Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - TAURIGA SCIENCES, INC. | Financial_Report.xls |
| EX-32.1 - CERTIFICATION - TAURIGA SCIENCES, INC. | nvnc_ex321.htm |
| EX-31.1 - CERTIFICATION - TAURIGA SCIENCES, INC. | nvnc_ex311.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
þ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended March 31, 2012
OR
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ______ to ______.
Commission File Number:000-53723
IMMUNOVATIVE, INC.
(f/k/a Novo Energies Corporation)
(Exact name of registrant as specified in its charter)
|
Florida
|
65-1102237
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(IRS Employer Identification No.)
|
|
|
417, Rue St-Pierre, Suite 804
Montreal, QC
|
H2Y 2M3
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
Registrant’s telephone number, including area code: (514) 840-3697
Securities registered under Section 12(b) of the Exchange Act:
None
Securities registered under Section 12(g) of the Exchange Act:
Common Stock, $.00001 Par Value
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. o Yes þ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.o Yes þ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the issuer was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. þ Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).x Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company filer. See definition of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).o Yes þ No
On September 30, 2011, the last business day of the registrant’s most recently completed second quarter, the aggregate market value of the Common Stock held by non-affiliates of the registrant was $11,781,807, based upon the closing price on that date of the Common Stock of the registrant on the OTC Bulletin Board system of $0.175. For purposes of this response, the registrant has assumed that its directors, executive officers and beneficial owners of 5% or more of its Common Stock are deemed affiliates of the registrant.
As of as of July 9, 2012 the registrant had 134,546,457 shares of its Common Stock, $0.00001 par value, outstanding.
TABLE OF CONTENTS
|
Page
|
|||||
|
PART I.
|
|||||
|
Item 1.
|
Business
|
4 | |||
|
Item 1.A.
|
Risk Factors
|
17 | |||
|
Item 1.B.
|
Unresolved Staff Comments
|
25 | |||
|
Item 2.
|
Properties
|
25 | |||
|
Item 3.
|
Legal Proceedings
|
25 | |||
|
Item 4.
|
Mine Safety Disclosures
|
25 | |||
|
PART II.
|
|||||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
26 | |||
|
Item 6.
|
Selected Financial Data
|
28 | |||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operation
|
28 | |||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
32 | |||
|
Item 8.
|
Financial Statements and Supplementary Data
|
33 | |||
|
Item 9.
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
|
33 | |||
|
Item 9A.
|
Controls and Procedures
|
34 | |||
|
Item 9B.
|
Other Information
|
34 | |||
|
PART III.
|
|||||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
36 | |||
|
Item 11.
|
Executive Compensation
|
37 | |||
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
39 | |||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
40 | |||
|
Item 14.
|
Principal Accounting Fees and Services
|
40 | |||
|
PART IV.
|
|||||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
41 | |||
| Signatures | 42 | ||||
| Exhibits | |||||
2
FORWARD LOOKING STATEMENTS
This report on Form 10-K contains forward-looking statements within the meaning of Rule 175 of the Securities Act of 1933, as amended, and Rule 3b-6 of the Securities Act of 1934, as amended, that involve substantial risks and uncertainties. These forward-looking statements are not historical facts, but rather are based on current expectations, estimates and projections about our industry, our beliefs and our assumptions. Words such as “anticipate,” “expects,” “intends,” “plans,” “believes,” “seeks” and “estimates” and variations of these words and similar expressions are intended to identify forward-looking statements. These statements are not guarantees of future performance and are subject to risks, uncertainties and other factors, some of which are beyond our control and difficult to predict and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this Form 10-K. Investors should carefully consider all of such risks before making an investment decision with respect to the Company’s stock. The following discussion and analysis should be read in conjunction with our consolidated financial statements for Immunovative, Inc. Such discussion represents only the best present assessment from our Management.
3
PART I
Background
We are a Florida corporation formed on April 8, 2001. We were originally organized to be a blank check company.
On June 8, 2009, the Board of Directors approved the change of name to “Novo Energies Corporation”. As described in a report filed with the U.S. Securities and Exchange Commission on June 26, 2009, a majority of shareholders executed a written consent in lieu of an Annual Meeting (the “Written Consent”) effecting the change of the name of our business from “Atlantic Wine Agencies, Inc.” to “Novo Energies Corporation” on June 8, 2009 to better reflect what we then intended to be our future operations. We filed an amendment to our Articles of Incorporation on June 8, 2009 with the Florida Secretary of State to affect this name change after receiving the requisite corporate approval.
On June 23, 2009, the Board of Directors approved a 3-for-1 forward stock split. Accordingly, all share and per share amounts have been retroactively adjusted in the accompanying financial statements.
On July 30, 2009, Novo Energies Corporation(“Novo”) formed a wholly-owned subsidiary - WTL Renewable Energy, Inc. (“WTL”). WTL was established as a Canadian Federal Corporation whose business is to initially research available technologies capable of transforming plastic and tires into useful energy commodities. Simultaneously, WTL also intended to plan, build, own, and operate renewable energy plants throughout Canada utilizing a third party technology and using plastic and tire waste as feedstock. On May 8, 2012, the name was changed to Immunovative Canada, Inc.
On May 17, 2011, Novo entered into an exclusive memorandum of understanding with Immunovative Clinical Research, Inc. (“ICRI”), a Nevada corporation and wholly-owned subsidiary of Immunovative Therapies, Ltd. (“ITL”), an Israeli corporation pursuant to which the Company and ICRI intended to pursue a merger resulting in Novo owning ICRI.
Present
License Agreement:
The planned merger with ICRI was dropped in favor of a licensing agreement with ITL. On December 12, 2012, the Company entered into a License Agreement (the “License Agreement”) with ITL, pursuant to which Novo received an immediate exclusive and worldwide license to commercialize all the Licensed Products based on ITL’s current and future patents and a patent in-licensed from the University of Arizona. The license granted covers two experimental products for the treatment of cancer in clinical development called AlloStim™ and Allo Vax™ (“Licensed Products”). Accordingly, Novo abandoned its endeavors into the clean energy business and changed its name to Immunovative, Inc. (“Immunovative,” or the “Company”) on May 8, 2012 to develop the next generation of immunotherapies to treat cancer
4
In exchange for the license, the Company has undertaken an obligation to provide the financial support for ITL to conduct a Phase II/III clinical trial designed to provide evidence in support of the efficacy of ITL’s lead Licensed Product called “AlloStimTM” (“The Pivotal Trial”). The Company committed to provide $10 million in support of the Pivotal Trial to be paid over a period from the date of the License Agreement until the date that is two years after receiving notice from a regulatory agency in the US, Canada, EU or Thailand of approval to commence the Pivotal Trial.
Upon successful completion of the Pivotal Trial, the Company and ITL have agreed to consummate a merger transaction (either directly or through a subsidiary of the Company) with ITL’s shareholders immediately prior to the merger owning 75% of the post-merger shares and the shareholders of the Company immediately prior to the merger owning 25% of the post-merger shares on a fully diluted basis. The successful completion of the Pivotal Trial shall be defined as the date that the treatment protocol for the number of evaluable subjects necessary to conduct a statistical analysis comparing a placebo control group with a Licensed Product is completed, whereby there is sufficient power to detect a statistically significant (p<0.10) increase in overall survival of 50% or greater of the Licensed Product as compared to the placebo.
The License Agreement provides that the percentage of the post-merger shares that the shareholders of the Company immediately prior to the merger may increase in certain circumstances, including if the Company provides ITL more than the $10 million set out in the License Agreement and if ITL has outstanding debt (excluding any liabilities owed to patent attorneys or for patent maintenance fees) at the time of the merger. Likewise the License Agreement provides that the percentage of the post-merger shares that the shareholders of the Company immediately prior to the merger may decrease in certain circumstances, including if ITL raises funds on its own or if the Company has outstanding debts at the time of the merger.
If there is a successful completion of a Pivotal Trial but the Company has not paid the full $10 million, the parties may agree to merge or the Company may receive shares of ITL based on the amount of funds the Company has provided ITL and the license will terminate. If there is not a successful completion of a Pivotal Trial and the Company decides to continue to fund the clinical trials, the Company will receive shares in ITL for any additional payments more than $10 million. In each of these instances, the shares that the Company will receive will be based on a valuation (prior to the funds provided by the Company) of ITL of $30 million, which can be decreased for any outstanding debts (with the exception of patent related debts and trade liabilities) of ITL or increased for any funds raised by ITL on its own.
If the Company pays all amounts due under the License Agreement, but there is no successful completion of a Pivotal Trial, The Company and ITL may nevertheless agree to merge. If they do not merge, the Company shall maintain the license granted under the License Agreement.
General Overview
The mission of the Company is to develop innovative biological drug products and treatment protocols which harness the power of the human immune system to provide patients with improved quality of life and curative or life-extending treatment options.
The Company serves as the commercial arm of ITL, a private Israeli biopharmaceutical company headquartered in Jerusalem, Israel. However, the Company’s current objective is to fund ITL sufficient funds to complete the Pivotal Trial and then consummate a merger. The intent is that the merged company would pursue the licensing and commercialization of the Licensed Products.
ITL was founded by Dr. Michael Har-Noy, ITL and has developed a novel approach for the treatment of cancer. The Licensed products are designed to mimic an immune mechanism already proven to be clinically effective in humans called the “graft vs. tumor” effect which occurs after allogeneic bone marrow transplant procedures while eliminating the need for a matched donor, chemotherapy conditioning and without any of the type of toxicity associated with transplant procedures. From this novel approach, two products called AlloStimTM (entering phase II/III) and AlloVaxTM (entering phase I/II) have been developed by ITL.
“Immunotherapy” is the science of harnessing the power of the human immune system to target and eliminate tumors wherever they reside in the body and then to protect against the return of the disease without further need for treatment.
5
Immunotherapy is an emerging type of cancer treatment that holds great promise and we believe is the only treatment method known that has the ability to hunt down and kill the very last microscopic tumor cell in the body. Surgery, chemotherapy, radiation and targeted therapies do not have this fine specificity at the single cell level. For this reason, immunotherapy is often referred to as the only known anti-cancer treatment that has a curative potential. In addition, cancer vaccine immunotherapy have been shown to have very mild side-effects, making cancer vaccines a welcome modality to patients that suffer from the side-effects of chemotherapy, radiation and surgery.
The Company has the worldwide, exclusive rights to commercialize AlloStimTM and AlloVaxTM, including any future improvements. AlloStimTM is a patented living cell product derived from normal blood donors. AlloVaxTM is an individualized anti-cancer vaccine derived from a sample of a patient’s own tumor.
AlloStimTM is the lead immunotherapy drug developed by ITL and licensed to the Company. Management believes that the available clinical data on the use of this drug supports that it may be the first immunotherapy drug with evidence that it can debulk chemotherapy-resistant metastatic cancer.
About Dr. Michal Har-Noy, ITL’s Founder
Dr. Michael Har-Noy has over 25 years of experience in immunotherapy drug development, GMP manufacturing and management of early stage biopharmaceutical operations. He is the author of numerous scientific publications and holder of numerous patents in the field of immunotherapy. He is the founder of ITL, an Israeli biopharmaceutical company focused on the development of minimally toxic cancer therapies where the active ingredient is living immune cells.
ITL was founded in May 2004 as a joint venture between the Israel Office of the Chief Scientist, Hadassah‐Hebrew University Medical Center Department of Bone Marrow Transplantation and Dr. Har-Noy. Dr. Har-Noy has lead ITL through pre-clinical toxicology/pharmacology studies, animal efficacy studies and human Phase I/II clinical trials on two immunotherapy drug candidates called AlloStim™ and AlloVax™. ITL operates a wholly-owned subsidiary, Immunovative Clinical Research, Inc., a Nevada Corporation, headquartered in Carlsbad, CA responsible for coordinating all worldwide clinical trial data analysis and GCP regulatory compliance. Prior to founding ITL, Dr. Har-Noy served as the President and CEO of MedCell Biologics from 1995‐2003 where he developed an immunotherapy for HIV/AIDS and Renal Cell Carcinoma that completed Phase I clinical trials.
Dr. Har-Noy attended an MD‐PhD program at Rush University Medical School in Chicago and conducted graduate and post-graduate studies at University of Minnesota, Harvard University Medical School‐Beth Israel hospital and the National Cancer Institute in the development of manufacturing technology for immune cells, such as LAK cells, TIL cells and other autologous cellular immunotherapies, development of immunomonitoring technology. Currently at Hadassah Medical Center, his research focus has been on investigating the mechanism of action of immune‐mediated graft vs. tumor and graft vs. host effects.
The Company announced on June 5, 2012, the publication of abstract reporting the results of the Company’s licensor, ITL’s Food and Drug Administration (“FDA”)-approved phase I/II clinical trial of 42 refractory metastatic solid tumor patients with a variety of indications. Although no objective tumor response was observed, there was evidence of enhanced survival and immune-mediated tumor debulking without graft-versus-host disease. Responsive Evaluation Criteria in Solid Tumors (RESIST) criteria which is usually used to determine response of chemotherapy drugs to cancer by measuring the change in size of tumors after treatment was reported to overestimate tumor burden after treatment with AlloStimTM, as responding tumors swelled and appeared larger on CT. This explained the reason why patients seemed to improve in health status and live longer after AlloStimTM treatment but were not scored as having objective tumor responses. While traditional RECIST criteria did not seem to accurately predict treatment response, multi-parameter analysis identified serum IL-12 as a biomarker predictor of enhanced survival. In the Phase I/II trial 50% were IL-12+ and survived a median of 211 days vs. 131 days for IL-12 negative patients (p < .009). Subset analysis determined that the patients with Her2+ metastatic breast cancer had the highest IL-12 response rate. The Abstract (Number e13013) titled: "Response of HER2+ Breast Cancer Patients to Allogenic Cell Immunotherapy" is now available on on-line at the American Society of Clinical Oncology Meeting ("ASCO 2012") website at: http://abstract.asco.org/AbstView_114_92017.html .
Immunovative, Inc. (“IMUN”) is a public Florida Corporation headquartered in Montreal, Canada and with sales offices located in Paris, Monaco and New York.
IMUN is responsible to fund the ITL’s pivotal Phase II/III clinical trial (estimated to require US$10 million) in late stage metastatic breast cancer to be conducted primarily at the National Cancer Institute of Thailand.
6
The Value Proposition
Management believes that the novel approach to treat cancer developed by ITL clearly represents a unique opportunity to fulfill an unmet medical need. Currently approved therapies to treat cancer provide minimal efficacy at the expense of high toxicity. The probabilities of success for a therapy providing significant survival benefits and little or no toxicity are extremely high in this sector where the efficacy hurdles for market approval are relatively very low.
IMUN has a locked value of $30 million to acquire its parent, ITL, in exchange of $10 million in financing.
The Business Model
ITL has GMP production facilities in Jerusalem. AlloStimTM is produced in batches from the blood of normal blood donors and aliquoted into single dose vials and stored frozen in liquid nitrogen as an intermediate product called T-StimTM. At current scale, one blood donor currently produces about 100 doses of T-StimTM. T-StimTM is believed to be stable for at least two years in liquid nitrogen. Prior to use in the clinic, T-StimTM is thawed and activated in a 4h process and then formulated into syringes and shipped by courier service to the point-of-care. There is no need to match a donor with a patient.
INVESTIGATOR-INITIATED CLINICAL TRIALS AND SUB-LICENSING
Under the Licensing Agreement, IMUN has the right to sub-license marketing rights to the Licensed Products. These products are believed to have broad applicability to a variety of cancer and infectious disease indications. In order to develop initial feasibility data on other indications, IMUN intends to establish collaborations with academic medical centers to conduct Phase I/II clinical studies. It is expected that the funding for a majority of the costs for these studies will come from grants or private donations to the academic medical centers. IMUN intends to use any positive data from these studies to support a sub-licensing program where the marketing rights to the indication will be sub-licensed to a marketing partner. These sub-licenses are intended to be negotiated by indication and by territory.
It is expected that the sub-licensee would fund continued clinical development in the indication an territory with the aim to obtain regulatory approval in the licensed territory. It is possible that such sub-licensing agreements could provide for upfront licensing fees, milestone payments or other strategic assets. It is also expected that such sub-licensing would include an agreement on royalties an/or transfer pricing which would provide revenues to IMUN upon successful commercialization.
Development Plans and Capital Requirements
IMUN plans to primarily be engaged in fund raising to support the $10 million requirement to conduct the Pivotal Trial over the next two years and subsequently to execute collaborative agreements with academic medical centers and then pursue a sub-licensing strategy. The main mode of fund raising is expected to be through sale of our common stock at a discount to our public price through sales to private and institutional investors. ITL will use the funding to support the completion of a pivotal, randomized, controlled, Phase II/III clinical trial of AlloStimTM in advanced metastatic breast cancer.
The pivotal breast cancer clinical trial will be conducted at the National Cancer Institute of Thailand (NCIT) located in Bangkok, Thailand. A complete Investigational New Drug Application (“IND”) application has been submitted to the Thai FDA and the NCI Institutional Review Board detailing the plans for this Phase II/III pivotal study. The trial is anticipated to be approved in the fourth quarter of 2012, with. first patient accruals in the first quarter of 2013. The Pivotal Trial is estimated to complete accrual in 24 months and the data is estimated to be mature for analysis after 32 months. There are many factors that can negatively influence these timing projections and many are not within our control (see “Risk Factors”).
7
After completion of the $10 million financing for the Pivotal Trial, additional funds will be required for scale-up of manufacturing and building of commercialization infrastructure. It is estimated that approximately $30 million will be required for this purpose. The Company intends to begin plans for raising this additional $30 million shortly after completion of the $10 million fund raising obligation for financing the Pivotal Trial. IMUN plans to merge with ITL upon successful completion of the Pivotal Trial. Management believes that if the survival data indicates statistically significant extension of survival of AlloStimTM vs. Placebo, that it may be possible to conduct an IPO at the time of, or shortly after, the completion of the merger. After the merger, IMUN will own 25% of the merged entity and 75% will be owned by ITL (subject to adjustment).
Management intends to list this newly merged entity on the NASDAQ market system concurrent with the announcement of successful pivotal trials. IPO will be sized to provide sufficient capital to finance the launch of the AlloStimTM product into the market. It is estimated that at least $100 million may be required for this purpose.
Once the overall survival end-point of the Pivotal Study is reached and a pilot manufacturing facility has been built and validated, a Biological License Application (BLA) is intended to be submitted in the third and/or fourth quarter of 2016 seeking US FDA marketing approval for AlloStimTM. Under expected fast track approval status, the BLA could be approved by as early as the first or second quarter of 2017.
OVERVIEW IMMUNOTHERAPY
Immunotherapy is a new modality for cancer treatment that potentially holds great promise for becoming a curative therapy with minimal toxicity. The human immune system is capable of seeking out and destroying cancers cells wherever they reside in the body. Harnessing the power of the immune system may hold one of the greatest potentials for winning the battle against cancer.
The immune system, if properly stimulated and educated, is capable of eliminating every last tumor cell. The concept of vaccine immunotherapy against cancer is based on the body's natural defense system, which protects against a variety of diseases. Vaccine immunotherapy has proven to be one of the most effective treatment strategies for prevention of certain infectious diseases. Vaccines using killed or attenuated pathogens have revolutionized public health by preventing the development of many important infectious diseases, including poliomyelitis, small pox, diphtheria, rabies, typhoid, cholera, plague, measles, mumps, hepatitis B, diphtheria toxin and tetanus. Application of these vaccination concepts to cancer could similarly potentially revolutionize cancer treatment.
However, attempts to develop cancer vaccines for treatment of existing tumors have proven to be much more difficult than developing vaccines for prevention of infectious diseases. Attempts to develop immunotherapies such as cancer vaccines, despite many decades of experimental work, have yet to consistently reach their curative potential in human clinical trials.
8
Immunotherapy Failure
There are two main reasons we believe why prior immunotherapies have failed in clinical trials in the past: the stimulation of the wrong immune response and the Inability to overcome tumor immunoavoidance mechanisms.
Stimulation of wrong immune response
Cancer patients by definition are immunocompromised, therefore many immunotherapy strategies have focused on methods to stimulate or strengthen the immune system in order to eradicate cancer. We believe this is a failed strategy because the immune system in fact does respond to cancer. Most tumors are infiltrated with large numbers of immune cells. Stimulating an immune response that has already failed to eradicate the tumor only enhances this wrong immune response.
Inability to overcome tumor immunoavoidance mechanisms
Attempts to educate the immune system to generate the correct immune response through cancer vaccination failed because they may not able to overcome the tumor’s ability to avoid the specific immune response created. Some tumors have evolved very sophisticated methods to avoid the correct immune response. Many tumors can avoid the immune system in the same manner as a fetus is protected from the mother’s immune system in the womb.
Despite all the previous failures of immunotherapies in clinical trials, there has been one major exception.
BONE MARROW TRANSPLANT
The one exception to the failure of immunotherapy protocols to produce significant anti-tumor effects in patients is the immune response that occurs in patients that undergo allergenic bone marrow/stem cell transplant procedures (BMT). BMT is a proven curative therapy for hematological malignancy and also has shown application for the treatment of solid tumors. The curative effect of BMT is mediated by transplanted immune cells derived from a normal (tissue matched) donor. The immune mediated anti-tumor effect of these transplanted immune cells is known as the graft vs. tumor (GVT) effect.
The powerful GVT immune response observed in BMT procedures is capable of overcoming tumor immunoavoidance mechanisms, resulting in complete systemic eradication of cancer in many instances. This GVT effect has been shown to be capable of curing patients with large tumor burdens, including patients with tumors unresponsive to chemotherapy, radiation and other forms of immunotherapy.
The GVT effect is believed to be the most powerful and most effective anti-tumor mechanism ever observed in the treatment of human malignancy. However, the same immune cells (primarily T-cells) which mediate GVT also mediate a serious and often lethal side effect known as graft vs. host disease (GVHD). This is due to the fact that the transplanted immune T-cells recognize both normal and tumor cells as foreign and mount attacks against both indiscriminately. Despite the curative effect of BMT, the severe treatment related toxicity has prevented the wide spread application of BMT for cancer treatment.
ITL’s products are based upon “Mirror Effect™” technology which is designed to provide the same anti-tumor effect that has been proven to be curative in allergenic bone marrow transplant (BMT) procedures without the lethal toxicity.
Technology Overview
The “Mirror Effect™” technology developed by ITL is based on products and methods using T-cells of the immune system.
9
T-CELLS OF THE IMMUNE SYSTEM
T-cells are part of the adaptive immune system of humans. T cells or T lymphocytes belong to a group of white blood cells known as lymphocytes, and play a central role in cell-mediated immunity. They can be distinguished from other lymphocyte types, such as B cells and natural killer cells (NK cells) by the presence of a special receptor on their cell surface called T cell receptors (TCR). Several different subsets of T cells are known, each with a distinct function.
T helper cells (Th cells) are a subset of T-cells that assist other white blood cells in immunologic processes, including maturation of B cells into plasma cells and memory B cells, and activation of cytotoxic T cells and macrophages, among other functions. These T helper cells are also known as CD4+ T cells because they express the CD4 protein on their surface. Helper T cells become activated when they are presented with peptide antigens by MHC class II molecules that are expressed on the surface of Antigen Presenting Cells (APCs). Once activated, they divide rapidly and secrete small proteins called cytokines that regulate or assist in the active immune response.
Memory T cells are another subset of antigen-specific T cells that persist long-term after an infection has resolved. They quickly expand to large numbers of effector T cells upon re-exposure to their cognate antigen, thus providing the immune system with "memory" against past infections. Memory cells may be either CD4+ or CD8+.
THE “MIRROR EFFECTTM” TECHNOLOGY
At ITL, technology has been developed to separate the beneficial GVT effect from the detrimental GVH effect of BMT procedures. ITL products and methods use T-cell infusions to elicit mechanisms that mirror the mechanisms mediated by an allogeneic BMT. This concept is called the Mirror Effect™. In the Mirror Effect™, T-cells from a normal donor are infused into a patient and instead of these foreign cells mediating the GVT effect, these cells instead stimulate the patient's own immune system to attack the tumor. This effect is known as the host-vs-tumor (HVT) effect and it is the mirror image of the GVT effect. The patient's immune system is alerted by the infusion of foreign cells and raises up to reject the foreign cells, an effect known as host-vs-graft (HVG). The HVG effect is the mirror image of the GVH effect. However, unlike the GVH effect the HVG effect is not toxic to a patient.

The reason that the GVT effect maybe such a powerful immune mechanism for curing cancers is that the interaction between the host and donor creates the release of an array of inflammatory cytokines that signal the body of an imminent danger. These danger signals shut down the ability of the tumor to avoid an immune attack and enable immune-mediated killing of tumors disseminated throughout the body. The Mirror Effect™ creates these same danger signals in the context of a rejection response to a foreign cell infusion (HVG) rather than as an attack against normal tissues (GVH). Thus the Mirror Effect™ has the potential to cause a proven curative anti-tumor effect of BMT without the extremely toxic side-effects. This may represent a new concept in the treatment of cancer.
10
The products developed at ITLgenerate the Mirror Effect™ in patients by utilizing immune cells from healthy donors. T-cells from healthy donors are activated and administered to a patient who has not been immunosuppressed.All of the therapies developed at ITLare dependent on the patient having a robust immune system because the desired Mirror Effect™ is based on the rejection response elicited by the patient’s immune system against the administered donor T-cells. The donor T-cells are not HLA-matched to the patient and it is preferable that the HLA-mismatch is maximized between donor T-cells and the patient.
Activation of T-cells occurs by engagement of cell surface proteins, i.e. CD3 and CD28, on the T-cells. Binding of the CD3 protein and costimulation of the CD28 protein on the CD4+ T-cells is required for initiating an effective T-cell mediated immune response. Although activation of T-cells is well known in the art, the methods of activating T-cells at Immunovative are unique and have been patented and result in T-cell compositions with unique characteristics that have also been patented.
The procedures used at ITLresults in enhanced activation of the T-cells. “Activated” T-cells at ITL have one or more agents, i.e. anti-CD3 and anti-CD28 antibodies, bound to the T-cell surface proteins and these bound agents are in turn, cross-linked by the use of a cross-linking agent. The cross-linking agent can be attached to a support.
Product Details:
The main products in the product portfolio of ITLare AlloStim™ and AlloVax™. The process of developing these products involves a separate intermediate product called T-Stim™. T-Stim™ and AlloStim™ are products of two sequential production processes. AlloVax™ is a customized vaccine product in which the disease antigens of a patient are administered along with AlloStim™.
ALLOSTIM™
AlloStim™ is the product of two sequential production processes:
1.T-Stim™
T-Stim™ cells are produced ex-vivo in a 9 day proprietary culture process (patents pending) in a bioreactor. T-Stim™ cells are produced from CD4+ CD45RA+ naïve T-cell precursors that are purified from the blood of normal donors.
After the 9 days in culture approximately 10 billion T-Stim™ cells are produced. There is no need to match the donor to the recipient as is required in BMT procedures. The AlloStim™ product is an intentional mismatch to the recipient.
The T-Stim™ production process involves:
|
I.
|
Purifying precursor immune cells from the blood (from leukapheresis source material) of normal screened donors;
|
|
II.
|
Culturing the precursors in the presence of custom monoclonal antibodies (mAbs) conjugated to a biodegradable tissue-like matrix simulating a lymph node;
|
|
III.
|
Aliquoting the resulting cultured T-Stim™ cells into single dosage forms;
|
|
IV.
|
Freezing the aliquoted T-Stim™ cells in liquid nitrogen for long-term inventory storage.
|
In culture, the mAbs (anti-CD3 and anti-CD28) together with the cell-to cell contact due to the high density of the cells in culture, causes the activation, expansion and maturation of the naïve CD4 cells. The cells expand approximately 100-fold over the culture period resulting in one donor producing enough T-Stim™ cells for approximately 100 doses.
11
Under these controlled conditions, T-Stim™ cells mature into memory CD4 cells that produce extraordinary high amounts of IL-2, IFN-gamma and TNF-alpha upon activation. Additionally, T-Stim™ cells express high density CD40L and FasL on the cell surface. CD40L interacts with CD40 expressed on innate immune cells and activates these cells to produce Th1 cytokines and kill tumors. FasL enables these cells to directly kill tumors.
The frozen product can be distributed through hospital pharmacies and remains stable for over 24 months.
|
I.
|
Activation
|
In order to produce the Mirror Effect™ in a patient, the T-Stim™ cells require activation prior to infusion into the patient. The second production process involves converting T-Stim™ to AlloStim™. T-Stim™ cells are activated with monoclonal antibody-coated particles that remain attached during infusion. When T-Stim™ cells are activated and formulated for infusion the product is called AlloStim™.
ALLOVAX™
AlloVax™ is a new product under development that combines AlloStim™ with a vaccine formulation containing chaperone proteins (also known as heat shock proteins) isolated from a sample of a patient’s cancer. This product is applicable to patients with both solid tumors and hematological (blood) cancers that have been recommended for first line chemotherapy/radiation treatment to induce remission. A high percentage of patients can be induced into remission with first line chemotherapy/radiation, but almost all patients eventually have a recurrence of the cancer. There is no curative therapy for patients that have a recurrence of a blood cancer except for allogeneic bone marrow/stem cell transplant (BMT) procedures. However, the high toxicity associated with the procedure, requirements for a matched tissue donor and the often lethal side effects limit the clinical application.
AlloVax™ is individualized anti-cancer vaccine that is designed to educate the immune system during the period of cancer remission to prevent the tumor from recurring. Chaperone proteins isolated from cancer cells have been shown to carry hidden tumor antigens that can be used to educate the immune system to identify and kill the cancer cells from which they were derived. ITL has purchased the exclusive rights to a patented process developed by Dr. EmmanualKatsanis and scientists at the University of Arizona to purify these chaperone proteins from a small sample of tumor tissue. (See U.S. patent no. 6,875,849).
The AlloVax™ treatment protocol involves first removing a sample of the cancer cells from the patient prior to chemotherapy/radiation therapy. After the patient is in remission, the patient receives intradermal injections of AlloStim™ which are rejected by the patient immune system making the patient immune to the AlloStim™ cells. The cancer sample obtained prior to chemotherapy/radiation treatment is then processed in the laboratory to isolate the chaperone proteins containing the unique tumor antigens. The chaperone protein sample is then combined with AlloStim™ and injected intradermally. In this setting, AlloStim™ acts as an adjuvant for the chaperone protein vaccine formulation. This causes an immune response to reject the AlloStim™ and an immune response against the unique tumor antigens carried by the chaperone proteins. The combination of these immune responses serves to educate the immune system that the tumor antigens are a danger to the body. In this manner, if the tumor recurs the immune system is primed to destroy the tumor without any further treatment in order to keep the patient in remission.
Patent Portfolio Protection Summary:
The patent portfolio of ITL includes a number of claims in different patents and patent applications that protect T-Stim™ and AlloStim™. These claims cover a number of embodiments including compositions of activated T-cells, methods of making these compositions and methods of using these compositions to elicit the Mirror Effect™ in a patient. Some of the broader embodiments of the claims encompass engendering the Mirror Effect™ or aspects of the Mirror Effect™ by using allogeneic cells. Claims have been pursued that are tiered as well as overlapping in order to maximize the protection of Immunovative products. Claims are still being prosecuted that encompass different aspects of these unique cell products.
12
Clinical Trials:
PRIOR HUMAN DATA
Protocol planned for use in taxane-anthracycline metastatic breast cancer (MBC) has successfully completed a Phase I/II clinical trial in 42 advanced metastatic cancer patients, including 16 MBC patients, under a US FDA cleared IND application. The results from this clinical trial provided radiological, pathological and immunological evidence of immune-mediated anti-tumor killing (debulking) activity correlating with survival. 11 of these 42 patients (26%) survived over 1 year, with 9 of the 42 (21%) alive at 18 months. Of the 16 MBC patients accrued in the trial, 5 of the 16 (31%) were still stable and surviving after 1 year. The patient population in this trial were heavily pre-treated, high disease burdened, low performance status metastatic cancer patients that had exhausted all standard treatment options and have a life expectancy of less than 60 days. The average ECOG score (0-4) at baseline was 2.143 ± 0.1208 (N=42). At 60 days, the average ECOG score improved significantly (p< 0.0001) to 1.250 ± 0.1421 (N=40). The Kaplan-Meyer curve for all accrued and evaluable patients in the Phase I/II clinical trial is shown below. Patients were accrued between September 2009 and May 2010 and followed for survival until May 2011.

PATIENTS CHARACTERISTICS
The average age was 60.5 years (range 44-89 years) with 18 male and 24 female. Patients were heavily pre-treated with an average of 2.7 lines of prior chemotherapy with an average of 7 prior courses, 52% had prior radiotherapy and 90% had prior surgical excision of either the primary tumor or metastatic disease lesions. The most common indication was breast cancer (38%), followed by colorectal cancer (16.6%) and also including metastatic ovarian, sarcoma, squamous cell carcinoma, lung, bladder/ureter, pancreas, melanoma and esophageal cancers. The patients had high tumor burdens with an average of 21.2 metastatic lesions per patient (patients with innumerable lesions in an organ were scored as 10 lesions). The most common metastatic tumor sites were lung/pleural (63% of accrued patients), liver (63%), lymph node (53%) and bone (50%). In addition, 60% of patients presented with pleural effusion and/or atelectasis and 13% with malignant ascites.
The following table presents details about the clinical trial design as well as the patients characteristics prior to initiating the therapy.
13
Clinical Trial Design:

Next Milestones:
ALLOSTIMTM
PHASE II/III:Metastatic Breast Cancer
A pivotal metastatic breast cancer clinical trial will be conducted at the National Cancer Institute of Thailand (NCIT) located in Bangkok. A complete IND application has been submitted to the Thai FDA and the NCI Institutional Review Board detailing the plans for this Phase II/III pivotal study.
The trial is anticipated to be cleared in the fourth quarter of 2012, with the accrual beginning first in the fourth quarter of 2013 and completed by the fourth quarter of 2014.The survival data is expected to mature and possibly hit statistical significance by the fourth quarter of 2015.
Trial Specifications:
|
·
|
Randomized, controlled clinical trial anthracycline/taxane resistant metastatic breast cancer
|
|
·
|
InSituVaxTM protocol : AlloStimTM(cells + beads) vs. beads alone combined with ablation
|
|
·
|
208 patients randomized 1:1 treatment vs control
|
|
·
|
Powered to detect 80% overall survival advantage (control=8 months)
|
ALLOVAXTM
PHASE I/II: Advanced Head & Neck Cancer
AlloVaxTM is expected to be submitted at the Thai and US FDA during the the fourth quarter of 2012. The trial design proposed is for a phase I/II randomized, placebo-controlled clinical trial of advanced head and neck cancer. The study is expected to last 1 year and will accrue 52 patients randomized 3:1 treatment to placebo. It will be conducted at the National Cancer Institute of Thailand.
Funding for this trial is anticipated to be covered under the same $10 million IMUN will pay for the Pivotal Trial.
14
Overall Cancer Situation/ Recent Trends
Over recent decades, the incidence of cancer has escalated to epidemic proportions, now striking nearly one in two men (44%) and more than one in three women (39%). This increase translates into approximately 56% more cancer in men and 22% more cancer in women over the course of a single generation. The National Cancer Institute estimates that the number of cancer cases will increase still further because of the growth and aging of the population, dramatically doubling by 2050.
Despite decades of research and new treatment approaches, reversal in overall mortality rates has been minimal and due largely to a reduction in lung cancer deaths from reduced smoking in men, rather than to advances in treatment. Overall five-year survival rates for all cancers have remained virtually static since 1970, from 49 to 54 percent for all races combined.
Current therapies for cancer have significant emotional and physical side effects. Many patients view the treatments to be worse than the disease. The monetary costs for treating cancer are also staggering. The annual direct costs of cancer treatment has more than quadrupled over the past 20 years from $18 billion in 1985 to $41 billion in 1995 to over $120 billion in 2010. Additionally, indirect costs from loss of wages, taxes, earnings and productivity were estimated at $100 billion in 1999.Cancer costs are projected to reach $158 billion, in 2010 dollars, by the year 2020, because of a growing population of older people who are more likely to develop cancer.
Each year, 226,000 women develop breast cancer in the United States and 30% of women with early stage breast cancer may eventually develop metastatic breast cancer. The 5-year survival rate is 21% for women with metastatic cancer and 96% without metastatic cancer. Traditional first/second line treatments still result in recurrences in 50% of women with metastatic breast cancer. These individuals are the targets of the first Clinical Trial.
PROBLEMS WITH CURRENT THERAPIES
Despite dramatic advances in our understanding of cancer cell biology, conventional cancer therapy has remained fundamentally unchanged for decades. The three major forms of cancer therapy remain to be surgery, radiation and chemotherapy. Surgery and radiation therapies have reached their logical limits, and chemotherapy remains the current mainstay of cancer management.
Most chemotherapy drugs are broad-spectrum cytotoxic agents. These drugs are designed to inflict greater damage on cancer cells than on normal cells. Nonetheless, all chemotherapy drugs affect normal cells and cause severe side effects. Chemotherapy is an attempt to kill the tumor before the drug kills the patient.
The major limitation of all current cancer therapies is the inability to eliminate the last tumor cell. This means that current cancer therapies, for the most part, can only extend survival but rarely can actually cure the disease. While current therapies can often initially eradicate measurable evidence of disease, they generally fail to eliminate all the tumor cells. Therefore, any remaining cells may proliferate and cause a relapse of cancer. In this common scenario, the first set of remaining cells has resisted chemotherapy/radiation. The offspring of these tumor cells that were not destroyed by the chemotherapy/radiation have a selective advantage, leaving the person with a recurrence of cancer that is often widespread and resistant to chemotherapy/radiation and other techniques.
Breast Cancer
Breast cancer is the most prevalent cancer in women worldwide and is also the principle cause of cancer death among women. In 2010, nearly 1.5 million women were newly diagnosed with breast cancer (Worldwide Breast Cancer, 2011). Breast cancer accounts for about 10% of worldwide cancer.
15
According to the National Cancer Institute, in 2012 for the United States only, the number of new breast cancer cases for females is expected to reach 226,870 (2,190 males) with expected related deaths of 39,510 (410 males) (NCI, 2012). From a daily perspective, this represent 621 new female breast cancer cases and 108 related deaths every day.
North America, Eastern and Northern Europe, together with Australia and New Zealand have the highest breast cancer incidence rates worldwide, making these countries an attractive opportunity for a novel treatment approach such as AlloStimTM. The world map below presents the number of new breast cancer cases occurring worldwide per 100,000 of population.
Map: number of new breast cancer cases occurring worldwide per 100,000 of population.

Despite innovative therapeutic advances such as the introduction of molecularly targeted therapies Avastin, Herceptin, and Tyverb/Tykerb, there is still a clear unmet need across all lines of therapy in the metastatic setting. This, combined with the potential size of the patient population, strong advocacy framework, and treatment across multiple lines of therapy, makes breast cancer a very attractive commercial opportunity.
Metastatic Breast Cancer
Breast cancer can be divided in different sub-types of breast cancers, with a great variability in the disease characteristics, development rate and survival rate for each of them. Even if there is not a single type of cancer someone would wish for, metastatic breast cancer is certainly one of the most devastating and feared types of cancer.
Nearly 30% of women with early stage breast cancer will eventually develop metastatic breast cancer (O’Shaughnessy, 2005). According to theAmerican Cancer Society statistics (2000-2011), despite all the medical advances and decrease in cancer overall mortality rates over the past 20 years, deaths caused by metastatic breast cancer have remained stable with around 40,000 victims each year. The median survival rate after metastatic breast cancer diagnosis is 3 years and there has been no statistically significant improvement since the 1990’s (American Society of Clinical Oncology [ASCO] Report - 2008).
16
Inefficient Therapies
ITL will target anthracycline-taxane resistant metastatic breast cancer in the pivotal Phase II/III clinical trial for AlloStimTM. Anthracyclines and taxanes are the most active and widely used chemotherapeutic agents for treating breast cancer in hormone receptor-negative patients and those whose disease progresses while they are taking hormone therapy. These agents are commonly used in the adjuvant setting (after surgical removal of a lump or the breast), either in combination or sequentially. Incorporating taxanes into anthracycline-based regimens significantly improves disease-free survival and overall survival (OS) rates.
This benefit is evident regardless of hormone receptor status, degree of nodal involvement, age, menopausal status, and type of taxane or administration schedule. Anthracyclines and taxanes, either alone or in combination, are also the preferred option for hormone receptor-negative patients with metastatic breast cancer (MBC). Response rates of 25% to 69% have been reported when taxanes (paclitaxel or docetaxel) are used as first-line treatment of MBC.
Accordingly, anthracycline- and taxane-containing regimens have been established as the most effective chemotherapeutic agents for first- and second-line therapies in MBC. Because of this, more women are receiving anthracyclines and taxanes early in the treatment of the disease. Even despite this recent trend toward aggressive treatment of early stage breast cancer with anthracyclines and taxanes, nearly half of these women will have metastatic recurrence. The prior exposure to anthracycline and taxane drugs leaves these women facing first-line therapy for metastatic disease resistant to anthracycline and taxane drugs and, thus, with limited treatment options. Anthracycline-taxane resistant metastatic breast cancer is an unmet medical need. There are no effective treatment options available for these women. This is the target of ITL’s pivotal trial.
Competitive Landscape
MARKET SIZE & PARTICIPANTS
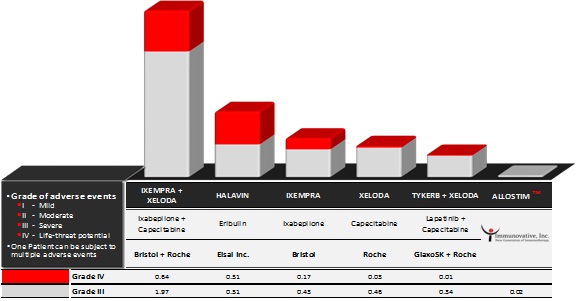
The market for currently commercialized third-line treatments for anthracycline/taxane resistant metastatic breast cancer represents close to $2.2 Billion in annual sales and is led by 4 fours companies (products): Roche (Xeloda), GlaxoSmithKline (Tykerb), Bristol Myers Squibb (Ixempra), Eisai Inc. (Halavin)
ITEM 1A. RISK FACTORS
The following important factors among others, could cause our actual operating results to differ materially from those indicated or suggested by forward-looking statements made in this Form 10-K or presented elsewhere by management from time to time.
There are numerous and varied risks, known and unknown, that may prevent us from achieving our goals. If any of these risks actually occur, our business, financial condition or results of operation may be materially adversely affected. In such case, the trading price of our common stock could decline and investors could lose all or part of their investment.
17
Risks Related to Our Business
Early Stage Biopharmaceutical Research Company
The success of the Company’s business plan depends not only on its own success, but also on the success of ITL. ITL is in the early stages of development, faced with all the initial and inherent risks associated with the commencement of a new business. To date, ITL has not generated any material revenues. ITL will continue to conduct research activities, including human clinical trials, for an additional at least three years before having the possibility of realizing any material revenues from operations. ITL's technology and research activities may never lead to a successful therapy for human disease, and there may never be any revenues generated from those activities. To date, ITL's resources have been dedicated to the research and development of the AlloStimTM and AlloVaxTM products.
The commercialization of AlloStimTM and AlloVaxTM will require significant additional investment, research and development, clinical testing, and regulatory approvals. ITL and the Company may not be able to develop, produce at a reasonable cost, or market AlloStimTM or AlloVaxTM successfully. Further, AlloStimTM or AlloVaxTM may prove to have undesirable or unintended side effects that may prevent or limit the commercial use and stability. AlloStimTM and AlloVaxTM will require regulatory approval before they can be commercialized and will be subject to regulatory oversight upon commencement of commercial use. If any products are ultimately developed, they may not generate substantial revenues and the Company may never be profitable.
The Company’s business could suffer if the FDA does not lift the hold it has placed on AlloStimTM
On September 26, 2011, ITL received a letter from the U.S. Department of Health & Human Services, FDA in which the FDA proposed to terminate the IND related to AlloStimTM. If that IND is terminated or the clinical hold is continued, ITL will not be able to proceed with clinical trials in the United States. ITL provided responses to the FDA in January 2012 and in June 2012 and does not believe that the IND will be terminated. However, the clinical hold placed by the U.S. FDA still remains in place.
ITL plans to continue with clinical phase II/III trials for AlloStimTM in other jurisdictions outside the US and, plans to conduct these foreign trials under a U.S. IND. However, a U.S. IND is not required to initiate the foreign trials and ITL believes that as long as the clinical trials are conducted under international standards for Good Clinical Practices (“GCP”), and meet FDA regulations for acceptance of foreign clinical data (21 CFR 312.120) that the results could be used at a later date in support of a marketing application to the U.S. FDA even if the clinical hold remains on the current IND.
However, there may be negative perceptions from investors and potential licensing partners with respect to the regulatory status of ITL's products with the U.S. FDA. These negative perceptions could have serious negative impact on the ability of ITL to advance its clinical development and for the Company to raise funds for this advancement. There are no provisions in the License Agreement for a reduction in terms if the FDA terminates the IND or continues the clinical hold.
We require additional financing to meet our obligations under the License Agreement, to fund our operations and to commercialize the product.
The Company needs significant additional financing to meet the terms of the License Agreement and to reach the stage of commercialization of at least one product candidate. In addition to funds necessary to support ITL’s clinical trials, we will also have to raise funds to continue the Company’s operations. We may not be able to raise such funds on favorable terms, if at all.
If clinical tests establish both the safety and efficacy of AlloStimTM in phase II/III clinical trials, significantly more capital will be required to manufacture and market the product. Significant capital requirements will be required for each indication that the Company may seek regulatory approval. If adequate funds are not available, the Company may lose the exclusive license or be required to delay, scale back or eliminate one or more of product development programs, or obtain funds through arrangements with collaborative partners or others that may require the Company to relinquish rights to certain of its products, product candidates or technologies that the Company might not otherwise relinquish.
Other unfavorable factors may also require the Company to seek additional financing, such as unexpected extended pre-clinical or clinical data required by the FDA, difficulty in obtaining regulatory approvals, competition, unforeseen market developments, unforeseen or unexpected difficulties scaling-up and manufacturing AlloStimTM or AlloVaxTM, or unexpected expenditures relating to its operations. Any of these could result in the Company needing more funds than anticipated, which could add significant unexpected dilution to existing shareholders. The Company cannot assure investors that it will be able to raise any required additional funds, or that even if the Company will be able to raise those funds that they can be raised in a timely manner, or on terms which are favorable to the Company.
18
Dilution.
Investors may experience dilution due to future equity issuances and will experience dilution if the Company completes a merger with ITL.
There is a need for significant additional financing to meet the terms of the License Agreement. We must raise significant funds to meet our outstanding obligations under the License Agreement, and intend to do so through the use of sales of our equity. Additionally, we will have to raise funds to continue the Company’s operations and to eventually commercialize ITL's technology. We do not know the terms of any future placements of equity, but any future placements at a purchase price below current market value on a per share basis will dilute the value of current shareholders investments.
Under the terms of the License Agreement, the Company and ITL will merge upon the successful completion of clinical trials. In such an instance, the shareholders of the Company at the time of the Merger will own 25% of the post-merger company, subject to certain adjustments. The investors in this placement will experience a dilution in their share ownership of the Company and, depending on the net tangible book value of ITL at the time of the merger, may experience a dilution in their net tangible book value per share. If there has been a successful completion of clinical trials and the merger does not occur, we will need to raise additional funds to commercialize the products and such raises will likely further dilute the equity ownership of existing stockholders.
Technologies Ultimately May Not Prove Successful
ITL will focus its research and development activities on areas that are highly technical and extremely advanced. As a result, the outcome of any research and development and clinical testing programs are highly uncertain. Historically, only a very small fraction of such programs sponsored by pharmaceutical or biotechnology companies ultimately result in commercial products. Product candidates that initially appear promising, often fail to yield successful products after human clinical testing. In many cases, clinical studies will show that a product candidate does not work, or that it raises safety concerns, or has other side effects that outweigh the intended benefit. Success in pre-clinical or early clinical trials that generally focus on safety issues may not translate into success in large-scale clinical trials designed to demonstrate statistically significant efficacy for reasons that often are not fully understood. Even after a product is approved and launched, general usage or post-marketing studies may identify safety or other previously unknown problems with the product that may result in regulatory approvals being suspended, limited to more narrow indications, or revoked.
The Company’s success relies upon IT.L
The Company’s success relies on the successful execution of clinical trials by ITL. How ITL conducts its clinical trials, appoints, retains and replaces management, handles its expenses and operates other matters related to its day-to-day business are outside of the Company’s control. If ITL mishandles any of these matters, the Company does not have the authority or capability to correct such mistakes.
Key Employee.
The Company is materially dependent upon the continued service, technical knowledge, expertise and abilities of Dr. Michael Har-Noy, as founder and Chief Executive Officer for ITL. If the services of Dr. Har-Noy should become unavailable for any reason, business would be seriously and adversely affected. Dr. Har-Noy currently does not have an employment contract with the ITL nor any key man life insurance.
Biomanufacturing.
The FDA has determined that the current product candidates will be classified as biological drugs. Manufacturing of biological drugs is complex. Unlike traditional chemical pharmaceutical products, a biological drug cannot be characterized in terms of its physical and chemical properties to a degree that would enable an assay of the finished product alone to ensure that the product will perform in the intended manner. Accordingly, it is essential for the manufacturing of a biological drug to be able to both validate and control the manufacturing process in order to demonstrate that the finished product is made strictly and consistently in compliance with pre-established product release criteria. Slight deviations in the manufacturing process may result in unacceptable changes in the final products resulting in rejection of the finished product. For these reasons, manufacturing of biological products is subject to extensive government regulations.
Manufacturing processes which are used to produce smaller quantities of biological products for research and development purposes or initial small clinical trials can not always be successfully scaled-up to allow production of commercial quantities of the product at a reasonable cost, or at all. All of these difficulties are compounded when dealing with novel biological products that require novel-manufacturing processes such as ITL intends to employ. Even minor changes in the manufacturing process may require regulatory approval, which in turn may require additional clinical testing.
ITL could encounter technical problems during the design, scale-up and validation of its planned cell processing system. ITL has attempted to anticipate potential problems in the scale-up of its cell processing system design. However, it is possible that unanticipated problems with the manufacturing system could delay the planned start of clinical trials and could cause ITL to run out of cash before completing the validation of its cell processing system and the clinical trials
Even if ITL is successful in validating a cell processing system, it cannot be assured that the system will be able to produce product from every donor’s blood, or even a majority of donor’s blood. It is possible that some patients may require multiple infusions of cells, increasing its manufacturing costs. It is also possible that ITL could expend considerable funds in attempts to produce product from some donor’s blood and not be successful in producing a product.
19
Dependence Upon Suppliers
Some of the reagents and components ITL intends to use in its proposed cell processing system are purchased from a single source. It is possible, due to the time and expense it might take to change a component or reagent and re-validate the manufacturing process, and receive regulatory approval for the change, that vendors could take advantage of their single source status and raise the price of their products or delay delivery of their products, forcing ITL to accept inflated vendors’ terms. To mitigate this risk, ITL plans to attempt to validate second sources of critical reagents. However, ITL cannot assure that it will be able to second source all the reagents and components used in its manufacturing process.
Need for Technical and Management Personnel
The Company and ITL will need to hire technical and management personnel with relevant biopharmaceutical experience to assist the Company in its efforts to develop and commercialize its products. People with the requisite technical and management background are in high demand and may be difficult to find, hire and retain. The Company’s inability to recruit such personnel could have a materially adverse effect on its ability to develop, manufacture and test its proposed products or obtain the necessary regulatory approvals or raise additional financing.
Government Regulation
The Company’s proposed production of biological products for use in the treatment of human diseases and disorders is subject to extensive regulation by the FDA, as well as comparable agencies in foreign countries. The process of obtaining regulatory approvals, which may eventually allow the Company to produce and market its proposed products, will be time consuming and expensive. The Company cannot assure that such approvals will be granted. In addition, even if approval is granted, it could be limited, or it could be withdrawn for any number of reasons, including its failure to comply with certain regulatory standards. Currently, the testing of ITL's products in the United States is on clinical hold. There is no assurance that the clinical hold will be lifted in a timely manner or ever.
Assuming the Company eventually engages in the commercial production of AlloStimTM or AlloVaxTM, the FDA and various State and foreign regulatory agencies will inspect the Company and its facilities from time to time to determine whether the Company is in compliance with regulations relating to manufacturing practices of biological products, including validation, testing and quality control data and documentation. A determination that the Company is in violation of any such regulations could lead to the imposition of civil penalties, including fines, product recalls or product seizures and, in extreme cases, criminal sanctions.
Patents and Licenses
The pharmaceutical industry places considerable importance on obtaining patent and trade secret protection for new technologies, products and processes. Its success will depend in large part on its ability to file for and obtain patent protection for many of its principal products and procedures, to defend existing or future patents, to maintain trade secrets, and to operate without infringing upon the proprietary rights of others. ITL has applied for several patents involving compositions, methods and uses relating to AlloStimTM and AlloVaxTM in the United States and in certain foreign countries. The Company cannot assure that a patent will be issued in response to all these applications.
Patent protection is highly uncertain and involves complex legal and factual questions and issues. The patent application and issuance process can be expected to take several years and will entail considerable expense. ITL cannot assure that patents will issue as a result of any applications or that the existing patents, or any patents resulting from such applications, will be sufficiently broad to afford protection against competitors with similar or competing technology. Patents ITL obtains may be challenged, invalidated or circumvented, or the rights granted under such patents may not provide the Company with any competitive advantages.
A United States patent application is maintained under conditions of confidentiality while the application is pending, so the Company cannot determine the inventions being claimed in pending patent applications filed by third parties, if any. Litigation may be necessary to defend or enforce its patent rights or to determine the scope and validity of the proprietary rights of others. Defense and enforcement of patent claims can be expensive and time consuming, even in those instances in which the outcome is favorable, and could result in the diversion of substantial resources and management time and attention from its other activities. An adverse outcome could subject Immunovative to significant liability to third parties, which shall require the Company to obtain additional licenses from third parties, require the Company to alter its products or processes, or require that the Company cease altogether any related research and development activities or product sales.
Competition
Pharmaceutical and biotechnology companies which have far greater technical and financial resources than the Company are known to be conducting research into cures and therapies for diseases targeted by the Company. Many of these competing entities are developing alternative immunotherapy. Even if the Company is successful in developing AlloStimTM for disease, any one of the competing pharmaceutical or biotechnology companies could at any time develop a cure or therapy which is superior to, or comparable and less costly than, any therapy developed by the Company.
Lack of Public Market; Limited Liquidity and Transferability; Offering Price
There is currently limited market for any of the Company’s shares, and no assurances are given that a market will develop or, if such a market develops, that it will be sustained with sufficient liquidity to permit investors to sell their Shares at any time. Accordingly, investors may have difficulty in selling their Shares in the future, and the Company can give no assurance that the Shares can ever be resold at or near the offering price, or at all, even in an emergency. Investors must be prepared to hold the Shares for an unlimited period of time.
Authorization of Share Rights
The Company’s Articles of Association authorize the issuance of shares with such rights and the board of directors may determine preferences as from time to time. Accordingly, the board of directors may, without shareholder approval, issue capital stock with dividend, liquidation, conversion, voting or other rights that could adversely affect the voting power or other rights of the holders of the Company’s ordinary stock. In addition, the issuance of such capital stock may have the effect of rendering more difficult or discouraging an acquisition of the Company or changes in control of the Company. Although the Company does not currently intend to issue any special rights shares, there can be no assurance that the Company will not do so in the future.
Product Liability
The Company may, during clinical development and in the future, be subject to claims for product liability. The Company anticipates that the Company will be named on ITL’s clinical trial and product liability insurance at such time as its operations warrant such coverage. A successful claim against the Company in excess of any such coverage could have a material adverse effect on the Company. Additionally, such insurance is expensive and may not be available to the Company in the future on reasonable terms, if at all.
The Company has not paid dividends to date and does not intend to pay any dividends in the near future.
The Company has never paid dividends on its common stock and presently intends to retain future earnings, if any, to finance the operations of our business. You may never receive any dividends on the shares.
20
We have sustained recurring losses since inception and expect to incur additional losses in the foreseeable future.
We were formed on April 8, 2001 and have reported annual net losses since inception. For our year ended March 31, 2012 and 2011, we experienced net losses of $6,245,879 and $2,811,538, respectively. We used cash in operating activities of $2,222,296 and $736,909 in 2012 and 2011, respectively. As of March 31, 2012, we had a combined accumulated deficit of $16,244,237 from prior operations and $4,595,168 from the period December 11, 2011 (inception of development) to March 31, 2012. In addition, we expect to incur additional losses in the foreseeable future, and there can be no assurance that we will ever achieve profitability. Our future viability, profitability and growth depend upon our ability to successfully operate, expand our operations and obtain additional capital. There can be no assurance that any of our efforts will prove successful or that we will not continue to incur operating losses in the future.
We do not have substantial cash resources and if we cannot raise additional funds or generate more revenues, we will not be able to pay our vendors and will probably not be able to continue as a going concern.
As of March 31, 2012, our available cash balance was $619,624. We will need to raise additional funds to pay outstanding vendor invoices and execute our business plan. Our future cash flows depend on our ability to market and sell our common stock and into sublicensing. There can be no assurance that additional funds will be available when needed from any source or, if available, will be available on terms that are acceptable to us.
We may be required to pursue sources of additional capital through various means, including joint-venture projects and debt or equity financings. Future financings through equity investments will be dilutive to existing stockholders. Also, the terms of securities we may issue in future capital transactions may be more favorable for our new investors. Newlyissued securities may include preferences, superior voting rights, the issuance of warrants or other convertible securities, which will have additional dilutive effects. Further, we may incur substantial costs in pursuing future capital and/or financing, including investment banking fees, legal fees, accounting fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we may issue, such as convertible notes and warrants, which will adversely impact our financial condition and results of operations.
Our ability to obtain needed financing may be impaired by such factors as the weakness of capital markets and the fact that we have not been profitable, which could impact the availability or cost of future financings. If the amount of capital we are able to raise from financing activities, together with our revenues from operations, is not sufficient to satisfy our capital needs, even to the extent that we reduce our operations accordingly, we may be required to cease operations.
We have a limited operating history, and it may be difficult for potential investors to evaluate our business.
We began operations in in the area of Immunotherapy on December 12, 2011, the date of inception for the new development stage company. Our limited operating history in the Immunonotherapy business makes it difficult for potential investors to evaluate our business or prospective operations. We have not generated any revenues to date. As an early-stage company, we are subject to all the risks inherent in the initial organization, financing, expenditures, complications and delays inherent in a relatively new business. Investors should evaluate an investment in us in light of the uncertainties encountered by such companies in a competitive environment. Our business is dependent upon the implementation of our business plan, as well as the ability of our merchants to enter into agreements with consumers for their respective products and/or services. There can be no assurance that our efforts will be successful or that we will be able to attain profitability.
21
Competition may increase in the cancer therapy market.
We may in the future compete for potential customers with other cancer therapies.
There can be no assurance that we will be able to compete successfully against future competitors. If we are unable to compete effectively, or if competition results in a deterioration of market conditions, our business and results of operations would be adversely affected.
The success of our business depends on the continuing contributions of Dr. Michael Har-Noy, founder of ITL, and other key personnel who may terminate their employment with us at any time, and we will need to hire additional qualified personnel.
We rely heavily on the services of Dr. Michael Har-Nov, founder, and Antonio Treminio, chairman of the board of directors and our chief executive officer, as well as several other management personnel. Loss of the services of any such individual would adversely impact our operations. In addition, we believe our technical personnel represent a significant asset and provide us with a competitive advantage over many of our competitors and that our future success will depend upon our ability to retain these key employees and our ability to attract and retain other skilled financial, marketing, technical and managerial personnel.
If we are unable to attract, train and retain highly qualified personnel, the quality of our services may decline and we may not successfully execute our internal growth strategies.
Our success depends in large part upon our ability to continue to attract, train, motivate and retain highly skilled and experienced employees, including technical personnel. Qualified technical employees periodically are in great demand and may be unavailable in the time frame required to satisfy our customers’ requirements. While we currently have available technical expertise sufficient for the requirements of our business, expansion of our business could require us to employ additional highly skilled technical personnel.
There can be no assurance that we will be able to attract and retain sufficient numbers of highly skilled technical employees in the future. The loss of personnel or our inability to hire or retain sufficient personnel at competitive rates of compensation could impair our ability to secure and complete customer engagements and could harm our business.
22
Risks Relating to Our Industry
Our Company has experienced, and continues to experience, rapid growth in operations, which has placed, and will continue to place, significant demands on its management, operational and financial infrastructure.
If the Company does not effectively manage its growth, the quality of its products and services could suffer, which could negatively affect the Company’s brand and operating results. To effectively manage this growth, the Company will need to continue to improve its operational, financial and management controls and its reporting systems and procedures. Failure to implement these improvements could hurt the Company’s ability to manage its growth and financial position.
The Company treats its proprietary information as confidential and relies on internal nondisclosure safeguards and on laws protecting trade secrets, all to protect its proprietary information.
There can be no assurance that these measures will adequately protect the confidentiality of the Company's or ITL’s proprietary information or that others will not independently develop products or technology that are equivalent or superior to those of the Company or ITL. The Company and ITL’s patents, trademarks, trade secrets, copyrights and/or other intellectual property rights are important assets to the Company and ITL. Various events outside of the Company’s and ITL’s control pose a threat to its intellectual property rights as well as to the Company’s and ITL’s products and services. Although the Company and/or ITL seek to obtain patent protection for its products, it is possible that the Company and/or ITL may not be able to protect some of these innovations. There is always the possibility, despite the Company’s and ITL’s efforts that the scope of the protection gained will be insufficient or that an issued patent may be deemed invalid or unenforceable.
Risks Relating to Our Organization and Our Common Stock
In 2001, we became a publicly registered company that is subject to the reporting requirements of federal securities laws, which can be expensive and may divert resources from other projects, thus impairing our ability to grow.
In 2001, we became a public reporting company and, accordingly, subject to the information and reporting requirements of the Exchange Act and other federal securities laws, including compliance with the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”). The costs of preparing and filing annual and quarterly reports, proxy statements and other information with the SEC and furnishing audited reports to stockholders will cause our expenses to be higher than they would have been if we remained private.
If we fail to establish and maintain an effective system of internal control, we may not be able to report our financial results accurately or to prevent fraud. Any inability to report and file our financial results accurately and timely could harm our reputation and adversely impact the trading price of our common stock.
It may be time consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act. We may need to hire additional financial reporting, internal controls and other finance personnel in order to develop and implement appropriate internal controls and reporting procedures. Effective internal control is necessary for us to provide reliable financial reports and prevent fraud. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as we would if an effective control environment existed, and our business and reputation with investors may be harmed. In addition, if we are unable to comply with the internal controls requirements of the Sarbanes-Oxley Act, then we may not be able to obtain the independent accountant certifications required by such act, which may preclude us from keeping our filings with the SEC current and may adversely affect any market for, and the liquidity of, our common stock.
Public company compliance may make it more difficult for us to attract and retain officers and directors.
The Sarbanes-Oxley Act and new rules subsequently implemented by the SEC have required changes in corporate governance practices of public companies. As a public company, we expect these new rules and regulations to increase our compliance costs and to make certain activities more time consuming and costly. As a public company, we also expect that these new rules and regulations may make it more difficult and expensive for us to obtain director and officer liability insurance in the future and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve on our board of directors or as executive officers.
Because we became public by means of a merger, we may not be able to attract the attention of major brokerage firms.
There may be risks associated with us becoming public through a merger. Securities analysts of major brokerage firms may not provide coverage of us since there is no incentive to brokerage firms to recommend the purchase of our common stock. No assurance can be given that brokerage firms will, in the future, want to conduct any secondary offerings on behalf of our Company.
23
Our stock price may be volatile.
The market price of our common stock is likely to be highly volatile and could fluctuate widely in price in response to various factors, many of which are beyond our control, including the following:
|
●
|
changes in our industry;
|
|
●
|
competitive pricing pressures;
|
|
●
|
our ability to obtain working capital financing;
|
|
●
|
additions or departures of key personnel;
|
|
●
|
limited “public float” in the hands of a small number of persons whose sales or lack of sales could result in positive or negative pricing pressure on the market price for our common stock;
|
|
●
|
sales of our common stock;
|
|
●
|
our ability to execute our business plan;
|
|
●
|
operating results that fall below expectations;
|
|
●
|
loss of any strategic relationship;
|
|
●
|
regulatory developments;
|
|
●
|
economic and other external factors; and
|
|
●
|
period-to-period fluctuations in our financial results.
|
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
We may not pay dividends in the future. Any return on investment may be limited to the value of our common stock.
We do not anticipate paying cash dividends in the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as our board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
There is currently a limited liquid trading market for our common stock.
To date there has not been a liquid trading market for our common stock. We cannot predict how liquid the market for our common stock might become. As soon as is practicable after becoming eligible, we anticipate applying for listing of our common stock on either the NYSE Amex Equities, the NASDAQ Capital Market or other national securities exchange, assuming that we can satisfy the initial listing standards for such exchange. We currently do not satisfy the initial listing standards for any of these exchanges, and cannot ensure that we will be able to satisfy such listing standards or that our common stock will be accepted for listing on any such exchange. Should we fail to satisfy the initial listing standards of such exchanges, or our common stock is otherwise rejected for listing and remains quoted on the OTC Bulletin Board or is suspended from the OTC Bulletin Board, the trading price of our common stock could suffer and the trading market for our common stock may be less liquid and our common stock price may be subject to increased volatility.
Furthermore, for companies whose securities are quoted on the OTC Bulletin Board, it is more difficult (i) to obtain accurate quotations, (ii) to obtain coverage for significant news events because major wire services generally do not publish press releases about such companies and (iii) to obtain needed capital.
24
Our common stock is currently considered a “penny stock,” which may make it more difficult for our investors to sell their shares.
Our common stock is currently considered a “penny stock” and may continue in the future to be subject to the “penny stock” rules adopted under Section 15(g) of the Exchange Act. The penny stock rules generally apply to companies whose common stock is not listed on The NASDAQ Stock Market or other national securities exchange and trades at less than $5.00 per share, other than companies that have had average revenue of at least $6,000,000 for the last three years or that have tangible net worth of at least $5,000,000 ($2,000,000 if the company has been operating for three or more years). These rules require, among other things, that brokers who trade penny stock to persons other than “established customers” complete certain documentation, make suitability inquiries of investors and provide investors with certain information concerning trading in the security, including a risk disclosure document and quote information under certain circumstances. Many brokers have decided not to trade penny stocks because of the requirements of the penny stock rules and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. Since our securities are subject to the penny stock rules, investors may find it more difficult to dispose of our securities.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
If our stockholders sell substantial amounts of our common stock in the public market, or upon the expiration of any statutory holding period under Rule 144, or issued upon the exercise of outstanding options or warrants, it could create a circumstance commonly referred to as an “overhang” and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are occurring, also could make more difficult our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
Antonio Treminio, our chief executive officer and chairman of our board of directors, beneficially owns a substantial portion of our outstanding common stock and preferred stock, which enables him to influence many significant corporate actions and in certain circumstances may prevent a change in control that would otherwise be beneficial to our stockholders.
Antonio Treminio beneficially owns approximately 9.3% of our outstanding shares of common stock. As such, he has a substantial impact on matters requiring the vote of the stockholders, including the election of our directors and most of our corporate actions. This control could delay, defer, or prevent others from initiating a potential merger, takeover or other change in our control, even if these actions would benefit our stockholders and us. This control could adversely affect the voting and other rights of our other stockholders and could depress the market price of our common stock.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 2. PROPERTIES
On January 31, 2012, the Company entered into a three year lease for its corporate office. The lease requires a monthly payment of $2,150 per month.
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
25
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
The Company's Common Stock is traded on the OTC-Bulletin Board under the symbol IMUN.QB (formerly NVNC.QB). As of March 31, 2012, the Company’s common stock was held by 1,134 shareholders of record, which does not include shareholders whose shares are held in street or nominee name.
The following sets forth the range of the closing bid prices for the Company's Common Stock for the period April 1, 2010 through March 31, 2012. Such prices represent inter-dealer quotations, do not represent actual transactions, and do not include retail mark-ups, mark-downs or commissions.
|
For the Years Ended March 31,
|
||||||||||||||||
|
2012
|
2011
|
|||||||||||||||
|
High
|
Low
|
High
|
Low
|
|||||||||||||
|
First Quarter
|
$ | 0.16 | $ | 0.070 | $ | 0.20 | $ | 0.10 | ||||||||
|
Second Quarter
|
$ | 0.195 | $ | 0.091 | $ | 0.19 | $ | 0.04 | ||||||||
|
Third Quarter
|
$ | 0.169 | $ | 0.091 | $ | 0.07 | $ | 0.04 | ||||||||
|
Fourth Quarter
|
$ | 0.149 | $ | 0.0964 | $ | 0.09 | $ | 0.04 | ||||||||
The Company’s transfer agent isPacific Stock Transfer Co., 4045 South Spencer St, #403Las Vegas, NV89119.
Dividend Distributions
No cash dividends were declared by the Company during the fiscal year ended March 31, 2012. While the payment of dividends rests within the discretion of the Board of Directors, it is not anticipated that cash dividends will be paid in the foreseeable future, as the Company intends to retain earnings, if any, for use in the development of its business. The payment of dividends is contingent upon the Company's future earnings, if any, the Company's financial condition and its capital requirements, general business conditions and other factors.
Securities authorized for issuance under equity compensation plans
No shares were available for issuance under any equity compensation plan at March 31, 2012.
Penny Stock
Our common stock is considered "penny stock" under the rules the Securities and Exchange Commission (the "SEC") under the Securities Exchange Act of 1934. The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ Stock Market System, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or quotation system. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document prepared by the Commission, that:
|
-
|
contains a description of the nature and level of risks in the market for penny stocks in both public offerings and secondary trading;
|
|
-
|
contains a description of the broker's or dealer's duties to the customer and of the rights and remedies available to the customer with respect to a violation to such duties or other requirements of Securities' laws; contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between the bid and ask price;
|
|
-
|
contains a toll-free telephone number for inquiries on disciplinary actions;
|
|
-
|
defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and
|
|
-
|
contains such other information and is in such form, including language, type, size and format, as the Commission shall require by rule or regulation.
|
26
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, the customer with:
|
-
|
bid and offer quotations for the penny stock;
|
|
-
|
the compensation of the broker-dealer and its salesperson in the transaction;
|
|
-
|
the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the marker for such stock; and
|
|
-
|
monthly account statements showing the market value of each penny stock held in the customer's account.
|
In addition, the penny stock rules that require that prior to a transaction in a penny stock not otherwise exempt from those rules; the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written acknowledgement of the receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written suitable statement.
These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our stock.
Related Stockholder Matters
None.
Recent Sales of Unregistered Securities
On June 1, 2011, the Company’s convertible debenture, under a debt assignment agreement, was transferred to Green Eagle Capital Corp. who was acting as principal and agent to certain investors for the transaction. Green Eagle Capital Corp. is owned by a shareholder of the Company. On July 11, 2011, the convertible debenture was converted into 10,000,000 shares of the Company’s common stock.
On July 1, 2011, the Company converted $78,000 of unpaid rent for its headquarters to common stock. The conversion rate was at $0.11 per share the date on which the conversion occurred. Accordingly, the Company issued 709,090 shares.
On July 15, 2011, the Company issued 465,000 shares of its common stock to a former consultant in settlement of a consulting agreement. The shares were valued at $0.10 per share. Accordingly, stock based compensation in the amount of $46,500 was recorded.
On February 15 and 28, 2012, the Company entered into a consulting agreements with Bridgeview Capital to assist the Company in developing a business strategy, assist in capital introductions and other mutually agreed upon services. In consideration for these services, the Company issued 3,000,000 shares of its common stock valued at $0.14 per share, the fair market value at the date of commitment. The shares vested immediately.
On February 15, 2012, the Company entered into a consulting agreement with an individual to assist the Chief Executive Officer with day to day operating activities. In consideration for these services, the Company, in addition to paying bi-weekly compensation, issued 250,000 shares vesting immediately. The shares were valued at $0.14, the fair market value at the date of commitment.
On February 28, 2012, the Company entered into a consulting agreement with an individual to provide capital introduction and other services as mutually agreed upon with the Company. In consideration for these services, the Company issued 1,195,000 shares of its common stock valued at $0.14 per share. The shares vested immediately.
On February 28, 2012, the Company entered into a consulting agreement with Rubicon Capital Advisors, LLC to assist the Company in developing marketing and investor relations strategies and other services as mutually agreed to by the Company and consultant. In consideration for these services, the Company issued 2,500,000 shares of its common stock valued at $0.14 per share. The shares are considered earned as of the date of this agreement.
27
On February 28, 2012, the Company entered into a consulting agreement with Sirton International, Inc. to assist the company in developing a marketing and investor relations, assist the Company in developing an acquisition strategy and structure with the European market and other services as mutually agreed to by the Company and consultant. In consideration for these services, the Company issued 5,400,000 shares of its common stock valued at $0.14 per share, the fair value of the stock at the date of commitment. The shares are considered earned as of its date of agreement.
In connection with settlement agreements dated February 21 and 23, 2012, the Company issued 1,100,000 shares of its common stock valued at $0.14 per share to Satellite Advisors Group, LLC and Dr. Stella Snug.
Subsequent to the year ended March 31, 2012, the Company through various private placements sold approximately 9,268,000, shares of its common stock at $0.10 per share aggregating $926,800.
On May 15, 2012, the Company entered into a consulting agreement with an individual for a period of 36 months to assist the Company in securing a qualified management team, develop acquisition strategies and other mutually agreed services. In consideration for such services, the Company agreed to pay $6,000 per month and issued 2,500,000 shares of its common stock vesting immediately and considered earned upon issuance.
On May 15, 2012, the Company amended the Chief Executive Officer’s employment agreement awarding him an additional 2,500,000 shares of the Company’s common stock.
We issued the equity securities described in this section in reliance on the registration exemption provided by Section 4(2) of the Securities Act of 1933.
As a smaller reporting company, the Company is not required to provide this information. .
Our financial statements are prepared in accordance with U.S. Generally Accepted Accounting Principles (GAAP). The preparation of the financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses, and related disclosures. Though we evaluate our estimates and assumptions on an ongoing basis, our actual results may differ from these estimates.
Certain or our accounting policies that we believe are the most important to the portrayal of our financial condition and results of operations and that require management’s subjective judgments are described below to facilitate a better understanding of our business activities. We base our judgments on our experience and assumptions that we believe are reasonable and applicable under the circumstances.
28
Foreign Currency Translation
The Company considers the Canadian dollar to be its functional currency. Assets and liabilities were translated into US dollars at year-end exchange rates. Statement of operations amounts were translated using the average rate during the year. Gains and losses resulting from translating foreign currency financial statements were included inaccumulated other comprehensive gain or loss, a separate component of stockholders’ deficit.
Stock-Based Compensation
The Company accounts for Stock-Based Compensation under ASC 718 “Compensation-Stock Compensation,” which addresses the accounting for transactions in which an entity exchanges its equity instruments for goods or services, with a primary focus on transactions in which an entity obtains employee services in share-based payment transactions. ASC 718-10 requires measurement of cost of employee services received in exchange for an award of equity instruments based on the grant-date fair value of the award (with limited exceptions). Incremental compensation costs arising from subsequent modifications of awards after the grant date must be recognized.
The Company accounts for stock-based compensation awards to non-employees in accordance with ASC 505-50, Equity-Based Payments to Non-Employees. Under ASC 505-50, the Company determines the fair value of the warrants or stock-based compensation awards granted as either the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable. Any stock options or warrants issued to non-employees are recorded in expense and an offset to additional paid-in capital in shareholders’ equity/(deficit) over the applicable service periods using variable accounting through the vesting dates based on the fair value of the options or warrants at the end of each period.
The Company issues stock to consultants for various services. The costs for these transactions are measured at the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable. The value of the common stock is measured at the earlier of (1) the date at which a firm commitment for performance by the counterparty to earn the equity instruments is reached or (2) the date at which the counterparty’s performance is complete. The Company recognized consulting expense and a corresponding increase to additional paid-in-capital related to stock issued for services.
Comprehensive Income
The Company adopted ASC 220-10, Reporting Comprehensive Income, (formerly SFAS No. 130), which requires the reporting of comprehensive income in addition to net income from operations.
Comprehensive income is a more inclusive financial reporting methodology that includes disclosure of information that historically has not been recognized in the calculation of net income.
Impairment of Long-Lived Assets
Long-lived assets, primarily fixed assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets might not be recoverable. The Company does perform a periodic assessment of assets for impairment in the absence of such information or indicators. Conditions that would necessitate an impairment assessment include a significant decline in the observable market value of an asset, a significant change in the extent or manner in which an asset is used, or a significant adverse change that would indicate that the carrying amount of an asset or group of assets is not recoverable. For long-lived assets to be held and used, the Company recognizes an impairment loss only if its carrying amount is not recoverable through its undiscounted cash flows and measures the impairment loss based on the difference between the carrying amount and estimated fair value.
29
Research and Development
The Company expenses research and development costs as incurred.
Comparison of the Fiscal Years Ended March 31, 2012 and 2011
Revenues
During the year ended March 31, 2012, the Company is considered a development stage company and accordingly, did not have any revenues.
Operating Expenses
Commencing December 12, 2011, we changed the direction of the company from that of a Renewable Energy Company to a company focused on the development and commercialization of cancer research and the next generation of Immunotherapy Treatments. During the new development stage period which commenced December 12, 2011 to March 31, 2012, the Company realized a loss of $4,595,168. The loss is attributable to (a) share based compensation to consultants of $3,305,300, (b) payments under the license agreement aggregating $819,164, (c) professional fees approximating $225,000, (d) executive salaries of approximately $50,000 and (e) other expenditures including travel related expenditures of $195,704.
For the full year ended March 31, 2012, our operating expenses consist primarily of share based compensation to consultants, compensation costs and professional services. Total operating expenses for the year ended march 31, 2011 were $5,842,250 compared to $2,237,745 for the year ended March 31, 2012, an increase of $3,604,505. This increase is primarily attributable to (a) an increase in share based compensation of $2,454,013, (b) payments made under the License agreement to Immunovative Therapies, Ltd., an Israeli Corporation of $819,164, (c) increased professional fees aggregating $139,566, (d) increased consulting fees of $273,860 to help develop business and marketing strategies, (e) increased travel and related expenditures of $176,141 and (f) general reductions in other operating expenses of $258,239, resulting from the change in direction of our business.
Interest expense for the year ended March 31, 2012 was $66,793 compared to $573,793 at March 31, 2011, a decrease of $507,000. This decrease is attributable to: (a) reduction of discount amortization of $379,784, (b) elimination of the convertible debenture in July 2012 resulting in $37,500 less interest for the year and in 2011, a loan default fee of 15% was accrued aggregating $75,000 and (c) repayment of a portion of the loan to the Chief Executive Officer resulting in reduced interest of $14,700.
During the year ended March 31, 2012, the Company entered into an agreement with the holders of the convertible debenture whereby the Company was able to transfer the debt to Green Eagle Capital Corp. through a debt assignment agreement. In connection with the debt assignment, the Company allowed Green Eagle Corp. to convert the debt to common stock of the Company at an agreed upon number of shares significantly in excess of the original conversion agreement resulting in a loss on extinguishment of debt of $336,836.
Comparison of the Fiscal Years Ended March 31, 2011 and 2010
Revenues
During the year ended March 31, 2011 the Company is considered a development stage company and accordingly, did not have any revenues.
30
Operating Expenses
During the years ended March 31, 2011 and March 31, 2010, the Company was in the Renewable Energy business and, accordingly, the following discussion pertains to that business.
For the full year ended March 31, 2011, our operating expenses were $2,811,538 compared to $2,935,831 for the year ended March 31, 2010, a decrease of $124,293. The decrease is primarily attributable to (a) a decrease of in-general and administrative expense of $583,604 resulting from a $81,000 decrease in share based compensation, a decrease of approximately $250,000 in consulting fees, a decrease of approximately $100,000 in salaries and related expenditure and a general reduction of approximately $150,000 in other general and administrative expenditures. These expenditure reductions are directly related to the winding down of the renewable energy operations; (b) research and development expenditures decreased $44,206 in 2011 from $515,279 in 2010. Such decrease is attributable to the winding down of the renewable energy business. (c) Interest expense for the year ended March 31, 2011 was $573,793 compared to $72,390, an increase of $501,403. This increase is attributable to a full year discount amortization of $334,872, the recording of a default premium on the convertible debenture of $75,000 and a full year’s interest on all other indebtedness.
Liquidity and Capital Resources
We continue to fund our operations through private placement offerings and financings.
During the fiscal year ending March 31, 2012, we initiated private placement offering of our equity securities to a number of accredited investors. Through these private placement offerings, we sold 13,450,000 shares of our common stock at $0.05 per share aggregating $672,500 and 22,853,356 shares of our common stock at $0.10 per share aggregating $2,285,356. During the period December 12, 2011 (inception of the development stage) to March 31, 2012, we sold 2,056, 356 shares of our common stock at $0.10 per share aggregating $2,065,356.
At March 31, 2012, we had cash and cost equivalents of $619,624 compared to $8,730 at March 31, 2011. The increase of $610,894 is primarily attributable to the sale of common stock net of payments under the license agreement and operating expenditures.
Cash Flows
Net cash used in operating activities amounted to ($1,519,266) for the period from December 2, 2011 (inception of Development Stage) to March 31, 2012. Net cash provided by used in operating activities amounted to ($2, 222,296) and ($736,900) for the fiscal years ended March 31, 2012 and 2011, respectively. Net cash from operations which was a negative $1,519,266 was impacted primarily by stock based compensation awards amounting to $3,152,300, company shares issued in settlement agreements aggregating $153,000 and payments under the license agreement of $819,164.
Net cash used in operating activities for the years ended March 31, 2012 and 2011 was ($2,222,296) and ($736,909) a decrease of $1,485,387 primarily from share based compensation to consultants.
During the period from inception December 12, 2011 (inception of the Development Stage), we generated 2,065,356 from private placement sales of our common stock and repaid the loan to our Chief Executive Officer of $75,000.
During fiscal year 2012, we generated cash from financing activates of $2,957,856 from private placement sales of our common stock and made loan repayments of $120,000 to our Chief Executive Officer. During fiscal 2011, we generated cash from financing activities of $698,796 from private placement sales of our common stock.
We do not believe that our cash on hand at March 31, 2011 will be sufficient to fund our license agreement requirements if all the conditions of the license agreement required of the licensor are met. We will continue to seek additional equity financing. However, there is no assurance that we will be successful in our equity private placements.
31
Going Concern Qualifications
The Company’s financial statements as of March 31, 2011 and for the year then ended have been prepared assuming the Company will continue as a going concern. As discussed in Note A to the financial statements, since inception of the Development Stage (December 12, 2012) the Company had losses of $4,595,168 and from prior operations a loss of $16,244,237 at March 31, 2012, has experienced negative cash flows from operations, and there are existing uncertain conditions which the Company faces relative to its obtaining financing and capital in the equity markets. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
Contractual Obligations
Not Applicable
Off-Balance Sheet Arrangements
As of March 31, 2012, we had no off-balance sheet arrangements as defined in Item 303(a)(4) of Regulation S-K.
Recent Accounting Pronouncements
In June 2011, the FASB issued ASU No. 2011-05 which amends ASC Topic 220, Comprehensive Income. Under the amendment, an entity has the option to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. In both choices, an entity is required to present each component of net income along with total income, each component of other comprehensive income along with a total for other comprehensive income, and a total amount for comprehensive income. This ASU eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity. The amendments in this ASU do not change the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income. The amendments in this ASU should be applied retrospectively. Additionally, the FASB issued a second amendment to ASC Topic 220 in December 2011, ASU No. 2011-12, which allows companies the ability to defer certain aspects of ASU 2011-05. For public entities, these amendments are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. The amendments do not require any transition disclosures.
On September 15, 2011, the FASB issued ASU 2011-08, Intangibles-Goodwill and Other, which simplifies how an entity is required to test Goodwill for impairment. This ASU will allow an entity to first assess qualitative factors to determine whether it is necessary to perform the two-step quantitative goodwill impairment test. Under the ASU, an entity would not be required to calculate the fair value of a reporting unit unless the entity determines, based upon qualitative assessment, that it is more likely than not that its fair value is less than its carrying amount. The ASU includes a number of factors to consider in conducting the qualitative assessment. The ASU is effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. Early adoption is permitted. The Company does not expect the provisions of ASI 2-11-08 to have a material effect on the financial position, results of operations, or cash flows of the Company.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a smaller reporting company, the Company is not required to provide this information.
32
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A Development Stage Company)
AUDITED FINANCIAL STATEMENTS
FOR THE YEARS ENDED MARCH 31, 2012 AND 2011
33
|
CONTENTS
|
||||
|
Page
|
||||
|
Report of Independent Registered Public Accounting Firm
|
F - 1 | |||
|
Consolidated Balance Sheets
|
F - 2 | |||
|
Consolidated Statements of Operations
|
F - 3 | |||
|
Consolidated Statements of Cash Flows
|
F - 4 | |||
|
Consolidated Statements of Stockholders’ Deficit
|
F - 6 | |||
|
Notes to Consolidated Financial Statements
|
F - 8 | |||
MEYLER & COMPANY, LLC
CERTIFIED PUBLIC ACCOUNTANTS
ONE ARIN PARK
1715 HIGHWAY 35
MIDDLETOWN, NJ 07748
Report of Independent Registered Public Accounting Firm
To the Board of Directors
Immunovative, Inc.
Montreal, Canada
We have audited the accompanying consolidated balance sheets of Immunovative, Inc. and Subsidiary, formerly known as Novo Energies Corporation and Subsidiary, (a Development Stage Company) as of March 31, 2012 and 2011 and the related consolidated statements of operations, and cash flows for each of the years in the two-year period ended March 31, 2012 and for the period December 12, 2011 (inception of Development Stage) to March 31, 2012 and the statement of stockholders’ deficit for each of the years in the two year period ended March 31. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Immunovative, Inc. and Subsidiary (a Development Stage Company) as of March 31, 2012 and 2011, and the results of its operations and its cash flows for each of the years in the two-year period ended March 31, 2012 and for the period December 12, 2011 (inception of Development Stage) to March 31, 2012, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note A to the financial statements, the Company incurred a net loss of $6,245,879 for the year ended March 31, 2012, has an accumulated deficit from prior operations of $16,244,237, an accumulated deficit during development stage of $4,595,168 and negative working capital of $106,445 at March 31, 2012 and there are existing uncertain conditions the Company faces relative to its’ ability to obtain capital and operate profitably. These conditions raise substantial doubt about it’s ability to continue as a going concern. Management’s plans regarding these matters are also described in Note A. The financial statements do not include any adjustments that may result from the outcome of this uncertainty.
|
|
|
/s/ Meyler & Company, LLC | |
| Middletown, NJ | |||
| July 16, 2012 |
F-1
|
IMMUNOVATIVE, INC. AND SUBSIDIARY
|
||||||
|
(Formerly Novo Energies Corporation and Subsidiary)
|
||||||
|
(A DEVELOPMENT STAGE COMPANY)
|
||||||
|
CONSOLIDATED BALANCE SHEETS
|
|
ASSETS
|
||||||||
|
March 31,
|
||||||||
|
2012
|
2011
|
|||||||
|
CURRENT ASSETS
|
||||||||
|
Cash
|
$ | 619,624 | $ | 8,730 | ||||
|
Prepaid expense
|
12,264 | - | ||||||
|
Total current assets
|
631,888 | 8,730 | ||||||
|
Equipment - net
|
36,468 | 8,353 | ||||||
|
TOTAL ASSETS
|
$ | 668,356 | $ | 17,083 | ||||
|
LIABILITIES AND STOCKHOLDERS' DEFICIT
|
||||||||
|
CURRENT LIABILITIES
|
||||||||
|
Note Payable Caete Invest & Trade, S.A.
|
$ | 179,572 | $ | 179,572 | ||||
|
Convertible Debenture
|
575,000 | |||||||
|
Accounts Payable
|
208,644 | 196,019 | ||||||
|
Accrued Interest
|
77,847 | 124,263 | ||||||
|
Accrued Expenses
|
18,172 | |||||||
|
Accrued Professional Fees
|
145,822 | 256,858 | ||||||
|
Related party payables
|
||||||||
|
Accrued Rent
|
78,000 | |||||||
|
Accrued Consulting
|
26,773 | |||||||
|
Accrued Salaries and Taxes
|
39,412 | 46,880 | ||||||
|
Due to Chairman and CEO
|
16,500 | 20,312 | ||||||
|
Note Payable to Chief Executive Officer
|
52,364 | 161,371 | ||||||
|
Total current liabilities
|
$ | 738,333 | $ | 1,665,048 | ||||
|
STOCKHOLDERS' DEFICIT
|
||||||||
|
Common stock, par value $0.00001; 1,000,000,000 shares
|
||||||||
|
authorized, 116,667,888 and 53,245,238 issued and
|
||||||||
|
outstanding at March 31, 2012 and March 31, 2011,
|
||||||||
|
respectively
|
1,166 | 532 | ||||||
|
Additional paid-in capital
|
20,770,505 | 12,976,186 | ||||||
|
Accumulated deficit from prior operations
|
(16,244,237 | ) | (14,593,526 | ) | ||||
|
Accumulated deficit during development stage
|
(4,595,168 | ) | - | |||||
|
Accumulated other comprehensive loss
|
(2,243 | ) | (31,157 | ) | ||||
|
Total Stockholders' Deficit
|
(69,977 | ) | (1,647,965 | ) | ||||
|
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT
|
$ | 668,356 | $ | 17,083 | ||||
See accompanying notes to consolidated financial statements.
F-2
|
IMMUNOVATIVE, INC. AND SUBSIDIARY
|
||||||||
|
(Formerly Novo Energies Corporation and Subsidiary)
|
||||||||
|
(A DEVELOPMENT STAGE COMPANY)
|
||||||||
|
CONSOLIDATED STATEMENTS OF OPERATIONS
|
|
Period from
|
||||||||||||
|
December 12, 2011
|
||||||||||||
|
(Inception of
|
||||||||||||
|
For the Year ended
|
Development)
|
|||||||||||
|
March 31,
|
to March 31,
|
|||||||||||
|
2012
|
2011
|
2012
|
||||||||||
|
OPERATING EXPENSES
|
||||||||||||
|
General and Administrative
|
$ | 5,016,621 | $ | 1,763,076 | $ | 3,767,432 | ||||||
|
Research and Development
|
820,164 | 471,073 | 819,164 | |||||||||
|
Depreciation Expense
|
5,465 | 3,596 | 2,668 | |||||||||
|
Total Expenses
|
5,842,250 | 2,237,745 | 4,589,264 | |||||||||
|
LOSS FROM OPERATIONS
|
5,842,250 | 2,237,745 | 4,589,264 | |||||||||
|
OTHER EXPENSE
|
||||||||||||
|
Interest Expense
|
66,793 | 573,793 | 5,904 | |||||||||
|
Loss on Extinguishment of Debt
|
336,836 | |||||||||||
|
Total Other Expense
|
403,629 | 573,793 | 5,904 | |||||||||
|
NET LOSS
|
(6,245,879 | ) | (2,811,538 | ) | (4,595,168 | ) | ||||||
|
OTHER COMPREHENSIVE INCOME
|
||||||||||||
|
Translation Adjustment
|
28,914 | 5,517 | ||||||||||
|
COMPREHENSIVE LOSS
|
$ | (6,216,965 | ) | $ | (2,806,021 | ) | $ | (4,595,168 | ) | |||
|
NET LOSS PER SHARE
|
||||||||||||
|
Basic and Diluted
|
$ | (0.09 | ) | $ | (0.07 | ) | ||||||
|
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING
|
||||||||||||
|
BASIC AND DILUTED
|
73,039,842 | 41,433,771 | ||||||||||
See accompanying notes to consolidated financial statements.
F-3
|
IMMUNOVATIVE, INC. AND SUBSDIARY
|
||||||||
|
(Formerly Novo Energies Corporation and Subsidiary)
|
||||||||
|
(A DEVELOPMENT STAGE COMPANY)
|
||||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
Period from
|
||||||||||||
|
December 12, 2011
|
||||||||||||
|
(Inception of
|
||||||||||||
|
Development)
|
||||||||||||
|
For the Year Ended March 31,
|
to March 31,
|
|||||||||||
|
2012
|
2011
|
2012
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||||||
|
Net loss
|
$ | (6,245,879 | ) | $ | (2,811,538 | ) | $ | (4,595,168 | ) | |||
|
Adjustments to reconcile net loss to cash provided
|
||||||||||||
|
by (used in) operating activities:
|
||||||||||||
|
Stock based compensation
|
3,592,047 | 1,291,034 | 3,152,300 | |||||||||
|
Shares issued in Settlement Agreement
|
153,000 | - | 153,000 | |||||||||
|
Loss on extinguishment of debt
|
336,836 | |||||||||||
|
Note Payable Discount Amortization
|
10,993 | 390,777 | ||||||||||
|
Convertible Debenture Repayment Premium
|
75,000 | |||||||||||
|
Depreciation
|
5,465 | 3,596 | 2,797 | |||||||||
|
Decrease (increase) in assets
|
||||||||||||
|
Miscellaneous Receivable
|
3,674 | |||||||||||
|
Prepaid expense
|
(12,264 | ) | 4,494 | |||||||||
|
Increase (decrease) in liabilities
|
||||||||||||
|
Accounts Payable
|
12,624 | 58,441 | (16,443 | ) | ||||||||
|
Accrued Interest
|
55,799 | 107,809 | 11,448 | |||||||||
|
Accrued expense
|
18,172 | 18,172 | ||||||||||
|
Accrued Professional Fees
|
(111,036 | ) | 233,688 | (153,295 | ) | |||||||
|
Related party payables
|
(38,053 | ) | (89,390 | ) | (96,571 | ) | ||||||
|
Cash used in operating activities
|
(2,222,296 | ) | (736,909 | ) | (1,519,266 | ) | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
||||||||||||
|
Purchase of Equipment
|
(33,580 | ) | (2,445 | ) | (21,009 | ) | ||||||
|
Cash used in investing activities
|
(33,580 | ) | (2,445 | ) | (21,009 | ) | ||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||||||
|
Repayment of Note Payable to Chief Executive
|
||||||||||||
|
Officer
|
(120,000 | ) | - | (75,000 | ) | |||||||
|
Sale of Common Stock
|
2,957,856 | 698,796 | 2,065,356 | |||||||||
|
Cash provided by financing activities
|
2,837,856 | 698,796 | 1,990,356 | |||||||||
|
Foreign Currency Translation Effect
|
28,914 | (5,518 | ) | |||||||||
|
NET INCREASE / (DECREASE) IN CASH
|
610,894 | (46,076 | ) | 450,081 | ||||||||
|
CASH, BEGINNING OF PERIOD
|
8,730 | 54,806 | 169,543 | |||||||||
|
CASH, END OF PERIOD
|
$ | 619,624 | $ | 8,730 | $ | 619,624 | ||||||
See accompanying notes to consolidated financial statements.
F-4
|
IMMUNOVATIVE, INC. AND SUBSDIARY
|
|||||||||
|
(Formerly Novo Energies Corporation and Subsidiary)
|
|||||||||
|
(A DEVELOPMENT STAGE COMPANY)
|
|||||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
Period from
|
||||||||||||
|
December 12, 2011
|
||||||||||||
|
(Inception of
|
||||||||||||
|
Development)
|
||||||||||||
|
For the Year Ended March 31,
|
to March 31,
|
|||||||||||
|
2012
|
2011
|
2012
|
||||||||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW
|
||||||||||||
|
INFORMATION:
|
||||||||||||
|
Interest and Taxes Paid
|
- | - | - | |||||||||
|
NON CASH ITEMS
|
||||||||||||
|
Conversion of convertible debenture
|
$ | (575,000 | ) | |||||||||
|
Conversion of accrued interest related to
|
||||||||||||
|
Convertible debenture
|
(102,215 | ) | ||||||||||
|
Conversion of accrued rent to common stock
|
(78,000 | ) | ||||||||||
|
Additional paid-in capital
|
755,141 | |||||||||||
|
Common stock
|
74 | |||||||||||
See accompanying notes to consolidated financial statements.
F-5
|
IMMUNOVATIVE, INC. AND SUBSIDIARY
|
|
(Formerly Novo Energies Corporation and Subsidiary)
|
|
(A DEVELOPMENT STAGE COMPANY)
|
|
CONSOLIDATED STATEMENT OF STOCKHOLDERS' DEFICIT
|
|
For the two years ended March 31, 2012
|
|
Deficit
|
|||||||||||||||||||||||||
|
Deficit
|
accumulated
|
Accumulated
|
|||||||||||||||||||||||
|
Additional
|
accumulated
|
during the
|
Other
|
Total
|
|||||||||||||||||||||
|
Number of
|
Paid-in
|
from prior
|
development
|
comprehensive
|
stockholders'
|
||||||||||||||||||||
|
shares
|
Amount
|
Capital
|
operations
|
stage
|
income (loss)
|
deficit
|
|||||||||||||||||||
|
Balance March 31, 2010
|
34,345,152 | $ | 344 | $ | 10,986,545 | $ | (11,781,988 | ) | $ | (25,640 | ) | $ | (820,739 | ) | |||||||||||
|
During the period April 1, 2010 to September 30,
|
|||||||||||||||||||||||||
|
2010, sale of common stock under private
|
|||||||||||||||||||||||||
|
placement agreements at $0.10 per share.
|
1,550,000 | 16 | 154,984 | 155,000 | |||||||||||||||||||||
|
During the period October 1, 2010 to March 31,
|
|||||||||||||||||||||||||
|
2010, sale of common stock under private
|
|||||||||||||||||||||||||
|
placement agreements at $0.05 per share.
|
10,278,500 | 103 | 543,693 | 543,796 | |||||||||||||||||||||
|
Issuance of shares on June 7, 2010 under
|
|||||||||||||||||||||||||
|
a consulting agreement at $0.05 per share.
|
30,000 | 1 | 1,499 | 1,500 | |||||||||||||||||||||
|
Issuance of shares on August 1, 2010 under
|
|||||||||||||||||||||||||
|
a consulting agreement at $0.11 per share.
|
1,500,000 | 15 | 134,985 | 135,000 | |||||||||||||||||||||
|
Amendment of chief executive officer employment
|
|||||||||||||||||||||||||
|
contract to include 1,200,000 shares of common
|
|||||||||||||||||||||||||
|
stock valued at $0.18 per share.
|
1,200,000 | 12 | 71,988 | 72,000 | |||||||||||||||||||||
|
Cancellation of Elso consulting agreement and
|
|||||||||||||||||||||||||
|
retirement of shares.
|
(900,000 | ) | (9 | ) | (89,991 | ) | (90,000 | ) | |||||||||||||||||
|
Shares issued for consulting services vested
|
|||||||||||||||||||||||||
|
during the year at $0.10 to $1.00.
|
1,241,586 | 10 | 682,523 | 682,533 | |||||||||||||||||||||
|
Issuance of shares under a consulting contract
|
|||||||||||||||||||||||||
|
dated February 1, 2011 at $0.08 per share.
|
1,000,000 | 10 | 79,990 | 80,000 | |||||||||||||||||||||
|
Fair value of Affiliate shares used to compensate
|
|||||||||||||||||||||||||
|
consultants for services performed.
|
140,000 | 140,000 | |||||||||||||||||||||||
|
Modification of consulting contract
|
|||||||||||||||||||||||||
|
on March 28, 2011 to issue additional
|
|||||||||||||||||||||||||
|
shares at $0.09 per share.
|
3,000,000 | 30 | 269,970 | 270,000 | |||||||||||||||||||||
|
Net loss for year ended March 31, 2011
|
(2,811,538 | ) | (2,811,538 | ) | |||||||||||||||||||||
|
Translation adjustment
|
(5,517 | ) | (5,517 | ) | |||||||||||||||||||||
|
Balance March 31, 2011 (Forward)
|
53,245,238 | 532 | 12,976,186 | (14,593,526 | ) |
-
|
(31,157 | ) | (1,647,965 | ) | |||||||||||||||
See accompanying notes to consolidated financial statements.
F-6
|
IMMUNOVATIVE, INC. AND SUBSIDIARY
|
||||||||||||||
|
(Formerly Novo Energies Corporation and Subsidiary)
|
||||||||||||||
|
(A DEVELOPMENT STAGE COMPANY)
|
||||||||||||||
|
CONSOLIDATED STATEMENT OF STOCKHOLDERS' DEFICIT
|
||||||||||||||
|
For the two years ended March 31, 2012
|
|
Deficit
|
|||||||||||||||||||||||||
|
Deficit
|
accumulated
|
Accumulated
|
|||||||||||||||||||||||
|
Additional
|
accumulated
|
during the
|
Other
|
Total
|
|||||||||||||||||||||
|
Number of
|
Paid-in
|
from prior
|
development
|
comprehensive
|
stockholders'
|
||||||||||||||||||||
|
shares
|
Amount
|
Capital
|
operations
|
stage
|
income (loss)
|
deficit
|
|||||||||||||||||||
|
Balance March 31, 2011
|
53,245,238 | 532 | 12,976,186 | (14,593,526 | ) | (31,157 | ) | (1,647,965 | ) | ||||||||||||||||
|
Sale of common stock under private
|
|||||||||||||||||||||||||
|
placement agreements at $0.10 per share.
|
22,853,560 | 229 | 2,285,127 | 2,285,356 | |||||||||||||||||||||
|
Sale of common stock under private
|
|||||||||||||||||||||||||
|
placement agreements at $0.05 per share.
|
13,450,000 | 134 | 672,366 | 672,500 | |||||||||||||||||||||
|
Issuance of shares under consulting agreement
|
|||||||||||||||||||||||||
|
between $0.10 and $0.14 per share
|
14,845,000 | 148 | 2,008,152 | 2,008,300 | |||||||||||||||||||||
|
Issuance of shares in connection with
|
|||||||||||||||||||||||||
|
settlement agreements at $0.14 per share.
|
1,565,000 | 16 | 199,484 | 199,500 | |||||||||||||||||||||
|
Vesting of stock based compensation.
|
137,247 | 137,247 | |||||||||||||||||||||||
|
Conversion of accrued expense to common stock.
|
709,090 | 7 | 77,993 | 78,000 | |||||||||||||||||||||
|
Conversion of convertible debt to common stock.
|
10,000,000 | 100 | 1,013,950 | 1,014,050 | |||||||||||||||||||||
|
Issuance of stock options.
|
1,400,000 | 1,400,000 | |||||||||||||||||||||||
|
Net loss for the period from April 1, 2011
|
|||||||||||||||||||||||||
|
to December 11, 2011.
|
(1,650,711 | ) | (1,650,711 | ) | |||||||||||||||||||||
|
Net loss for the period from December 12, 2011
|
|||||||||||||||||||||||||
|
(inception of development) to March 31, 2012.
|
(4,595,168)
|
(4,595,168 | ) | ||||||||||||||||||||||
|
Translation adjustment.
|
28,914 | 28,914 | |||||||||||||||||||||||
|
Balance March 31, 2012
|
116,667,888 | $ | 1,166 | $ | 20,770,505 | $ | (16,244,237 | ) |
$ (4,595,168)
|
$ | (2,243 | ) | $ | (69,977 | ) | ||||||||||
See accompanying notes to consolidated financial statements.
F-7
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE A - NATURE OF BUSINESS AND GOING CONCERN
Nature of Business
The Company, prior to December 12, 2012, was involved in the business of exploiting new technologies for the production of clean energy. In May 2011, the Company had entered into an exclusive memorandum of understanding with Immunovative Therapies, Ltd. (“ITL”) (an Israeli company) whereby the Company would acquire a subsidiary of ITL. On December 12, 2011, the Company terminated this memorandum of understanding and entered into a License Agreement (the “License Agreement”) with ITL, pursuant to which the Company received an immediate exclusive and worldwide license to commercialize all the Licensed Products based on ITL’s current and future patents and a patent in-licensed from the University of Arizona. The license granted covers two experimental products for the treatment of cancer in clinical development called AlloStim TM and AlloVax TM (“Licensed Products”). On May 8th, 2012, the Company changed its name to Immunovative, Inc. to better reflect its new direction on the development and commercialization of the next generation of immunotherapy treatments.
Going Concern
As indicated in the accompanying financial statements, the Company has incurred cumulative net operating losses of $4,595,168 since inception of the development stage and has negative working capital of $106,445. Management’s plans include the raising of capital through equity markets to fund future operations, a successful merger with ITL and generating of revenue by sub-licensing its commercialization rights with ITL to other companies as well as potentially generating revenue once all drugs or treatments are eligible for commercialization. Failure to raise adequate capital, complete a successful merger and generate adequate sales revenues could result in the Company having to curtail or cease operations. Additionally, even if the Company does raise sufficient capital to support its operating expenses, complete a successful merger and generate adequate revenues, there can be no assurances that the revenues will be sufficient to enable it to develop business to a level where it will generate profits and cash flows from operations. These matters raise substantial doubt about the Company’s ability to continue as a going concern. However, the accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. These financial statements do not include any adjustments relating to the recovery of the recorded assets or the classification of the liabilities that might be necessary should the Company be unable to continue as a going concern.
NOTE B – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Effective July 1, 2009, the Company adopted Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 105-10 Generally Accepted Accounting Principles-Overall (“ASC 105-10”). ASC 105-10 establishes the FASB Accounting Standards Codification (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied to nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority. The Codification superseded all existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the codification is non-authoritative. The FASB will not issue new standards in the form of Statements, FASB Positions or Emerging Issue Task Force Abstracts. Instead, it will issue Accounting Standards Updates (“ASUs”). The FASB will not consider ASUs as authoritative in their own right. ASUs will serve only to update
F-8
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE B – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
the Codification, provide background information about the guidance and provide the basis for conclusions on the change(s) in the Codification.
Use of Estimates
The preparation of the financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Foreign Currency Translation
The Company considers the Canadian dollar to be its functional currency. Assets and liabilities were translated into US dollars at year-end exchange rates. Statement of operations amounts were translated using the average rate during the year. Gains and losses resulting from translating foreign currency financial statements were included in accumulated other comprehensive gain or loss, a separate component of stockholders’ deficit.
Cash Equivalents
For purposes of reporting cash flows, cash equivalents include investment instruments purchased with an original maturity of three months or less. There were no cash equivalents in 2012 or 2011.
Equipment and Depreciation
Equipment is stated at cost and is depreciated using the straight line method over the estimated useful lives of the respective assets. Routine maintenance, repairs and replacement costs are expensed as incurred and improvements that extend the useful life of the assets are capitalized. When equipment is sold or otherwise disposed of, the cost and related accumulated depreciation are eliminated from the accounts and any resulting gain or loss is recognized in operations.
Consolidated Financial Statements
The financial statements include the accounts and activities of Immunovative, Inc. and its wholly-owned Canadian subsidiary, Immunovative Canada, Inc. (formerly known as WTL Renewable Energies, Inc.) All inter-company transactions have been eliminated in consolidation.
Net Loss Per Common Share
The Company computes per share amounts in accordance with ASC Topic 260 Earnings per Share (EPS) which requires presentation of basic and diluted EPS. Basic EPS is computed by dividing the income (loss) available to Common Stockholders by the weighted-average number of common shares outstanding for the period. Diluted EPS is based on the weighted-average number of shares of Common Stock and Common Stock equivalents outstanding during the periods. A fully diluted calculation is not presented since the results would be anti-dilutive.
F-9
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE B – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Stock Based Compensation
The Company accounts for Stock-Based Compensation under ASC 718 “Compensation-Stock Compensation”, which addresses the accounting for transactions in which an entity exchanges its equity instruments for goods or services, with a primary focus on transactions in which an entity obtains employee services in share-based payment transactions. ASC 718-10 requires measurement of cost of employee services received in exchange for an award of equity instruments based on the grant-date fair value of the award (with limited exceptions). Incremental compensation costs arising from subsequent modifications of awards after the grant date must be recognized.
The Company accounts for stock-based compensation awards to non-employees in accordance with ASC 505-50, Equity-Based Payments to Non-Employees. Under ASC 505-50, the Company determines the fair value of the warrants or stock-based compensation awards granted as either the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable. Any stock options or warrants issued to non-employees are recorded in expense and an offset to additional paid-in capital in shareholders’ equity/(deficit) over the applicable service periods using variable accounting through the vesting dates based on the fair value of the options or warrants at the end of each period.
The Company issues stock to consultants for various services. The costs for these transactions are measured at the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable. The value of the common stock is measured at the earlier of (1) the date at which a firm commitment for performance by the counterparty to earn the equity instruments is reached or (2) the date at which the counterparty’s performance is complete. The Company recognized consulting expense and a corresponding increase to additional paid-in-capital related to stock issued for services.
Comprehensive Income
The Company has adopted ASC 211-05 effective January 1, 2012 which requires entities to report comprehensive income within a continuous statement of comprehensive income.
Comprehensive income is a more inclusive financial reporting methodology that includes disclosure of information that historically has not been recognized in the calculation of net income.
Income Taxes
The Company accounts for income taxes utilizing the liability method of accounting. Under the liability method, deferred taxes are determined based on differences between financial statement and tax bases of assets and liabilities at enacted tax rates in effect in years in which differences are expected to reverse. Valuation allowances are established, when necessary, to reduce deferred tax assets to amounts that are expected to be realized.
F-10
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE B – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Impairment of Long-Lived Assets
Long-lived assets, primarily fixed assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets might not be recoverable. The Company will perform a periodic assessment of assets for impairment in the absence of such information or indicators. Conditions that would necessitate an impairment assessment include a significant decline in the observable market value of an asset, a significant change in the extent or manner in which an asset is used, or a significant adverse change that would indicate that the carrying amount of an asset or group of assets is not recoverable. For long-lived assets to be held and used, the Company would recognize an impairment loss only if its carrying amount is not recoverable through its undiscounted cash flows and measures the impairment loss based on the difference between the carrying amount and estimated fair value.
Research and Development
The Company expenses research and development costs as incurred.
Fair Value Measurements
ASC 820 Fair Value Measurements defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosure about fair value measurements.
The following provides an analysis of financial instruments that are measured subsequent to initial recognition at fair value, grouped into Levels 1 to 3 based on the degree to which fair value is observable:
Level 1- fair value measurements are those derived from quoted prices (unadjusted in active markets for identical assets or liabilities);
Level 2- fair value measurements are those derived from inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices); and
Level 3- fair value measurements are those derived from valuation techniques that include inputs for the asset or liability that are not based on observable market data (unobservable inputs).
Financial instruments classified as Level 1 - quoted prices in active markets include cash.
These consolidated financial instruments are measured using management’s best estimate of fair value, where the inputs into the determination of fair value require significant management judgment to estimation. Valuations based on unobservable inputs are highly subjective and require significant judgments. Changes in such judgments could have a material impact on fair value estimates. In addition, since estimates are as of a specific point in time, they are susceptible to material near-term changes. Changes in economic conditions may also dramatically affect the estimated fair values.
F-11
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE B – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Fair Value Measurements (Continued)
Fair value estimates discussed herein are based upon certain market assumptions and pertinent information available to management as of March 31, 2012. The respective carrying value of certain financial instruments approximated their fair values due to the short-term nature of these instruments. These financial instruments include cash, accounts payable, accrued expenses and due to related parties.
Uncertainty in Income Taxes
Income taxes are accounted for under the liability method of accounting for income taxes. Under the liability method, future tax liabilities and assets are recognized for the estimated future tax consequences attributable to differences between the amounts reported in the financial statement carrying amounts of assets and liabilities and their respective tax bases. Future tax assets and liabilities are measured using enacted or substantially enacted income tax rates expected to apply when the asset is realized or the liability settled. The effect of a change in income tax rates on future income tax liabilities and assets is recognized in income in the period that the change occurs. Future income tax assets are recognized to the extent that they are considered more likely than not to be realized.
ASC 740 “Income Taxes” clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements. This standard requires a company to determine whether it is more likely than not that a tax position will be sustained upon examination based upon the technical merits of the position. If the more-likely-than-not threshold is met, a company must measure the tax position to determine the amount to recognize in the financial statements.
As a result of the implementation of this standard, the Company performed a review of its material tax positions in accordance with recognition and measurement standards established by ASC 740 and concluded that the tax position of the Company does not meet the more-likely-than-not threshold as of March 31, 2012.
Reclassification
Certain amounts at March 31, 2011 have been reclassified to conform to the presentation used in the March 31, 2012 financial statements.
Recent Accounting Pronouncements
In June 2011, the FASB issued ASU No. 2011-05 which amends ASC Topic 220, Comprehensive Income. Under the amendment, an entity has the option to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. In both choices, an entity is required to present each component of net income along with total income, each component of other comprehensive income along with a total for other comprehensive income, and a total amount for comprehensive income. This ASU eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity. The amendments in this ASU do not change the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income. The amendments in this ASU should be applied retrospectively. Additionally, the FASB issued a second amendment to ASC
F-12
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE B – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Recent Accounting Pronouncements (Continued)
Topic 220 in December 2011, ASU No. 2011-12, which allows companies the ability to defer certain aspects of ASU 2011-05. For public entities, these amendments are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. The amendments do not require any transition disclosures. The Company has adopted this ASC retroactively.
On September 15, 2011, the FASB issued ASU 2011-08, Intangibles-Goodwill and Other, which simplifies how an entity is required to test Goodwill for impairment. This ASU will allow an entity to first assess qualitative factors to determine whether it is necessary to perform the two-step quantitative goodwill impairment test. Under the ASU, an entity would not be required to calculate the fair value of a reporting unit unless the entity determines, based upon qualitative assessment, that it is more likely than not that its fair value is less than its carrying amount. The ASU includes a number of factors to consider in conducting the qualitative assessment. The ASU is effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. Early adoption is permitted. The Company does not expect the provisions of ASU 2011-08 to have a material effect on the financial position, results of operations, or cash flows of the Company.
Management does not believe any other recently issued but not yet effective accounting pronouncements, if adopted, would have an effect on the accompanying consolidated financial statements.
NOTE C – EQUIPMENT
|
The Company’s equipment is as follows:
|
March 31,
|
Estimated
|
||||||||
|
2012
|
2011
|
Life
|
||||||||
|
Computer and office equipment
|
$ | 47,011 | $ | 13,431 |
5 years
|
|||||
|
Less: accumulated depreciation
|
10,543 | 5,078 | ||||||||
| $ | 36,468 | $ | 8,353 | |||||||
NOTE D – LICENSE AGREEMENT
On December 12, 2011, the Company entered into a License Agreement (the “License Agreement”) with Immunovative Therapies, Ltd., an Israeli Corporation (“ITL”), pursuant to which the Company received an immediate exclusive and worldwide license to commercialize all product candidates (the “Licensed Products”) based on ITL’s current and future patents and a patent in-licensed from the University of Arizona. The license granted covers two experimental products for the treatment of cancer in clinical development called AlloStim TM and AlloVaz TM (“Licensed Products”).
In exchange for the license, the Company has undertaken an obligation to pay ITL $10 million from the date of the License Agreement until the date that is two years after receiving notice from a regulatory agency in the US, Canada, EU or Thailand of approval to commence a Phase II/III clinical trial (“Regulatory Notice”). The $10 million due from the Company under the License Agreement is to be paid in the following installments: (i) $450,000 upon the signing of the Agreement (paid in February 2012), (ii) $150,000 at the start of each month after ITL submits to a peer-reviewed journal a manuscript for publication describing the results of the Phase I/II clinical trial conducted pursuant to IND 13,9361 until the $10 million has been paid, (iii) $2 million within 60 days of receiving Regulatory Notice and (iv) at any time that the Company chooses prior to the dates above.
F-13
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE D – LICENSE AGREEMENT (CONTINUED)
Upon successful completion of a randomized Phase II/III clinical trial (the “Clinical Trial”) designed to prove the efficacy of at least one of the Licensed Products, the Company and ITL have agreed to consummate a merger transaction (either directly or through a subsidiary of the Company) with the ITL shareholders immediately prior to the merger owning 75% of the post-merger shares and the shareholders of the Company immediately prior to the merger owning 25% of the post-merger shares on a fully diluted basis. The successful completion of the Clinical Trial shall be defined as the date that the treatment protocol for the number of evaluable subjects necessary to conduct a statistical analysis comparing a placebo control group with a Licensed Product is completed, whereby there is sufficient power to detect a statistically significant (p<0.10) increase in overall survival of 50% or greater of the Licensed Product as compared to the placebo.
The License Agreement provides that the percentage of the post-merger shares that the shareholders of the Company immediately prior to the merger will increase in certain circumstances, including if the Company provides ITL more than the $10 million set out in the License Agreement and if ITL has outstanding debt (excluding any liabilities owed to patent attorney or for patent maintenance fees) at the time of the merger. Likewise the License Agreement provides that the percentage of the post-merger shares that the shareholders of the Company immediately prior to the merger will decrease in certain circumstances, including if ITL raises funds on its own or if the Company has outstanding debts at the time of the merger.
If there is a successful completion of a Clinical Trial but the Company has not paid the full $10 million, the parties may agree to merge or the Company may receive shares of ITL based on the amount of funds that the Company has provided ITL and the license will terminate. If there is not a successful completion
of a Clinical Trial and the Company decides to continue to fund the clinical trials, the Company will receive shares in ITL for any additional payments more than $10 million. In each of these instances, the shares that the Company will receive will be based on a valuation (prior to the funds provided by the Company) of ITL of $30 million, which can be decreased for any outstanding debts (with the exception of patent related debts and trade liabilities) of ITL or increased for any funds raised by ITL on its own.
If the Company pays all amounts due under the License Agreement, but there is no successful completion of a Clinical Trial, the Company and ITL may nevertheless agree to merge. If they do not, the Company shall maintain the license granted under the License Agreement. As of March 31. 2012, the Company has paid ITL $819,164 and recorded the payment as Research and Development expenses.
NOTE E – NOTE PAYABLE TO CAETE INVEST & TRADE, S.A.
On November 1, 2009, the Company issued a $242,000 promissory note to Caete Invest & Trade, S.A. maturing on October 31, 2010. The note bears interest at the rate of 10% per annum and is payable at maturity. The face amount of the note plus accrued interest is convertible into unregistered common stock of the company at the lesser of 100% of the volume weighted average price (“VWAP”) of common stock as reported by Bloomberg L.P. on the day prior to the conversion date and a 15% discount to the lowest daily closing “VWAP” of common stock during the five days prior to the conversion date. The Company, in accordance with EITF 98-5 and 00-27, utilized the Market approach to value the debt instrument and concluded that a beneficial conversion feature exists since the effective conversion price of shares was less than the stock price at commitment date. The 15% discount created a beneficial conversion feature at the commitment date aggregating $36,300 which is being accreted monthly from
F-14
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE E – NOTE PAYABLE TO CAETE INVEST & TRADE, S.A. (CONTINUED)
the issuance date of the promissory note through maturity and is being recorded as additional interest expense. On February 4, 2010, $62,428 of the loan was repaid. The loan balance is $179,572 at March 31, 2012 and 2011, respectively. On April 26, 2012, through an assignment of the Debt Agreement, Caete Invest & Trade, S.A. agreed to sell and/or assign the debt, including interest owed by the Company to a third party investor/shareholder of the Company who repaid Caete Invest & Trade, S.A.. The assignment transferred to the individual any and all rights, interests and claim arising under the original note agreement. On May 21, 2012, the note was converted into 2,500,000 shares of the Company’s common stock.
NOTE F – NOTE PAYABLE TO CHIEF EXECUTIVE OFFICER
On January 21, 2010, the Company owed its Chief Executive Officer approximately $376,560 for salary and expenditures paid by him on behalf of the company. The company and its Chief Executive Officer agreed to formalize a portion of the debt and issued a $172,364 promissory note maturing on January 21, 2012. The note bears interest at the rate of 10% per annum and is payable at maturity. The face amount of the loan plus accrued interest is convertible into unregistered common stock of the company at the lesser of 100% of the volume weighted average price (“VWAP”) of common stock as reported by Bloomberg L.P. on the day prior to the conversion date and a 15% discount to the lowest daily closing “VWAP” of common stock during the five days prior to the conversion date. The Company, in accordance with EITF 98-5 and 00-27, utilized the Market approach to value the debt instrument and concluded that a beneficial conversion feature exists since the effective conversion price of shares was less than the stock price at commitment date. The 15% discount created a beneficial conversion feature at the commitment date aggregating $55,923 which will be accreted monthly from the issuance date of the promissory note through maturity and will be recorded as additional interest expense. During the year ended March 31, 2012, $120,000 of the note was repaid. Accordingly, at March 31, 2012 and 2011,the loan balance is $52,364 and $161,371, respectively, net of the unamortized discount of $10,993 at March 31, 2012.
NOTE G – CONVERTIBLE DEBENTURE
On January 26, 2010, the Company issued at par, a $500,000 Secured Convertible Debenture maturing on January 26, 2011. The debenture bore interest at the rate of 10% per annum and is payable monthly. The Company had granted a security interest in substantially all of the assets of the Company as collateral for the debenture. The face amount of the loan plus accrued interest is convertible into unregistered common stock of the company at the lesser of 100% of the volume weighted average price (“VWAP”) of common stock as reported by Bloomberg L.P. on the day prior to the conversion date and a 15% discount to the lowest daily closing “VWAP” of common stock during the five days prior to the conversion date. Additionally, the Company issued commitment shares totaling 6,085,193 equivalent to $1,500,000 at the closing date to obtain the loan. The Company in accordance with APB 14 utilized the Market Approach to value the debt instrument and allocated the net proceeds from the issuance of the debenture based upon the pro rata portion of the face value of the debentures and the undiscounted value of the commitment shares. Additionally, 15% of the Debenture was allocated to a beneficial conversion feature in accordance with EITF 98-5 and EITF 00-27. The Company concluded that the 15% discount created a beneficial conversion feature at the commitment date since the effective conversion price of the
F-15
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE G – CONVERTIBLE DEBENTURE (CONTINUED)
shares was less than the stock price at the commitment date. The beneficial conversion feature and the pro rata value of the commitment shares aggregated $395,521 and was accreted monthly from the issuance date of the Debenture through maturity and was recorded as additional interest expense.
At March 31, 2011, the Company recorded the 15% redemption premium which is required upon repayment, and the additional 5% interest penalty resulting from the loan being in default for non-payment of interest.
On June 1, 2011, the Company’s secured convertible debenture was transferred to Green Eagle Capital Corp., a corporation controlled by a shareholder of the Company through a debt assignment instrument. Green Eagle Capital Corp., in a negotiated settlement, liquidated the debt. The assignment transferred to Green Eagle Capital Corp. any and all rights interests and claims arising under the original debenture agreement.
On July 11, 2011, Green Eagle Capital Corp., as principal agent for a group of investors, converted the secured convertible debenture into 10,000,000 shares of the Company’s common stock. In connection with the conversion, the Company incurred a loss on the extinguishment of debt in accordance with ASC Topic 470-60 Debt. Under the terms of the original conversion agreement, the Company would have issued 6,678,320 shares based upon the conversion provisions. Under the debt assignment and new conversion agreement, an additional 3,321,680 shares were issued valued at the conversion price in the original conversion agreement. The additional shares represent a loss on extinguishment of debt aggregating $336, 836.
NOTE H – STOCKHOLDERS’ DEFICIT
On June 7, 2010, the Company entered into a six month consulting agreement with an individual to assist the Company in developing a public relations strategy, new investor awareness strategies and communications. The consulting contract called for a monthly cash payment of $2,000 and 5,000 shares per month. The shares were to be valued at $0.05 per share, the value at commitment date. On December 1, 2010 the Company terminated the agreement. As a result, the Company agreed to issue 30,000 common shares in settlement.
On August 1, 2010, the Company entered into a consulting agreement with an individual to assist the Company in developing a business strategy, an acquisition strategy and other services. The term of agreement is one year commencing July 15, 2010. The individual received 1,500,000 shares at $0.11 per share aggregating $165,000. The compensation is being recorded on a monthly basis. On March 28, 2011, the contract was modified increasing the compensation to 4,500,000 shares. The additional 3,000,000 shares were valued at $0.09, the fair value on date of commitment and vested immediately.
On August 18, 2010, the Board approved the issuance of 1,200,000 shares of its common stock to its Chief Executive in accordance with his amended employment contract. The shares vest at the rate of 50,000 shares per month over a 24 month period commencing June 18, 2010 and have been valued at $0.18 per share, the fair market value at the date of commitment.
F-16
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE H - STOCKHOLDERS’ DEFICIT (CONTINUED)
During the twelve months ended March 31, 2011, the Company, under various private placement agreements, sold 1,550,000 shares of its common stock at $0.10 per share aggregating $155,000 and 10, 278,500 shares of its common stock at $0.05 aggregating $543,796.
On February 1, 2011, the Company entered into a consulting agreement with an individual to assist the Company in developing business and acquisition strategies and any other services mutually agreed to between the Company and consultant. The term of the agreement is for one year. The compensation, received was 1,000,000 shares of the Company’s common stock which vested immediately. The shares were valued at $0.08 per share, the fair market value of the stock as at the date of commitment.
During the year ended March 31, 2011, the Company recorded stock based compensation of $682,533 for 1,241,586 shares vested relating to consulting agreements at a price range of $0.10 to $1.00 per share.
During the year ended March 31, 2012, the Company sold 13,450,000 shares of its common stock, under private placement agreements, at $0.05 per share aggregating $672,500 and 22,853,560 shares of its common stock @ $0.10 per share aggregating $2,285,356.
On June 15, 2011, the Company entered into a consulting agreement with CSIR Group, Inc. to assist in general business strategies. In consideration for the services to be rendered, the Company issued 500,000 shares of its common stock at $0.12 per share, the commitment date value aggregating $60,000. The amount has been recorded as stock based compensation. The agreement was terminated in August 2011 and the shares are currently under administrative hold waiting for settlement between the parties.
On June 15, 2011, the Company entered into a consulting agreement with SARLA Group, SA to assist in general business strategies. In consideration for the services to be rendered, the Company issued 1,000,000 shares of its common stock at $0.11 per share, the commitment date value aggregating $110,000. The amount has been recorded as stock based compensation.
On June 15, 2011, the Company entered into a consulting agreement with Octave LG Investment, Inc. to assist in general business strategies. In consideration for the services to be rendered, the Company issued 1,000,000 shares of its common stock at $0.11 per share, the commitment date value aggregating $110,000. The amount has been recorded as stock based compensation.
On July 1, 2011, the Company converted $78,000 of unpaid rent for its headquarters to common stock. The conversion rate was at $0.11 per share the date on which the conversion occurred. Accordingly, the Company issued 709,090 shares.
On July 15, 2011, the Company issued 465,000 shares of its common stock to a former consultant in settlement of a consulting agreement. The shares were valued at $0.10 per share. Accordingly, stock based compensation in the amount of $46,500 was recorded.
On February 15 and 28, 2012, the Company entered into a consulting agreements with Bridgeview Capital to assist the Company in developing a business strategy, assist in capital introductions and other mutually agreed upon services. In consideration for these services, the Company issued 3,000,000 shares of its common stock valued at $0.14 per share, the fair market value at the date of commitment. The shares vest immediately.
F-17
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE H - STOCKHOLDERS’ DEFICIT (CONTINUED)
On February 15, 2012, the Company entered into a consulting agreement with an individual to assist the Chief Executive Officer with day to day operating activities. In consideration for these services, the Company, in addition to paying bi-weekly compensation, issued 250,000 shares vesting immediately. The shares were valued at $0.14, the fair market value at the date of commitment.
On February 28, 2012, the Company entered into a consulting agreement with an individual to provide capital introduction and other services as mutually agreed upon with the Company. In consideration for these services, the Company issued 1,195,000 shares of its common stock valued at $0.14 per share. The shares vest immediately.
On February 28, 2012, the Company entered into a consulting agreement with Rubicon Capital Advisors, LLC to assist the Company in developing marketing and investor relations strategies and other services as mutually agreed to by the Company and consultant. In consideration for these services, the Company issued 2,500,000 shares of its common stock valued at $0.14 per share. The shares are considered earned as of the date of this agreement.
On February 28, 2012, the Company entered into a consulting agreement with Sirton International, Inc. to assist the company in developing a marketing and investor relations, assist the Company in developing an acquisition strategy and structure with the European market and other services as mutually agreed to by the Company and consultant. In consideration for these services, the Company issued 5,400,000 shares of its common stock valued at $0.14 per share, the fair value of the stock at the date of commitment. The shares are considered earned as of its date of agreement.
In connection with settlement agreements dated February 21 and 23, 2012, the Company issued 1,100,000 shares of its common stock valued at $0.14 per share to Satellite Advisors Group, LLC and Dr. Stella Snug.
NOTE I - PROVISION FOR INCOME TAXES
Deferred income taxes are determined using the liability method for the temporary differences between the financial reporting basis and income tax basis of the Company’s assets and liabilities. Deferred income taxes are measured based on the tax rates expected to be in effect when the temporary differences are included in the Company’s tax return. Deferred tax assets and liabilities are recognized based on anticipated future tax consequences attributable to differences between financial statement carrying amounts of assets and liabilities and their respective tax bases.
|
Deferred tax assets consist of the following:
|
March 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Net operating losses
|
$ | (2,300,000 | ) | 960,000 | ||||
|
Valuation allowance
|
(2,300,000 | ) | (960,000 | ) | ||||
| $ | - | $ | - | |||||
F-18
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE I - PROVISION FOR INCOME TAXES (CONTINUED)
At March 31, 2012, the Company had a U.S. net operating loss carryforward in the approximate amount of $7,700,000 available to offset future taxable income through 2031. The Company established valuation allowances equal to the full amount of the deferred tax assets due to the uncertainty of the utilization of the operating losses in future periods. The Company also has a Canadian carry forward loss which approximates $500,000 and is available to offset future taxable income through 2032.
A reconciliation of the Company’s effective tax rate as a percentage of income before taxes and federal statutory rate for the periods ended March 31, 2012 and 2011 is summarized as follows:
|
2012
|
2011
|
|||||||
|
Federal statutory rate
|
(34.00 | )% | (34.00 | )% | ||||
|
State income taxes, net of federal benefits
|
3.3 | 3.3 | ||||||
|
Valuation allowance
|
30.7 | 30.7 | ||||||
| 0 | % | 0 | % | |||||
NOTE J - WARRANTS
The following table summarizes the activity of the warrants outstanding as at March 31, 2012:
|
|
Number of
Warrants
|
Exercise
Price
|
Expiration
Date
|
||||||||
|
Balance March 31, 2010
|
- | ||||||||||
|
Issued
|
|||||||||||
|
Private placements
|
194,465 | $ | 0.75 |
7/2012 to 11/2012
|
|||||||
|
Consulting contracts
|
400,000 | $ | 0.35 to $0.40 |
8/2014
|
|||||||
|
Cancelled-consulting contract
|
(200,000 | ) | $ | 0.35 | |||||||
|
Exercised
|
- | ||||||||||
|
Balance March 31, 2011 and 2012
|
394,465 | ||||||||||
Under the private placements agreements, each warrant entitles the holder to purchase one share of the Company’s common stock for $0.75 per share and the warrants expire three years from the date of issuance.
The warrants were valued utilizing the following assumption employing the Black-Scholes Pricing Model:
|
Consulting
|
Private
|
|||||||
|
Agreements
|
Placements
|
|||||||
|
Volatility
|
84.23% to 190.65%
|
94.99% to 195.52%
|
||||||
|
Risk-free rate
|
2.51% to 2.68%
|
1.42% to 1.52%
|
||||||
|
Dividend
|
- | - | ||||||
|
Expected life of warrants
|
5 | 3 | ||||||
NOTE K - STOCK OPTIONS
On February 1, 2012, the Company awarded 5,000,000 options to purchase common shares to its’ Chief Executive Officer and 5,000,000 options to purchase common shares to a consultant. These options
F-19
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE K - STOCK OPTIONS (CONTINUED)
vested immediately and were for services performed. The Company recorded stock-based compensation expense of $1,400,000 for the issuance of these options. The following weighted average assumptions were used for Black-Scholes option-pricing model to value these stock options:
|
Volatility
|
220 | % | ||
|
Expected dividend rate
|
- | |||
|
Expected life of options in years
|
10 | |||
|
Risk-free rate
|
1.87 | % |
A summary of option activity as of March 31, 2012, and changes during the period then ended, is presented below:
|
Weighted
|
||||||||||||||||
|
Weighted
|
Average
|
|||||||||||||||
|
Average
|
Remaining
|
Aggregate
|
||||||||||||||
|
Exercise
|
Number of
|
Contractual
|
Intrinsic
|
|||||||||||||
|
Options
|
Price
|
Shares
|
Term
|
Value
|
||||||||||||
|
Balance March 31, 2011
|
- | - | - | - | ||||||||||||
|
Options granted
|
$ | 0.10 | 10,000,000 | |||||||||||||
|
Options exercised
|
||||||||||||||||
|
Options cancelled/forfeited
|
- | - | - | - | ||||||||||||
|
Balance at March 31, 2012
|
$ | 0.10 | 10,000,000 | 9.85 | $ | 400,000 | ||||||||||
|
Exercisable at March 31, 2012
|
$ | 0.10 | 10,000,000 | 9.85 | $ | 400,000 | ||||||||||
The weighted-average grant-date fair value of options granted during the year ended March 31, 2012 was $0.14.
NOTE L - COMMITMENTS AND CONTINGENCIES
On June 18, 2010, the Company amended the Chief Executive’s employment contract whereby he will receive 1,200,000 shares of Company common stock and vest at the rate of 50,000 shares per month over a 24 month period commencing June 18, 2010.
On January 31, 2012, the Company entered into a three year lease for its corporate office. This requires a monthly payment of $2,150 per month. Required annual payments are as follows: 2013-$19,350; 2014-$25,800; and 2015-$6,450.
NOTE M - SUBSEQUENT EVENTS
Subsequent to the year ended March 31, 2012, the Company through various private placements sold approximately 9,268,000, shares of its common stock at $0.10 per share aggregating $926,800.
On May 15, 2012, the Company entered into a consulting agreement with an individual for a period of 36 months to assist the Company in securing a qualified management team, develop acquisition strategies and other mutually agreed services. In consideration for such services, the Company agreed to pay $6,000 per month and issued 2,500,000 shares of its common stock vesting immediately and considered earned upon issuance. Additionally, should the Company raise a total of $7,500,000 in equity financing,
F-20
IMMUNOVATIVE, INC. AND SUBSIDIARY
(Formerly Novo Energies Corporation and Subsidiary)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2012
NOTE M - SUBSEQUENT EVENTS (CONTINUED)
the consultant shall receive an additional 2,500,000 shares of the Company’s common stock at current prices.
On May 15, 2012, the Company amended the Chief Executive Officer’s employment agreement awarding him an additional 2,500,000 shares of the Company’s common stock. Additionally, should the Company raise a total of $7,500,000 in equity financing, he will earn an additional 2,500,000 shares of the Company’s common stock at $0.10 per share.
F-21
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
We have had no disagreements with our principal independent accountants.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
The Company’s Chief Executive Officer and Chief Financial Officer have evaluated the effectiveness of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the year ended March 31, 2012 covered by this Form 10-K. Based upon such evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that, as of the end of such period, the Company’s disclosure controls and procedures were not effective as required under Rules 13a-15(e) and 15d-15(e) under the Exchange Act.
Management’s Annual Report on Internal Control Over Financial Reporting
The management of the Company is responsible for the preparation of the consolidated financial statements and related financial information appearing in this Annual Report on Form 10-K. The consolidated financial statements and notes have been prepared in conformity with accounting principles generally accepted in the United States of America. The management of the Company is also responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. A company's internal control over financial reporting is defined as a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that:
|
▪
|
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company;
|
|
▪
|
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the issuer are being made only in accordance with authorizations of management and directors of the Company; and
|
|
▪
|
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company's assets that could have a material effect on the financial statements.
|
Management, including the Chief Executive Officer and Chief Financial officer, does not expect that the Company's disclosure controls and internal controls will prevent all error and all fraud. Because of its inherent limitations, a system of internal control over financial reporting can provide only reasonable, not absolute, assurance that the objectives of the control system are met and may not prevent or detect misstatements. Further, over time, control may become inadequate because of changes in conditions or the degree of compliance with the policies or procedures may deteriorate.
With the participation of the Chief Executive Officer and Chief Financial Officer, our management evaluated the effectiveness of the Company's internal control over financial reporting as of March 31, 2012 based upon the framework in Internal Control –Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on that evaluation, our management has concluded that, as of March 31, 2012, our internal controls over financial reporting were not effective. Specifically, management identified the following material weaknesses at March 31, 2012:
|
1.
|
Lack of oversight by independent directors in the establishment and monitoring of required internal controls and procedures;
|
|
2.
|
Lack of functioning audit committee, resulting in ineffective oversight in the establishment and monitoring of required internal controls and procedures;
|
|
3.
|
Insufficient personnel resources within the accounting function to segregate the duties over financial transaction processing and reporting and to allow for proper monitoring controls over accounting;
|
|
4.
|
Insufficient written policies and procedures over accounting transaction processing and period end financial disclosure and reporting processes.
|
34
To remediate our internal control weaknesses, management intends to implement the following measures:
|
▪
|
The Company will add sufficient number of independent directors to the board and appoint an audit committee.
|
|
▪
|
The Company will add sufficient knowledgeable accounting personnel to properly segregate duties and to effect a timely, accurate preparation of the financial statements.
|
|
▪
|
Upon the hiring of additional accounting personnel, the Company will develop and maintain adequate written accounting policies and procedures.
|
The additional hiring is contingent upon the Company’s efforts to obtain additional funding through equity or debt for its continued operational activities and corporate expenses. Management expects to secure funds in the coming fiscal year but provides no assurances that it will be able to do so.
We understand that remediation of material weaknesses and deficiencies in internal controls are a continuing work in progress due to the issuance of new standards and promulgations. However, remediation of any known deficiency is among our highest priorities. Our management will periodically assess the progress and sufficiency of our ongoing initiatives and make adjustments as and when necessary.
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant rules of the SEC that permit us to provide only management’s report in this annual report. On July 21, 2010, President Obama signed the Dodd-Frank Wall Street Reform and Consumer Protection Act. Included in the Act is a provision that permanently exempts smaller public companies that qualify as either a Non-Accelerated Filer or Smaller Reporting Company from the auditor attestation requirement of Section 404(b) of the Sarbanes-Oxley Act of 2002.
Changes in Internal Control over Financial Reporting
Except as set forth above, there were no changes in our internal control over financial reporting that occurred during the period covered by this report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on the Effectiveness of Controls
The Company’s management, including the CEO and CFO, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of the control system must reflect that there are resource constraints and that the benefits must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
ITEM 9B. OTHER INFORMATION
On May 15, 2012, the Company amended the Chief Executive Officer’s employment agreement awarding him an additional 2,500,000 shares of the Company’s common stock. Additionally, should the Company raise a total of $7,500,000 in equity financing, he will earn an additional 2,500,000 shares of the Company’s common stock at $0.10 per share.
35
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS; COMPLIANCE WITH SECTION 16(A) OF THE EXCHANGE ACT
OFFICERS AND DIRECTORS
Mr. Antonio Treminio is the sole director of the Company. The Company's directors are elected at each Annual Meeting of Shareholders. The sole director currently serving on the Company's Board and the executive officers are set forth in the table below:
|
Name
|
Age
|
Positions and Offices With The Company
|
||
|
Antonio Treminio
|
41
|
Chief Executive Officer and Chairman of the Board
|
||
|
David E. Price, Esq.
|
48
|
Secretary |
No director holds any directorship in a company with a class of securities registered pursuant to Section 12 of the Securities Exchange Act of 1934 or subject to the requirements of Section 15(d) of such Act. No director holds any directorship in a company registered as an investment company under the Investment Company Act of 1940.
As the Board of Directors only has one director and two employees, no Audit or Strategy Committee has been established. The Company does not have a standing nominating committee or any committee performing a similar function. For the above reasons, the Company has not adopted a code of ethics.
The following is a biographical summary of the directors and officers of the Company:
Antonio Treminio
Since 1996, Mr. Antonio Treminio has been involved as a consultant to public traded companies, participating in structuring mergers and acquisition, re-capitalization, financing in the mining / precious metals & energy sector. Since 2003, Mr. Treminio has been the president of Lusierna Asset Management Ltd. In 1993, after finishing his attending his studies in Business Administration at Loyalist College in Belleville, Ontario, Mr. Treminio started his career in the private banking sector with Dean Witter Reynolds. In 1995, he joined PaineWebber to further his career while focusing on establishing Strategic Alliances and/or Referral Agreements with top-tier Latin American financial institutions.
David E. Price, Esq.
On June 1 2012, David E. Price Esq., was appointed as Company Secretary. Mr. Price is a Corporate Securities Attorney located in Washington, DC. He has broad experience in US and international corporate transactions. He is a member of the Corporate Lawyer’s Association; Euro-American Lawyers Group; Association of US Securities Attorneys; and the American Bar Association. He is also a Bar member of Maryland; United States District Court (District of Maryland); Court of Appeals, District of Columbia; United States District Court for the District of Columbia; United States Court of Appeals, 4th Circuit; and the Supreme Court of the United States.
36
COMPLIANCE WITH SECTION 16(a) OF THE
SECURITIES EXCHANGE ACT OF 1934
Section 16(a) of the Securities Exchange Act of 1934 requires executive officers and directors who beneficially own more than ten percent (10%) of the Company's Common Stock to file initial reports of ownership and reports of changes of ownership with the Securities and Exchange Commission. Executive officers, directors and greater than ten percent (10%) beneficial owners are required by Commission regulations to furnish the Company with copies of all Section 16(a) forms they file.
The information required to be compliant with Section 16(a) is found herein. However, at the present time the required individuals have not filed the appropriate Section 16(a) forms although it has been represented to the Company that such are being prepared and will be filed shortly after the filing of this annual report.
Except as set out below, the Company has not paid in either 2011 or 2010 any annual or long-term compensation through the latest practicable date to the Chief Executive Officer of the Company and sole director of the Company or to any executive officers of the Company or directors of the Company who held such positions during 2011.
|
SUMMARY COMPENSATION TABLE
|
|||||||||||||||||||||||||||||||
|
Name and
principal position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive
Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
All Other
Compensation
($)
|
Total ($)
|
||||||||||||||||||||||
|
Antonio Treminio
|
2010
|
120,000 | 120,000 | ||||||||||||||||||||||||||||
|
2011
|
120,000 | 120,000 | |||||||||||||||||||||||||||||
|
2012
|
120,000 | 108,000 | 700,000 | 928,000 | |||||||||||||||||||||||||||
|
Faisal Butt
|
2011
|
24,802 | 24,802 | ||||||||||||||||||||||||||||
|
Andre L’Heureux
|
2010
|
25,500 | 91,667 | 208,333 | 325,000 | ||||||||||||||||||||||||||
|
Hakim Zahar
|
2010
|
40,000 | 88,600 | 128,600 | |||||||||||||||||||||||||||
37
Employment Contracts
On May 1, 2009, the Company issued 3,000,000 shares of its common stock to Andre L’Heureux, President of the Company, in connection with his employment contract. The original contract specified that these shares vest at the rate of 83,333 per month over a three year period. On October 21, 2009, Mr. L’Heureux resigned as President and became Chief Technical Officer. On October 1, 2010, the Board modified the agreement to provide for the immediate vesting of all unearned shares. The shares were valued at $0.10 per share utilizing May 1, 2009 as the measurement date.
Effective October 23, 2009, the Company entered into an employment agreement with Hakim Zahar as the President of the Company. The agreement called for a base salary of $10,000 per month with payments starting November 15, 2009. The executive was to receive a minimum of 50,000 shares of the Company’s common stock per month starting on the effective date of this agreement. This agreement could be terminated by either party at will. On May 28, 2010, the Company terminated the contract and agreed to compensate Mr. Zahar through February 15, 2010. In accordance with the settlement agreement, 200,000 shares of the Company’s common stock were issued at a price of $0.44 per share based upon the commitment debt.
On July 10, 2009, the Company executed an employment agreement with Antonio Treminio to be the Company’s Chief Executive Officer and Chairman of the Board of Directors. The agreement can be terminated by either party. The agreement calls for a base salary of $10,000 per month effective September 22, 2008 and the issuance of incentive stock options equal to Five Percent (5%) of all of the Company’s issued and outstanding common stock on September 22, 2008 (the “Stock Options”). Twenty Percent (20%) of the Stock Options shall vest for each plastic and/or tire facility constructed during the five years subsequent to the date herein so long as each plastic facility has a capacity to process 15 tons of plastic waste per day and/or each tire facility has the capacity to process 30 tons of tire waste per day. One Hundred Percent (100%) of the Stock Options shall vest upon the occurrence of a Change of Control as it is defined in the employment agreement. The Company will create an incentive stock option plan and award stock options equal to 5% of the Company’s issued and outstanding common stock at the date of the agreement. As a result of the Company changing direction to Immune Therapy, the previous stock option plan is considered null and void. On June 18, 2010, the Company amended the agreement to award 1,200,000 shares of its common stock to be vested over the succeeding 24 months.
On January 21, 2010, the Company owed its Chief Executive Officer approximately $376,560 for salary and expenditures paid by him on behalf of the company. The company and its Chief Executive Officer agreed to formalize a portion of the debt and issued a $172,364 promissory note maturing on January 21, 2012. The note bears interest at the rate of 10% per annum and is payable at maturity. The face amount of the loan plus accrued interest is convertible into unregistered common stock of the company at the lesser of 100% of the volume weighted average price (“VWAP”) of common stock as reported by Bloomberg L.P. on the day prior to the conversion date and a 15% discount to the lowest daily closing “VWAP” of common stock during the five days prior to the conversion date. The Company, in accordance with EITF 98-5 and 00-27, utilized the Market approach to value the debt instrument and concluded that a beneficial conversion feature exists since the effective conversion price of shares was less than the stock price at commitment date. The 15% discount created a beneficial conversion feature at the commitment date aggregating $55,923 which will be accreted monthly from the issuance date of the promissory note through maturity and will be recorded as additional interest expense. During the year ended March 31, 2012, $120,000 of the note was repaid. Accordingly, at March 31, 2012 and 2011, the loan balance was $52,364 and $161,371, respectively, net of amortized discount of $10,993 at March 31, 2012.
38
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Security Ownership of Certain Beneficial Owners
The following table sets forth information regarding the beneficial ownership of the shares of the Common Stock (the only class of shares previously issued by the Company) at March 31, 2011 by (i) each person known by the Company to be the beneficial owner of more than five percent (5%) of the Company’s outstanding shares of Common Stock, (ii) each director of the Company, (iii) the executive officers of the Company, and (iv) by all directors and executive officers of the Company as a group. Each person named in the table, has sole voting and investment power with respect to all shares shown as beneficially owned by such person and can be contacted at the address of the Company.
|
Amount and Nature of Beneficial Owner (1)
|
Percent
of Class
|
|||||||
|
Antonio Treminio
|
12,509,524
|
9.3
|
%
|
|||||
|
650 Notre Dame West
|
||||||||
|
Apt 1101
|
||||||||
|
Montreal, QUE H3C 1J2
|
||||||||
|
Canada
|
||||||||
|
All executive officers and directors as a group (1 person)
|
12,509,524
|
9.3
|
%
|
|||||
|
Major Stockholders:
|
||||||||
|
Trafalgar Capital Specialized Investment Fund
|
8,585,193
|
6.4
|
%
|
|||||
|
The Dickens, Kirk Street
|
||||||||
|
16 Northington Street
|
||||||||
|
London WC1N 2DG
|
||||||||
(1) Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person’s actual ownership or voting power with respect to the number of shares of common stock actually outstanding as of the date of this Annual Report. As of the date of this Annual Report, there were 134,546,457 shares of common stock issued and outstanding.
Changes in Control
We are unaware of any contract, or other arrangement or provision, the operation of which may at a subsequent date result in a change of control of our Company.
39
On January 21, 2010, the Company owed its Chief Executive Officer approximately $376,560 for salary and expenditures paid by him on behalf of the company. The company and its Chief Executive Officer agreed to formalize a portion of the debt and issued a $172,364 promissory note maturing on January 21, 2012. The note bears interest at the rate of 10% per annum and is payable at maturity. The face amount of the loan plus accrued interest is convertible into unregistered common stock of the company at the lesser of 100% of the volume weighted average price (“VWAP”) of common stock as reported by Bloomberg L.P. on the day prior to the conversion date and a 15% discount to the lowest daily closing “VWAP” of common stock during the five days prior to the conversion date. The Company, in accordance with EITF 98-5 and 00-27, utilized the Market approach to value the debt instrument and concluded that a beneficial conversion feature exists since the effective conversion price of shares was less than the stock price at commitment date. The 15% discount created a beneficial conversion feature at the commitment date aggregating $55,923 which will be accreted monthly from the issuance date of the promissory note through maturity and will be recorded as additional interest expense. During the year ended March 31, 2012, $120,000 of the note was repaid. Accordingly, at March 31, 2012 and 2011, the loan balance was $52,364 and $161,371, respectively, net of the unamortized discount of $10,993 at March 31, 2012.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
|
Year Ended
March 31, 2012
|
Year Ended
March 31, 2011
|
|||||||
|
Audit Fees
|
$
|
85,000
|
$
|
100,000
|
||||
|
Audit Related Fees
|
$
|
0
|
$
|
0
|
||||
|
Tax Fees
|
$
|
0
|
$
|
0
|
||||
|
All Other Fees
|
$
|
0
|
$
|
0
|
||||
|
$
|
85,000
|
$
|
100,000
|
|||||
Audit Fees
Audit fees are the aggregate fees billed for professional services rendered by our independent auditors for the audit of our annual financial statements, the review of the financial statements included in each of our quarterly reports and services provided in connection with statutory and regulatory filings or engagements.
Audit Related Fees
Audit related fees are the aggregate fees billed by our independent auditors for assurance and related services that are reasonably related to the performance of the audit or review of our financial statements and are not described in the preceding category.
Tax Fees
Tax fees are billed by our independent auditors for tax compliance.
All Other Fees
All other fees include fees billed by our independent auditors for products or services other than as described in the immediately preceding three categories.
40
41
SIGNATURES
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
IMMUNOVATIVE, INC. (f/k/a NOVO ENERGIES CORPORATION)
|
/s/ Antonio Treminio
|
|
|
Name: Antonio Treminio
|
|
|
Title: Chairman of the Board, Chief Executive Officer,
|
|
|
Chief Financial Officer and Secretary
|
|
|
Date: July 16, 2012
|
42
