Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - INFINITY PHARMACEUTICALS, INC. | d433419d8k.htm |
| EX-99.1 - PRESS RELEASE - INFINITY PHARMACEUTICALS, INC. | d433419dex991.htm |
 Building a Fully Integrated
Biopharmaceutical Company
November 2012
Exhibit 99.2 |
 Forward Looking Statements
2
•
This presentation contains forward-looking statements within the meaning of The
Private Securities Litigation Reform Act of 1995.
•
Such forward-looking statements include those regarding research and
development plans, the expected timing for reporting data on the IPI-145
and Hsp90 programs, the therapeutic potential of Infinity’s product candidates, Infinity’s
financial guidance with respect to year end cash for 2012, and Infinity’s
expectations with respect to the availability of cash to fund its operations
into 2014. •
Such
statements
are
subject
to
numerous
important
factors,
risks
and
uncertainties
that
may
cause
actual
events
or
results to differ materially from the company’s current expectations.
•
For
example,
there
can
be
no
guarantee
that
Infinity
will
report
data
in
the
timeframes
it
has
estimated,
that
any
product
candidate Infinity is developing will successfully complete necessary preclinical
and clinical development phases or that development of any of
Infinity’s product candidates will continue. Further, there can be no guarantee that any positive
developments in Infinity’s product portfolio will result in stock price
appreciation. Management’s expectations could also be affected by risks
and uncertainties relating to: Infinity’s results of clinical trials and preclinical studies, including
subsequent analysis of existing data and new data received from ongoing and future
studies; the content and timing of decisions
made
by
the
U.S.
FDA
and
other
regulatory
authorities,
investigational
review
boards
at
clinical
trial
sites
and
publication review bodies; Infinity’s ability to enroll patients in its
clinical trials; unplanned cash requirements and expenditures; development
of agents by Infinity’s competitors for diseases in which Infinity is currently developing its
product candidates; and Infinity’s ability to obtain, maintain and enforce
patent and other intellectual property protection for any product candidates
it is developing. •
These and other risks which may impact management’s expectations are described
in greater detail under the caption “Risk Factors”
included in Infinity’s Annual Report on Form 10-K for the year ended
December 31, 2011 and subsequent filings filed by Infinity with the
Securities and Exchange Commission. •
Any
forward-looking
statements
contained
in
this
presentation
speak
only
as
of
November
6,
2012,
and
Infinity
expressly disclaims any obligation to update any forward-looking statements,
whether as a result of new information, future events or otherwise.
|
 Building a Fully Integrated
Biopharmaceutical Company
Sustainable Model for Value Creation
Focused
pipeline
Near-term
value
inflection
points
Global rights
to all products
Substantial
market
opportunities
3 |
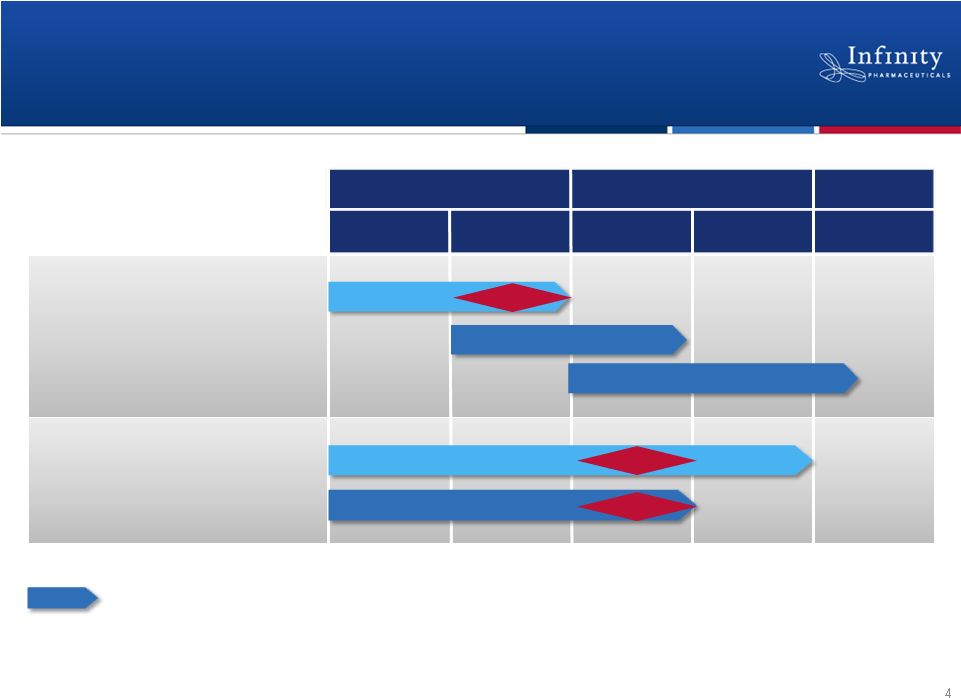 Pipeline: Multiple Near-Term Data Readouts
4
1H’12
1H’13
2H’13
2H’12
PI3K: IPI-145
NSCLC (mKRAS)
NSCLC (Heavy smokers)
Hematologic Malignancies
2012
2013
2014+
Hsp90: Retaspimycin HCl
Asthma
Rheumatoid Arthritis
Randomized, double-blind, placebo-controlled trial
Phase 1b/2
Data
Phase 2
Data
Phase
1
Data
Phase 2a
Phase 2 |
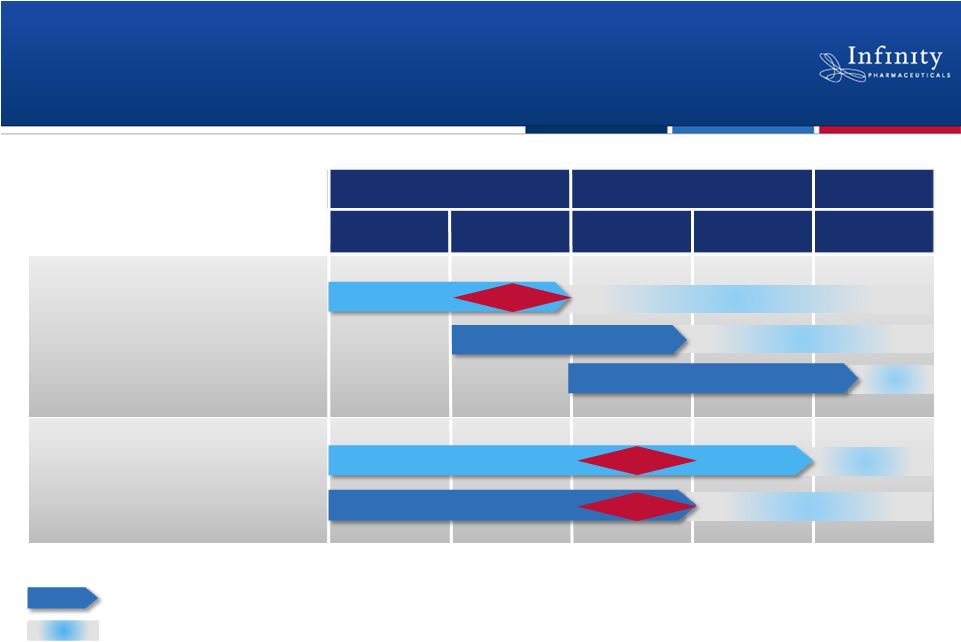 Pipeline: Multiple Near-Term Data Readouts
5
1H’12
1H’13
2H’13
2H’12
PI3K: IPI-145
NSCLC (mKRAS)
NSCLC (Heavy smokers)
Hematologic Malignancies
2012
2013
2014+
Hsp90: Retaspimycin HCl
Asthma
Rheumatoid Arthritis
Randomized, double-blind, placebo-controlled trial
Phase 3
Phase 3
Next step and approximate timing, if data are positive.
Phase 2
Phase 1b/2
Phase 2
Data
Data
Phase
1
Data
Expansions >>> Phase 2
Phase 2
Phase 3
Phase 2a |
 IPI-145:
Only
PI3K-
,
Inhibitor
in Clinical Development |
 IPI-145: First-
and Best-in-Class Potential
Profile:
7
•
Potent oral PI3K-
,
inhibitor
Therapeutic Focus:
•
Dual development path in hematologic malignancies and inflammation
Intellectual Property:
•
Composition patent issued in U.S.; pending broadly ex-U.S.
•
U.S. patent expiry: 2030, excluding patent term extension
Commercial Rights:
•
Global rights to IPI-145 |
 IPI-145 Targets PI3K-
and PI3K-
•
Insulin signaling
•
Mutated in solid tumors
PI3K-
•
Platelet activation
•
Insulin signaling
•
Neutrophil function
•
Solid tumors
PI3K-
•
Mast cell activation
•
Innate immune function
•
Immune cell trafficking (chemokines)
•
Solid tumors
PI3K-
•
B-cell activation and function
•
T-cell activation and function
•
Fc receptor signaling in mast cells
PI3K-
Ali et al., J Immunol 2008; Ferrandi et al., JPET, 2007; Fruman, D.,
Current Opinion in Immunology, 2004; Hirsch et al., Journal of
Endocrinology, 2007; Kulkarni et al., Sci Signaling 2011; Ni et al., Cancer Disc, 2012; Schmid et al., Cancer Cell, 2011
8 |
 IPI-145:
A
Potent
PI3K-
,
Inhibitor
PI3K-
PI3K-
PI3K-
PI3K-
k
off
(h
-1
)
0.9
3.0
15.5
> 1800
k
on
(10
6
M
-1
s
-1
)
15.2
3.4
2.7
4.2
t
1/2
(min)
46
14
2.7
0.1
K
d
=(
k off
/k
on
)
23 pM
243 pM
1,564 pM
25,900 pM
9
Palombella. New York Academy of Sciences 2012. |
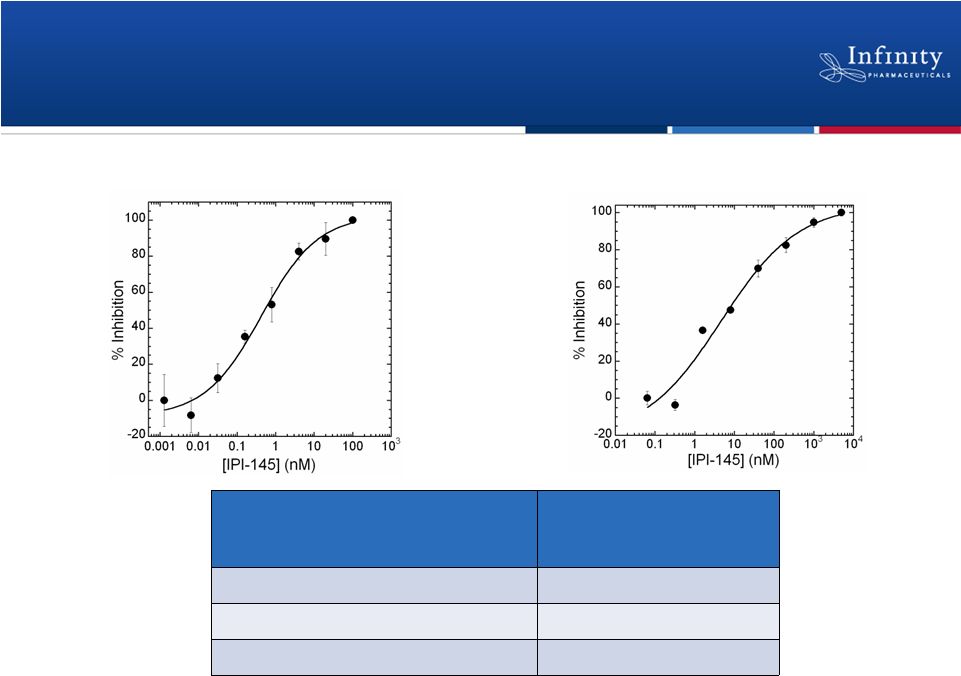 IPI-145 Inhibits B-cell and T-cell Proliferation
Functional Cellular Activity
IPI-145 (EC
50
)
Murine B-cell proliferation
0.5 nM
Human B-cell proliferation
0.5 nM
Human T-cell proliferation
9.5 nM
Human B-cell
proliferation Human T-cell
proliferation Anti-IgM and anti-CD40 stimulation
Concanavalin A stimulation
10
Palombella. New York Academy of Sciences 2012. |
 PI3K Franchise: Potential to Develop Portfolio in
Heme Malignancies and Inflammation
Heme Malignancies
Inflammation
CLL
iNHL
Others
RA
Asthma
Others
DLBCL
T-Cell
11
Potential Best-in-Class
Potential First-in-Class |
 IPI-145: First-
and Best-in-Class
Opportunities in Hematologic
Malignancies |
 Phase 1 Trial in Hematologic Malignancies
8 mg
BID
15 mg
BID
25 mg
BID
50 mg
BID
Ongoing
escalation
25 mg BID expansion (n=30)
•
Chronic lymphocytic leukemia
•
Indolent non-Hodgkin’s lymphoma
•
Mantle cell lymphoma
13
Potential
cohort
expansions
1
•
Diffuse large B-cell lymphoma
•
T-cell lymphomas
•
Acute lymphocytic leukemia
•
Myeloproliferative neoplasms
•
CLL, iNHL, MCL
MTD
Pre-
MTD
1
Up to 5 MTD cohorts of up to 30 patients each.
35 mg
BID
Escalation
as
of
ASH
abstract
data
cutoff
on
July
16
th |
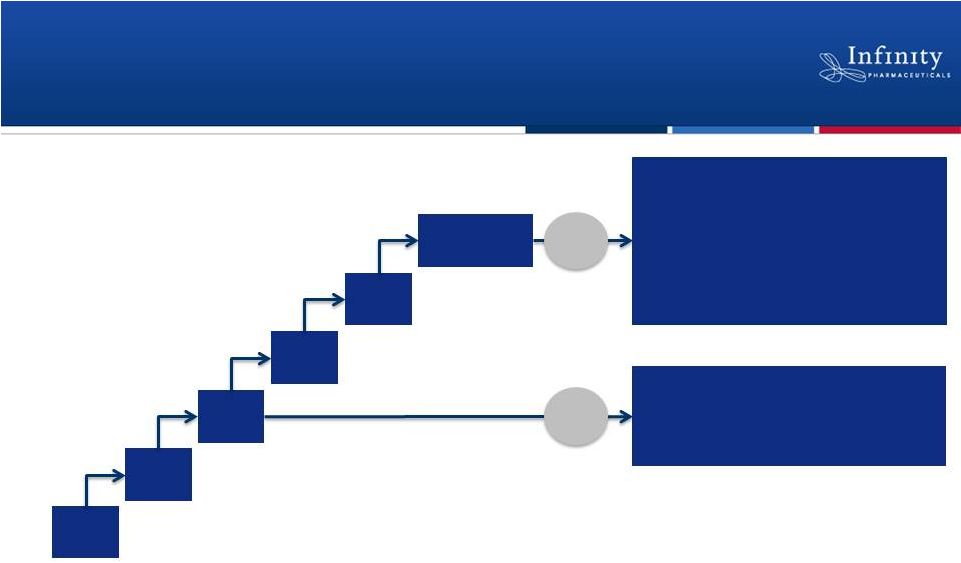 Phase 1 Trial in Hematologic Malignancies
8 mg
BID
15 mg
BID
25 mg
BID
50 mg
BID
Ongoing
escalation
25 mg BID expansion (n=30)
•
Chronic lymphocytic leukemia
•
Indolent non-Hodgkin’s lymphoma
•
Mantle cell lymphoma
14
Potential
cohort
expansions
1
•
Diffuse large B-cell lymphoma
•
T-cell lymphomas
•
Acute lymphocytic leukemia
•
Myeloproliferative neoplasms
•
CLL, iNHL, MCL
MTD
Pre-MTD
1
Up to 5 MTD cohorts of up to 30 patients each.
35 mg
BID |
 IPI-145 Generally Well Tolerated To Date
•
MTD has not been reached
•
Dose escalation to 50 mg BID and beyond
•
1 DLT: transient Grade 4 neutropenia at 15 mg BID
15 |
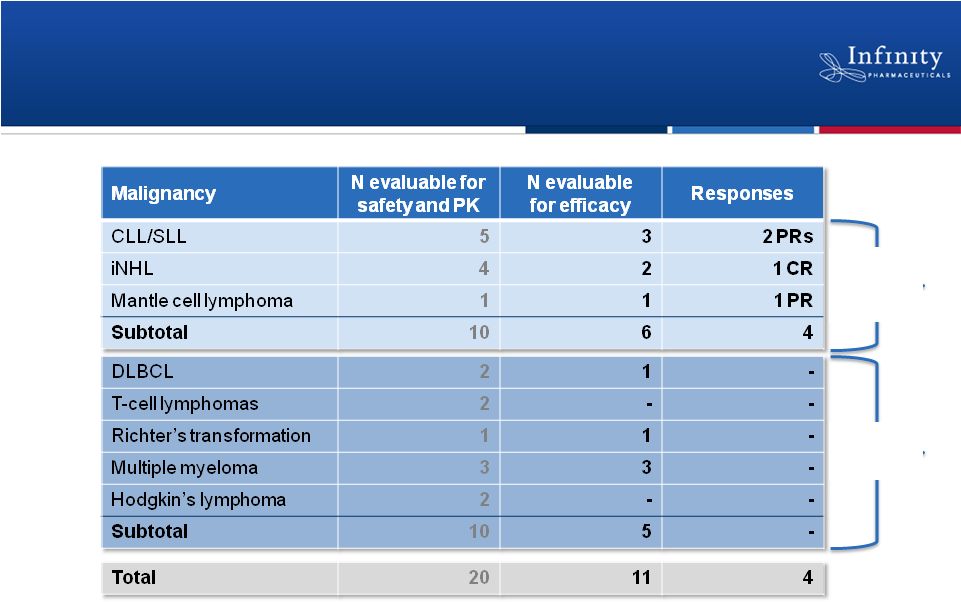 Phase 1 Dose-Escalation Trial
Clinical Responses Observed at Low Doses
16
Responses observed at every one of the lowest doses
evaluable for response: 8 mg, 15 mg and 25 mg BID
Included in
ongoing
escalation
Focus of 25
mg BID
expansion
cohort |
 Summary of Phase 1 of IPI-145 Trial in
Hematologic Malignancies
•
Generally well tolerated
•
Responses observed at every one of the lowest doses evaluable
for response: 8 mg, 15 mg and 25 mg BID
•
Dose escalation ongoing
•
Completed enrollment in 25 mg BID expansion cohort in patients
with CLL, iNHL and mantle cell lymphoma
•
Updated data expected at ASH on December 10
CONFIDENTIAL
17 |
 IPI-145: First-in-Class Opportunity in
Inflammatory Diseases |
 Completed Phase 1 Trial in Healthy Subjects
•
Phase 1 single ascending and multiple ascending dose study
completed
–
Proportional increase in plasma exposure with increased dose
–
Rapid, dose dependent, and durable inhibition of ex vivo basophil
activation at all dose levels
–
Well tolerated
•
Data to be presented at American College of Rheumatology
Annual
Scientific
Meeting
in
Washington,
D.C.
November
11
th
19
ClinicalTrials.gov NCT01549106. |
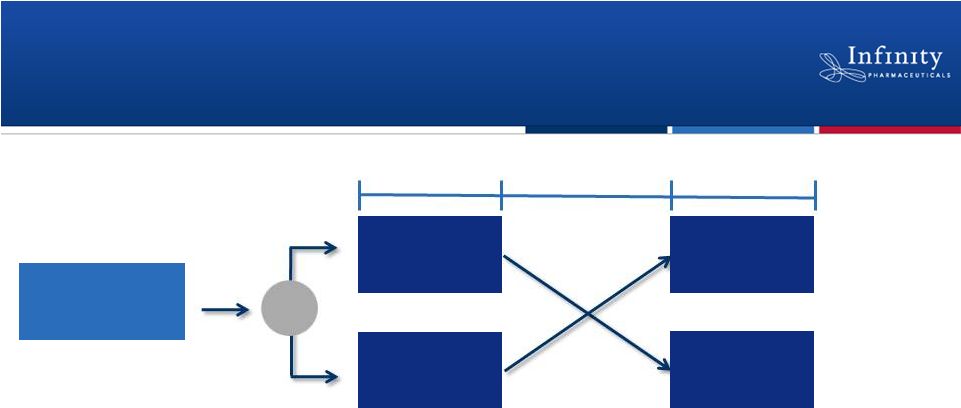 Initiated Phase 2a Trial in Asthma
•
Double-blind, randomized, placebo-controlled, crossover study
•
Approximately 30 subjects with mild, allergic asthma
•
Multiple-dose study designed to evaluate safety, PK, and activity
–
Efficacy endpoints include FEV
1
, markers of inflammation
and airway hyperresponsiveness
20
~30 subjects
IPI-145
Placebo
IPI-145
Placebo
14 Days
14 Days
7-12 Day Washout
ClinicalTrials.gov NCT01653756.
R |
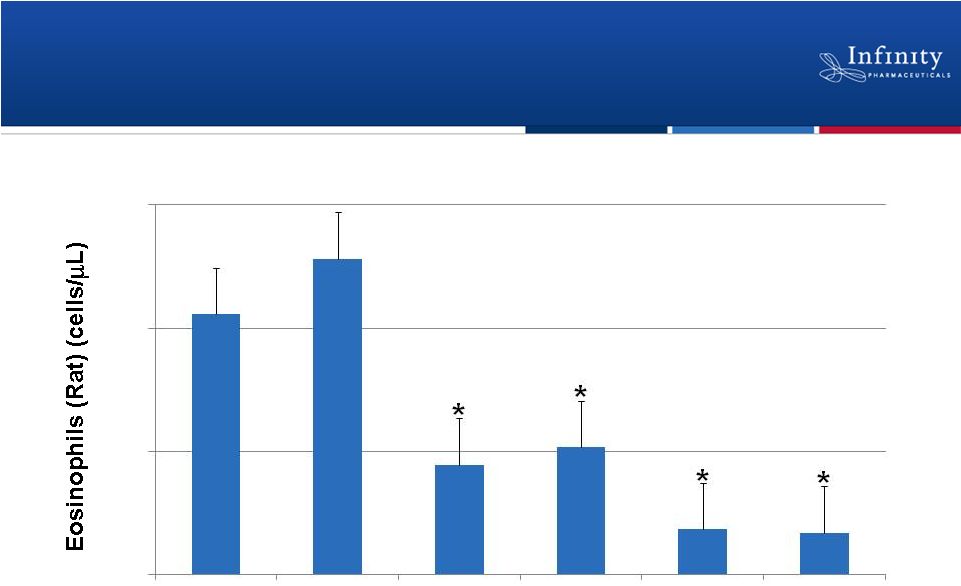 IPI-145 Inhibits Leukocyte Migration in
Preclinical Allergic Asthma Model
*p <0.05 compared to vehicle
21
0.1 mg/kg
IPI-145
0.3 mg/kg
IPI-145
1.0 mg/kg
IPI-145
10 mg/kg
IPI-145
Palombella. New York Academy of Sciences 2012.
Vehicle
750
500
250
0
10 mg/kg
Dexamethasone |
 Planning Phase 2 Trial in RA
•
Double-blind, randomized, placebo-controlled
•
Designed to evaluate safety and activity of multiple doses
22
Patients with moderate
to severe RA
Multiple doses of IPI-145
Placebo
R |
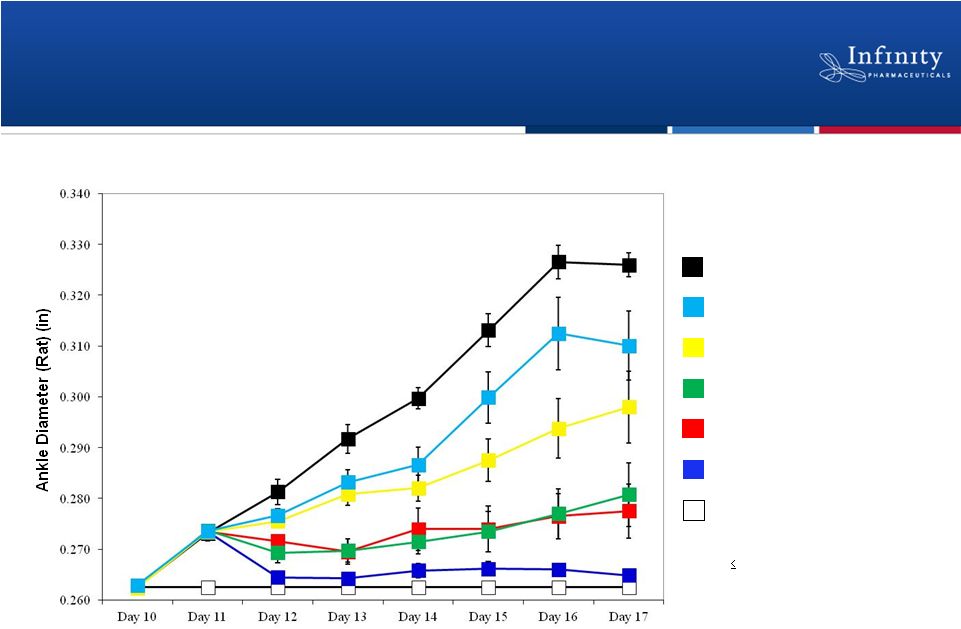 IPI-145 Demonstrates Dose-Dependent Effect in
Preclinical Model of Collagen Induced Arthritis
n=4/Normal Controls
n=8/Treatment Group
Vehicle
0.1 mg/kg IPI-145 (*day 14)
0.5 mg/kg IPI-145 (*day 13-17)
5.0 mg/kg IPI-145 (*day 12-17)
10.0 mg/kg etanercept (*day 12-17)
10.0 mg/kg IPI-145 (*day 12-17)
Normal control (*day 11-17)
*p
0.05
23
Palombella. New York Academy of Sciences 2012. |
 1
Decision Resources, Non-
Hodgkin’s Lymphoma, Apr 2011.
2
Decision Resources Immune and
Inflammatory Disorders Study –
Rheumatoid Arthritis, May 2012.
3
Decision Resources Immune and
Inflammatory Disorders Study –
Asthma Dec 2011.
Prevalence in G7 regions: US, UK,
IT, DE, ES, FR, JP.
24
PI3K Franchise: Potential
First-in-Class and
Best-in-Class Opportunities
RA
2
5.2M
Other
Multiple Large Opportunities
Heme
Malignancies
>600,000
Severe Asthma
3
7.1M
1 |
 Retaspimycin HCl (IPI-504):
Potent, Selective Inhibitor of Heat
Shock Protein 90 (Hsp90) |
 Retaspimycin HCl: Addressing Unmet Need
in NSCLC
Profile:
Therapeutic Focus:
Intellectual Property:
Commercial Rights:
26
•
Selective and potent Hsp90 inhibitor
•
Non-small cell lung cancer (NSCLC), with robust biomarker strategy
•
Composition and methods patents issued broadly worldwide
•
U.S. patent expiry: 2025, excluding patent term extension
•
Global rights to retaspimycin HCl |
 Hsp90 Plays an Important Role in Cancer
Cell Survival
Function of Hsp90
•
Chaperone necessary for stability
and function, to maintain protein
homeostasis
Function of Hsp90 in Cancer Cells
•
Many oncoproteins are hyper-
dependent on Hsp90 for function
•
Hsp90 is elevated in cancer cells
and buffers cancer specific stress
ATP
Retaspimycin HCl
Oncoprotein
Hsp90
27 |
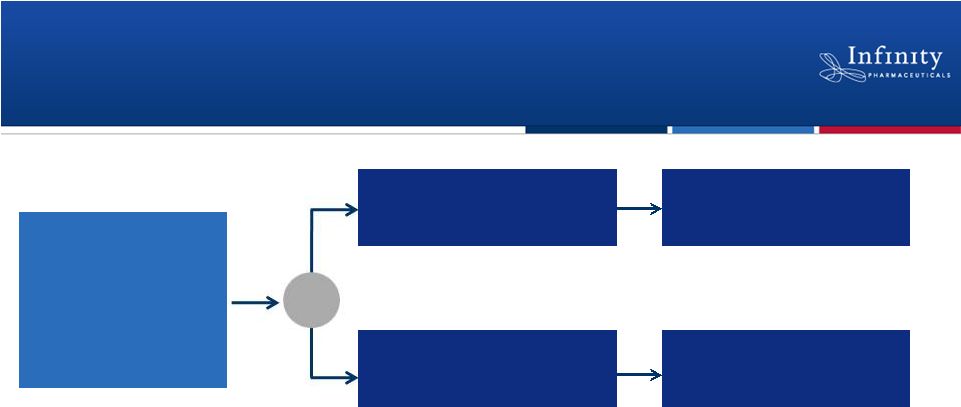 Phase 2 Trial in NSCLC Patients with a
Smoking History: Enrollment Complete
•
Randomized, double-blind, placebo-controlled trial
•
Anticipate topline overall survival data in 1H’13
28
Smokers
w/ 2
nd
-
or 3
rd
-line
NSCLC
(docetaxel naïve)
N = 226
Follow-up for OS
Follow-up for OS
Docetaxel +
Retaspimycin HCl
(N = ~105)
Docetaxel +
placebo
(N = ~105)
R
ClinicalTrials.gov NCT01362400.
–
Co-primary endpoints: Overall survival in total population and squamous
cell –
Secondary endpoints: Predictive biomarkers, progression free survival,
overall response rate |
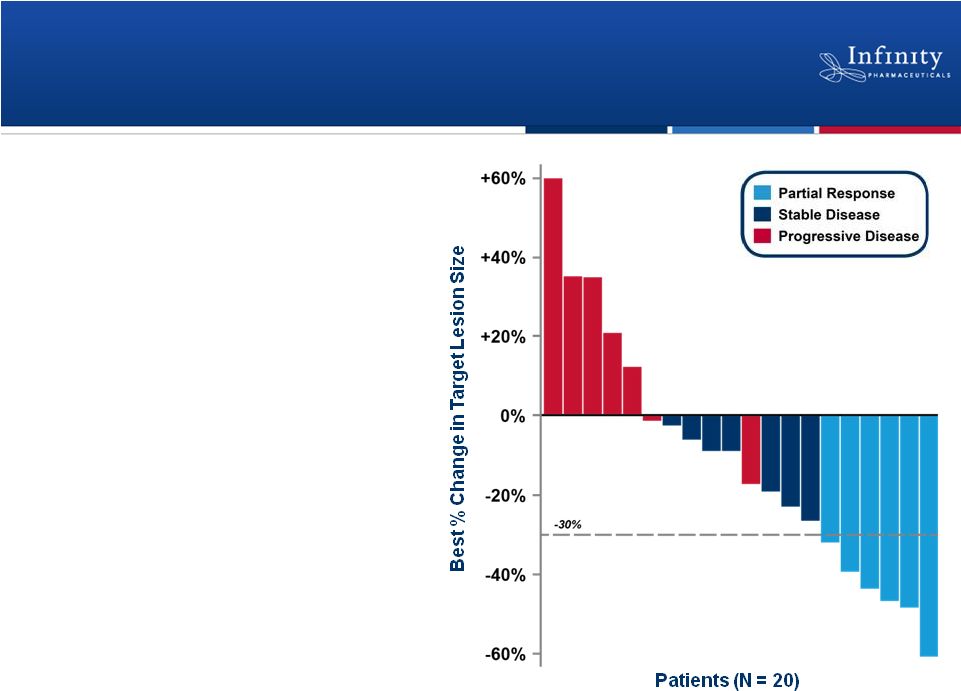 Phase 1b Trial: Clinically Active in
Combination with Docetaxel
29
•
Partial response in 6 patients
(ORR = 26%)
•
Stable disease in 7 patients
•
Well tolerated
–
No unexpected or overlapping
toxicities
–
No dose reductions or
discontinuations due to liver
function tests
–
No significant visual
disturbances
Riely et al., ASCO 2011.
PR
PR
PR
PR
PR
PR |
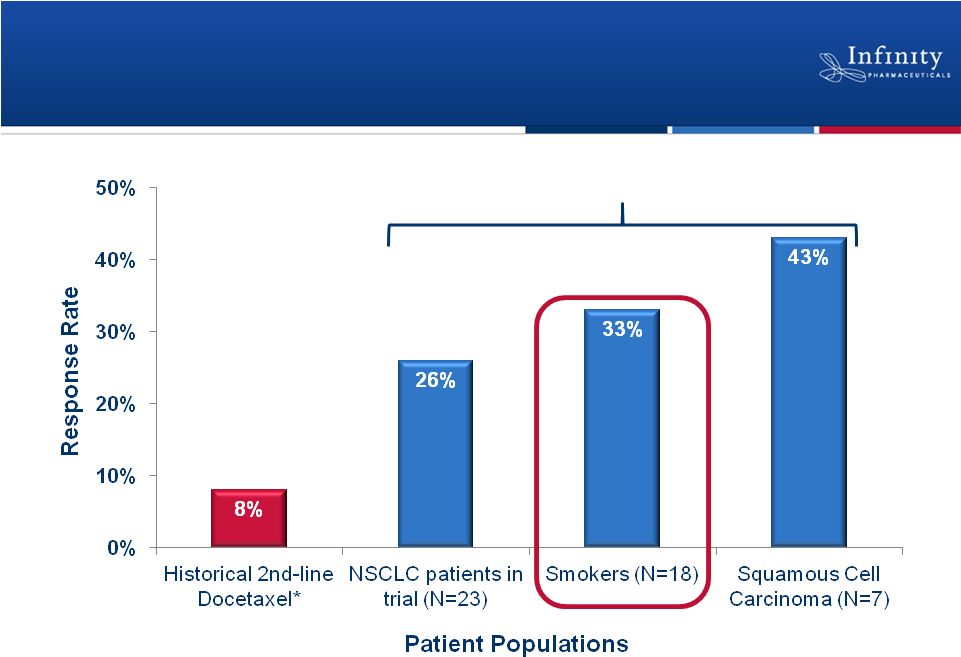 Phase 1b Trial: Responses Observed in
Patients with Historically Poor Prognoses
30
*Hanna et al, J Clin Oncol, 22:1589-97.
Riely et al., ASCO 2011.
Retaspimycin HCl Plus Docetaxel: Response Rate |
 Retaspimycin HCl:
Significant Commercial Potential
Current NSCLC market is ~ $4.0B and is projected to grow to $6.3B in 2019*
*Decision Resources NSCLC Pharmacor Report, March 2011. G7 regions: US, UK, IT, DE, ES, FR,
JP Roberts
et
al.,
2010;
J
Clin
Oncol
28(31):4769-4777.
Janjigian
et
al.,
2010;
Cancer
116(3):670-675.
31
Patient
Population
% of Overall
NSCLC
Population
Number of
Patients
Number of
Stage IIIb/IV
Patients
Heavy smokers
Squamous cell
KRAS mutant
70%
35%
30%
~291,000
~145,000
~125,000
~180,000
~90,000
~80,000 |
 2012 Financial Highlights
•
$189.4M in cash and investments as of September 30, 2012 (unaudited)
•
Year-end
2012
cash
and
investments:
$155
million
to
$165
million
¹
•
Cash
runway
into
2014
²
32
1
Financial guidance provided as of November 6, 2012.
2
Exclusive of additional funding or business development activities and based on
Infinity’s current operating plans. |
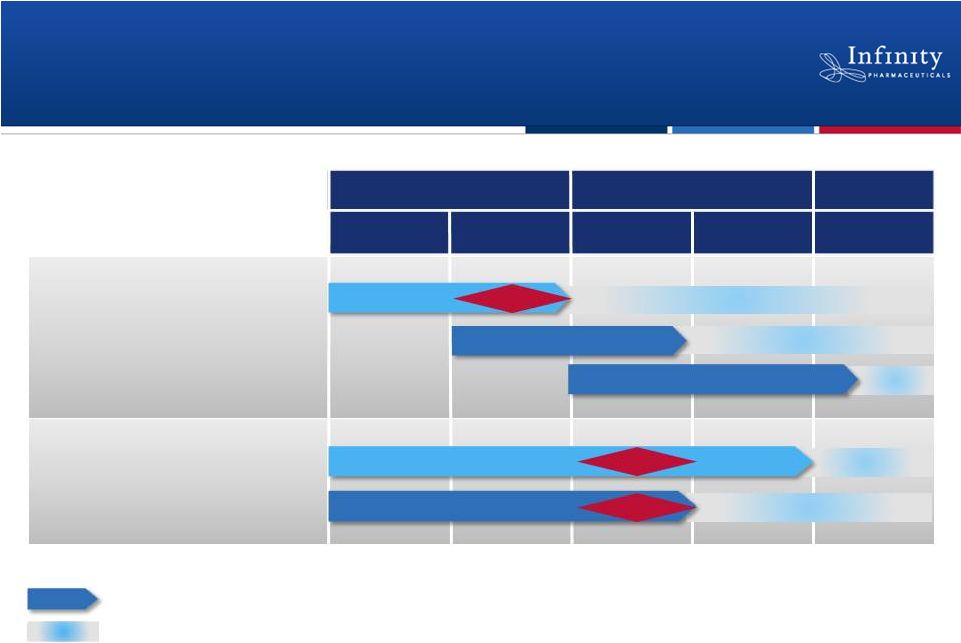 Pipeline: Multiple Near-Term Data Readouts
33
1H’12
1H’13
2H’13
2H’12
PI3K: IPI-145
NSCLC (mKRAS)
NSCLC (Heavy smokers)
Hematologic Malignancies
2012
2013
2014+
Hsp90: Retaspimycin HCl
Asthma
Rheumatoid Arthritis
Randomized, double-blind, placebo-controlled trial
Phase 3
Phase 3
Expansions >>> Phase 2
Phase 2
Phase 3
Next step and approximate timing, if data are positive.
Data
Data
Data
Phase
1
Phase 2a
Phase 2
Phase 1b/2
Phase 2 |
 Building a Fully Integrated
Biopharmaceutical Company
November 2012 |
 Third Party Obligations
Millennium
•
Up to $21M remaining in pre-NDA filing milestones for two distinct
product candidates
•
Royalties on net sales of IPI-145 ranging from single-
to low-double digits
•
For next-generation PI3K inhibitors
–
Milestones and royalties, possible co-development and co-commercial rights
on certain products in U.S. only
Mundipharma/Purdue
•
Infinity
pays
4%
aggregate
royalty
on
worldwide
net
sales,
until
$260M
in
royalties is paid
•
Thereafter, INFI pays 1% royalty on U.S. net sales
•
Applies to PI3K, FAAH and early discovery programs
Astra Zeneca
•
INFI pays single-digit royalty on worldwide net sales of retaspimycin HCl
35 |
