Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a13-2128_18k.htm |
Exhibit 99.1
|
|
J.P. Morgan Healthcare Conference 2013 January 7-9, 2013 - San Francisco, California INNOVATIVE THERAPIES NOVEL PRODUCTS ©2013 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
FORWARD LOOKING STATEMENTS Certain statements in this presentation are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as "anticipate," "believe," "forecast," "estimate," "expect," "intend," "likely," "may," "plan," "potential," "predict," "opportunity" and "should," among others. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, our lack of commercial experience with Qsymia in the U.S.; the timing of initiation and completion of the clinical studies required as part of the approval of Qsymia by the United States Food and Drug Administration, or FDA; the response from the FDA to the data that VIVUS will submit relating to post-approval clinical studies; the impact of the indicated uses and contraindications contained in the Qsymia label and the REMS requirements; the impact of distribution of Qsymia through a certified pharmacy network; whether or not the FDA approves our amendment to the REMS for Qsymia, which, if approved, would allow dispensing through select retail pharmacies to increase access while meeting all requirements of the REMS; that we may be required to provide further analysis of previously submitted clinical trial data; our appeal of the negative opinion of the European Medicines Agency's, or EMA, Committee for Medicinal Products for Human Use, or CHMP, for the Marketing Authorization Application, or MAA, for Qsymia; our ability to successfully commercialize or establish a marketing partnership for avanafil, which will be marketed in the U.S. under the name STENDRA, or the ability of our partners to maintain regulatory approvals to manufacture and adequately supply our products to meet demand; our history of losses and variable quarterly results; substantial competition; risks related to the failure to protect our intellectual property and litigation in which we may become involved; uncertainties of government or third party payer reimbursement; our reliance on sole source suppliers; our limited sales and marketing and manufacturing experience; our reliance on third parties and our collaborative partners; our failure to continue to develop innovative investigational drug candidates and drugs; risks related to the failure to obtain FDA or foreign authority clearances or approvals and noncompliance with FDA or foreign authority regulations; our ability to demonstrate through clinical testing the safety and effectiveness of our investigational drug candidates; the timing of initiation and completion of clinical trials and submissions to foreign authorities; the volatility and liquidity of the financial markets; our liquidity and capital resources; and our expected future revenues, operations and expenditures. As with any pharmaceutical in development, there are significant risks in the development, the regulatory approval, and commercialization of new products. There are no guarantees that the product will receive regulatory approval outside the United States for any indication or prove to be commercially successful. VIVUS does not undertake an obligation to update or revise any forward-looking statements. Investors should read the risk factors set forth in VIVUS' Form 10-K for the year ending December 31, 2011, and periodic reports filed with the Securities and Exchange Commission. ©2013 VIVUS Inc. All rights reserved | www.vivus.com 1 |
|
|
INNOVATIVE THERAPIES; NOVEL PRODUCTS Two newly approved products in high potential markets: Qsymia™ for chronic weight management Stendra™ for erectile dysfunction ©2013 VIVUS Inc. All rights reserved | www.vivus.com 2 |
|
|
QSYMIA PRESCRIBING INFORMATION ©2013 VIVUS Inc. All rights reserved | www.vivus.com 3 |
|
|
TRANSFORMING THE OBESITY MARKET 4 ©2013 VIVUS Inc. All rights reserved | www.vivus.com 4 |
|
|
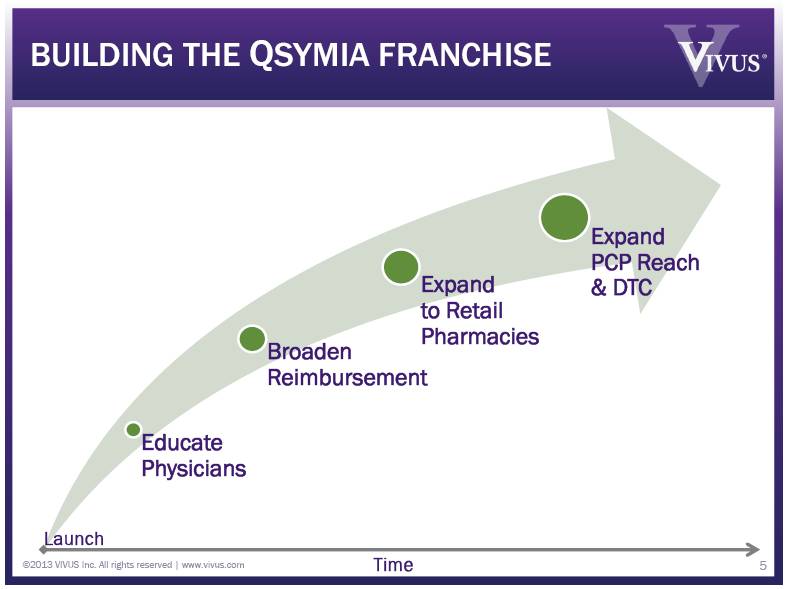
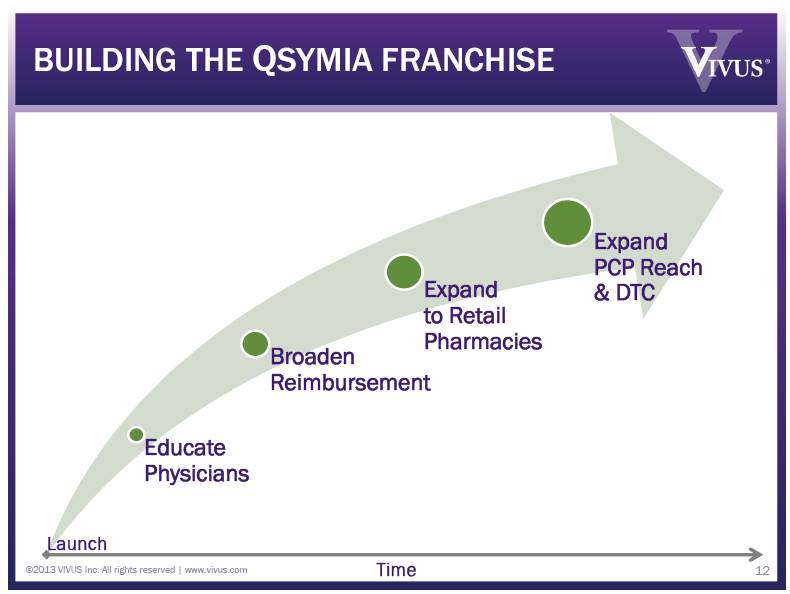
Educate Physicians Broaden Reimbursement Expand to Retail Pharmacies Expand PCP Reach & DTC BUILDING THE QSYMIA FRANCHISE ©2013 VIVUS Inc. All rights reserved | www.vivus.com 5 Launch Time |
|
|
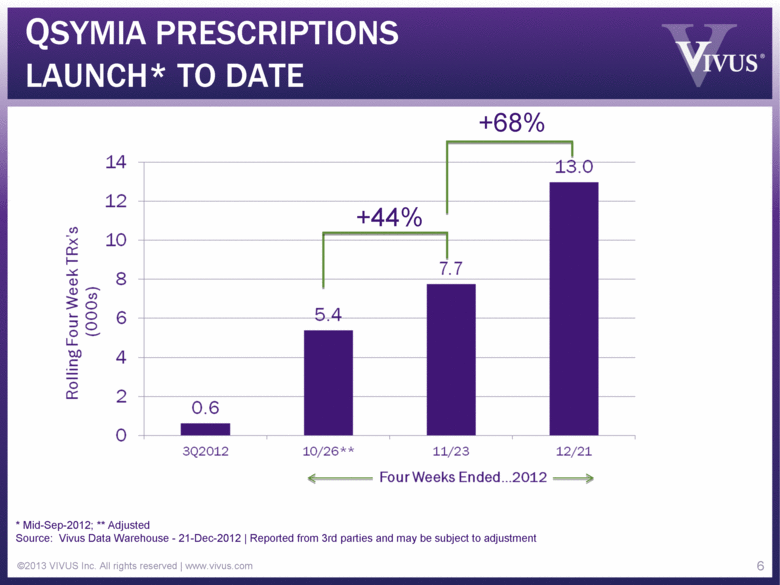
QSYMIA PRESCRIPTIONS LAUNCH* TO DATE ©2013 VIVUS Inc. All rights reserved | www.vivus.com 6 Rolling Four Week TRx’s (000s) 0 2 4 6 8 10 12 14 0.6 5.4 7.7 13.0 3Q2012 10/26** 11/23 12/21 * Mid-Sep-2012; ** Adjusted Source: Vivus Data Warehouse - 21-Dec-2012 | Reported from 3rd parties and may be subject to adjustment +44% +68% |
|
|
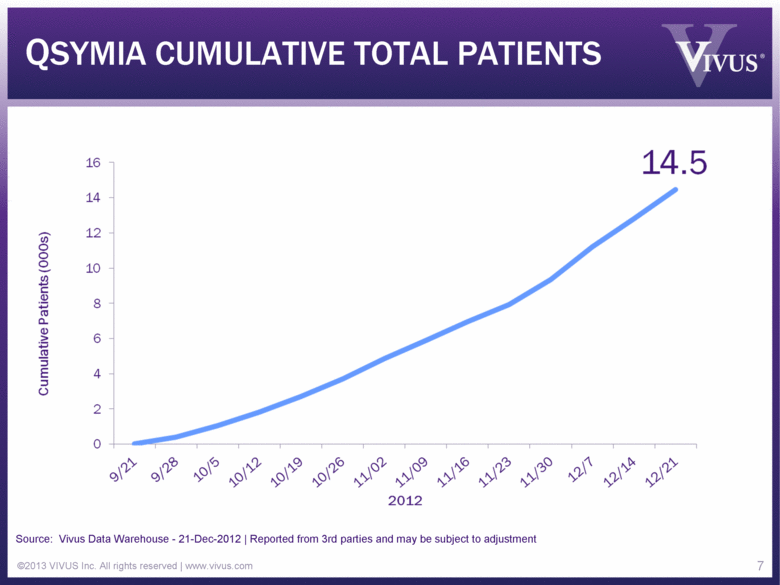
QSYMIA CUMULATIVE TOTAL PATIENTS ©2013 VIVUS Inc. All rights reserved | www.vivus.com 7 Cumulative Patients (000s) 0 2 4 6 8 10 12 14 16 9/21 9/28 10/5 10/12 10/19 10/26 11/02 11/09 11/16 11/23 11/30 12/7 12/14 12/21 14.5 Source: Vivus Data Warehouse - 21-Dec-2012 | Reported from 3rd parties and may be subject to adjustment |
|
|
MAJOR PBM VALIDATES QSYMIA Qsymia now on ESI National Preferred Formulary Among the largest Pharmacy Benefit Managers (PBM) Manages pharmacy benefit for 26.3MM lives U.S.* Tier 3; Prior Authorization Co-pay: ~$50 - $60 ©2013 VIVUS Inc. All rights reserved | www.vivus.com 8 *HealthLeaders InterStudy (Jan-2012) |
|
|
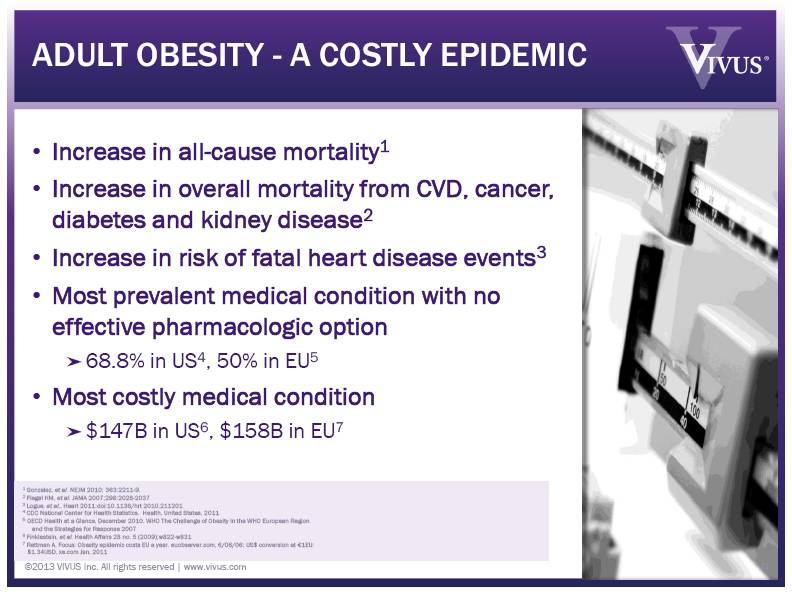
1 Gonzalez, et al. NEJM 2010; 363:2211-9. 2 Flegal KM, et al. JAMA 2007;298:2028-2037 3 Logue, et al., Heart 2011:doi:10.1136/hrt 2010.211201 4 CDC National Center for Health Statistics. Health, United States, 2011 5 OECD Health at a Glance, December 2010, WHO The Challenge of Obesity in the WHO European Region and the Strategies for Response 2007 6 Finklestein, et al. Health Affairs 28 no. 5 (2009);w822-w831 7 Rettman A, Focus: Obesity epidemic costs EU a year. euobserver.com, 6/06/06; US$ conversion at €1EU: $1.34USD, xe.com Jan, 2011 ADULT OBESITY - A COSTLY EPIDEMIC Increase in all-cause mortality1 Increase in overall mortality from CVD, cancer, diabetes and kidney disease2 Increase in risk of fatal heart disease events3 Most prevalent medical condition with no effective pharmacologic option 68.8% in US4, 50% in EU5 Most costly medical condition $147B in US6, $158B in EU7 ©2013 VIVUS Inc. All rights reserved | www.vivus.com 9 |
|
|
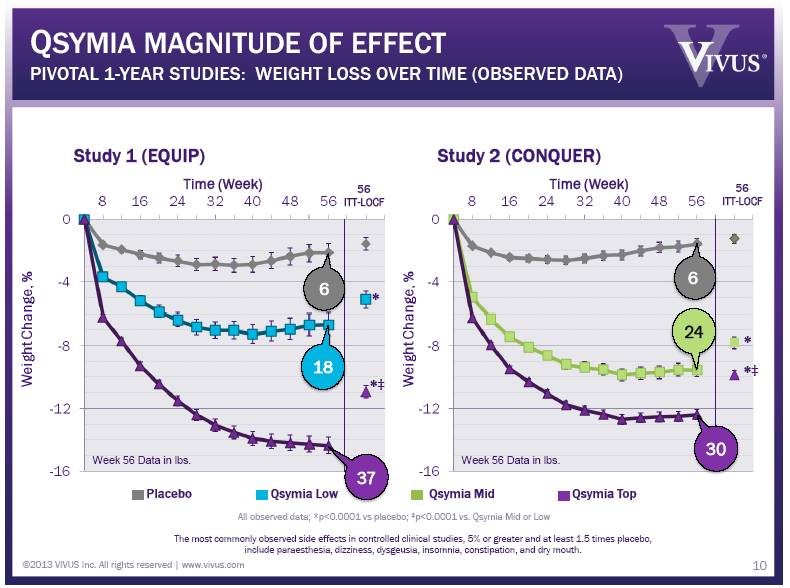
©2013 VIVUS Inc. All rights reserved | www.vivus.com QSYMIA MAGNITUDE OF EFFECT PIVOTAL 1-YEAR STUDIES: WEIGHT LOSS OVER TIME (OBSERVED DATA) 10 Study 1 (EQUIP) Study 2 (CONQUER) Time (Week) Time (Week) 56 ITT-LOCF 56 ITT-LOCF All observed data; *p<0.0001 vs placebo; ‡p<0.0001 vs. Qsymia Mid or Low The most commonly observed side effects in controlled clinical studies, 5% or greater and at least 1.5 times placebo, include paraesthesia, dizziness, dysgeusia, insomnia, constipation, and dry mouth. Placebo Qsymia Top * *‡ * *‡ 6 24 30 37 18 6 Week 56 Data in lbs. Week 56 Data in lbs. |
|
|
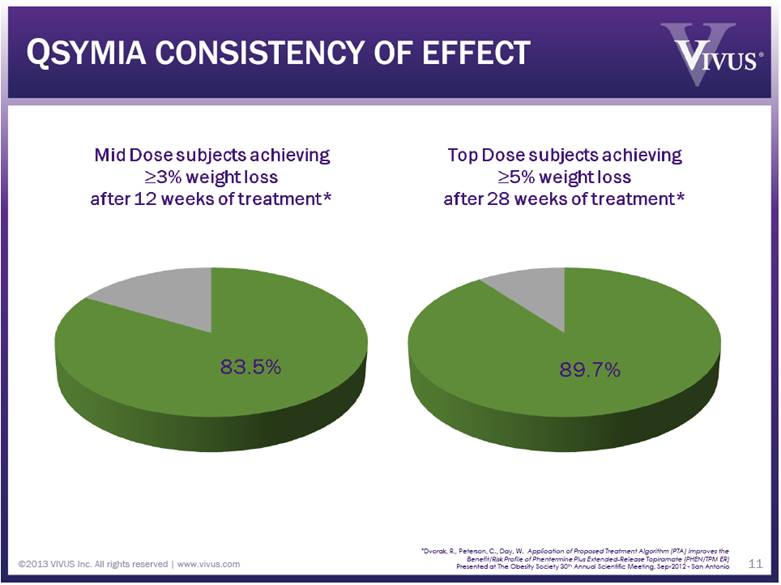
QSYMIA CONSISTENCY OF EFFECT ©2013 VIVUS Inc. All rights reserved | www.vivus.com 11 *Dvorak, R., Peterson, C., Day, W. Application of Proposed Treatment Algorithm (PTA) Improves the Benefit/Risk Profile of Phentermine Plus Extended-Release Topiramate (PHEN/TPM ER) Presented at The Obesity Society 30th Annual Scientific Meeting, Sep-2012 - San Antonio |
|
|
Educate Physicians Broaden Reimbursement Expand to Retail Pharmacies Expand PCP Reach & DTC BUILDING THE QSYMIA FRANCHISE ©2013 VIVUS Inc. All rights reserved | www.vivus.com 12 Launch Time |
|
|
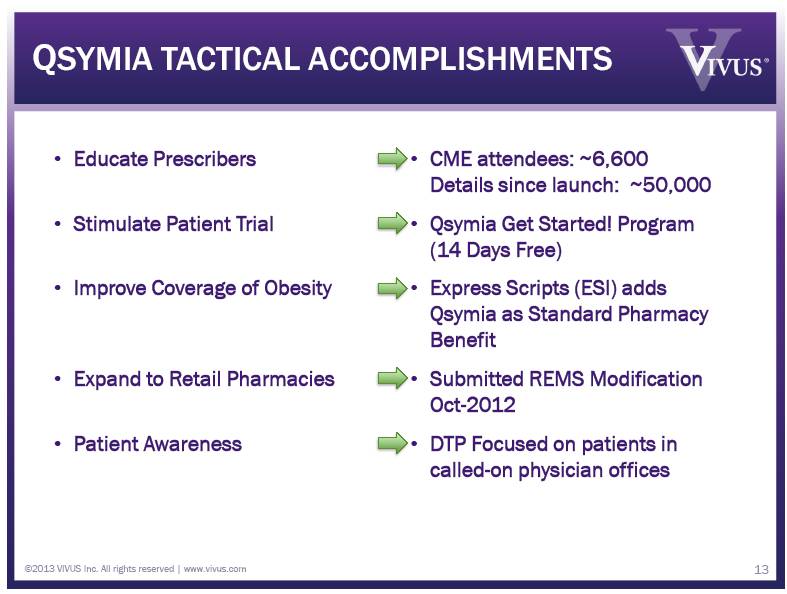
QSYMIA TACTICAL ACCOMPLISHMENTS 13 Educate Prescribers Stimulate Patient Trial Improve Coverage of Obesity Expand to Retail Pharmacies Patient Awareness CME attendees: ~6,600 Details since launch: ~50,000 Qsymia Get Started! Program (14 Days Free) Express Scripts (ESI) adds Qsymia as Standard Pharmacy Benefit Submitted REMS Modification Oct-2012 DTP Focused on patients in called-on physician offices ©2013 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
QSYMIA: EXPANSION TO RETAIL REMS Modification Submission to FDA: “...we request that you submit a REMS modification that will facilitate dispensing through a broader range of pharmacies...” (NDA Approval Letter) Submitted Oct-2012; six-month review expected GOAL: Expand from mail order-only to include retail pharmacies 14 ©2013 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
Qsymia Home Delivery Network ©2013 VIVUS Inc. All rights reserved | www.vivus.com 15 Walmart Pharmacy QSYMIA CERTIFIED PHARMACIES Kaiser Permanente (For Kaiser members only) |
|
|
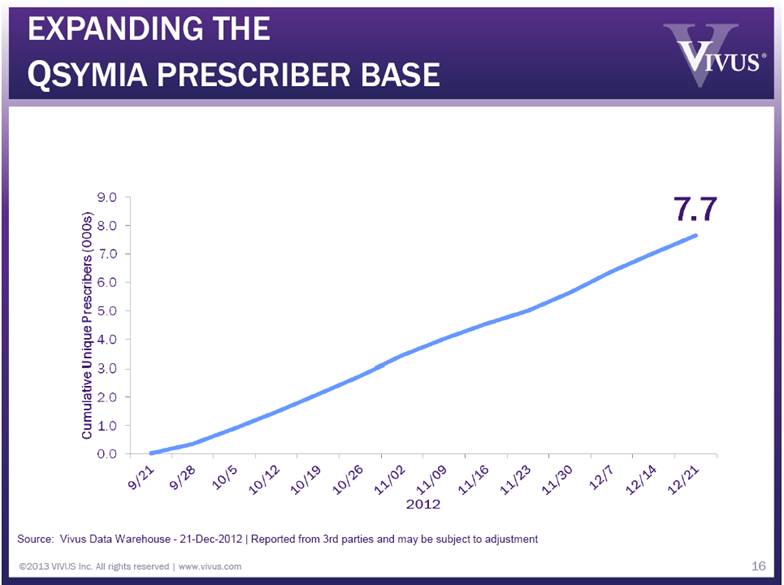
EXPANDING THE QSYMIA PRESCRIBER BASE ©2013 VIVUS Inc. All rights reserved | www.vivus.com 16 Source: Vivus Data Warehouse - 21-Dec-2012 | Reported from 3rd parties and may be subject to adjustment |
|
|
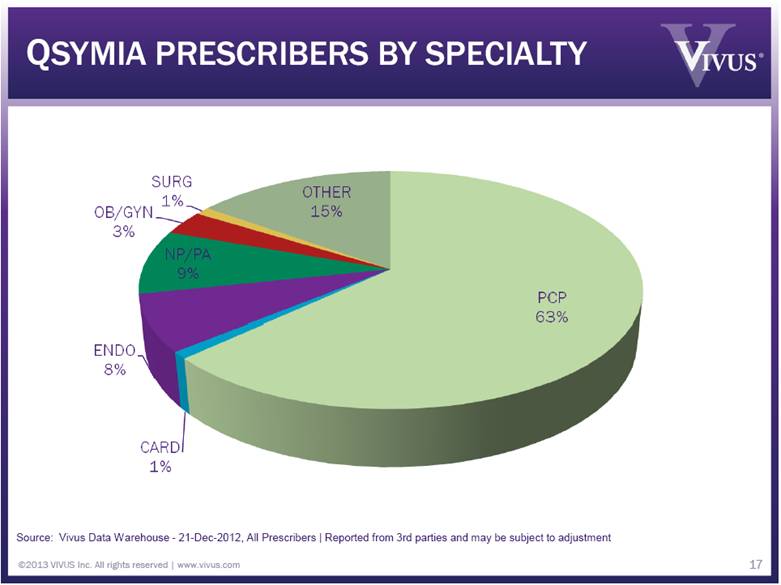
QSYMIA PRESCRIBERS BY SPECIALTY ©2013 VIVUS Inc. All rights reserved | www.vivus.com 17 Cumulative Unique Prescribers (000s) 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 9/21 9/28 10/5 10/12 10/19 10/26 11/02 11/09 11/16 11/23 11/30 12/7 12/14 12/21 2012 Source: Vivus Data Warehouse - 21-Dec-2012, All Prescribers | Reported from 3rd parties and may be subject to adjustment |
|
|
QSYMIA: A VISION FOR THE BRAND Obesity treatment becomes mainstream medicine Widely accepted by primary care physicians Broadly reimbursed Substantial consumer awareness & acceptance Qsymia will become the product of choice Double digit efficacy Well tolerated Improvements in co-morbidities ©2013 VIVUS Inc. All rights reserved | www.vivus.com 18 |
|
|
STENDRA™ PRESCRIBING INFORMATION ©2013 VIVUS Inc. All rights reserved | www.vivus.com 19 |
|
|
STENDRA™ Highly selective PDE5i with rapid onset EMA decision anticipated 1Q2013 Opportunity to commercialize in the US, EU and ROW Partnering discussions underway ©2013 VIVUS Inc. All rights reserved | www.vivus.com 20 |
|
|
VIVUS INVESTMENT HIGHLIGHTS Two FDA approved products Making significant progress in building the Qsymia brand Educating physicians Expanding reimbursement Working to expand distribution Growing Qsymia prescriptions ©2013 VIVUS Inc. All rights reserved | www.vivus.com 21 |
|
|
INNOVATIVE THERAPIES NOVEL PRODUCTS ©2013 VIVUS Inc. All rights reserved | www.vivus.com |