Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REVA Medical, Inc. | d355631d8k.htm |
 2012 Annual
General Meeting 21 May 2012
Sydney, Australia
Exhibit 99.1 |
 2012 REVA
Medical, Inc. 1
1
Important Notice
Not an Offer for Securities
This presentation has been prepared by REVA Medical, Inc. (“REVA” or
the “Company”) solely for its use at presentations to be made by the Company. This
presentation does not constitute an offer, invitation, solicitation or recommendation with
respect to the purchase or sale of any security in the Company nor does it constitute
financial product advice nor take into account your investment objectives, taxation situation, financial situation or needs. An investor must
not act on the basis of any matter contained in this presentation but must make its own
assessment of the Company and conduct its own investigations and analysis. Information is
a synopsis only
This presentation only contains a synopsis of information on the Company and accordingly no
reliance may be placed for any purpose whatsoever on the sufficiency or completeness of
such information The information presented in this presentation is subject to change without notice and the Company does not
have any responsibility or obligation to inform you of any matter arising or coming to their
notice, after the date of this presentation, which may affect any matter referred to in
this presentation.
Currency references
Financial amounts in this presentation are expressed in US Dollars., except
where specifically noted.
Forward looking Statements
This presentation contains or may contain forward-looking statements that
are based on management's beliefs, assumptions and expectations and on information
currently available to management. All statements that are not historical, including those statements that address future operating performance
and events or developments that we expect or anticipate will occur in the future, are
forward-looking statements. You should not place undue reliance on these
forward-looking statements. Although management believes these forward-looking statements are reasonable as and when made, forward-looking
statements are subject to a number of risks and uncertainties that may cause our actual
results to vary materially from those expressed in the forward-looking statements,
including our ability to obtain the regulatory approvals required to market our ReZolve® scaffold, our ability to timely and successfully complete
our clinical trials, our ability to protect our intellectual property position, our ability to
commercialize our products if and when approved, our ability to develop and
commercialize new products, and our estimates regarding our capital requirements and financial performance, including profitability. Other risks and
uncertainties that may cause our actual results to vary materially from any
forward-looking statements are described in the "Risk Factors" section of our Annual
Report on Form 10-K filed with the United States Securities and Exchange Commission (the
“SEC”) on 29 February 2012, as updated in our Quarterly Report on Form
10-Q filed with the SEC for the period ended 31 March 2012. We may update our risk factors from time to time in our periodic reports or other current
reports filed with the SEC. Any forward-looking statements in this announcement speak only
as of the date when made. REVA does not assume any obligation to publicly update or
revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
Disclaimer
This presentation has been prepared by the Company based on information which is available to
it. The information contained in this presentation is an overview and does not contain
all information necessary to make an investment decision. Although reasonable care has been taken to ensure that the facts
stated in this presentation are accurate and that the opinions expressed are fair and
reasonable, no representation or warranty, express or implied, is made as to the
fairness, accuracy, completeness or correctness of the information and opinions contained in this presentation and no reliance should be placed on such
information or opinions. To the maximum extent permitted by law, none of the Company, or any
of its members, directors, officers, employees or agents, advisers nor any other
person accepts any liability whatsoever for any loss, however arising, from the use of the presentation or its contents or otherwise
arising in connection with it, including, without limitation, any liability arising from fault
or negligence on the part of the Company or any of its directors, officers, employees or
agents. |
 2012 REVA
Medical, Inc. 2
2
Introductions
•
Board of Directors
–
Brian H. Dovey
–
Anne Keating
–
Gordon E. Nye
–
James J. Schiro
–
Robert Thomas
•
Executive Officers
–
Robert K. Schultz, PhD; COO
–
Katrina Thompson; CFO
•
DLA Piper –
Legal Counsel
–
Michael Kagnoff
–
David Morris
•
Ernst & Young LLP -
Auditors
–
Gamini Martinus
•
Inteq
Limited
–
Corporate
Advisers
–
David Allen |
 2012 REVA
Medical, Inc. 3
3
Annual General Meeting
of Stockholders
10:30 a.m. AEST
Monday 21 May 2012 |
 2012 REVA
Medical, Inc. 4
4
Establish Quorum
Call to Order |
 2012 REVA
Medical, Inc. 5
5
Proxy Proposals
1.
Elect
two
Class
II
directors
–
Gordon
E.
Nye
and
Robert
Thomas
2.
Ratify appointment of independent registered accounting firm
3.
Approve grant of 12,500 options to Brian H. Dovey
4.
Approve grant of 12,500 options to Anne Keating
5.
Approve grant of 12,500 options to Gordon E. Nye
6.
Approve grant of 12,500 options to James J. Schiro
7.
Approve grant of 12,500 options to Robert Thomas
8.
Approve, on advisory basis, executive compensation |
 2012 REVA
Medical, Inc. 6
6
Results of Voting
1.
Collection of ballots/voting
2.
Inspector report on voting
3.
Other business
4.
Adjournment |
 2012 REVA
Medical, Inc. 7
7
Operations Update
since AGM 2011 |
 2012 REVA
Medical, Inc. 8
8
Operations Update
RESTORE Clinical Trial Underway
•
Completed all preclinical and other testing
•
Encountered some challenges and delays that we successfully
overcame
•
Commenced the pilot RESTORE trial in Brazil Dec. 2011
•
Expanded patient enrollment into Europe
–
Additional sites in Germany, Austria and Poland |
 2012 REVA
Medical, Inc. 9
9
Operations Update
New resources support manufacturing and CE Mark trial needs
•
Directors of Operations and Quality Assurance hired
•
Doubled facility size (to 37,000 sq. ft)
•
Increased workforce by 32% (engineering, assembly, quality)
|
 2012 REVA
Medical, Inc. 10
10
Operations Update
New resources, cont.
•
Polymer manufacturing now in house
–
State of the art polymer synthesis facility |
 2012 REVA
Medical, Inc. 11
11
Operations Update
ReZolve2
Lower profile system
–
Sheathless
–
6 French guide catheter compatible
–
Optimized retention
CE Mark and commercial device |
 2012 REVA
Medical, Inc. 12
12
Operations Update
Additional patents -
to further protect our technology
•
Nine US Patents related to stent design and polymers issued
or allowed May 2011 to May 2012
–
REVA -
US 7,939,611 , issued 5/10/11 -
Side-Chain Crystallizable Polymers
–
REVA -
US 7,947,071 , issued 5/24/11 -
Expandable Slide &Lock Stent
–
REVA -
US 7,988,721 , issued 8/2/11 -
Axially, Radially Nested Stent
–
Rutgers -
US 8,008,528 , issued 8/30/11 -
N-Substituted Monomers & Polymers
–
REVA -
US 8,034,365 , issued 10/11/11 -
N-Substituted Monomers & Polymers
–
REVA -
US 8,124,700 , issued 2/28/12 -
Side-Chain Crystallizable Polymers
–
REVA -
US 8,133,959 , issued 3/13/12 -
Side-Chain Crystallizable Polymers
–
Rutgers –
No. 12/577,205, allowed 4/6/12 -
Inherently Radio-Opaque Polymers
–
REVA -
US 8,172,894 , issued 5/8/12 -
Circumferentially Nested Slide & Lock Stent
REVA’s technology now protected by more than 280 patents |
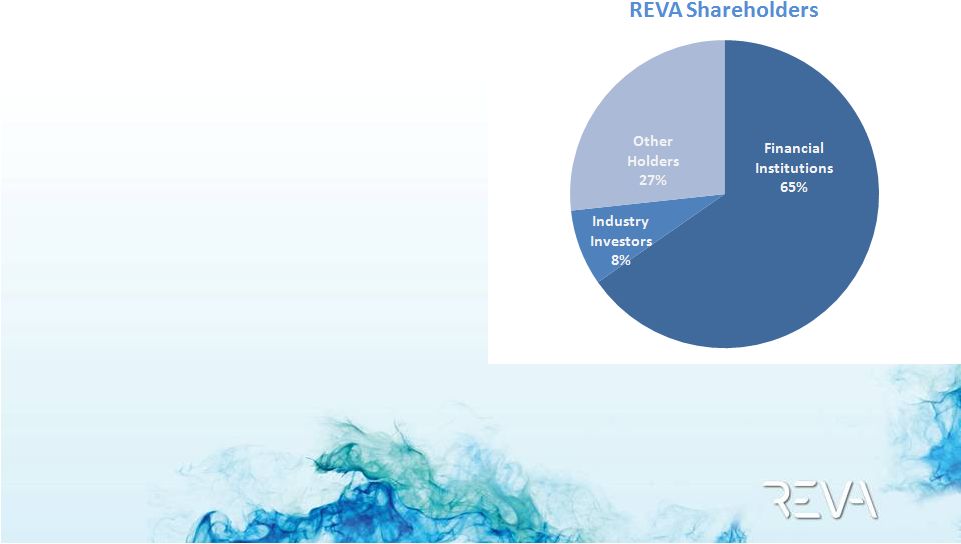 2012 REVA
Medical, Inc. 13
13
Corporate Update
Private Share Transaction March 2012
•
US$24m traded
•
Liquidity for legacy funds who
still retain substantial holdings
•
Buyers led by Elliott
Management
($17mm)
,
IPO
investors, and Medtronic
•
REVA has a strong base of
institutional support
New large sophisticated US investor helps support our belief of long-term value
Medtronic increases stake to 8% |
 2012 REVA
Medical, Inc. 14
14
2012 Coronary Stent Market
•
$4.6 billion WW*
•
Dominated by three major players
–
Abbott
–
Boston Scientific
–
Medtronic
•
Drug Eluting Stents (DES) at parity
–
Xience / Promus share leaders
•
Bioresorbable scaffolds poised to enter EU market this year
* JP Morgan Interventional Cardiology Market Model,
Feb. 2012 |
 2012 REVA
Medical, Inc. 15
15
Bioresorbable Competition*
•
Abbott –
the market leader
–
Commercially
launching
their
BVS
product
in
EU
-
Q4
2012
–
Premium-pricing strategy
–
US trial plans in discussion with FDA
•
Biotronik
–
Positive
6-month
DREAMS
data
(4.7%
TLR,
no
stent
thrombosis)
–
Improvement over first-generation device
–
Switching to –limus drug, adding radiopaque markers
•
Others
–
ARTS –
FIM approved for 2012
–
Elixir -
FIM Complete, CE trial initiated Nov 2011
* Based solely on public announcements made by the above referenced parties
|
 2012 REVA
Medical, Inc. 16
16
Finance and Administration |
 2012 REVA
Medical, Inc. 17
17
Financial Results
12/31/10
12/31/11
3/31/11
3/31/12
Balance Sheet:
Cash/Investments
81,747
$
64,387
$
77,734
$
58,804
$
Assets
83,475
67,320
79,081
62,540
Equity
81,947
64,583
77,557
60,041
Statement of Ops:
R&D Exps.
6,826
$
13,401
$
3,241
$
3,799
$
G&A Exps.
3,292
7,695
1,928
1,922
Net Loss
(23,507)
(20,908)
(5,133)
(5,698)
As of &
3 Mos. Ended
As of &
12 Mos. Ended
(U.S. Dollars, in thousands) |
 2012 REVA
Medical, Inc. 18
18
Corporate Governance
•
Sarbanes-Oxley Compliant as of 12/31/11
–
Internal control over financial reporting
–
U.S. Securities and Exchange Commission requirement
•
Diversity Policy Adopted 12/31/11
–
Diversity among workforce, executives, and directors
–
ASX Corporate Governance Principles requirement |
 2012 REVA
Medical, Inc. 19
19
RESTORE
Clinical Trial Update |
 2012 REVA
Medical, Inc. 20
20
RESTORE Clinical Trial
ReZolve Sirolimus-Eluting Bioresorbable Coronary Scaffold
Initiated December 2011
•
Up to 50 patients
–
Sites in Brazil & Europe
–
Principal Investigator:
–
Dr. Alexandre Abizaid
•
Primary Endpoint(s):
–
Freedom from ischemic-driven target lesion
revascularization at 6 months
–
Quantitative measurements at 12 months
(QCA/IVUS) |
 2012 REVA
Medical, Inc. 21
21
RESTORE Clinical Trial –
Device
ReZolve
Sirolimus-Eluting Bioresorbable Coronary Scaffold
Drug-eluting
(Sirolimus)
Radiopaque
Strong and Resilient
Polymer
Unique Slide & Lock
Design |
 2012 REVA
Medical, Inc. 22
22
RESTORE Clinical Trial
Investigators
•
Germany
–
Prof. Dr. med. Björn Andrew
Remppis,
Bad Bevensen
–
Prof. Dr. med. Johannes Brachmann, Coburg
–
Prof. Dr. med. Volker Schächinger, Fulda
–
PD Dr. med. Stephan Fichtlscherer, Frankfurt
–
Professor Dr. med. Axel Schmermund,
Frankfurt
–
Prof. Dr. med. Norbert Frey, Kiel
–
Prof. Dr. med. Nikos Werner, Bonn
•
Brazil
–
Dr. Alexandre Abizaid, Sao Paulo
Principal Investigator
•
Austria
–
Dr. med. Matthias Heigert, Salzburg
–
Dariusz Dudek, MD PhD, Krakow
•
Poland |
 2012 REVA
Medical, Inc. 23
23
RESTORE Clinical Trial
Interim Update
•
13 patients implanted with ReZolve®
as of 14 May 2012
•
Enrollment impacted by:
–
Strict inclusion criteria
–
7 Fr. sheath profile
–
IVUS requirement
No reported Material Adverse
Coronary Events (MACE) to date |
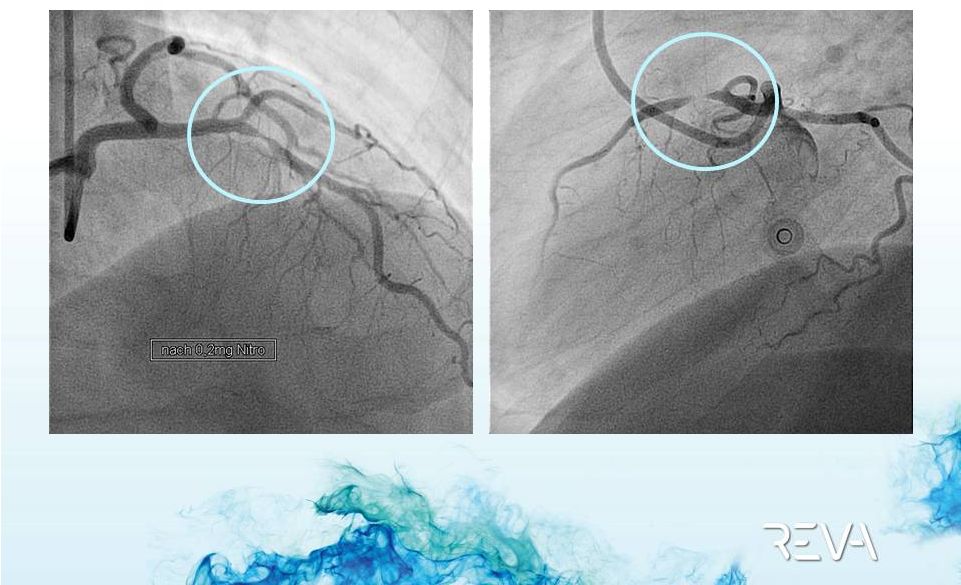 2012 REVA
Medical, Inc. 24
24
Case Example
Pre-implant
90% Occlusion
95%+ occlusion of the artery |
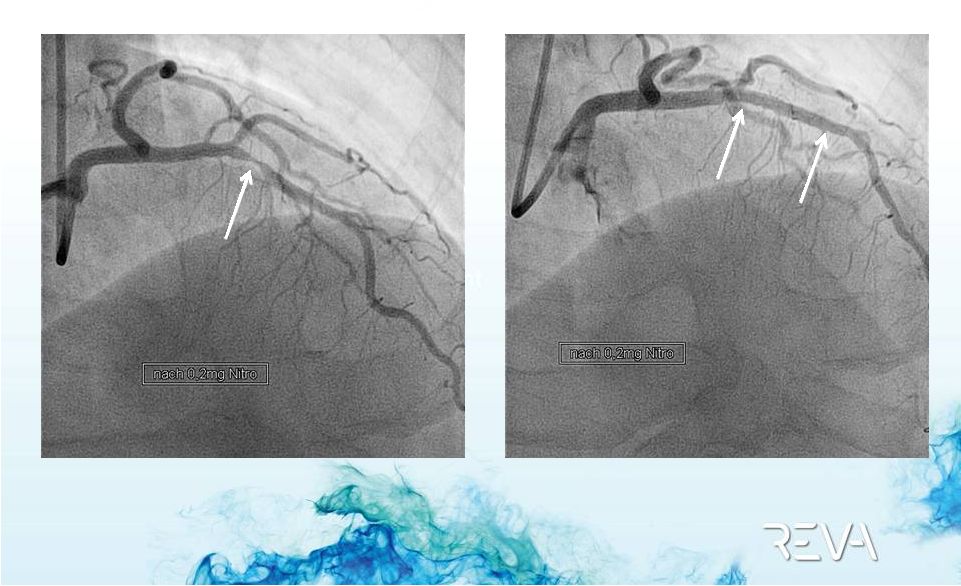 2012 REVA
Medical, Inc. 25
25
Case Example
Final Result after ReZolve
Implant
Post Implant
Pre-Implant
ReZolve
Implant |
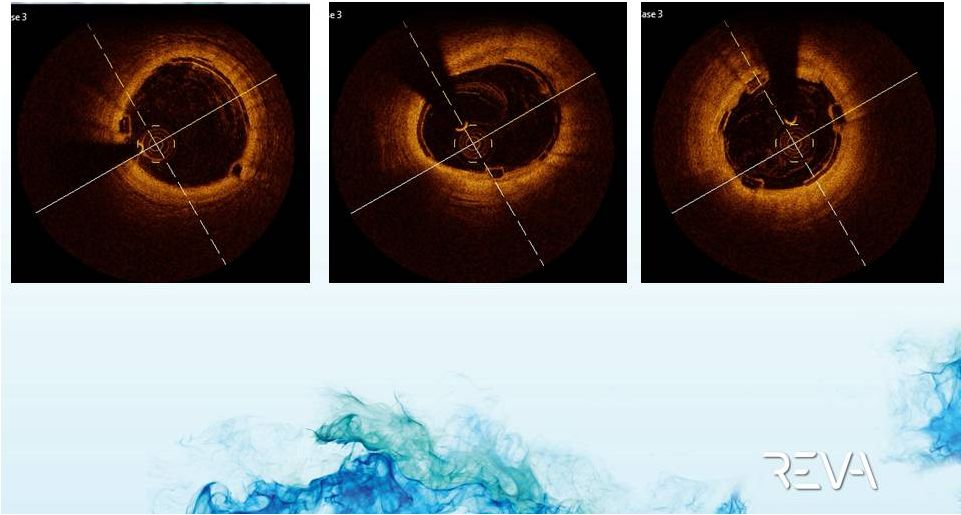 2012 REVA
Medical, Inc. 26
26
Case Example
OCT Imaging of ReZolve
Implant
Distal Scaffold
Mid Scaffold
Proximal Scaffold |
 2012 REVA
Medical, Inc. 27
27
RESTORE Clinical Trial
Conclusions
•
All treated patients have patent arteries
•
Radiopacity of ReZolve®
appreciated by physicians
•
Delivery and deployment did not require special procedures
•
Patient enrollment in pilot will continue through Q2 2012
•
CE Mark clinical trial planned for Q4 2012 with ReZolve2
|
 2012 REVA
Medical, Inc. 28
28
Development Update |
 2012 REVA
Medical, Inc. 29
29
ReZolve2
Commercial Product
ReZolve (1.83 mm profile)
ReZolve2 (1.47 mm profile)
Ahead of Schedule
•
Lower
Profile
(6
Fr.)
•
No sheath
–
Improved retention
•
Increased strength |
 2012 REVA
Medical, Inc. 30
30
2007
•
Modular Design
•
7 Fr. delivery
•
Radiopaque
ReZolve
Device Evolution
•
Spiral Design
•
retractable sheath
•
Sirolimus
ReZolve
First in man
•
Lower Profile (6 Fr.)
•
Sheathless
•
Improved retention
ReZolve2
ReZolve |
 2012 REVA
Medical, Inc. 31
31
REVA’s Polymer Evolution
I
2
DAT
Tyrosine
Ethyl-
Ester
PCL
PCL
85%
2009
I
2
DAT
I
2
DAT
TYR
90%
2012
PEG
Tyrosine
I
2
DAT
I
2
DAT
Tyrosine
Ethyl-
Ester
2007
90% |
 2012 REVA
Medical, Inc. 32
32
ReZolve2
Example of Deliverability and Strength
6
French
“sheathless”
system
implanted in lesion animal model |
 2012 REVA
Medical, Inc. 33
33
Pivotal CE Mark Clinical Trial
•
Multi-Center Global Trial
–
Up to 30 sites in Brazil, Europe, Australia and New Zealand
•
Enrollment planned to begin Q4 2012
–
Broadened inclusion criteria
–
IVUS not required
–
Additional size (diameter/length)
•
Up to 200 patients
•
Lower profile (6 Fr.) ReZolve2 system |
 2012 REVA
Medical, Inc. 34
34
Final Comments
•
Pilot study has demonstrated initial safety of ReZolve®
scaffold
–
no reported MACE
•
ReZolve2
is REVA’s commercial product
–
Improved deliverability and scaffold performance
•
CE Mark trial planned to commence Q4 2012
•
A special thanks to our world-class investigators
•
6-month data on initial patient cohort will assess clinical
endurance of ReZolve®
–
October 2012 |
 2012 REVA
Medical, Inc. 35
35
Thank you |
