Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Chelsea Therapeutics International, Ltd. | v329747_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Chelsea Therapeutics International, Ltd. | v329747_ex99-1.htm |

© 2004 - 2012 Chelsea Therapeutics, Inc. Northera ™ ( droxidopa ) Study 306B Preliminary Results 4 December 2012

NASDAQ: CHTP 2 Forward - Looking Statements This presentation is being provided for informational and discussion purposes . This presentation is not intended to provide and should not be relied upon as investment advice or an opinion regarding the appropriateness or suitability of any investment . Nothing herein should be construed to be an offer to sell, or a solicitation of an offer to buy, any securities . This presentation contains forward - looking statements regarding future events including our intention to pursue the development of Northera . These statements are subject to risks and uncertainties that could cause the actual events or results to differ materially . These include reliance on key personnel and our ability to attract and/or retain key personnel ; risks of distraction of the Board and management at this critical time ; the risk that the FDA will not accept our proposal regarding any trial or other data to support a NDA for Northera ; the risk that we will not be able to resubmit the NDA for Northera and that the FDA will not approve a resubmitted NDA ; the risk that our resources will not be sufficient to conduct any study of Northera that will be acceptable to the FDA ; the risk that we cannot complete any additional study for Northera without the need for additional capital ; the risks and costs of drug development and that such development may take longer or be more expensive than anticipated ; our need and ability to raise additional operating capital in the future ; our reliance on our lead drug candidate Northera ; risk of regulatory approvals of Northera or our other drug candidates for additional indications ; risk of volatility in our stock price, related litigation, and analyst coverage of our stock ; reliance on collaborations and licenses ; intellectual property risks ; our history of losses ; competition ; and market acceptance for our products if any are approved for marketing .

Study 306B: a Phase III Trial Evaluating the Safety and Efficacy of Northera ™ ( droxidopa ) for the Treatment of Symptomatic Neurogenic Orthostatic Hypotension (NOH) Associated with Parkinson’s Disease (PD) NASDAQ: CHTP 3
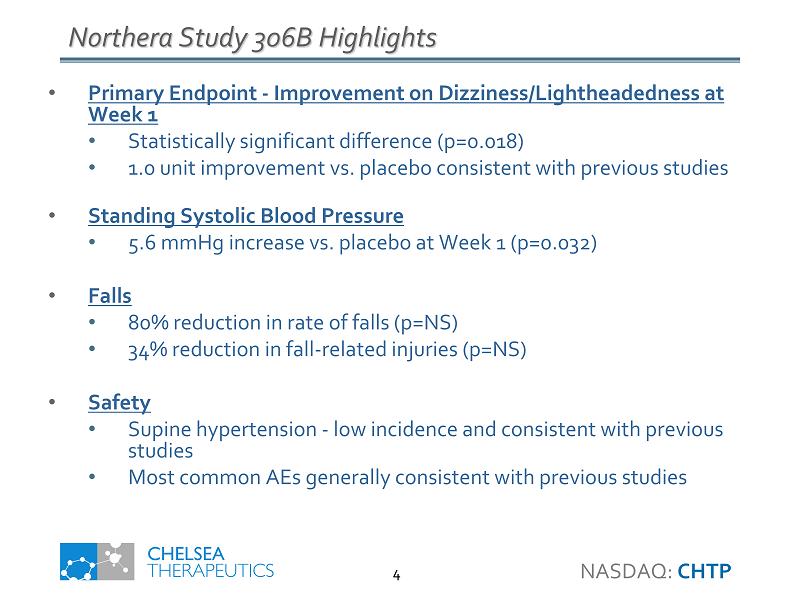
Northera Study 306B Highlights • Primary Endpoint - Improvement on Dizziness/Lightheadedness at Week 1 • Statistically significant difference (p=0.018) • 1.0 unit improvement vs. placebo consistent with previous studies • Standing Systolic Blood Pressure • 5.6 mmHg increase vs. placebo at Week 1 (p=0.032) • Falls • 80% reduction in rate of falls (p=NS) • 34% reduction in fall - related injuries (p=NS) • Safety • Supine hypertension - low incidence and consistent with previous studies • Most common AEs generally consistent with previous studies NASDAQ: CHTP 4
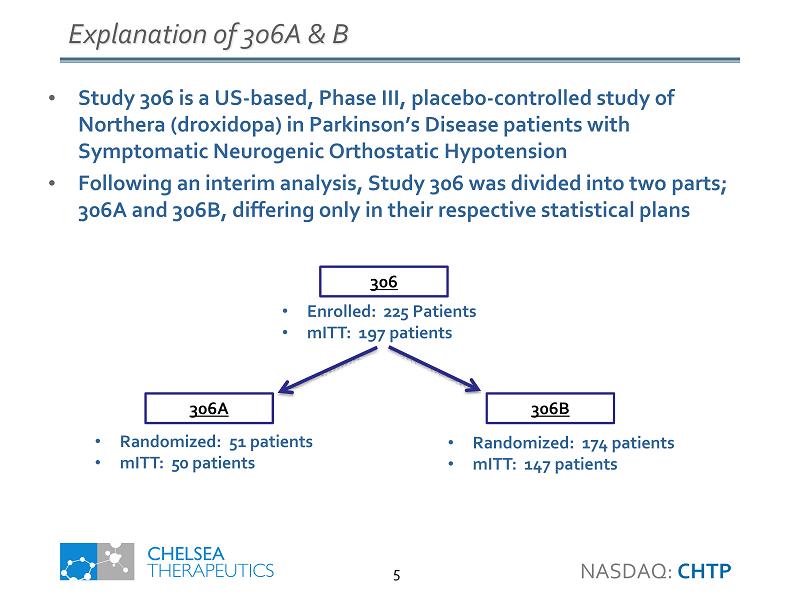
Explanation of 306A & B • Study 306 is a US - based, Phase III, placebo - controlled study of Northera ( droxidopa ) in Parkinson’s Disease patients with Symptomatic Neurogenic Orthostatic Hypotension • Following an interim analysis, Study 306 was divided into two parts; 306A and 306B, differing only in their respective statistical plans • Randomized: 174 patients • mITT : 147 patients 306A • Enrolled: 225 Patients • mITT : 197 patients • Randomized: 51 patients • mITT : 50 patients 306B 306 NASDAQ: CHTP 5
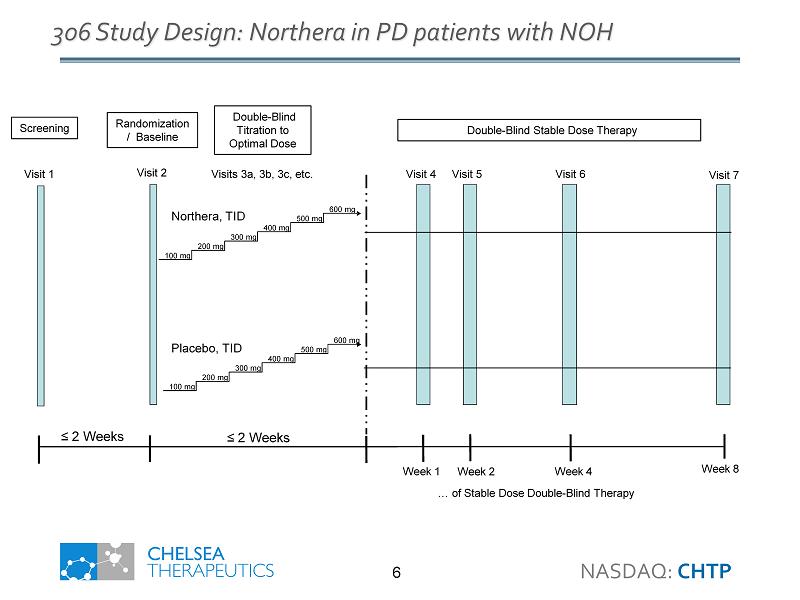
NASDAQ: CHTP 6 306 Study Design : Northera in PD patients with NOH Visit 2 Northera , TID Placebo, TID Visits 3a, 3b, 3c, etc. Visit 4 Visit 5 Visit 6 Visit 7 600 mg 500 mg 400 mg 300 mg 200 mg 100 mg 600 mg 500 mg 400 mg 300 mg 200 mg 100 mg Week 1 Week 2 Week 4 Week 8 Randomization / Baseline Double - Blind Titration to Optimal Dose Double - Blind Stable Dose Therapy ≤ 2 Weeks ≤ 2 Weeks Visit 1 Screening … of Stable Dose Double - Blind Therapy
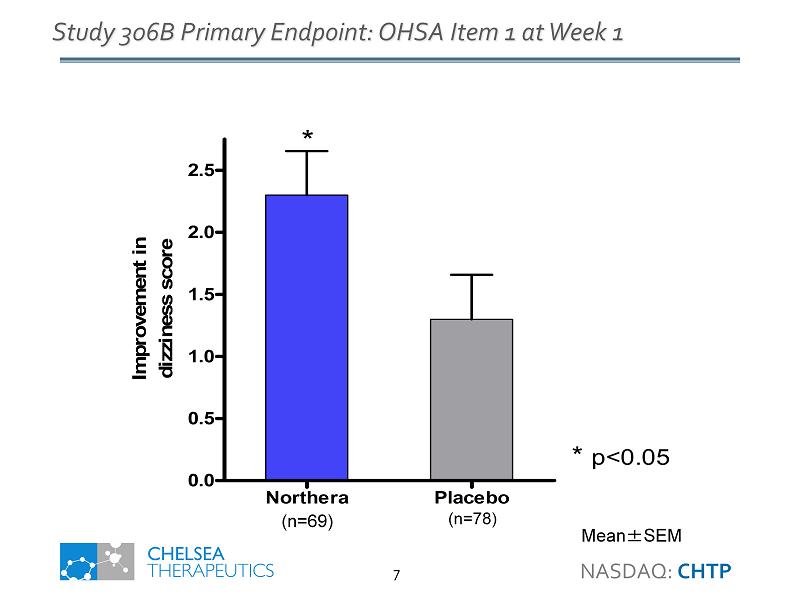
Northera Placebo 0.0 0.5 1.0 1.5 2.0 2.5 * *p<0.05 Improvement in dizziness score Study 306B Primary Endpoint: OHSA Item 1 at Week 1 Mean ± SEM (n=78) (n=69) NASDAQ: CHTP 7
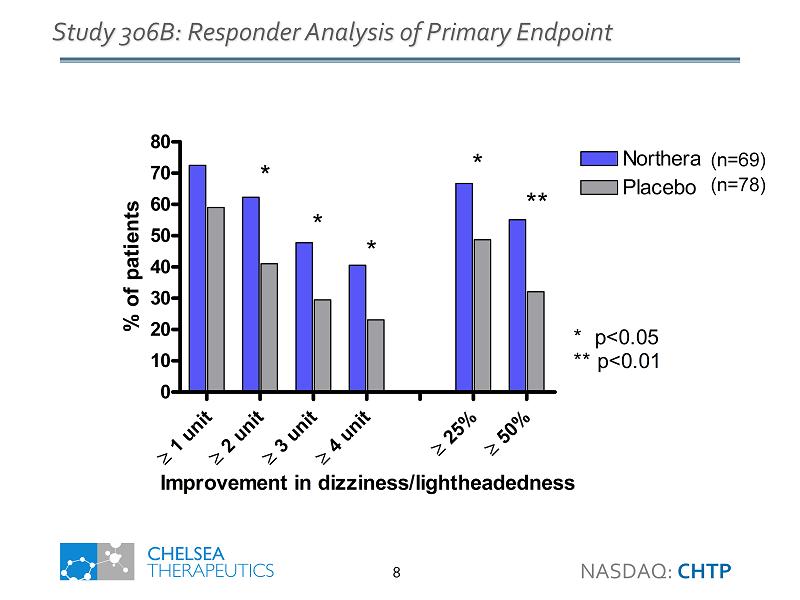
1 unit 2 unit 3 unit 4 unit 25% 50% 0 10 20 30 40 50 60 70 80 Northera Placebo * p<0.05 ** p<0.01 * * * * ** Improvement in dizziness/lightheadedness % of patients Study 306B: Responder Analysis of Primary Endpoint (n=69) (n=78) NASDAQ: CHTP 8
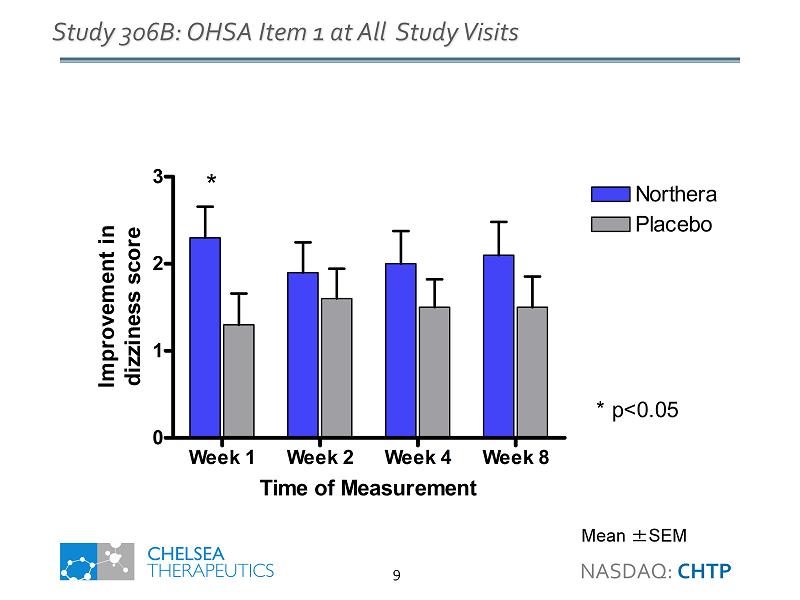
Week 1 Week 2 Week 4 Week 8 0 1 2 3 Northera Placebo * p<0.05 * Time of Measurement Improvement in dizziness score Study 306B: OHSA Item 1 at All Study Visits Mean ± SEM NASDAQ: CHTP 9
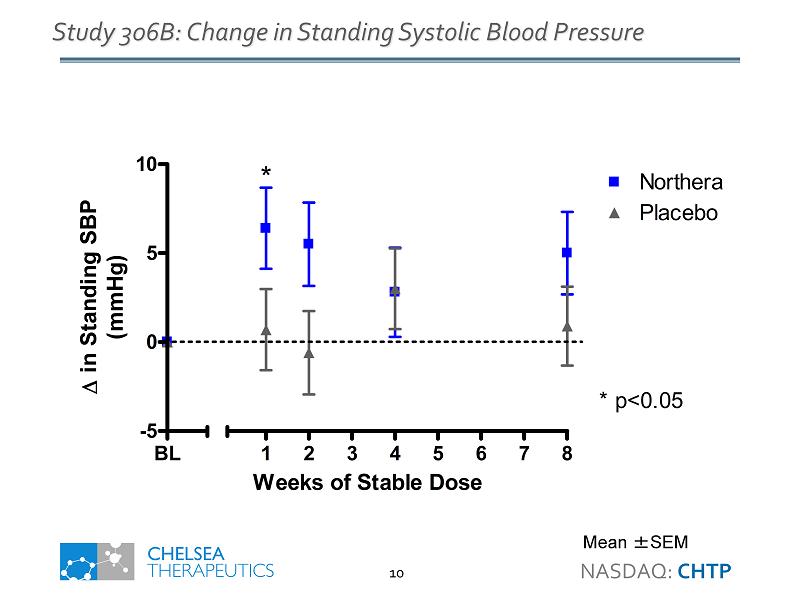
BL -5 0 5 10 Northera Placebo 1 2 3 4 5 6 7 8 * p<0.05 * Weeks of Stable Dose in Standing SBP (mmHg) Study 306B: Change in Standing Systolic Blood Pressure Mean ± SEM NASDAQ: CHTP 10
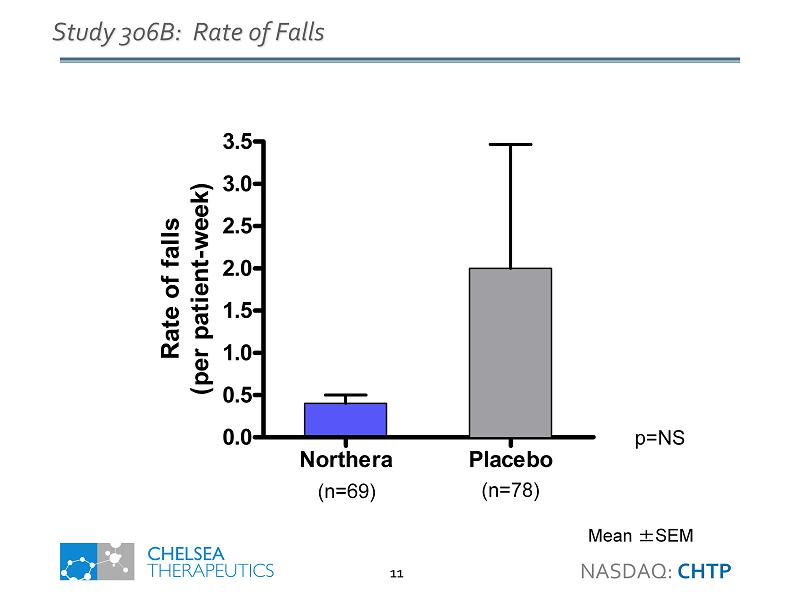
Northera Placebo 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 Rate of falls (per patient-week) Study 306B: Rate of Falls Mean ± SEM (n=78) (n=69) p=NS NASDAQ: CHTP 11
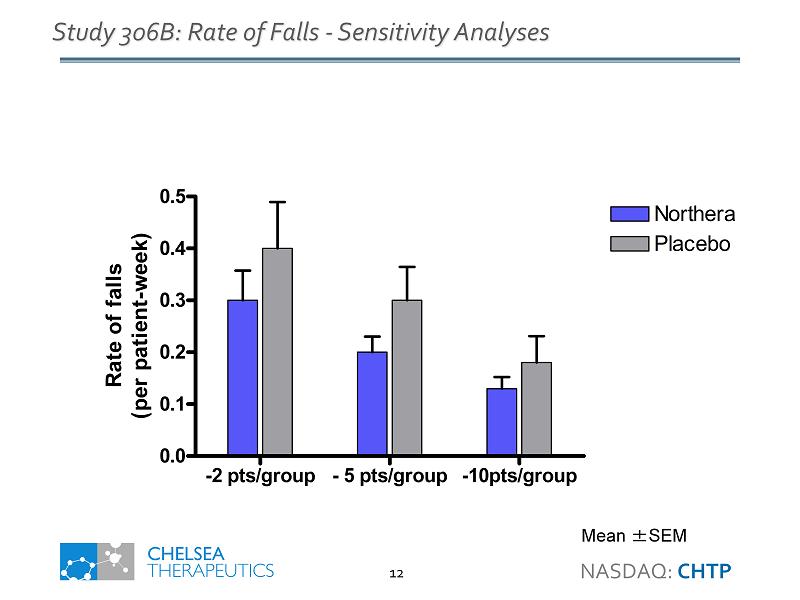
-2 pts/group - 5 pts/group -10pts/group 0.0 0.1 0.2 0.3 0.4 0.5 Northera Placebo Rate of falls (per patient-week) Study 306B: Rate of Falls - Sensitivity Analyses Mean ± SEM NASDAQ: CHTP 12
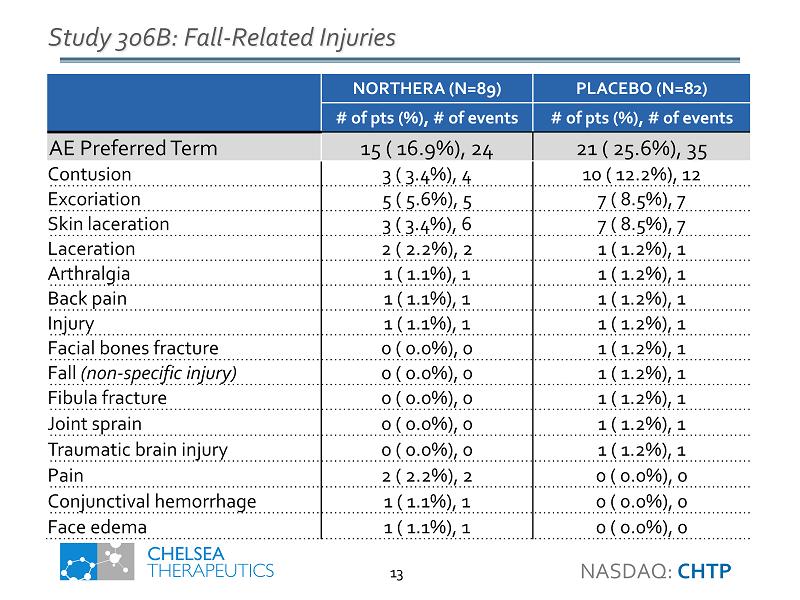
Study 306B: Fall - Related Injuries NORTHERA (N=89) PLACEBO (N=82) # of pts (%), # of events # of pts (%), # of events AE Preferred Term 15 ( 16.9%), 24 21 ( 25.6%), 35 Contusion 3 ( 3.4%), 4 10 ( 12.2%), 12 Excoriation 5 ( 5.6%), 5 7 ( 8.5%), 7 Skin laceration 3 ( 3.4%), 6 7 ( 8.5%), 7 Laceration 2 ( 2.2%), 2 1 ( 1.2%), 1 Arthralgia 1 ( 1.1%), 1 1 ( 1.2%), 1 Back pain 1 ( 1.1%), 1 1 ( 1.2%), 1 Injury 1 ( 1.1%), 1 1 ( 1.2%), 1 Facial bones fracture 0 ( 0.0%), 0 1 ( 1.2%), 1 Fall (non - specific injury) 0 ( 0.0%), 0 1 ( 1.2%), 1 Fibula fracture 0 ( 0.0%), 0 1 ( 1.2%), 1 Joint sprain 0 ( 0.0%), 0 1 ( 1.2%), 1 Traumatic brain injury 0 ( 0.0%), 0 1 ( 1.2%), 1 Pain 2 ( 2.2%), 2 0 ( 0.0%), 0 Conjunctival hemorrhage 1 ( 1.1%), 1 0 ( 0.0%), 0 Face edema 1 ( 1.1%), 1 0 ( 0.0%), 0 NASDAQ: CHTP 13
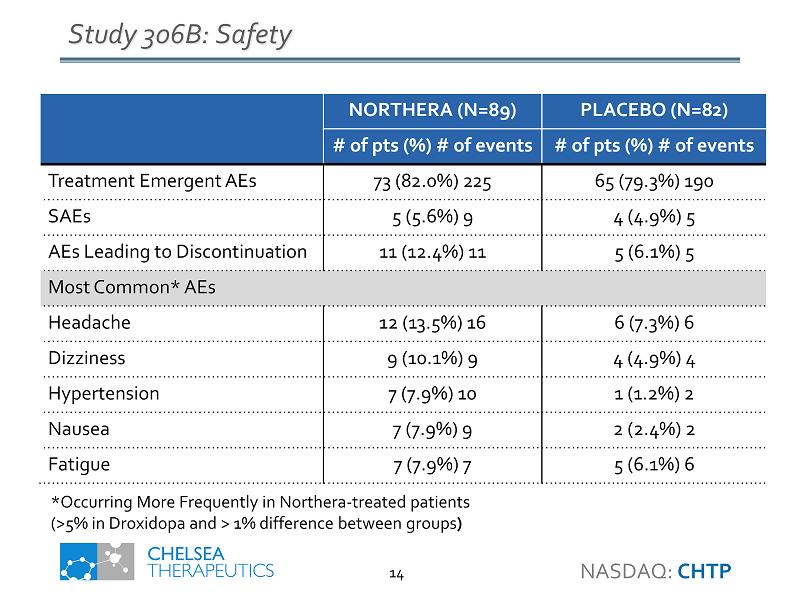
Study 306B: Safety NORTHERA (N=89) PLACEBO (N=82) # of pts (%) # of events # of pts (%) # of events Treatment Emergent AEs 73 (82.0%) 225 65 (79.3%) 190 SAEs 5 (5.6%) 9 4 (4.9%) 5 AEs Leading to Discontinuation 11 (12.4%) 11 5 (6.1%) 5 Most Common* AEs Headache 12 (13.5%) 16 6 (7.3%) 6 Dizziness 9 (10.1%) 9 4 (4.9%) 4 Hypertension 7 (7.9%) 10 1 (1.2%) 2 Nausea 7 (7.9%) 9 2 (2.4%) 2 Fatigue 7 (7.9%) 7 5 (6.1%) 6 *Occurring More Frequently in Northera - treated patients (>5% in Droxidopa and > 1% difference between groups ) NASDAQ: CHTP 14
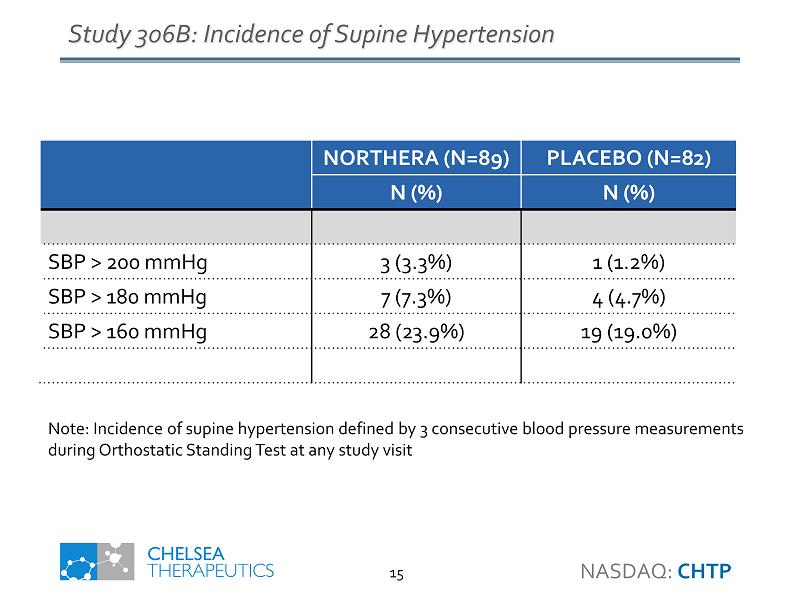
Study 306B: Incidence of Supine Hypertension NORTHERA (N=89) PLACEBO (N=82) N (%) N (%) SBP > 200 mmHg 3 (3.3%) 1 (1.2%) SBP > 180 mmHg 7 (7.3%) 4 (4.7%) SBP > 160 mmHg 28 (23.9%) 19 (19.0%) Note: Incidence of supine hypertension defined by 3 consecutive blood pressure measurements during Orthostatic Standing Test at any study visit NASDAQ: CHTP 15
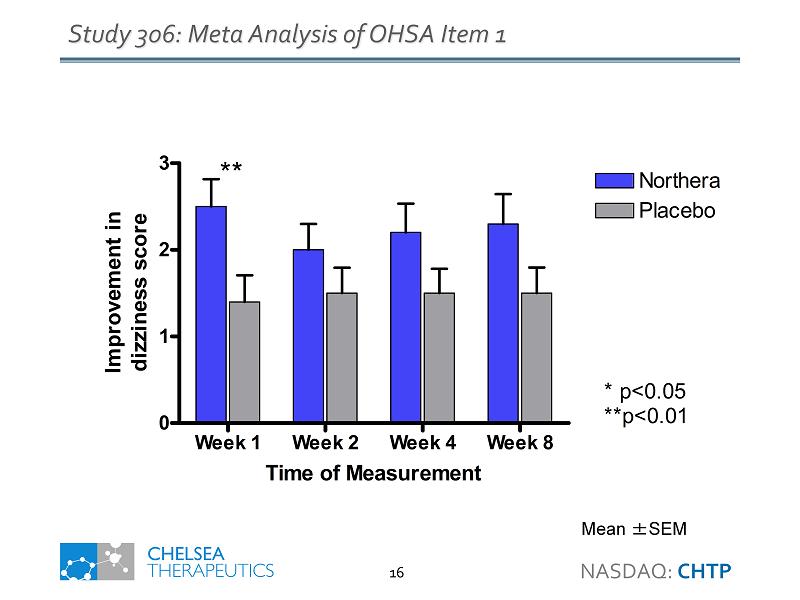
Week 1 Week 2 Week 4 Week 8 0 1 2 3 Northera Placebo ** * p<0.05 **p<0.01 Time of Measurement Improvement in dizziness score Study 306: Meta Analysis of OHSA Item 1 Mean ± SEM NASDAQ: CHTP 16
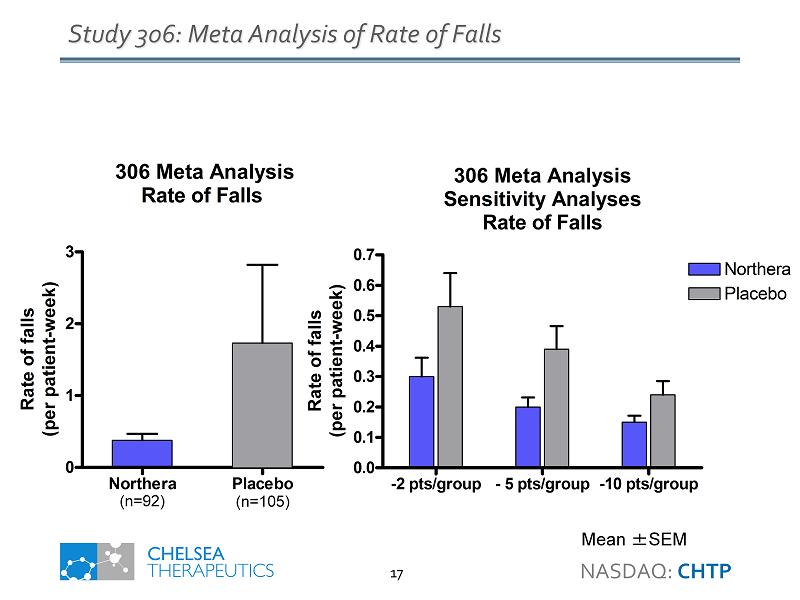
Study 306: Meta Analysis of Rate of Falls Mean ± SEM 306 Meta Analysis Rate of Falls Northera Placebo 0 1 2 3 Rate of falls (per patient-week) 306 Meta Analysis Sensitivity Analyses Rate of Falls -2 pts/group - 5 pts/group -10 pts/group 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 Northera Placebo Rate of falls (per patient-week) ( n=92) ( n=105) NASDAQ: CHTP 17
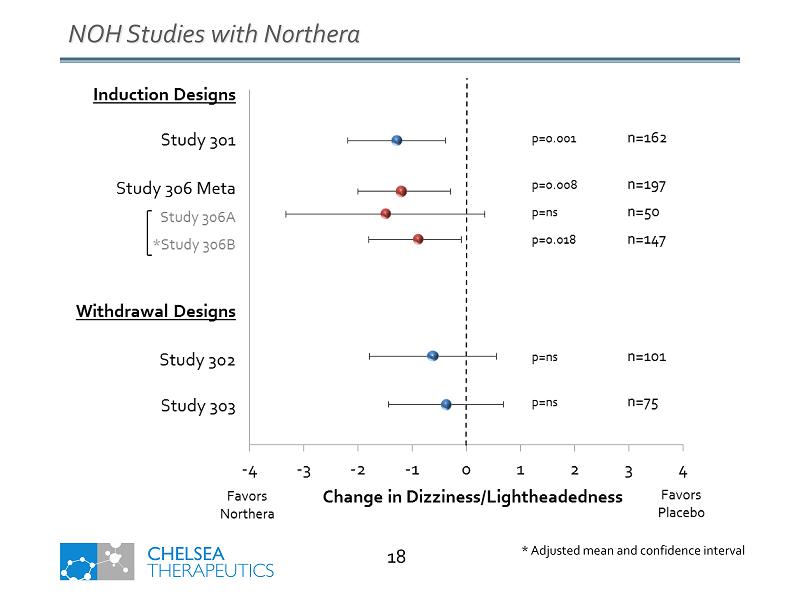
* Adjusted mean and confidence interval NOH Studies with Northera 18
Summary Study 306B Results • Primary Endpoint: Improvement on Dizziness/ Lightheadedness • Secondary Endpoints: Standing Systolic Blood Pressure, Falls and Falls - related Injuries • Safety and Tolerability Next Steps • Further Analyses of 306B • Extensive Database of 650 NOH patients • Inform Our Next Steps NASDAQ: CHTP 19

Questions for Dr. Hauser • What are your thoughts on Study 306B and what the efficacy results tell us? • Can you speak to what the data informs you on the tolerability of Northera ? • In your opinion, how could a therapy like Northera help your patients with NOH? NASDAQ: CHTP 20

©
2004 - 2012 Chelsea Therapeutics, Inc. Northera ™ ( droxidopa ) Study 306B Preliminary Results 4 December 2012 21
