Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - QUESTCOR PHARMACEUTICALS INC | d340254d8k.htm |
| EX-99.2 - TRANSCRIPT OF CONFERENCE - QUESTCOR PHARMACEUTICALS INC | d340254dex992.htm |
| EX-99.1 - PRESS RELEASE - QUESTCOR PHARMACEUTICALS INC | d340254dex991.htm |
 First Quarter
2012 Conference Call
First Quarter 2012
Conference Call
NASDAQ:
QCOR
NASDAQ:
QCOR
Exhibit 99.3
1 |
 2
•
Today’s webcast, accompanying slide presentation
and archived replay is available online at
http://ir.questcor.com/events.cfm
•
Telephone replay is available by dialing:
–
U.S.: 855-859-2056
–
International: 404-537-3406
–
Replay Passcode: 70200329
•
Today’s webcast, accompanying slide presentation
and archived replay is available online at
http://ir.questcor.com/events.cfm
•
Telephone replay is available by dialing:
–
U.S.: 855-859-2056
–
International: 404-537-3406
–
Replay Passcode: 70200329
Conference Call Logistics
2 |
 3
Safe Harbor Statement
Note: Except for the historical information contained herein, this press release contains
forward-looking statements that have been made pursuant to the Private Securities
Litigation Reform Act of 1995. These statements relate to future events or our future
financial performance. In some cases, you can identify forward-looking statements by terminology such as
"believes," "continue," "could," "estimates," "expects,"
"growth," "may," "plans," "potential," "should," "substantial" or
"will" or the negative of such terms and other comparable terminology. These statements
are only predictions. Actual events or results may differ materially. Factors that could
cause or contribute to such differences include, but are not limited to, the following:
Our reliance on Acthar for substantially all of our net sales and profits; Reductions in vials used
per prescription resulting from changes in treatment regimens by physicians or patient
compliance with physician recommendations; The complex nature of our manufacturing
process and the potential for supply disruptions or other business disruptions; The lack
of patent protection for Acthar; and the possible FDA approval and market introduction of
competitive products; Our ability to continue to generate revenue from sales of Acthar to treat
on-label indications associated with NS, and our ability to develop other therapeutic
uses for Acthar; Research and development risks, including risks associated with
Questcor's work in the area of NS and potential work in the area of Lupus, and our reliance on third-
parties to conduct research and development and the ability of research and development to
generate successful results; Our ability to comply with federal and state regulations,
including regulations relating to pharmaceutical sales and marketing practices;
Regulatory changes or other policy actions by governmental authorities and other third parties in
connection with U.S. health care reform or efforts to reduce federal and state government
deficits; Our ability to receive high reimbursement levels from third party payers; An
increase in the proportion of our Acthar unit sales comprised of Medicaid-eligible
patients and government entities; Our ability to estimate reserves required for Acthar used by
government entities and Medicaid-eligible patients and the impact that unforeseen
invoicing of historical Medicaid prescriptions may have upon our results; Our ability to
effectively manage our growth, including the expansion of our NS selling effort, and our
reliance on key personnel; The impact to our business caused by economic conditions; Our ability to
protect our proprietary rights; The risk of product liability lawsuits; Unforeseen business
interruptions and security breaches; Volatility in Questcor's monthly and quarterly
Acthar shipments, estimated channel inventory and end-user demand, as well as
volatility in our stock price; and Other risks discussed in Questcor's annual report on Form 10-K for the
year ended December 31, 2011 as filed with the Securities and Exchange Commission, or SEC, on
February 22, 2012, and other documents filed with the SEC. The risk
factors and other information contained in these documents should be considered in evaluating Questcor's
prospects and future financial performance. |
 4
•
238 paid NS scripts
•
1,000 paid MS scripts
–
Up 97% YOY
•
112 paid IS scripts
•
Record financial performance
–
4,111 vials shipped, up 105% YOY
–
$96.0M in net sales, up 161% YOY
–
$0.58 GAAP EPS (diluted), up 241% YOY
•
238 paid NS scripts
•
1,000 paid MS scripts
–
Up 97% YOY
•
112 paid IS scripts
•
Record financial performance
–
4,111 vials shipped, up 105% YOY
–
$96.0M in net sales, up 161% YOY
–
$0.58 GAAP EPS (diluted), up 241% YOY
Strong First Quarter Results |
 5
Paid Rxs
Paid Rxs
5
NS Scripts-Strong Continued Growth
28
Yellow numbers in the bars show the number of NS sales
representatives making calls for the majority of the quarter. Q3 ‘11
included expansion and training of new sales people.
Notes: Historical trend information is not necessarily indicative of future results.
Chart includes "Related Conditions" - diagnoses that are either alternative
descriptions of the condition or are closely related to the medical condition which is the focus of the chart. |
 6
Notes: Historical trend information is not necessarily indicative of future
results. Acthar is marketed for the on-label indication of MS
exacerbations
in
adults,
though
the
chart
includes
"Related
Conditions"
-
diagnoses
that
are
either
alternative
descriptions
of
the
condition
or
are closely related to the medical condition which is the focus of the chart. About
5% of the prescriptions in the tables are for related conditions. Yellow
numbers in the bars show the number of MS sales representatives making calls for the majority of the quarter.
Notes: Historical trend information is not necessarily indicative of future
results. Acthar is marketed for the on-label indication of MS
exacerbations
in
adults,
though
the
chart
includes
"Related
Conditions"
-
diagnoses
that
are
either
alternative
descriptions
of
the
condition
or
are closely related to the medical condition which is the focus of the chart. About
5% of the prescriptions in the tables are for related conditions. Yellow
numbers in the bars show the number of MS sales representatives making calls for the majority of the quarter.
77
30
38
MS Scripts-Record of Consistent Growth
Paid Rxs |
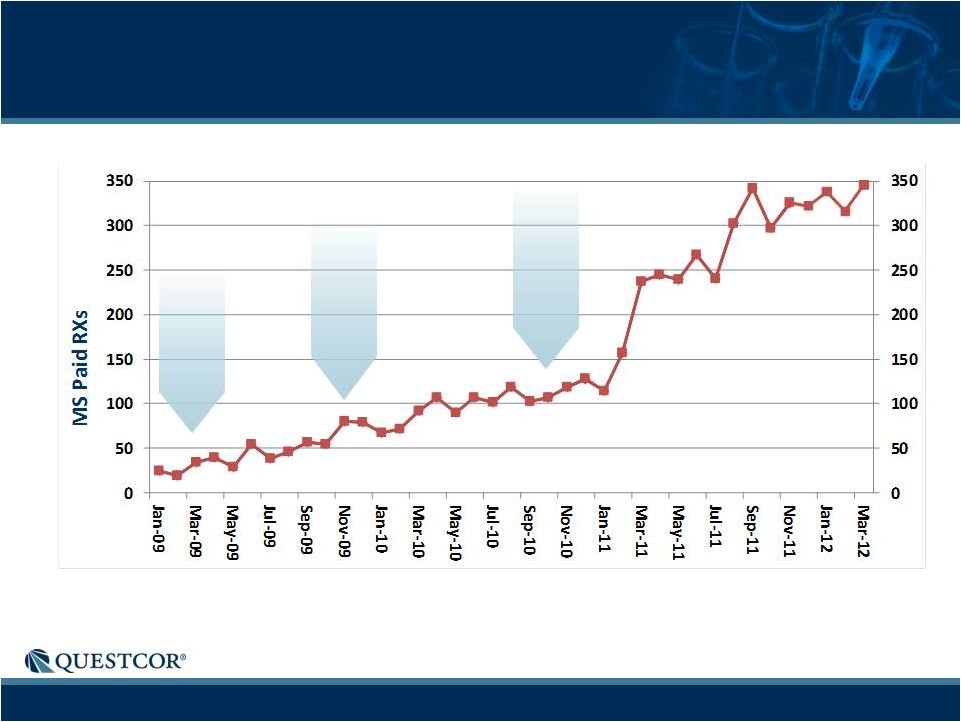 7
15-30
reps
30-38
reps
38-77
reps
Monthly MS Scripts History
Notes: Historical trend information is not necessarily indicative of future
results. Acthar is marketed for the on-label indication of MS
exacerbations
in
adults,
though
the
chart
includes
"Related
Conditions"
-
diagnoses
that
are
either
alternative
descriptions
of
the
condition
or
are closely related to the medical condition which is the focus of the chart. About
5% of the prescriptions in the tables are for related conditions. Notes:
Historical trend information is not necessarily indicative of future results. Acthar is marketed for the on-label indication of MS
exacerbations
in
adults,
though
the
chart
includes
"Related
Conditions"
-
diagnoses
that
are
either
alternative
descriptions
of
the
condition
or
are closely related to the medical condition which is the focus of the chart. About
5% of the prescriptions in the tables are for related conditions. |
 8
Growth in Shipped Vials |
 9
•
Generating more data
for on-label indications
–
NS
–
MS
–
IS
–
Lupus
•
Generating more data
for on-label indications
–
NS
–
MS
–
IS
–
Lupus
•
Investigating Acthar in
new indications
–
Diabetic nephropathy
–
Autism
–
Traumatic brain injury
–
ALS
–
Migraine
•
Investigating Acthar in
new indications
–
Diabetic nephropathy
–
Autism
–
Traumatic brain injury
–
ALS
–
Migraine
Over 40 Acthar R&D and Investigator
Initiated Research Studies
Understanding Acthar:
Understanding Acthar:
the science of how it works
the science of how it works |
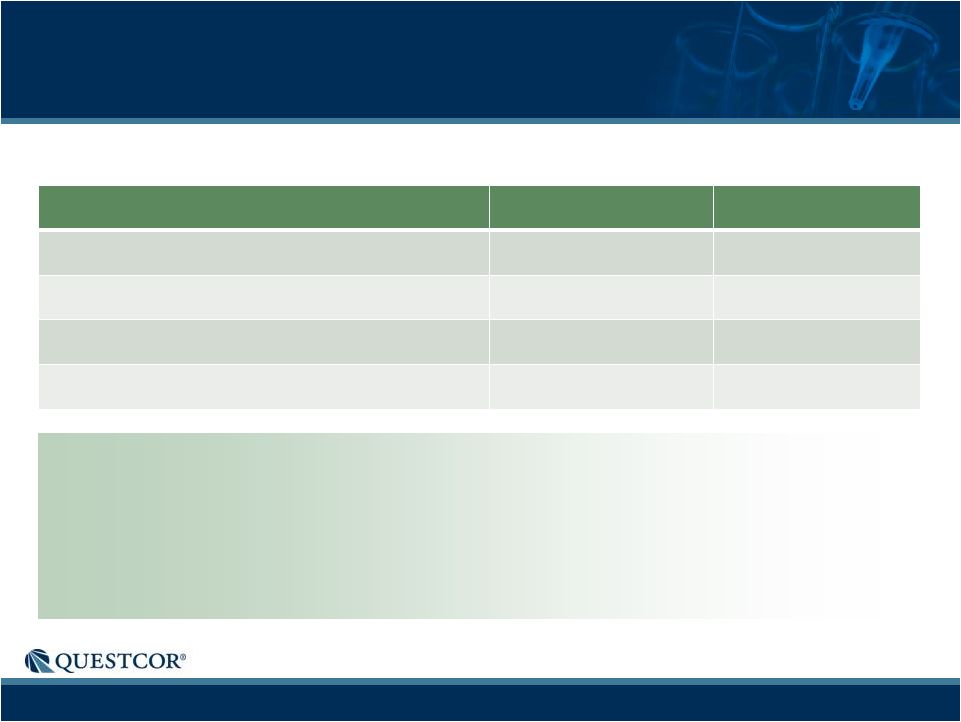 10
Q1 –
2012
Q1 –
2011
Net Sales ($M)
$96.0
$36.8
Gross Margin
94%
95%
Operating Income ($M)
$57.3
$16.4
Fully Diluted, GAAP EPS
$0.58
$0.17
Q1-2012 Financial Results
•
First quarter vials shipped: 4,111
•
First quarter cash flow from operations: $40.9M
•
Channel inventory estimated to be higher than fourth quarter
•
Medicaid reserves continue to appear adequate
•
798,285 shares repurchased during Q1-2012
•
First quarter vials shipped: 4,111
•
First quarter cash flow from operations: $40.9M
•
Channel inventory estimated to be higher than fourth quarter
•
Medicaid reserves continue to appear adequate
•
798,285 shares repurchased during Q1-2012
Record Net Sales (up 161%) and Solid Earnings (EPS up 241%)
Record Net Sales (up 161%) and Solid Earnings (EPS up 241%) |
 11
04/20/12
Cash / ST Investments
$248M*
Accounts Receivable
$37M
Questcor is Cash Flow Positive
*After return of $107 million of cash to shareholders through share repurchases.
*After return of $107 million of cash to shareholders through share repurchases.
Debt-free balance sheet |
 12
Acthar has sustainable competitive
Acthar has sustainable competitive
advantages-without FDA approval risk
advantages-without FDA approval risk
Acthar is approved for 19 indications-many
Acthar is approved for 19 indications-many
in large markets with sizable unmet need
in large markets with sizable unmet need
Sales in NS and MS are growing rapidly,
Sales in NS and MS are growing rapidly,
yet market penetration is low
yet market penetration is low
Developing new vertical market -
Developing new vertical market -
Rheumatology
Rheumatology
High margins provide good
High margins provide good
operating leverage
operating leverage
Profitable, cash flow positive, no debt
Profitable, cash flow positive, no debt
Investment Highlights
12 |
