Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REPROS THERAPEUTICS INC. | v315344_8k.htm |

Developing clinical stage small molecule therapeutics to treat hormonal and reproductive system disorders

Repros Disclaimer Any statements that are not historical facts contained in this release are forward - looking statements that involve risks and uncertainties, including Repros ' ability to have the partial hold on Proellex ® lifted and to determine a safe and effective dose for Proellex ®, raise needed additional capital on a timely basis in order for it to continue to fund its operations and pursue its development activities, and such other risks which are identified in the Company's most recent Annual Report on Form 10 - K and in any subsequent quarterly reports on Form 10 - Q. These documents are available on request from Repros Therapeutics or at www.sec.gov. Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise.

Investment Highlights • Focused strategy: small molecule therapeutics for reproductive disorders • Two late stage clinical programs each with +$1B sales potential • Androxal ® : oral treatment for Low Testosterone with pending patent/ patent life to the mid 2020’s(growing +$1B market) – Restoration of testicular function and testosterone levels in treatment of 2 º hypogonadism (most common cause of low T) • Proellex : treatment for uterine fibroids and endometriosis with pending patent/ patent life to the mid 2020’s (+$5B market) – Chronic relief of uterine fibroid symptoms – Fibroid de - bulking – Chronic relief of the symptoms associated with endometriosis • Key late stage clinical & regulatory events driven news flow in 2012
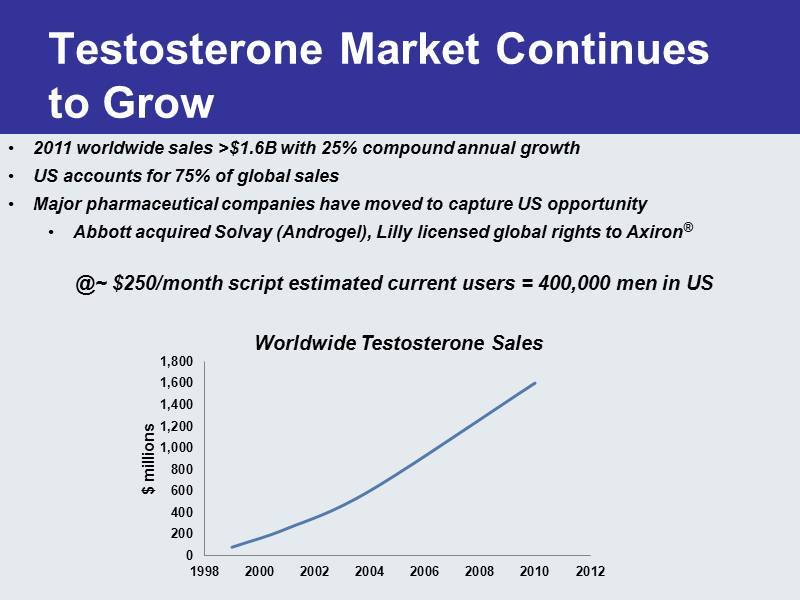
Testosterone Market Continues to Grow 0 200 400 600 800 1,000 1,200 1,400 1,600 1,800 1998 2000 2002 2004 2006 2008 2010 2012 $ millions • 2011 worldwide sales >$1.6B with 25% compound annual growth • US accounts for 75% of global sales • Major pharmaceutical companies have moved to capture US opportunity • Abbott acquired Solvay ( Androgel ), Lilly licensed global rights to Axiron ® Worldwide Testosterone Sales @~ $250/month script estimated current users = 400,000 men in US
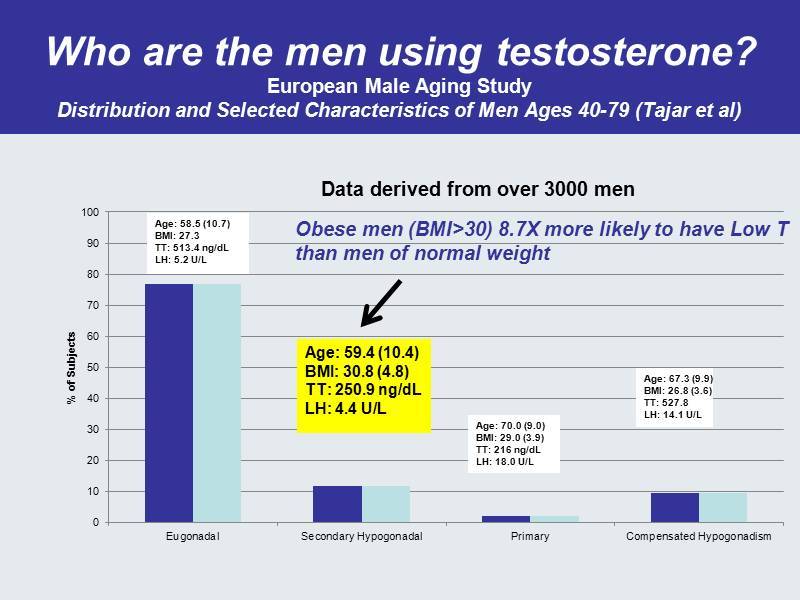
Who are the men using testosterone? European Male Aging Study Distribution and Selected Characteristics of Men Ages 40 - 79 ( Tajar et al) 0 10 20 30 40 50 60 70 80 90 100 Eugonadal Secondary Hypogonadal Primary Compensated Hypogonadism % of Subjects Age: 58.5 (10.7) BMI: 27.3 TT: 513.4 ng / dL LH: 5.2 U/L Age: 59.4 (10.4) BMI: 30.8 (4.8) TT: 250.9 ng / dL LH: 4.4 U/L Age: 70.0 (9.0) BMI: 29.0 (3.9) TT: 216 ng / dL LH: 18.0 U/L Age: 67.3 (9.9) BMI: 26.8 (3.6) TT: 527.8 LH: 14.1 U/L Data derived from over 3000 men Obese men (BMI>30) 8.7X more likely to have Low T than men of normal weight

The Average American Male at All Ages Was Overweight in 2000 22 23 24 25 26 27 28 29 30 20-29 30-39 40-49 50-59 60-74 BMI Age by Decade Mean BMI 1960 2000 Overweight BMI > 25 (6’ 190# male BMI=25.8) Obese BMI > 30 (6’ 230# male BMI =31.2) In 2010 there were ~90 million men in the US between the ages of 20 and 65 32% are obese
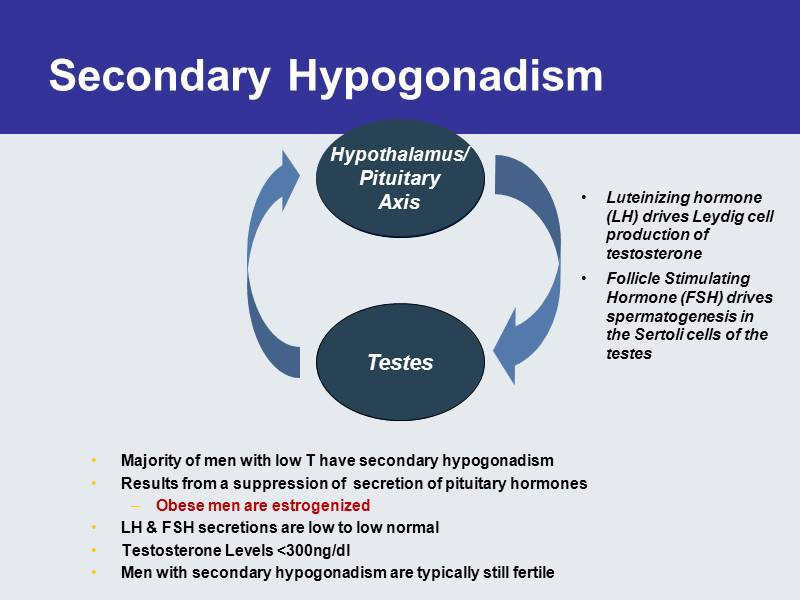
Secondary Hypogonadism • Majority of men with low T have secondary hypogonadism • Results from a suppression of secretion of pituitary hormones – Obese men are estrogenized • LH & FSH secretions are low to low normal • Testosterone Levels <300ng/dl • Men with secondary h ypogonadism are typically s till f ertile Testes Hypothalamus/ Pituitary Axis • Luteinizing hormone (LH) drives Leydig cell production of testosterone • Follicle Stimulating Hormone (FSH) drives spermatogenesis in the Sertoli cells of the testes Testes Hypothalamus / Pituitary Axis
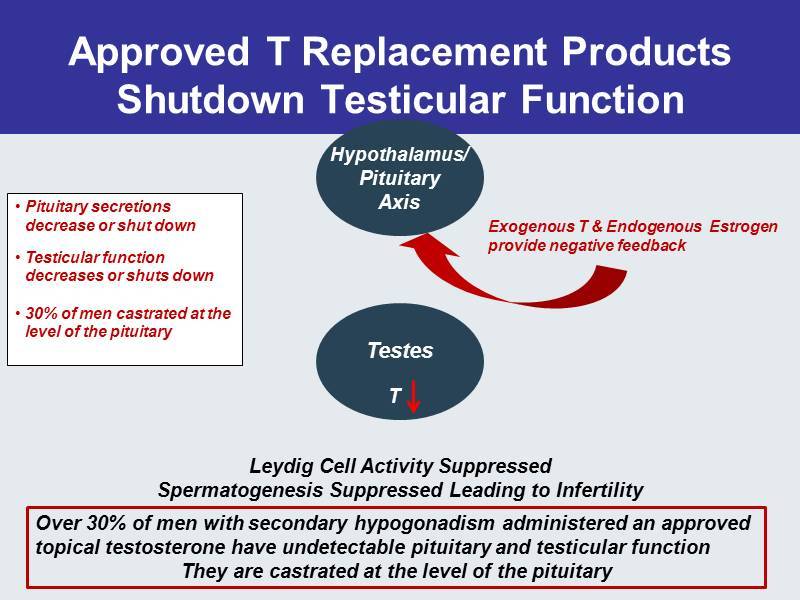
Approved T Replacement Products Shutdown Testicular Function Testes Hypothalamus / Pituitary Axis • Pituitary secretions decrease or shut down • Testicular function decreases or shuts down • 30% of men castrated at the level of the pituitary Leydig Cell Activity Suppressed Spermatogenesis Suppressed Leading to Infertility T Over 30% of men with secondary hypogonadism administered an approved topical testosterone have undetectable pituitary and testicular function They are castrated at the level of the pituitary Exogenous T & Endogenous Estrogen provide negative feedback

Androxal Restores Normal Testicular Function Testes Increased T estosterone Secretion Androxal • Androxal blocks estrogen at the level of the pituitary • Allows responsiveness to low T conditions • Leads to increased LH & FSH to stimulate normal testicular function • Increased LH and FSH levels T Hypothalamus / Pituitary Axis

FDA Recommendation for NDA May 9, 2012 • Phase 3 pivotal studies to be conducted under SPA – 2 identical trials • 154 subjects in each trial (114 on Androxal , 38 on placebo) – Men with morning T<300 ng / dL assessed twice on two separate days • Up - titration from 12.5 to 25 • 3 month duration – Co - primary endpoints • 75% of men achieve T in normal range (300 - 1040 ng / dL ) • Non inferior to placebo regarding change in sperm counts • Safety Requirements – >100 for one year (anticipate reaching goal late summer 2012) – >800 for 6 months (~200 by late summer, 500 subject 6 month study enrolling) – 100 subject one year DEXA study (bone marker data suggests Androxal builds bone) • Goal to Submit NDA Q1 2014 or earlier

Previous Androxal Experience ZA - 003 Testosterone Distribution (6 Month Data) 0 10 20 30 40 50 60 70 80 <300 300-400 400-800 >800 Testosterone Range (ng/dl) Percent of Patients 25 mg 12.5 mg Androgel Placebo n=162 patients

Briefing Package Excerpt ( Androxal data from ZA - 204) Correlation of Morning T with 24 Hour Average & Tmax Androxal Arms Both correlations significant (p<0.0001) with a correlation coefficient of ~ 0.9

ZA - 204 End of Dosing Outcome 0 200 400 600 800 1000 1200 Total T ng / dL End of Dosing Morning T Values 0 200 400 600 800 1000 1200 24 hour Avg. TT ( ng / dL ) 24 Hour Average T Values 0 500 1000 1500 2000 2500 3000 Maximum TT ( ng / dL ) Maximum Recorded T 6.25 mg 12.5 mg 25 mg Androgel Androgel Androgel Above FDA recommended Upper limit

Androxal Preserves Testicular Function Approved Topical Products Suppress the Hypothalamic - Pituitary - Testes Axis

24 Hour T & LH 25 mg Androxal Subject Study ZA - 204 Subject 2 - 003 Age: 55, BMI: 32 0 2 4 6 8 10 12 14 16 0 100 200 300 400 500 600 700 800 0 5 10 15 20 T @ Baseline T @ 6 Weeks LH @ Baseline LH @ 6 Weeks Baseline 0 time: 8:09 AM Week 6 0 time: 7:25 AM
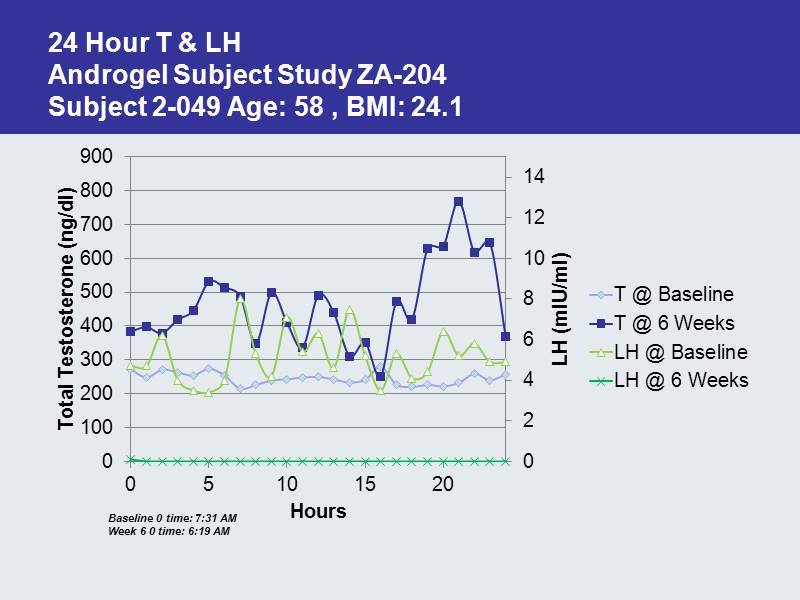
24 Hour T & LH Androgel Subject Study ZA - 204 Subject 2 - 049 Age: 58 , BMI: 24.1 0 2 4 6 8 10 12 14 0 100 200 300 400 500 600 700 800 900 0 5 10 15 20 LH ( mIU /ml) Total Testosterone ( ng /dl) Hours T @ Baseline T @ 6 Weeks LH @ Baseline LH @ 6 Weeks Baseline 0 time: 7:31 AM Week 6 0 time: 6:19 AM

0 100 200 300 400 500 600 700 Morning TT (ng/dL) Fig. 14: Mean Morning TT Over Time (ZA - 204) 6.25mg Androxal 12.5mg Androxal 25mg Androxal Androgel Week 2 Week 4 Week 6 Last Day of Dosing One Week Follow - up One week after dosing all Androxal arms were better than baseline One week after dosing the Androgel arm was worse than baseline Men on Androxal exhibit continued improvement in T even after dosing has stopped
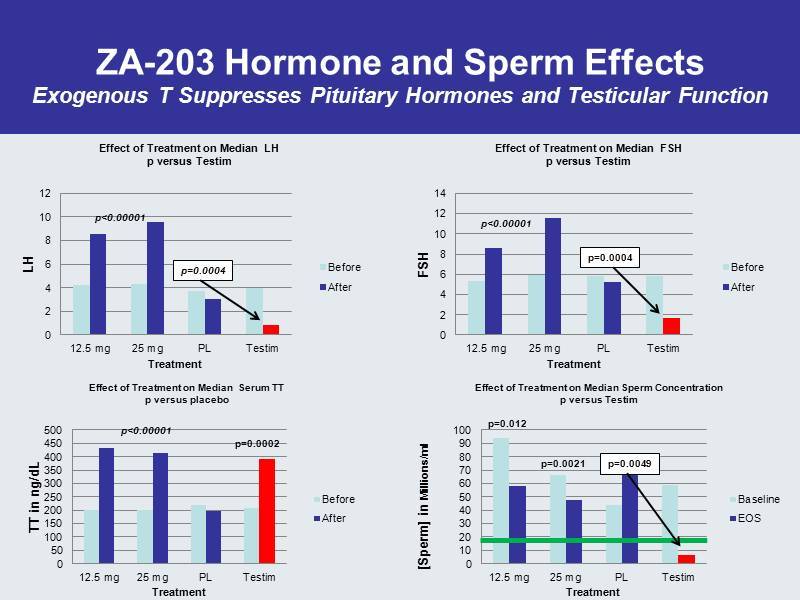
ZA - 203 Hormone and Sperm Effects Exogenous T Suppresses Pituitary Hormones and Testicular Function 0 2 4 6 8 10 12 14 12.5 mg 25 mg PL Testim FSH Treatment Effect of Treatment on Median FSH p versus Testim Before After p<0.00001 p=0.0004 0 10 20 30 40 50 60 70 80 90 100 12.5 mg 25 mg PL Testim [Sperm] in Millions/ml Treatment Effect of Treatment on Median Sperm Concentration p versus Testim Baseline EOS p=0.012 p=0.0021 p=0.0049 0 2 4 6 8 10 12 12.5 mg 25 mg PL Testim LH Treatment Effect of Treatment on Median LH p versus Testim Before After p=0.0004 0 50 100 150 200 250 300 350 400 450 500 12.5 mg 25 mg PL Testim TT in ng/dL Treatment Effect of Treatment on Median Serum TT p versus placebo Before After p<0.00001 p=0.0002 p<0.00001
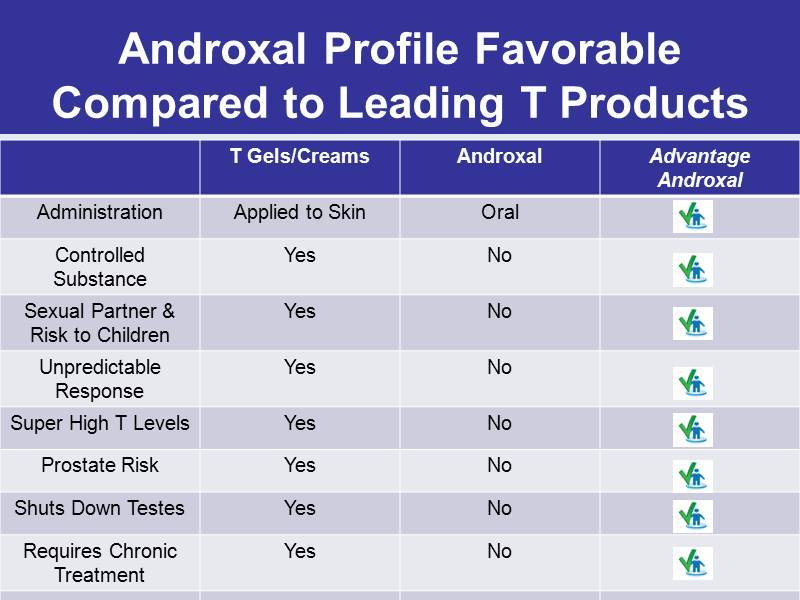
Androxal Profile Favorable Compared to Leading T Products T Gels/Creams Androxal Advantage Androxal Administration Applied to Skin Oral Controlled Substance Yes No Sexual Partner & Risk to Children Yes No Unpredictable Response Yes No Super High T Levels Yes No Prostate Risk Yes No Shuts Down Testes Yes No Requires Chronic Treatment Yes No

Third Party Study Indicates Favorable Reimbursement Potential for Androxal Majority of payers believe Androxal’s oral administration and non - chronic use may offer overall cost savings – Third party assessment of payers indicates vast majority (>90%) would add Androxal to formularies • Cost will be key for tier placement • 50% of plans indicated they would require a PA(Prior Authorization) to show proper diagnosis • 62 % of respondents expect Androxal to be priced at parity to Androgel • Anticipated Androxal pricing of $170 - 350/month would be competitive with Androgel

Androxal Take Home Message • Because of Obesity, 30% of American Males are at Risk of Secondary Hypogonadism – Co - morbidities include diabetes and cardiovascular disease • Approved T Products Worsen the Underlying Condition • Only Androxal + Diet + Exercise can reverse this disorder

Proellex for the Treatment of Uterine Fibroids and Endometriosis Over 30 million women of reproductive age in the US afflicted with symptomatic uterine fibroids or endometriosis Over 300,000 hysterectomies performed every year in the US to treat these two disorders No acceptable chronic therapeutic options available today
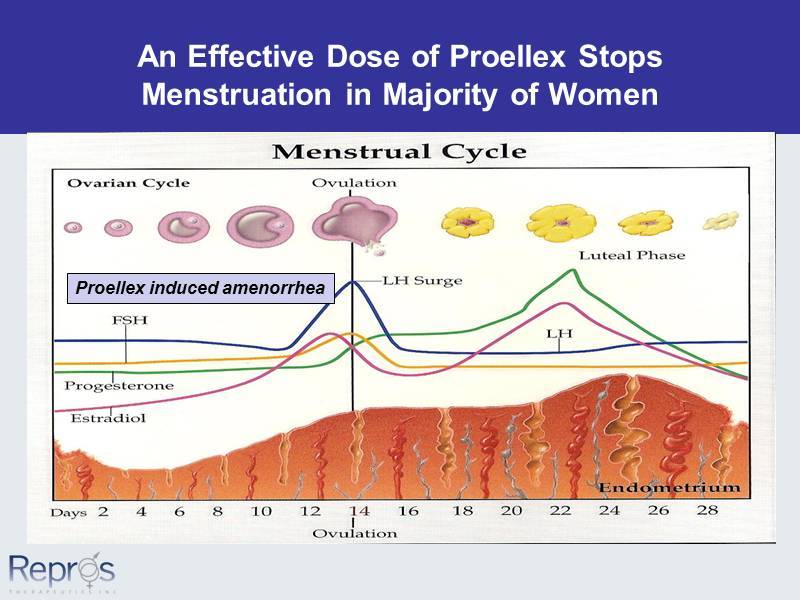
An Effective Dose of Proellex Stops Menstruation in Majority of Women Proellex induced amenorrhea

% of Women Experiencing Proellex Induced Amenorrhea in Low Dose Study 0 10 20 30 40 50 60 70 80 90 100 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg Proposed Doses for Phase 3 Endometriosis Study Doses safely tested in Phase 2 trial

Can a Low Dose Work? Key Symptom Driving Women to Seek Therapy for Uterine Fibroids “Excessive Menstrual Bleeding” 0 20 40 60 80 100 120 140 160 BL Mo 1 Mo 2 Mo 3 PBAC Score Placebo Proellex 12.5 mg Proellex 25 mg US Phase IIb (n=127 ) P<0.0001 Menorrhagia =PBAC>80 Amenorrhea drives outcomes
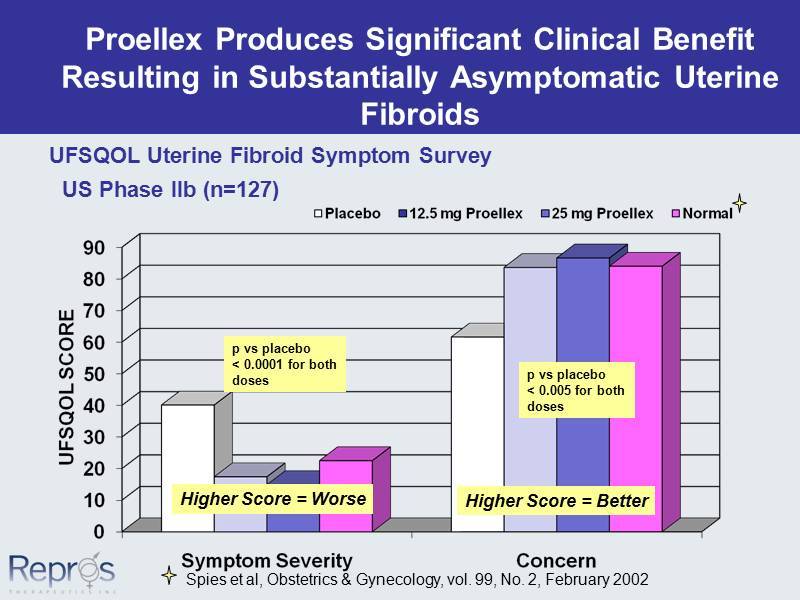
Proellex Produces Significant Clinical Benefit Resulting in Substantially Asymptomatic Uterine Fibroids UFSQOL Uterine Fibroid Symptom Survey p vs placebo < 0.0001 for both doses p vs placebo < 0.005 for both doses Spies et al, Obstetrics & Gynecology, vol. 99, No. 2, February 2002 Higher Score = Worse Higher Score = Better US Phase IIb (n=127)
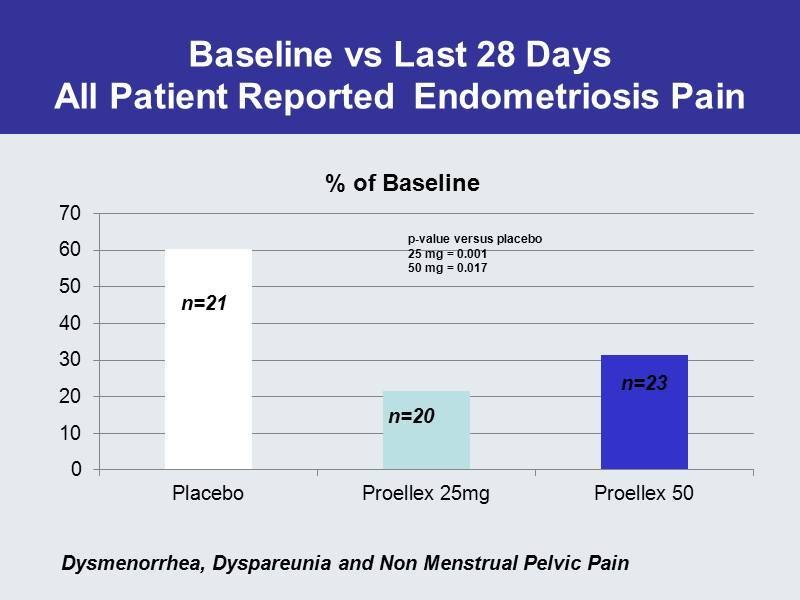
Baseline vs Last 28 Days All Patient Reported Endometriosis Pain 0 10 20 30 40 50 60 70 Placebo Proellex 25mg Proellex 50 % of Baseline n=23 p - value versus placebo 25 mg = 0.001 50 mg = 0.017 n=20 n=21 Dysmenorrhea , Dyspareunia and Non Menstrual Pelvic Pain

Doses That Stop Menses Have Significant Impact on Analgesic Use in the Control of the Pain Symptoms of Endometriosis 88.9 33.4 36.4 0 10 20 30 40 50 60 70 80 90 100 Placebo 25mg Proellex 50mg Proellex % of Subjects Requiring Narcotic or Non Narcotic Analgesics at End of Study Baseline End of Study 55.5 22.2 13.6 0 10 20 30 40 50 60 70 80 90 100 Placebo 25mg Proellex 50mg Proellex % of Subjects Requiring Narcotics at End of Study Baseline End of Study Repros advised by FDA to submit safety assessment for FDA review before conducting any additional oral studies P<0.01 P<0.001

Vaginal Proellex to Eliminate the Need for Hysterectomy – New IND effective • Unaffected by oral outcomes – Initial Phase 2 study to test four doses of vaginal administration in the treatment of uterine fibroids • Assess reduction of fibroid size and elimination of symptoms • Company anticipates efficacy effects greater than highest dose tested in humans (50mg) with systemic exposure less than 3 mg oral.
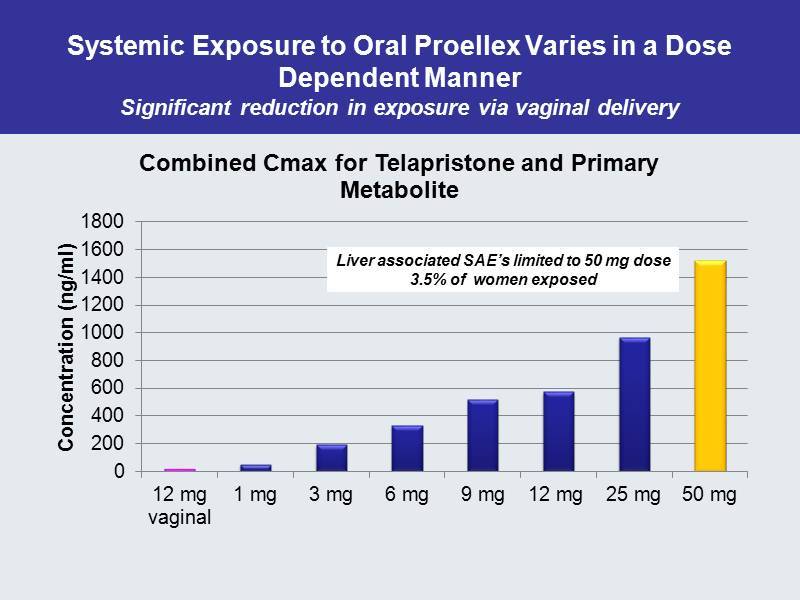
Systemic Exposure to Oral Proellex Varies in a Dose Dependent Manner Significant reduction in exposure via vaginal delivery 0 200 400 600 800 1000 1200 1400 1600 1800 12 mg vaginal 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg Concentration ( ng /ml) Combined Cmax for Telapristone and Primary Metabolite Liver associated SAE’s limited to 50 mg dose 3.5% of women exposed

Early Efficacy Signal Detected for Proellex - V 0 25 50 75 100 125 150 175 200 225 250 275 PBAC Score Baseline Cycle 1 Menorraghia PBAC=80 0 10 20 30 40 50 60 70 80 90 UFSQOL Score Baseline Cycle 1 Normal Score for Women w/o fibroids Steady State Drug Concentrations Subject 1: 14.2 ng /ml Subject 2: not detected Subject 3: 14.8 ng /ml Subject 4: 20.0 ng /ml Subject 5: 22.4 ng /ml Subject 6: 5.6 ng /ml
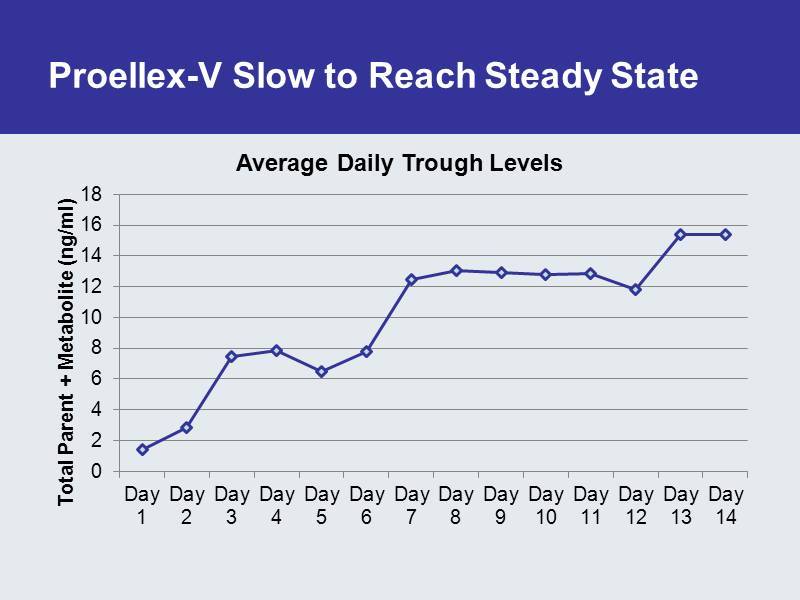
Proellex - V Slow to Reach Steady State 0 2 4 6 8 10 12 14 16 18 Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Day 8 Day 9 Day 10 Day 11 Day 12 Day 13 Day 14 Total Parent + Metabolite ( ng /ml) Average Daily Trough Levels

At Steady State Proellex - V Yields Flat Exposure Profile 0 100 200 300 400 500 600 0 4 8 12 16 20 24 Conc. (ng/ml) Time (Hr) Average: PK Oral (1, 3, 6, 9 and 12 mg vs Vaginal 12 mg) 1 mg oral 3 mg oral 6 mg oral 9 mg oral 12 mg oraql 12 mg vaginal
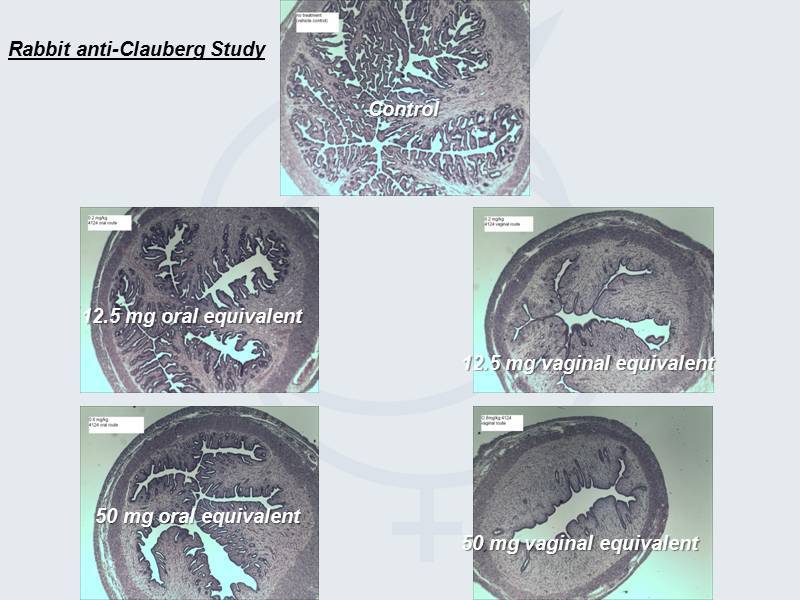
Control 12.5 mg oral equivalent 50 mg oral equivalent 12.5 mg vaginal equivalent 50 mg vaginal equivalent Rabbit anti - Clauberg Study

Increasing Oral Dose Continues to Shrink Fibroids Repros Expects Vaginal Delivery to Exceed Shrinkage Effects of Highest Oral Dose Tested 0 10 20 30 40 50 60 70 80 90 100 Baseline 3 month treatment @ 25mg 4 month treatment @ 50mg Four women initially treated for 3 months at 25 mg dose Follow - up study with same women treated for 4 months at 50 mg

Financial Summary • Cash and equivalents (as of 6/1/12, unaudited) ~ $10.8M • Cash burn = 2012 = ~$12 M) • Cash runway = Q2 2013 • 14.82 MM shares outstanding – Warrants outstanding = 1.75MM Series A (Purchased in unit deal @$2.46) + 1.69MM Series B (@$2.49 with cashless exercise provision) – Forced warrant strike at $8/share converts B warrants to ~ 1.16 million shares with cashless exercise provision

Recently Completed & Upcoming Milestones • Reported results for two Phase 2 Androxal Studies Q4 - 11 • Reported results for low dose Phase 2 Proellex Study Q4 - 11 • Reported effective IND for Vaginal Proellex Phase 2 Q1 - 12 • Commence Dosing Phase 2 UF Vaginal Proellex Q1 - 12 • Held Meeting with FDA for Phase 3 Androxal May ’12 • Anticipate 1 st SPA review for Androxal Phase 3 July’12 • Commence Phase 3 Pivotal Androxal Studies Q3 - 12 • Report outcome of FDA review of low dose Proellex Q3 - 12 • Report Phase 2 UF Vaginal Proellex Study Q4 - 12
