Attached files
| file | filename |
|---|---|
| 8-K - SUCAMPO PHARMACEUTICALS, INC. 8-K - Sucampo Pharmaceuticals, Inc. | a50471809.htm |
| EX-99.1 - EXHIBIT 99.1 - Sucampo Pharmaceuticals, Inc. | a50471809ex99_1.htm |
Exhibit 99.2

Third Quarter 2012 Results 1
Agenda Introductions and Forward-Looking Statements Silvia Taylor Highlights of the Quarter Ryuji Ueno, MD, PhD, PhD Commercial Update Andrew Smith, Stanley G. Miele Pipeline and R&D Update Peter Lichtlen, MD, PhD Financial Performance Cary J. Claiborne Closing Remarks Ryuji Ueno, MD, PhD, PhD 2

Forward-Looking Statements This presentation contains “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements are based on management’s current expectations, and involve risks and uncertainties that may cause results to differ materially from those set forth in the statements. The forward-looking statements may include statements regarding product development, product potential, future financial and operating results, and other statements that are not historical facts. The following factors, among others, could cause actual results to differ from those set forth in the forward-looking statements: the impact of pharmaceutical industry regulation and healthcare legislation; Sucampo’s ability to accurately predict future market conditions; dependence on the effectiveness of Sucampo’s patents and other protections for innovative products; the risk of new and changing regulation and health policies in the US and internationally and the exposure to litigation actions internationally, and/or regulatory actions. No forward-looking statement can be guaranteed and actual results may differ materially from those projected. Sucampo undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise. Forward-looking statements in this presentation should be evaluated together with the many uncertainties that affect Sucampo’s business, particularly those mentioned in the risk factors and cautionary statements in Sucampo’s Form 10-Q, August 9, 2012 and Form 10-K for the year ended Dec 31, 2011, which the company incorporates by reference. 3

Q3 2012 Highlights Ryuji Ueno, MD, PhD, PhD Chairman, Chief Executive Officer, Chief Scientific Officer, and Co-founder
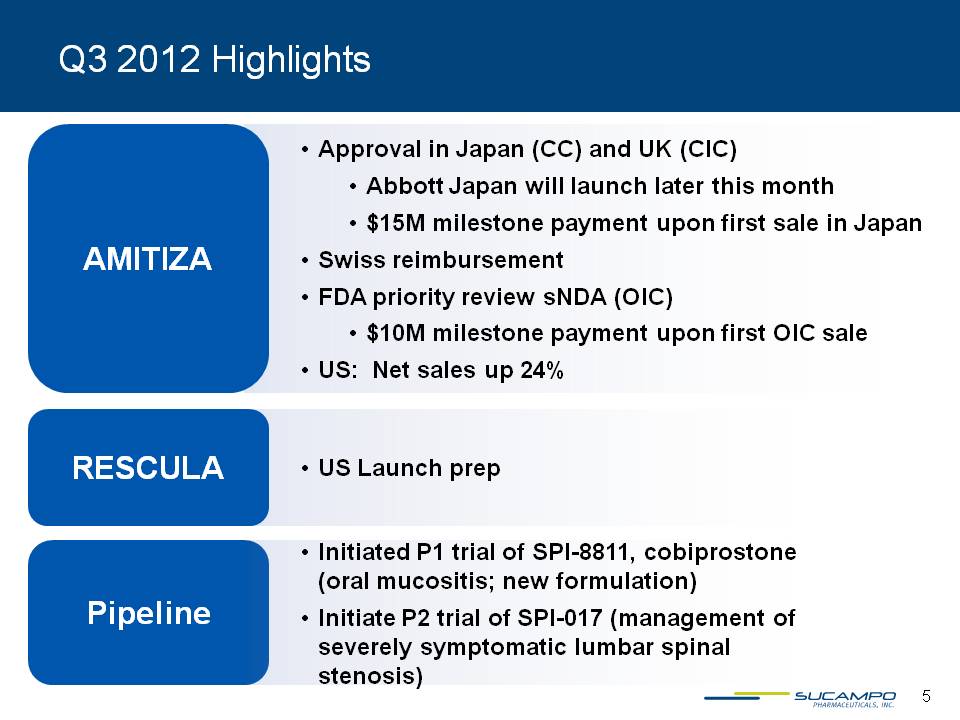
Q3 2012 Highlights AMITIZA Approval in Japan (CC) and UK (CIC) Abbott Japan will launch later this month $15M milestone payment upon first sale in Japan Swiss reimbursement FDA priority review sNDA (OIC) $10M milestone payment upon first OIC sale US: Net sales up 24% RESCULA US Launch prep Pipeline Initiated P1 trial of SPI-8811, cobiprostone (oral mucositis; new formulation) Initiate P2 trial of SPI-017 (management of severely symptomatic lumbar spinal stenosis) 5

Commercial Update Andrew Smith Vice President of Operations and Finance Stanley G. Miele President, Sucampo Pharma Americas and SVP, Sales and Marketing
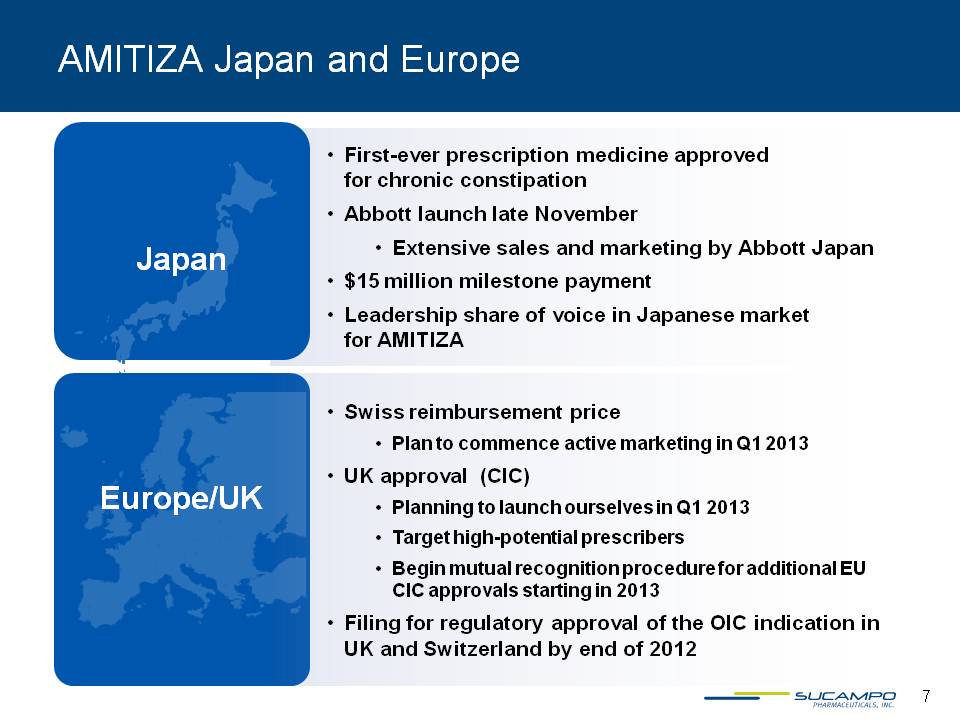
AMITIZA Japan and Europe Japan First-ever prescription medicine approved for chronic constipation Abbott launch late November Extensive sales and marketing by Abbott Japan $15 million milestone payment Leadership share of voice in Japanese market for AMITIZA Europe/UK Swiss reimbursement price Plan to commence active marketing in Q1 2013 UK approval (CIC) Planning launch ourselves in Q1 2013 Target high-potential prescribers Begin mutual recognition procedure for additional EU CIC approvals starting in 2013 Filing for regulatory approval of the OIC indication in UK and Switzerland by end of 2012 7
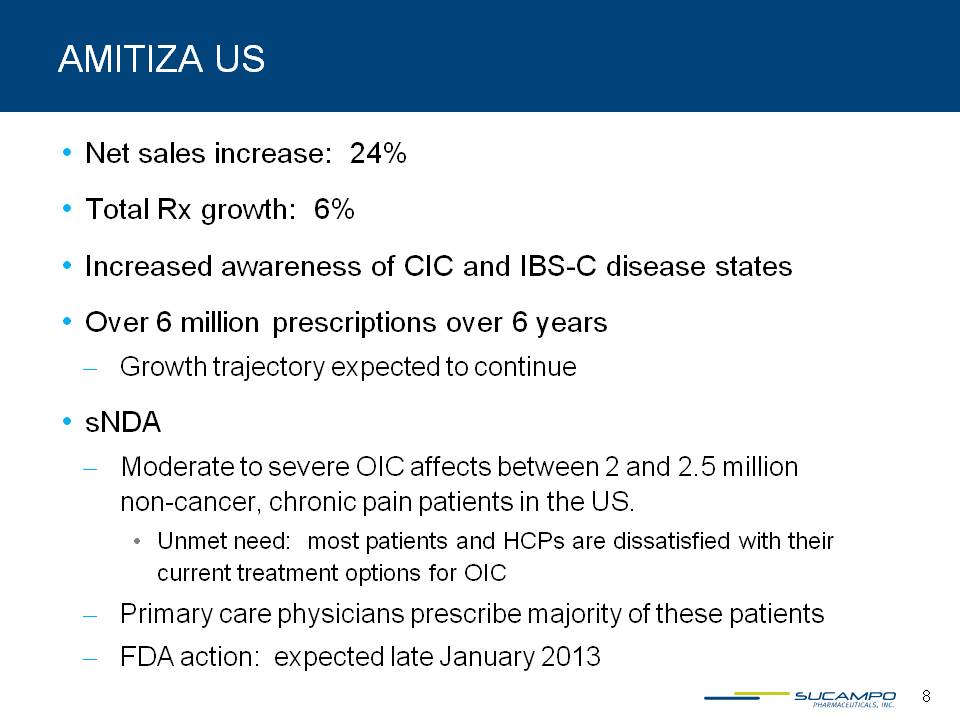
AMITIZA US Net sales increase: 24% Total Rx growth: 6% Increased awareness of CIC and IBS-C disease states Over 6 million prescriptions over 6 years Growth trajectory expected to continue sNDA Moderate to severe OIC affects between 2 and 2.5 million non-cancer, chronic pain patients in the US. Unmet need: most patients and HCPs are dissatisfied with their current treatment options for OIC Primary care physicians prescribe majority of these patients FDA action: expected late January 2013 8

RESCULA US sNDA approval expected Q4 2012 New label: reflect current scientific understanding of unique mechanism of action be approved for first-line treatment Launch RESCULA upon getting the sNDA approval 9

Pipeline and & R&D Update Peter Lichtlen, MD, PhD Senior Medical Officer and Vice President, European Operations
AMITIZA sNDA in OIC Priority review granted Orally-administered drug with strong safety record Laxatives have not been shown to be effective in treating OIC FDA decision early 2013 P3 pediatric trials Q1 2013 New pediatric functional constipation indication Development of new liquid formulation Takeda funding significant amount of development costs for pediatric indication, and 100% of development costs for liquid formulation 11
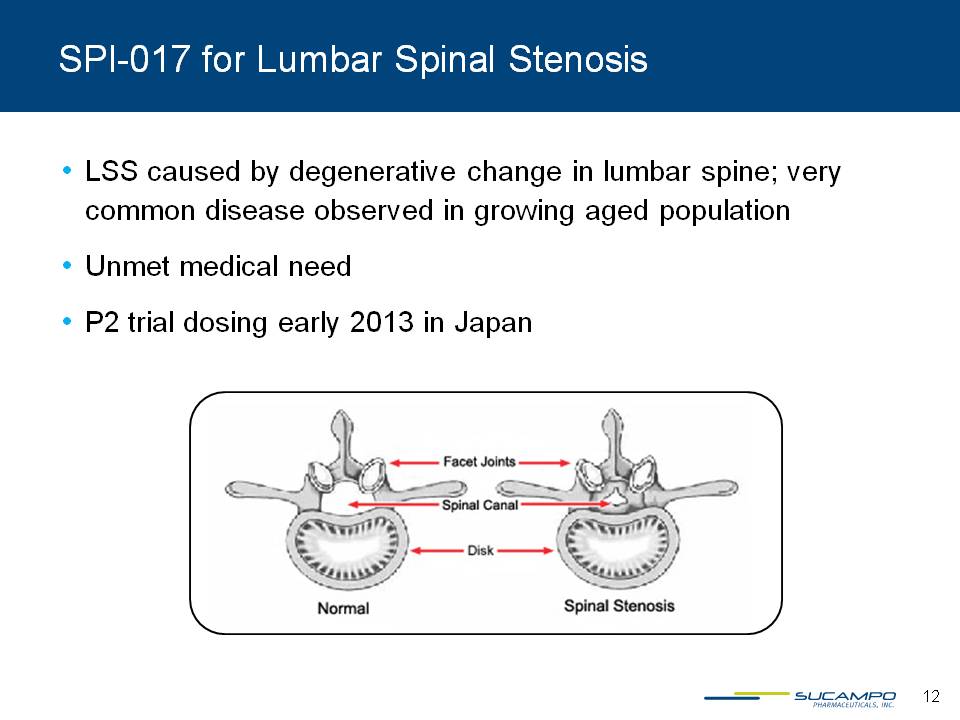
SPI-017 for Lumbar Spinal Stenosis LSS caused by degenerative change in lumbar spine; very common disease observed in growing aged population Unmet medical need P2 trial dosing early 2013 in Japan 12
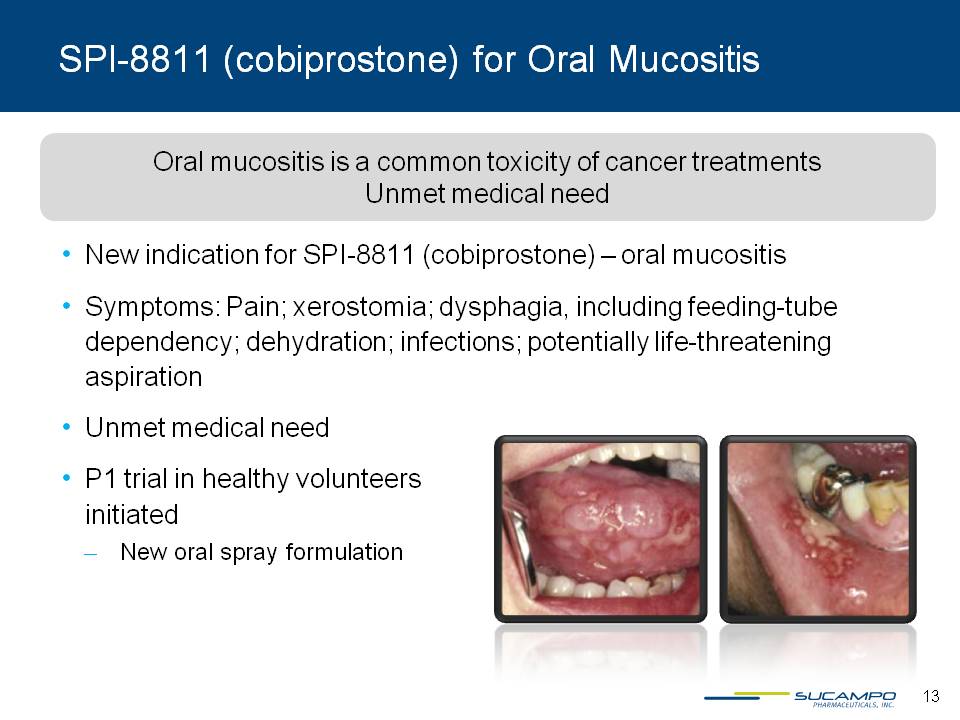
SPI-8811 (cobiprostone) for Oral Mucositis Oral mucositis is a common toxicity of cancer treatments Unmet medical need New indication for SPI-8811 (cobiprostone) – oral mucositis Symptoms: Pain; xerostomia; dysphagia, including feeding-tube dependency; dehydration; infections; potentially life-threatening aspiration Unmet medical need P1 trial in healthy volunteers initiated New oral spray formulation 13

Financial Performance Cary J. Claiborne Chief Financial Officer
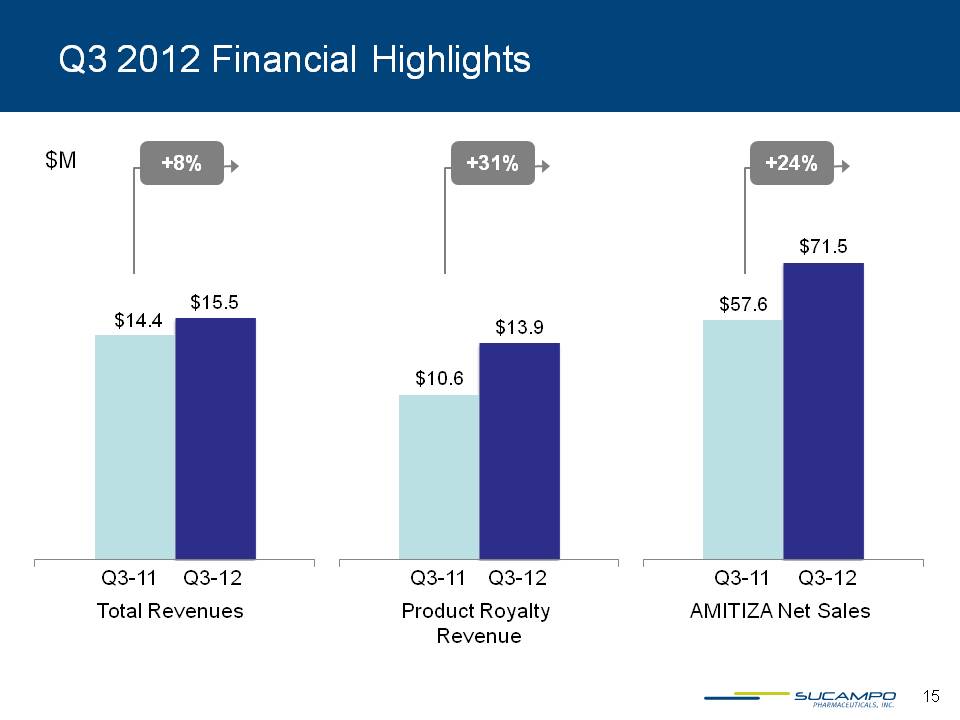
Q3 2012 Financial Highlights $M +8% +31% +24% $14.4 $15.5 $10.6 $13.9 $57.6 $71.5 Q3-11 Q3-12 Q3-11 Q3-12 Q3-11 Q3-12 Total Revenues Product Royalty Revenue AMITIZA Net Sales 15
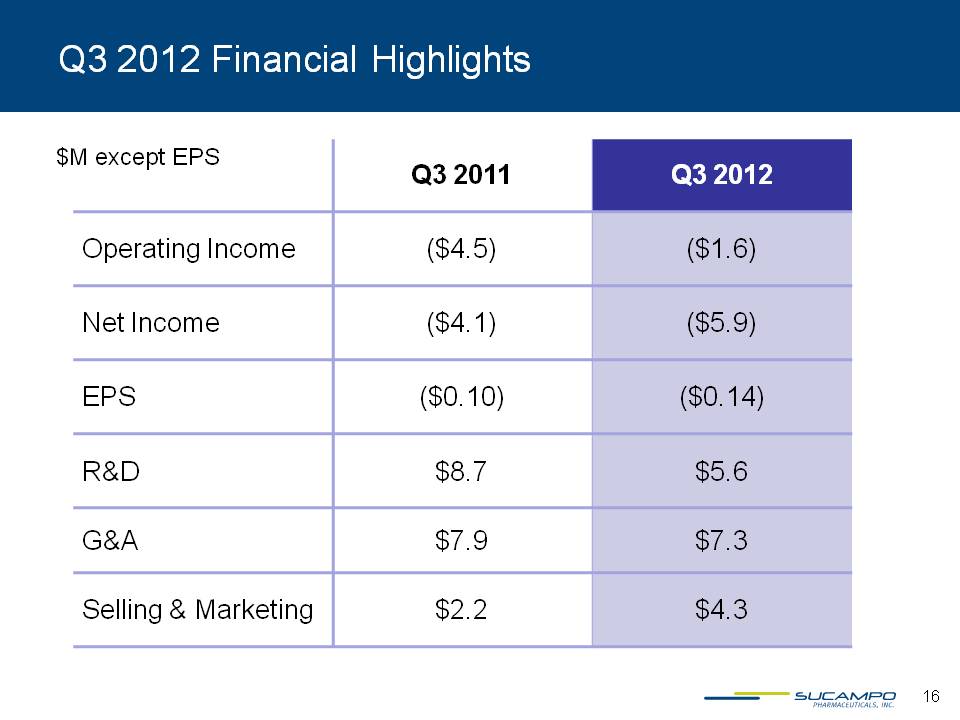
Q3 2012 Financial Highlights $M except EPS Q3 2011 Q3 2012 Operating Income ($4.5) ($1.6) Net Income ($4.1) ($5.9) EPS ($0.10) ($0.14) R&D $8.7 $5.6 G&A $7.9 $7.3 Selling & Marketing $2.2 $4.3 16

Q3 2012 Financial Highlights Cash position $82.1 million as of September 30, 2012 Repurchased 123,135 shares during quarter Recently raised authorized amount to $5,000,000 One class of common stock and staggered board 17

Closing Remarks Ryuji Ueno, MD, PhD, PhD Chairman, Chief Executive Officer, Chief Scientific Officer, and Co-founder
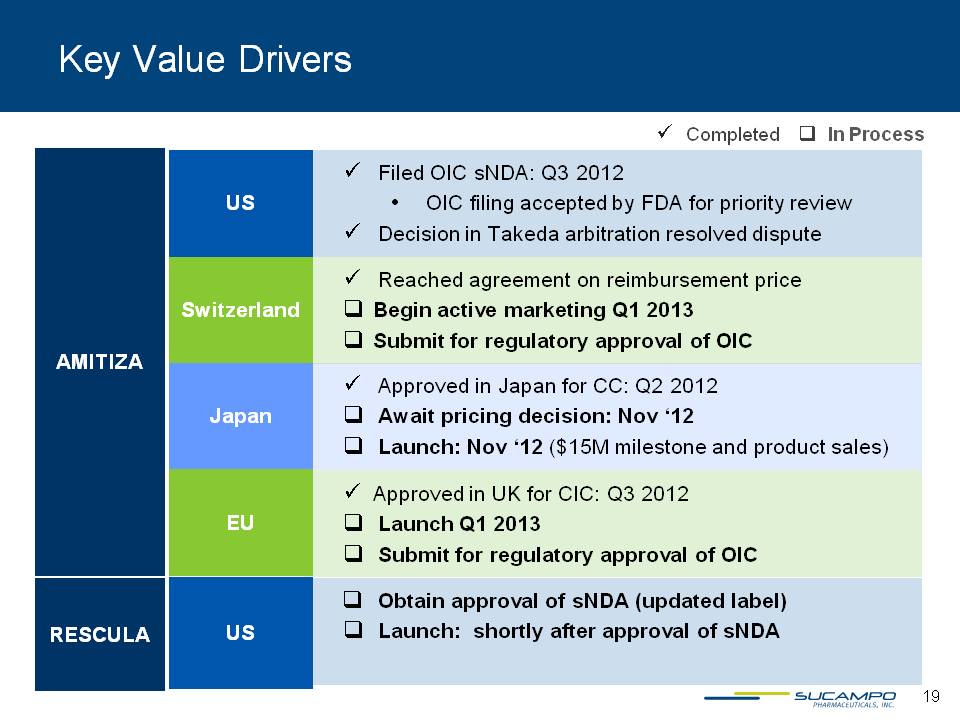
Key Value Drivers Completed In Process AMITIZA US Filed OIC sNDA: Q3 2012 OIC filing accepted by FDA for priority review Decision in Takeda arbitration resolved dispute Switzerland Reached agreement on reimbursement price Begin active marketing Q1 2013 Submit for regulatory approval of OIC Japan Approved in Japan for CC: Q2 2012 Await pricing decision: Nov ‘12 Launch: Nov ‘12 ($15M milestone and product sales) EU Approved in UK for CIC: Q3 2012 Launch Q1 2013 Submit for regulatory approval of OIC RESCULA US Obtain approval of sNDA (updated label) Launch: shortly after approval of sNDA 19

Q&A
