Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - InspireMD, Inc. | v326541_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - InspireMD, Inc. | v326541_ex99-1.htm |
Exhibit 99.2

The MASTER Trial A Prospective , Randomized, Multicenter Evaluation of a PET Micronet Mesh Covered Stent (MGuard) in STEMI Gregg W. Stone, MD Columbia University Medical Center NewYork - Presbyterian Hospital Cardiovascular Research Foundation

Disclosure Statement of Financial Interest • Consulting Fees/Honoraria • Abbott Vascular, Boston Scientific, Medtronic, InspireMD, Atrium Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company

Background • Suboptimal myocardial reperfusion after PCI in STEMI is common, and results in increased infarct size and mortality • The MGuard Embolic Protection Stent (EPS) s a novel thin - strut metallic stent with a PET micronet covering designed to trap and exclude thrombus and friable atheromatous debris to prevent distal embolization

The MGuard and MGuard Prime Embolic Protection Stent (EPS) *InspireMD, Tel Aviv, Israel; ** Polyethyleneterephthalate MGuard MGuard Prime Metallic frame 316L stainless steel L605 cobalt chromium Strut width 100 µm 80 µm Crossing profile 1.1 – 1.3 mm 1.0 – 1.2 mm Shaft dimensions 0.65 – 0.86 mm 0.65 – 0.86 mm Mesh sleeve PET** PET** - Fiber width 20 µm 20 µm - Net aperture size 150 - 180 µm 150 - 180 µm

Thrombus Entrapment by the MGuard in STEMI Jain AK and Rothman MT. JACC 2011;58;e39 Pre Post aspiration Residual thrombus

Thrombus Entrapment by the MGuard in STEMI Jain AK and Rothman MT. JACC 2011;58;e39 Thrombus trapped behind mesh Post MGuard Mesh

M GUARD for A cute ST E levation R eperfusion The MASTER Trial STEMI with symptom onset within 12 hours at 432 pts at 50 sites in 9 countries Substudies: Cardiac MRI : 60 pts (30 pts in each arm) at 3 - 5 days Angio FU: 5 0 pts in MGuard arm at 13 months Follow - up: 30 days, 6 months, 1 year Primary endpoint: ST - segment resolution at 60 - 90 minutes PCI with BMS or DES PCI with MGuard R Stratified by infarct vessel and thrombus aspiration

Principal Inclusion Criteria • Symptoms consistent with STEMI within 12 hours of symptom onset • ≥ 2 mm of ST - segment elevation in ≥ 2 contiguous leads • PCI of a single de novo lesion with RVD ≥ 3.0 to ≤4.0 mm and length ≤ 33 mm (capable of being covered by a single study stent)

Principal E xclusion Criteria • LBBB, paced rhythm, etc. • Prior PCI within 6 months or prior CABG anytime • LVEF ≤20%, cardiogenic shock or CPR • ≥50% left main stenosis present • Infarct lesion ostial or bifurcation with ≥ 2.0 mm sidebranch • T arget vessel or infarct lesion excessively tortuous, angulated or with moderate to heavy calcification • Prior stent proximal or w/i 10 mm distal to the target
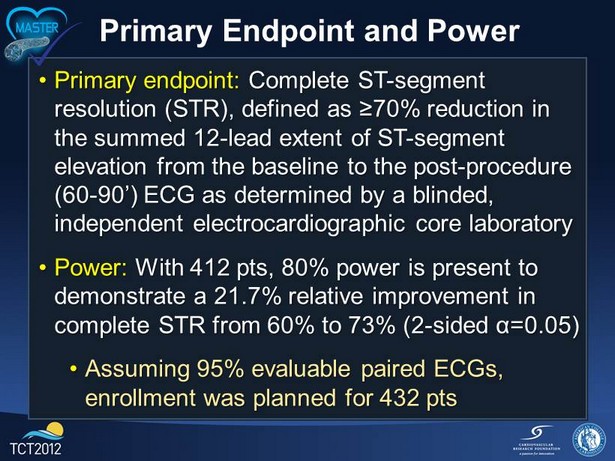
Primary Endpoint and Power • Primary endpoint: Complete ST - segment resolution (STR), defined as ≥70 % reduction in the summed 12 - lead extent of ST - segment elevation from the baseline to the post - procedure (60 - 90’) ECG as determined by a blinded, independent electrocardiographic core laboratory • Power : With 412 pts, 80 % power is present to demonstrate a 21.7% relative improvement in complete STR from 60% to 73% (2 - sided α=0.05) • Assuming 95 % evaluable paired ECGs, enrollment was planned for 432 pts

Study Organization Principal investigators: Alexandre Abizaid, Dariusz Dudek, Sigmund Silber Study chairman: Gregg W. Stone Executive committee: GW Stone , A Abizaid , D Dudek , S Silber, C Lotan, MB Leon, E Bar, E Yaacoby, M Ivenshitz Data monitoring: K CRI, Poland; MedPass Int, France; CRC, Brazil; Tal Yerushalmi , Israel; Modestas Jarutis , Ireland; Adele Liebenberg and Brendalynne Bezuidenhout , South Africa Data management: InspireMD , Tel Aviv, Israel . Data analysis Cardiovascular Research Foundation (CRF), NY, NY; and biostatistics: Helen Parise ( Director ), Ovidiu Dressler Event adjudication : Cardiovascular Research Center (CRC), Sao Paulo, Brazil; Andrea Abizaid, MD (Director) STR and MRI core labs: CRF; S Wolff, A Maehara, E Cristea, P Genereux (Directors) Angio core labs: CRC; Ricardo Costa ( Director ), and CRF; Sorin J. Brener (myocardial blush analysis ) DSMB: B Gersh (Chair), D Faxon, S Pocock Sponsor and funding: InspireMD, Tel Aviv, Israel

Top 12 Enrolling Sites 1. Bela Merkely, Semmelweis University, Budapest, Hungary 37 2. Dariusz Dudek, University Hospital in Krakow, Krakow, Poland 33 3 . Ran Kornowski, Rabin Medical Center, Petach Tiqva , Israel 31 4. Roman Wojdyła, Krakow Center of Invasive Cardiology, Electrotherapy and Angiology, Krakow, Poland 23 5 . Dezső Apró , State Hospital for Cardiology, Balatonfured , Hungary 19 6. Haim Danenberg , Hadassah U Medical Center, Jerusalem, Israel 19 7. Itzhak Herz , Laniado Hospital, Netanya, Israel 18 8. Bogdan Januś , E . Szczeklik Specialized Hospital, Tarnow , Poland 16 9. Marc A. Ohlow , Zentralklinik Bad Berka , Bad Berka , Germany 15 10. Krystrof Żmudka , John Paul II Hospital, Krakow, Poland 15 11. Jacek Legutko , INTERCARD, Nowy Targ , Nowy Targ , Poland 15 Between July 22, 2011 and May 29, 2012, 433 pts were randomized at 50 sites in 9 countries

Baseline Characteristics MGuard stent (n=217) Control stent (n=216) Age (years) 60 [52, 68] 58 [51, 67] Male 75.1% 76.9% Hypertension 42.3% 47.4% Hyperlipidemia 27.4% 27.1% Diabetes mellitus 12.0% 18.1% Cigarette smoking 55.3% 46.8% Prior MI 3.7% 8.8% Prior PCI 3.7% 5.6% Symptoms to device, mins 207 [156, 308] 240 [140, 383] Infarct artery = LAD 40.1% 40.3% Baseline TIMI flow = 0/1 66.5% 74.0% Baseline RVD, mm 3.15 [2.87, 3.38] 3.06 [2.87, 3.40] Baseline DS % 100 [85, 100] 100 [88, 100]
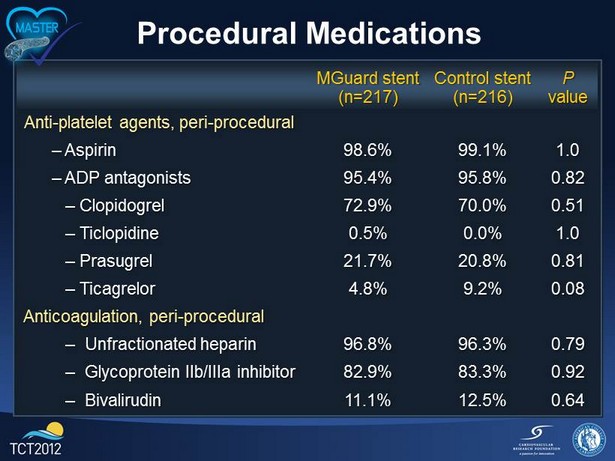
Procedural Medications MGuard stent (n=217) Control stent (n=216) P value Anti - platelet agents, peri - procedural – Aspirin 98.6% 99.1% 1.0 – ADP antagonists 95.4% 95.8% 0.82 – Clopidogrel 72.9% 70.0% 0.51 – Ticlopidine 0.5% 0.0% 1.0 – Prasugrel 21.7% 20.8% 0.81 – Ticagrelor 4.8% 9.2% 0.08 Anticoagulation, peri - procedural – Unfractionated heparin 96.8% 96.3% 0.79 – Glycoprotein IIb/IIIa inhibitor 82.9% 83.3% 0.92 – Bivalirudin 11.1% 12.5% 0.64
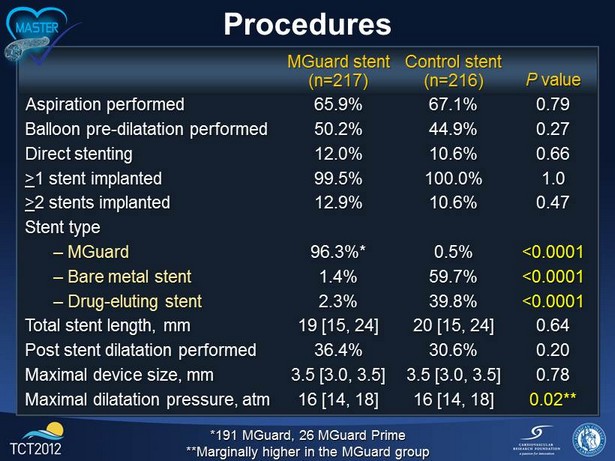
Procedures *191 MGuard, 26 MGuard Prime **Marginally higher in the MGuard group MGuard stent (n=217) Control stent (n=216) P value Aspiration performed 65.9% 67.1% 0.79 Balloon pre - dilatation performed 50.2% 44.9% 0.27 Direct stenting 12.0% 10.6% 0.66 > 1 stent implanted 99.5% 100.0% 1.0 > 2 stents implanted 12.9% 10.6% 0.47 Stent type – MGuard 96.3%* 0.5% <0.0001 – Bare metal stent 1.4% 59.7% <0.0001 – Drug - eluting stent 2.3% 39.8% <0.0001 Total stent length, mm 19 [15, 24] 20 [15, 24] 0.64 Post stent dilatation performed 36.4% 30.6% 0.20 Maximal device size, mm 3.5 [3.0, 3.5] 3.5 [3.0, 3.5] 0.78 Maximal dilatation pressure, atm 16 [14, 18] 16 [14, 18] 0.02**

Device Success Device success: < 50% final residual stenosis using only the randomized stent Lesion success: < 50% final residual stenosis using any percutaneous method Angiographic success: < 50% final residual stenosis and final TIMI 3 flow 95.9 95.9 100 91.7 91.7 100 99.1 99.5 82.9 82.4 0 25 50 75 100 Reach and cross lesion with study stent Device success Lesion success TIMI-3 flow Angiographic success MGuard (n=217) Control (n=216) P=0.006 P=0.004 P<0.001 P=0.03 P=0.50 * *9/217 cases (4.1%), including 9/191 (4.7%) and 0/26 (0%) cases in which the original MGuard and MGuard Prime devices were used, respectively

Procedural Results MGuard stent (n=217) Control stent (n=216) P value TIMI flow = 3 91.7% 82.9% 0.006 TIMI flow = 2 6.5% 11.6% 0.06 TIMI flow = 0/1 1.8% 5.6% 0.01 Corrected TIMI frame count 17 [12, 23] 18 [13,22] 0.23 Myocardial blush = 2/3 83.9% 84.7% 0.81 IPTE 21.7% 22.3% 0.87 RVD, mm 3.20 [2.90, 3.46] 3.16 [2.91, 3.46] 0.99 MLD, in - stent, mm 2.99 [2.73, 3.25] 2.99 [2.69, 3.31] 0.91 MLD in - lesion, mm 2.64 [2.40, 2.96] 2.64 [2.36, 2.95] 0.82 DS%, in - stent 6.9 [4.2, 10.5] 6.4 [3.9, 10.3] 0.56 DS%, in - lesion 15.3 [9.6, 21.2] 15.4 [10.8, 21.2] 0.66 IPTE = intraprocedural thrombotic events

Primary Endpoint: Complete ST - segment resolution MGuard (n=204) Control (n=206) 44.7% 38.3% 17.0% 57.8% 25.5% 16.7% Difference [95%CI] = 13.2% [3.1, 23.3 ] P=0.008

Primary Endpoint: Complete ST - segment resolution Multivariable predictors Variables: age , male , BMI, hypertension, hyperlipidemia, diabetes, current smoking, congestive heart failure, prior angina, prior MI, prior PCI, randomized group OR (95%CI) P value MGuard vs. control 1.73 (1.14 - 2.62 ) 0.01 Age ( yrs ) 1.03 (1.00 - 1.05) 0.02 Male 0.42 (0.25 - 0.71) 0.001 Current smoking 2.07 (1.31 - 3.27) 0.002
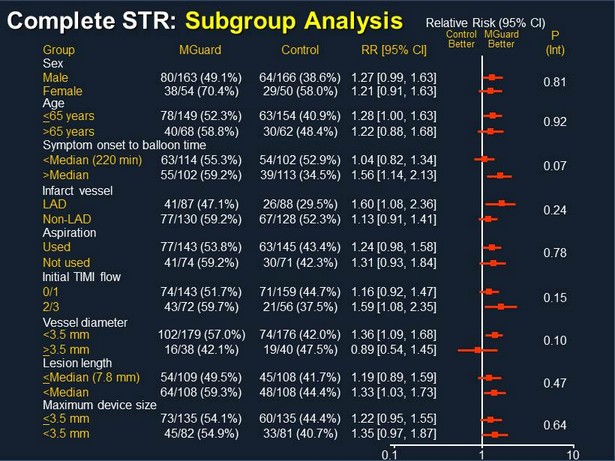
Complete STR: Subgroup Analysis Group MGuard Control RR [95% CI] Control Better MGuard Better P (Int) 0.81 Relative Risk (95% CI) 0.1 10 0.64 1 Male 80/163 (49.1%) 64/166 (38.6%) 1.27 [0.99, 1.63] Sex Female 38/54 (70.4%) 29/50 (58.0%) 1.21 [0.91, 1.63] 0.92 < 65 years 78/149 (52.3%) 63/154 (40.9%) 1.28 [1.00, 1.63] Age >65 years 40/68 (58.8%) 30/62 (48.4%) 1.22 [0.88, 1.68] 0.07 <Median (220 min) 63/114 (55.3%) 54/102 (52.9%) 1.04 [0.82, 1.34] Symptom onset to balloon time >Median 55/102 (59.2%) 39/113 (34.5%) 1.56 [1.14, 2.13] 0.24 LAD 41/87 (47.1%) 26/88 (29.5%) 1.60 [1.08, 2.36] Infarct vessel Non - LAD 77/130 (59.2%) 67/128 (52.3%) 1.13 [0.91, 1.41] 0.78 Used 77/143 (53.8%) 63/145 (43.4%) 1.24 [0.98, 1.58] Aspiration Not used 41/74 (59.2%) 30/71 (42.3%) 1.31 [0.93, 1.84] 0.15 0/1 74/143 (51.7%) 71/159 (44.7%) 1.16 [0.92, 1.47] Initial TIMI flow 2/3 43/72 (59.7%) 21/56 (37.5%) 1.59 [1.08, 2.35] 0.10 <3.5 mm 102/179 (57.0%) 74/176 (42.0%) 1.36 [1.09, 1.68] Vessel diameter > 3.5 mm 16/38 (42.1%) 19/40 (47.5%) 0.89 [0.54, 1.45] 0.47 < Median (7.8 mm) 54/109 (49.5%) 45/108 (41.7%) 1.19 [0.89, 1.59] Lesion length <Median 64/108 (59.3%) 48/108 (44.4%) 1.33 [1.03, 1.73] < 3.5 mm 73/135 (54.1%) 60/135 (44.4%) 1.22 [0.95, 1.55] Maximum device size <3.5 mm 45/82 (54.9%) 33/81 (40.7%) 1.35 [0.97, 1.87]
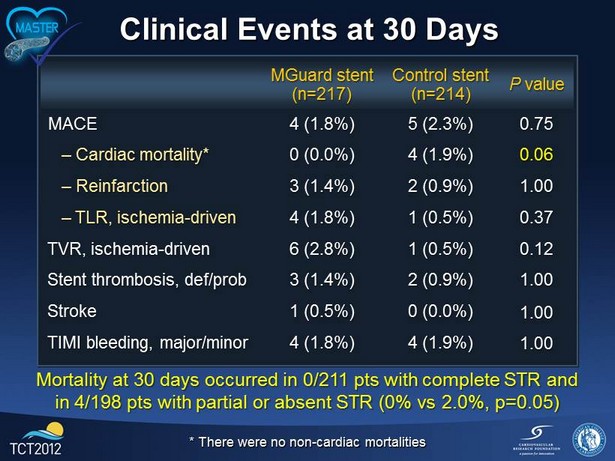
Clinical Events at 30 Days MGuard stent (n=217) Control stent (n=214) P value MACE 4 (1.8%) 5 (2.3%) 0.75 – Cardiac mortality* 0 (0.0%) 4 (1.9%) 0.06 – Reinfarction 3 (1.4%) 2 (0.9%) 1.00 – TLR, ischemia - driven 4 (1.8%) 1 (0.5%) 0.37 TVR, ischemia - driven 6 (2.8%) 1 (0.5%) 0.12 Stent thrombosis, def/prob 3 (1.4%) 2 (0.9%) 1.00 Stroke 1 (0.5%) 0 (0.0%) 1.00 TIMI bleeding, major/minor 4 (1.8%) 4 (1.9%) 1.00 * There were no non - cardiac mortalities Mortality at 30 days occurred in 0/211 pts with complete STR and in 4/198 pts with partial or absent STR (0% vs 2.0%, p=0.05)

3 - 5 Day MRI Substudy Results MGuard stent (n=30) Control stent (n=29) P value Total LV myocardial mass, gms 141 [117, 163] 147 [118, 174] 0.41 Infarct mass, grams 17.1 [10.0, 30.0] 22.3 [15.7, 30.1] 0.27 Infarct mass (% total LV mass) 13.3 [7.9, 25.0] 16.6 [10.0, 22.6] 0.48 Total MVO, grams 0.3 [0.0, 1.6] 1.0 [0.2, 2.8] 0.14 MVO (% total LV mass) 0.4 [0.0, 1.4] 0.8 [0.2, 1.9] 0.39 Abnormal wall motion score 22.5 [20.0, 26.0] 25.0 [21.0, 27.0] 0.48 LVEF (%) 48.3 [44.5, 52.3] 47.3 [42.0, 54.5] 0.79 MVO = microvascular obstruction

Limitations • Single - blind only • Underpowered for infarct size and clinical events, and subgroup analyses should be considered hypothesis - generating. • More experience with the MGuard Prime in STEMI is required • Long - term clinical and angiographic follow - up is ongoing • Discordance between TIMI flow, STR, infarct size, and death (improvement) vs. blush and IPTE (no significant change) is noted
Conclusions and Implications • Among pts with acute STEMI undergoing emergent PCI, the MGuard micronet mesh covered stent compared to conventional metallic stents resulted in superior rates of epicardial coronary flow and complete STR • A larger randomized trial is warranted to verify these findings, and determine whether these benefits result in reduced infarct size and/or improved clinical outcomes (MASTER II)

The MASTER Trial JACC image Stone GW et al. J Am Coll Cardiol 2012;
