Attached files
| file | filename |
|---|---|
| 8-K - INSMED INCORPORATED 8-K 7-9-2012 - INSMED Inc | form8k.htm |
Exhibit 99.1

Developing Innovative Inhaled Treatments for Serious
Lung Infections
Lung Infections
July 2012

This presentation contains forward-looking statements which are made pursuant to provisions of Section 21E
of the Securities Exchange Act of 1934. Investors are cautioned that such statements in this presentation,
including statements relating to our financial position, projected year end cash and cash runway, the status
and the results of preclinical studies and clinical trials and preclinical and clinical data described herein, the
timing of responses to information and data requests from FDA, the development of our products, our
estimates of the size of the potential markets for our product candidates, and the business strategies,
evaluations, plans and objectives of management, constitute forward-looking statements which involve risks
and uncertainties that could cause actual results to differ materially from those anticipated by the forward-
looking statements. Our results may be affected by such factors as the receipt and timing of FDA and other
regulatory reviews and approvals, if at all, competitive developments affecting our product development,
delays in product development or clinical trials, and patent disputes involving currently developing products.
The risks and uncertainties include, without limitation, we may experience unexpected regulatory actions,
delays or requests, our future clinical trials may not be successful, we may be unsuccessful in developing our
product candidates or receiving necessary regulatory approvals, we may experience delays in our product
development or clinical trials, our product candidates may not prove to be commercially successful, our
expenses may be higher than anticipated and other risks and challenges detailed in our filings with the U.S.
Securities and Exchange Commission, including our Annual Report on Form 10-K for the year ended
December 31, 2011 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2012.
Investors are cautioned not to place undue reliance on any forward-looking statements which speak only as
of the date of this presentation. We undertake no obligation to publicly release the results of any revisions to
these forward-looking statements that may be made to reflect events or circumstances that occur after the
date of this release or to reflect the occurrence of unanticipated events.
of the Securities Exchange Act of 1934. Investors are cautioned that such statements in this presentation,
including statements relating to our financial position, projected year end cash and cash runway, the status
and the results of preclinical studies and clinical trials and preclinical and clinical data described herein, the
timing of responses to information and data requests from FDA, the development of our products, our
estimates of the size of the potential markets for our product candidates, and the business strategies,
evaluations, plans and objectives of management, constitute forward-looking statements which involve risks
and uncertainties that could cause actual results to differ materially from those anticipated by the forward-
looking statements. Our results may be affected by such factors as the receipt and timing of FDA and other
regulatory reviews and approvals, if at all, competitive developments affecting our product development,
delays in product development or clinical trials, and patent disputes involving currently developing products.
The risks and uncertainties include, without limitation, we may experience unexpected regulatory actions,
delays or requests, our future clinical trials may not be successful, we may be unsuccessful in developing our
product candidates or receiving necessary regulatory approvals, we may experience delays in our product
development or clinical trials, our product candidates may not prove to be commercially successful, our
expenses may be higher than anticipated and other risks and challenges detailed in our filings with the U.S.
Securities and Exchange Commission, including our Annual Report on Form 10-K for the year ended
December 31, 2011 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2012.
Investors are cautioned not to place undue reliance on any forward-looking statements which speak only as
of the date of this presentation. We undertake no obligation to publicly release the results of any revisions to
these forward-looking statements that may be made to reflect events or circumstances that occur after the
date of this release or to reflect the occurrence of unanticipated events.
Safe Harbor Statement
2
Insmed: Value Proposition
Attractive
Late-Stage
Opportunity
Late-Stage
Opportunity
w ARIKACE (liposomal amikacin for inhalation), is in Phase 3 (CLEAR-108) for
cystic fibrosis (CF) Pseudomonas (Pa) lung infections and Phase 2 (TARGET-
NTM) for non-TB mycobacteria (NTM) lung infections
cystic fibrosis (CF) Pseudomonas (Pa) lung infections and Phase 2 (TARGET-
NTM) for non-TB mycobacteria (NTM) lung infections
w ARIKACE has strong Phase 2 efficacy and safety data in CF
w Amikacin is an FDA-approved antibiotic, long recognized as one of the most
effective treatments for gram-negative infections
effective treatments for gram-negative infections
Compelling
Business Model
Business Model
w Two orphan indications with high unmet need and combined global market
potential of over $1 billion
potential of over $1 billion
w Limited commercial infrastructure required
w Strong IP and potential for extended exclusivity
Strong Balance
Sheet &
Experienced
Management
Sheet &
Experienced
Management
w As of 3/31/12, company reported ~$73 million in cash, investments & CD
w We believe cash is sufficient to take Company through the availability of
top-line data for both CF CLEAR-108 trial and TARGET-NTM trial
top-line data for both CF CLEAR-108 trial and TARGET-NTM trial
w Management has extensive anti-infective development, regulatory, and
commercial experience
commercial experience
ARIKACE®* is a highly differentiated product that offers a compelling
business opportunity in two orphan diseases
business opportunity in two orphan diseases
* ARIKACE® is a registered trademark of Insmed Incorporated
3
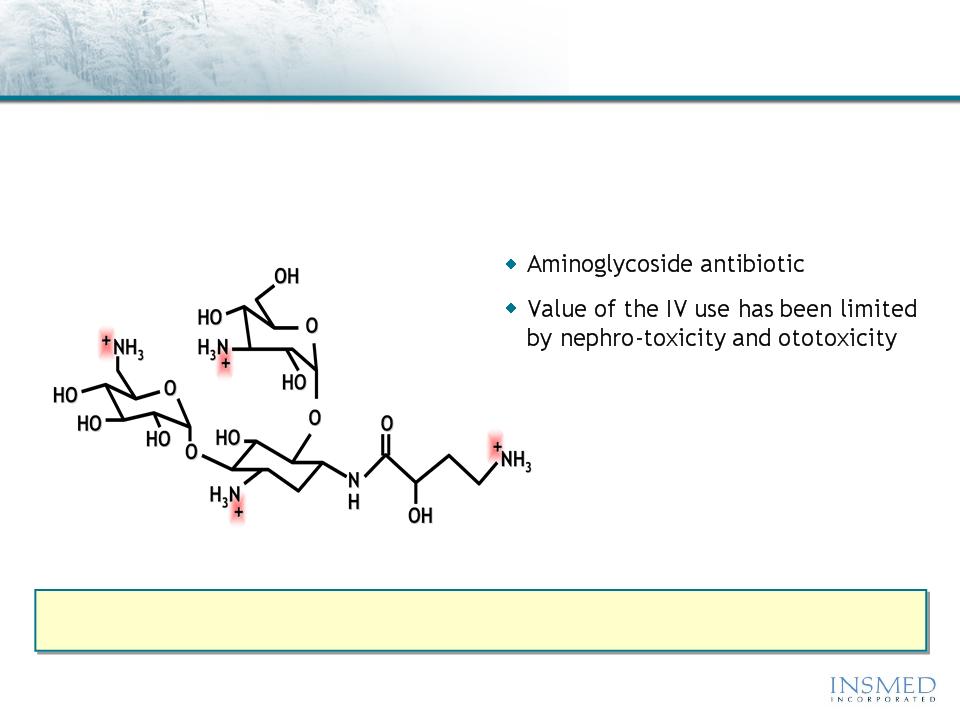
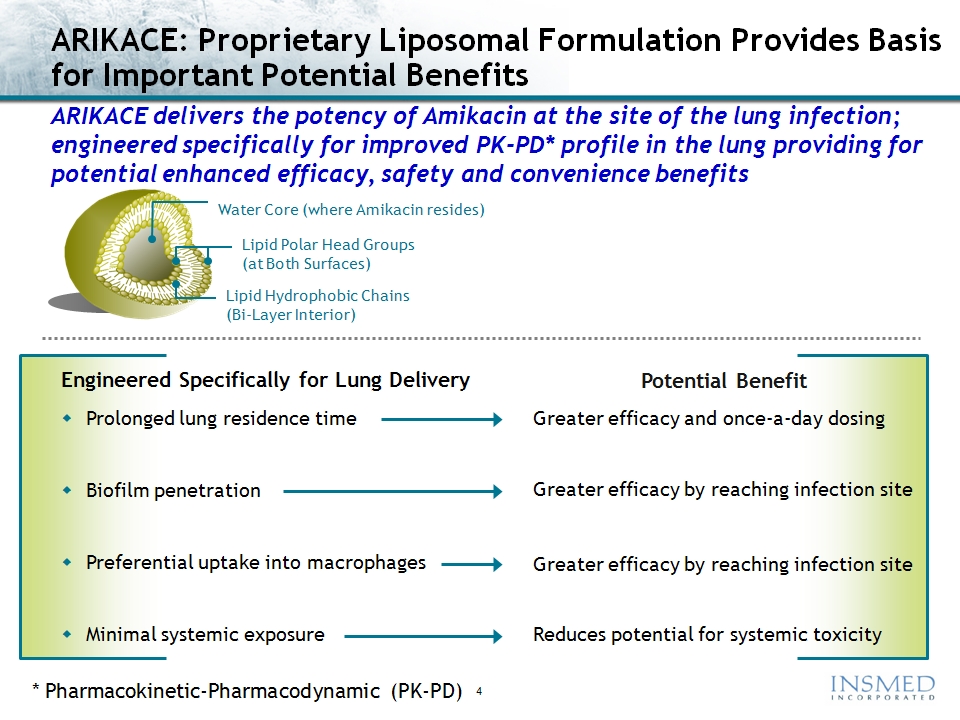
ARIKACE: Amikacin Summary
Amikacin is an FDA-approved antibiotic with proven efficacy in the
treatment of gram-negative infections, including Pseudomonas and NTM
treatment of gram-negative infections, including Pseudomonas and NTM
ARIKACE (liposomal amikacin for inhalation) delivers high, sustained levels of drug to
the lung while reducing systemic exposure to well below established toxicity levels
the lung while reducing systemic exposure to well below established toxicity levels

5
ARIKACE: Delivery Using Proprietary eFlow® Technology
ARIKACE is delivered once daily via the state-of-the-art PARI Optimized,
Investigational eFlow Nebulizer System with Advanced Mesh Technology
Investigational eFlow Nebulizer System with Advanced Mesh Technology
w Fast drug delivery with efficient
lung deposition
lung deposition
w Small, portable, silent and
cordless device weighs less than
10 ounces.
cordless device weighs less than
10 ounces.
w eFlow Technology Device
exclusivity from PARI Pharma for
15 years after first commercial
sale of ARIKACE
exclusivity from PARI Pharma for
15 years after first commercial
sale of ARIKACE
* eFlow® is a registered trademark of PARI Pharma GmbH
6
ARIKACE: Development Plan
Target-NTM
Study in U.S.
Study in U.S.
w ARIKACE vs. placebo in recalcitrant patients who are on a stable ATS/IDSA
guidelines-based multi-drug treatment regimen; N ≈ 100
guidelines-based multi-drug treatment regimen; N ≈ 100
w No inhaled antibiotics approved for treating NTM lung infections and little
known competitive activity in clinic
known competitive activity in clinic
w Study initiated in May-2012 → top-line results from randomized portion of
trial projected in 4Q13
trial projected in 4Q13
CLEAR-109
CF Pseudomonas
Study for U.S.
CF Pseudomonas
Study for U.S.
w FDA removed the clinical hold for CF Pa Phase 3 study in May
w Insmed will defer plans to initiate a Phase 3 study of ARIKACE in the U.S. for
CF patients until the Company reviews top-line results from CLEAR-108
CF patients until the Company reviews top-line results from CLEAR-108
Insmed is focusing on CLEAR-108 (CF Pa Phase 3 Study) and TARGET-NTM
(NTM Phase 2 Study)
(NTM Phase 2 Study)
CLEAR-108
CF Pseudomonas
Study for
EU/Canada
CF Pseudomonas
Study for
EU/Canada
w ARIKACE vs. Tobi® (inhaled tobramycin solution); N ≈ 300
w Builds off of strong Phase 2 efficacy and safety data
w Broad population with preferred trial design
w Trial initiated in April 2012 → top-line results projected in mid-2013
w Eligible patients roll-over into open-label ARIKACE® long term safety and
tolerability study, CLEAR-110
tolerability study, CLEAR-110
* Tobi® is a Registered Trademark of Novartis Pharmaceuticals Corporation
7
Arikace—Cystic Fibrosis
Epidemiology and Disease Description
Epidemiology and Disease Description
Cystic fibrosis is a life-threatening disease with significant unmet needs
that is growing in prevalence
that is growing in prevalence
7
w Affects about 70,000 children
and adults worldwide (30,000 in
U.S. and Europe, each)
and adults worldwide (30,000 in
U.S. and Europe, each)
w Inherited disease that causes
thick, sticky mucus to build up
in the lungs
thick, sticky mucus to build up
in the lungs
w Despite expanded use of current
products, lung function often
continues to decline
products, lung function often
continues to decline
w High treatment burden →
major compliance issue
major compliance issue
Source: Adapted from Cystic Fibrosis Foundation, Patient Registry
Annual Data Reports 2010
Annual Data Reports 2010
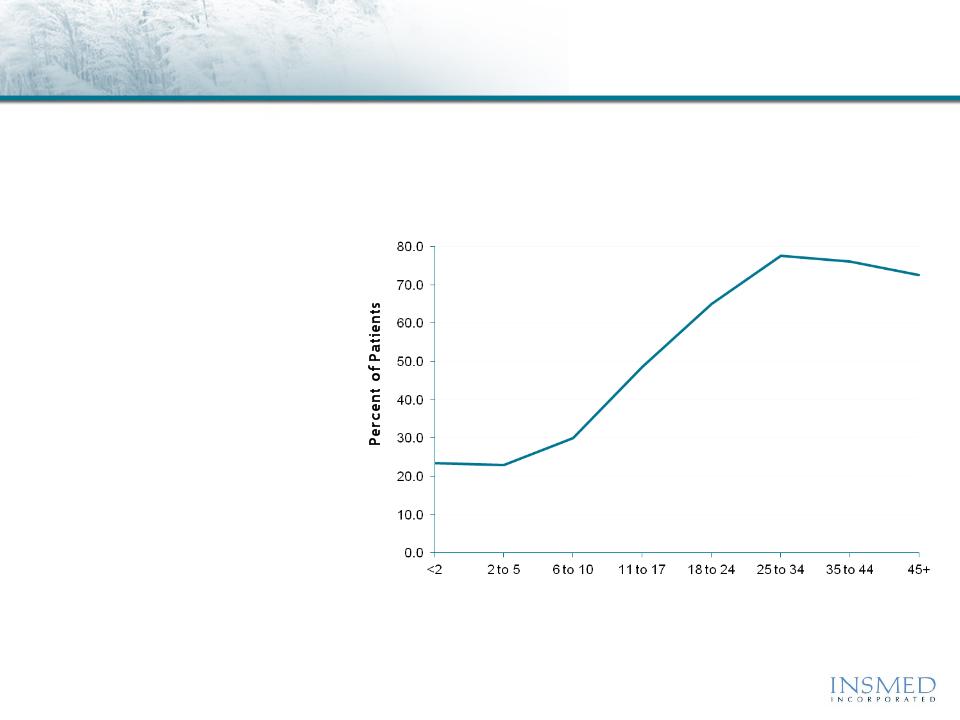
Mean = 51.2%
Pseudomonas Lung Infections Increase with Patient Age
Age (Years)

8
ARIKACE: Cystic Fibrosis
Need for New Inhaled Antibiotics
Need for New Inhaled Antibiotics
Current inhaled antibiotics produce modest efficacy in a limited patient
population providing an opportunity for ARIKACE to become first-line
treatment
population providing an opportunity for ARIKACE to become first-line
treatment
w Current inhaled antibiotics are not indicated for a significant segment of the
CF population -- patients with FEV-1 % predicted of greater than 75%
CF population -- patients with FEV-1 % predicted of greater than 75%
w Improvement in lung function with current inhaled antibiotics is not sustained
in the off-treatment period, and appears to decline over multiple cycles
in the off-treatment period, and appears to decline over multiple cycles
w Lung function continues to decline at an average rate of 1% to 3% per year
with some patients experiencing much greater declines
with some patients experiencing much greater declines
9
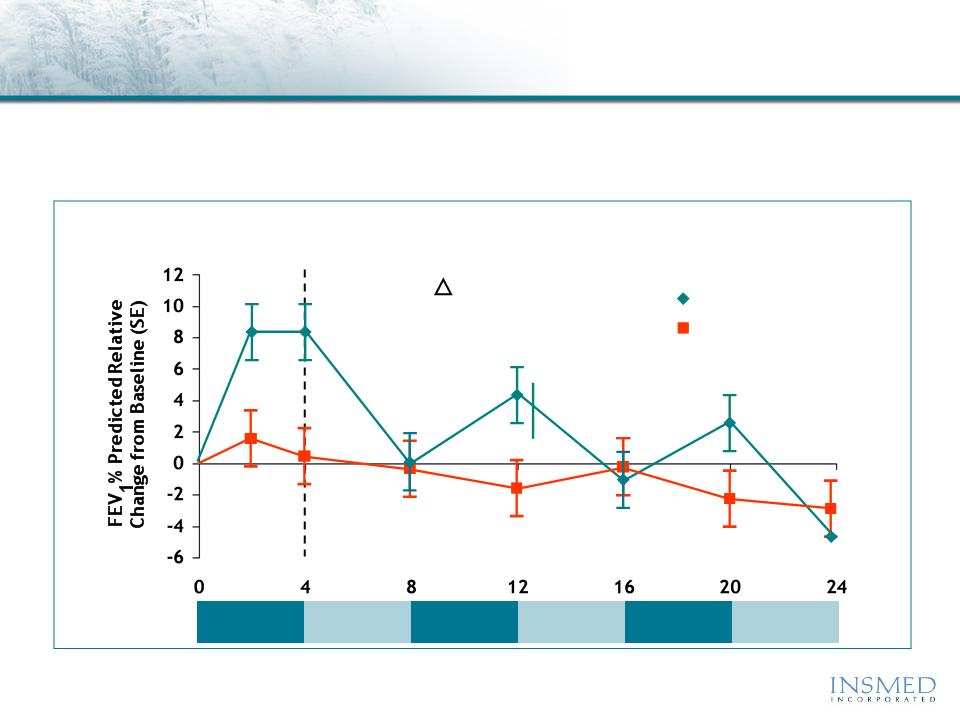
Cayston® vs. Tobi®
CF Phase 3 Trial Results: Pulmonary Function
Lung Function
Adjusted Mean Relative Change in FEV1 % Predicted
Source: 2010 North American CF Conference Poster 305 and Slide Presentation, 10/10.
* Cayston® (aztreonam for inhalation solution) is a registered trademark of Gilead Sciences.
** Tobi® (Tobramycin Inhalation Solution) is a registered trademark of Novartis.
*** AZLI = Cayston; TIS = Tobi
Lung function returned to baseline or lower during each off treatment
period and at the end of 24 weeks, both treatment groups showed a
decline in lung function from baseline
period and at the end of 24 weeks, both treatment groups showed a
decline in lung function from baseline
Week:
2
AZLI
TIS
+ 7.8
P = 0.0001
95% CI (3.86, 11.73)
|
AZLI/ TIS
28 Days
|
|
AZLI/ TIS
28 Days
|
|
AZLI/ TIS
28 Days
|
|
10
P = 0.033
P = 0.003
(36/36)
(36/35)
(33/36)
(32/35)
(34/35)
(34/34)
(N=ARIKACE/Placebo)
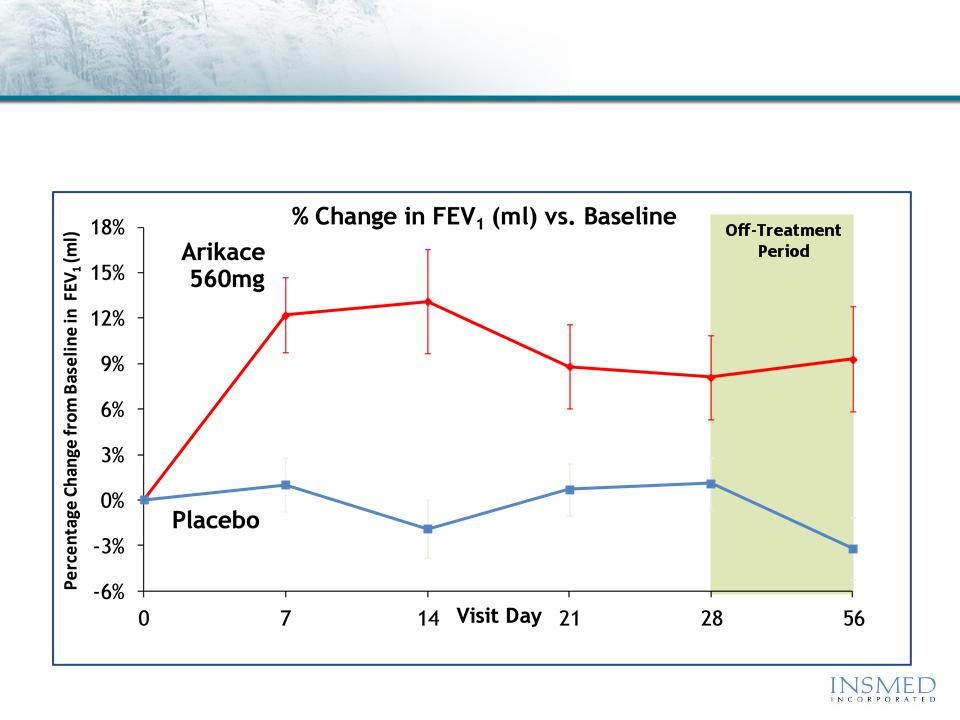
ARIKACE: Cystic Fibrosis
Phase 2 Pooled Results (560mg QD): Pulmonary Function
Phase 2 Pooled Results (560mg QD): Pulmonary Function
(N)
Mean (SE)
ARIKACE demonstrated statistically significant and clinically meaningful
improvement in pulmonary function throughout the 28-day treatment
period that was sustained through the off-treatment period
improvement in pulmonary function throughout the 28-day treatment
period that was sustained through the off-treatment period
11
Visit Days
Patients Receiving 560 mg ARIKACE Once Daily for 28 Days and Off-Treatment for 56 Days
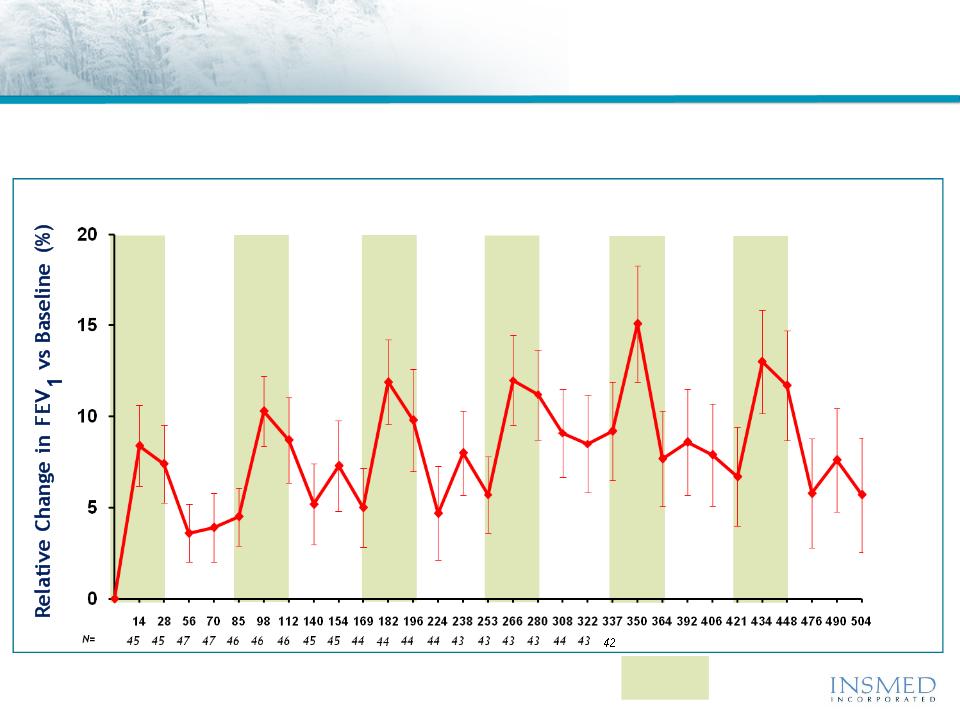
ARIKACE: Cystic Fibrosis
Open Label Extension (TR02-105): Durability of Response
Open Label Extension (TR02-105): Durability of Response
42
41
42
41
41
41
41
41
45
Treatment
Period
* Significance at end of treatment over 6 cycles
** Significance 56 days off-treatment over 6 cycles
p=0.0001**
p<0.0001*
41
47
Cycle
1
Cycle
2
Cycle
3
Cycle
4
Cycle
5
Cycle
6
An open label extension study demonstrated the sustained efficacy
of ARIKACE during and between multiple cycles of therapy

12
ARIKACE: Cystic Fibrosis
Phase 3 Program Has Been Initiated in Europe and Canada
Phase 3 Program Has Been Initiated in Europe and Canada
Insmed has reached agreement with EMA and Health Canada on pivotal study
requirements for CF patients with Pseudomonas lung infections
requirements for CF patients with Pseudomonas lung infections
w CLEAR-108: Phase 3 Primary Efficacy Study (vs. Tobi®, N ≈ 300)*
– Primary End-Point: Relative Change in FEV-1 at week 24
• Key Secondary End-Point: Time to First Pulmonary Exacerbation
– Patient Population: Patients ages 6 and above with FEV-1 % Predicted ≥ 25%
– Approximately 260 patients required to demonstrate non-inferiority at agreed upon
margin with 80% power
margin with 80% power
– Top-Line results projected in mid-2013
* Patients who complete CLEAR-108 are eligible to participate in CLEAR-110, which is a long term open
-label extension study in which patients receive ARIKACE every other month for up to 2 years
-label extension study in which patients receive ARIKACE every other month for up to 2 years
13
ARIKACE: Non-TB Mycobacteria
Disease Description and High Unmet Need
Disease Description and High Unmet Need
w NTM are intracellular organisms that invade and multiply chiefly within macrophages in
the lung and are characteristically resistant to most antibiotics
the lung and are characteristically resistant to most antibiotics
w NTM lung infections occurs commonly in patients with structural lung disease (e.g.
COPD, bronchiectasis and CF) and in postmenopausal women without clear risk factors
COPD, bronchiectasis and CF) and in postmenopausal women without clear risk factors
w NTM lung infections are often debilitating and progressive
Ø Virtually all patients experiencing chronic or recurring cough
Ø Other frequent symptoms including sputum production, fatigue, malaise, dyspnea,
fever, hemoptysis, chest pain and weight loss
fever, hemoptysis, chest pain and weight loss
Non-TB mycobacteria (NTM) are intracellular pathogens that can cause
severe, chronic pulmonary disease with limited effective treatment options
severe, chronic pulmonary disease with limited effective treatment options
ATS - American Thoracic Society;
IDSA - Infectious Disease Society of America
“Current treatment for NTM lung disease requires lengthy multi-drug regimens that
can be poorly tolerated and have limited efficacy, especially in patients with severe
disease or in those who have failed prior treatment attempts,”
can be poorly tolerated and have limited efficacy, especially in patients with severe
disease or in those who have failed prior treatment attempts,”
David E. Griffith, M.D., Lead author of the ATS/IDSA's diagnosis and treatment guidelines for NTM, and
Professor of Medicine at the University of Texas Health Science Center at Tyler
Professor of Medicine at the University of Texas Health Science Center at Tyler
14
ARIKACE: Non-TB Mycobacteria
Market Opportunity
Market Opportunity
“The prevalence of this debilitating chronic disease continues to grow, and
the current NTM treatment paradigm lacks acceptable treatment options”*
the current NTM treatment paradigm lacks acceptable treatment options”*
Sources: 1. Clarity Pharma Research, Patient Chart Study, 2012.
2. Adjemian et al. 2011 ATS Poster: Prevalence of Pulmonary Nontuberculous Mycobacterial
Disease among Medicare Beneficiaries, USA, 1997-2007.
Disease among Medicare Beneficiaries, USA, 1997-2007.
3. SDI Healthcare Database, July 2009.
*MAC - Mycobacterium avium Complex; M. abscessus - Mycobacterium abscessus
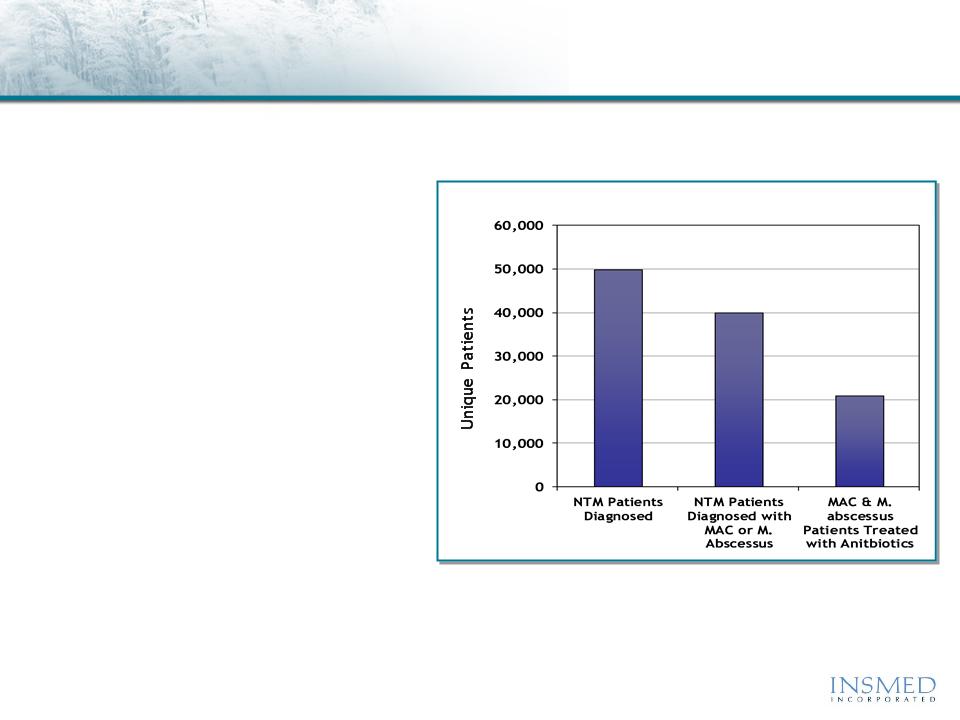
U.S. Patients Diagnosed with NTM Lung Infections in 20111
50K
40K
21K
w Diagnosis growing at~ 8% annually2
w MAC and M. abscessus* account for
75%-85% of NTM lung disease in U.S.
75%-85% of NTM lung disease in U.S.
w Mean age is ~ 57 years with 53%
treated with antibiotics1
treated with antibiotics1
w Treated patients use an average of
7.6 antibiotic courses per year3
7.6 antibiotic courses per year3
w Average length of inpatient hospital
stay is 10.2 days3
stay is 10.2 days3
* Mark Rolfe, M.D. FCCP, President of New Lung Associates P.A., Medical Director of the Lung Transplant and Adult Cystic F
Fibrosis Programs at Tampa General Hospital

15
ARIKACE: Non-TB Mycobacteria
Rationale for ARIKACE
Rationale for ARIKACE
w NTM lung infections are difficult to treat since NTM are taken
up and multiply inside lung macrophages and most antibiotics
have poor macrophage penetration
w Amikacin IV is a recommended treatment for MAC and
M. abscessus in the ATS/IDSA's NTM diagnosis and treatment
guidelines1 but use is limited due to nephro- and oto-toxicity
w The proprietary liposomal formulation enables ARIKACE to be
preferentially taken up and concentrated in the lung macrophages while potentially
decreasing systemic exposure and related toxicities
decreasing systemic exposure and related toxicities
§ ARIKACE was shown to have superior in vitro activity against MAC and M. abscessus vs.
“free” amikacin2
“free” amikacin2
§ ARIKACE is well positioned to become the first drug approved for NTM lung infections
ARIKACE opportunity: achieve superior efficacy in NTM treatment by
better penetrating lung macrophages where NTM bacteria reside while
limiting systemic drug exposure
better penetrating lung macrophages where NTM bacteria reside while
limiting systemic drug exposure
Sources: 1. Griffith et al. ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of NTM
Diseases, American Journal of Respiratory and Critical Care Medicine, 2007.
Diseases, American Journal of Respiratory and Critical Care Medicine, 2007.
2. Study conducted by L. E. Bermudez at Oregon State University.
ARIKACE: Non-TB Mycobacteria
TARGET-NTM Clinical Study Initiated in Mid-2012
TARGET-NTM Clinical Study Initiated in Mid-2012
w Trial Design and Patient Population (N ≈ 100):
– Randomized, double-blind, placebo controlled Phase 2 study in patients with
recalcitrant/persistent NTM lung infections who are on a stable ATS/IDSA
guidelines-based multi-drug treatment regimen
recalcitrant/persistent NTM lung infections who are on a stable ATS/IDSA
guidelines-based multi-drug treatment regimen
– Patients receive ARIKACE or placebo daily for 84 days; then all patients can
receive ARIKACE 560 mg in an open-label manner for an additional 84 days
receive ARIKACE 560 mg in an open-label manner for an additional 84 days
– Study population: patients ages 18 to 75
w Key Inclusion Criteria: History of chronic infection with either Mycobacterium avium
complex (MAC) or Mycobacterium abscessus or mixed infection with both species
complex (MAC) or Mycobacterium abscessus or mixed infection with both species
w Primary endpoint: Change in mycobacterial culture results from baseline to end of
treatment [Time Frame: 84 days]
treatment [Time Frame: 84 days]
Insmed appears to be the only company with an NTM clinical program; top
-line Phase 2 data projected in 4Q 2013
-line Phase 2 data projected in 4Q 2013
w There have been very few clinical trials to support current NTM treatment
recommendations, and no new drugs have been assessed in randomized trials
for NTM lung disease in many years.
recommendations, and no new drugs have been assessed in randomized trials
for NTM lung disease in many years.
– according to Kenneth N. Olivier, M.D., M.P.H., Principal Investigator of the study and
staff pulmonologist at the NIAID, part of NIH
staff pulmonologist at the NIAID, part of NIH
17
Projected
Cash at year
end 2012
(including
Cash at year
end 2012
(including
cash, investments & CD)
w Approximately $60 to $64 million currently forecast
w We believe cash is sufficient to take Company through the
availability of top-line data for both CLEAR-108 and TARGET-NTM
top-line results
availability of top-line data for both CLEAR-108 and TARGET-NTM
top-line results
Current Overview: Capital Structure and Key Financials
Balance Sheet
w Cash of ~$73 million as of March 31, 2012 consisting of cash,
investments & CD
investments & CD
Present Capital
Structure
Structure
(INSM)
w 26.5 million fully diluted shares:
ü 24.9 million Common Shares
ü 1.6 million options and restricted stock units
Insmed has a strong cash position
