Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CTI BIOPHARMA CORP | d402988d8k.htm |
 1
MPN
Pacritinib
Phase 3*
MDS/AML
Tosedostat
Phase 3*
NHL
Pixuvri™
Marketed (EU)
Cell Therapeutics, Inc.
Company Overview
* Phase 3 ready.
Covering a Spectrum
of Blood Related
Cancers
Exhibit 99.1 |
 2
Forward Looking Statement
This presentation includes forward-looking statements that involve a number of
risks and uncertainties, the outcome of which could materially and/or
adversely affect actual future results and the market price of CTI's securities. Specifically, the risks and uncertainties that could affect
the development of Pixuvri, OPAXIO, pacritinib, tosedostat and brostallicin include
risks associated with preclinical and clinical developments in the
biopharmaceutical industry in general and with Pixuvri, OPAXIO, pacritinib, tosedostat or brostallicin in particular including, without
limitation, the potential failure of Pixuvri to prove safe and effective for the
treatment of relapsed or refractory non-Hodgkin's lymphoma and/or other
tumors as determined by the U.S. Food and Drug Administration (the “FDA”), that Pixuvri may not be available to patients in the E.U.
beginning
in
September
2012,
that
CTI
may
not
market
and
commercialize
Pixuvri
as
planned,
that
CTI
may
not
launch
Pixuvri
in
the
E.U.
this
year
as
planned
or
in
any
of
the
specific
countries
in
the
E.U.
we
are
currently
planning
to
launch,
that
CTI
may
not
be
able
to
complete
the
PIX306 clinical trial of Pixuvri-rituximab compared to
gemcitabine-rituximab in patients who have relapsed after 1 to 3 prior regimens for
aggressive B-cell NHL and who
are not eligible for autologous stem cell transplant by June 2015 or at all as required by the European
Medicines
Agency
(the
“EMA”)
or
have
the
results
of
such
trial
available
by
June
2015
or
at
all,
that
CTI
may
not
be
able
complete
a
post-
marketing study aimed at confirming the clinical benefit observed in the PIX301
trial, that the conditional marketing authorization for Pixuvri may not be
renewed, the potential failure of OPAXIO to prove safe and effective for treatment of non-small cell lung and ovarian cancers, that
the interim survival results for the phase 3 clinical trial for OPAXIO may not be
ready in 2012, the potential that phase III studies of pacritinib
might
not
begin
in
the
fourth
quarter
of
2012,
the
potential
that
pacritinib
may
fail
to
prove
safe
and
effective
for
primary
MF
and
MF
secondary
to other MPNs, the potential that phase III studies of tosedostat may not occur as
planned, the potential failure of tosedostat to prove safe and effective for
the treatment of elderly patients with newly-diagnosed AML or high-risk myelodysplastic syndrome ("MDS") (including when
administered in combination with cytarabine or decitabine) as determined by the FDA
and/or the EMA, the potential failure of combination studies of tosedostat
with hypomethylating agents in treating AML and/or MDS, that the studies of tosedostat may not achieve their primary
and/or secondary objectives, that the development of tosedostat may be delayed by
disputes with Chroma Therapeutics Ltd. (“Chroma”) under CTI’s
co-development and license agreement with Chroma, that OPAXIO, pacritinib, tosedostat and/or brostallicin may not be approved by the
FDA and/or the EMA, that CTI cannot predict or guarantee what actual sales of its
product candidates will be, that CTI cannot predict or guarantee the pace or
geography of enrollment of its clinical trials or the total number of patients enrolled, that CTI may not achieve a burn
rate of an average of $4.5 million per month through the remainder of the year,
that CTI's average net operating burn rate may increase, that CTI cannot
guarantee that it will not exceed it estimated net operating burn rate for 2013, that CTI cannot guarantee that it will not incur any
debt
or
maintain
its
current
cash
amount
of
cash
available,
that
CTI
cannot
guarantee
that
its
current
amount
of
cash
available
will
be
sufficient
to sustain CTI’s operations until January 2013, that CTI cannot predict or
guarantee when CTI will achieve profitability, if ever, or if CTI will
achieve cash flow break even in the fourth quarter of 2014, and that CTI may not be
able to continue to raise capital as needed to fund its operations
in
general,
and,
including,
without
limitation,
competitive
factors,
technological
developments,
costs
of
developing,
producing,
and
selling its product candidates, and the risk factors listed or described from time
to time in CTI's filings with the Securities and Exchange Commission
including, without limitation, CTI's most recent filings on Forms 10-K, 8-K, and 10-Q. Except as may be required by law, CTI does
not intend to update or alter its forward-looking statements whether as a
result of new information, future events, or otherwise. |
 3
Company Overview
Hematology/Oncology-focused biotechnology company
1 commercial product
•
Pixuvri™
:
for
the
treatment
of
non-Hodgkin's
lymphoma
(“NHL”)
-
Conditional
Marketing
Authorization
May
2012-
E.U.
launch
September
2012
-
Attractive “niche”
market ~16,000 patients
(1)
; 10 yrs market exclusivity in E.U.
3 late-stage product candidates:
•
Pacritinib,
:
highly
selective
JAK2
inhibitor,
Phase
3-
expected
Q4
2012
-
Addresses unmet need in MF market post ruxolitinib approval
•
Tosedostat
: novel aminopeptidase inhibitor anticipating Phase 3 in 2013
for MDS/AML
•
Opaxio
:
protein-paclitaxel
conjugate
completing
Phase
3
trial
in
1
st
line
maintenance therapy for Ovarian Cancer
~$24mm cash and no debt
on the balance sheet
(2)
•
Estimated cash burn of an average of ~$4.5mm per month through the remainder of
2012. (1) European Cancer Observatory, Publications on NHL subgroup and
relapse rates includes 4th line. (2) Pro forma cash balance as of
August 1,2012. |
 4
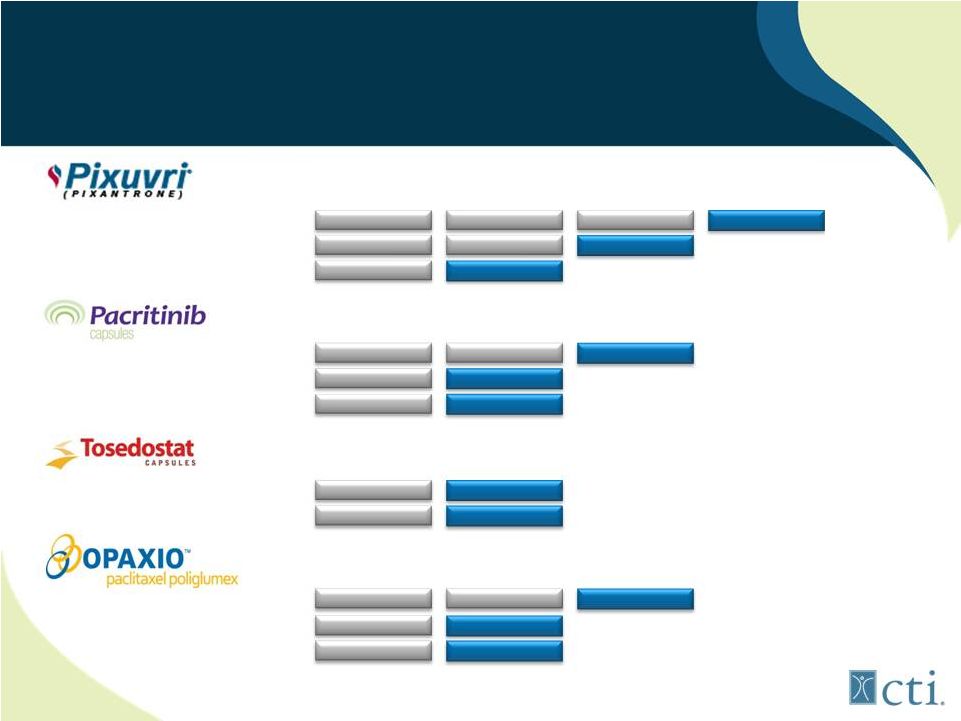
Current Pipeline
Third-line r/r aggressive NHL (PIX301)
Second-line r/r aggressive NHL (PIX306)
First-line aggressive NHL (PIX203)
Myelofibrosis (<100,000plts)
(1)
Primary Myelofibrosis
Relapsed Lymphoma
First-line or r/r Myelodysplastic Syndrome
Elderly high-risk r/r AML (OPAL)
Maintenance therapy for ovarian cancer
First-line GBM +XRT vs standard of care
Head and neck cancer w/ Erbitux+XRT
Phase 1
Phase 2
Phase 3
Commercial
(1)
Phase 3 study is expected to initiate in 4Q 2012. |
 5
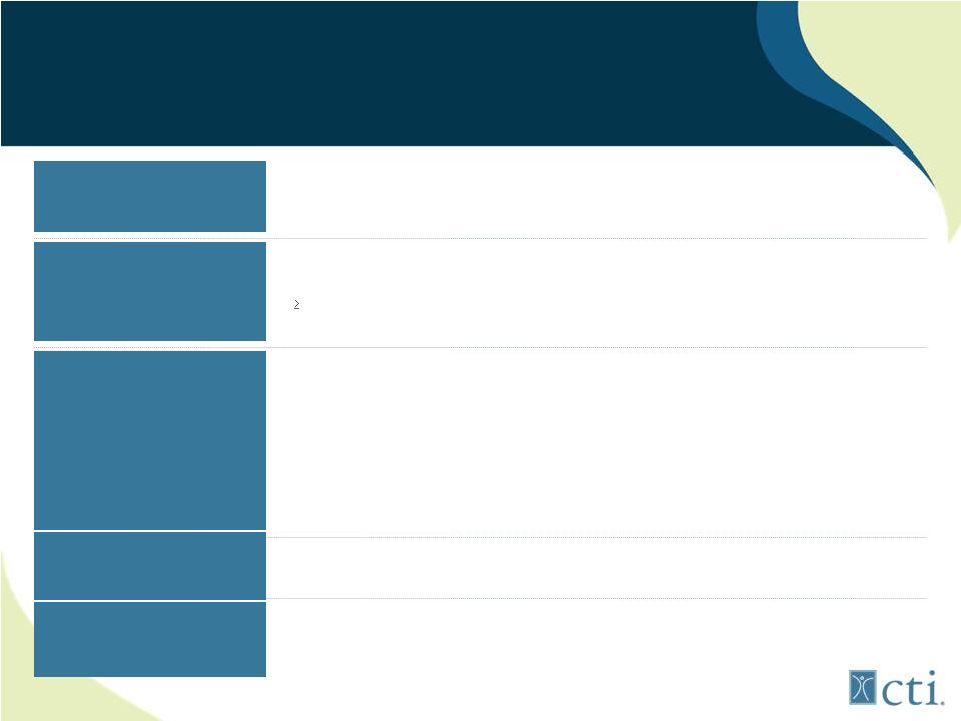
Investment Highlights
Pixuvri Approved in E.U.
Pacritinib is a Differentiated
JAK2 Inhibitor
Leading, Late-Stage
Oncology Franchise
Tosedostat has Shown
Encouraging Data in
AML/MDS
Significant Upcoming
Milestones
•
Late-stage portfolio focused on blood-related cancers
•
Deep pipeline with 1 product approved in the E.U., 1 product in Phase 3 trials, 2
products planning to initiate pivotal Phase 3 trials in 2012/2013
•
Received
E.U.
conditional
marketing
approval
in
May
2012;
launch
roll-out
September
2012
•
Engaged Quintiles to hire Pixuvri dedicated sales force and MSL’s
•
$240mm potential peak sales in E.U., pricing –reimbursement studies support
reasonable price •
Pixuvri
sales
can
help
fund
pipeline
development
and
potentially
lead
to
profitability
by
Q4-2014
•
Highly selective JAK2 inhibitor (does not affect the JAK1 pathway, unlike
competitors) •
Positioned to be the first JAK2 inhibitor for MF patients with platelet counts
below 100,000/uL, and potentially the second JAK2 inhibitor to enter the
market after Incyte’s JAKAFI (peak worldwide sales of JAKAFI in
MF are estimated at $1bn) -
Only grade 3 side effect is diarrhea
-
Potential competitive advantage over JAKAFI in patients with thrombocytopenia (27%
-33% of MF market)
•
Expected to enter pivotal Phase 3 trial in Q4 2012, target enrollment completion:
12 months •
The only aminopeptidase inhibitor in advanced clinical development
•
Demonstrating results in Phase 2 trials
•
September 2012: Pixuvri launch in E.U.
•
Q4 2012: Expected to Initiate pacritinib Phase 3 trial in
myelofibrosis •
Q1-2013 Expected interim survival analysis on Opaxio (GOG212) phase 3
trial |
 6
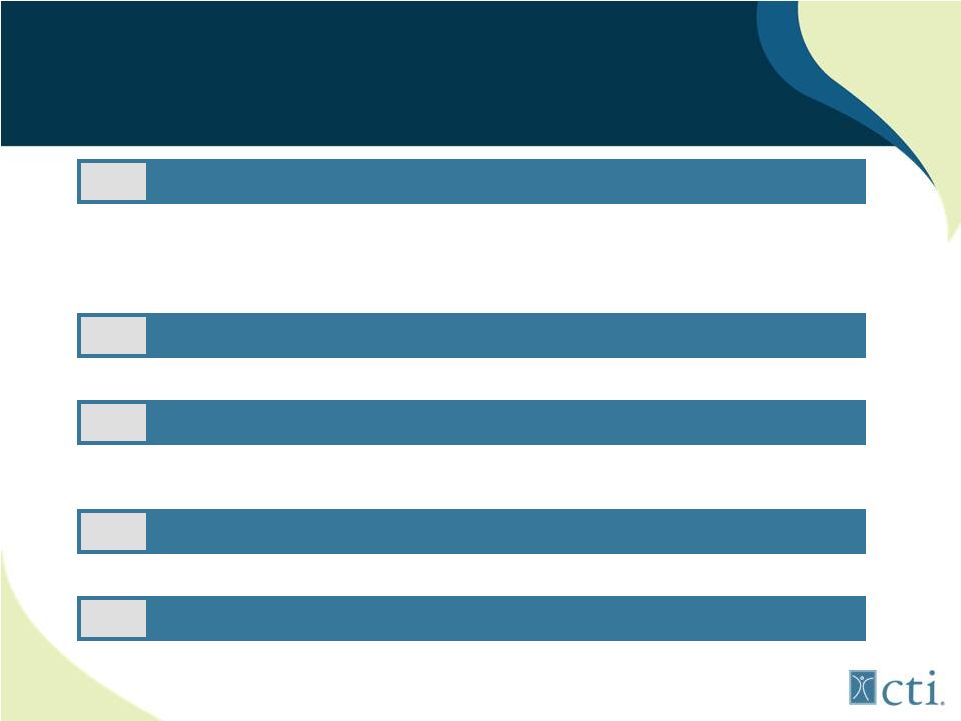
Recent Strategic Initiatives
Reduced Projected Monthly Cash Burn from an average of ~$5.5mm to
~$4.5mm 1
•
Do not plan to resubmit Pixuvri US NDA in the near-term
•
Discuss options for PIX306 trial with FDA/EMA for earlier data (PFS)
•
Delaying
initiation
of
tosedostat
Phase
3
trial
until
1
st
line
phase
2
results
•
Reduced advisory, consulting, legal fees and occupancy costs
Initiation of Pacritinib Phase 3 Trial to Q4 2012
4
Pixuvri Pre-launch Activities in Support of a September 2012 Launch
3
•
Engaged Quintiles to hire CTI’s Pixuvri dedicated sales force and medical
specialists •
EU General Manager, Market Access Specialist, and VP of Medical Affairs in
place New Hires with Significant Industry and Operational Expertise
2
•
Recently hired new CMO and EVP, Corporate Development
Implemented Reverse Stock Split
5
•
Current shares outstanding ~56.6 million
•
Engaged CRO that conducted COMFORT II trial
•
Planned initiation Q4 2012 |
 7
Executive Management Team
Officers
Position
Experience
James Bianco, MD
Principal Founder,
President & Chief
Executive Officer, and
Director
•
Chief architect of CTI’s portfolio strategy and has secured more than $1.6bn
in operating capital
•
Previously was an Assistant Member in the clinical research division of the Fred
Hutchinson Cancer Research Center and an Assistant Professor of Medicine at
the University of Washington and Director Bone Marrow Transplant Unit
SVAMC •
Received B.S. in Biology and Physics from New York University and M.D. from
Mount Sinai School of Medicine
Steven Benner,
MD, MHS
EVP, Chief Medical
Officer
•
Joined CTI as EVP and CMO in June 2012
•
Previously was SVP and CMO at OncoMed and CMO at Protein Design Labs
•
Industry experience Bristol –Myers Squibb
•
Completed his training in medical oncology at Johns Hopkins University, where he
also earned an M.H.S. in Clinical Epidemiology from the University’s
school of Public Health
Matt Plunkett. PhD
EVP, Corporate
Development
•
Former CFO California Institute Regenerative Medicine, iPierian
•
Previously Managing Director, Head West Coast Biotechnology, CIBC
•
70 transactions, $4.3bn equity linked transactions, $1bn M&A
•
Received PhD. from UC Berkeley |
 8 |
 9
Pixuvri™: Addresses an Unmet Need
Anthracyclines: most active class of chemotherapy
•
Cornerstone agents for NHL, AML, sarcoma, breast
•
Cumulative cardiac damage is dose limiting
Pixuvri™
•
Lacks structural motifs that lead to cardiotoxicity
-
Significant reduction in biochemical, echo-cardiographic and severe
clinical
symptoms
(CHF)
vs
doxorubicin
in
randomized
P2
clinical
trial |
 10
Pixuvri™:
Conditional
Marketing
Authorisation
3
rd
/4
th
line aggressive B-cell NHL |
 11
Lancet Oncology: May 2012
11 |
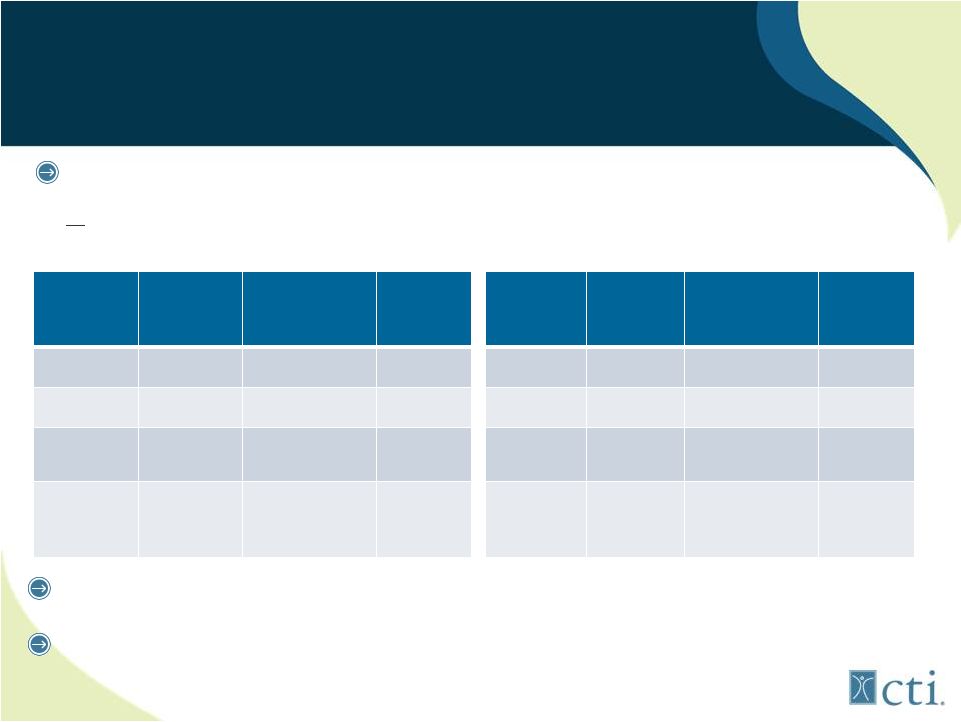 12
Pixuvri™:
First to Market for r/r
aggressive B-cell NHL
Pixuvri
(n=70)
Comparator
(n=70)
P Value
CR/CRu
20%
5.7%
0.021
ORR
40%
14.3%
0.001
PFS
5.3mos
2.6mos
HR=0.60
0.005
OS
10.2mos
7.6mos
HR=0.79
0.25
Pixuvri
(n=50)
Comparator
(n=49)
P value
CR/CRu
28%
4.1%
0.002
ORR
48%
12.2%
<0.001
PFS
5.7mos
2.8mos
HR=0.50
0.002
OS
13.9mos
7.8mos
HR=0.76
0.27
Basis: PIX301 trial, only randomized controlled trial in
>3rd
line
aggressive
NHL
(n=140
patients)
Manageable toxicities (neutropenia)
Lower than expected incidence of cardiac events (7%)
Intent to Treat Results
On Label 3
rd
/4
th
line B-cell Results |
 Pixuvri™: EU Commercial Opportunity
Market research supports potential peak
penetration ~8,125 patients/year*
Market access (pricing) strategy*
•
Quantitative benefit over physician choice
•
CR statistically correlative with OS
•
EU
List
Price
corridor
$29,500
-
$35,000
-
Allows for potential net ex-factory price $29,500
•
Incremental cost/QALY (ICER) favorable
Currently plan to launch in free market countries
Q4 2012
•
Nordic, Austria/Norway, Germany/UK/Netherlands
•
Balance of major market countries expected in 2013
13
*Market Access, Reimbursement and Market penetration projections provided by, UBC, and Kantar
Research respectively |
 14
Pixuvri™: U.S. Regulatory Status
FDA appeal: Office New Drugs (OND) provided path for
resubmission
-
2 Independent
Radiographic
Panel
confirmed
1st
panels
results
-
Document Interim analysis was not conducted then PIX301 trial would be
considered a positive study
EU launch and pacritinib acquisition driving portfolio
dynamics and priorities
•
NDA strategy under review
•
Considering potential to use PFS from PIX306 trial to provide data for
accelerated approval while trial completes and survival data matures
•
Could
potentially
fulfill
EMA-PMC
and
serve
as
2
trial
for
US
submission
in 2014
•
Plan to seek FDA and Scientific Advice on the above in 2013
nd
nd
14 |
 A
highly differentiated JAK2 inhibitor 15 |
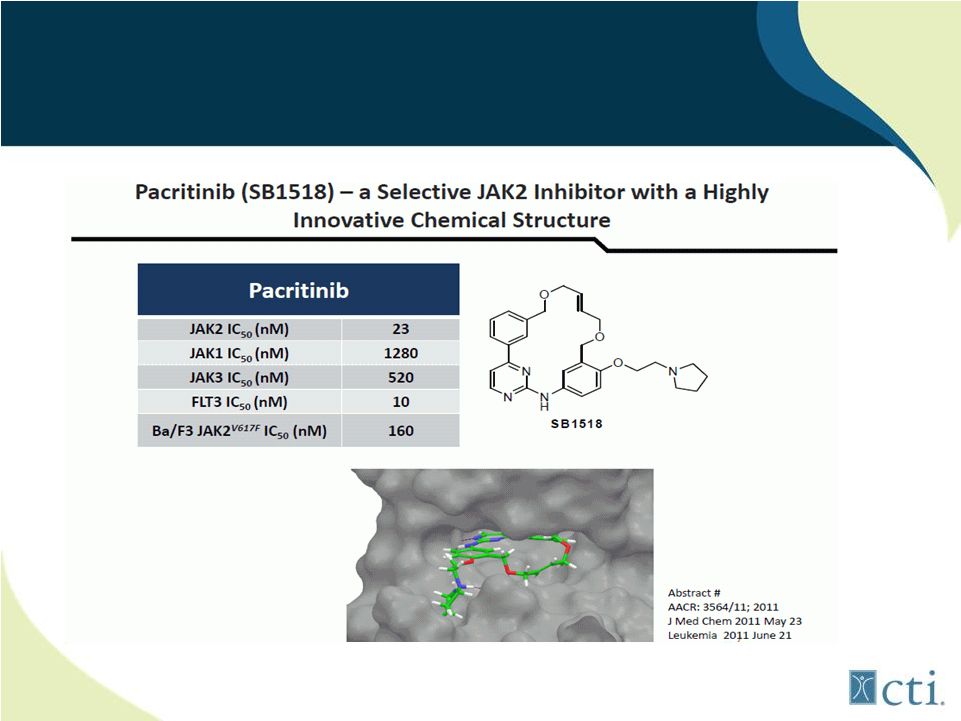 16
Pacritinib:
Inhibits
2
important
activating
mutations
(JAK2
+
FLT3) |
 17
Initial
Target
Market:
MF
patients
with
thrombocytopenia
(platelets
<100
x10
9
)
Table 2: Maximum Restarting Doses for Jakafi After
Safety Interruption* Current Platelet Count
Maximum Dose When
Restarting Jakafi Treatment *
Greater than or equal to 125 X 109/L
20 mg twice daily
100 to less than 125 X 109/L
15 mg twice daily
75 to less than 100 X 109/L
10 mg twice daily for at least 2 weeks;
if stable, may increase to 15 mg twice
daily
50 to less than 75 X 109/L
5 mg twice daily for at least 2 weeks; if
stable, may increase to 10 mg twice
daily
Less than 50 X 109/L
Continue hold
*Maximum
doses
are
displayed.
When
restarting,
begin
with
a
dose
at
least
5
mg
twice
daily
below
the dose at interruption.
JAKAFI™
(ruxolitinib label for patients with low platelets)
|
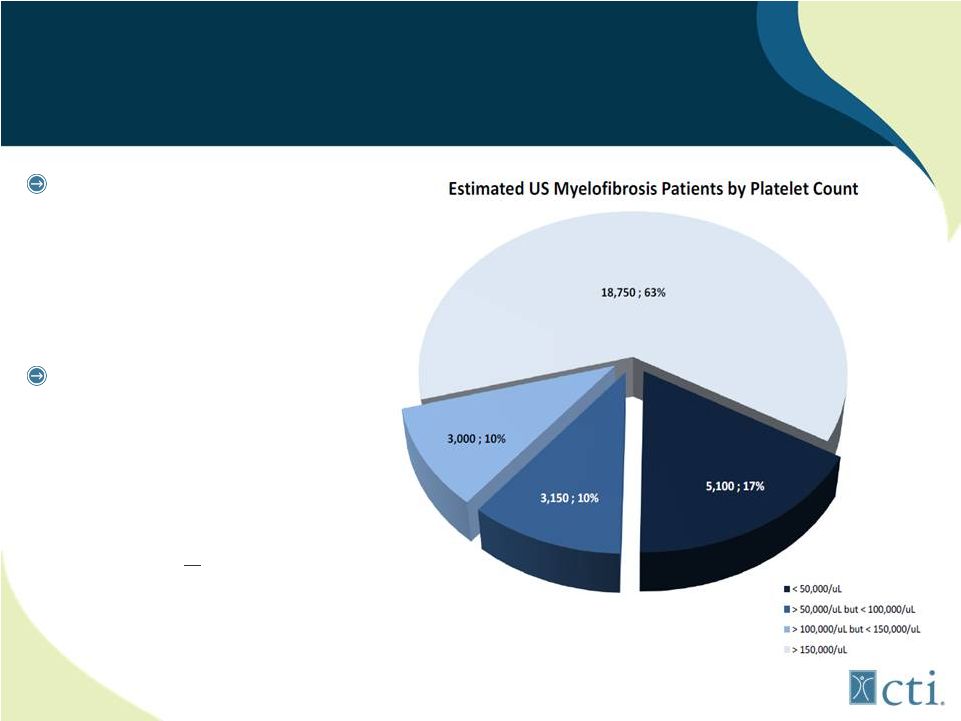 18
27% MF Patients Have Severe Thrombocytopenia
JAK1/JAK2 inhibitors
(ruxolitinib) associated
with treatment emergent
thrombocytopenia and
anemia
Pacritinib: selective
JAK2 inhibitor lacks
myelosupporession;
potentially first to market
for MF patients with low
platelets (<100,000/uL)
Source: MPN Research Foundation.
|
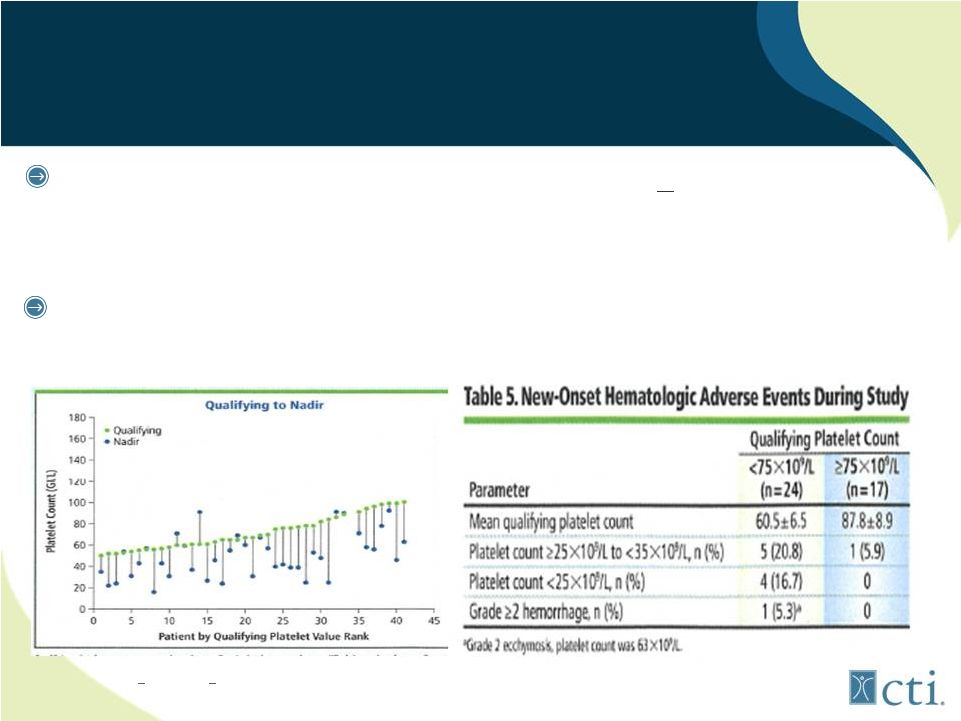 19
Pacritinib: Targets patient population
unserved by ruxolitinib
Ruxolitinib
approval
trials
excluded
patients
<100,00/uL
platelets
-
93% COMFORT II had normal platelets, median COMFORT I, 244,000/uL
-
However, MF patients with low platelet counts are higher risk population
Ruxolitinib study* (n=41) MF in patients with low platelets*
•
75% tolerated up to 10mg bid by 24 weeks, but TET* still problematic
*<100,000/uL >
50,000/uL ASCO June 2012 abstract #6630 preliminary data.
TET: treatment emergent thrombocytopenia |
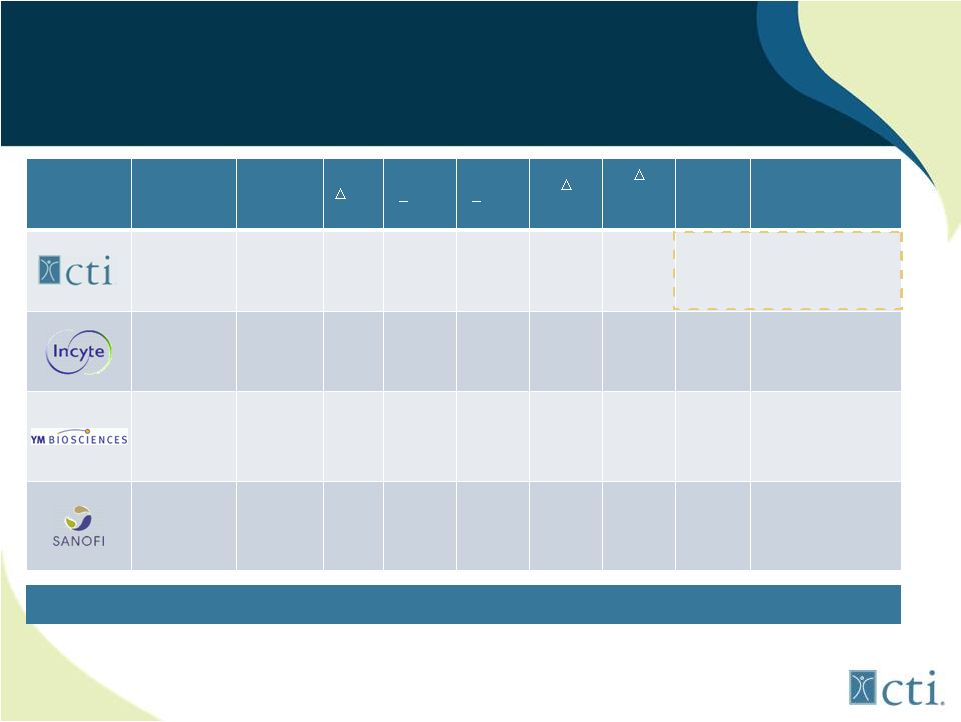 20
Summary Clinical Trial Results
JAK Inhibitors in MF
Company
Drug
Stage
SX
Spleen
>50%
PE
Spleen
>35%
MRI
RBC
JAK2
V617F*
Platelet
cutoff
Side
Effects
Pacritinib
JAK2-wt
JAK2V617F
FLT-3
Phase 3
(Phase 2
n=65)**
>50%
42%
32%
NR
>30%
NONE
Nausea/diarrhea
Ruxolitinib
JAK1
JAK2-wt
Marketed
>50%
29-42%
14%
NA
100,000
Anemia,
thrombocytopenia,
withdrawal
syndrome
CYT387
JAK1
JAK2-wt
TYK2
Phase 3
(Phase 2
n=60)
>50%
45%
50%
NA
50,000
Thrombocytopenia,
headaches, 1
st
dose
effect, neuropathy,
lipase/amylasemia
SAR302503
JAK2-wt
JAK2V617F
FLT-3
Phase 3
(Phase ½
n=59)
>50%
39%
0%
21%
50,000
Nausea/diarrhea,
anemia,
thrombocytopenia,
lipase/amylasemia
Chart taken from Terferri, Supplement to Blood ASH 2012
*Median allele burden reduction at 24 cycles.
** Data combined from two Phase 2 trials.
Pacritinib Demonstrates a Truly Differentiated Side-Effect Profile
|
 21
Pacritinib: Phase 2 Studies
Source: ASH 2011. |
 22
Pacritinib: Phase 2 Studies (combined)
Source: ASH 2011. |
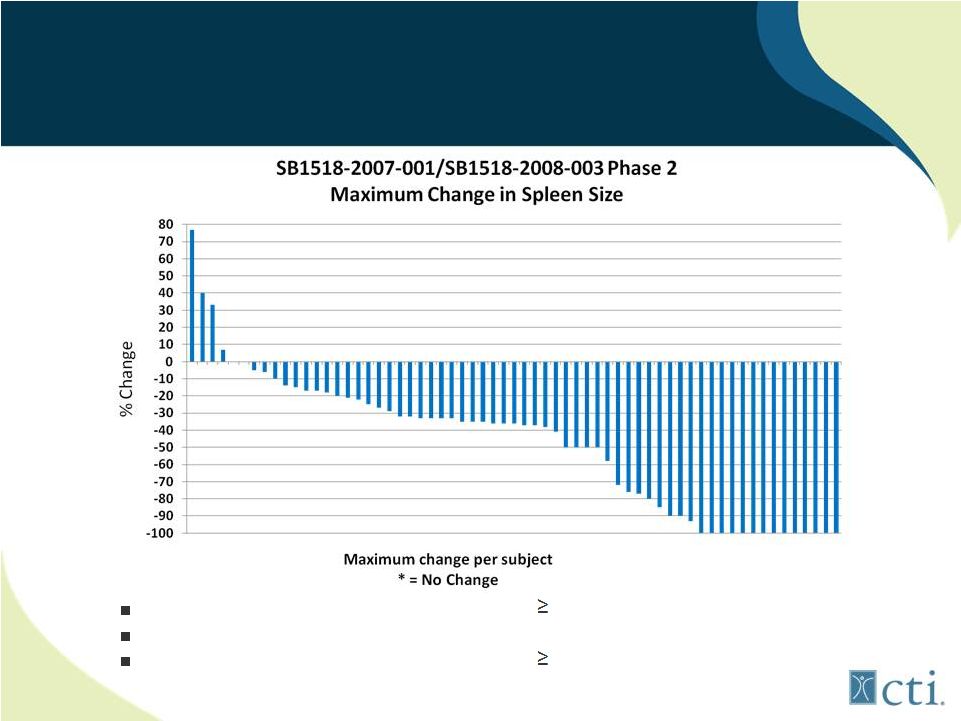 23
Pacritinib: Phase 2 Efficacy (combined)
27 of 65 (42%) patients had response of 50%
14 of 65 (21%) had complete response with resolution of splenomegaly
8 of 26 (32%) treated with SB1518 had 35% reduction by
MRI* Source: ASH 2011.
*Among patients who had MRI measurements (8 patients did not have baseline
MRI’s). |
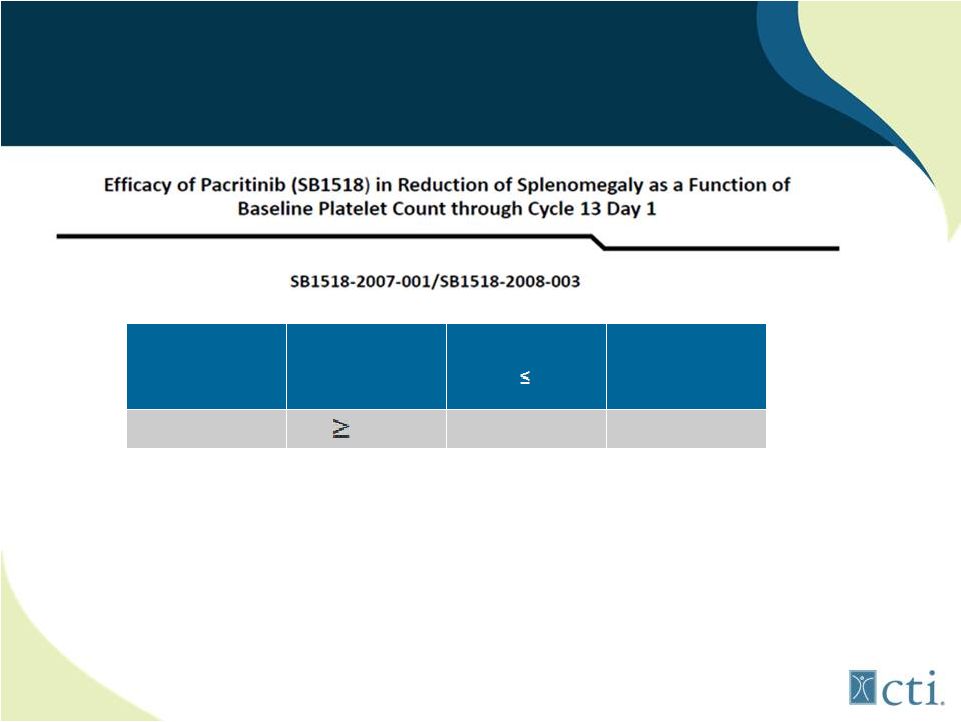 24
Pacritinib: Benefit Independent
of Platelet Count
Assessment
Maximum
decrease from
baseline
Patients with
baseline platelet
counts
150,000
N=36
All Evaluable
Patients
N=65
PE
50%
14 (39%)
27 (42%)
Source: ASH 2011.
*
Reduction of splenomegaly was observed with similar frequency in patients with normal or low platelet
counts |
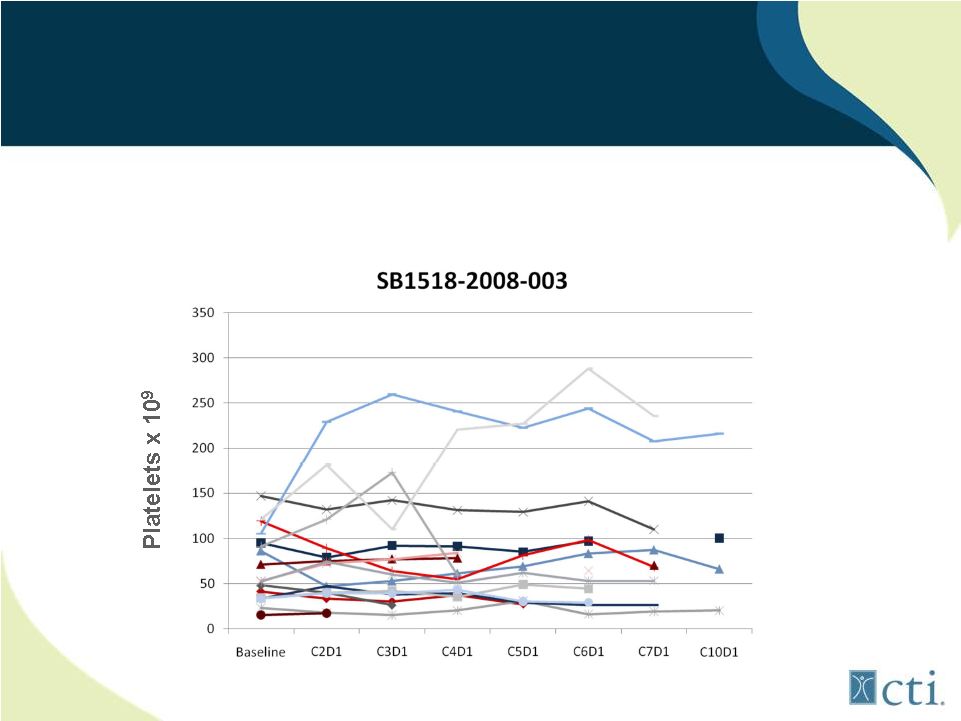 25
Lack of treatment emergent thrombocytopenia observed in patients
with low starting platelet counts
Pacritinib: Minimal Effect on Platelet
Counts
Source: Komrokji. R., et al |
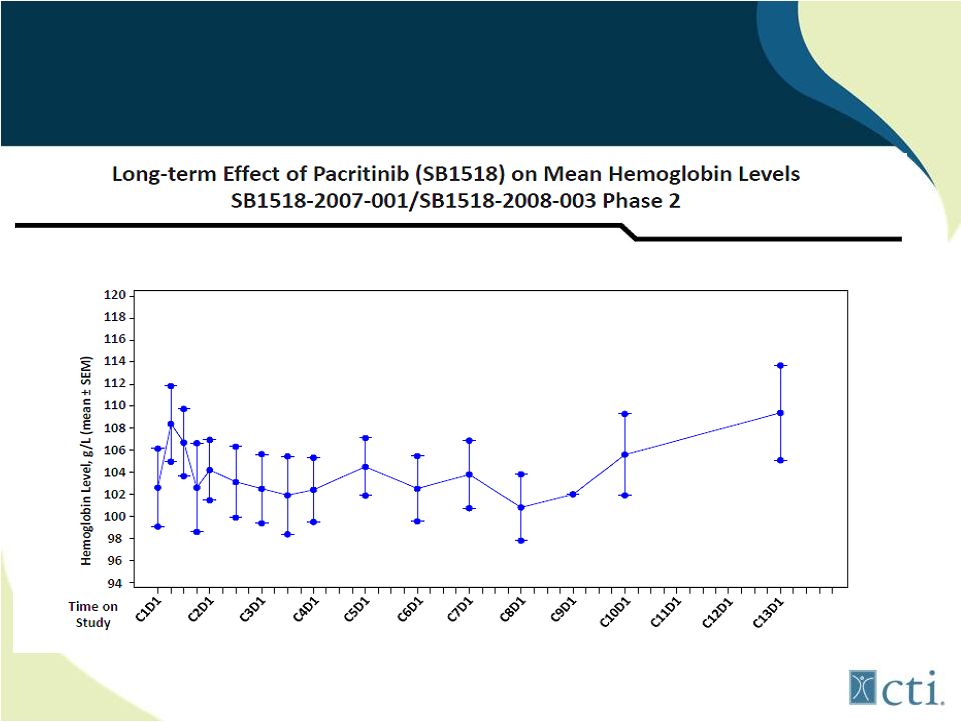 26
Pacritinib: Lack of treatment associated
anemia
Source: Komrokji. R., et al |
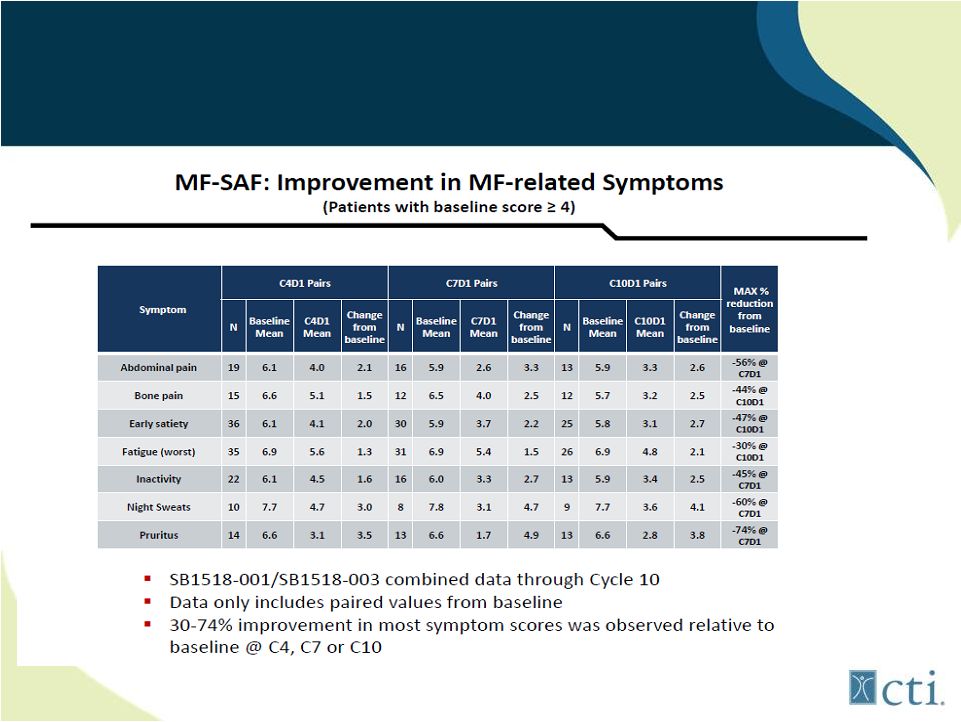 27
Pacritinib: Durable Improvement
in MF Related Symptoms
Source: ASH 2011. |
 28
Contemplated Phase 3 Trial Designs
Target Indication:
•
Patients with PMF, PPV-MF, PET-MF with splenomegaly and
thrombocytopenia
•
Two phase 3 trials, each approximately 270 patients
-
Pacritinib Vs BAT
-
Pacritinib Vs BAT including patients on low dose ruxolitonib
(5,10mg) with treatment emergent thrombocyopenia (<100,000/uL
platelets)
Target Initiation: Q4 2012
•
Estimated enrollment time-
12 months + 6 months follow-up
•
Potential topline results mid 2014
Contemplated Phase 3 Trial Design(s) |
 29
Pacritinib: Certain Key Deal Terms
Acquired molecule from S*Bio
•
S*BIO was established as a joint venture between The Singapore Economic
Development Board Investments and Chiron Corporation as Singapore’s first
fully- integrated, drug discovery company
Upfront payment $30mm
•
$15 million cash
•
$15mm total in stock
Up to $132.5mm in potential approval and sales milestones
Sliding scale, low single digit royalty on sales |
 30
A new class of cancer targeted agents
AADR inducers
30 |
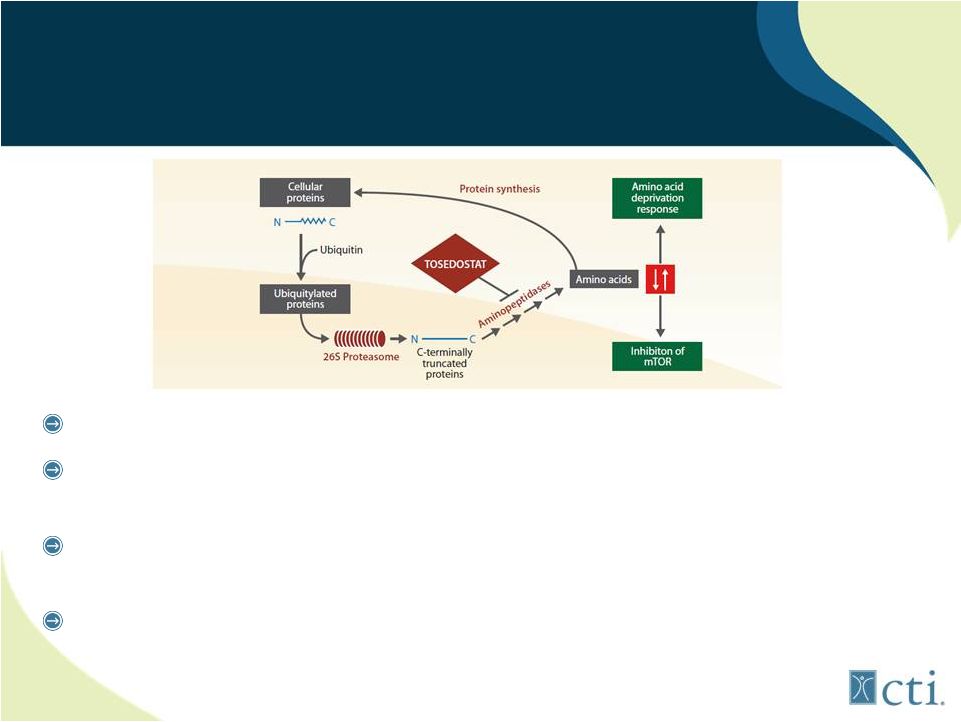 31
Tosedostat:
Novel Mechanism of Action AADR Inducer
Targets members of aminopeptidase family
Induces genes characteristic of Amino Acid Deprivation
Response (AADR) resulting in cell death
AADR changes only seen in transformed (tumor) cells and not
normal cells
Demonstrated clinical activity across range of solid and liquid
tumors |
 32
Tosedostat: OPAL Study Results
Encouraging Phase 2 data -
r/r elderly AML (n=73)
•
Oral once-a-day dosing for 6 months
•
16 of 50 (32%) evaluable patients had bone marrow response
-
9 (12%) of which were complete bone marrow responses
•
9 of 25 (36%) patients who previously failed therapy with HMA
had a bone marrow response
Well-tolerated
•
Most common grade > 3 febrile neutropenia (29%), fatigue (21%)
|
 33
Tosedostat: Phase 3 Study
Data support pivotal trial: high risk MDS/secondary AML
Phase 3 trial options:
•
Relapsed/refractory setting combination with LDAC
-
High risk heterogenous patient population
-
Several drugs currently targeting this population
•
1
st
line setting combination with HMA
-
Substantial investigator interest
-
Higher probability of success and shorter enrollment time
Ongoing phase 2 data (1
st
line HMA + tosedostat in MDS)
will drive decision in 2013 |
 34
Partnership with Chroma Therapeutics
Exclusive co-development and marketing rights in the Americas
•
Chroma Therapeutics has ROW rights
Cost-sharing
•
CTI bears 75% of agreed upon direct development expenses
•
Registration trials aimed at US/EU regulatory approval
Initial Payments
•
$5mm upon execution of license
•
$5mm upon initiation of first pivotal trial
Development and sales based milestones
Royalty rate based on net sales volume |
 35
Financial
Overview |
 36
Financial Overview
Exchanges / Tickers
•
NASDAQ: CTIC and MTA: CTIC.MI
Market Capitalization
•
~$125mm
Shares Outstanding
•
~56.6mm
Cash
•
$24mm
(1)
Debt
•
None
Projected Cash Burn
•
~$4.5mm / month
Cash Remaining
•
January 2013
Estimated
2013
Cash
Burn
(2)
•
$58mm net of Pixuvri product margin contribution
Targeted Cash Flow Break
Even
(2)
•
Q4 2014
Financing Need
•
~$75mm
of
additional
financing
is
needed
to
reach
potential
CFBE and
maintain a level of cash reserves
Capital Structure and Financial Statistics
(1)
Estimated cash balance as of August 1, 2012.
(2)
Assumes Pixuvri price reimbursement and market penetration within corridors and
percentages noted in Kantar Market Research and this presentation and also
assumes burn rate stays in line with expectations |
 37
Expected News Flow
Upcoming Planned Events
•
Pixuvri launch in the EU
•
Pacritinib phase 3 trial initiation in Myelofibrosis
•
Brostallicin-
SABC triple negative breast cancer
•
OPAXIO interim survival analysis Phase 3 ovarian cancer trial
•
IQWiG innovation score assignment
Upcoming publications
•
Journal Clinical Oncology
Pacritinib in lymphoma
•
Blood
Pacritinib in AML
•
Journal Clinical Oncology
Pacritinib MF phase 2
•
Journal Immunology
SB1578 in RA |
