Attached files
| file | filename |
|---|---|
| 8-K - 8-K - OPKO HEALTH, INC. | d364036d8k.htm |
 Jefferies 2012 Global Healthcare Conference
June 5, 2012
Exhibit 99.1 |
| Cautionary Statement
2
This
presentation
contains
"forward-looking
statements,”
as
that
term
is
defined
under
the
Private
Securities
Litigation
Reform
Act
of
1995
(PSLRA),
which
statements
may
be
identified
by
words
such
as
“expects,”
“plans,”
“projects,”
“will,”
“may,”
“anticipates,”
“believes,”
“should,”
“intends,”
“estimates,”
"potential"
and
other
words
of
similar
meaning,
including
statements
regarding
our
estimated
revenues
and
financial
projections,
our
ability
to
achieve
high
levels
of
growth,
the
potential
for
our
products
under
development
to
be
game-changing,
the
potential
of
our
next
generation
prostate
markers
to
reduce
biopsies
and
increase
detection
of
cancer,
our
ability
to
develop
a
lead
Alzheimer’s
test
and
additional
diagnostic
products,
the
ability
of
our
Alzheimer’s
diagnostic
test
to
distinguish
Alzheimer’s
disease
from
other
dementias,
our
ability
to
develop
certain
therapeutics,
new
drugs
and
vaccines,
including
for
the
treatment
of
genetic
diseases,
cancers,
neurological
and
metabolic
disorders,
our
ability
to
develop
and
commercialize
compounds
that
prevent
the
progression
of
neurodegenerative
diseases,
our
ability
to
develop,
test
and
launch
new
products,
the
expected
timing
of
the
studies
and
trials
relating
to
our
products
under
development,
the
outcome
of
our
clinical
trials
and
validation
studies
and
that
such
outcomes
will
support
commercialization,
the
expected
size
of
the
market
for
certain
of
our
products
under
development,
the
potential
benefits
of
our
products
under
development,
our
ability
to
successfully
develop
and
commercialize
our
product
candidates
such
as
simple
blood
tests
for
Alzheimer’s,
Neuromylelitis
Optica,
Multiple
Sclerosis
and
other
neurological
disorders,
lung,
pancreatic,
and
other
cancers
and
autoimmune
diseases,
as
well
as
products
for
other
markets
such
as
urology,
women’s
health,
cardiology
and
infectious
disease,
our
ability
to
develop
and
commercialize
next
generation
prostate
markers,
and
the
timing
of
clinical
trials
and
expected
U.S.
and
European
regulatory
approvals
for
our
product
candidates
and
the
commercial
launch
of
our
product
candidates,
as
well
as
other
non-historical
statements.
These
forward-looking
statements
are
only
predictions
and
reflect
our
views
as
of
the
date
they
were
made,
and
we
undertake
no
obligation
to
update
such
statements.
Such
statements
are
subject
to
many
risks
and
uncertainties
that
could
cause
our
activities
or
actual
results
to
differ
materially
from
the
activities
and
results
anticipated
in
forward-looking
statements,
including
risks
inherent
in
funding,
developing
and
obtaining
regulatory
approvals
of
new,
commercially-viable
and
competitive
products
and
treatments,
general
market
factors,
competitive
product
development,
product
availability,
federal
and
state
regulations
and
legislation,
delays
associated
with
development
of
novel
technologies,
unexpected
difficulties
and
delays
in
validating
and
testing
product
candidates,
the
regulatory
process
for
new
products
and
indications,
manufacturing
issues
that
may
arise,
the
cost
of
funding
lengthy
research
programs,
the
need
for
and
availability
of
additional
capital,
the
possibility
of
infringing
a
third
party’s
patents
or
other
intellectual
property
rights,
the
uncertainty
of
obtaining
patents
covering
our
products
and
processes
and
in
successfully
enforcing
them
against
third
parties,
and
the
possibility
of
litigation,
among
other
factors,
including
all
of
the
risks
identified
under
the
heading
Risk
Factors
in
our
Annual
Report
on
Form
10-K
and
other
filings
with
the
Securities and
Exchange Commission. |
 Company
Overview
Discovery
Earlier Clinical
Commercial
Mid / Late Clinical
Opportunistic pharmaceutical and diagnostics company focused on
large, high growth markets:
–
New PLATFORM to develop diagnostic tests for cancer and
neurodegenerative diseases
–
New microfluidics SYSTEM for rapid, lab-quality point of care tests
–
Next generation prostate cancer markers
–
New neuroprotective drugs for neurodegenerative diseases.
–
New drugs based on gene up-regulation, e.g. genetic diseases
–
New drugs for asthma, COPD and cystic fibrosis
–
Emerging markets pharmaceutical business
–
Investments in other hi-tech companies
3
Cash, cash equivalents and marketable securities of approximately $62 million
as of 3/31/2012 |
 Rolapitant Overview
4
Rolapitant out-licensed to Tesaro in December 2010
Schering-Plough (S-P) divested to OPKO as FTC condition for S-P
merger with Merck Payments of up to $121MM
Double digit tiered royalties
Potentially best-in-class cancer supportive care product
Potent neurokinin-1(NK-1) receptor antagonist for
chemotherapy-induced nausea and vomiting (CINV)
Single dose
Rapid onset
Long acting
Phase 3 program initiated following positive Phase 2 results
Efficacy and dose identified in Phase 2 trial (n=454)
Over 1,000 patients and healthy volunteers evaluated in Phase 1 and 2
Well accepted regulatory endpoints and clinical trial designs
Three global Phase 3 studies ongoing; 2 HEC (n=530) and 1 MEC (n=1350) with
results expected during second half of 2013
Five-day
activity
following
single
oral
dose
in
patients
treated
with
highly
emetogenic
chemotherapy (HEC) |
 Rolapitant Market Opportunity
5
U.S. market opportunity of ~ $1.25 billion
6.6 million annual CINV patient treatments in 2011
NCCN and ASCO guideline recommendations could lead to 70%
penetration by the NK-1 class
Merck -
EMEND®
currently only NK-1 receptor antagonist on market
EMEND®
2011 global sales -
$419 million
Strong IP portfolio with U.S. exclusivity expected through 2028
|
| AntagoNAT
Natural Antisense Transcript Inhibitors
6
•
Chemically modified single-strand oligonucleotides
•
Up-regulate expression of endogenous proteins
•
Extensive screening of over 400 gene targets
•
Demonstrated up-regulation of genes related to genetic
diseases, cancer, neurological,metabolic disorders
•
Animal studies confirm increases of
–
Target mRNA
–
Proteins expressed by the target gene
•
8 AntagoNATs have been tested in animals |
| 7
New Class of Compounds Prevent
Neurodegenerative Disease Progression
•
Licensed world-wide rights from The Scripps Research
Institute to novel compounds that inhibit jun-N-terminal
kinases (JNK) (important for neuron survival).
•
Lead compound, a small molecule (SR-3306), could be
first drug to protect brain in Parkinson’s disease, MS,
ALS, and AD.
•
Current therapies are symptomatic, have untoward side
effects, lose effectiveness over time, or have risks
associated with surgery.
•
Inhibition of JNK to treat PD would be paradigm shifting
by preventing neurodegeneration and disease
progression. |
 8
Therapeutic Potential of JNK Inhibitors
Orally bioavailable, brain penetrant JNK inhibitors have
shown efficacy in two animal models of Parkinson’s disease
and represent first in class neuroprotective agents for the
treatment of PD
These JNK inhibitors have potential in all neurodegenerative
disease including AD, MS, ALS, and stroke |
 9 |
 |
 |
 12 |
 13 |
 14 |
 15 |
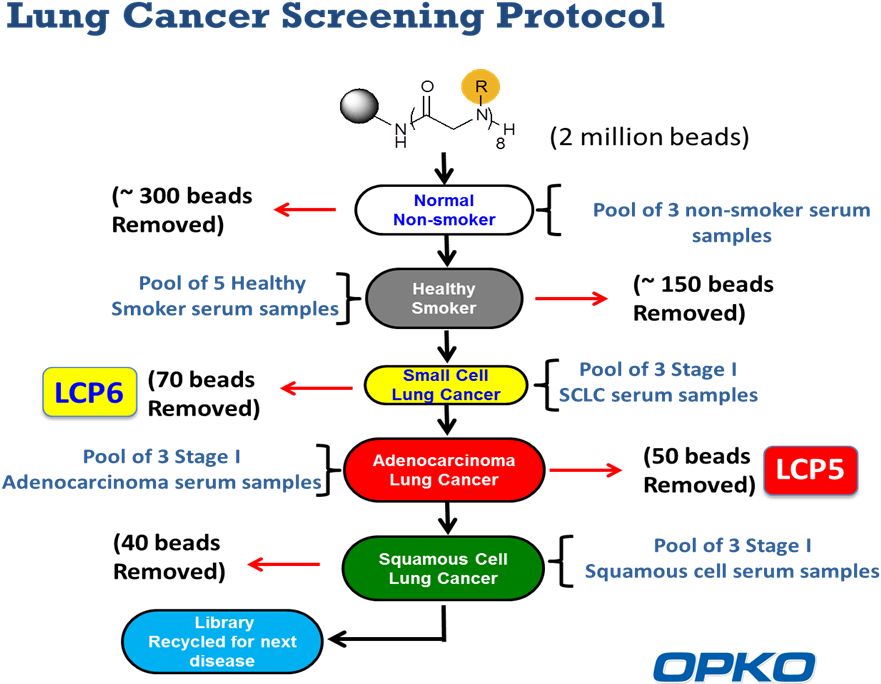 16 |
 17 |
 18 |
 19 |
 20 |
 21
OPKO Diagnostics Platform
Product:
Immunoassay System Yielding Rapid Lab-Quality Quantitative
Test Results with Finger-Stick Drop of Blood (OPKO
Dx
Card)
Technology
Acquired Claros Diagnostics in October 2011
Proprietary Microfluidics and Amplification Chemistry
Pre-loaded Reagents on a Disposable Cassette
Sensitive Single Marker or Multiplex Assays
Inexpensive Analyzer
Initial Target Applications
Urology / CE Mark Approval in Europe / US Clinical Trial Underway
Next Generation Prostate Cancer Markers (4Kscore™)
Specialty Tests: Vitamin D
OPKO Molecular Diagnostic Tests Applications
Strong IP Portfolio |
 OPKO Dx Card Technology Applications
Urology
Women’s
Health
Cardiology
Infectious
Disease
Other
Urologist
Office
Ob/Gyn
Office
Emergency
Room
Clinic and
Blood Bank
GP Office
PCa Panel:
•
total-PSA
•
free-PSA
•
Testosterone
•
Novel Markers
Panel Tests:
•
Pre-term bleed
•
Fertility
•
Menopause
Cardio Panel:
•
CTnI
•
BNP
•
D-dimer
STD Panel:
•
HIV
•
Hepatitis B
•
Syphilis
Key Tests:
•
TSH, T4
•
Vitamin D
•
Folic Acid
Liquid Sample Capabilities
Whole Blood
Semen
Spinal Fluid
Serum
Saliva
Tears
Urine
Amniotic Fluid
Sweat
GPs
22 |
 Overview of OPKO Diagnostics Technology
Inexpensive Disposable
Injection-Molded Microfluidics
On-Board Delivery of Multiple Reagents
Multiplex Platform
Robust Signal Detection
Sensitive Proprietary Amplification Chemistry for Microfluidics
Inexpensive Hardware Unit (OPKO Dx Reader)
Reliable Standard Optics
Ease of Use and Safety
No Sample Prep
User-Friendly Sample Introduction
Biohazard Containment
Key Attributes –
High Quantitative Performance / Low Cost
23 |
 Easy to Use
1. Collect Blood
3. Insert into OPKO
Dx Reader
2. Snap into Cassette
4. Read Results in less
than 10 minutes
24 |
 OPKO Diagnostics
2012 Plans
25
Urology
U.S. FDA Trial Completion / Filing
Proprietary Next Generation Prostate Markers
Signed license agreement in May, 2012 for launch of OPKO’s novel
panel of Kallikrein markers in UK, Ireland, Sweden and Denmark
70 Million WW Tests Per Year
$2 Billion Market
Vitamin D
EU Approval Expected in 2012
U.S. Approval Expected in 2013
Complementary Multiplex Panel Potential
100 Million WW Tests Per Year
$5 Billion Market |
| Next
Generation Prostate Markers (4Kscore™)
•
1.2 Million prostate biopsies performed a year in U.S.
–
750,000 of these are unnecessary
–
Costs in excess of $2.5 billion
•
4Kscore™
combines PSA and two novel kallikrein markers to
provide significantly greater accuracy
–
Tested in over 10,000 patients to predict the probability of cancer-
positive biopsy
–
Can dramatically reduce, up to 60%, of unnecessary biopsies now
performed
26 |
| OPKO
Emerging Markets 27
OPKO Chile —
Pharma Genexx, S.A.
•
Rapidly growing sales from >100 products
•
Revenues: $21.5 MM and $18.0 MM for 2011and 2010, respectively
•
Acquired ALS Distribution Limited, the exclusive product distributor of
Arama Laboratories, in April, 2012
•
Estimated 2012 Revenues of $26-$30 MM
OPKO Mexico —
Pharmacos Exakta S.A. de C.V.
•
25 products across a range of therapeutic indications
-
Primarily branded ophthalmics, with expanding proprietary focus
•
Acquired in December, 2011
•
Develops and produces high value, high potency active pharmaceutical
ingredients
•
FDA registered state of the art facility in Nesher, Israel
•
Revenue $1.6 MM for the first three months ended March 31, 2012
•
Revenues: $6.4 and $3.8 MM for year end 2011 and 2010, respectively
OPKO Israel —
Fine Tech Pharmaceuticals, Ltd. |
 Strategic Investments
28
CoCrystal
Discovery, Inc. (~16% equity interest*)
–
Founded by Nobel Laureate, Roger Kornberg, Ph.D.
–
New approach to develop broad spectrum anti-viral drugs
–
Development program with Teva for Hepatitis C drug
Sorrento
Therapeutics (~23% equity interest*)
–
New technology to produce human monoclonal antibodies libraries that are more
complete and more efficient
Fabrus,
LLC
(~13%
equity
interest*)
–
Next gen technology to identify therapeutic antibody targets
–
Founded by Vaughn Smider, M.D., Ph.D. Scientist at The Scripps Research
Institute Tesaro,
Inc.
(~2%
equity
interest*)
–
Oncology-focused biopharmaceutical company
–
Founded by former executives of MGI Pharma
–
Currently in registration for initial public offering
–
Reducer™
for refractory angina undergoing multicenter clinical trial
–
Tiara™
pre-clinical novel transcutaneous mitral valve
ChromaDex,
Inc.
(~2%
equity
interest)
–
Dietary supplement BlueScience and antioxidant pterostilbene pTeroPure
–
OPKO distribution rights in Latin America
* As of March 31, 2012
Proprietary technologies with significant upside potential
–
Developing innovative vascular devices
Neovasc,
Inc.
(~
4%
equity
interest*) |
