Attached files
| file | filename |
|---|---|
| 8-K - BOSTON THERAPEUTICS, INC. 8-K - Boston Therapeutics, Inc. | boston8k.htm |
Exhibit 99.1

Investor Presentation April 2012 Our Mission Boston Therapeutics, Inc. (BTI) mission is to research, develop and bring to market applied carbohydrate technologies that address unmet medical needs in the areas of diabetes and inflammatory diseases. Company: Boston Therapeutics, Inc. Sector: Pharmaceutical Ticker Symbol: BTHE.PB Shares Outstanding: 16,223,206 Incorporate: 2009 Fiscal Year End: December 31 Innovators in Complex Carbohydrate Chemistry™ Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)

Forward-Looking Statement Any statements about any possible future expectations, plans and prospects for the Company, including statements containing the words "believes," "anticipates," "plans," "expects," and similar expressions, constitute forward-looking statements, which are subject to the safe harbor for such statements in the Private Securities Litigation Reform Act of 1995. Future events could cause actual results to differ materially from those indicated by such statements. Reference is made to the factors discussed in the “Management Discussion and Analysis" and "Risk Factors" sections of the Company's most recent business plan or report filed with the Securities and Exchange Commission. The forward-looking statements herein represent the Company's views and possibilities as of the date of this presentation and should not be relied upon to represent the Company's views as of a subsequent date. While the Company anticipates that subsequent events may cause the Company's views to change, the Company disclaims any obligation to update such forward-looking statements. For a more comprehensive risk disclosure we direct you to our web site: www.bostonti.com Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)
Investment Proposition OTC Consumer Product: SugarDown®—BTI is in a unique position to capitalize on consumer demand for a non-systemic product that can help with blood glucose management after a meal. SugarDown® is already in direct sales channel in the U.S. and being sold through strategic partners in the Italian and Chinese Markets in addition to receiving new requests for new retail distribution in the U.S. and International markets weekly. Pharmaceutical Products: PAZ320 is a non-systemic, chewable drug candidate to be prescribed for reduction of blood glucose in diabetics. The compound is a phase II clinical trial asset, currently in clinical trial at Dartmouth-Hitchcock Medical Center in New Hampshire. PAZ320 is a non-toxic compound for prevention of diabetes and management of post-prandial glucose excursion by delaying glucose absorption in the intestine. IPOXYN™ is an injectable phase III asset anti-hypoxia drug candidate for Limb Ischemia. IpoxynTM is a glycoprotein drug candidate formulated to function as a universal carrier of oxygen. It was specifically developed for Lower Limb Ischemia associated with diabetes. IpoxynTM is an oxygen therapy and non-biologic as a New Chemical Entity (NCE). Oxyfex™ is a veterinary counterpart to Ipoxyn™. Boston Therapeutics, Inc. is seeking to raise $3 million to meet working capital needs, including inventory build, marketing investment spending and clinical trials. Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)

Market Approach Complex Carbohydrate FDA approved GRAS* Chemistry Glycemic Health SugarDown™ Consumer Brand OTC Initial Target Markets U.S. China Italy Phase I Trial Complete 6/2011 Product Available PAZ320 Pharmaceutical PAZ320 Clinical Trials Validation in clinical trials Dartmough-Hitchcock Medical Center Phase II Trial Commenced 12/2011 75% Enrollment Boston Therapeutics approach to the market is two simultaneous paths: Consumer brand OTC : Near Term Revenue Longer-term drug development (*GRAS Generally Regarded as Safe) Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)
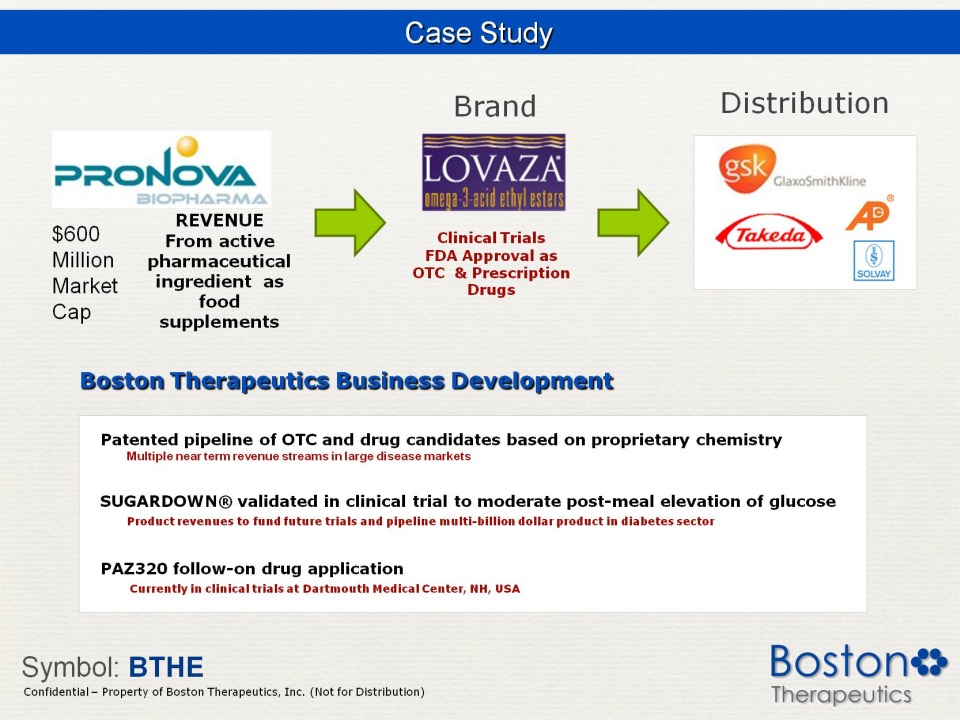
Case Study Brand Distribution Pronova Biopharma $600 Million Market Cap REVENUE From active Pharmaceutical ingredient as food supplements Lovaza omega-3 acid ethyl esters Clinical Trials FDA Approval as OTC & Prescription Drugs gsk GlaxoKlineSmith Takeda AP Solvay Boston Therapeutics Business Development Patented pipeline of OTC and drug candidates based on proprietary chemistry Multiple near term revenue streams in large disease markets SUGARDOWN® validated in clinical trial to moderate post-meal elevation of glucose Product revenues to fund future trials and pipeline multi-billion dollar product in diabetes sector PAZ320 follow-on drug pplication
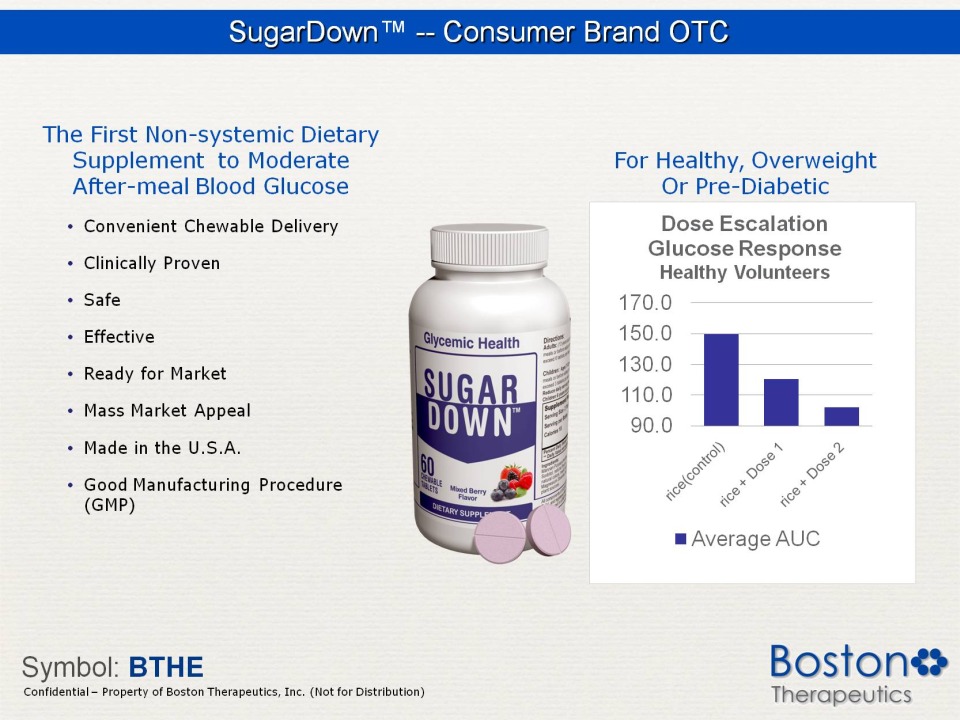
SugarDown™ -- Consumer Brand OTC The First Non-systemic Dietary Supplement to Moderate After-meal Blood Glucose Convenient Chewable Delivery Clinically Proven Safe Effective Ready for Market Mass Market Appeal Made in the U.S.A. Good Manufacturing Procedure (GMP) For Healthy, Overweight Or Pre-Diabetic Dose Escalation Glucose Response Healthy Volunteers 170.0 150.0 130.0 110.0 90.0 rice (control) + Dose 1 2 Average AUC Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)

Clinical Trials The University of Sydney SugarDown Research Group: University of Sydney Study: Phase I Determination of the postprandial glucose and insulin responses of white rice alone and white rice consumed with SUGARDOWN Status: Completed 2011 Findings: No serious adverse events, No withdrawals Postprandial glucose: 19% and 32% reduction, respectively. Postprandial insulin: 16% and 24% reduction, respectively. Plasma glucose responses were significantly associated with their corresponding insulin iAUC responses (r = 0.78, p = 0.0001). “ The study showed that with both doses of mannan polysaccharide, there was a significant and incremental reduction in iAUC for both plasma glucose and insulin”. - Dr. Nicholas J. Taylor Darthmouth- Hitchcock PAZ320 Research Group: Dartmouth-Hitchcock Medical Center Study: Phase II Study to Evaluate the Safety and Efficacy of PAZ320 when added to Oral Agents or Insulin regimen in Patients with Type 2 Diabetes Status: 75% enrolled April 2012. Independent Review Board (IRB) approved July 2011. Findings: no safety concerns from interim data analysis Type 2 Diabetics: Expecting >40% reduction in iAUC postprandial glucose response Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)
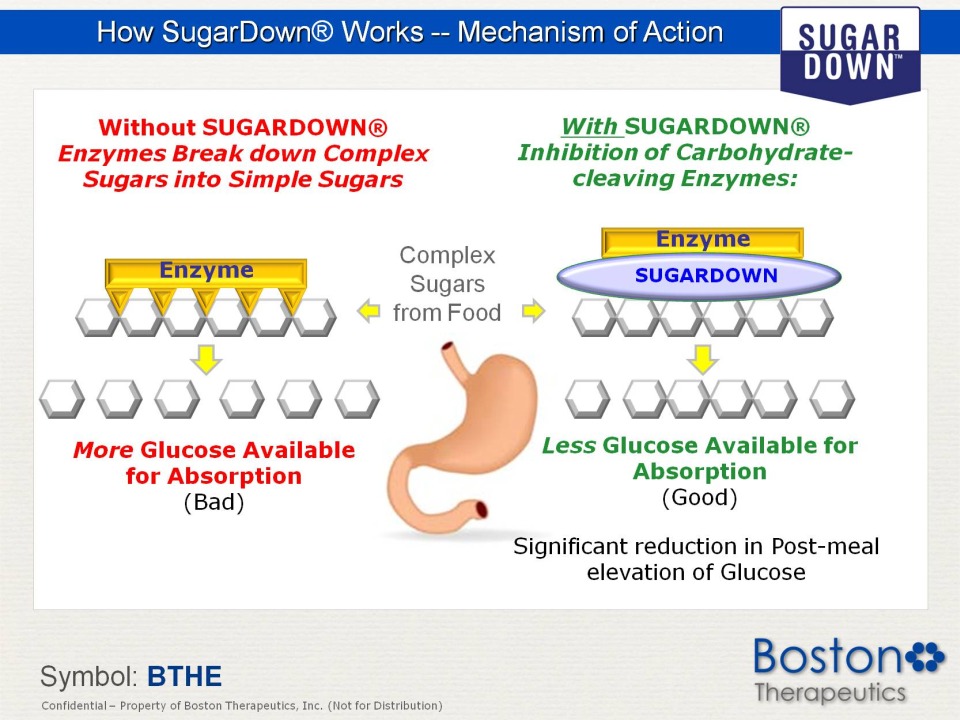
How SugarDown® Works -- Mechanism of Action Without SUGARDOWN® Enzymes Break down Complex Sugars into Simple Sugars Enzyme More Glucose Available for Absorption (Bad) With SUGARDOWN® Inhibition of Carbohydrate-cleaving Enzymes: Complex Sugars from Food Less (Good) Significant reduction in Post-meal elevation of Glucose Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)
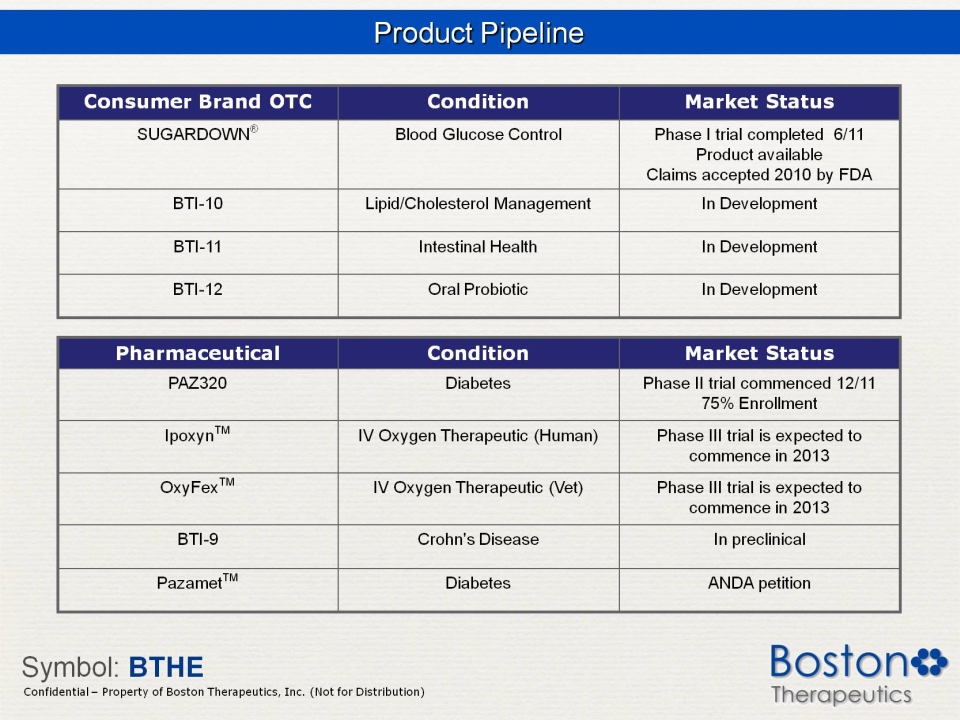
Product Pipeline Consumer Brand OTC Condition Market Status SUGARDOWN® Blood Glucose Control Phase I trial completed 6/11 Product available Claims accepted 2010 by FDA BTI-10 Lipid/Cholesterol Management In Development BTI-11 Intestinal Health BTI-12 Oral Probiotic Pharmaceutical PAZ320 Diabetes Phase II trial commenced 12/11 75% Enrollment Ipoxyn™ IV Oxygen Therapeutic (Human) Phase III trial is expected to commence in 2013 OxyFex™ (Vet) BTI-9 Crohn’s Disease In preclinical Pazamet™ Diabetes ANDA petition Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)
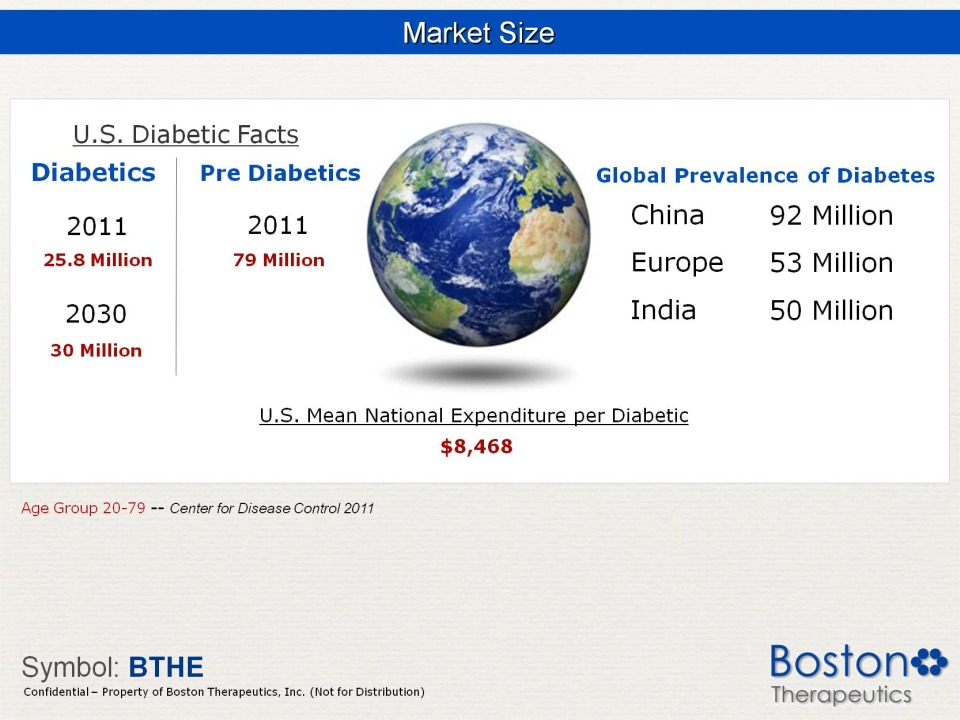
Market Size U.S. Diabetic Facts Pre 2011 25.8 Million 79 Million 2030 30 Million Global Prevalence of Diabetes China 92 Million Europe 53 Million India 50 Million Mean National Expenditure per Diabetic $8,468 Age Group 20-79 -- Center for Disease Control 2011 Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)

What Consumers Have to Say... “I've been entertaining friends from France, and have been eating out more these past few days, up to 2x a day. With SUGARDOWN®, I've been getting totally normal blood sugar levels, 95 fasting, and under 130 post- meal. I took 2 tablets of SUGARDOWN® before eating a bowl of pretzels and checked my glucose 30 minutes later and it actually went down to 262. It usually goes to 400 after a snack like that. “My energy level is much more consistent and not as impacted by meals - highs and lows are much less dramatic.” “I have noticed after 10 days of taking SUGARDOWN that my digestion is much better as well as overall improvement of my intestinal functions. Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)

Executive Team and Advisors Our Executive Team can move products through FDA and into Consumer Markets David Platt, Ph.D. Chairman, Chief Executive Officer, Chief Financial Officer and Treasurer Dr. Platt is a world-renown expert in carbohydrate chemistry and has founded three publicly-traded companies, creating nearly $1B for investors. He has raised $150M directly in public markets in the US, and has lead two drug candidates from concept to phase II clinical trials. Prior to Boston Therapeutics, from 2001 to 2009, Dr. Platt was the Founder, Chief Executive Officer and Chairman of the Board at Pro-Pharmaceuticals, Inc., a publicly-traded company. Kenneth A. Tassey, Jr., President, Chief Operating Officer and Director Mr. Tassey has served as president of Boston Therapeutics, Inc. since November 2010. Prior to that, Mr. Tassey co-founded Boston Therapeutics, Inc. and served as Chief Executive Officer and President since its inception in June 2009 until its merger with Avanyx Therapeutics, Inc., now Boston Therapeutics, Inc.From 2007 to 2009, Mr. Tassey was President of TKCI, a consulting firm for commercial finance projects. Prior to TKCI, from 2005 to 2007, Mr. Tassey served as President of Liberty Shore LLC, as a consultant to businesses and to commercial and residential lenders. Mr. Tassey has a background in business management and operations. John Corella, Director of Marketing Mr. Corella has been working in the P.R. marketing, media and technology arenas for over 25 years, both domestically and internationally. Mr. Corella is the founder of CMME, a targeted communications company focused on product brand marketing, direct and retail sales working with Whole Foods Market, Costco, Safeway and host of regional retailers. CMME’s unique approach involves coordinated initiatives that combine the fusion of consumer analytics and media services, with a sophisticated consumer approach that embraces street credible solutions. Mr. Corella has been actively involved in effective market research in the general and the emerging acculturated market as to product adaptation and brand strategies of media. Anthony Squeglia, Director of Investor Relations (Advisor) From 2007 to 2012 Anthony Squeglia served as Chief Financial Officer of Galectin Therapeutics, Inc, (GALT) a NASDAQ company. From 2003 to 2007 Mr. Squeglia was Vice President of Investor Relations for the Galectin Therapeutics, Inc, and was instrumental in the Company’s listing on the Amex (Pro-Pharmaceuticals), as well as in its fund-raising activities. From 2001 to 2003, Mr. Squeglia was a Partner in JFS Advisors, a management consulting firm that delivered strategic services to entrepreneurial businesses that included raising funds, business planning, positioning, branding, marketing and sales channel development. Previously, Mr. Squeglia helped to successfully launch an IPO for Summa Four and held senior management positions with Unisys, AT&T, Colonial Penn and ITT. Mr. Squeglia received an M.B.A. from Pepperdine University and a B.B.A. from The Wharton School, University of Pennsylvania. Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)

Executive Team and Advisors (cont’d) Our Executive Team can move products through FDA and into Consumer Markets Carl L. Lueders, Audit Committee Chairman and Director Mr. Lueders has been a Director of the Company since September 2009, has a broad range of experience in finance, operations, short- and long-term planning, forecasting, performance measurement, SEC reporting, and controls. He is currently Chief Financial Officer (CFO) for Micronetics, Inc. a manufacturer of microwave and radio frequency products for commercial wireless, defense and aerospace products. Prior to that he was acting CFO for Pro-Pharmaceuticals and before that CFO for R.F. Morse & Son, a privately held agri-based company. Prior to that Mr. Lueders spent 22 years with publicly held Polaroid in various finance positions, including Vice President and Controller, Treasurer and acting Chief Financial Officer. Mr. Lueders is a CPA and received his B.A. in Economics from the University of Massachusetts at Amherst and his M.B.A. from Babson College. Dale H. Conaway, D.V.M., Director Mr. Conaway, a Director of the Company since September 2009, is the Chief Veterinary Medical Officer for the Office of Research Oversight, an office within the Veterans Health Administration under the U.S. Department of Veterans Affairs. From 2001 to 2006, Dr. Conaway was the Deputy Regional Director (Southern Region). From 1998 to 2001, Dr. Conaway served as Manager of the Equine Drug Testing and Animal Disease Surveillance Laboratories for the Michigan Department of Agriculture. From 1994 to 1998, he was Regulatory Affairs Manager for the Michigan Department of Public Health Vaccine Production Division. Dr. Conaway received a D.V.M. degree from Tuskegee Institute and an M.S. degree in pathology from the College of Veterinary Medicine at Michigan State University. Mr. Conaway also serves on the Board of Directors of Pro-Pharmaceuticals. Henry J. Esber, Ph.D., Director Dr. Esber, has been a Principal in Esber D&D consulting since 2005. From 2003 to 2005, Dr. Esber was a Senior Consultant, Business Development at Charles River Labs, Discovery and Development Services. From 2005 to 2006, Dr. Esber was a consultant and from 2006, he was Senior Vice President and Chief Business Officer for Bio-Quant. Dr. Esber is the co-founder of BioSignature Diagnostics, Inc. and Advanced Drug Delivery, Inc. Dr. Rom E. Eliaz, Ph.D., MBA, a Director Dr. Eliaz, a Director of the Company since September 2009, has been a CEO of Nasvax, an Israel-based biotech company and President and CEO of JJ Pharma Inc. since September 2009. He has also been CEO and Managing Director, Elrom Ventures Corp. since May 2007. From January 2007 to October 2007 Dr. Eliaz was a Senior Director of Development at, Intradigm Corp. From March 2004 to December 2006 Dr. Eliaz was a Director of Development, Pfizer Inc., (Rinat Neuroscience). Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)

Executive Team and Advisors (cont’d) Yehuda Inbar, Vice President of IT/Media Mr. Inbar has been responsible for developing major online media projects for Microsoft, HP, Intel, AT&T and other major corporations. From 1997 to 2009, he was Information Solutions Architect and 3D Expert with Show & Tell, Inc. In 1988, Mr. Inbar founded and continues to serve as President of 3DMedia Network. Hana Chen-Walden, M.D., Chief Medical Director Dr. Chen-Walden is an Endocrinologist and has specialized in regulatory affairs in the pharmaceutical industry in the U.S and Europe. She has thirty years of regulatory experience with the EMEA and in individual European countries Since 2004 until present Dr. Chen-Walden consulted for European Clinical and Regulatory Consultancy in medical monitoring, quality assurance and regulatory input for clinical studies in the fields of oncology, cardiology, diabetes, neurology, respiratory diseases and medical devices. From 2000 – 2003 Dr. Chen-Walden was Director of International Regulatory Affairs Covalent Group Ltd. From 1997 – 2000 she was Medical, Drug Safety and Regulatory Director at CRC, a clinical CRO in France. Dr. Chen Walden received her Doctorate of Medicine from University of Tel Aviv, Israel. . Dr. Chen-Walden has practiced medicine in Germany and France. Dr. Peter Sheehan, M.D., Advisor, Medical Director Dr. Sheehan is an internationally respected specialist in the field of diabetes. Areas of clinical and research interest for Dr. Sheehan include peripheral artery disease, diabetic neuropathy, and wound healing. He has served as the American Diabetes Association (ADA) Foot Council Chairman, on the national Board of Directors, and currently as Chairman of the Cardiometabolic Risk Initiative. Dr. Sheehan was on the Board of Directors of the Wound Healing Society and served as President of the Wound Healing Foundation. He is also a member of the Steering Committee of the P.A.D. Coalition (peripheral artery disease). He is a graduate of the SUNY-Downstate School of Medicine, where he also completed his residency in Internal Medicine. Dr. Sheehan continued his training at the Yale University School of Medicine in New Haven where he completed a fellowship in Endocrinology and Metabolism. David Liu, Ph.D., Scientific Advisor Dr. Liu currently serves as Vice President, Research and Development at HDM Systems Corp. and manages the development of power electronic devices for battery-based systems used in transportation and green energy storage applications. In his previous work as a research scientist in the Biomedical Research Department at St. Elizabeth’s Medical Center, Tufts Medical School, Dr. Liu contributed to the fundamental understanding of the red blood cell membrane architecture, the molecular pathophysiology of various hereditary hemolytic anemia and identification of critical protein domains of red blood cell surface receptor for malaria invasion, He has published over sixty research papers on these subjects in a number of prestigious journals. He subsequently served as Chairman of the Board of International Power Devices, Inc. (IPD), a producer of high density DC/DC power supply devices used by telecommunication and data communication companies, such as Cisco and Nortel Networks; in January of 1999, Power-One acquired IPD for $48 million. Since 1992, Dr Liu has been an Associate Professor of Medicine, Tufts University School of Medicine, Boston, MA.† Dr. Liu received his Ph.D. in 1975 in Biochemistry from Carnegie-Mellon University. Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)

Boston Therapeutics, Inc. News April 18, 2012 Boston Therapeutics Receives First Purchase Order From Advance Pharmaceutical: SUGARDOWN® Sales in Hong Kong April 10, 2012 Boston Therapeutics PAZ320 Interim Clinical Trial Safety Results March 22, 2012 Boston Therapeutics Is Quoted as BTHE on the OTC Bulletin Board January 24, 2012 Boston Therapeutics Announces Clinical Study Safety Results With SUGARDOWN® at University of Sydney, Australia December 29, 2011 Boston Therapeutics Gets First Sales of SUGARDOWN® in Italy December 22, 2011 Boston Therapeutics Elects Dr. Henry Esber to Board of Directors October 11, 2011 Boston Therapeutics to Start Clinical Trial in Type 2 Diabetes at Dartmouth Hitchcock Medical Center in New Hampshire to test Press releases available upon request Symbol: BTHE Confidential Property of Boston Therapeutics, Inc. (Not for Distribution)
