, direction, date, and time.
The second part of this Data Collection System is customer specific. Many customers have additional types of data that they want to know or want collected relevant to a location. An example of this is, every time a child enters or leaves a school bus or the time and location that the bus stops. This data input system can therefore be customized to meet the exact needs of a customer.
Another kind of data collection technology we use in some of our products is called RFID, which stands for Radio Frequency Identification. RFID devices are small radios that could be used to track information about people or objects. RFID provides a low-cost solution for certain kinds of tracking activities, especially short-range activities, typically less than a hundred feet. RFID devices are primarily used to determine if the object is close to a given location.
Another kind of data collection technology we use in some of our products is called MDVR, which stands for mobile digital video recorder. MDVR devices use a small digital camera that takes up to 30 pictures per second and then stores these images electronically. When these individual pictures are ‘played back’, you see a movie of the recorded event. Schools are particularly interested in this type of technology in conjunction with GPS to monitor what is happening on their buses.
Data Control & Processing System
This system can be thought of as the “brains” of the product. A technical term for this system is an embedded system, meaning one that lives deep inside the overall product. An embedded system is a small special-purpose computer system built into a larger device. The reason we use an embedded system in our products is to keep cost to us low, so our products stay more competitive in price. Simple embedded systems can cost us as little as a few dollars each and use very little power compared to the desktop computers that many people are familiar with, which usually cost much more. On our embedded systems there is typically no disk drive, operating system, keyboard, or screen. Our embedded systems instead communicate with other computers by radio. These other computers typically have a keyboard and screens, and they are used to display our information.
The programs that we run on these systems are custom designed and built by our software engineers. These programs are called firmware. The firmware controls how the data is collected, what data should be collected and what events should be monitored and reported, what should be ignored, and how, when and what data should be sent back to the user. As mentioned above, the system is relatively easy to customize, and the firmware is also easy to customize as it has been written in a modular fashion that allows changes to one section to be implemented without the need to completely re-write the program. This also helps us keep costs down.
Data Transport System
This system can be thought of as the “mouth” of the product. It communicates to the outside world where it is and what has happened. There are many different types of technology that can be used to transport this data back to the user, generally using some kind of wireless technology based on radios. We currently use cellular radios, satellite radios, Wi-Fi radios, and other special purpose radios.
We use a cellular radio based on GSM cellular technology. GSM, or Global System for Mobile Communications, is a second-generation digital mobile telephone standard. GSM was initially developed as pan-European collaboration, intended to enable mobile roaming between member countries.
The cellular radios typically operate in “real time.” When an event occurs, the data is immediately transported back to a user at a remote location.
While cellular coverage and reception is good in urban areas, it is less effective in rural areas and is non-existent in most wilderness areas. Sometimes our products are used in areas where there is poor or no cellular coverage. To overcome this we sometimes use a special radio that communicates with satellites orbiting the earth. This form of communication has the advantage that our products can be used in very remote areas almost anywhere in the world. The major downside is that these radios are large and expensive and the airtime usage costs can be high. Another problem associated with this technology is that, like GPS, these satellite radios work best when there is a clear line of site to the satellites; as such they may not work well indoors, or under dense foliage or in deep valleys. The satellite radios typically operate in “real time.” When an event occurs, the data is immediately transported back to the user. Some of our customers do not want to have the expense of a real time cellular or satellite connection, nor are they interested in having the data in real time. For this we use either a special purpose radio or a Wi-Fi radio.
The Wi-Fi radio operates in a very similar manner to cordless phones found in many households. These phones typically consist of a base station and handset. Our system is very similar; it consists of a base station unit that receives data from the mobile unit that would be on the asset or vehicle being tracked. The base station is typically attached to a personal computer that takes the raw data from the radio and re-formats it into information that can be displayed by other computers on the Internet. Wi-Fi stands for “Wireless Fidelity” and is a technology in very common use to wirelessly connect personal computers to other computer networks, including the Internet.
Our special purpose radios work in a very similar manner to the Wi-Fi radio, except that they can sometimes transmit data over longer distances.
Wi-Fi radios operate in an unlicensed part of the radio spectrum and as such do not have any special government licensing fees associated with them. Because they operate in a license-free spectrum, the Federal Communications Commission (FCC) imposes some restrictions on the use of these radios. One major restriction is that the radio signal range is limited to about 300 feet.
Since most of the time our product operates well beyond 300 feet from the base station, all the collected data is stored within the device for later transmission when the product comes back into that range again. When the vehicle or asset comes back into base-station range, the mobile units automatically download their information. We call this mode of operation “near time.” These “near time” products do not incur any special airtime usage charges. As such, they can be significantly cheaper to operate than the cellular or satellite equivalents.
Once the data is transmitted back to the user they can either display the information on our software or on some other computer application.
Mobile DVR Customers
Our ideal customers for Travado IBUS™ are school districts that have fleet in excess of 30 buses, where they can gain significant savings in time and effort in maintaining their video recording equipment and tape libraries. We reach these potential customers through a variety of avenues mentioned above. We also have had interest in this product from both the military and homeland security who are both interested in a more rugged version of our product.
Location Tools Products - Government Regulation
Since our location-based products are based on the use, in most cases, of radio technology, we are required to comply with a variety of Federal, State, and local regulations regarding the use of radio devices. The primary set of regulations that concerns our products are those promulgated by the Federal Communication Commission, or FCC, which is tasked with managing the use of the radio spectrum in the United States. We have two strategies for compliance with these regulations. First, we have certain products, such as our Gotcha! ® child safety product, which we have independently tested and certified for compliance by the FCC or their approved third party laboratories. In the case of Gotcha!®, we achieved this certification, called a Grant of Equipment Authorization, on July 26, 2003, and we were issued FCC identifier number Q79-703 as a result. This process typically takes several months and creates testing costs of between $5,000 and $10,000 per device certification. In other cases, we elect to purchase complete and certified radio systems to incorporate into our products that have already achieved this certification. This is the case with our Travado IBUS™ product, which uses 2.4GHz Wi-Fi radios manufactured by a supplier of ours that has already achieved this certification. Using this second approach typically accelerates time to market over the first approach outlined, but may add to the cost of manufacturing the product over the long term.
In November 2004, we received radio frequency use approval from the government of Canada for our Gotcha! ® product, known as “CA” approval, IC: 5521A-P and IC: 5522A-C for the parent unit and child unit, respectively. This is one of the steps required to sell products like Gotcha! ® in Canada.
For markets outside the United States, we may be required to comply with other government regulations regarding the use of radios. For example, the “CE” certification, formally called the “European Union EMC” program, has similarities in part to the FCC certification program in the United States. While we design our products to meet this and other important international regulations, we have not formally applied for the “European Union EMC” certification on any of our products, but may choose to do so in the future, when we believe we have more time to devote to developing sales channels in those international markets. We may also be required to comply with government regulations regarding the export of certain kinds of technology. To date, we have no plans to export our technology to areas where such special U.S. government export restrictions might be in force, such as to Cuba.
An important additional aspect of FCC government regulation that affects our location tools products is the management of the cellular airwaves. In particular, the FCC mandate entitled E911-Phase II puts a burden on cellular network operators to provide position information of cellular phone users to within approximately 100 feet of accuracy in the very near future. Some cellular network operators can provide this today. We believe that some of our products based on cellular devices and GPS can in the future be manufactured without the GPS unit, while still being able to accurately determine location. This may allow us to penetrate new markets by lowering the cost and size of some of our products.
Location Tools - Patents and Trademarks
We were granted a United States trademark on our Gotcha! ® location tool for child safety applications in May of 2004.
Location Tools Products and Services Competition
The market for communications and information products related to location-based services is highly competitive and we expect competition to increase in the general marketplace. We believe that the principal competitive factors that will differentiate the various competitors in this marketplace will be product features, quality, customer service, brand, advertising, price positioning, time-to-market, and availability. The market for GPS-based products is relatively recent as a direct result of the U.S. Government’s removal of the GPS based filters in May 2000 to allow for more accurate tracking. Many of the companies in the vehicle tracking market have expensive systems that are permanently installed into the vehicle and often involve a fee-based monthly subscription service. Most of our products are removable, and some can be used without incurring any monthly airtime charges. Many GPS device vendors have been offering products to the marine, aviation, and outdoor enthusiast markets for several years. We do not generally compete in those markets. For the personal safety locating products markets, we consider our principal competitors in the consumer market to be Wherify, Digital Angel, Angel Alert, and Child Guard. For our commercial vehicle and asset tracking markets, our principal competitors include @Road, Axiom Navigation, ALK, and Thales Navigation. There are approximately 100 other smaller companies offering similar products and services that we presently offer. There is significant other competition from large competitors in the GPS/Navigation market, including General Motors-OnStar® system. We have no current plans to directly compete with major automotive suppliers for in-vehicle navigation solutions, due to the amount of resources required to successfully compete.
Regarding the School Bus market there are three distinct segments in which we operate. These are Bus Tracking, Video Recording, and Student Tracking.
In the Bus Tracking segment there are currently 13 companies offering GPS based school bus tracking and another 22 dabbling in the periphery of tracking systems, such as route and fleet optimization. The most notable competitors in this segment are @Roads, Every Day Wireless, and Honeywell Video Systems. Most of these systems currently require the user to go to the bus and plug in a computer drive of some kind in order to download the data; others only display the GPS co-ordinates and speed on a tape or DVR recording. Our system allows users to automatically download and map the route as well as displaying speed and location on a DVR recording.
In the Video Recording segment there are at least 22 competitors all offering a conventional tape recorder style product. When you look at those companies offering DVR the number drops to 12. These products all require the ‘video technician’ to go out to the bus to collect the hard drive and then download the contents at a special workstation. With our product users do not need to go out to the bus to get video. A user can be at a different location and request and get the desired video. Notable competitors in this segment are Honeywell Video Systems, Gatekeeper, REI, and Seon.
In Student Tracking we were only able to determine one competitor of note, Every Day Wireless, however a few mobile phone companies are beginning to offer products in this area.
When you consider that each of these three components bought individually as standalone products can cost well in excess of $6,000 per bus, the NSC Travado™ product offers significant competitive advantage as our retail price is significantly less than this figure, often less than ½ of this amount, depending on the volume requirements and features requested by a given customer.
As of the date of this report we have not been able to find anyone who can offer a bundled product like the Travado IBUS™.
Personnel
As of the date of this report, we have six full time employees.
WHERE YOU CAN FIND MORE INFORMATION
You are advised to read this Form 10-K in conjunction with other reports and documents that we file from time to time with the SEC. In particular, please read our Quarterly Reports on Form 10-Q and Current Reports on Form 8-K that we file from time to time. You may obtain copies of these reports directly from us or from the SEC at the SEC’s Public Reference Room at 100 F. Street, N.E. Washington, D.C. 20549, and you may obtain information about obtaining access to the Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains information for electronic filers at its website http://www.sec.gov.
In an effort to keep our stockholders and the public informed about our business, we may make “forward-looking statements.” Forward-looking statements usually relate to future events and anticipated revenues, earnings, cash flows or other aspects of our operations or operating results. As indicated previously, forward-looking statements are often identified by words, “will”, “may”, “should”, “continue”, “anticipate”, “believe”, “expect”, “plan”, “forecast”, “project”, “estimate”, “intend” and words of similar nature. Forward-looking statements generally include statements containing:
| • | projections about accounting and finances; |
| • | plans and objectives for the future; |
| • | projections or estimates about assumptions relating to our performance; or |
| • | our opinions, views or beliefs about the effects of current or future events, circumstances or performance. |
You should view those statements with caution. Those statements are not guarantees of future performance, circumstances or events. They are based on facts and circumstances known to us as of the date the statements are made. All phases of our business are subject to uncertainties, risks and other influences, many of which we do not control. Any of these factors either alone or taken together, could have a material adverse effect on us and could change whether any forward-looking statement ultimately turns out to be true. Additionally, we assume no obligation to update any forward-looking statement as a result of future events, circumstances or developments.
Outlined below are some of the risks that we believe could affect our business and financial statements for 2011 and beyond. An investment in our common stock involves a high degree of risk. You should carefully consider the following information about these risks, together with the other information contained in this Annual Report on Form 10-K, before investing in our common stock. If any of the events anticipated by the risks described below occur, our results of operations and financial condition could be adversely affected which could result in a decline in the market price of our common stock, causing you to lose all or part of your investment.
The Report Of Our Independent Registered Public Accounting Firm Contains Explanatory Language That Substantial Doubt Exists About Our Ability To Continue As A Going Concern
The independent auditor’s report on our financial statements contains explanatory language that substantial doubt exists about our ability to continue as a going concern. We have a net loss for the year ended September 30, 2008 of $249,607, an accumulated deficit at September 30, 2008 of $25,491,084, cash flows used in operating activities of $(4,107) and need additional cash flows to maintain our operations. We depend on the continued contributions of our executive officers to finance our operations and need to obtain additional funding sources to explore potential strategic relationships and to provide capital and other resources for the further development and marketing of our products and business. If we are unable to obtain sufficient financing in the near term or achieve profitability, then we would, in all likelihood, experience severe liquidity problems and may have to curtail or cease our operations altogether. If we curtail our operations or cease our operations, we may be placed into bankruptcy or undergo liquidation, the result of which will adversely affect the value of our common shares.
Because We Are Quoted On The OTCBB “OTC Markets “formerly known as “Pinks Sheets” Instead Of An Exchange Or National Quotation System, Our Investors May Have A Tougher Time Selling Their Stock Or Experience Negative Volatility On The Market Price Of Our Stock.
Our common stock is traded on the OTCBB “OTC Markets”. The OTCBB “OTC Markets” is often highly illiquid, in part because it does not have a national quotation system by which potential investors can follow the market price of shares except through information received and generated by a limited number of broker-dealers that make markets in particular stocks. There is a greater chance of volatility for securities that trade on the OTCBB “OTC Markets” as compared to a national exchange or quotation system. This volatility may be caused by a variety of factors, including the lack of readily available price quotations, the absence of consistent administrative supervision of bid and ask quotations, lower trading volume, and market conditions. Investors in our common stock may experience high fluctuations in the market price and volume of the trading market for our securities. These fluctuations, when they occur, have a negative effect on the market price for our securities. Accordingly, our stockholders may not be able to realize a fair price from their shares when they determine to sell them or may have to hold them for a substantial period of time until the market for our common stock improves.
We depend significantly upon the continued involvement of our present management.
The Company’s success depends significantly upon the involvement of our present management, who is in charge of our strategic planning and operations. We may need to attract and retain additional talented individuals in order to carry out our business objectives. The competition for individuals with expertise in this industry could be intense and there are no assurances that these individuals will be available to us.
Our Common Stock Is Subject To Penny Stock Regulation
Our shares are subject to the provisions of Section 15(g) and Rule 15g-9 of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), commonly referred to as the "penny stock" rule. Section 15(g) sets forth certain requirements for transactions in penny stocks and Rule 15g-9(d)(1) incorporates the definition of penny stock as that used in Rule 3a51-1 of the Exchange Act. The Commission generally defines penny stock to be any equity security that has a market price less than $5.00 per share, subject to certain exceptions. Rule 3a51-1 provides that any equity security is considered to be penny stock unless that security is: registered and traded on a national securities exchange meeting specified criteria set by the Commission; authorized for quotation on the NASDAQ Stock Market; issued by a registered investment company; excluded from the definition on the basis of price (at least $5.00 per share) or the registrant's net tangible assets; or exempted from the definition by the Commission. Since our shares are deemed to be "penny stock", trading in the shares will be subject to additional sales practice requirements on broker/dealers who sell penny stock to persons other than established customers and accredited investors.
Compliance with changing regulation of corporate governance and public disclosure will result in additional expenses and pose challenges for our management.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act and the rules and regulations promulgated there under, the Sarbanes-Oxley Act and SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the U.S. public markets. Our management team will need to devote significant time and financial resources to comply with both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion of management time and attention from revenue generating activities to compliance activities.
We rely heavily on international third parties to manufacture subsystems of our available on acceptable terms, if at all. If such financing is not available on satisfactory terms, we may be unable to continue, products.
We currently rely on international third-party contractors to manufacture key sub assemblies to satisfy the needs of such customers. Reliance on one or more international third-party manufacturers exposes us to the risk that delivery schedules cannot be met, and that we cannot fulfill orders for some of our products in a timely way at the right price in U.S. dollars. This risk includes the concern that:
| • | Third-party manufacturers might be unable to manufacture our products in the volume and of the quality required to meet customers’ needs; |
| • | Our existing and future contract manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply our customers; |
| • | If any third-party manufacturer makes improvements in the manufacturing process for our products, we may not own, or may have to share, the intellectual property rights to the innovation. |
Each of these conditions could delay the shipments to our customers, approvals required by regulatory authorities, and the commercialization of some of our customers’ products. These risks could also result in higher costs to the customer or could deprive us of potential product revenues.
Risks Relating To Our Industry
There are substantial inherent risks in attempting to commercialize new technological applications, and, as a result, we may not be able to successfully develop products or technology for commercial use.
Our company conducts ongoing development on our medical billing software. Our product development team is working on developing technology and products in various stages. Software development requires significant amounts of capital and takes an extremely long time to reach commercial viability, if at all. During the development of our software, we may experience technological barriers that we may be unable to overcome. Because of these uncertainties, it is possible that none of our product candidates will be successfully developed.
Risks Related to our Securities
The market price for our common stock may be volatile, and you may not be able to sell our stock at a favorable price or at all.
Many factors could cause the market price of our common stock to rise and fall, including:
| • | actual or anticipated variations in our quarterly results of operations; |
| • | changes in market valuations of companies in our industry; |
| • | changes in expectations of future financial performance; |
| • | fluctuations in stock market prices and volumes; |
| • | issuances of dilutive common stock or other securities in the future; |
| • | the addition or departure of key personnel; |
| • | announcements by us or our competitors of acquisitions, investments or strategic alliances; and |
| • | it is possible that the proceeds from sales of our common stock may not equal or exceed the prices you paid for the shares after including the costs and fees of making the sales |
Substantial sales of our common stock, or the perception that such sales might occur, could depress the market price of our common stock.
We cannot predict whether future issuances of our common stock or resale in the open market will decrease the market price of our common stock. The consequence of any such issuances or resale of our common stock on our market price may be increased as a result of the fact that our common stock is thinly, or infrequently, traded. The exercise of any options, or the vesting of any restricted stock that we may grant to directors, executive officers and other employees in the future, the issuance of common stock in connection with acquisitions and other issuances of our common stock may decrease the market price of our common stock.
Holders of our common stock have a risk of potential dilution if we issue additional shares of common stock in the future.
Although our Board of Directors intends to utilize its reasonable business judgment to fulfill its fiduciary obligations to our then existing stockholders in connection with any future issuance of our common stock, the future issuance of additional shares of our common stock would cause immediate, and potentially substantial, dilution to the net tangible book value of those shares of common stock that are issued and outstanding immediately prior to such transaction. Any future decrease in the net tangible book value of our issued and outstanding shares could have a material adverse effect on the market value of our shares.
We do not intend to pay cash dividends to our stockholders, so you will not receive any return on your investment in our Company prior to selling your interest in the Company.
The Company has never paid any cash dividends to our stockholders. We currently intend to retain any future earnings for funding growth and, therefore, do not expect to pay any cash dividends in the foreseeable future. As a result, you will not receive any return on your investment prior to selling your shares in our Company and, for the other reasons discussed in this “Risk Factors” section, you may not receive any return on your investment even when you sell your shares in our Company.
Certain shares of our common stock are restricted from immediate resale. The lapse of those restrictions, coupled with the sale of the related shares in the market, or the market’s expectation of such sales, could result in an immediate and substantial decline in the market price of our common stock.
Most of our shares of common stock are restricted from immediate resale in the public market. The restricted shares are restricted in accordance with Rule 144, which states that if unregistered, restricted securities are to be sold, a minimum of one year must elapse between the later of the date of acquisition of the securities from the issuer or from an affiliate of the issuer, and any resale of those securities in reliance on Rule 144. The Rule 144 restrictive legend remains on the stock until the holder of the stock holds the stock for longer than six months (unless an affiliate) and meets the other requirements of Rule 144 to have the restriction removed. The sale or resale of those shares in the public market, or the market’s expectation of such sales, may result in an immediate and substantial decline in the market price of our shares. Such a decline will adversely affect our investors, and make it more difficult for us to raise additional funds through equity offerings in the future.
Our common stock is subject to restrictions on sales by broker-dealers and penny stock rules, which may be detrimental to investors.
Our common stock is subject to Rules 15g-1 through 15g-9 under the Exchange Act, which imposes certain sales practice requirements on broker-dealers who sell our common stock to persons other than established customers and “accredited investors” (as defined in Rule 501(a) of the Securities Act). For transactions covered by this rule, a broker-dealer must make a special suitability determination for the purchaser and have received the purchaser’s written consent to the transaction prior to the sale. This rule adversely affects the ability of broker-dealers to sell our common stock and purchasers of our common stock to sell their shares of our common stock.
Additionally, our common stock is subject to SEC regulations applicable to “penny stocks.” Penny stocks include any non-Nasdaq equity security that has a market price of less than $5.00 per share, subject to certain exceptions. The regulations require that prior to any non-exempt buy/sell transaction in a penny stock; a disclosure schedule proscribed by the SEC relating to the penny stock market must be delivered by a broker-dealer to the purchaser of such penny stock. This disclosure must include the amount of commissions payable to both the broker-dealer and the registered representative and current price quotations for our common stock. The regulations also require that monthly statements be sent to holders of a penny stock that disclose recent price information for the penny stock and information of the limited market for penny stocks. These requirements adversely affect the market liquidity of our common stock.
Our Articles of Incorporation allow us to sell preferred stock without shareholder approval.
Our Board of Directors has the authority to issue up to 4,000,000 shares of preferred stock and to determine the price, rights, preferences, privileges and restrictions, including voting rights, of those shares without any additional vote or action by our shareholders. The rights of the holders of the common stock will be subject to, and could be materially adversely affected by, the rights of the holders of any preferred stock that may be issued in the future. For example we could issue preferred stock that has superior rights to dividends or is convertible into shares of common stock. This might adversely affect the market price of the common stock.
If we experience delays and/or defaults in customer payments, we could be unable to recover all expenditures.
Because of the nature of our contracts, at times we commit resources to projects prior to receiving payments from the customer in amounts sufficient to cover expenditures on projects as they are incurred. Delays in customer payments may require us to make a working capital investment. If a customer defaults in making their payments on a project in which we have devoted resources, it could have a material negative effect on our results of operations.
If we do not effectively manage our growth, our existing infrastructure may become strained, and we may be unable to increase revenue growth.
Our past growth that we have experienced, and in the future may experience, may provide challenges to our organization, requiring us to expand our personnel and our operations. Future growth may strain our infrastructure, operations and other managerial and operating resources. If our business resources become strained, our earnings may be adversely affected and we may be unable to increase revenue growth. Further, we may undertake contractual commitments that exceed our labor resources, which could also adversely affect our earnings and our ability to increase revenue growth.
SHOULD ONE OR MORE OF THE FOREGOING RISKS OR UNCERTAINTIES MATERIALIZE, OR SHOULD THE UNDERLYING ASSUMPTIONS PROVE INCORRECT, ACTUAL RESULTS MAY DIFFER SIGNIFICANTLY FROM THOSE ANTICIPATED, BELIEVED, ESTIMATED, EXPECTED, INTENDED OR PLANNED
ITEM 1B. UNRESOLVED STAFF COMMENTS
This Item is not applicable to us as we are not an accelerated filer, a large accelerated filer, or a well-seasoned issuer.
The Company leased 1,601 square feet of office space at 8361 E Evans Rd, Suite 106, Scottsdale, Arizona 85260-6951. The lease for the Scottsdale facility expired October 31, 2008 and is at a rental rate of $2,028 per month for the remainder of the lease.
Since September 2011 the Company uses the Chief Financial Officers office as the Corporation’s office at no cost to the Company.
We are currently not involved in any litigation that we believe could have a material adverse effect on our financial condition or results of operations. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of the executive officers of our company or any of our subsidiaries, threatened against or affecting our company, our common stock, any of our subsidiaries or of our company’s or our company’s subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect.
ITEM 4 - MINING SAFETY DISCLOSURES
N/A
ITEM 5. MARKET FOR REGISTANT’S COMMON STOCK, RELATED STOCKHOLDER MATTERS AND ISSUERS PURCHASES OF EQUITY SECURITIES.
Cloud common stock is traded in the over-the-counter market, and quoted in the National Association of Securities Dealers Inter-dealer Quotation System (“Electronic Bulletin Board) and can be accessed on the Internet at OTCmarkets.com under the symbol “NSCT.”
At September 30, 2008, there were 137,276,879 shares of common stock of Cloud outstanding and there were in excess of 8,000 shareholders of record of the Company’s common stock.
The following table sets forth for the periods indicated the high and low bid quotations for Cloud’s common stock. These quotations represent inter-dealer quotations, without adjustment for retail markup, markdown or commission and may not represent actual transactions.
| Periods | High | Low | |||||
| Fiscal Year 2008 | |||||||
| First Quarter (October – December 2007) | $ | 0.06 | $ | 0.01 | |||
| Second Quarter (January – March 2008) | $ | 0.09 | $ | 0.03 | |||
| Third Quarter (April - June 2008) | $ | 0.07 | $ | 0.04 | |||
| Fourth Quarter (July – September 2008) | $ | 0.06 | $ | 0.02 | |||
| Fiscal Year 2007 |
|||||||
| First Quarter (October – December 2006) | $ | 0.035 | $ | 0.020 | |||
| Second Quarter (January – March 2007) | $ | 0.061 | $ | 0.024 | |||
| Third Quarter (April - June 2007) | $ | 0.050 | $ | 0.020 | |||
| Fourth Quarter (July – September 2007) | $ | 0.028 | $ | 0.010 |
On December 2, 2012, the closing bid price of our common stock was $0.02
Dividends
To date, the Company has never declared or paid any cash dividends on our capital stock and we do not expect to pay any cash dividends in the foreseeable future. Payment of future dividends, if any, will be at the discretion of our Board of Directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs and plans for expansion.
Transfer Agent
Cloud’s Transfer Agent and Registrar for the common stock is Pacific Stock Transfer located in Las Vegas, Nevada.
Recent sales of Unregistered Securities
2012
During the ten months ended July 31, 2012 the Company issued 30,000,000 common shares for the reduction of debt from our CEO and reduced debt of the company by $3,325,949. The Company also issued 19,000 common shares for services rendered by professionals and recorded the expenses based upon the trading price of the common shares of $0.0102 and expensed $204 as consulting fees. The Company issued 1,055,333 common shares to third parties that have purchased our medical billing software at the trading price of the common stock of $0.0160 and recorded a revenue contra account of $20,871.
The Company issued 90,000 common shares for $18,000 in cash.
The Company issued 200,000 common shares to Kroos Medical Management in accordance with the acquisition agreement and recorded it at the trading price of the common shares of $0.020 and recorded an investment in Kroos Medical Management of $4,000.
2011
In September 30, 2011 the Company issued 15,000,000 common shares at the trading price of the common stock of $0.007. The Stock was issued for compensation to new management and recorded as expense of $105,000.
The Company issued 250,000 in common stock for $25,000 in cash.
2010
No common stock was issued.
2009
In September 30, 2009 the Company issued 28,000,000 common shares at the trading price of the common stock of $0.001. The Stock was issued for unpaid compensation to officers and reduced accrued compensation by $35,800.
2008
In September 30, 2008 the Company issued 15,193,966 common shares at the trading price of the common stock of $0.001. The Stock was issued for unpaid compensation to officers and reduced accrued compensation by $63,000. The Company issued 87,078 common shares at a trading price of the common stock of $.0229 and the Company expensed
2007
As of September 30, 2007 we are authorized to issue up to 187,000,000 shares of common stock, par value $0.01 per share. As of September 30, 2007, there were 121,995,835 shares of common stock outstanding. We had 868 shareholders of record of common stock on September 30, 2007. Each holder of common stock is entitled to one vote for each share held on all matters. Our articles of incorporation and bylaws do not provide for cumulative voting in elections of directors or any other matters brought before shareholder meetings.
Common Stock
The holders of our common stock are entitled to receive such dividends, if any, as may be declared by our board of directors from time to time out of legally available funds. The dividend rights of our common stock are junior to any preferential dividend rights of any outstanding shares of preferred stock. The holders of our common stock also are entitled to receive distributions upon our liquidation, dissolution or winding up of our assets that are legally available for distribution, after payment of all debt and other liabilities and distribution in full of preferential amounts, if any, to be distributed to holders of our preferred stock.
The holders of our common stock are not entitled to preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
The Company has authorized 4,000,000 shares of preferred stock, at $.001 par value and zero are issued and outstanding as of September 30, 2008. The Corporation established and designates the rights and preferences of a Series A Convertible Preferred Stock, and reserves 4,000,000 shares of preferred stock against its issuance, such rights, preferences and designations. Each share of the Preferred Stock shall have 150 votes on all matters presented to be voted by the holders of our common stock. All 4,000,000 shares of preferred stock that have been granted to our CEO & CFO on November 30, 2010 and issued on April 11, 2012 which was valued at the trading price of the common stock of $0.0095 and recorded as an expense of $38,000.
The issuance of preferred stock by our board of directors could adversely affect the rights of holders of the common stock by, among other things, establishing preferential dividends, liquidation rights or voting powers. See “Risk Factors” above.
Warrants and Options
All warrants issued have expired.
Stock Option Plan
Our board of directors adopted the 2000 Stock Option Plan effective January 1, 2001. Our stockholders formally approved the 2000 Stock Option Plan on February 14, 2001. The 2000 Stock Option plan terminates in accordance with the term on December 1, 2010. The Current Board of Directors has not approved nor extended the Stock Option Plan therefore it is terminated.
ITEM 6. SELECTED FINANCIAL DATA
The following information has been summarized from financial information included elsewhere and should be read in conjunction with such financial statements and notes thereto.
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following is management’s discussion and analysis of certain significant factors that have affected our financial position and operating results during the periods included in the accompanying financial statements, as well as information relating to the plans of our current management. This report includes forward-looking statements. Undue reliance should not be placed on these forward-looking statements which speak only as of the date hereof. We undertake no obligation to update these forward-looking statements.
The following discussion and analysis should be read in conjunction with our financial statements and the related notes thereto and other financial information contained elsewhere in this Form 10-K.
Business
The Company was incorporated in Texas on June 22, 1953 as American Mortgage Company. On May 16, 1996, the Company changed its name to National Scientific Corporation. On April 3, 2012, the Company changed its name to Cloud Medical Doctor Software Corporation. During 1996, the Company acquired the operations of Eden Systems, Inc. (Eden) as a wholly owned subsidiary. Eden was engaged in water treatment and the retailing of cleaning products. Eden’s operations were sold on October 1, 1997. From September 30, 1997 through the year ended September 30, 2001, we aimed our efforts in the research and development of semiconductor proprietary technology and processes and in raising capital to fund its operations and research. Beginning in calendar 2002, we focused our efforts on the development, acquisition, enhancement and marketing of location device technologies. Our revenue is derived from sales of electronic devices, recognized as the product is delivered.
In February 4, 2010 the prior Board members Mr. Michael Grollman and Mr. Greg Szabo, decided to discontinue and wind down the operations of the Company's Mobile DVR and Location Products business and operate these remaining Company assets in a Limited Liability Company named NSC Labs, LLC controlled and owned by Mr. Grollman. Mr. Grollman and NSC Labs, LLC was to pay the Company 2% of revenues and $100,000. Mr. Grollman nor NSC Labs, LLC never paid consideration for this transaction.
In November 19, 2010, the prior management was terminated and changed management and began working on operations related to the Company’s medical billing software.
Critical Accounting Policies
Use of Estimates and Assumptions
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (“GAAP”) requires management to make estimates and assumptions that affect (i) the reported amounts of assets and liabilities, (ii) the disclosure of contingent assets and liabilities known to exist as of the date the financial statements are published, and (iii) the reported amount of net revenues and expenses recognized during the periods presented. Adjustments made with respect to the use of estimates often relate to improved information not previously available. Uncertainties with respect to such estimates and assumptions are inherent in the preparation of financial statements; accordingly, actual results could differ from these estimates.
These estimates and assumptions also affect the reported amounts of revenues, costs and expenses during the reporting period. Management evaluates these estimates and assumptions on a regular basis. Actual results could differ from those estimates.
Revenue Recognition
Revenue includes product sales and provision of services. The Company recognizes revenue from product sales and provision of services at the time evidence of an arrangement exists, fees are contractually fixed or determinable, collection is reasonably assured through historical collection results and regular credit evaluations, and there are no uncertainties regarding customer acceptance.
Share-Based Compensation
The Company measures the cost of services received in exchange for an award of an equity instrument based on the grant-date fair value of the award. Compensation cost is recognized over the vesting or requisite service period. The Black-Scholes option-pricing model is used to estimate the fair value of options or warrants granted.
RESULTS OF OPERATIONS FROM DISCONTINUED MOBILE DVR AND LOCATION PRODUCTS BUSINESS
Fiscal Year Ended September 30, 2008, Compared to Fiscal Year Ended September 30, 2007
Revenues increased to $668,796 from $624,793 for the years ended September 30, 2008 and 2007 respectively. This increase is primarily attributable to increase in sales for 2008.
Cost of goods sold increased to $365,178 from $317,606 for the years ended September 30, 2008 and 2007, respectively. Our cost of goods sold is directly related to the increase in revenue.
General and administrative expense decreased to $619,763 from $611,924 for the years ended September 30, 2008 and 2007, respectively. The decrease in general and administrative expense was related to the decrease in research and development costs of the Company.
Depreciation and amortization decreased to $622 from $415 for the years ended September 30, 2008 and 2007, respectively. The increase in depreciation and amortization was due to the write off of all remaining office assets.
Interest Expense decreased to $46,064 from $166,725 for the years ended September 30, 2008 and 2007, respectively. Our interest expense is related to the cost of debt and our cost of debt was reduced by repayment of loans of $80,875 and related party loans of $28,138. Also prior management was accruing interest on back pay and there was no agreement to support that accrual.
Net loss for the years ended September 30, 2008 and 2007 decreased to $249,607 from $445,722 respectively.
Liquidity and Capital Resources
The Company has material notes, one from our prior Chief Executive Officer for approximately $97,992 , one convertible note with an investor relationship with Strategic Working Capital Fund, LP $149,361 net of discount and beneficial conversion feature of $25,639 with a total balance due of $175,000, and three notes with shareholders of $35,170. In September 30, 2008 the Company was in compliance with all note requirements but as of today’s date all note agreements are in default.
Our cash provided by (used in) operating activities were $(4,647) and $23,986 for the years ended September 30, 2008 and 2007, respectively. The increase (decrease) in cash flows provided by (used in) operations was primarily attributable to the changes in operating assets and liabilities, primarily related to increases in amounts payable, in 2008 as compared to the 2007 period.
Cash provided by (used in) financing activities was ($73,638) and $18,002 for the years ended September 30, 2008 and 2007, respectively. We repaid notes payables of $45,500 and related party notes payables of $28,138 as compared to repayment of notes payables of $125,823 and increase in notes payables of 34,025 and sale of common stock of $109,800 for year ended September 30, 2007.
We are currently investigating opportunities regarding the acquisition or leasing of companies in the software sector and are actively pursuing acquisitions.
There is a strong possibility that, in attempting to accomplish the above, we may not be able to satisfy our cash requirements over the next twelve months and may be required to raise additional cash from outside sources.
In the event that we are required to raise additional cash from outside source, we may issue equity securities or incur additional debt. There is no assurance that such funding, if required will be available to us or, if available, will be available upon terms favorable to us.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We do not hold any derivative instruments and do not engage in any hedging activities.
CLOUD MEDICAL DOCTOR SOFTWARE CORPORATION
S.E.Clark & Company, P.C.
Registered Firm: Public Company Accounting Oversight Board
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
Cloud Medical Doctor Software Corporation,
fka National Scientific Corporation
Phoenix, Arizona
We have audited the accompanying balance sheet of Cloud Medical Doctor Software Corporation, fka National Scientific Corporation (the “Company”), as of September 30, 2008, and the related statements of operations, stockholders’ deficit and cash flows for the year then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit. The financial statements for the year ended September 30, 2007, were audited by other auditors whose report expressed an unqualified opinion with a going concern paragraph on those financial statements on December 19, 2007. On December 13, 2011 the former auditor resigned and notified the Company (See, Exhibit 23, below).
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Cloud Medical Doctor Software Corporation, fka National Scientific Corporation, as of September 30, 2008 and the results of their operations and their cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has a net loss for the year ended September 30, 2008 of $249,607, an accumulated deficit at September 30, 2008 of $25,491,084, cash flows used in operating activities of $4,107 and needs additional cash flows to maintain its operations. Those conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to those matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ S.E.Clark & Company, P.C.
Tucson, Arizona
December 14, 2012
| CLOUD MEDICAL DOCTOR SOFTWARE CORPORATION | ||||||||
| (Formerly National Scientific Corporation) | ||||||||
| BALANCE SHEET | ||||||||
| September 30, | ||||||||
| 2008 | 2007 | |||||||
| ASSETS | ||||||||
| Cash | $ | 7,602 | $ | 85,887 | ||||
| Accounts receivable, net | 29,957 | 6,216 | ||||||
| Inventory | 660 | 22,900 | ||||||
| Total current assets | 38,219 | 115,003 | ||||||
| PROPERTY AND EQUIPMENT, net | — | 622 | ||||||
| Deposit | 355 | 2,408 | ||||||
| Deferred offering costs, net | 10,000 | 14,800 | ||||||
| TOTAL ASSETS | $ | 48,574 | $ | 132,833 | ||||
| LIABILITIES AND STOCKHOLDERS DEFICIT | ||||||||
| Accounts payables and accrued expenses | $ | 164,275 | $ | 195,004 | ||||
| Accounts payables related party | — | 419 | ||||||
| Disputed Accrued expenses - related party | 1,056,742 | 883,400 | ||||||
| Due to factors | — | 2,602 | ||||||
| Accrued interest | 4,667 | — | ||||||
| Notes payable - related party | 133,162 | 161,300 | ||||||
| Notes payable - current portion | — | 80,875 | ||||||
| Total current liabilities | 1,358,846 | 1,323,600 | ||||||
| Convertible note payable - less discount and beneficial conversion feature of $25,639 and $37,945 | 149,361 | 137,055 | ||||||
| TOTAL LIABILITIES | 1,508,208 | 1,460,655 | ||||||
| CONTINGENCIES AND COMMITMENTS | — | — | ||||||
| STOCKHOLDERS' DEFICIT: | ||||||||
| Preferred stock, $.010 par value, 4,000,000 shares authorized; none issue and outstanding as of September 30, 2008 and 2007 | — | — | ||||||
| Common stock, $.01 par value, 187,000,000 shares authorized; 137,276,879 and 121,995,835 issued and outstanding as of September 30, 2008 and 2007 | 1,372,770 | 1,219,959 | ||||||
| Additional paid in capital | 22,658,682 | 22,693,696 | ||||||
| Accumulated deficit | (25,491,084 | ) | (25,241,477 | ) | ||||
| Total stockholders' deficit | (1,459,633 | ) | (1,327,822 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $ | 48,574 | $ | 132,833 | ||||
| THE LINE OF BUSINESS REPORTED ON THESE FINANCIAL STATEMENTS | ||||||||
| WAS DISCONTINUED IN FEBRUARY 2010. SEE NOTE 1 | ||||||||
| The accompanying notes are an integral part of these financial statements. | ||||||||
CLOUD MEDICAL DOCTOR SOFTWARE CORPORATION
NOTES TO FINANCIAL STATEMENTS
FOR THE YEAR ENDED SEPTEMBER 30, 2008
NOTE 1 - DESCRIPTION OF BUSINESS
THE COMPANY PREVIOUSLY HAD INSUFFICIENT WORKING CAPITAL TO PAY FOR THE PROFESSIONAL SERVICES REQUIRED TO PREPARE, AUDIT, AND FILE THE QUARTERLY AND ANNUAL REPORTS REQUIRED BY THE SECURITIES ACT OF 1934. AS A RESULT, THE AUDITORS WHO AUDITED THE SEPTEMBER 30, 2007 FINANCIAL STATEMENTS AND REVIEWED THE QUARTERLY REPORTS THROUGH JUNE 30, 2008 RESIGNED EFFECTIVE DECEMBER 13, 2011.
IN FEBRUARY 2010 THE BOARD OF DIRECTORS VOTED TO DISCONTINUE THE MOBILE DVR AND LOCATION PRODUCTS LINE OF BUSINESS REPORTED IN THESE FINANCIAL STATEMENTS DUE TO THE CUMULATIVE EFFECTS OF SEVERELY DELCLINING REVENUES RESULTING FROM THE 2008 RECESSION. SINCE THIS DECISION WAS MADE SUBSEQUENT TO THE YEARS ENDED SEPTEMBER 30, 2008 AND 2009, THE QUARTERLY AND YEAR END STATEMENTS FOR THOSE YEARS ARE BEING REPORTED ON A GOING CONCERN BASIS RATHER THAN AS DISCONTINUED OPERATIONS. THEY WILL, HOWEVER, BE REPORTED AS DISCONTINUED OPERATIONS COMMENCING WITH THE FISCAL 2010 FILINGS.
NEW MANAGEMENT HAS SUBSEQUENTLY INFUSED SUFFICIENT WORKING CAPITAL TO BRING THE 1934 ACT FILINGS CURRENT. ADDITIONALLY, A NEW LINE OF BUSINESS HAS ALSO COMMENCED WHICH WILL BE REPORTED ON IN THE FISCAL 2011 AND 2012 FILINGS.
ACCORDINGLY, THESE FINANCIAL STATEMENTS ARE BEING SUBMITTED ONLY TO COMPLY WITH SEC RULES AND REGULATIONS AND SHOULD NOT BE RELIED UPON FOR YOUR INVESTMENT DECISIONS. THE OPERATIONS REPORTED ON IN THESE FINANCIAL STATEMENTS HAVE BEEN DISCONTINUED AS OF FEBRUARY 4, 2010. NEW MANAGEMENT ENCOURAGES THE READERS OF THESE FINANCIAL STATEMENTS TO SUSPEND ANY INVESTMENT DECISIONS PERTAINING TO THE STOCK OF THIS COMPANY UNTIL ALL REQUIRED 1934 ACT FILINGS ARE BROUGHT CURRENT.
The Company was incorporated in Texas on June 22, 1953 as American Mortgage Company. On May 16, 1996, the Company changed its name to National Scientific Corporation. On April 3, 2012, the Company changed its name to Cloud Medical Doctor Software Corporation. During 1996, the Company acquired the operations of Eden Systems, Inc. (Eden) as a wholly owned subsidiary. Eden was engaged in water treatment and the retailing of cleaning products. Eden’s operations were sold on October 1, 1997. From September 30, 1997 through the year ended September 30, 2001, we aimed our efforts in the research and development of semiconductor proprietary technology and processes and in raising capital to fund its operations and research. Beginning in calendar 2002, we focused our efforts on the development, acquisition, enhancement and marketing of location device technologies. Our revenue is derived from sales of electronic devices, recognized as the product is delivered.
In February 4, 2010 the prior Board members Mr. Michael Grollman and Mr. Greg Szabo, decided to discontinue and wind down the operations of the Company's Mobile DVR and Location Products business and operate the remaining Company assets in a Limited Liability Company named NSC Labs, LLC controlled and owned by Mr. Grollman. Mr. Grollman and NSC Labs, LLC was to pay the Company 2% of revenues and $100,000. However, Mr. Grollman nor NSC Labs, LLC never paid consideration for this transaction.
In November 19, 2010, the prior management was terminated and changed management and began working on operations related to the Company’s medical billing software.
NOTE 2 - BASIS OF PRESENTATION
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (“GAAP”) requires management to make estimates and assumptions that affect (i) the reported amounts of assets and liabilities, (ii) the disclosure of contingent assets and liabilities known to exist as of the date the financial statements are published, and (iii) the reported amount of net revenues and expenses recognized during the periods presented. Adjustments made with respect to the use of estimates often relate to improved information not previously available. Uncertainties with respect to such estimates and assumptions are inherent in the preparation of financial statements; accordingly, actual results could differ from these estimates.
In managements’ opinion, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation have been included.
NOTE 3 - GOING CONCERN ISSUES
The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern. However, the Company has a net loss for the twelve months ended September 30, 2008 of $249,607, an accumulated deficit at September 30, 2008 of $25,491,084, cash flows used in operating activities of $4,107 and needs additional cash to maintain its operations.
These factors raise doubt about the Company’s ability to continue as a going concern. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty. The Company’s continued existence is dependent upon management’s ability to develop profitable operations, continued contributions from the Company’s executive officers to finance its operations and the ability to obtain additional funding sources to explore potential strategic relationships and to provide capital and other resources for the further development and marketing of the Company’s products and business.
NOTE 4 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The Company prepares its financial statements in accordance with accounting principles generally accepted in the United States of America. Significant accounting policies are as follows:
Use of Estimates and Assumptions
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (“GAAP”) requires management to make estimates and assumptions that affect (i) the reported amounts of assets and liabilities, (ii) the disclosure of contingent assets and liabilities known to exist as of the date the financial statements are published, and (iii) the reported amount of net revenues and expenses recognized during the periods presented. Adjustments made with respect to the use of estimates often relate to improved information not previously available. Uncertainties with respect to such estimates and assumptions are inherent in the preparation of financial statements; accordingly, actual results could differ from these estimates.
Revenue Recognition
Revenue includes provision of services. The Company recognizes revenue from provision of services at the time evidence of an arrangement exists, fees are contractually fixed or determinable, collection is reasonably assured through historical collection results and regular credit evaluations, and there are no uncertainties regarding customer acceptance.
Accounts Receivable and Allowance for Uncollectible Accounts
Substantially all of the Company’s accounts receivable balance is related to trade receivables. Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses in its existing accounts receivable. The Company will maintain allowances for doubtful accounts for estimated losses resulting from the inability of its customers to make required payments for services. Accounts with known financial issues are first reviewed and specific estimates are recorded. The remaining accounts receivable balances are then grouped in categories by the number of days the balance is past due, and the estimated loss is calculated as a percentage of the total category based upon past history. Account balances are charged off against the allowance when it is probable the receivable will not be recovered. The Company had no charge offs for year ended September 30, 2008.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less to be cash equivalents. At September 30, 2008, cash and cash equivalents include cash on hand and cash in the bank and the FDIC insures these deposits up to $250,000.
Inventory
The Company’s inventory is carried at the lower of cost or net realizable value using the first-in, first-out (“FIFO”) method. The Company’s inventory is comprised of supplies and parts used in the Company’s operations. The Company evaluates the need to record adjustments for obsolescence for inventory on a regular basis. At September 30, 2008, the Company’s inventory balance was $662 and at September 30, 2007, the Company’s inventory balance was $22,900.
Property and Equipment
Property and equipment is recorded at cost and depreciated over the estimated useful life, ranging from three to ten years, using the straight-line method. When items are retired or otherwise disposed of, loss or gain is charged or credited for the difference between the net book value and proceeds realized. Ordinary maintenance and repairs are charged to expense as incurred, and replacements and betterments are capitalized.
Impairment of Long-Lived Assets
Property and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the estimated fair value of the asset. As of September 30, 2008 the Company impaired $20,596 of assets of the Company.
Income Taxes
Deferred income taxes are provided to reflect the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company adopted the provisions of ASC Topic 740, Accounting For Uncertainty In Income Taxes-An Interpretation of ASC Topic 740 ("ASC Topic 740"). ASC Topic 740 contains a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates it is more likely than not, that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount, which is more than 50% likely of being realized upon ultimate settlement. The Company considers many factors when evaluating and estimating the Company's tax positions and tax benefits, which may require periodic adjustments. At September 30, 2008, the Company did not record any liabilities for uncertain tax positions.
Share-Based Compensation
The Company measures the cost of services received in exchange for an award of an equity instrument based on the grant-date fair value of the award. Compensation cost is recognized over the vesting or requisite service period. The Black-Scholes option-pricing model is used to estimate the fair value of options or warrants granted.
Basic and Diluted Net Loss Per Share
Basic income (loss) per share is computed by dividing net income (loss) available to common shareholders by the weighted average number of common shares outstanding during the reporting period. The weighted average number of shares is calculated by taking the number of shares outstanding and weighting them by the amount of time that they were outstanding. Diluted earnings per share reflects the potential dilution that could occur if stock options, warrants, and other commitments to issue common stock were exercised or equity awards vest resulting in the issuance of common stock that could share in the earnings of the Company.
Diluted loss per share is the same as basic loss per share during periods where net losses are incurred since the inclusion of the potential common stock equivalents would be anti-dilutive as a result of the net loss.
Concentration of Credit Risk
All of the Company’s cash and cash equivalents are maintained in regional and national financial institutions. The Company has exposure to credit risk to the extent that its cash and cash equivalents exceed amounts covered by the U.S. federal deposit insurance; however, the Company has not experienced any losses in such accounts. In management’s opinion, the capitalization and operating history of the financial institutions are such that the likelihood of material loss is remote.
Fair Value of Financial Instruments
The Company's financial instruments consist primarily of cash, accounts payable and accrued expenses, and debt. The carrying amounts of such financial instruments approximate their respective estimated fair value due to the short-term maturities and approximate market interest rates of these instruments.
The Company adopted ASC Topic 820, Fair Value Measurements (“ASC Topic 820”), which defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements. The standard provides a consistent definition of fair value which focuses on an exit price that would be received upon sale of an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The standard also prioritizes, within the measurement of fair value, the use of market-based information over entity specific information and establishes a three-level hierarchy for fair value measurements based on the nature of inputs used in the valuation of an asset or liability as of the measurement date.
The three-level hierarchy for fair value measurements is defined as follows:
| • | Level 1 – inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets; liabilities in active markets; |
| • |
Level 2 – inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability other than quoted prices, either directly or indirectly, including inputs in markets that are not considered to be active; or directly or indirectly including inputs in markets that are not considered to be active; |
| • | Level 3 – inputs to the valuation methodology are unobservable and significant to the fair value measurement |
Reclassifications
Certain prior period amounts have been reclassified to conform to current year presentations.
Recent Accounting Pronouncements
No accounting standards or interpretations issued recently are expected to a have a material impact on the Company’s financial position, operations or cash flows.
NOTE 5- ACCOUNT RECEIVABLES
| 2008 | 2007 | ||||||
| Trade receivables | $ | 29,957 | $ | 6,216 | |||
| Less: reserves | — | — | |||||
| Total receivables | $ | 29,957 | $ | 6,216 | |||
On September 7, 2005, we entered into a factoring agreement with United Capital Funding (UCF) of Florida. UCF is a specialized financial services firm offering Accounts Receivable Management and working capital funding via factoring. We expect to improve our cash flow with this arrangement.
Under the arrangement, we sell to UCF as absolute owner, invoices that we submit to UCF to be factored. Upon purchase, UCF assumes the risk of non-payment on purchased invoices, so long as the cause of non-payment is solely due to the occurrence of an insolvency event. As collateral securing the obligations, we grant UCF a continuing first priority security interest in all accounts and related inventory. Notwithstanding the creation of the above security interest, our relationship with UCF is one of Seller and Purchaser of accounts, and not that of lender and borrower. However, as there is certain recourse for non-payment, the accounts receivable are recorded at the estimated realizable value, net of allowances and the net advanced amount from UCF is recorded as an obligation at the end of fiscal 2007 and 2006. The initial and periodic factoring fee is .45% of the face amount of factored invoices. The factoring period is five days and the purchase price is 80% of the face amount, excluding sales tax.
UCF typically advances to us 80% of the total amount of invoices factored, excluding sales tax. UCF retains 20% of the outstanding factored invoices as a reserve, which it holds until the customer pays the factored invoice to UCF.
As of September 30, 2007, Trade Receivables of $6,216 included factored invoices totaling $3,470.
NOTE 6- INVENTORY
Inventory, net consists of the following at September 30, 2008 and 2007:
| 2008 | 2007 | ||||||
| Inventory, gross | $ | 660 | $ | 22,900 | |||
| Less: reserve for obsolescence | — | — | |||||
| Inventory, net | $ | 660 | $ | 22,900 | |||
We marked down our inventory of Gotcha!® products, during the year ended September 30, 2006 by $17,627, for estimated obsolescence based on assumptions about age of the inventory and current demand. Our inventory of Gotcha!® products was fully written off against the reserve of $64,602 in fiscal 2007.
NOTE 7- PROPERTY AND EQUIPMENT
Property and equipment consists of the following at September 30, 2008 and 2007:
| 2008 | 2007 | |||||||
| Computer equipment | $ | 2,074 | $ | 2,074 | ||||
| Total property and equipment | 2,074 | 2,074 | ||||||
| Less: accumulated depreciation | (2,074 | ) | (1,452 | ) | ||||
| Net property and equipment | $ | — | $ | 622 | ||||
Depreciation expense for the years ended September 30, 2008 and 2007 was $622 and $415, respectively.
NOTE 8- DISPUTED ACCRUED EXPENSES – RELATED PARTY
Disputed accrued expenses from related parties consist of the following at September 30, 2008 and 2007:
| 2008 | 2007 | |||||||
| Disputed salaries & vacation pay – former management and staff | $ | 786,653 | $ | 575,727 | ||||
| Disputed salaries & vacation pay - former employee | 29,375 | 29,375 | ||||||
| Disputed payroll taxes for back pay | 62,224 | 40,781 | ||||||
| Disputed interest expense | 178,490 | 156,271 | ||||||
| Disputed employee stock retainage pool | — | 50,250 | ||||||
| Disputed other liabilities | — | 30,996 | ||||||
| Total Disputed Accrued Expenses – Related Party | $ | 1,056,742 | $ | 883,400 | ||||
NOTE 9 – NOTES PAYABLE
As of September 30, 2008 and 2007, all long-term debt consisted of the following notes payable:
| 2008 | 2007 | |||||||
| Non-interest bearing note payable, due June 2006; unsecured; repayment made by the Company with either cash or its restricted common stock or a combination of cash and stock | $ | — | $ | 43,250 | ||||
| 8% ( 6% in 2006) note payable to an Officer of the Company; principal and interest payable on demand | 97,992 | 161,300 | ||||||
| Non- interest bearing note payable; unsecured; payable on demand | — | — | ||||||
| 12% note payable; secured; payable on demand | 11,625 | 11,625 | ||||||
| 12% note payable; secured; payable on demand | 20,000 | 20,000 | ||||||
| 8% note payable; unsecured; principal payable in full in November 2010; with semi- annual interest payments in May and November | 175,000 | 175,000 | ||||||
| 6% note payable; unsecured; payable on demand | 3,545 | 6,000 | ||||||
| 308,162 | 417,175 | |||||||
| Less: | ||||||||
| Current portion of long term debt | (133,162 | ) | (242,175 | ) | ||||
| Discount | (21,500 | ) | (31,820 | ) | ||||
| Beneficial conversion feature | (4,139 | ) | (6,125 | ) | ||||
| Long-term debt, net of current portion | $ | 149,361 | $ | 137,055 | ||||
On November 1, 2005, as an important phase in the current year’s financing plan, we entered into a financing program with Strategic Working Capital Fund, LP. The terms of this program include a five-year Note payable at maturity in November 2010 for $175,000, at an effective annual interest rate of 8%. Interest is due and payable semi-annually on May 1st and November 1st for each year in which the note is outstanding The transaction also included 1,200,000 restricted common shares and a conversion/exchange option to convert the principal amount and any unpaid interest of the Note into common shares at a per share conversion price of $0.0525. These shares include weighted average anti-dilution provisions, as well as piggyback registration rights. Additionally, the Note has various put and call rights, and has a right to early payment under certain conditions after 2 years. The 1,200,000 restricted common shares were recorded at $0.043, which was the five-day average market closing price of our stock. The note and common stock were issued with a debt discount of $51,600 and a beneficial conversion feature of $9,933. The discount and beneficial conversion feature are being amortized to interest expense over the term of the note, which is approximately 60 months. The issuance of the shares resulted in deferred financing costs of $24,000. The deferred financing costs are being amortized over term of the note, which is approximately 60 months, and are included in the statement of operations as offering costs.
On February 24, 2005, March 28, 2005, May 2, 2005, and May 27, 2005 our Chairman Michael Grollman made new personal loans to the Company totaling $159,000 to assist us with working capital needs. The loans are evidenced by a demand note that provides for repayment within five business days of a demand notice from Mr. Grollman, with interest of 6% compounded annually from June 1, 2005. These loans were secured by an interest in the copyrights in the Company’s iBus software and designs. During the quarter ended September 30, 2007, these loans were paid down by $11,000. As of September 30, 2007, these loans had a balance outstanding of $148,000 and the interest rate going forward was adjusted to 8% compounded annually from October 1, 2007. On September 30, 2007, the Company converted unpaid interest of $13,300 on demand notes payable to Michael Grollman into a new demand note of $13,300 that provides for repayment within five business days of a demand notice from Mr. Grollman, with interest of 8% compounded annually on October 1st. The balance of the note at September 30, 2008 is $97,992 and the note was repaid in the amount of $50,000.
On April 27, 2006, we secured a short-term loan of $16,625 from a shareholder. The loan carries an origination/placement fee of $500 and has a perfectible security interest a) prior to delivery in any assets purchased for the fulfillment of a customer’s order dated March 16, 2006, and b) in any receivable resulting from the fulfillment of the customer’s purchase order. The interest rate on the loan is 12%. The transaction also included 100,000 warrants to purchase our common stock at $0.036 at any time before April 27, 2009. The note had an approximate maturity date of June 15, 2006. At September 30, 2006 and September 30, 2007 this note was in default. A partial payment towards principal and interest of $5,258 was made on October 6, 2006. As of September 30, 2008 $11,625 of this loan was outstanding.
On May 31, 2006, we secured a short-term loan of $20,000 from a shareholder. The loan carries an origination/placement fee of $500 and has a perfectible security interest a) prior to delivery in any assets purchased for the fulfillment of Clover Park, WA’s order and b) in any receivable resulting from the fulfillment of the Clover Park purchase order. The interest rate on the loan is 12%. The transaction also included 100,000 warrants to purchase our common stock at $0.036 at any time before May 31, 2009. The note had a maturity date of July 10, 2006. At September 30, 2008 and September 30, 2007 this note was in default.
On December 27, 2006, we signed a loan agreement with an employee for $8,000. The loan is unsecured and carries an interest rate of 6%. The note is payable on demand with 30 days prior notice On February 27, 2007 we paid $2,000 towards the principal amount. During September 30, 2008 $2,455 was paid towards the principal amount and the balance was $3,544 for fiscal year ended.
The aggregate maturities of long-term debt at September 30, 2007 were as follows:
| 2009 | 133,162 | ||
| 2010 | 175,000 | ||
| $ | 308,162 |
NOTE 10 – COMMITMENT AND CONTINGENCIES
On August 27, 2004 the Company signed a twenty-six month non-cancelable operating lease agreement for an office in Scottsdale, Arizona. The lease for the Scottsdale facility expired October 31, 2006. On October 20, 2006 NSC executed a lease for 1,601 square feet of office/warehouse space at 8361 E. Evans Road, Suite 106, Scottsdale, Arizona 85260. The lease for this Scottsdale facility commenced on November 1, 2006 and expires October 31, 2008 and is at a base rental rate of $1,963 per month for the first twelve months and $ 2,028 per month for the final twelve months. In 2009 the Company lease was on a month to month basis.
Future minimum lease obligations at September 30, 2008 are as follows:
| Year Ending September 30, | Amount | |
|---|---|---|
| 2009 | 2028 | |
| $ | 2028 |
Rent expense for the years ended September 30, 2008 and 2007 was approximately $39,000 and $25,000, respectively.
NOTE 11– RELATED PARTY TRANSACTIONS
On July 14, 2008, Mr. Grollman agreed to convert approximately $25,000 of his back pay to 5,000,000 shares of our restricted common stock, at the rate of the average market price per share of $0.005.
On July 14, 2008, Mr. Clark agreed to convert approximately $25,000 of his back pay to 5,000,000 shares of our restricted common stock, at the rate of the average market price per share of $0.005.
On September 8, 2008, Mr. Clark agreed to convert approximately $10,000 of his back pay to 5,000,000 shares of our restricted common stock, at the rate of the average market price per share of $0.002.
During September 30, 2008 the Company issued 196,933 for services provided by the Board of Directors to the Company valued at a weighted trading price of $.015 valued at $3,000.
NOTE 12 - EQUITY
As of September 30, 2008 the Company had 137,276,879 common shares outstanding at a par value of $.01 and no preferred shares outstanding at a par value of $.01.
2008
In September 30, 2008 the Company issued 15,193,966 common shares at the trading price of the common stock of $0.001. The Stock was issued for unpaid compensation to officers and reduced accrued compensation by $63,000. The Company issued 87,078 common shares at a trading price of the common stock of $.0229 and the Company expensed $1,994.
2007
As of September 30, 2007 we are authorized to issue up to 187,000,000 shares of common stock, par value $0.01 per share. As of September 30, 2007, there were 121,995,835 shares of common stock outstanding. We had 868 shareholders of record of common stock on September 30, 2007. Each holder of common stock is entitled to one vote for each share held on all matters. Our articles of incorporation and bylaws do not provide for cumulative voting in elections of directors or any other matters brought before shareholder meetings.
Common Stock
The holders of our common stock are entitled to receive such dividends, if any, as may be declared by our board of directors from time to time out of legally available funds. The dividend rights of our common stock are junior to any preferential dividend rights of any outstanding shares of preferred stock. The holders of our common stock also are entitled to receive distributions upon our liquidation, dissolution or winding up of our assets that are legally available for distribution, after payment of all debt and other liabilities and distribution in full of preferential amounts, if any, to be distributed to holders of our preferred stock.
The holders of our common stock are not entitled to preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
The Company has authorized 4,000,000 shares of preferred stock, at $.001 par value and zero are issued and outstanding as of September 30, 2008. The Corporation established and designates the rights and preferences of a Series A Convertible Preferred Stock, and reserves 4,000,000 shares of preferred stock against its issuance, such rights, preferences and designations. Each share of the Preferred Stock shall have 150 votes on all matters presented to be voted by the holders of our common stock. All 4,000,000 shares of preferred stock that have been issued to our CEO & CF on April 11, 2011 valued at the trading price of the common stock of $0.0095 and recorded as an expense of $38,000.
The issuance of preferred stock by our board of directors could adversely affect the rights of holders of the common stock by, among other things, establishing preferential dividends, liquidation rights or voting powers. See “Risk Factors” above.
Warrants and Options
Options
The Prior board of directors adopted the 2000 Stock Option Plan effective January 1, 2001. Our stockholders formally approved the 2000 Stock Option Plan on February 14, 2001. The Current Board of Directors has terminated the Stock Option Plan.
Under the 2000 Stock Option Plan, the purchase price must be at least 100% of the fair market value of our common stock (if the option is an incentive stock option), or at least 25% of the fair market value of our common stock at the time the option is granted (if the option is a nonqualified grant), or such higher price as may be determined by the Board of Directors at the time of grant. If however, an incentive stock option is granted to an individual who would, immediately before the grant, directly or indirectly own more than 10% of the total combined voting power of all our classes of stock, the purchase price of the shares of common stock covered by such incentive stock option may not be less than 110% of the fair market value of such shares on the day the incentive stock option is granted. As the price of the Company’s common stock is currently quoted on the OTC Bulletin Board, the fair market value of the common stock underlying options granted under the 2000 Stock Option Plan shall be the last closing sale price of the common stock on the day the options are granted. If there is no market price for the common stock, then our Board of Directors may, after taking all relevant facts into consideration, determine the fair market value of our common stock.
As of September 30, 2008, we have granted options to purchase an aggregate of 5,271,756 shares of our common stock, under the plan, leaving a balance of 1,728,244 available for grant. Also as of September 30, 2008, 5,271,756 options are exercisable and 5,271,756 are vested. We have reserved the right to issue a total of 7,000,000 shares of our common stock for issuance under the 2000 Stock Option Plan.
On October 9, 2007, 20,000 options were granted to Greg Szabo, a director, for board services. The weighted average grant date fair value of these options was $0.008. Stock based compensation of $158 was recognized in the financial statements using the Black-Scholes option pricing model value on the grant date. The options have a life of ten years, an exercise price of $0.0212 and vested immediately.
On November 14, 2007, 225,000 options were granted to Graham Clark, a director. The compensation committee as an incentive to retain key staff members approved this grant. The weighted average grant date fair value of these options was $0.016. Stock based compensation of $3,579 was recognized in the financial statements using the Black-Scholes option pricing model value on the grant date. The options have a life of ten years, an exercise price of $0.03 and vested immediately.
On November 14, 2007, 100,000 options were granted to Greg Szabo, a director. The compensation committee approved this grant. The weighted average grant date fair value of these options was $0.016. Stock based compensation of $1,591 was recognized in the financial statements using the Black-Scholes option pricing model value on the grant date. The options have a life of ten years, an exercise price of $0.03 and vested immediately.
On November 14, 2007, 100,000 options were granted to Michael Grollman, a director. The compensation committee as an incentive to retain key staff members approved this grant. The weighted average grant date fair value of these options was $0.016. Stock based compensation of $1,591 was recognized in the financial statements using the Black-Scholes option pricing model value on the grant date. The options have a life of ten years, an exercise price of $0.03 and vested immediately. Also, on November 14, 2007, in lieu of taking a larger grant, 450,000 options granted to Michael Grollman on December 1, 2000 at an exercise price of $1.84 were canceled and exchanged for 450,000 options having an exercise price of $0.03.The incremental cost of the modified award recognized in the financial statements was $4,785.
On January 14, 2008, 20,000 options were granted to Greg Szabo, a director, for board services. The weighted average grant date fair value of these options was $0.0189. Stock based compensation of $379 was recognized in the financial statements using the Black-Scholes option pricing model value on the grant date. The options have a life of ten years, an exercise price of $0.019 and vested immediately.
On April 11, 2008, 20,000 options were granted to Greg Szabo, a director, for board services. The weighted average grant date fair value of these options was $0.0118. Stock based compensation of $236 was recognized in the financial statements using the Black-Scholes option pricing model value on the grant date. The options have a life of ten years, an exercise price of $0.016 and vested immediately.
During the year ended September 30, 2008, no options were exercised.
The Company values all warrants using the Black-Scholes option-pricing model. Critical assumptions for the Black-Scholes option-pricing model include the market value of the stock price at the time of issuance, the risk-free interest rate corresponding to the term of the warrant, the volatility of the Company’s stock price, dividend yield on the common stock, as well as the exercise price and term of the warrant. The warrants are not subject to any form of vesting schedule and, therefore, are exercisable by the holders anytime at their discretion during the life of the warrant. No discounts were applied to the valuation determined by the Black-Scholes option-pricing model for the fiscal year ended September 30, 2008 and 2007:
| 2008 | 2007 | |||
| Risk - free interest rate | 2.66% to 4.23% | 4.46% to 5.03% | ||
| Expected life (years) | 5 | 5 | ||
| Expected volatility | 116.0% to 126.6% | 91.4% to 184.8% | ||
| Expected dividends | None | None | ||
| Forfeitures assumed | None | None | ||
| Weighted average grant date fair value | $0.0157 | $0.0280 |
The following table summarizes the stock option activity during the fiscal year ended 2008:
| Number of Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term (1) | Aggregate Intrinsic Value (2) | ||||||||||||
| Options Outstanding, September 30, 2007 | 4,786,756 | $ | 0.74 | 6.16 | |||||||||||
| Granted | 935,000 | 0.03 | |||||||||||||
| Exercised | — | — | |||||||||||||
| Forfeited or expired | (450,000 | ) | 1.84 | ||||||||||||
| Options Outstanding, September 30, 2008 | 5,271,756 | $ | 0.43 | 5.97 | $ | — | |||||||||
| Options Exercisable September 30, 2008 | 5,271,756 | $ | 0.43 | 5.97 | — | ||||||||||
| (1) | Remaining contractual term is presented in years | |
| (2) | The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the closing price of our common stock as of September 30, 2008, for those awards that have an exercise price currently below the closing price as of September 30, 2008. Awards with an exercise price above the closing price above the closing price of $0.015 as of September 30, 2008 are considered to have no intrinsic value |
Warrants
During the year ended September 30, 2008, no awards were granted, no share purchase warrants were exercised, and no warrants were forfeited.
On June 24, 2008, three year warrants previously issued to purchase 135,000 shares of common stock with an exercise price of $0.062 expired.
On June 25, 2008, our board of directors resolved to instruct management to extend the expiration date of 2,550,000 warrants previously set to expire on September 30, 2008. The warrants, issued to purchase 2,550,000 shares of common stock exercisable at prices ranging from $0.35 to $0.75 per share were issued between July 2003 and March 2004 in connection with the June 2003 Private Placement Memorandum. The fair value of these warrants, on September 30, 2008, determined to be $9,099 was expensed as stock based compensation.
The following assumptions were utilized to value warrants during the fiscal year ended September 30, 2008 and 2007:
| 2008 | 2007 | ||
| Risk - free interest rate | 2.36% | 4.60% to 4.91% | |
| Expected life (years) | 1 | 2 to 5 | |
| Expected volatility | 228.0% | 83.5% to 184.8% | |
| Expected dividends | None | None | |
| Forfeitures assumed | None | None | |
| Weighted average grant date fair value | $0.0037 | $0.0147 |
The following table summarizes the warrant activity during the year ended September 30, 2008:
| Number of Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term (1) | Aggregate Intrinsic Value (2) | |||||||||||
| Warrants Outstanding, September 30, 2007 | 17,714,197 | $ | 0.17 | 1.56 | ||||||||||
| Granted | - | - | - | |||||||||||
| Exercised | - | - | - | |||||||||||
| Expired | (135,000 | ) | - | - | ||||||||||
| Warrants Outstanding, September 30, 2008 | 17,579,197 | $ | 0.18 | 1.18 | $ | - | ||||||||
| (1) | Remaining contractual term is presented in years | |
| (2) | The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the closing price of our common stock as of September 30, 2008, for those awards that have an exercise price currently below the closing price as of September 30, 2008. Awards with an exercise price above the closing price as of September 30, 2008 of $0.015 are considered to have no intrinsic value. |
Currently all warrants and options issued have expired as of the filing of this 10K.
NOTE 13 - INCOME TAXES
The provision (benefit) for income taxes from continued operations for the years ended September 30, 2008 and 2007 consist of the following:
| September 30, | |||||||
| 2008 | 2007 | ||||||
| Current: | |||||||
| Federal | $ | 0 | $ | 0 | |||
| State | 0 | 0 | |||||
| $ | 0 | $ | 0 | ||||
| Deferred: | |||||||
| Federal | $ | 6,948,525 | $ | 6,839,000 | |||
| State | 209,859 | 178,000 | |||||
| 7,158,384 | 7,017,000 | ||||||
| Valuation allowance | (7,158,384 | ) | (7,017,000 | ) | |||
| Provision benefit for income taxes, net | $ | - | $ | - | |||
The difference between income tax expense computed by applying the federal statutory corporate tax rate and actual income tax expense is as follows:
| September 30, | |||||||
| 2008 | 2007 | ||||||
| Statutory federal income tax rate | 34.0 | % | 34.0 | % | |||
| State income taxes and other | 9.0 | 9.0 | |||||
| Valuation allowance | (43.0 | ) | (43.0 | ) | |||
| Effective tax rate | 0.0 | % | 0.0 | % | |||
Deferred income taxes result from temporary differences in the recognition of income and expenses for the financial reporting purposes and for tax purposes. The tax effect of these temporary differences representing deferred tax asset and liabilities result principally from the following:
| September 30, | |||||||
| 2008 | 2007 | ||||||
| Net operating loss carryforward | $ | 24,026,525 | $ | 23,917,000 | |||
| Valuation allowance | (24,026,525 | ) | (23,917,000 | ) | |||
| Deferred income tax asset | $ | - | $ | - | |||
The Company has a net operating loss carryforward of approximately $24.0 million available to offset future taxable income through 2030. For income tax reporting purposes, the Company’s aggregate unused net operating losses are subject to limitations of Section 382 of the Internal Revenue Code, as amended. Under the Tax Reform Act of 1986, the benefits from net operating losses carried forward may be impaired or limited on certain circumstances. Events which may cause limitations in the amount of net operating losses that the Company may utilize in any one year include, but are not limited to, a cumulative ownership change of more than 50% over a three-year period. The consolidation of any limitations that may be imposed for future issuances of equity securities, including issuances with respect to acquisitions have not been determined. The Company has provided a valuation reserve against the full amount of the net operating loss benefit, because in the opinion of management based upon the earning history of the Company; it is more likely than not that the benefits will not be realized.
ASC 740 requires the consideration of a valuation allowance to reflect the likelihood of realization of deferred tax assets. Significant management judgment is required in determining any valuation allowance recorded against deferred tax assets. In evaluating the ability to recover deferred tax assets, the Company considered available positive and negative evidence, giving greater weight to its recent cumulative losses and its ability to carry-back losses against prior taxable income and lesser weight to its projected financial results due to the challenges of forecasting future periods. The Company also considered, commensurate with its objective verifiability, the forecast of future taxable income including the reversal of temporary differences. At that time the Company continued to have sufficient positive evidence, including recent cumulative profits, a reduction in operating expenses, the ability to carry-back losses against prior taxable income and an expectation of improving operating results, showing a valuation allowance was not required. At the end of the year ended September 30, 2007, changes in previously anticipated expectations and continued operating losses necessitated a valuation allowance against the tax benefits recognized in this quarter and prior quarters since they are no longer “more-likely-than-not” realizable. Under current tax laws, this valuation allowance will not limit the Company’s ability to utilize U.S. federal and state deferred tax assets provided it can generate sufficient future taxable income in the U.S.
The Company anticipates it will continue to record a valuation allowance against the losses of certain jurisdictions, primarily federal and state, until such time as it is able to determine it is “more-likely-than-not” the deferred tax asset will be realized. Such position is dependent on whether there will be sufficient future taxable income to realize such deferred tax assets. The Company’s effective tax rate may vary from period to period based on changes in estimated taxable income or loss by jurisdiction, changes to the valuation allowance, changes to federal, state or foreign tax laws, future expansion into areas with varying country, state, and local income tax rates, deductibility of certain costs and expenses by jurisdiction.
NOTE 14 – SUBSEQUENT EVENTS
In accordance with the Subsequent Events Topic of the FASB ASC 855, Management has evaluated subsequent events, and has determined that the following events are reasonably likely to impact the financial statements:
2012
During the ten months ended July 31, 2012, the Company issued the following shares:
The Company issued 30,000,000 common shares for there the reduction of debt from our CEO and reduced debt of the company by $3,325,949.
The Company issued 19,000 common shares for services rendered by professionals and recorded the expenses based upon the trading price of the common shares of $0.0102 and expensed $204 as consulting fees.
The Company issued 1,055,333 common shares to third parties that have purchased our medical billing software at the trading price of the common stock of $0.0160 and recorded a revenue contra account of $20,871.
The Company issued 90,000 common shares for $18,000 in cash.
The Company issued 200,000 common shares to Kroos Medical Management in accordance with the acquisition agreement and recorded it at the trading price of the common shares of $0.020 and recorded an investment in Kroos Medical Management of $4,000.
2011
During the year ended September 30, 2011, the Company issued 15,000,000 common shares at the trading price of the common stock of $0.007. The Stock was issued for compensation to new management and recorded and expense of $105,000.
The Company issued 250,000 in common stock for $25,000 in cash.
2010
No common stock was issued.
2009
In September 30, 2009 the Company issued 28,000,000 common shares at the trading price of the common stock of $0.001. The Stock was issued for unpaid compensation to officers and reduced accrued compensation by $35,800.
Software asset purchases:
On June 22, 2012 the Company acquired Doctors Network of America in Flowood, Mississippi for 500,000 common shares and the acquisition was valued at $10,000.
The fair value of the consideration and the assets acquired is based on the aggregate value of the common stock issued in exchange for the software as shown below:
| June 22, 2012 | ||||
| Fair Value of Consideration: | ||||
| Common Stock (500,000 common shares valued at $.008) | $ | 10,000 | ||
| Total Purchase Price | $ | 10,000 | ||
Fair Value of Assets acquired: |
||||
| Assets: | ||||
| Software | $ | 10,000 | ||
| Fair value of total assets | $ | 10,000 | ||
The acquisitions terms are as follows:
| 1. | Cloud-MDs will provide medical billing services under Cloud-MDs Corporation. No need for a wholly owned subsidiary to be formed to accommodate medical billing. |
| 2. | DNA will provide personnel, network access, facilities and expertise to deliver contracted billing service support to Cloud-MDs for clients that Cloud-MDs sends to DNA for billing support. DNA will be compensated on an agreed upon schedule. All revenue will be reported as Cloud-MDs revenue and tracked separately. |
| 3. | Goal is to grow DNA related gross revenue within Cloud-MDs to 3X, or greater, current DNA gross revenue over the next 3 years. |
| 4. | Cloud-MDs will acquire the assets of Krooss Medical management Systems, LLC once the revenue goals in item #3 are achieved. As consideration to Bill Krooss and Marie Krooss (collectively “Ownership”) of Krooss Medical Management Systems, LLC for the acquisition of DNA, Cloud-MDs proposes the following: |
| a. | Current Ownership will remain in their current roles at Krooss Medical Management Systems, LLC with current responsibilities and will take on additional responsibilities of sales and marketing in the territory of the state of Mississippi and surrounding states and will assume new responsibilities within Cloud-MDs corporate. | |
| b. | Current executive management will remain for a period of not less than 1 year after the acquisition and Ownership salary will be established as $200,000.00. | |
| c. | Current Ownership will be awarded NSCT class 144 stock in the amount of 500,000 common shares, with anti-dilution clause, that is restricted for 12 months during which time Ownership may not sell the awarded stock. These shares will be awarded as follows: |
| i. | Upon acquisition, 200,000 shares of class 144 common stock with a 12 month restriction with applicable anti-dilution clause. This stock is non-refundable to NSCT in the event of any misrepresentations by NSCT to Krooss Medical Management Systems, LLC during the acquisition or the first 90 days following the acquisition; | |
| ii. | After the first 90 days following the acquisition, NSCT will award to the Ownership 300,000 shares of class 144, common stock with a 12 month restriction with applicable anti-dilution clauses. |
| d. | For one (1) year after the acquisition, current Ownership will have the opportunity to earn up to 125,000 shares, per calendar quarter, of addition shares of NSCT common stock based on meeting certain performance criteria. The shares will be issued with applicable anti-dilution clause. |
| 5. | The acquired assets and customer base of Krooss Medical Management Systems, LLC will be merged into the current asset and customer base of Cloud-MDs. | |
| 6. | Upon the purchase of DNA by Cloud-MDs, all liabilities of DNA as of the date of asset transfer will be the responsibility of Krooss Medical management Systems, LLC. | |
| 7. | DNA cannot add new clients. New clients will be sent to Cloud-MDs. | |
| 8. | DNA ownership may be called upon to act as a reference for Cloud-MDs and/or work with Cloud-MDs senior management in an advisory capacity. | |
| 9. | DNA will become a Cloud-MDs software (PM/EMR/RM) reseller and will acquire an enterprise license with unlimited use: |
| a. | Enterprise license is $100,000 and includes all updates, hosting, etc. and DNA receives 100,000 shares of Cloud-MDs 144 class stock if done prior to stock roll-back. If after roll-back, stock award shall equal $100,000/then current share price. Leasing is available. | |
| b. | DNA can resell licenses to current DNA clients or other clients: |
| i. | MSRP of software license is $50,000/provider, leasing is available | |
| ii. | A commission of up to 40% of revenue on each software license sold goes to DNA + up to 3,000 shares of stock per software license sold and support will be provided by DNA staff after training. | |
| iii. | All revenue other than commissions goes to Cloud-MD | |
| iv. | DNA takes 1st support call on all software licenses it sells | |
| v. | Primary, non-exclusive, territories are the states of Mississippi, Alabama and Louisiana with additional non-exclusive territories permissible based on a specific sales opportunity |
| c. | DNA will become official beta site for Cloud-MDs PM/EMR/RM software releases. |
| 10. | Upon acquisition of DNA by Cloud-MDs, Cloud-MDs will assume employer responsibilities for all current Krooss Medical Management Systems, LLC employees. All employees of Krooss Medical Management Systems, LLC will remain employed by Cloud-MDs in their current positions for at least 180 days after the acquisition after which time each will be evaluated for their then current responsibilities or considered for new responsibilities. | |
| 11. | Independent Medical Practice Support, LLC (“IMPS”) assets and customer base are not part of this acquisition discussion or negotiation and any IMPS related expenses or liabilities must be removed from DNA accounting records and will not be considered in future acquisition discussions or negotiations. |
On August 14, 2012
The Company through a comprehensive agreement with MediSouth, LLC, has purchased a complete source code license and will integrate and enhance this feature set as part of its ever expanding Cloud-MD Office product suite.
The fair value of the consideration and the assets acquired is based on the aggregate value of the common stock issued in exchange for the software as shown below:
| August 14, 2012 | ||||
| Fair Value of Consideration: | ||||
| Common Stock (100,000 common shares valued at $.025) | $ | 2,500 | ||
| Total Purchase Price | $ | 2,500 | ||
Fair Value of Assets acquired: |
||||
| Assets: | ||||
| Software source code | $ | 2,500 | ||
| Fair value of total assets | $ | 2,500 | ||
2.4. Principal Conditions and Consideration.
Subject to all of the terms and conditions set forth in this Agreement, and in consideration of the sale, assignment, transfer and delivery of the Purchased Assets by Licensor to Licensee, as of the Effective Date of this agreement Licensee and Licensor agree to the following (the "Purchase Consideration"):
| a) | MediSouth will provide Cloud-MD with the software source code for the License, expertise to install and integrate the License into Cloud-MD product offerings and deliver the MediSouth software source code to Cloud-MD. |
| b) | For as long as Licensee makes use of the License, Licensor agrees to provide Licensee with all software updates for the License within 5 working days of Licensor implementing those same software updates in its own production version of the License. |
| c) | Licensor agrees that Licensee shall have the right to modify the software contained in the License to meet Licensee's operational needs. |
| d) | Cloud-MD shall provide back to MediSouth, with the permission of each affected Cloud-MD client, the de-identified purchasing data it collects as a result of Cloud-MD clients utilizing the embedded Medical Supplies and Pharmaceutical Inventory Management functionality provided by the License. |
| e) | MediSouth shall receive 100,000 shares of non-diluteable, Class 144, Cloud-MD stock with a one (1) year trading restriction if this agreement is executed prior to Licensee's planned stock roll-back. If this agreement is executed after the Licensee's stock roll-back, the stock award shall equal $300, OOO/then current share price of Licensee's publicly traded stock. MediSouth shall complete the Subscription Agreement. |
| f) | MediSouth will grant Cloud-MD all necessary rights to use the License and related patents and those rights shall be transferable to any Licensee who may purchase Cloud-MD and its assets. |
| g) | In the event that Cloud-MD is acquired by another entity, the use of the License shall transfer to the acquiring entity with the provision that the License may be used only within the Cloud-MD software application as it was used at the time of the acquisition. |
| h) | Other than 2.4.g above, Cloud-MD may not sell the License, transfer the License, allow any other entity to use the License as part of that entity's software application or transfer any rights to another party to use the License separately from Cloud-MD products in which the License is embedded. |
| i) | If from the Effective Date of this agreement through the end of the 1st year following the Permanent Transfer, Licensee discovers any misrepresentations on the part of Licensor to Licensee or Licensor, Licensee or Licensor will have the right to dissolve this agreement. Should this occur, Licensor will be required to forfeit the stock awarded to it as described in item 2.4.e above with that stock having an established per share value equal to the value of a share of Licensee's stock as officially recorded on the date of Licensee's stock rollback and any assets from the original acquisition that still remain under the control of Licensee will be returned to Licensor and in their then current condition or status and both parties will exit the relationship holding each other harmless in all matters. |
| j) | Licensor agrees to provide a software device in the licensed software that will offer an automatic logon from Licensee's software. |
| k) | Licensor agrees to provide software operations, communications and hosting services for the licensed software in same computing facility as Licensee has its operational software until Licensee and Licensor mutually agree that this service is no longer necessary, |
| 1) | Licensee and Licensor agree to execute sales agreements for each other's products and services. |
| m) | Licensee agrees that if due to Licensee modifications to the Licensed software, data from the modified Licensed software cannot be processed by Licensor, Licensee shall provide data in original Licensed software format and make that data available to Licensor as in 2.4.d. |
| n) | Licensee shall have the right to copy and use all forms of documentation, marketing information and data related to the License. |
| o) | Licensee shall have the right to brand the License with a product name selected by the Licensee and Licensor. |
| p) | Licensor shall change the appearance of the License's end-user computer display screens to match the appearance of the Licensee's computer application's end-user computer displays, including the branding described in 2.4.0 above. |
| q) | In the event that Licensor should cease doing business, Licensee shall have the right to continue usage of the License and all associated documentation and related marketing material without recourse from Licensor or any entity representing Licensor's interests. |
| r) | The "powered by MediScan" text or graphic must always be displayed on modules and application web pages provided by Licensor. |
2.5 Holdback Stock
Pursuant to the terms and subject to the conditions of this Agreement, no provisions shall be made for the purpose of withholding previously awarded stock by Licensee to Licensor other than those already cited in section 2.4 of this Agreement.
Cloud-MD Inventory Management System is a ground breaking Cloud-based Medical Supply Inventory Management System, specifically designed for small and medium Medical Practices, DME's, Home Health, Long-term Care, and Surgery Centers that will offer:
1. Centralized management control
over medical supply and drug inventories
2. Real time utilization and financial inventory summary
3. Low price notifications
4. Par level/reorder tracking
5. RX expiration tracking
6. Auto supply re-ordering from a practices established suppliers
Based on a barcode scanning technology, Cloud-MD Inventory Management is designed to reduce workloads by automating inventory control processes. The Cloud-MD's Inventory Management System requires no installation, on-site software or special hardware. The new Cloud-MD Office application suite, of which Cloud-MD Inventory Management is a part, is fully delivered via the Internet and requires no special computing environment at the end-user facility.
Manual inventory is time consuming, labor intensive and leaves room for error. Benefiting healthcare professionals across the country, Cloud-MD's Inventory Management System is a low-cost, low-maintenance, easy to use automated system that can provide effective solutions.
On January 1, 2011 the Company agreed to purchase the software and the source code from Absolute Medical Software Systems, LLC a company controlled by Michael De La Garza. The Company purchased the software for a $5,000,000 promissory note for 8% interest rate the note matures on April 30, 2021. On October 5, 2011 the Company issued Absolute Medical Software Systems, LLC 30,000,000 common shares in full payment of this promissory note. The Company valued the software and source code on the development cost of the software provided by Absolute Medical Software Systems, LLC of $4,296,000.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
Effective May 26, 2012, the Registrant engaged S. E. Clark & Company, P.C. The previous auditor Semple, Marchal & Cooper, LLP resigned as auditors on December 16, 2011 a letter was delivered to the Company via certified mail.
During the period January 26, 2007 through December 16, 2011 through the date they resigned Semple, Marchal & Cooper, LLP there have been no disagreements with Semple, Marchal & Cooper, LLP (as defined in Item 304(a)(1)(iv) of Regulation S-K) on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of Semple, Marchal & Cooper, LLP would have caused them to make reference thereto in their report on financial statements for such years.
During the period January 26, 2007 through December 16, 2011 through the date of the dismissal of Semple, Marchal & Cooper, LLP, there were no reportable events as defined in Item 304(a)(1)(v) of Regulation S-K.
(b) New independent registered public accounting firm.
On May 26, 2012, and effective the same date, on the recommendation of the Registrant’s Board of Directors, the Registrant engaged S. E. Clark & Company, P.C as its independent registered public accounting firm to audit the Registrant’s financial statements for the fiscal year ended September 30, 2008 and to perform procedures related to the financial statements included in the Registrant’s quarterly reports on Form 10-Q, beginning with the quarter ending December 31, 2008 through current.
During the two fiscal years ended September 30, 2007 and 2006, and through the date of the engagement of S. E. Clark & Company, P.C, neither the Registrant nor anyone on its behalf has consulted with S. E. Clark & Company, P.C, regarding either:
| (a) | The application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Registrant’s financial statements, and neither was a written report provided to the Registrant nor was oral advice provided that S. E. Clark & Company, P.C, concluded was an important factor considered by the Registrant in reaching a decision as to an accounting, auditing, or financial reporting issue; or |
| (b) | Any matter that was either the subject of a disagreement or a reportable event, as each term is defined in Items 304(a)(1)(iv) or (v) of Regulation S-K, respectively. |
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and that such information is accumulated and communicated to our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Our disclosure controls and procedures were designed to provide reasonable assurance that the controls and procedures would meet their objectives. As required by SEC Rule 13a-15(b), our Chief Executive Officer and Chief Financial Officer carried out an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this report. Based on the foregoing, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not effective at the reasonable assurance level.
Management’s Annual Report on Internal Control over Financial Reporting
Our Chief Executive Officer and Chief Financial Officer are responsible for establishing and maintaining adequate internal control over our financial reporting. In order to evaluate the effectiveness of internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act, management has conducted an assessment, including testing, using the criteria in Internal Control — Integrated Framework, issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Our system of internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Management has used the framework set forth in the report entitled Internal Control-Integrated Framework published by the Committee of Sponsoring Organizations of the Treadway Commission, known as COSO, to evaluate the effectiveness of our internal control over financial reporting. Based on this assessment, our Chief Executive Officer and Chief Financial Officer have concluded that our internal control over financial reporting was not effective as of September 30, 2008.
The Company’s material weaknesses in financial reporting were:
The inability of the Company to prepare and file its financial statements timely due to its limited financial and personnel resources and delays in the Company’s ability to respond to SEC inquiries regarding financial and accounting presentation. Further, the Company is delinquent in filings for fiscal year ended 2011 and 2012.
There were no changes in our internal control over financial reporting that occurred during the year ended September 30, 2008 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
It should be noted that any system of controls, however well designed and operated, can provide only reasonable and not absolute assurance that the objectives of the system are met. In addition, the design of any control system is based in part upon certain assumptions about the likelihood of certain events. Because of these and other inherent limitations of control systems, there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions, regardless of how remote.
There are no events required to be disclosed by the Item.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS; COMPLIANCE WITH SECTION 16(a) OF THE EXCHANGE ACT.
Director and Executive Officer
Set forth below is information regarding the Company’s current directors and executive officers. There are no family relationships between any of our directors or executive officers. The directors are elected annually by stockholders. The executive officers serve at the pleasure of the Board of Directors.
| Name | Age | Title | ||
| Michael De La Garza | 54 | Chairman, CEO, President | ||
| Pamela J. Thompson | 49 | Chief Financial Officer, Secretary, Treasurer, Directors |
The chief executive officer and director and officer of the Company will hold office until additional members or officers are duly elected and qualified. The background and principal occupations of the sole officer and director of the Company is as follows:
Michael De La Garza
Cloud Medical Doctor Software Corporation – 2010-Present. President, CEO, Chairman of the Board,
Card Activation Technology Corporation. - President, CEO, Chairman of the Board a gift card technology company- 2008-2009
MedCom USA, Corporation. -President, CEO, Chairman of the Board of a medical software company - 2008-2009
PayMed USA, LLC President and co-founder of a medical software company -2003-2012,
Providers Solution, Corporation. President and co-founder of a medical software company -2000-2012,
Accident and Injury Medical Centers, Corporation, President and co-founder of a medical rehab company 1993-1999
Medical Synergies, Corporation. COO, President, a medical billing company. 1991-1999
The Center’s for Diagnostic Medical Services, Corporation. COO and President, a medical imaging group company 1991-1999
Pamela Thompson
Cloud Medical Doctor Software Corporation; 2010-Present; Chief Financial Officer
Ms. Pamela J. Thompson, CPA, P.C. has been Chief Financial Officer, Treasurer, Secretary and Director of Cloud Medical Doctor Software Corporation since November 19, 2010. Ms. Thompson is the Founder of Pamela J. Thompson CPA PC and serves as its Principle Executive Officer.
Ms. Thompson formed Pamela J. Thompson, CPA, P.C. in 1990 and has over 20 years of experience in SEC compliance, tax and accounting for publicly traded companies. Her Sarbanes-Oxley Section compliance implementation experience is focused on assisting publicly traded companies with performing risk assessment on critical business process and documenting business process controls. She served as Chief Financial Officer, Secretary and Treasurer of several public companies. Ms. Thompson started her career in 1986 with the international accounting firm of Arthur Andersen and was subsequently with Pannell Kerr Forester, and one of the larger regional firms Eide, Bailey and Company. Ms. Thompson is featured in Arizona Republic, New Jersey Star, Arizona Women's Success Magazine, National Basketball Players Association Magazine, and Behind the Bench: National Basketball Wives Association Magazine and Wall Street Journal. She is a Certified Fraud Examiner Candidate. Ms. Thompson is also a Member of the Arizona Women's Society of Certified Public Accountants and Behind the Bench: National Basketball Wives Association. She is a Member of the American Institute of Certified Public Accountants. She is also a Member of the Association of Certified Fraud Examiners and Arizona Association of Certified Fraud Examiners. Ms. Thompson holds a Bachelor of Science from Minnesota State University - Moorhead in Accountancy in 1986 and holds her licenses as a Certified Public Accountant in the State of Arizona in 1993.
Audit Committee Financial Expert
The Company does not have an audit committee or a compensation committee of its board of directors. In addition, the Company’s board of directors has determined that the Company does not have an audit committee financial expert serving on the board. When the Company develops its operations, it will create an audit and a compensation committee and will seek an audit committee financial expert for its board and audit committee.
Conflicts of Interest
Members of our management are associated with other firms involved in a range of business activities. Consequently, there are potential inherent conflicts of interest in their acting as officers and directors of our company. Although the officers and directors are engaged in other business activities, we anticipate they will devote an important amount of time to our affairs.
Our officers and directors are now and may in the future become shareholders, officers or directors of other companies, which may be formed for the purpose of engaging in business activities similar to ours. Accordingly, additional direct conflicts of interest may arise in the future with respect to such individuals acting on behalf of us or other entities. Moreover, additional conflicts of interest may arise with respect to opportunities which come to the attention of such individuals in the performance of their duties or otherwise. Currently, we do not have a right of first refusal pertaining to opportunities that come to their attention and may relate to our business operations.
Our officers and directors are, so long as they are our officers or directors, subject to the restriction that all opportunities contemplated by our plan of operation which come to their attention, either in the performance of their duties or in any other manner, will be considered opportunities of, and be made available to us and the companies that they are affiliated with on an equal basis. A breach of this requirement will be a breach of the fiduciary duties of the officer or director. If we or the companies with which the officers and directors are affiliated both desire to take advantage of an opportunity, then said officers and directors would abstain from negotiating and voting upon the opportunity. However, all directors may still individually take advantage of opportunities if we should decline to do so. Except as set forth above, we have not adopted any other conflict of interest policy with respect to such transactions.
Compliance with Section 16(A) Of the Exchange Act 9.A. Directors and Executive Officers, Promoters, and Control Persons:
As of September 30, 2008, the Company is aware that all filings of Form 4 and 5 required of Section 16(a) of the Exchange Act of Directors, Officers or holders of 10% of the Company's prior management and board of directors have not filed the requirements timely.
Code of Ethics
We have adopted a code of ethics that applies to all of our executive officers, directors and employees. Code of ethics codifies the business and ethical principles that govern all aspects of our business. This document will be made available in print, free of charge, to any shareholder requesting a copy in writing from the Company. A form of the code of conduct and ethics was filed as Exhibit 14.1 to this Annual Report on Form 10-K for September 30, 2004.
ITEM 11. EXECUTIVE COMPENSATION
Executive Officers and Directors
THESE OFFICERS AND DIRECTORS ARE NO LONGER WITH THE COMPANY.
The following tables set forth certain information concerning all compensation paid, earned or accrued for service by (i) our Principal Executive Officer and Principal Financial Officer and (ii) all other executive officers who earned in excess of $100,000 in the three fiscal years ended September 30, 2008, and each of the other two most highly compensated executive officers of the Company who served in such capacity at the end of the fiscal year whose total salary and bonus exceeded $100,000 (collectively, the “Named Executive Officer”):
2008 and 2007 SUMMARY COMPENSATION TABLE
| Name and Principal Position |
Year | Salary ($) |
Bonus ($) |
Stock Awards ($) * |
Option Awards ($) * |
Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation ($) | All Other Compensation ($) |
Total ($) |
| Michal Grollman, Chairman of the Board, Chief Executive Officer, Principal Accountant | 2006 2007 2008 |
180,000 180,000 180,000 |
- - |
-25,000 | - - |
- - |
- - |
- - |
180,000 180,000 205,000 |
| Graham L. Clark Chief Operation Officer |
2006 2007 2008 |
150,000 150,000 150,000 |
- - 35,000 |
150,000 150,000 185,000 |
2008 and 2007 OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END TABLE
The following table sets forth certain information concerning outstanding stock awards held by the Named Executive Officers as of September 30, 2008:
| Option Awards | Stock Awards | ||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
Option Exercise Price ($) |
Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) |
Market Value of Shares or Units of Stock That Have Not Vested ($) |
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) |
| Michael Grollman | -0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- |
| Graham Clark | -0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- |
2008 and 2007 OPTION EXERCISES AND STOCK VESTED TABLE
| Option Awards | Stock Awards | ||||
| Name | Year | Number of Shares Acquired on Exercise (#) | Value Realized on Exercise ($) | Number of Shares Acquired on Vesting (#) | Value Realized on Vesting ($) |
| Michael Grollman | 2007 and 2008 | ||||
| Graham Clark | 2007 and 2008 | ||||
2008 and 2007 PENSION BENEFITS TABLE
| Name | Year | Plan Name | Number of Year of Credited Service | Present Value of Accumulated Benefit ($) | Payments During Last Fiscal Years (s) |
| Michael Grollman | 2007 and 2008 | ||||
| Graham Clark | 2007 and 2008 |
2008 and 2007 NONQUALIFIED DEFERRED COMPENSATION TABLE
| Name | Year | Executive Contribution in Last Fiscal Year ($) | Registrant Contributions in Last Fiscal Year ($) | Aggregate Earnings in Last Fiscal Year ($) | Aggregate Withdrawals/ Distributions | Aggregate Balance of Last Fiscal Year-End ($) |
| Michael Grollman | 2007 and 2008 | |||||
| Graham Clark | 2007 and 2008 |
2008 and 2007 DIRECTOR COMPENSATION TABLE
| Name | Fees Earned or Paid in Cash ($) |
Stock Awards ($) * |
Option Awards ($) * |
Non-Equity Incentive Plan Compensation ($) |
Nonqualified Deferred Compensation Earnings
($) |
All Other Compensation ($) |
Total ($) |
| Michael Grollman | - | - | - | - | - | - | - |
| Gregory Szabo | 2,000 | 5,000 | - | - | - | - | 7,000 |
| Graham Clark | - | - | - | - | - | - | - |
| * | Based upon the aggregate grant date fair value calculated in accordance with the Accounting Codification Standard Topic 718 “Share-Based Payments. Our policy and assumptions made in valuation of share based payments are contained in the notes to our financial statements. The monies shown in the “option awards” column is the total calculated value for each individual. |
2008 AND 2007 ALL OTHER COMPENSATION TABLE
| Name | Year | Perquisites and Other Personal Benefits ($) | Tax Reimbursement ($) | Insurance Premiums ($) | Company Contributions to Retirement and 401(k) Plans ($) | Severance Payments/ Accruals ($) | Change in Control Payments/ Accruals ($) | Total ($) |
| Michael Grollman | 2007 | |||||||
| 2008 |
2008 AND 2007 PERQUISITES TABLE
| Name | Year | Personal Use of Company Car/Parking ($) | Financial Planning/Legal Fees ($) | Club Dues ($) | Executive Relocation ($) | Total Perquisites and Other Personal Benefits ($) |
| Michael Grollman | 2007 | |||||
| 2008 | ||||||
| Graham Clark | 2007 | |||||
| 2008 |
2008 AND 2007 POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL TABLE
| Before Change in Control | After Change in Control | ||||||
| Name | Benefit | Termination w/o Cause or for Reason | Termination w/o Cause or for Good Reason | Voluntary Termination | Death | Disability | Change in Control |
| Michael Grollman | |||||||
| Graham Clark |
Employment Contracts
All Employment agreements have expired.
Compensation of Directors
We paid $7,000 to currently provide our directors with cash and stock compensation.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT.
The following table lists stock ownership of our Common Stock as of December 2, 2012 based on 211,276,212 shares of common stock issued and outstanding on a fully diluted basis. The information includes beneficial ownership by (i) holders of more than 5% of our Common Stock, (ii) each of our directors and executive officers and (iii) all of our directors and executive officers as a group. Except as noted below, to our knowledge, each person named in the table has sole voting and investment power with respect to all shares of our Common Stock beneficially owned by them.
| Name and Address of Owner | Title of Class | Number of Shares Owned (1) | Percentage of Class |
Michael De La Garza(1) c/o Cloud 736 East Braeburn Drive Phoenix, AZ 85022 |
Common Stock | 40,000,000 | 18.93% |
Pamela Thompson(2) c/o Cloud 736 East Braeburn Drive Phoenix, AZ 85022
|
Common Stock | 5,000,000 | 2.36% |
| All Officers and Directors | Common Stock | 45,000,000 | 21.29% |
| As a Group (2 persons) | Common Stock | 45,000,000 | 21.29% |
| (1) | Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. |
| (2) | Pamela Thompson does not have beneficial ownership to the stock issued to her; those shares are held in an irrevocable trust for the benefit of her minor children, that she does not have control or voting control of those shares. |
Changes in Control
We are not aware of any arrangements that may result in a change in control of the Company.
DESCRIPTION OF SECURITIES
General
The Company is authorized to issue 650,000,000 shares of common stock, $0.01 par value, of which 137,276,879 common shares were issued and outstanding as of September 30, 2008.
Common Stock
The holders of our common stock are entitled to receive such dividends, if any, as may be declared by our board of directors from time to time out of legally available funds. The dividend rights of our common stock are junior to any preferential dividend rights of any outstanding shares of preferred stock. The holders of our common stock also are entitled to receive distributions upon our liquidation, dissolution or winding up of our assets that are legally available for distribution, after payment of all debt and other liabilities and distribution in full of preferential amounts, if any, to be distributed to holders of our preferred stock.
The holders of our common stock are not entitled to preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
The Company has authorized 4,000,000 shares of preferred stock, at $.001 par value and zero are issued and outstanding as of September 30, 2008. The Corporation established and designates the rights and preferences of a Series A Convertible Preferred Stock, and reserves 4,000,000 shares of preferred stock against its issuance, such rights, preferences and designations. Each share of the Preferred Stock shall have 150 votes on all matters presented to be voted by the holders of our common stock. All 4,000,000 shares of preferred stock that have been issued to our CEO & CF on April 11, 2011 valued at the trading price of the common stock of $0.0095 and recorded as an expense of $38,000.
The issuance of preferred stock by our board of directors could adversely affect the rights of holders of the common stock by, among other things, establishing preferential dividends, liquidation rights or voting powers. See “Risk Factors” above.
Warrants and Options
All warrants and options issued have expired.
Stock Option Plan
Our board of directors adopted the 2000 Stock Option Plan effective January 1, 2001. Our stockholders formally approved the 2000 Stock Option Plan on February 14, 2001. The 2000 Stock Option plan terminates in accordance with the term on December 1, 2010. The Current Board of Directors has not approved nor extended the Stock Option Plan therefore it is terminated.
Voting Rights
Each holder of Common Stock is entitled to one vote for each share of Common Stock held on all matters submitted to a vote of stockholders.
Dividends
Subject to preferences that may be applicable to any then-outstanding shares of Preferred Stock, if any, and any other restrictions, holders of Common Stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by the Company’s board of directors out of legally available funds. The Company and its predecessors have not declared any dividends in the past. Further, the Company does not presently contemplate that there will be any future payment of any dividends on Common Stock.
Convertible Securities
The Company has one convertible security with Strategic Working Capital Fund, LP.
Amendment of our Bylaws
Our bylaws may be adopted, amended or repealed by the affirmative vote of a majority of our outstanding shares. Subject to applicable law, our bylaws also may be adopted, amended or repealed by our board of directors.
Transfer Agent
On August 31, 2011, the Company engaged Pacific Stock Transfer to serve in the capacity of transfer agent. Their mailing address and telephone number are as follows: Pacific Stock Transfer, 4045 South Spencer #403, Las Vegas, NV 89119 - (702) 361-3033.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
On July 14, 2008, Mr. Grollman agreed to convert approximately $25,000 of his back pay to 5,000,000 shares of our restricted common stock, at the rate of the average market price per share of $0.005.
On July 14, 2008, Mr. Clark agreed to convert approximately $25,000 of his back pay to 5,000,000 shares of our restricted common stock, at the rate of the average market price per share of $0.005.
On September 8, 2008, Mr. Clark agreed to convert approximately $10,000 of his back pay to 5,000,000 shares of our restricted common stock, at the rate of the average market price per share of $0.002.
During September 30, 2008 the Company issued 281,044 for services provided by the Board of Directors to the Company valued at a weighted trading price of $.02 valued at $3,000.The CEO and the President have employment contracts previously filed with the SEC that includes termination clauses that fully vest their ownership of shares. All employment agreements have expired.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Audit Fees. The aggregate fees billed by S. E. Clark & Company, P.C. for the audit of the Company’s annual financial statements for fiscal year ended September 30, 2008 was approximately $20,000. The aggregate fees billed by Semple, Marchal & Cooper P.C. for professional services rendered for the audit of the Company’s annual financial statements for fiscal year ended September 30, 2007 was approximately $40,000.
Audit-Related Fees. The aggregate fees billed by S. E. Clark & Company, P.C. for assurance and related services that are reasonably related to the performance of the audit or review of the Company’s financial statements for the fiscal year ended September 30, 2008, and that are not disclosed in the paragraph captioned “Audit Fees” above, were $0.The aggregate fees billed by Semple, Marchal & Cooper P.C for assurance and related services that are reasonably related to the performance of the audit or review of the Company’s financial statements for the fiscal year ended September 30, 2007, and that are not disclosed in the paragraph captioned “Audit Fees” above, were $0.00.
Tax Fees. The aggregate fees billed by S. E. Clark & Company, P.C. and Semple, Marchal & Cooper P.C for professional services rendered for tax compliance, tax advice and tax planning for the fiscal years ended September 30, 2008, and 2007 were $0, and $0, respectively.
All Other Fees. The aggregate fees billed by S. E. Clark & Company, P.C. and Semple, Marchal & Cooper P.C for products and services, other than the services described in the paragraphs “Audit Fees,” “Audit-Related Fees,” and “Tax Fees” above for the fiscal years ended September 30, 2008, and 2007 approximated $0 and $0, respectively.
The Board of Directors has received and reviewed the written disclosures and the letter from the independent registered public accounting firm required by Independence Standards Board Standard No. 1 (Independence Discussions with Audit Committees), and has discussed with its auditors its independence from the Company. The Board of Directors has considered whether the provision of services other than audit services is compatible with maintaining auditor independence.
Based on the review and discussions referred to above, the Board of Directors approved the inclusion of the audited financial statements be included in the Company’s Annual Report on Form 10-K for its 2010 fiscal year for filing with the SEC.
The Board of Directors pre-approved all fees described above.
ITEM 15. EXHIBITS AND REPORTS.
Exhibits
| 3.1 | Articles of Incorporation(1) | |
| 3.2 | Bylaws(2) | |
| 10.1 | Employment Agreement between National Scientific Corporation and Michael A. Grollman dated January 2001(4) | |
| 10.2 | Employment Agreement between National Scientific Corporation and Graham L. Clark dated January 2003(6) | |
| 10.3 | NSC Consulting Agreement dated August 2001, and Amendments dated August 2002 and July 2003, with Dr. El-Badawy El-Sharawy(6) | |
| 10.4 | Amended and Restated 2000 Stock Option Plan(3) | |
| 10.5 | Form of 2004 Stock Retainage Plan Agreement(6) | |
| 10.6 | Agreement Regarding Management Consulting Services with Stanton Walker of New York dated May 2003(6) | |
| 10.7 | Agreement Regarding Distribution and Marketing of Gotcha!® Child Safety Product and other products dated December 2002 with FutureCom Global, Inc. (6) | |
| 10.8 | Purchase Order from Verify Systems, Inc, dated March 2003 for IBUS™ School Child Tracking Systems(5) | |
| 10.9 | Letter of Understanding and Agreement dated April 2004 Regarding Sales and Distribution of Verify School safety products, and an Unlimited Software License with Anthony Grosso and CIS Services, LLC(6) | |
| 10.10 | Letter of Intent from Positus, Inc. dba Bike & Cycle Trak, dated February 2003 for Design of Power Sports Tracking System(6) | |
| 10.11 | Purchase Order from Positus, Inc. dba Bike & Cycle Trak, for Design of Power Sports Tracking System dated March 2003(6) | |
| 14 | Code of Ethics(7) | |
| 23 | Resignation of Semple, Marchal & Cooper, P.C. on December 7, 2011(8) | |
| 31 | Certification Pursuant to Section 302 of the Sarbanes-Oxley Act(8) | |
| 32 | Certification Pursuant to Section 906 of the Sarbanes-Oxley Act(8) |
| (1) | Incorporated by reference to the Registrant’s Form 10-SB filed on or about January 3, 2000. |
| (2) | Incorporated by reference to the Registrant’s Form 10-QSB for the quarter ended March 31, 2001 and filed on or about May 15, 2001. |
| (3) | Incorporated by reference to the Registrant’s Form 10-QSB for the quarter ended December 31, 2000 and filed on or about February 14, 2001. |
| (4) | Incorporated by reference to the Registrant’s Form 10-KSB for the year ended September 30, 2000 and filed on or about December 19, 2000. |
| (5) | Incorporated by reference to the Registrant’s Form S-8 filed on or around June 3, 2003. |
| (6) | Incorporated by reference to the Registrant’s Form SB2 filed on or around June 24, 2004. |
| (7) | Incorporated by reference to the Registrant’s Form 10-QSB for the quarter ended June 30, 2004 and filed on or about August 16, 2004. |
| (8) | Incorporated herein |
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, there unto duly authorized.
|
Registrant Date: January 16, 2013
|
Cloud Medical Doctor Software Corporation By: /s/ Michael De La Garza | |
| Michael De La Garza | ||
| Chairman, Chief Executive Officer (Principal Executive Officer), President |
|
Date: January 16, 2013
|
By: /s/ Pamela Thompson | |
| Pamela Thompson | ||
| Chief Financial Officer (Principal Accounting Officer), Secretary, Treasurer |
In accordance with the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Company and in the capacities and on the dates indicated.
Date: January 16, 2013
|
By: /s/ Michael De La Garza | |
| Michael De La Garza | ||
| Chairman |
Date: January 16, 2013
|
By: /s/ Pamela Thompson | |
| Pamela Thompson | ||
| Director |
EXHIBIT 23.

CERTIFICATION OF CHIEF EXECUTIVE OFFICER
PURSUANT TO RULES 13A-14 AND 15D-14
OF THE SECURITIES EXCHANGE ACT OF 1934
I, Michael De La Garza, certify that:
- I have reviewed this annual report on Form 10-K of Cloud Medical Doctor Software Corporation;
- Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
- Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the small business issuer as of, and for, the periods presented in this report;
- The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the small business issuer and have:
| a. | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the small business issuer, including its subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| b. | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| c. | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| d. | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
- The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
| a. | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| b. | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
By /s/ Michael De La Garza
Michael De La Garza
Chairman, Chief Executive Officer, President
January 16, 2013
CERTIFICATION OF CHIEF FINANCIAL OFFICER
PURSUANT TO RULES 13A-14 AND 15D-14
OF THE SECURITIES EXCHANGE ACT OF 1934
I, Pamela Thompson, certify that:
- I have reviewed this annual report on Form 10-K of Cloud Medical Doctor Software Corporation;
- Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
- Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the small business issuer as of, and for, the periods presented in this report;
- The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the small business issuer and have:
| a. | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the small business issuer, including its subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| b. | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| c. | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| d. | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
- The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
| a. | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| b. | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
By /s/ Pamela Thompson
Pamela Thompson
Chief Financial Officer, Secretary, Treasurer
January 16, 2013
Exhibit 32.1
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Cloud Medical Doctor Software Corporation (the “Company”) on Form 10-K for the period ended September 30, 2008 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Michael De La Garza, Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. section 1350, as adopted pursuant to section 906 of the Sarbanes-Oxley Act of 2002, That to the best of my knowledge:
(1) The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
By /s/ Michael De La Garza
Michael De La Garza
Chairmen, Chief Executive Officer, President
January 16, 2013
Exhibit 32.2
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Cloud Environmental Services, Inc, (the “Company”) on Form 10-K for the period ended September 30, 2008 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Pamela Thompson, Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. section 1350, as adopted pursuant to section 906 of the Sarbanes-Oxley Act of 2002, That to the best of my knowledge:
| (1) | The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| (2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
By /s/ Pamela Thompson
Pamela Thompson
Chief Financial Officer, Secretary, Treasurer
January 16, 2013
