Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AVEO PHARMACEUTICALS, INC. | d461381d8k.htm |
 2013 J.P. Morgan
Healthcare Conference
NASDAQ: AVEO
JANUARY 2013
1
Exhibit 99.1 |
 Forward-Looking Statements
This presentation contains forward-looking statements that involve substantial
risks and uncertainties, including among other things, statements
about: –
our planned development, commercialization and manufacturing plans, timelines and
strategies for tivozanib;
–
the potential therapeutic advantages and benefits of tivozanib and our other
product candidates; –
the timing and results of our ongoing and planned preclinical studies and clinical
trials; –
the potential benefits of our strategic partnership agreements, including our
agreement with Astellas, our ability to achieve additional payments under
these arrangements and our ability to enter into additional
arrangements;
–
our
plans
to
leverage
our
Human
Response
Platform™
to
inform
clinical
development;
–
our intellectual property position and strategies;
–
the expected RCC market and potential of tivozanib to obtain regulatory approval
and enter this market; –
our projections with respect to our achievement of corporate milestones, including
our anticipated plans for success in the oncology markets; and
–
AVEO having sufficient capital to fund its operations through 2013 and AVEO’s
estimates for 2012 expected yearend cash balance and cost savings from its
strategic restructuring. Actual
results
or
events
could
differ
materially
from
the
plans,
intentions
and
expectations
disclosed
in
the
forward-
looking
statements
we
make
due
to
a
number
of
important
factors,
including
risks
and
uncertainties
relating
to:
our
ability to successfully develop, test and gain regulatory approval of our
product candidates, including regulatory approval of tivozanib to
treat advanced RCC; our ability to obtain, maintain and enforce intellectual property rights;
competition; our dependence on our alliance partners and other third parties; our
ability to obtain necessary financing;
adverse
economic
conditions;
and
those
risk
factors
discussed
in
the
“Risk
Factors”
and
elsewhere
in
our most recent Form 10-Q and other periodic filings we make with the
Securities and Exchange Commission. All forward-looking statements
contained in this presentation speak only as of the date of this presentation, and we
undertake no obligation to update any of these statements, except as required by
law. 2 |
 Investment Thesis
3
•
Tivozanib in development for multiple
cancer indications
–
Successful TIVO-1 trial in 1
-line RCC
–
Target PDUFA date in July 2013
–
World-class partnership with Astellas
–
BATON Phase 2 studies in CRC & BC
•
Human Response Platform
™
supporting discovery and clinical
development
•
Significant commercial rights to all
oncology programs
•
Capital through 2013
Founded:
2002
HQ:
Cambridge, MA
Employees: ~225
NASDAQ:
AVEO
Tivozanib
is
an
investigational
compound
and
it
has
not
received
regulatory
approval
for
sale.
st |
 Anticipated Approval of Tivozanib
4 |
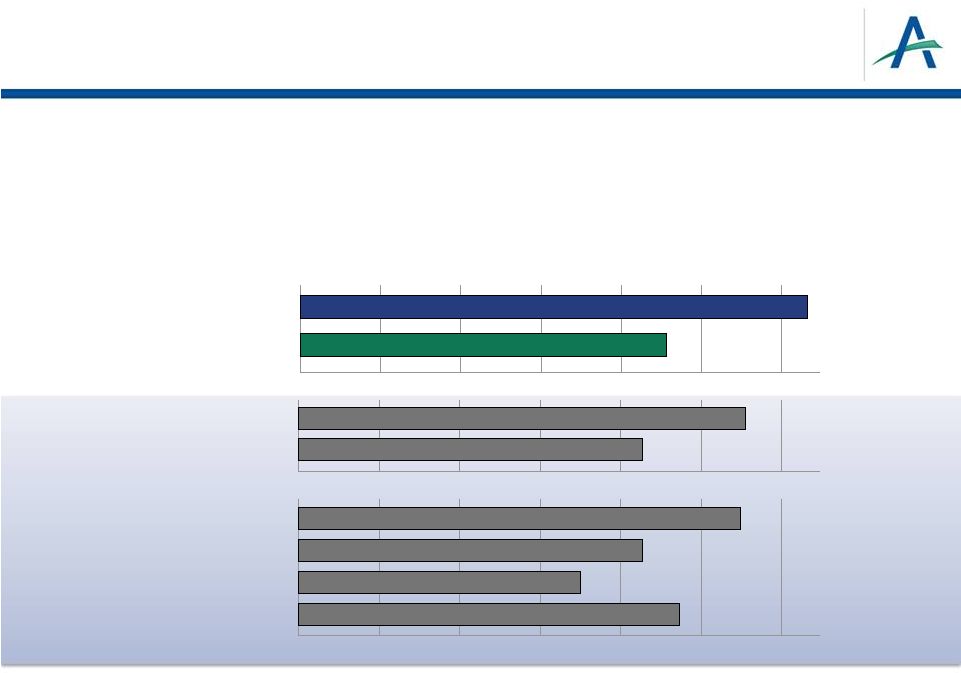 Basic
Criteria for Approval of New Anticancer Drug •
Positive outcome in adequate and well-controlled trials
–
Phase 3 TIVO-1 trial: superiority over sorafenib on primary endpoint of
PFS •
Median PFS (mos): 11.9 vs 9.1 in ITT; 12.7 vs 9.1 in treatment-naïve
subpopulation –
Phase 2 randomized discontinuation study: superiority over placebo
January 4, 2013
5
TIVO-1 Study
PFS in Treatment Naïve RCC Patients
Sunitinib
Pazopanib
Tivozanib
Sorafenib
0
12.7 months
9.1 months
2
8
10
12
4
6
Phase 3 Study
2
COMPARZ Study
5
11.1 months
8.4 months
0
4
6
8
10
12
2
Phase 3 Study
2
Standard Dose
4
Novel Dose
4
5
0
11
months
9.5 months
8.5 months
7 months
2
10
12
4
6
8
4. Motzer, et al. 2011 ASCO GU Symposium, Abstract
308. Standard dose (4/2), novel dose (continuous).
5. Motzer, et al. 2012 ESMO Congress, Abstract LBA8
1. Motzer et al, ASCO 2012
2. Votrient PI.
3. Sutent PI.
COMPARZ Study
1 |
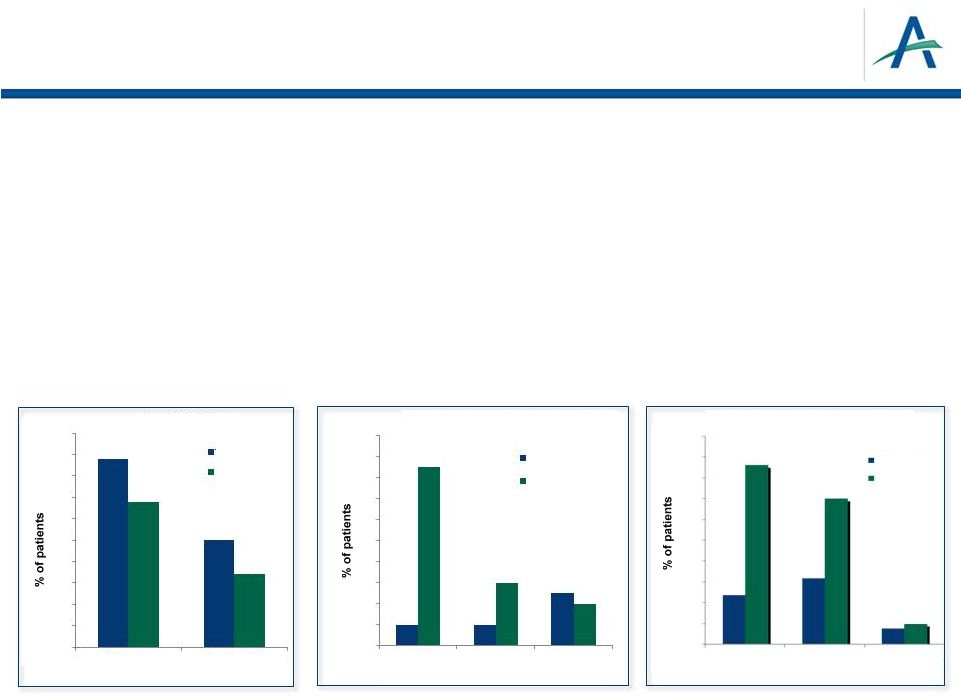 Basic
Criteria for Approval of New Anticancer Drug •
Positive outcome in adequate and well-controlled trials
–
Phase 3 TIVO-1 trial: superiority over sorafenib on primary endpoint of
PFS •
Median PFS (mos): 11.9 vs 9.1 in ITT; 12.7 vs 9.1 in treatment-naïve
subpopulation –
Phase 2 randomized discontinuation study: superiority over placebo
6
•
Acceptable safety profile
–
>1,000 subjects treated with tivozanib
–
Lower rates than sorafenib for common adverse events and dose
modifications –
Rate of fatal AEs consistent with reports from past pivotal RCC trials
tivozanib
sorafenib
50
45
40
35
30
25
20
15
10
5
0
44
34
25
17
All Grades
Grades 3/4
tivozanib
sorafenib
20
18
16
14
12
10
8
6
4
2
0
Hand-Foot
Syndrome
Diarrhea
Fatigue
17
2
2
6
5
4
50
45
40
35
30
25
20
15
10
5
0
tivozanib
sorafenib
43
35
12
16
Dose Reductions
Dose
Interruptions
4
5
Discontinuations
Hypertension
Other Common AEs (Grades 3/4)
Dose Adjustments Due to AEs
Motzer, et al., ASCO 2012 |
 Basic
Criteria for Approval of New Anticancer Drug •
Positive outcome in adequate and well-controlled trials
–
Phase 3 TIVO-1 trial: superiority over sorafenib on primary endpoint of
PFS •
Median PFS (mos): 11.9 vs 9.1 in ITT; 12.7 vs 9.1 in treatment-naïve
subpopulation –
Phase 2 randomized discontinuation study: superiority over placebo
January 4, 2013
7
•
Acceptable safety profile
–
>1,000 subjects treated with tivozanib
–
Lower rates than sorafenib for common adverse events and dose
modifications –
Rate of fatal AEs consistent with reports from past pivotal RCC trials
•
Established dose and regimen
–
Multiple
studies
conducted
using
1.5
mg/d
for
21
days,
repeating
every
28
days |
 Basic
Criteria for Approval of New Anticancer Drug •
Positive outcome in adequate and well-controlled trials
–
Phase 3 TIVO-1 trial: superiority over sorafenib on primary endpoint of
PFS •
Median PFS (mos): 11.9 vs 9.1 in ITT; 12.7 vs 9.1 in treatment-naïve
subpopulation –
Phase 2 randomized discontinuation study: superiority over placebo
January 4, 2013
8
•
Acceptable safety profile
–
>1,000 subjects treated with tivozanib
–
Lower rates than sorafenib for common adverse events and dose
modifications –
Rate of fatal AEs consistent with reports from past pivotal RCC trials
•
Established dose and regimen
–
Multiple
studies
conducted
using
1.5
mg/d
for
21
days,
repeating
every
28
days
•
Favorable benefit-risk profile |
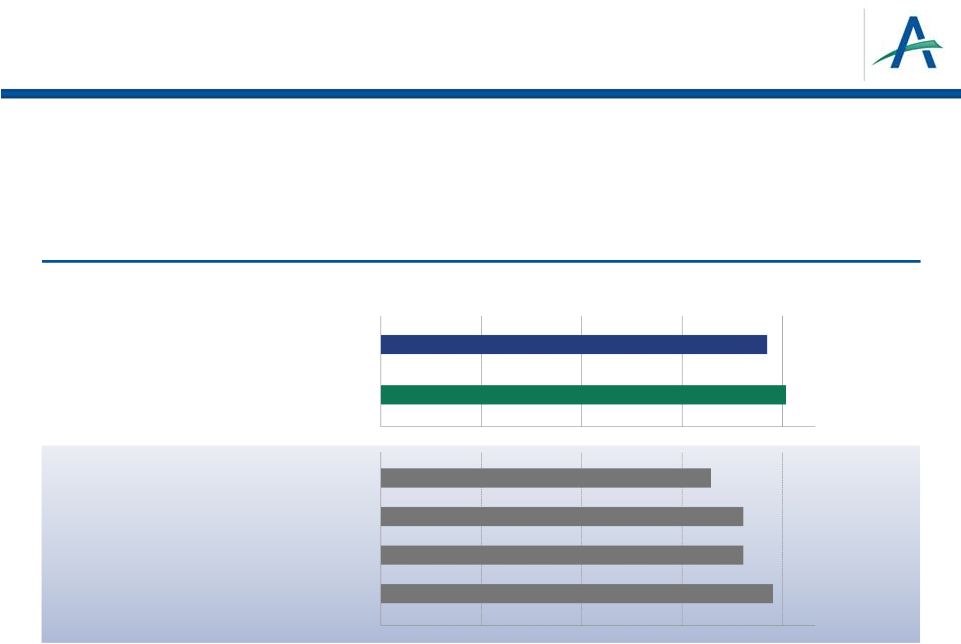 What About Overall
Survival? •
PFS is accepted registration endpoint in advanced RCC
•
OS is secondary endpoint in TIVO-1
–
Results not statistically significant
January 4, 2013
9
Pivotal Studies
for Currently
Available
Therapies
TIVO-1 Study
Sorafenib (TARGET): Escudier B, et al. J Clin Oncol 2009; 27:3312-8.
Bevacizumab: Escudier B, et al. J Clin Oncol 2010; 28: 2144-50.
Pazopanib: CHMP Assessment Report, 2010.
Sunitinib: Motzer RJ et al. J. Clin Oncol 2009; 27:3584-90.
Overall Survival Estimates at One Year (% of Pts)
Tivozanib (TIVO-1 Study)
Sorafenib (TIVO-1 Study)
0
20
40
60
80
77
81
Nexavar (TARGET
Study)
Votrient
Sutent
65
73
73
78
0
20
40
60
80
Avastin + Interferon |
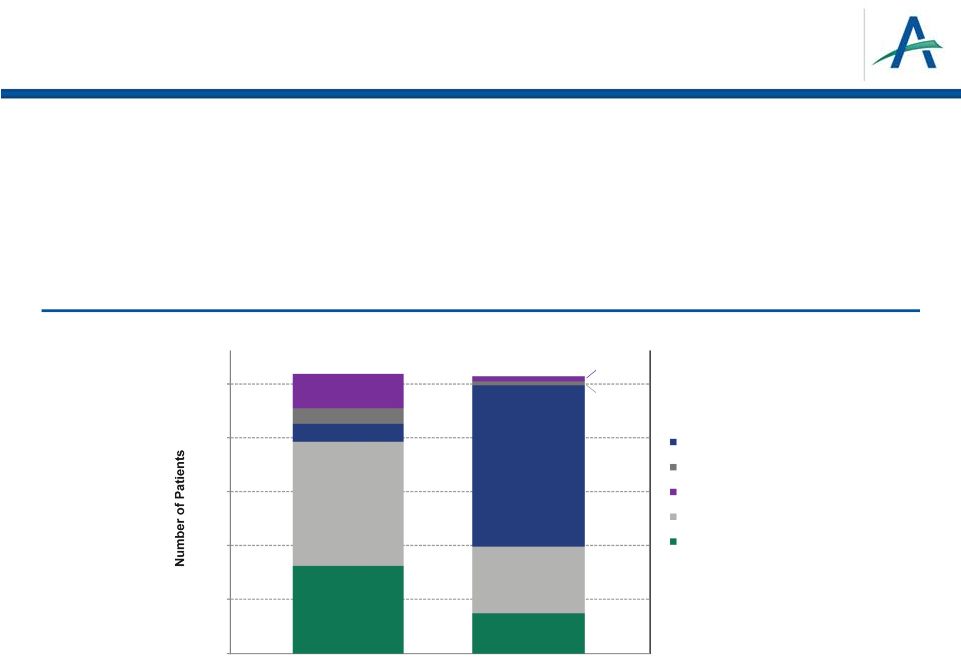 What About Overall
Survival? •
PFS is accepted registration endpoint in advanced RCC
•
OS is secondary endpoint in TIVO-1
–
Results not statistically significant
•
High use of one-way crossover confounded OS data
–
Patients on sorafenib able to receive tivozanib 2
-line
January 4, 2013
10
*Based on May 2012 data sweep in overall population
Use of 2
-Line Therapy in TIVO-1
nd
nd
0
50
100
150
200
250
Tivozanib Arm (n=260)
Sorafenib Arm (n=257)
Received radiotherapy or cytokine 2nd-line
Received mTOR inhibitor 2nd-line
Received VEGF inhibitor 2nd-line
Discontinued 1st-line, no 2nd-line
Still on 1st-line
81
116
16
15
32
37
62
(148 received
tivozanib)
150
4
4
* |
 Regulatory Status of Tivozanib in Advanced RCC
•
NDA submission included final OS analysis
–
Analysis conducted at 2 years after last patient enrolled
–
Final OS data to be presented at ASCO GU
•
NDA accepted for filing November 2012
•
ODAC anticipated 1H 2013
•
Target PDUFA action date set for July 28, 2013
11 |
 Addressing Medical Need in RCC
January 3, 2013
12 |
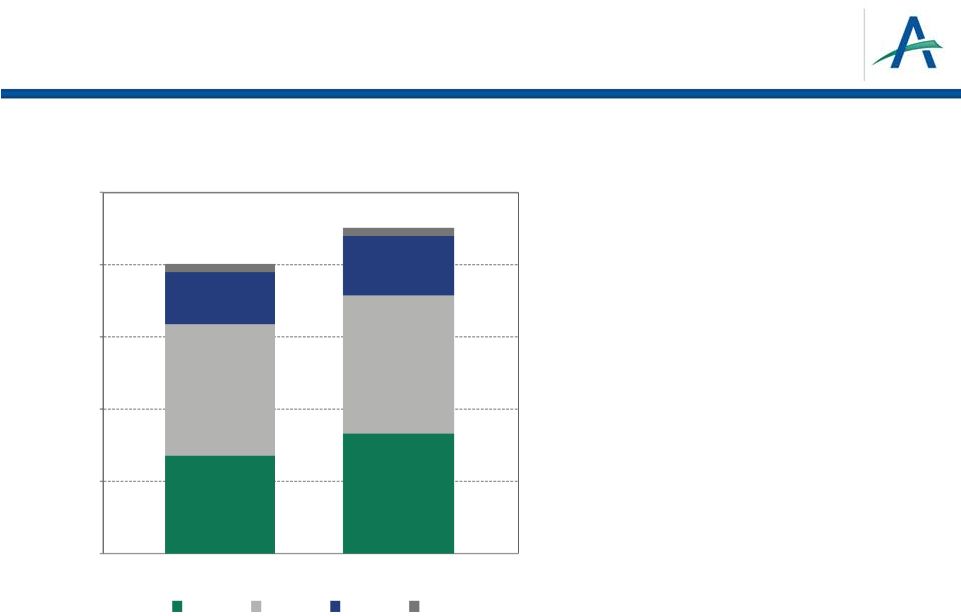 Global RCC
Sales Estimates for 12 Months Ending 9-30-12 RCC is Large and Growing Market
•
Global RCC sales grew 17% from MAT
Sep11 to MAT Sep12
•
US growth of 33% in same period
•
Growth drivers include
•
Expanded treatment options
•
Price increases (US)
•
Population growth
•
Increase in incidence
13
MAT = Moving Annual Total
Sources:
US
Wolters
Kluwer
/
Ex-US
IMS
Health
-
All
adjusted
for
estimated
%
of
sales
for
RCC
per
IMS.
Ex-US
adjustment
factor
triangulated
with
Decision
Resources
product
sales
estimates
for
2011.
Sorafenib
adjusted
for
IMS
and
WK
data
capture
based
on
reported
sales
0
500
$1,000
$1,500
$2,000
$2,500
MAT Sept 11
MAT Sept 12
$2.2B
$1.9B
ASIA
ROW
EU
US |
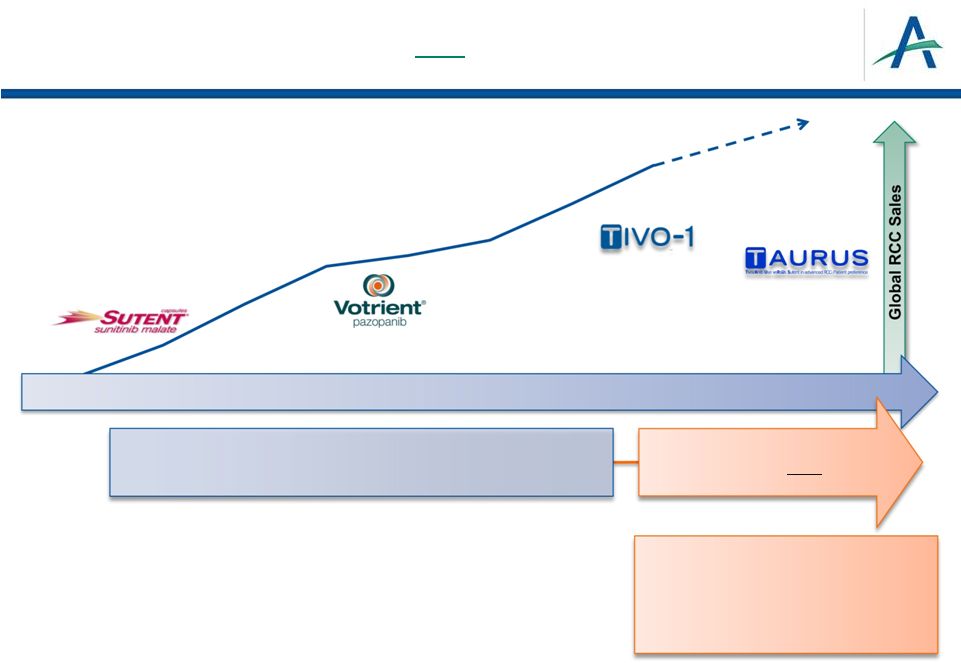
 Toxicities Impact Daily Living
Development of 1
-Line RCC Therapies
Targeted Therapies Approved: Focus on Efficacy
No
Targeted
Therapies
2013
14
•
>50%
of
patients
taking
sorafenib
experienced
hand-foot
syndrome
•
>50%
of
patients
taking
sunitinib
or
pazopanib
experienced
fatigue
•
>50%
of
patients
taking
sunitinib
or
pazopanib
experienced
diarrhea
1. Esudier, et al. J Clin Oncol. 2009;27(20):3312-3318
2. Sutent prescribing information revised 05.2011.
3. Votrient prescribing information revised 01.2012.
2005
2006
2007
2008
2009
2010
2011
2012
st
2
2
3
3
1 |
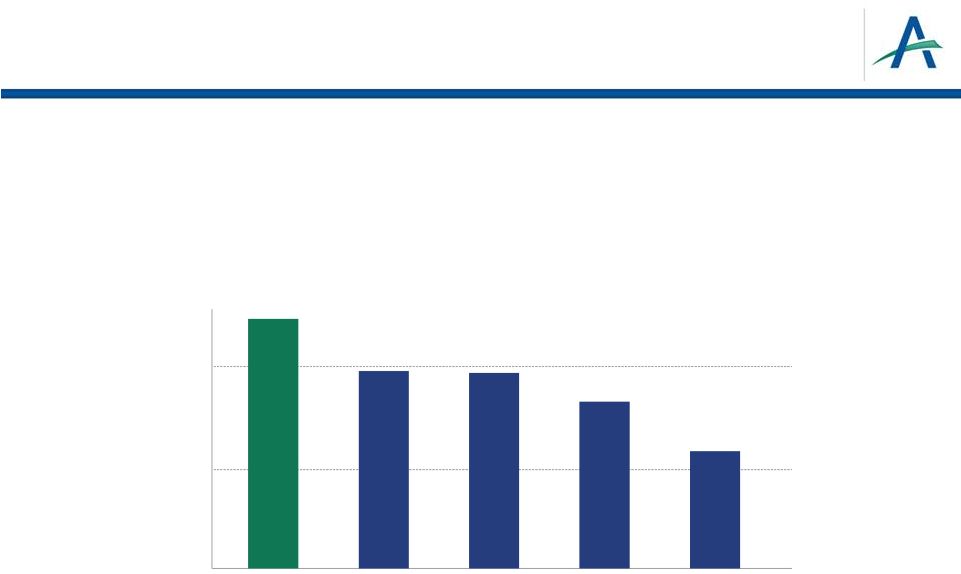 Patient Perspectives on
Toxicities: 272 Patient KCA Survey Reference: Wong MKK, Mohamed AF, Hauber AB, et al. Selecting
renal cell carcinoma therapy: Ranking of patient perspective on toxicities. J Clin Oncol 30,
2012 (suppl; abstr 4608). Patients report stomach problems, fatigue, mouth sores and
hand-foot syndrome as most troublesome side effects
15
Relative Importance for RCC Therapies
(10=most important)
10
8
6
4
2
0
PFS
Stomach
Problems
Fatigue
Mouth Sores
Hand-Foot
Syndrome |
 2012 was
transformational year for 1 -line RCC:
EFFICACY
Today, 1 -Line RCC Market in Flux
16
January 4, 2013
st
st
•
TIVO-1
data
demonstrate
tivozanib
is
only
agent
with
•
COMPARZ
data showed pazopanib PFS non-inferior to sunitinib
•
Median PFS (mos) of 8.4 vs 9.5
•
AGILE 1051
results report axitinib did not meet PFS superiority endpoint
compared
to
sorafenib
in
1
-line
st
>
profile distinguished from other agents
1 yr
of
PFS
and
safety |
 2012 was
transformational year for 1 -line RCC:
TOLERABILITY
Today, 1 -Line RCC Market in Flux
•
TIVO-1
data
demonstrate
low
rates
of
dose
reductions
(12%)
and
•
COMPARZ
data
showed
pazopanib
PFS
non-inferior
to
sunitinib
•
PISCES
data
reinforce
tolerability
matters
•
TAURUS
Phase
2
study
initiated
evaluating
patient
preference
for
tivozanib vs
sunitinib
17
January 4, 2013
st
st
•
Safety profile distinguished from other agents
•
45% and 60% dose
reductions
and
interruptions
by
both
agents,
respectively
•
70% of patients preferred pazopanib over sunitinib
•
61% of physicians preferred pazopanib over sunitinib
•
Randomized, double-blind cross-over study in 160 patients in US and Europe
interruptions (18%) with tivozanib
>
> |
 Future Focuses on Efficacy and
Tolerability
Targeted Therapies Approved: Focus on Efficacy
18
PISCES
COMPARZ
2005
2006
2007
2008
2009
2010
2011
2012
2014
2013
New Data:
Focus on Efficacy AND
Tolerability
•
Patient tolerability
•
Safety profile
•
Minimal dose modifications
•
Convenient dose and regimen |
 Launch Plan in RCC
19
19 |
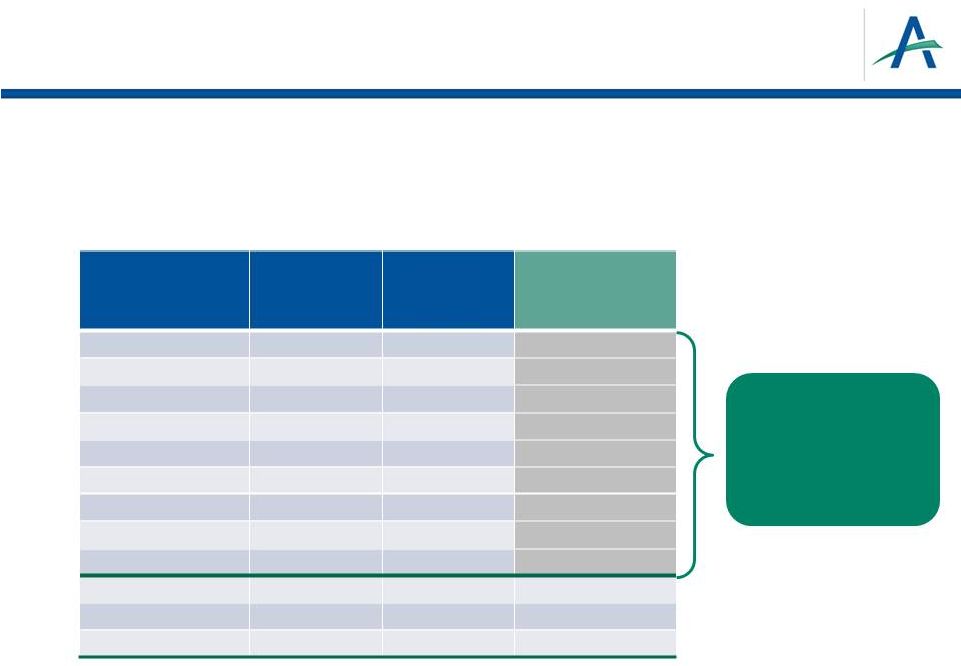 Key
Pre-Launch Commercial Objectives •
Refine understanding of RCC marketplace and opportunity for tivozanib
–
Comprehensive advisory board and market research plan
–
Customer targeting plan for launch
20
Analysis
supports ~80
Sales Reps can
cover top 85%
customers
RCC Decile
Customers
Total FTEs
Estimated
Patients per
Annual RCC
Customer
10
(10%)
7
(40%)
8
(30%)
9
(20%)
4
(70%)
5
(60%)
6
(50%)
3
(80%)
2a
(85%)
2b
(90%)
1a
(95%)
1b
(100%)
16
49
83
126
181
260
374
543
375
493
723
2,235
86.4
28.0
17.0
11.6
8.2
5.8
4.0
2.8
2.0
1.5
1.0
0.3
3.8
5.1
5.2
6.7
8.8
10.3
13.2
16.3
11.3
14.8
21.7
67.1 |
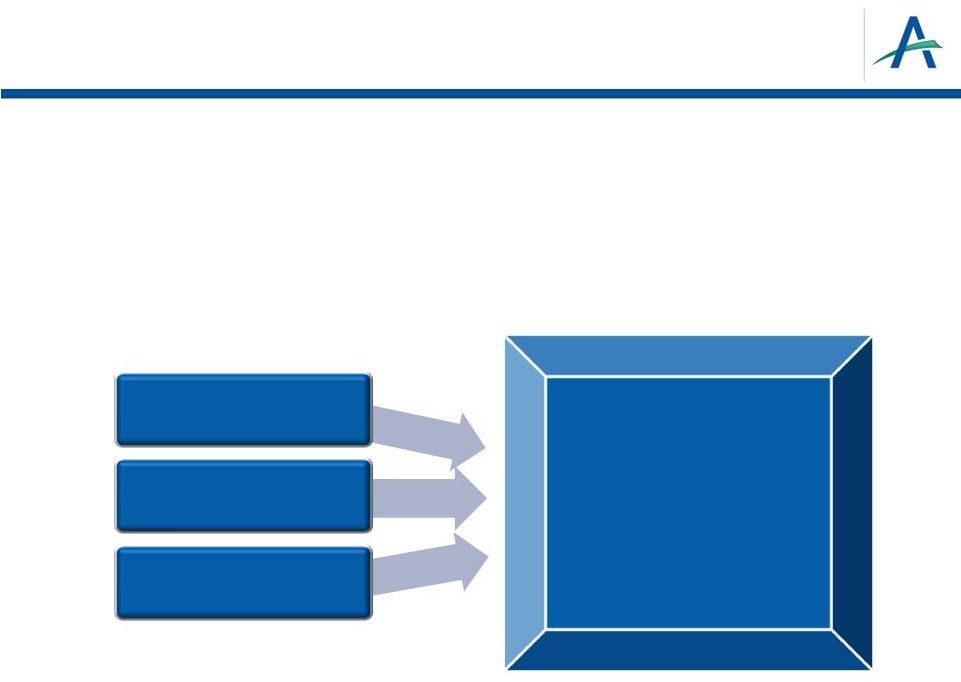 Key
Pre-Launch Commercial Objectives •
Refine understanding of RCC marketplace and opportunity for tivozanib
–
Comprehensive advisory board and market research plan
–
Customer targeting plan for launch
•
Optimize brand platform and positioning
–
Distinct global positioning and differentiated messaging for launch
21
Selective inhibition
of VEGFR 1,2,3
Potent inhibition
of VEGFR 1,2,3
Long half-life
Efficacy:
PFS
1
year
in
treatment-naïve patients
Safety:
Low rates of dose
reductions and interruptions
Dosing:
One tablet, once a
day
> |
 Key
Pre-Launch Commercial Objectives •
Refine understanding of RCC marketplace and opportunity for tivozanib
–
Comprehensive advisory board and market research plan
–
Customer targeting plan for launch
•
Optimize brand platform and positioning
–
Distinct global positioning and differentiated messaging for launch
•
Increase awareness of clinical unmet needs in RCC
–
Highlight challenges of disease and emphasize importance of communication
around patient concerns
22 |
 Key
Pre-Launch Commercial Objectives 23 |
 Key
Pre-Launch Commercial Objectives Refine understanding of RCC marketplace and opportunity for tivozanib
–
Comprehensive advisory board and market research plan
–
Customer targeting plan for launch
Optimize brand platform and positioning
–
Distinct global positioning and differentiated messaging for launch
Increase awareness of clinical unmet needs in RCC
–
Highlight challenges of disease and emphasize importance of communication
around patient concerns
Optimize patient access offerings
–
Patient services programs
–
Best-in-class reimbursement support
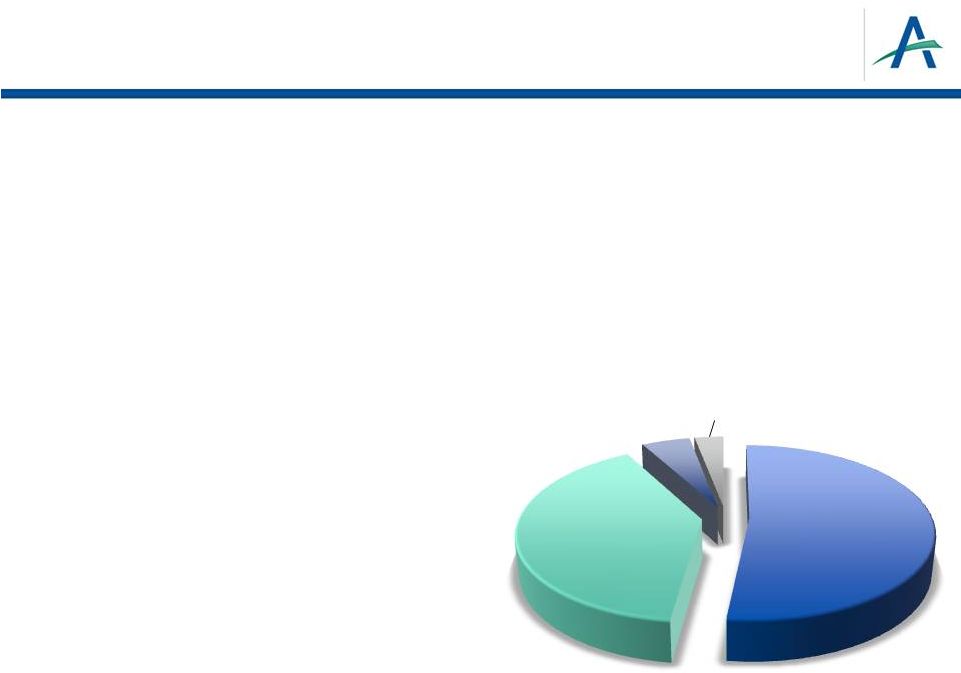
24
Medicaid
6%
Uninsured
7%
Medicare
26%
Commercial
Insurance
61%
•
•
•
• |
 Key
Pre-Launch Commercial Objectives Refine understanding of RCC marketplace and opportunity for tivozanib
–
Comprehensive advisory board and market research plan
–
Customer targeting plan for launch
Optimize brand platform and positioning
–
Distinct global positioning and differentiated messaging for launch
Increase awareness of clinical unmet needs in RCC
–
Highlight challenges of disease and emphasize importance of communication
around patient concerns
Optimize patient access offerings
–
Patient services programs
–
Best-in-class reimbursement support
Ensure launch readiness
–
Finalize distribution capabilities
–
Establish sales force infrastructure
25
Vice President, U.S. Sales
Brad Bailey, AVEO
Hired 3Q12
Area Directors
1Q13
Regional Managers
1Q13
Sales Specialists
2Q13
Sales Force Structure
•
•
•
•
• |
 Looking beyond RCC
26 |
 BATON: Biomarker Assessment of Tivozanib in ONcology
•
Phase 2 in
1
colorectal cancer (n=252)
•
Tivozanib
+ mFOLFOX6
vs
bevacizumab
+ mFOLFOX6
•
Primary endpoint: PFS
•
Assess predictive biomarkers
•
LDH; hypoxia-inducible factor
signature; VEGF A, C and D levels;
myeloid-specific markers (CD68)
•
Data anticipated in 2014
•
Phase 2 in 1
-line in metastatic triple
negative breast cancer (n=147)
•
Tivozanib + paclitaxel vs placebo +
paclitaxel
•
Primary endpoint: PFS
•
Assess predictive biomarkers
•
Hypoxia-inducible factor signature,
VEGF-A, VEGF-C, soluble VEGFR-1 and
-2; myeloid-specific markers (CD68)
•
Data anticipated in 2014
27
Biomarker Driven Approach to Tivozanib Development
st
st
-line metastatic |
 Financial Resources
Strategic restructuring conducted in October 2012
–
Expected to provide ~$100M in cost savings over 3 years, including
~$37M in 2013
Estimated
4Q12
cash
and
securities
of
~$160M*
Sufficient capital to fund operations through 2013
28
*Unaudited 4Q12 financial results as of 12/31/12
•
•
• |
 AVEO
Investment Thesis Tivozanib
approval
anticipated
in
2013
–
Anticipated ODAC in 1H13
–
Target PDUFA action date of July 28, 2013
–
MAA led by Astellas; anticipated submission in 2H13
BATON
studies
ongoing
Human
Response
Platform
supporting
development
Commercial
rights
to
all
oncology
programs
Capital
through
2013
29
•
•
•
•
•
™ |
