Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Adynxx, Inc. | alqa_8k.htm |

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
1
January, 7 2013
Biotech Showcase

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
2
This presentation contains forward-looking statements. Forward-looking statements are generally identifiable
by the use of words like "may," "will," "should," "could," "expect," "anticipate," "estimate," "believe," "intend,"
or "project" or the negative of these words or other variations on these words or comparable terminology. The
reader is cautioned not to put undue reliance on these forward-looking statements, as these statements are
subject to numerous factors and uncertainties outside of the our control that can make such statements
untrue, including, but not limited to, inadequate capital, adverse economic conditions, intense competition,
lack of meaningful research results, entry of new competitors and products, adverse federal, state and local
government regulation, termination of contracts or agreements, technological obsolescence of our products,
technical problems with our research and products, price increases for supplies and components, inability to
carry out research, development and commercialization plans, loss or retirement of key executives and
research scientists and other specific risks. We currently have no commercial products intended to diagnose,
treat, prevent or cure any disease. The statements contained in this presentation regarding our ongoing
research and development and the results attained by us to-date have not been evaluated by the Food and
Drug Administration. There can be no assurance that further research and development, and/or whether
clinical trial results, if any, will validate and support the results of our preliminary research and studies.
Further, there can be no assurance that the necessary regulatory approvals will be obtained or that we will be
able to develop new products on the basis of our technologies. In addition, other factors that could cause
actual results to differ materially are discussed in our Annual Report on Form 10-K filed with the SEC on
March 29, 2012 and our most recent Form 10-Q filings with the SEC. Investors and security holders are urged
to read these documents free of charge on the SEC's web site at www.sec.gov. We undertake no obligation to
publicly update or revise our forward-looking statements as a result of new information, future events or
otherwise.
by the use of words like "may," "will," "should," "could," "expect," "anticipate," "estimate," "believe," "intend,"
or "project" or the negative of these words or other variations on these words or comparable terminology. The
reader is cautioned not to put undue reliance on these forward-looking statements, as these statements are
subject to numerous factors and uncertainties outside of the our control that can make such statements
untrue, including, but not limited to, inadequate capital, adverse economic conditions, intense competition,
lack of meaningful research results, entry of new competitors and products, adverse federal, state and local
government regulation, termination of contracts or agreements, technological obsolescence of our products,
technical problems with our research and products, price increases for supplies and components, inability to
carry out research, development and commercialization plans, loss or retirement of key executives and
research scientists and other specific risks. We currently have no commercial products intended to diagnose,
treat, prevent or cure any disease. The statements contained in this presentation regarding our ongoing
research and development and the results attained by us to-date have not been evaluated by the Food and
Drug Administration. There can be no assurance that further research and development, and/or whether
clinical trial results, if any, will validate and support the results of our preliminary research and studies.
Further, there can be no assurance that the necessary regulatory approvals will be obtained or that we will be
able to develop new products on the basis of our technologies. In addition, other factors that could cause
actual results to differ materially are discussed in our Annual Report on Form 10-K filed with the SEC on
March 29, 2012 and our most recent Form 10-Q filings with the SEC. Investors and security holders are urged
to read these documents free of charge on the SEC's web site at www.sec.gov. We undertake no obligation to
publicly update or revise our forward-looking statements as a result of new information, future events or
otherwise.
Safe Harbor
2

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
3
Alliqua Strategic Vision
To improve patient care and quality of life
across a broad range of market segments,
including wound healing, through the
innovation and commercialization of topically
and systemically delivered biotherapeutics
using our proprietary hydrogel technology.
across a broad range of market segments,
including wound healing, through the
innovation and commercialization of topically
and systemically delivered biotherapeutics
using our proprietary hydrogel technology.
3

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
4
Corporate Snapshot
Went public 2010
Marketed products
SilverSeal® antimicrobial dressing
Hydress® non-medicated dressing
Expand portfolio via strategic in-licensing, internal
development
development
Three businesses
Alliqua Biomedical (drug delivery)
AquaMed Technologies (wound healing, contract research)
Hepalife (bio-artificial liver system)
4

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
5
Three Distinct Market Segments
5
Alliqua, Inc.
Hepalife
Biosystems
Biosystems
Hydrogel Platform
Businesses
Businesses
AquaMed
Technologies
Technologies
Wound Healing
Alliqua
Biomedical
Biomedical
Drug Delivery
Bio-Artificial
Liver
Liver

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
6
6
Hydrogel Platform:
Foundation of Alliqua and AquaMed
Cross-linked aqueous polymer gel on
colloidal sheets
colloidal sheets
Numerous chemical configurations
and physical properties
and physical properties
3D cross-linked networks of water-
soluble polymers
soluble polymers
Electron beam cross-linked
Up to 97% water composition
Proprietary manufacturing processes
Versatile Transdermal Drug
Delivery Technology
Delivery Technology

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
7
Alliqua Biomedical Business Unit
Current products: SilverSeal® and Hydress®
HCPCS reimbursement designations*
Next steps: expand infrastructure and product
lines
lines
Pre-operative and post-operative dressings
Gram positive antibiotics
Enyzmatic debridement agents
Biological modulators
Explore non-traditional markets: military, global
health initiatives
health initiatives
7
* Note: Local coverage determinations for all Medicare jurisdictions allow for daily use of product.
© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
8
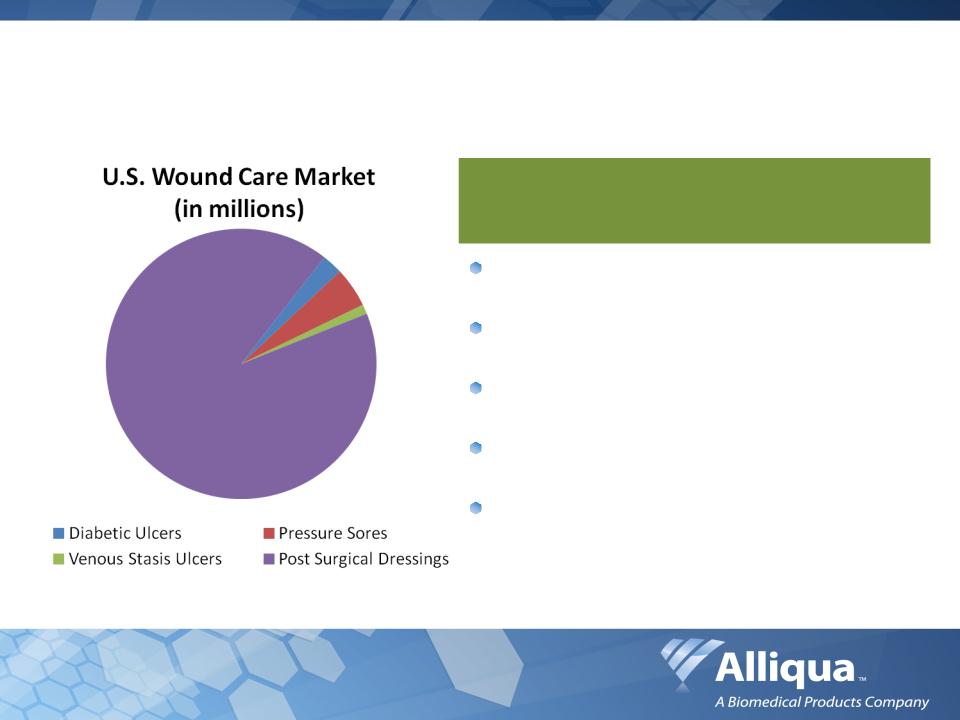
Wound Care:
Market Size
Market Size
1.3M new cases of diabetic foot
ulceration annually2
ulceration annually2
2.5M treated for pressure sores annually
(60,000 die from complications)3
(60,000 die from complications)3
600,000 Americans treated annually for
venous stasis ulcers4
venous stasis ulcers4
48M inpatient surgical procedures
performed annually5
performed annually5
400,000 moderate-severe burns
annually, 40,000 require hospitalization6
annually, 40,000 require hospitalization6
U.S. Wound Care Market
Increasing to $2.8B by 20161
Increasing to $2.8B by 20161
1 Millennium Research Group (MRG) US Markets for Wound Care Biomaterials 2012 report
2 GlobalData presentations 2012 ADA Scientific Sessions
3 American Geriatric Society: State of the Art: Pressure Ulcers; May 2012
4 Hankin etal, Clinical and Cost Efficacy of Advanced Wound Care Matrices for Venous Ulcers; Vol. 18, No. 5 June 2012
Journal of Managed Care Pharmacy
Journal of Managed Care Pharmacy
5 CDC National Hospital Discharge Survey: 2009 table, Procedures by selected patient characteristics - Number by procedure
category and age
category and age
6 WHO Burn Fact Sheet May 2012

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
9
Wound Care:
SilverSeal®
SilverSeal®
Manages bacterial burden
Continuous antimicrobial
protection
protection
Superior fluid management
No need to pre-wet or re-wet
7 day wear time
Provides moist environment
Very comfortable, painless
application and removal
application and removal
Cost effective
9
© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
10
Wound Care:
Hydress ®
Hydress ®
Non-medicated dressing
Improved wound healing
Highly breathable
Non-bonding
Thin physical profile
Very comfortable
Class I exempt device,
no FDA submission required
no FDA submission required
10

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
11
11
Manufacturing Facilities
16,500 sq. ft. facility in PA
1 of 2 manufacturers in the world
Can handle significant growth
FDA registered
cGMP compliant
High barrier to entry
Expensive to replace
2 - 3 year lead time
Proprietary processes

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
12
Contract Research Business Unit
Revenue generator poised for growth
Hydrogel is applicable to a wide range of
products:
products:
Wound healing: basic moist dressings, advanced
dressings incorporating actives or drugs
dressings incorporating actives or drugs
Cosmetics
Conductive adhesives
Temperature control
Drug delivery: passive, iontophoretic
Current revenue stream derived from CRO
business
business
12

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
13
Hepalife Business Unit
HepaMate™ extracorporeal cell-
based bioartificial liver system
based bioartificial liver system
Seeking to monetize asset
13

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
14
Business Unit Evolution
Five Year Plan
Five Year Plan
14
99%
10%

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
15
Seasoned Management
James Sapirstein- President and CEO
Eli Lilly, Hoffman-LaRoche, Bristol-Myers Squibb, Gilead,
Serono Labs, Tobira
Serono Labs, Tobira
Involved in launch and/or market positioning of 23
products including Viread, Toradol, Rocephin, Maxipime,
Videx EC
products including Viread, Toradol, Rocephin, Maxipime,
Videx EC
Steve Berger - CFO
Harborview Advisors, Global/CHC Worldwide,
Morgan Harris & Co,
Morgan Harris & Co,
Joseph Laudano - Medical Affairs
Hoffmann-LaRoche, Forest Labs
15

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
16
Board of Directors
Jerome Zeldis
Chairman, Alliqua BOD;
CMO, Celgene
CMO, Celgene
David Johnson
Exec Chair, AquaMed
Technologies; Former CEO,
Convatec
Technologies; Former CEO,
Convatec
Joe Leone
Audit committee chairman;
Former CFO, CIT Group
Former CFO, CIT Group
Ken Londoner
Endicott Management
Partners
Partners
Kenneth Pearsen, M.D.
Western New York
Radiology Associates
Radiology Associates
Richard Rosenblum
Harborview Advisors
James Sapirstein
Alliqua, Inc.
Jeffrey Sklar
Sklar, Heyman and
Company
Company
David Stefansky
Harborview Advisors
16

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
17
Expert Scientific Advisors
Stephen Brigido, DPM
Foot and ankle surgeon, Coordinated Health
Michael Goldberg, MD
Managing partner, Montaur Capital Partners
Michael Moore, MD, FACCWS
Medical Director, Wound Institute & Research Center
Harold Schoenhaus, DPM
Professor, Temple University School of Podiatric
Medicine
Medicine
Charles Wolff, DPM, FACFAS
Director, Podiatric Medicine & Surgery, Nyack Hospital
17

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
18
Investment Opportunity
Multibillion dollar market segments
Comparable companies in space have raised
significant funds at much higher valuations
significant funds at much higher valuations
GMP Manufacturing facility - primed for vertical
integration
integration
Experienced management and Board of Directors
Past investors participating in future rounds
Support of majority of shareholder base
Clean balance sheet; no debt
18

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
19
Thank you for your
attention
attention

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
20
Back Ups
20

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
21
21
Company History
Hydrogel Design Systems founded December
1996
1996
Company restructured and renamed Aquamed
Technologies 2009
Technologies 2009
Aquamed Technologies reverse merged into
Alliqua May 2010
Alliqua May 2010
Acquired 510k for SilverSeal® October 2011
Completed validation processes June 2012

© 2010 Copyright Alliqua, Inc. All Rights Reserved. The contents of this document are proprietary.
22
22
Reimbursement
The Company has received HCPCS designations
for the SilverSeal® and Hydress®:
for the SilverSeal® and Hydress®:
SilverSeal®
Size: 2X3
HCPCS: A6242
Minimum reimbursement amount: $6.51
Size: 4X5
HCPCS: A6243
Minimum reimbursement amount: $13.23
Hydress®
Size: 2X3
HCPCS: A6242
Minimum reimbursement amount: $6.51
Note: Local coverage determinations for all Medicare jurisdictions allow for daily use of product.
