Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - OPKO HEALTH, INC. | d464802d8k.htm |
| EX-99.1 - EX-99.1 - OPKO HEALTH, INC. | d464802dex991.htm |
 Corporate Presentation
January 2013
Exhibit 99.2 |
 ©
Cytochroma 2011
1
Forward-Looking Statements
Certain statements and information included in this presentation are “forward-looking
statements” under the Private Securities Litigation Reform Act of 1995 (PSLRA), including
expectations regarding (1) the market opportunity and growth potential of the CKD patient
population, (2) how to reach Cytochroma’s target physician audience, (3) new potential
indications for the CTAP101 Capsules, (4) the commercial opportunity for the CTAP101
Capsules, (5) the Phase 3 and NDA timelines for CTAP 101 through 2015, (6) the pipeline
for clinical programs for CKD patients and early stage pipeline products, and (7) the U.S.
market opportunity for Cytochroma’s product candidates generally. Many factors could
cause actual activities or results to differ materially from the activities and results
anticipated in these forward-looking statements. These factors include that the various
conditions to the closing of the transaction between Opko and Cytochroma may not be met,
as well as risks inherent in funding, developing and obtaining regulatory approvals of new,
commercially-viable and competitive products and treatments. In addition, these
forward- looking statements may also be adversely affected by scientific developments
relating to chronic kidney disease, general market factors, competitive product development,
product availability, federal and state regulations and legislation, the regulatory process for
new products and indications, manufacturing issues that may arise, patent positions and
litigation, among other factors. |
 ©
Cytochroma 2011
2
A clinical stage specialty pharmaceutical
company focused on Chronic Kidney Disease |
 ©
Cytochroma 2011
3
To Improve People’s Lives by Treating and
Preventing
Clinical Consequences of Vitamin D Insufficiency and Secondary
Hyperparathyroidism
Company Overview
Mission: |
 ©
Cytochroma 2011
4
To Improve People’s Lives by Treating and
Preventing
Clinical Consequences of Vitamin D Insufficiency and Secondary
Hyperparathyroidism
Company Overview
Mission: |
 ©
Cytochroma 2011
Product
Indication
Research
Preclinical
Phase I
Phase II
Phase III
Territorial
Rights
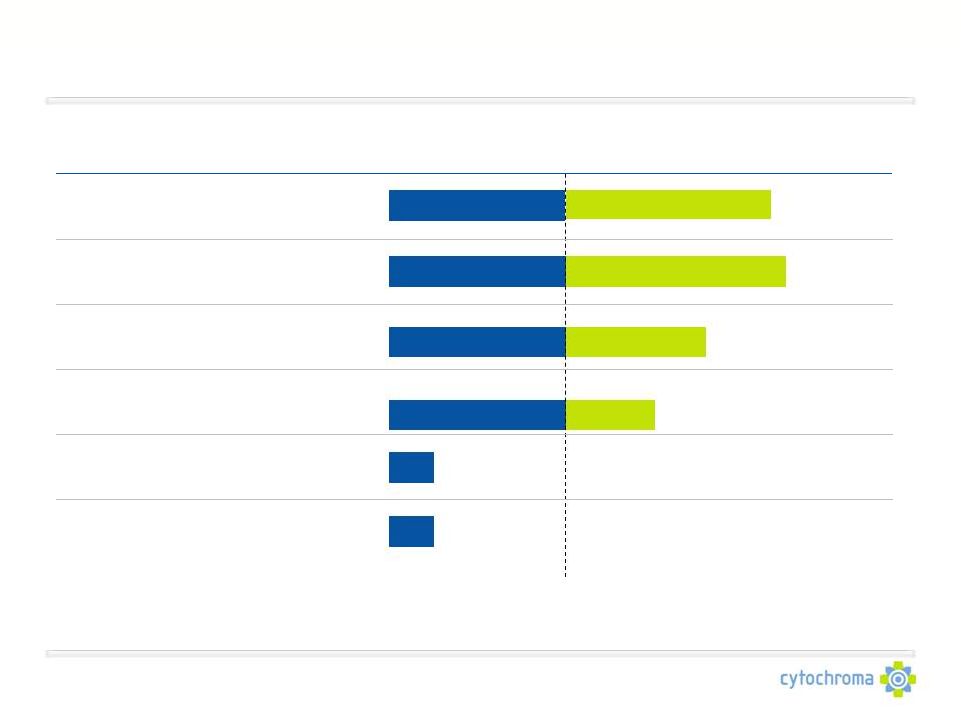
CTAP101
Capsules
Low 25D and SHPT*
(CKD Stage 3-4 Patients)
Worldwide
Fermagate
Tablets
Hyperphosphatemia
(CKD Stage 5 Patients)
Worldwide
CTA018
Injection
Moderate to Severe SHPT*
(CKD Stage 5 Patients)
Worldwide
CTAP201
Injection
Mild to Moderate SHPT*
(CKD Stage 5 Patients)
Worldwide
Phosphate Transport
Inhibitors
Hyperphosphatemia
Worldwide
CYP24 Inhibitors
CKD and pre-CKD
Worldwide
5
*
SHPT = Secondary Hyperparathyroidism
Cytochroma Product Pipeline
A clinical-stage specialty pharmaceutical company focused on CKD
|
 ©
Cytochroma 2011
6
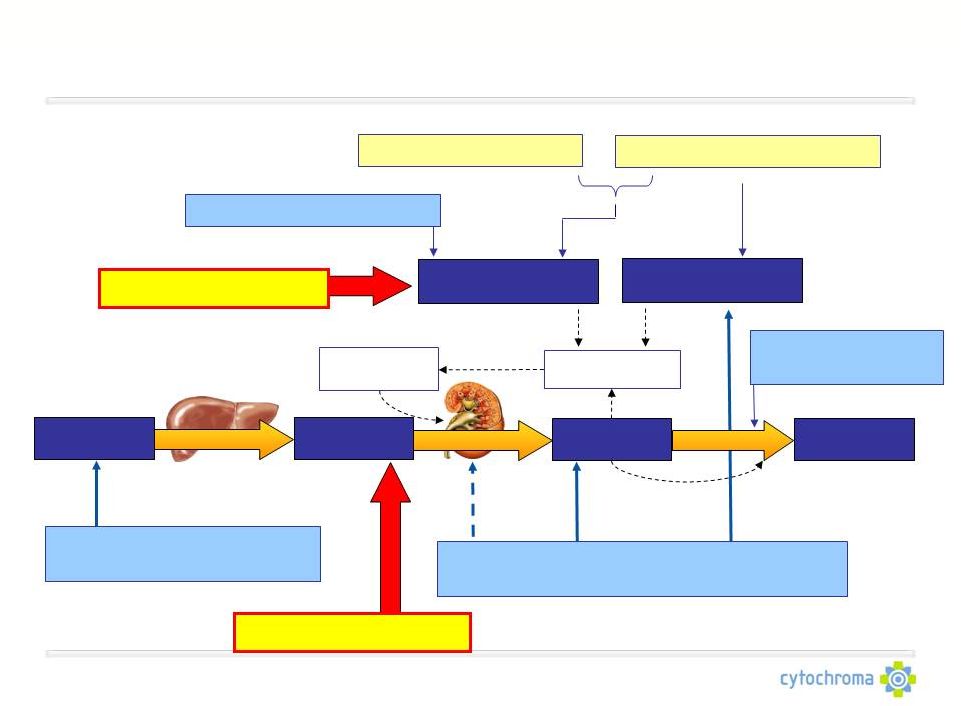
Vitamin D: Central To SHPT Etiology & Treatment
Vitamin D
Vitamin D
prohormone
Liver Enzyme
Vitamin D
hormone
Kidney Enzyme
Parathyroid
hormone
Target Tissues
(Parathyroid gland)
Vitamin D Hormone Replacement Therapies
(Paricalcitol, Doxercalciferol, Calcitriol)
Hyperphosphatemia
Hypocalcemia
Ca-Free Binder Therapies
CalcimimeticTherapies
Ca-Based Binder Therapies
Vitamin D Repletion
Therapies
CYP24 Inhibitor
Therapies
Inactivated
Vitamin D
CYP24 Enzyme
CTAP101 Capsules
Light blue boxes indicate
Cytochroma’s areas of focus
Fermagate Tablets |
 ©
Cytochroma 2011
7
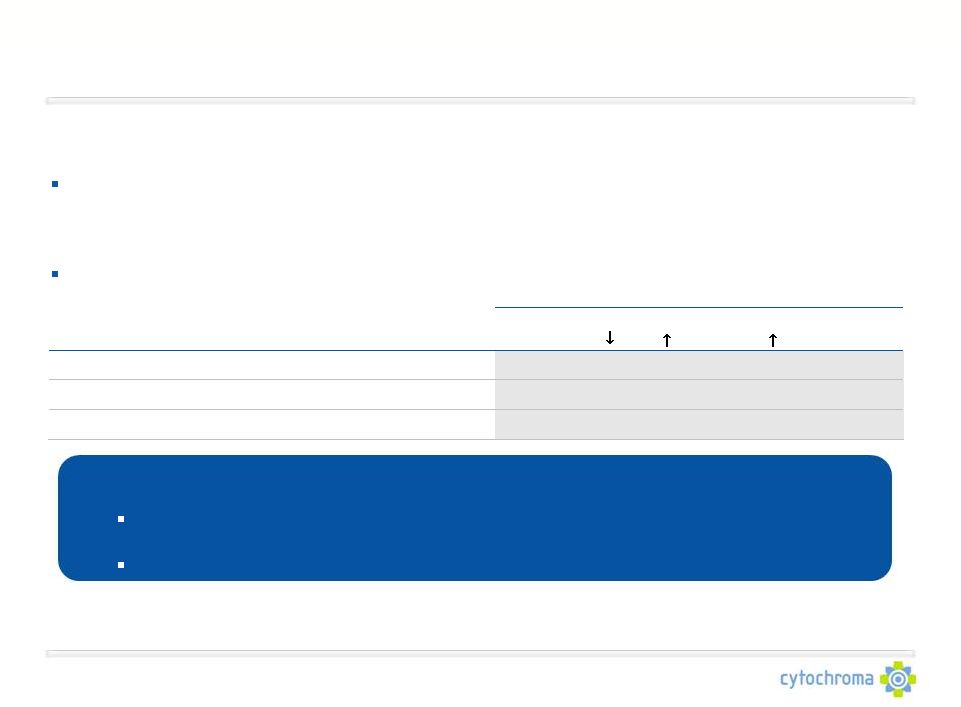
Market Opportunity: Chronic Kidney Disease (U.S.)
*National Kidney Foundation 2002
**US Renal Data Service 2009 Annual Data Report
Sources: Levin, A et al., Kidney International 2007; 71: pp.31-38.
Gonzalez, E et al. Am J Nephrol 2004;24:503-510.
LaClair, R et al. Am J Kidney Dis 2005;45:1026-1033.
A 75-100 person specialty salesforce can readily reach Cytochroma’s target
physician audience:
Primary commercialization targets include 4,500-6,000 office-based
nephrologists and 1,500- 3,000 endocrinologists
Other commercialization targets include geriatricians and 4,500 dialysis clinics
The CKD patient population is large and growing as a result of
cardiovascular disease, obesity and diabetes
Current U.S. sales are derived predominantly from Stage 5 CKD:
•
Approximately $1.5 billion for SHPT therapies
•
Approximately $0.8 billion for hyperphosphatemia therapies
CKD Stage 3-4 patients represent a significantly greater, and largely untapped
market opportunity % of CKD Patients with:
Stage
Kidney Function
CKD Prevalence
Vitamin D
SHPT
Hyperphosphatemia
(
Phosphorus)
3
Moderate impairment
7.6 Million*
70%
56%
37%
4
Severe impairment
0.4 Million*
80%
60%
50%
5
Failure
0.5 Million**
90%
90%
70%
Insufficiency (
25D)
(
PTH) |
 ©
Cytochroma 2011
8
Phase 2b study complete and reported strong
efficacy and safety data
High proportion of patients achieved increase
in 25D levels to target, as well as 30%
reduction in PTH from baseline
8
Modified-release
(MR) formulation of 25D*
Safe and effective treatment for elevated PTH
(SHPT) associated with low 25D levels in Stages
3-4 CKD
Achieves more reliable increases in serum 25D
and reductions in plasma PTH than nutritional
vitamin D
Lower risk of side effects compared to active
1,25D** products
Preserves protective renal feedback mechanism
Additional potential for new indications in:
•
Geriatric patients with low 25D levels and
elevated PTH
•
Patients with GI or malabsorptive disorders
•
Osteoporosis
•
Organ transplant recipients
CTAP101 Capsules
Product Overview
Clinical Status
Next Steps
End of Phase 2 meeting with FDA in Q1 2012
Phase 3 trials started in H2 2012 under SPA
505(b)(2) NDA filing in H1 2015
Intellectual Property
Formulation and method of use patents pending
CTAP101 US patent issued, protected through 2028
*
25-Hydroxyvitamin D3
**
1,25-Dihydroxyvitamin D |
 ©
Cytochroma 2011
9
9
Doxercalciferol Capsules
(Hectorol)
1,25D Hormones:
SHPT only
Nutritional Vitamin D:
SHPT and Low 25D
(Off-label)
* K/DOQI -
Kidney Disease Outcomes Quality Initiative is issued by the National
Kidney
Foundation
and
are
practice
guidelines
for
all
stages
of
chronic
kidney
disease
and related complications.
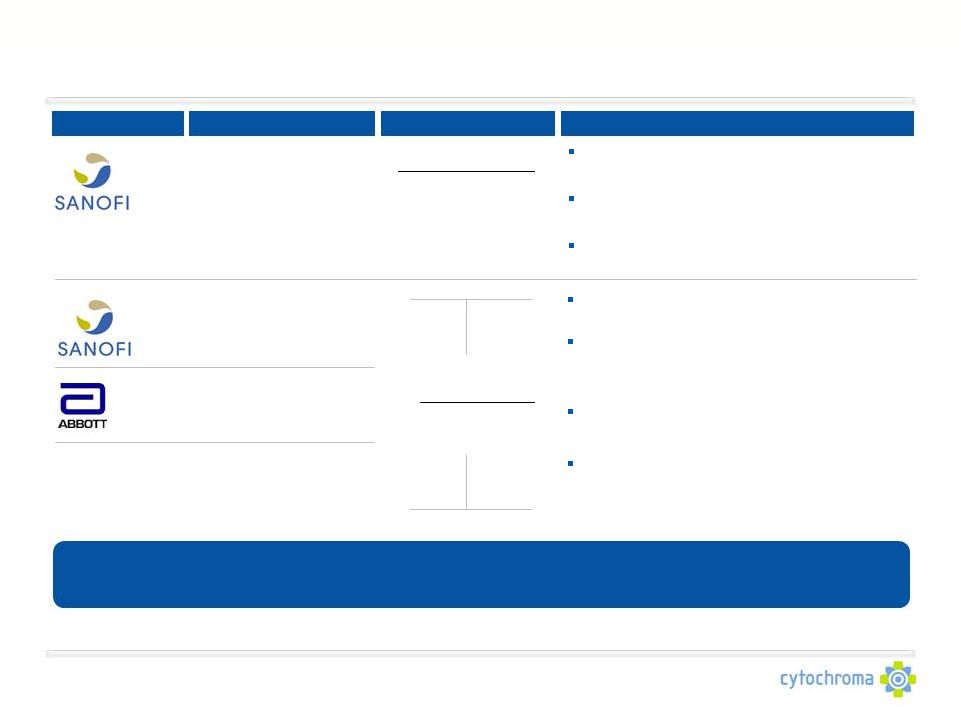
Limitations of Existing Therapies
Company
Product
Use
Limitations
Upregulate CYP24, which catabolizes both
administered therapy as well as endogenous 25D
and 1,25D
Limited systemic availability due to sequestering
in fat tissue
Inconsistent effects, dependant on GI functionality
and bile production
Limited efficacy to increase serum 25D levels and
lower plasma PTH to K/DOQI* target levels
Paricalcitol Capsules
(Zemplar)
Calcitriol Capsules
(Rocaltrol /Vectical)
Generic
Manufacturers
Ergocalciferol Capsules
(Drisdol)
Ergocalciferol Drops
(Drisdol)
Bypass protective renal feedback mechanism of
downregulating CYP27B1
Cause aberrations in local and systemic calcium
and phosphorus metabolism, leading to side
effects such as hypercalcemia,
hyperphosphatemia and soft tissue calcification
Cannot correct and may worsen low 25D levels
CTAP101 consistently increases serum 25D and achieves meaningful reductions in plasma
PTH without safety concerns |
 ©
Cytochroma 2011
10
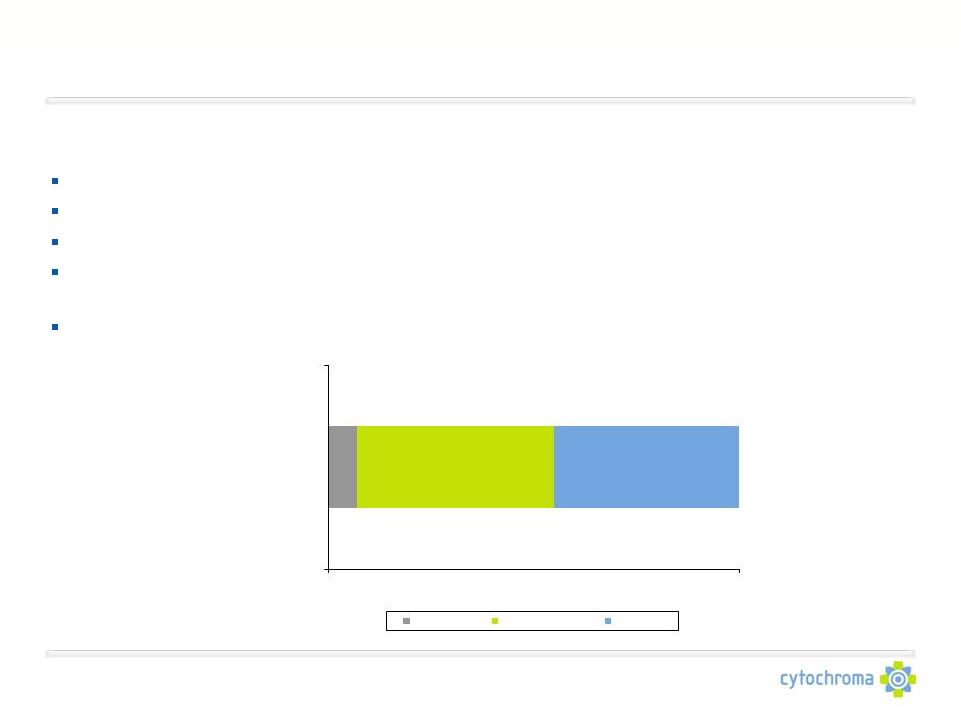
The Great Unmet Medical Need
Percent of Nephrologists
Nephrologists report a significant need for a therapy that can raise serum
25D levels and suppress plasma PTH levels
Nearly 60% of nephrologists frequently or always measure 25D levels in CKD Stage
3-4 patients Nearly 90% of nephrologists use nutritional vitamin D in CKD
Stage 3-4 patients Over 90% of nephrologists agree that nutritional
vitamin D has “limited efficacy to raise 25D and lower PTH” Over
90% of nephrologists agree that 1,25D hormones have “risks of side effects which limit dose and
efficacy”
Over 90% of nephrologists report a moderate-to-high need for a therapy that
can safely and effectively raise 25D levels and suppress PTH in CKD Stage
3-4 patients * As assessed through syndicated market research by BioTrends
and proprietary research by Mattson Jack 45%
7%
48%
0%
100%
How much need is
there for a single
therapy that can
safely and effectively
raise 25D levels AND
suppress PTH levels
to target in CKD-ND
patients?
Low Need
Moderate Need
High Need |
 ©
Cytochroma 2011
11
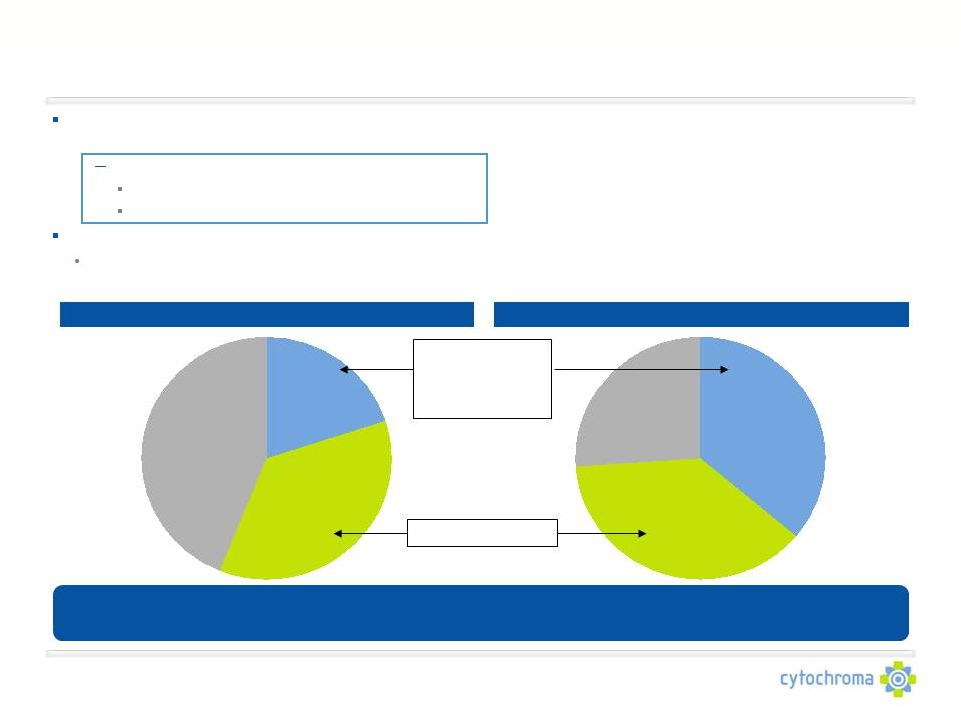
CTAP101: Significant Commercial Opportunity
BioTrends Research Group, Inc. December 2010
Low serum 25D and high plasma PTH are prevalent in CKD Stage 3-4 patients
•
8.0M CKD Stage 3-4 patients in the U.S.
4.0M patients with low serum 25D and high plasma PTH
~1.0M patients seen by nephrologist
~1.0M patients seen by endocrinologist
Existing
treatments
are
not
effective
or
have
significant
safety
issues
CTAP101’s efficacy and safety results compare favorably to both nutritional
vitamin D and 1,25D hormones in CKD stage 3-4 patients and will drive
untreated patients to start on CTAP101 Capsules Stage 3 CKD Treatment
Stage 4 CKD Treatment
Untreated
44%
1,25D
Hormone
20%
Nutritional
Vitamin D
36%
Nutritional
Vitamin D
38%
Untreated
26%
1,25D
Hormone
36%
Safety concerns
and exacerbates
vitamin D
insufficiency
Efficacy Concerns
CTAP101 Capsules is expected to take significant market share (35% to 50%) in the
CKD
Stage
3
and
4
market
–
a
potential
$1B
revenue
opportunity |
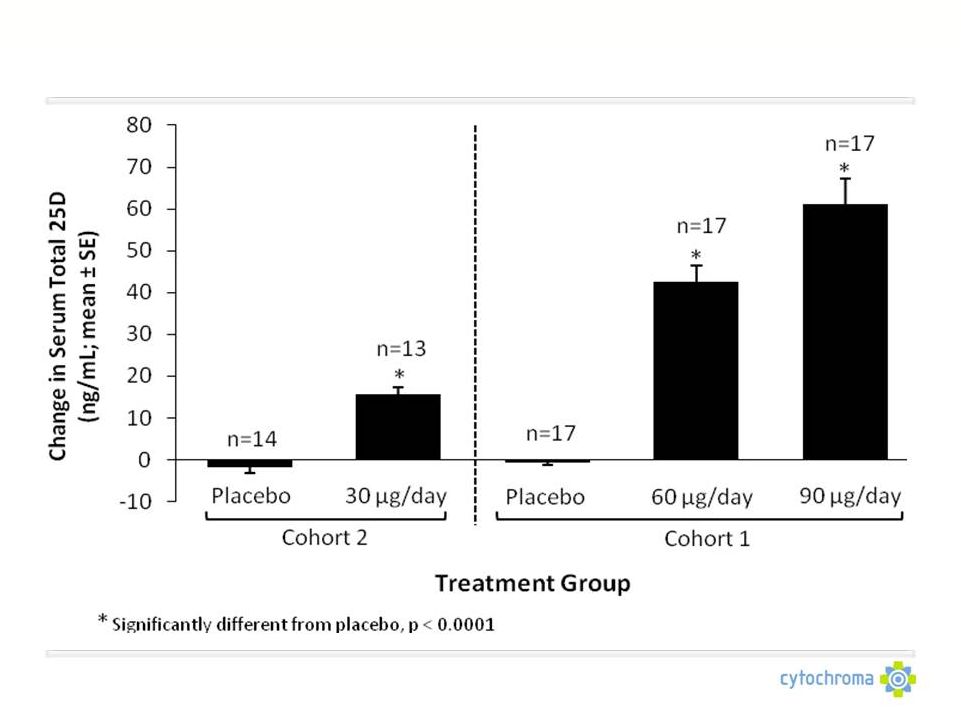 ©
Cytochroma 2011
CTAP101: Corrects Vitamin D Insufficiency
12 |
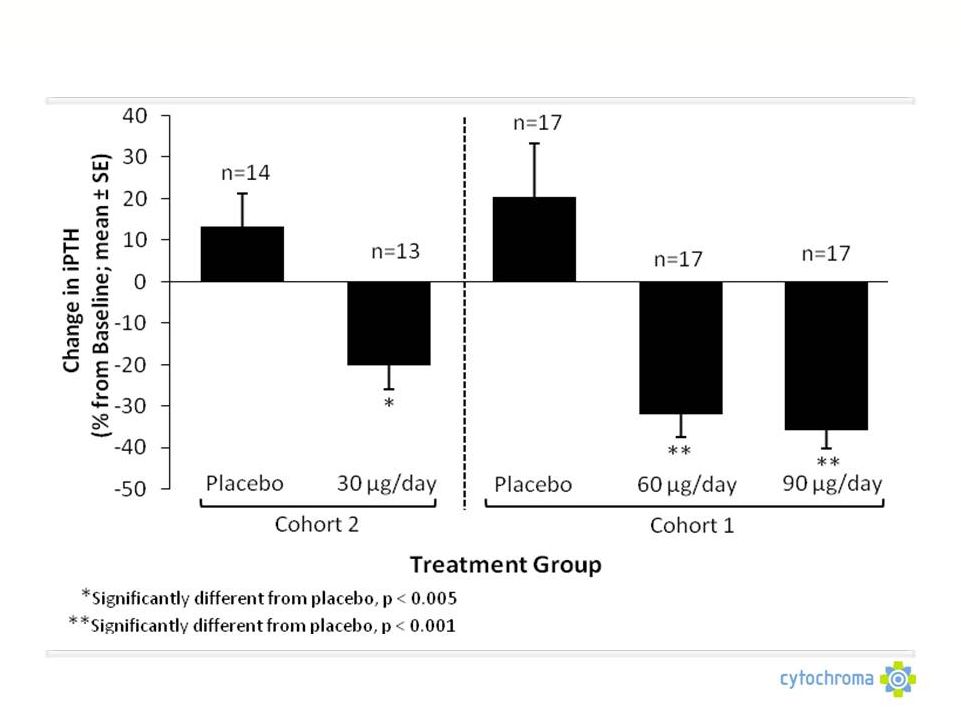 ©
Cytochroma 2011
CTAP101: Corrects SHPT (Elevated Plasma iPTH)
13 |
 ©
Cytochroma 2011
14
CTAP101 Capsules reliably increase serum 25D and reduce plasma PTH
CTAP101 Capsules are safe in CKD Stage 3-4 patients
CTAP101’s efficacy and safety compare favorably to competing products
No competing products can consistently normalize 25D levels and produce
significant reductions in PTH without safety concerns
The efficacy of OTC vitamin D at raising 25D or lowering PTH is modest; results
are mixed and a relatively low percentage of patients respond
1,25D (calcitriol) doses that significantly reduce PTH (0.5 mcg/day) produce
significant increases in serum calcium and cause hypercalcemia
CTAP101 Capsules: Phase 2b Summary
Repeat dose proof of concept
No clear dosing regimen for OTC vitamin D exists
Lower 1,25D doses (0.25 mcg/day) do not produce consistent, meaningful
reductions in PTH
1,25D analogues can significantly reduce PTH about the same amount as CTAP101
Capsules, but cause elevations in serum and urine calcium which raise
safety concerns and limit their use |
 ©
Cytochroma 2011
CTAP101: Phase 3 and NDA Timelines
2012
Q1
Q2
Q3
Q4
2013
Q1
Q2
Q3
Q4
2014
Q1
Q2
Q3
Q4
NDA
Filing
CL-3002
Data
CL-3001 trial
SPA
Open Label Extension
Q1
Q2
Q3
2015
Q4
CL-3002 trial
NDA
Safety
Update
NDA
Approval
TG Mouse Carcinogenicity Study
Dose ranging
SPA
CL-3001
Data |
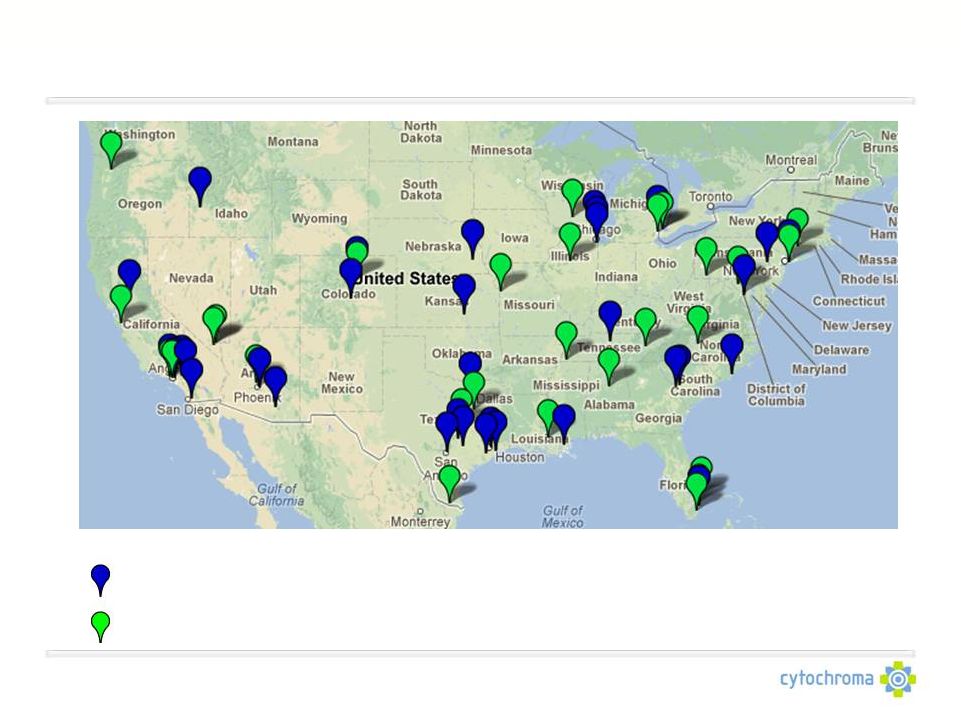 ©
Cytochroma 2011
CTAP101-CL-3001 & CL-3002 Combined Site Map
= Selected investigator sites for study 3001 (33 Sites)
= Selected investigator sites for study 3002 (30 Sites)
|
 ©
Cytochroma 2011
17
Corporate Highlights
CTAP101 Capsules is a Highly Differentiated Lead Asset
•
First-in-class modified-release vitamin D prohormone that both corrects
vitamin D insufficiency and controls SHPT* in patients with stage 3 or stage
4 CKD No
current
therapy
can
reliably
restore
adequate
serum
25D**
and
suppress
elevated
PTH***
•
Compelling Phase 2b data in hand; Phase 3 program ongoing under SPA.
•
USPTO recently issued a patent covering the product until ‘28
•
NDA filing expected in 1H’15
Broad and Deep Pipeline
•
Two Phase 2 and two Phase 3 clinical programs, all for CKD patients
•
Early stage pipeline includes new inhibitors of phosphate transport in the GI
tract, as well as CYP24 Large Market Opportunity
•
Cytochroma’s product candidates address $2.3 billion market in the U.S.
•
Vitamin D insufficiency affects 70-80% of the 8 million CKD Stage 3-4
patients in the U.S. *
SHPT = Secondary hyperparathyroidism
**
25D = 25-hydroxyvitamin D
***
PTH = parathyroid hormone |
 Corporate Presentation
January 2013 |
