Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REVA Medical, Inc. | d354388d8k.htm |
| EX-99.1 - INTERIM CLINICAL RESULTS ANNOUNCEMENT - REVA Medical, Inc. | d354388dex991.htm |
 0
0
ReZolve
A Polymer Scaffold to Restore the Vessel
Dr. Alexandre Abizaid
Exhibit 99.2 |
 2012 REVA
Medical, Inc. 1
1
Disclosure Statement of Financial Interest
•
Consulting Fees
•
REVA Medical, Inc.
Within the past 12 months, I have had a financial interest/arrangement or
affiliation with the organization(s) listed below.
Affiliation/Financial Relationship
Company |
 2012 REVA
Medical, Inc. 2
2
RESTORE Clinical Trial
ReZolve Sirolimus-Eluting Bioresorbable Coronary Scaffold
Initiated December 2011
•
Up to 50 patients
–
Sites in Brazil & Europe
•
Primary Endpoint(s):
–
Freedom from ischemic-driven target lesion
revascularization at 6 months
–
Quantitative measurements at 12 months
(QCA/IVUS) |
 2012 REVA
Medical, Inc. 3
3
RESTORE Clinical Trial –
Device
ReZolve
Sirolimus-Eluting Bioresorbable Coronary Scaffold
Drug-eluting
(Sirolimus)
Radiopaque
Strong and Resilient
Polymer
Unique Slide & Lock
Design
Tyrosine-derived polycarbonate material that is radiopaque
|
 2012 REVA
Medical, Inc. 4
4
ReZolve
Device Specifications
•
3.0 mm x 18 mm
•
Treatment range: 2.9 mm to 3.4 mm
•
Post-dilation up to 3.7 mm
•
7 French today
(6 Fr. planned)
•
80
g
Sirolimus
•
Fully radiopaque
•
Sheathed
rapid
exchange
delivery
system
(sheathless
planned)
•
Balloon expandable
•
No special storage or handling |
 2012 REVA
Medical, Inc. 5
5
ReZolve
Proprietary Technology
•
‘Slide & Lock’
design
–
Ratchet design
–
Strong
–
Minimal recoil
•
Tyrosine-derived polycarbonate
–
Tunable
–
Visible
–
Biocompatible
–
Drug compounded into tyrosine base
material
Our Ratchet
Concept
Metal
ReZolve |
 2012 REVA
Medical, Inc. 6
6
RESTORE Clinical Trial
Investigators
•
Germany
–
Prof. Dr. med. Björn Andrew
Remppis,
Bad Bevensen
–
Prof. Dr. med. Johannes Brachmann, Coburg
–
Prof. Dr. med. Volker Schächinger, Fulda
–
PD Dr. med. Stephan Fichtlscherer, Frankfurt
–
Professor Dr. med. Axel Schmermund,
Frankfurt
–
Prof. Dr. med. Norbert Frey, Kiel
–
Prof. Dr. med. Nikos Werner, Bonn
•
Brazil
–
Dr. Alexandre Abizaid, Sao Paulo
Principal Investigator
•
Austria
–
Dr. med. Matthias Heigert , Salzburg
•
Poland
–
Dariusz Dudek, MD PhD, Krakow |
 2012 REVA
Medical, Inc. 7
7
RESTORE Clinical Trial
Inclusion/Exclusion Criteria
•
Primary Inclusion Criteria
–
Clinical evidence of myocardial ischemia or positive function test
–
Visually estimated stenosis >50% and <100%
–
Reference vessel diameter 2.9 mm –
3.3 mm (confirmed by IVUS)
–
Lesion length
12mm
•
Primary Exclusion Criteria
–
Myocardial infarctions within 24 hours of the procedure
–
Ejection fraction <25%
–
Target vessel is totally occluded (TIMI 0 or 1)
–
Significant stenosis (>50%) proximal or distal to target lesion
–
Highly calcified lesion |
 2012 REVA
Medical, Inc. 8
8
RESTORE Clinical Trial
Progress Update
•
16 patients enrolled as of May 14, 2012
•
Enrollment distribution
–
4 patients in Brazil
–
6 patients in Austria
–
6 patients in Germany
No reported MACE to date |
 2012 REVA
Medical, Inc. 9
9
Clinical Case Examples |
 2012 REVA
Medical, Inc. 10
10
CASE 01
First ReZolve
Implant –
Baseline and Procedure Details
•
Baseline
–
56 year old caucasian female
–
CV risk factors: Hypertension, prior MI, smoker
–
LAD lesion >90% stenosis
–
Estimated reference vessel size 3.1 mm
–
Estimated lesion length 12
mm
•
Procedure
–
Intervention of LAD with ReZolve
on December 21, 2011
–
Predilation with 30 mm x 12 mm non-compliant balloon
–
3.0
mm
x
18
mm
ReZolve
scaffold
implant,
12
atm
–
Patient now past 4 month time point ; asymptomatic
Procedure performed by Dr. Alexandre Abizaid, Sao Paulo, Brazil
|
 2012 REVA
Medical, Inc. 11
11
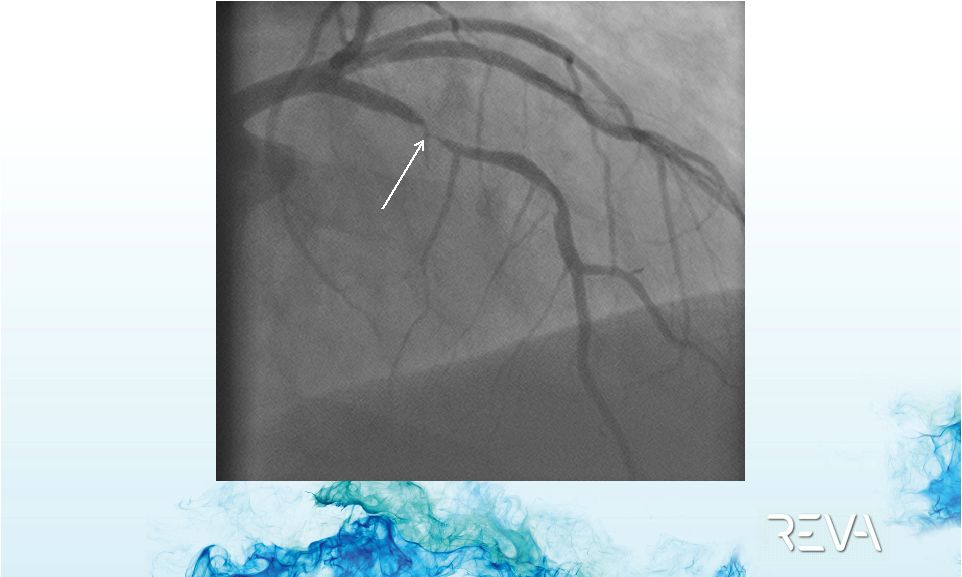
Case 01
First ReZolve
Implant –
Pre-implant
90% Stenosis
Procedure performed by Dr. Alexandre Abizaid, Sao Paulo, Brazil
|
 2012 REVA
Medical, Inc. 12
12
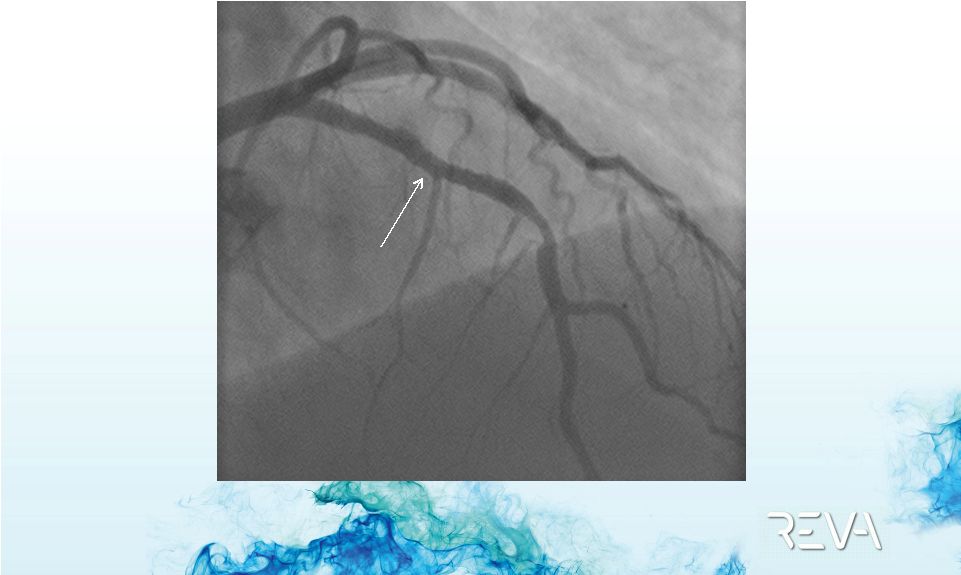
Case 01
First ReZolve
Implant –
Final ReZolve
Implant Result
Post ReZolve
Implant |
 2012 REVA
Medical, Inc. 13
13
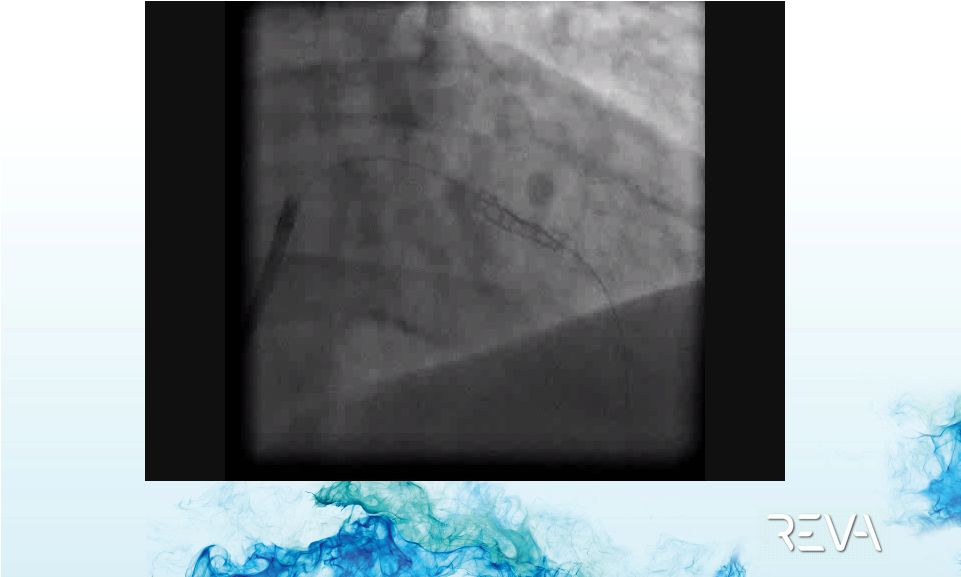
Case 01
First ReZolve
Implant –
Final ReZolve
Implant Result |
 2012 REVA
Medical, Inc. 14
14
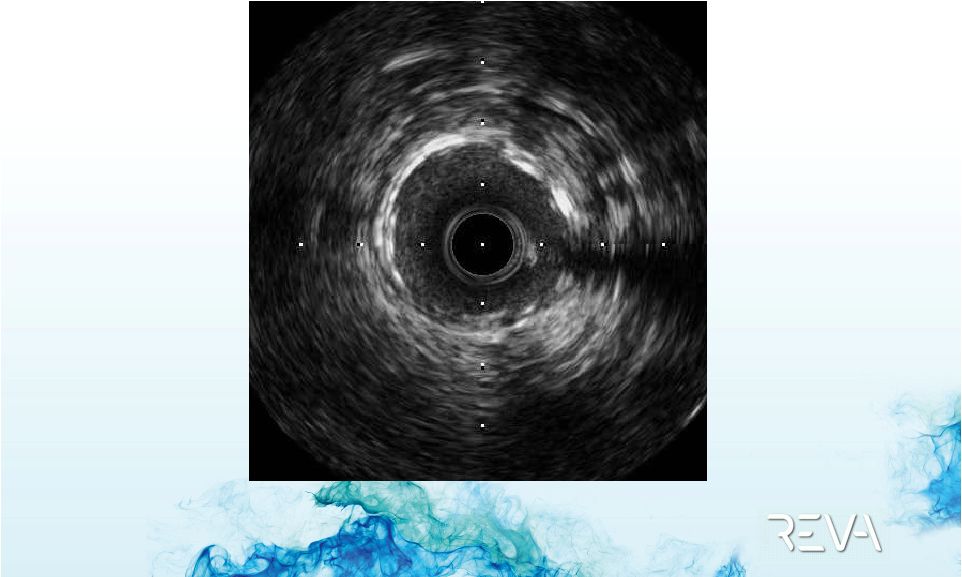
Case 01
First ReZolve
Implant –
IVUS Imaging |
 2012 REVA
Medical, Inc. 15
15
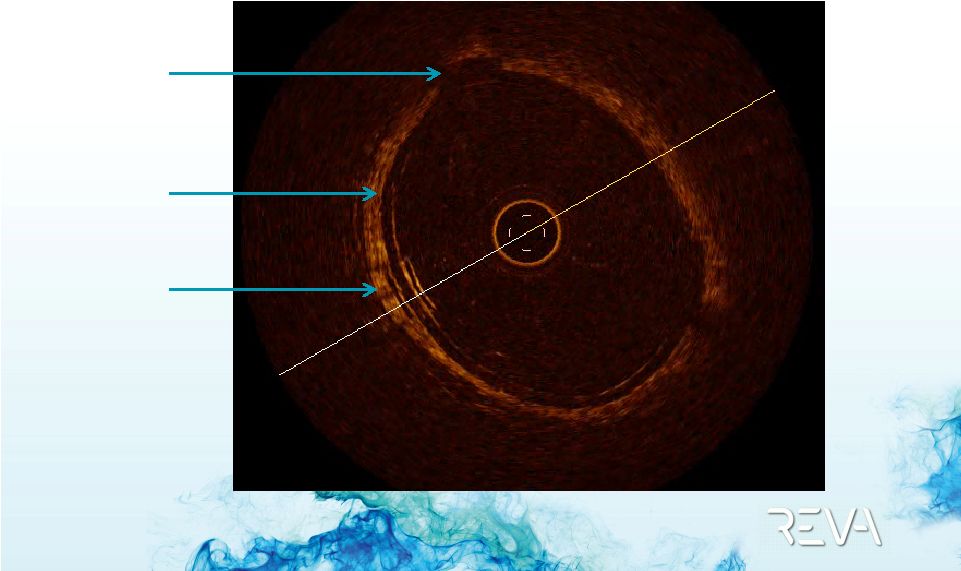
Case 01
First ReZolve
Implant –
OCT Imaging
Backbone
Sliding Slot
Rail –
“U” |
 2012 REVA
Medical, Inc. 16
16
CASE 02
Baseline and Procedure Details
•
Baseline
–
74 year old male
–
CV risk factors: Hypertension, Hypercholesterolemia
–
LAD lesion >95% Stenosis
–
Estimated reference vessel size 3.0 mm
–
Estimated lesion length 9 mm
•
Procedure
–
Intervention of LAD with ReZolve
on March 21, 2012
–
Predilation with 3.0 mm x 12 mm non-compliant balloon (13atm ,30
sec) –
3.0
mm
x
18
mm
ReZolve
scaffold
implanted
(12
atm,
20
sec)
–
Post-dilation with 3.25 mm x 20 mm non-compliant balloon (12 atm, 32
sec) –
Patient now past 1 month time point in FU-visit; asymptomatic
Procedure performed by Dr. med Matthias Heigert, II.Medizin,
University Clinic, Salzburg, Austria |
 2012 REVA
Medical, Inc. 17
17
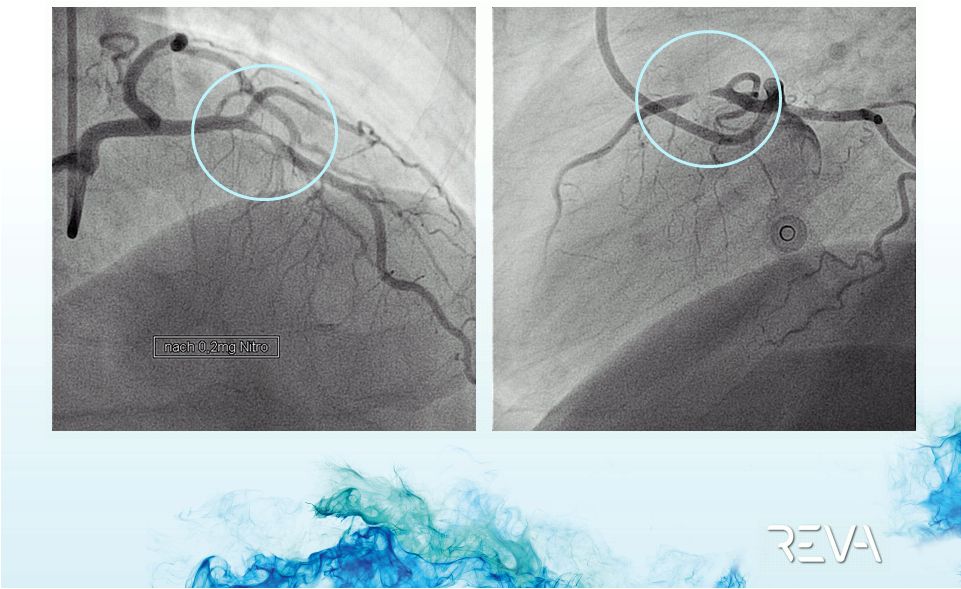
Case 02
Pre-implant
95+% Stenosis |
 2012 REVA
Medical, Inc. 18
18
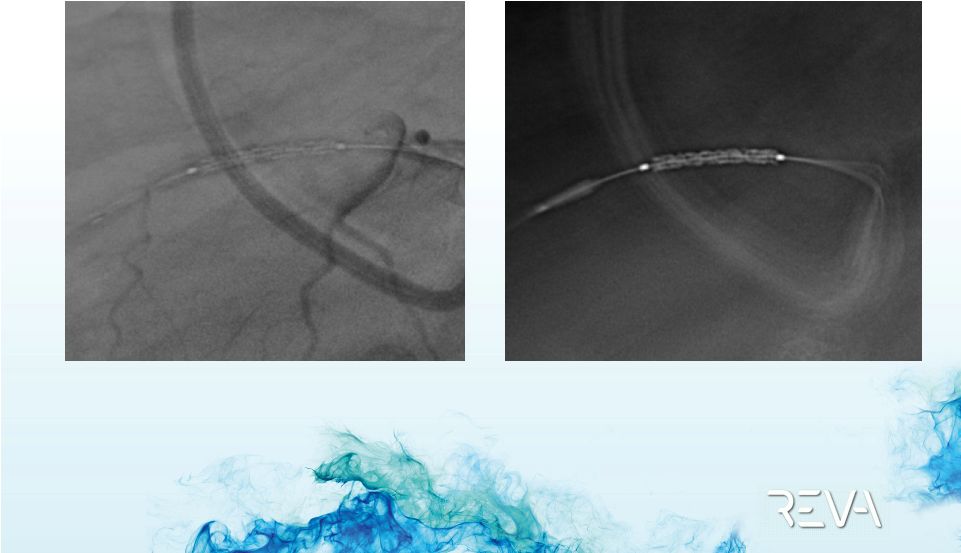
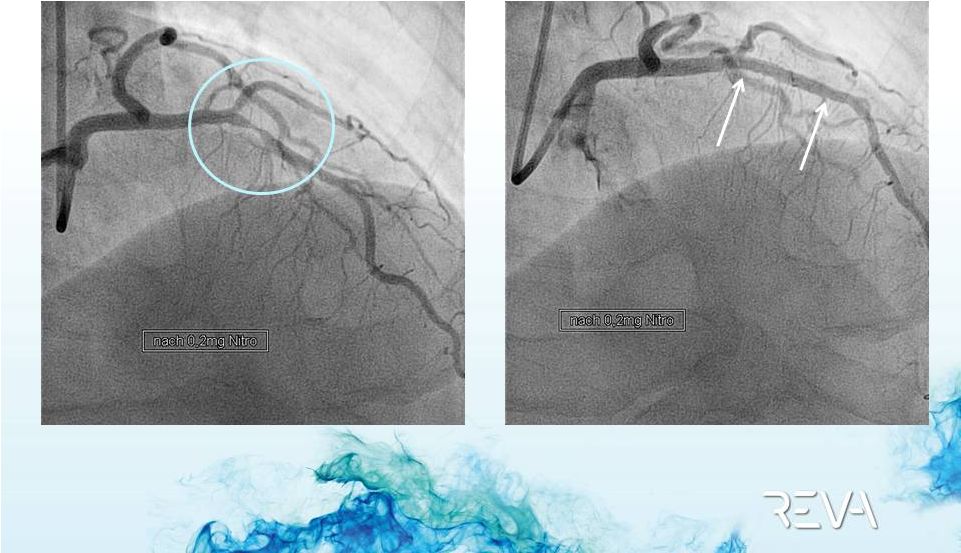
Case 02
Delivery to Lesion
Site |
 2012 REVA
Medical, Inc. 19
19
Case 02
Final ReZolve
Implant Result
Post-Implant
Pre-Implant
ReZolve
Implant |
 2012 REVA
Medical, Inc. 20
20
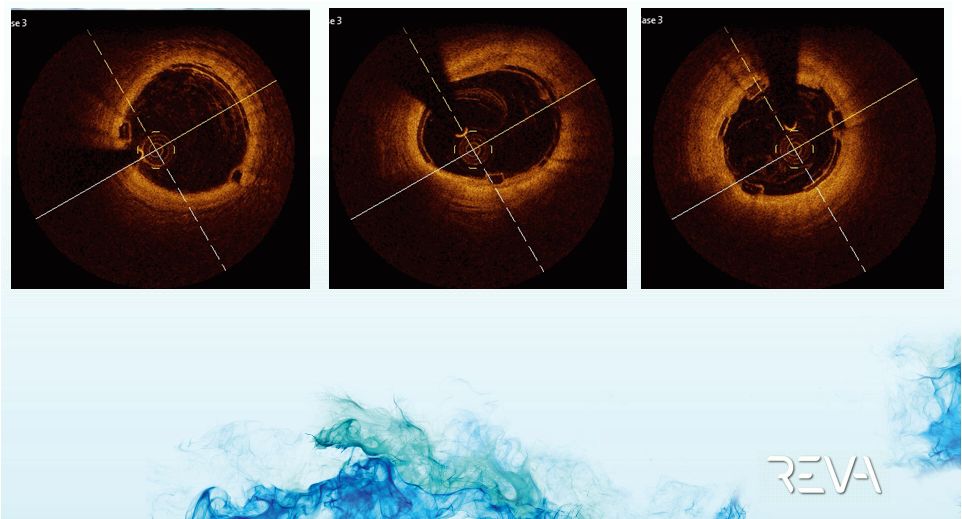
Case 02
OCT Imaging
Distal Scaffold
Mid Scaffold
Proximal Scaffold |
 2012 REVA
Medical, Inc. 21
21
RESTORE Clinical Trial
Observations
•
Acute Result: All patients have patent arteries
•
Scaffold is fully visible during procedure
•
ReZolve
deployed with continuous inflation pressure
•
All enrolled patients remain asymptomatic
(1-5 months post-implant)
Two stents could not reach lesion site due to current
pilot device profile and vessel tortuosity
Sheathed system limits deliverability to small and tortuous arteries
Therefore… |
 2012 REVA
Medical, Inc. 22
22
ReZolve2
Addresses Deliverability Needs
•
Lower Profile (6 Fr.)
•
No sheath
–
Improved retention
•
Increased radial strength
Will be used in upcoming CE Trial
ReZolve (1.83 mm profile)
ReZolve2 (1.47 mm profile) |
 2012 REVA
Medical, Inc. 23
23
Pivotal CE Trial
•
Multi-Center Global Trial
–
Up to 30 sites in Brazil, Europe, Australia and New Zealand
•
First enrollment planned for Q4 2012
•
ReZolve2
system
is
6
Fr.
and
sheathless |
 2012 REVA
Medical, Inc. 24
24
Thank you |
