Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Inspyr Therapeutics, Inc. | v329404_8k.htm |

Precise targeting of a potent, unique prodrug directly to tumors OTCBB: GNSZ INVESTOR PRESENTATION NOVEMBER 2012

SAFE HARBOR STATEMENT GNSZ | NOVEMBER 2012 PAGE 2 Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995: Any statements that are not historical facts are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward - looking statements, which may include, but are not limited to, factors related to GenSpera's anticipated growth strategies, future business development, ability to develop new products, expand to other related industries or markets in other geographical locations, and other information detailed from time to time in the filings and future filings with the United States Securities and Exchange Commission . Readers are advised that this information is intended for the use of investment professionals. Anyone interested in obtaining information on GenSpera should contact GenSpera, as set forth above, to receive GenSpera's most recent financial reports. This presentation was developed by GenSpera and is intended solely for informational purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy the Company's stock. This profile is based upon information available to the public, as well as other information from sources which management believes to be reliable, but is not guaranteed by the Company as being accurate nor does it purport to be complete. Opinions expressed herein are those of management as of the date of presentation and are subject to change without notice .

GENSPERA DEFINED Highly differentiated , novel therapeutic agent with a unique mechanism of action GNSZ | NOVEMBER 2012 PAGE 3 • Curative intent with potential for “complete kill” – active against both slowly and rapidly dividing cells • IP acquired from Johns Hopkins University – no milestones or royalties due • Lead drug candidate, G - 202, entering Phase II clinical trials in prostate cancer and hepatocellular carcinoma in Q4 2012 • Interim Phase II data expected in 2 indications in Q3 2013 • Achieved multiple clinical and regulatory milestones with total spend of only $ 15M • Most of invested capital from repeat investors
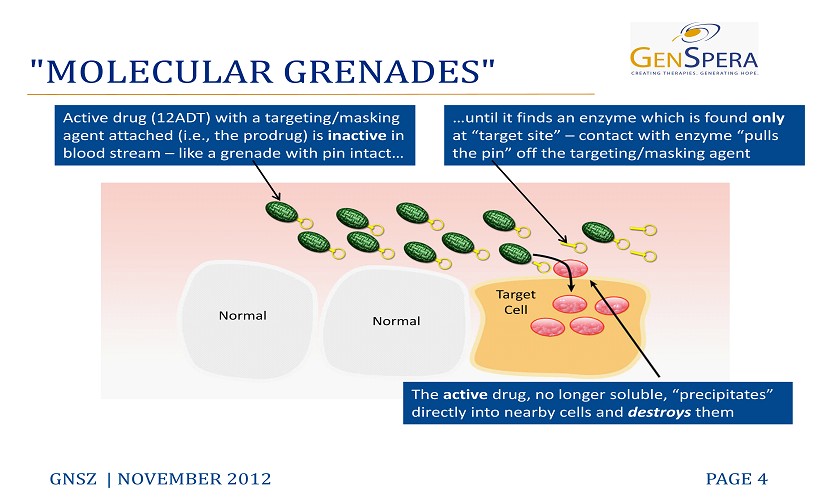
"MOLECULAR GRENADES" GNSZ | NOVEMBER 2012 PAGE 4 Active drug (12ADT) with a targeting/masking agent attached (i.e., the prodrug) is inactive in blood stream – like a grenade with pin intact… …until it finds an enzyme which is found only at “target site ” – contact with enzyme “pulls the pin” off the targeting/masking agent The active drug, no longer soluble, “precipitates” directly into nearby cells and destroys them Normal Normal Target Cell
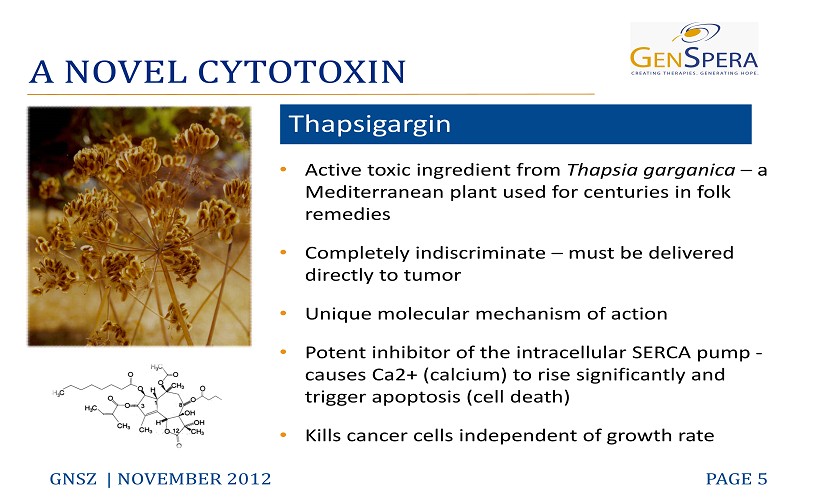
A NOVEL CYTOTOXIN GNSZ | NOVEMBER 2012 PAGE 5 • Active toxic ingredient from Thapsia garganica – a Mediterranean plant used for centuries in folk remedies • Completely indiscriminate – must be delivered directly to tumor • Unique molecular mechanism of action • Potent inhibitor of the intracellular SERCA pump - causes Ca2 + (calcium) to rise significantly and trigger apoptosis (cell death) • Kills cancer cells independent of growth rate Thapsigargin
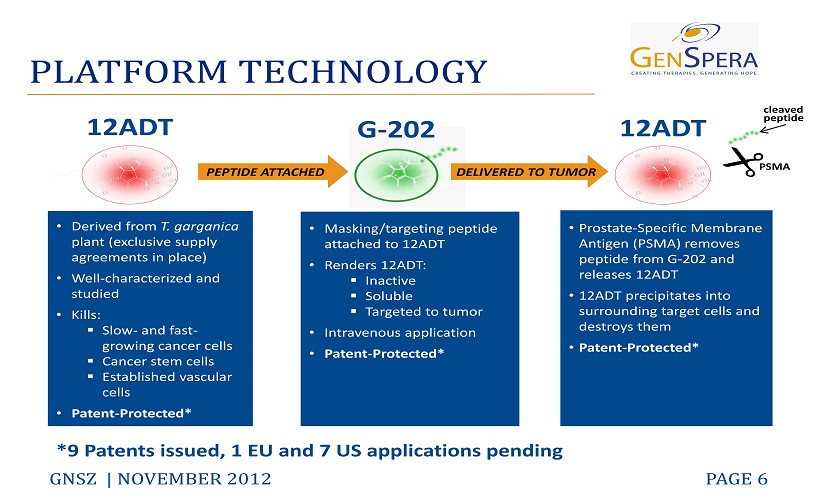
PLATFORM TECHNOLOGY GNSZ | NOVEMBER 2012 PAGE 6 12ADT • Derived from T. garganica plant (exclusive supply agreements in place) • Well - characterized and studied • Kills: ▪ Slow - and fast - growing cancer cells ▪ Cancer stem cells ▪ Established vascular cells • Patent - Protected* • Masking/targeting peptide attached to 12ADT • Renders 12ADT: ▪ Inactive ▪ Soluble ▪ Targeted to tumor • Intravenous application • Patent - Protected* • Prostate - Specific Membrane Antigen (PSMA) removes peptide from G - 202 and releases 12ADT • 12ADT precipitates into surrounding target cells and destroys them • Patent - Protected * PEPTIDE ATTACHED DELIVERED TO TUMOR G - 202 12ADT c leaved peptide PSMA * 9 Patents i ssued, 1 EU and 7 US applications pending

PSMA EXPRESSION GNSZ | NOVEMBER 2012 PAGE 7 G - 202 is expected to be useful against many tumor types, since most tumor vasculatures express PSMA. Denmeade, SR et al., Sci. Transl. Med. 4 , 140ra86 (2012) PSMA EXPRESSION IN NEOVASCULATURE OF SOLID TUMORS

G - 202 PHASE IB GNSZ | NOVEMBER 2012 PAGE 8 • Objectives: Refine dosing regimen and obtain more safety data in advance of Phase II • Eligibility: All solid tumors with failed prior treatments • Enrollment: 16 patients treated, 3 HCC still on study • Dosing regimen: 40 mg/m 2 on Day 1, 66.8 mg/m 2 on Days 2 and 3 of 28 - day schedule with hydration and standard pre - meds • Results: Easily managed minimal side effects and prolonged disease control in a few patients Cohort Expansion at Recommended Phase II Dose
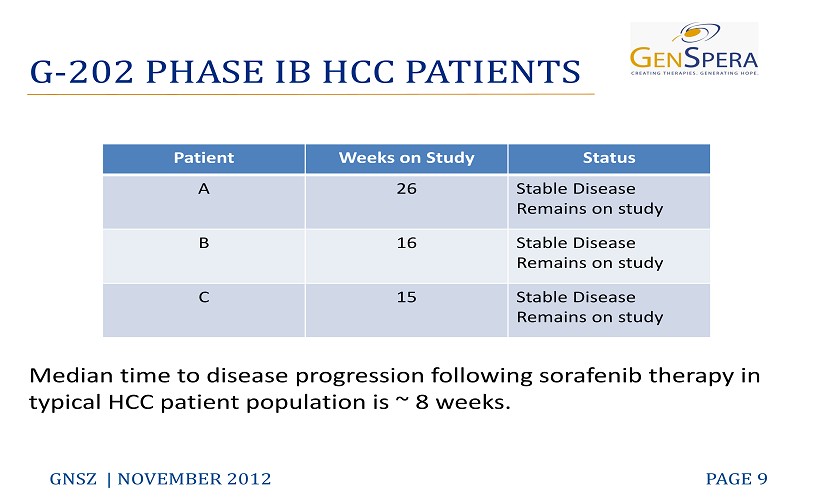
G - 202 PHASE IB HCC PATIENTS GNSZ | NOVEMBER 2012 PAGE 9 Median time to disease progression following sorafenib therapy in typical HCC patient population is ~ 8 weeks. Patient Weeks on Study Status A 26 Stable Disease Remains on study B 16 Stable Disease Remains on study C 15 Stable Disease Remains on study

G - 202 PHASE II HCC GNSZ | NOVEMBER 2012 PAGE 10 • Population: Patients with progressive advanced hepatocellular cancer following sorafenib therapy • Dosing Regimen: 40 mg/m 2 on Day 1, 66.8 mg/m 2 on Days 2 and 3 of 28 - day schedule • Primary Endpoint: Time to disease progression • Number of Patients and Sites: 29 patients at 10 sites in Texas (relationship with CTNeT) • Last Patient Enrolled: Expected Q4 2013 • Interim Data Available: Expected Q3 2013

G - 202 PHASE II PCa GNSZ | NOVEMBER 2012 PAGE 11 • Population: Patients with chemotherapy - naïve metastatic castrate - resistant prostate cancer • Dosing Regimen: 40 mg/m 2 on Day 1, 66.8 mg/m 2 on Days 2 and 3 of 28 - day schedule • Primary Endpoint: Percentage of patients who are progression - free after 24 weeks of treatment with G - 202 • Number of Patients and Sites: 34 patients at 5 sites in the US and UK • Last Patient Enrolled: Expected Q1 2014 • Interim Data Available: Expected Q3 2013

CORE DISTINCTIONS GNSZ | NOVEMBER 2012 PAGE 12 Highly differentiated , novel therapeutic agent with a unique mechanism of action • Curative intent – not simply intended to provide incremental improvement in median survival • Interim Phase II data expected in 2 indications in Q3 2013 • Focus is on rapidly achieving valuation milestones with minimal cost
SEASONED BIOTECH MANAGEMENT GNSZ | NOVEMBER 2012 PAGE 13 Craig A. Dionne, PhD Chairman & CEO • Over 23 years of experience in pharmaceutical industry • SVP Drug Discovery at Cephalon, Inc. (now Teva Pharmaceuticals) • EVP Prostate Cancer Foundation Nancy Jean Barnabei VP Finance & Treasurer • Over 20 years of experience in pharmaceutical industry • Previously CFO Corridor Pharmaceuticals, Inc. and Immune Control, Inc. • Founded Talkeetna Advisors, LLC • VP Finance, Treasurer and CFO of Locus Pharmaceuticals, Inc. • Controller of Cephalon, Inc. (now Teva Pharmaceuticals) Russell Richerson, PhD COO & Secretary • Over 25 years of experience in biotechnology industry • Previously COO Molecular Profiling Institute • VP Diagnostic R&D Prometheus Laboratories • Abbott Laboratories • Board member International Genomics Consortium

DIRECTORS GNSZ | NOVEMBER 2012 PAGE 14 Craig A. Dionne, PhD Chairman & CEO • Over 23 years of experience in pharmaceutical industry • SVP Drug Discovery at Cephalon, Inc. (now Teva Pharmaceuticals) • EVP Prostate Cancer Foundation Peter E. Grebow, PhD Director • Over 37 years of experience in pharmaceutical industry • Former EVP Technical Operations Cephalon, Inc. (now Teva Pharmaceuticals) • VP Drug Development Rorer Central Research • Board member Optimer Pharmaceuticals (NASDAQ: OPTR) and Q Holdings Bo Jesper Hansen, MD, PhD Director • Executive Chairman of Swedish Orphan Biovitrum AB (STO: SOBI) • Board member CMC AB, MipSalus Aps, TopoTarget A/S (NASDAQ OMX: TOPO), and Zymenex A/S Scott Ogilvie Director • President and CEO AFIN International, Inc. • Board member Neuralstem, Inc. (AMEX:CUR), Derycz Scientific, Inc. (OTCBB:DYSC), and Preferred Voice, Inc. (OTCBB:PRFV)

SCIENTIFIC ADVISORY BOARD GNSZ | NOVEMBER 2012 PAGE 15 John T. Isaacs, PhD • Professor at Johns Hopkins School of Medicine • Recognized authority in prostate cancer biology Samuel R. Denmeade , MD • Professor at Johns Hopkins and Board Certified Medical Oncologist • Extensive experience in development of prodrug therapies that are activated by cancer specific proteases Søren Brøgger Christensen, PhD • Professor at University of Copenhagen • Expert in chemistry of thapsigargin • Exploring further derivatives of thapsigargin compound Hans Lilja , MD, PhD • Clinical Chemist , Memorial Sloan - Kettering Cancer Center • Recognized authority on the biology of prostate specific antigen • Professor at Lund University

CONTACT GNSZ | NOVEMBER 2012 PAGE 16 Craig A. Dionne, PhD President & CEO cdionne@genspera.com GenSpera, Inc. 2511 N Loop 1604 W, Suite 204 San Antonio, TX 78258 www.genspera.com Phone: (210) 479 - 8112 Fax: (210) 479 - 8113
