Attached files
| file | filename |
|---|---|
| EX-32.1 - CERTIFICATION PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT - China BCT Pharmacy Group, Inc. | f10k2011a2ex32i_chinabct.htm |
| EX-31.1 - CERTIFICATION PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT - China BCT Pharmacy Group, Inc. | f10k2011a2ex31i_chinabct.htm |
| EX-32.2 - CERTIFICATION PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT - China BCT Pharmacy Group, Inc. | f10k2011a2ex32ii_chinabct.htm |
| EX-31.2 - CERTIFICATION PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT - China BCT Pharmacy Group, Inc. | f10k2011a2ex31ii_chinabct.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K/A
(Amendment No. 2 )
|
(Mark One)
|
|
þ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934.
|
For the fiscal year ended December 31, 2011
Or
|
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934.
|
For the transition period from ________ to __________
Commission file number 033-10893
China BCT Pharmacy Group, Inc.
(Exact Name of Registrant as Specified in Its Charter)
|
Delaware
|
20-8067060
|
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(I.R.S. Employer Identification No.)
|
No. 102, Chengzhan Road
Liuzhou City, Guangxi Province, P.R.C. 545007
(Address of Principal Executive Offices) (Zip Code)
Registrant’s telephone number, including area code: Tel.: (86) 772-363-8318
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act: Common Stock, par value $0.001 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer o
|
Accelerated filer o
|
Non-accelerated filer o
|
Smaller reporting company þ
|
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2). Yes o No þ
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter: $32,759,371 computed by reference to $2.09 as of June 30, 2011, which is less than $75 million.
As of March 29, 2012 there were 38,154,340 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE: None
CHINA BCT PHARMACY GROUP, INC.
ANNUAL REPORT ON FORM 10-K
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2010
TABLE OF CONTENTS
|
PART I
|
1
|
|
|
Item 1.
|
Business
|
1
|
|
Item 1A.
|
Risk Factors
|
28
|
|
Item 1B.
|
Unresolved Staff Comments
|
42
|
|
Item 2.
|
Properties
|
42
|
|
Item 3.
|
Legal Proceedings
|
44
|
|
Item 4.
|
Mine Safety Disclosures
|
44
|
|
PART II
|
44
|
|
|
Item 5.
|
Market for Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
44
|
|
Item 6.
|
Selected Consolidated Financial Data
|
46
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
46
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures about Market Risk
|
59
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
59
|
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
59
|
|
Item 9A.
|
Controls and Procedures
|
60
|
|
Item 9B.
|
Other Information
|
61
|
|
PART III
|
61
|
|
|
Item 10.
|
Directors and Executive Officers and Corporate Governance
|
61
|
|
Item 11.
|
Executive Compensation
|
64
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
73
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
75
|
|
Item 14.
|
Principal Accounting Fees and Services
|
79
|
|
PART IV
|
80
|
|
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
80
|
Explanatory Note
China BCT Pharmacy Group, Inc. (the “Company,” “we,” “us” or “our”) is filing this Amendment No. 2 on Form 10-K/A to our Annual Report on Form 10-K for the year ended December 31, 2011, originally filed with the Securities and Exchange Commission (the “SEC”) on March 30, 2012 (the “Original Form 10-K”) to (i) correct a typographical error in the Original 10-K and in Amendment No. 1 to the Original 10-K with regard to the date of the Report of Independent Registered Public Accounting Firm, (ii) to amend Item 13 of Part III to make certain corrections to amounts reported in tables relating to transactions with related companies and (iii) to correct typographical errors to certain date references that should have referenced the date of filing of the Original Form 10-K – March 30, 2012. The report, with the correct date of March 30, 2012, is included with this Amendment No. 2. No other changes to the Original Form 10-K or Amendment No. 1 have been made and this Amendment No. 2 does not modify or update in any way disclosures made in the Original Form 10-K or Amendment No. 1.
CAUTIONARY STATEMENT
This annual report contains forward-looking statements. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. Forward-looking statements involve risks and uncertainties. Forward-looking statements include statements regarding, among other things, (a) our projected sales, profitability and cash flows, (b) our growth strategies, (c) anticipated trends in our industries, (d) our future financing plans and (e) our anticipated needs for working capital. They are generally identifiable by use of the words “may,” “will,” “should,” “anticipate,” “estimate,” “plan,” “potential,” “projects,” “continuing,” “ongoing,” “expects,” “management believes,” “we believe,” “we intend” or the negative of these words or other variations on these words or comparable terminology. In particular, these include statements relating to future actions, future performance, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results.
Any or all of our forward-looking statements in this annual report may turn out to be inaccurate. They can be affected by inaccurate assumptions we might make or by known or unknown risks or uncertainties. Consequently, no forward-looking statement can be guaranteed. Actual future results may vary materially as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and matters described in this annual report generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this filing will in fact occur and you should not place undue reliance on these forward-looking statements.
PART I
ITEM 1. BUSINESS
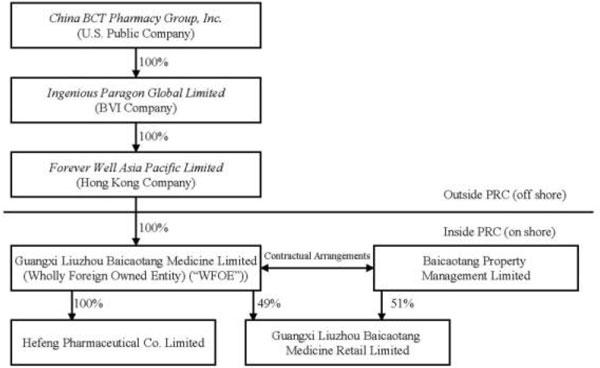
References in this annual report to the “PRC” or “China” are to the People’s Republic of China. Except as otherwise specifically stated or unless the context otherwise requires, the terms “Company,” “we,” “us” and “our” refer to collectively (i) China BCT Pharmacy Group, Inc. (f/k/a China Baicaotang Medicine Limited), a corporation incorporated in the State of Delaware; (ii) Ingenious Paragon Global Limited (“Ingenious”), a British Virgin Islands company which is a wholly-owned subsidiary; (iii) Forever Well Asia Pacific Limited (“Forever Well”), a Hong Kong company which is a wholly-owned subsidiary of Ingenious; (iv) Guangxi Liuzhou Baicaotang Medicine Limited (“Liuzhou BCT”), a PRC wholly foreign-owned enterprise (“WFOE”) which is a wholly-owned subsidiary of Forever Well; (v) Hefeng Pharmaceutical Co. Limited (“Hefeng Pharmaceutical”), a PRC company which is a wholly-owned subsidiary of Liuzhou BCT; and (vi) Guangxi Liuzhou Baicaotang Medicine Retail Limited (“BCT Retail”), a PRC company of which 49% of its registered capital was contributed by Liuzhou BCT and 51% of its registered capital was contributed by Baicaotang Property Management Limited (“Property Management”), an affiliated company.
References to BCT Retail’s “registered capital” are to the equity of BCT Retail, which under PRC law is measured not in terms of shares owned but in terms of the amount of capital that has been or will be contributed to a company by a particular shareholder or all shareholders. The portion of a limited liability company’s total capital contributed by a particular shareholder represents that shareholder’s ownership of the company and the total amount of capital contributed by all shareholders is the company’s total equity. Capital contributions are made to a company by deposits into a dedicated account in the company’s name, which the company may access in order to meet its financial needs. When a company’s accountant certifies to PRC authorities that a capital contribution has been made and the company has received the necessary government permission to increase its contributed capital, the capital contribution is registered with regulatory authorities and becomes a part of the company’s “registered capital”.
1
Summary
We are engaged in pharmaceutical distribution, retail pharmacy and manufacturing of pharmaceuticals through our three subsidiaries Liuzhou BCT, Hefeng Pharmaceutical, and BCT Retail, each of which is located in Guangxi Province, China.
We have integrated operations in the following business segments:
|
●
|
Pharmaceutical distribution
|
Pharmaceutical distribution is our principal business. We conduct our wholesale business through Liuzhou BCT by purchasing pharmaceutical products from suppliers and then distributing them to our wholesale customers, including hospitals, retail drug stores, other pharmaceutical wholesalers, clinics, medical centers, and individuals. Our pharmaceutical distribution business is focused on the market of Guangxi province, which includes major cities such as Nanning, Liuzhou and Guilin and which has a population of approximately 50 million people. We operate a large regional wholesale network in Guangxi Province supported by strategically placed warehouse facilities. For the year ended December 31, 2011, revenue generated from our pharmaceutical distribution segment was $190.7 million, or 74.0% of our total revenues for the year.
We distribute over 8,000 products from nearly 4,000 suppliers through our wholesale distribution in compliance with applicable PRC regulations. Hefeng Pharmaceutical, which is one of our wholly owned subsidiaries, is also one of our suppliers. In 2011 revenue derived from the distribution of third-party products constituted 99.99% of our pharmaceutical distribution segment revenue.
|
●
|
Retail pharmacy
|
Established in 2001, BCT Retail operates a large regional pharmaceutical retail network in Guangxi province, consisting of 194 directly owned retail stores in Guangxi province under the registered name “Baicaotang 百草堂.” Our retail stores provide convenient, high quality and professional pharmaceutical services and supply a wide variety of medicines, including western medicine, traditional Chinese medicine (“TCM”), Chinese herbal medicine, roughly processed Chinese herbal medicine, family planning products, and seasonal medicine. Among the 194 stores, there are 21 stores that are medi-care qualified stores, where customers are able to make their purchase either by cash or by using their medi-care insurance card for payment. For the year ended December 31, 2011, revenue generated from our retail pharmacy segment was $53.3 million, or 20.7% of our revenues for the year.
|
●
|
Manufacturing of pharmaceuticals
|
Hefeng Pharmaceutical has a manufacturing facility on approximately 40,000 square meters of land and manufactures four types of products:
|
●
|
A Chinese herbal medicine abstraction unit for raw material and medicine paste with 670 tons of annual abstraction capacity (Maximum daily unit production: 2.5 tons per day; maximum days of operation per year: 270 days);
|
|
●
|
A granular formulation unit with an annual production capacity of 0.25 billion packages (Maximum daily unit production: 768,960 packages per day; maximum days of operation per year: 324 days);
|
|
●
|
A pill formulation unit with an annual production capacity of 0.36 billion pills (Maximum daily unit production: 1,252,800 pieces per day; maximum days of operation per annum: 288 days), and
|
|
●
|
A liquid formulation unit with an annual production capacity of 0.1 billion injections (Maximum daily unit production : 347,500 pieces per day; maximum days of operation per annum : 288 days).
|
2
Hefeng Pharmaceutical produces and sells pharmaceutical products under the registered name “Asio (亚太)” including: traditional anti-inflammatory and antibacterial drugs, cancer treatment drugs, cardio-vascular disease drugs and hepatitis drugs. Hefeng Pharmaceutical’s best-selling products include:
|
●
|
Levodopa is used to treat the stiffness, tremors, spasms, and poor muscle control of Parkinson’s disease;
|
|
●
|
Tabellae Sarcandrae, a TCM protected drug, has similar anti-inflammatory and antibacterial effects as antibiotics in Western medicine;
|
|
●
|
Rotandine Sulfate is a non-prescription analgesic drug. Used for headaches, menstrual pain, and aiding sleep;
|
|
●
|
Corydalis Saxicola Bunting (Yanghuanglian) has been demonstrated to possess many pharmacological activities, including antibacterial, antiviral and anticancer activities; and
|
|
●
|
Ethacridine Lactate Injection is used for second trimester pregnancy termination from week 12-26 at hospitals.
|
In addition, Hefeng Pharmaceutical collaborates with several renowned medical research universities in China to continuously improve its raw material abstraction efficiency and production process, and to develop alternative formulas for existing drugs. For the year ended December 31, 2011, revenue generated from our manufacturing segment was $13.6 million, or 5.3% of our total revenues for the year.
The growth profile of Guangxi province is based on the following three factors:
|
●
|
According to data published by the National Bureau of Statistics, Guangxi Province’s GDP was RMB957 billion ($152 billion) in 2010. GDP per capita in Guangxi Province was RMB20,759 ($3,295) in 2010 as compared with GDP per capita between RMB39,906 ($6,334) to RMB74,538 ($11,831) in the coastal regions (such as, Fujian Province ($6,334), Guangdong Province ($6,995), Jiangsu Province ($8,356), Shandong Province ($6,485), Shanghai ($11,831), and Zhejiang Province ($8,079)). In 2010 Guangxi Province’s GDP growth rate was 14.2% as compared to an average GDP growth rate of 10.62% in the coastal regions in 2010. In 2010 Guangxi Province had a population of 46.10 million. In 2010 the population nature growth rate of Guangxi Province was 8.65 ‰ as compared with a population nature growth rate averaged at 4.67 ‰ in the coastal regions. (See http://www.stats.gov.cn/tjsj/ndsj/2011/indexce.htm).
|
|
●
|
The World Bank projected that the inflation rate in China in 2011 should moderate after having risen in March 2011 (World Bank “China Quarterly Update” from April 27, 2011)
(See http://www-wds.worldbank.org/external/default/WDSContentServer/WDSP/IB/2011/04/29/000356161 _20110429003758/Rendered/PDF/614000Replacem1Quarterly1April12011.pdf)
|
|
●
|
The general pharmaceutical industry growth rate resulting from the RMB850 billion healthcare reform bill passed by the Chinese government.
|
We were originally incorporated in the State of Delaware on November 30, 2006 under the name Purden Lake Resource Corp. to engage in the acquisition, exploration and development of natural resource properties. We ceased our operations because we had not commenced any exploration activities, and as a result prior to December 30, 2009 we were a “blank check” company with nominal assets. On December 24, 2009, we changed our name to China Baicaotang Medicine Limited and then to China BCT Pharmacy Group, Inc. on March 25, 2010.
3
Our wholly-owned subsidiary, Ingenious, was incorporated under the laws of the British Virgin Islands on May 29, 2008. Ingenious owns 100% of the issued and outstanding capital stock of Forever Well, a Hong Kong company incorporated on January 10, 2008. Forever Well is the sole shareholder of Liuzhou BCT a PRC limited company established on April 3, 1986. Liuzhou BCT contributed 100% of the registered capital of Hefeng Pharmaceutical, a PRC company established on September 18, 2000 and 49% of the registered capital of BCT Retail, a PRC company established on October 30, 2001. The remaining 51% of the registered capital of BCT Retail was contributed by Property Management, an affiliate of Liuzhou BCT.
The chart below illustrates the current structure of the Company:
The Reorganization
In 2008, the shareholders of Liuzhou BCT (the “Liuzhou BCT Shareholders”) and Xiaoyan Zhang, our CFO, developed a restructuring plan (the “Restructuring”). The goal of the Restructuring was to obtain the benefits of a U.S. public company by entering into a transaction with a public shell company in the United States by which we, the public shell company, would acquire operations based in the PRC, all in compliance with PRC law.
At that time, Ms. Zhang, who is a citizen of Hong Kong, was the sole shareholder of Ingenious, which had no assets or operations and owned 100% of Forever Well as the result of Ms. Zhang and the Liuzhou BCT Shareholders having completed the first two steps of a three-step Restructuring.
The first step was for Forever Well to acquire 100% of the equity interests of Liuzhou BCT and its subsidiaries (the “PRC Operating Companies”). Liuzhou BCT was owned at that time by the Liuzhou BCT Shareholders, certain former and current employees and directors of Liuzhou BCT.
In this step, Forever Well, a Hong Kong company formed by Mr. Ping-Ki Yue, was to acquire the PRC Operating Companies. The second step in the Restructuring was for Ingenious, which was 100% owned by Ms. Zhang, to acquire Forever Well and the third step was for Ingenious to enter into and complete a transaction with a U.S. public reporting company whereby that company would acquire Ingenious.
4
The first step was completed in conjunction with the second step so that as the PRC Companies became subsidiaries of Forever Well, Forever Well was acquired by Ingenious. As part of the second step of the Restructuring, the Liuzhou BCT Shareholders entered into an earn-in agreement (the “Earn-In Agreement”) which provided the Liuzhou BCT Shareholders with a process under which they could purchase for a nominal amount the shares of common stock held by Ms. Zhang. Thereafter Ingenious could undertake the third and final step of the Restructuring to enter into and complete a transaction with a U.S. public reporting company whereby that company would acquire Ingenious.
The Restructuring and the acquisition of Liuzhou BCT was structured to comply with the PRC M&A Laws. Under the PRC M&A Laws, the acquisition of PRC companies by foreign companies that are controlled by PRC citizens who are affiliated with the PRC companies is strictly regulated and requires approval from the Ministry of Commerce, which approval is burdensome to obtain. Such restrictions however, do not apply to foreign entities which are controlled by foreign persons. These restrictions apply only at a “snapshot in time” that occurs at the time PRC companies are acquired by a foreign entity. In our case, this was effective on August 4, 2008 when Forever Well acquired 100% of the equity interest of Liuzhou BCT from the 15 Liuzhou BCT Shareholders for aggregate consideration of RMB10,000,000 (approximately $1,470,588) which was the registered and fully paid up capital of Liuzhou BCT. At that time Forever Well was owned 100% by Ingenious, and Ingenious was owned 100% by Ms. Zhang, a Hong Kong citizen. Therefore this transaction was a pure cross-border transaction governed by and permitted under the 2006 PRC M&A regulations and the acquisition had been approved by the Ministry of Commerce on June 13, 2008.
Since PRC M&A Laws would have prohibited the Liuzhou BCT Shareholders who were PRC citizens from immediately receiving a controlling interest in Ingenious in a share exchange as consideration for the sale of their interest in Liuzhou BCT, Liuzhou BCT Shareholders holding a majority of the equity interest in Liuzhou BCT and Ms. Zhang instead agreed that they would enter into an Earn-In Agreement to grant those Liuzhou BCT Shareholders a call right to acquire up to all of Ms. Zhang’s interest in Ingenious (or a public parent company of Ingenious, as the case may be) after the acquisition of Liuzhou BCT was consummated in compliance with PRC law. Because all of the Liuzhou BCT shareholders were PRC citizens, a majority of Liuzhou BCT shareholders would not have been permitted to immediately receive shares in Forever Well or in Ingenious in exchange for their interests in Liuzhou BCT. However, there is no prohibition under PRC laws for those Liuzhou BCT shareholders to earn an interest in the Company after the acquisition of Liuzhou BCT was consummated.
As part of the first and second steps of Restructuring, the Liuzhou BCT Shareholders entered into an earn-in agreement. The earn-in agreement was succeeded by the earn-in agreement developed in connection with the Share Exchange (the “Earn-In Agreement”). It, like its successor, provided the Liuzhou BCT Shareholders with a process under which they could purchase for a nominal amount the shares held by Ms. Zhang. The Earn-In Agreement provides for the Liuzhou BCT Shareholders to obtain legal ownership of their proportionate number of our shares issued to Ms. Zhang in the Share Exchange. Ms. Zhang, however, has all of the governance rights to the shares and has elected the present directors of the Company.
On October 22, 2009 Ms. Zhang and certain Liuzhou BCT Shareholders entered into the Earn-In Agreement, which was amended on December 30, 2009 to extend the performance targets from 2009 and 2010 fiscal years to 2010 and 2011 fiscal years because the 2009 performance target was not going to be met. The Earn-in Agreement was further amended on May 19, 2010 to decrease the performance targets for the 2010 and 2011 fiscal years. The amendments to the Earn-in Agreement were made to increase the likelihood that the Liuzhou BCT Shareholders would all be able to earn back their shares in the Company, which was the goal and purpose of the reorganization. These amendments were made without additional consideration being paid
The Earn-in Agreement enables those Liuzhou BCT Shareholders to purchase shares of Ingenious (or its public parent company) from Ms. Zhang for a nominal amount per share provided that the Company meets certain performance targets for fiscal 2010 and 2011. For the 2010 and 2011 fiscal years the performance targets for the Company are $26 million and $28 million after tax audited net income, respectively. If the 2010 performance target is met, the Liuzhou BCT Shareholders have the right to acquire 50% of shares held by Ms. Zhang over which they have a call right. If the 2011 performance target is met, the Liuzhou BCT Shareholders have the right to acquire the other 50% of the shares held by Ms. Zhang over which they have a call right. The number of shares which can be acquired by the Liuzhou BCT Shareholders under the Earn-In Agreement is in proportion to their former relative ownership interest in Liuzhou BCT. Ms. Zhang may not transfer the shares during the five-year term of the Earn-In Agreement and in the event that all of the shares have not been delivered to the Liuzhou BCT Shareholders, Ms. Zhang can only transfer them as she and the Liuzhou BCT Shareholders agree. As the Company has met both of the 2010 and 2011 performance targets, the Liuzhou BCT Shareholders are entitled to the call right to purchase back their shares at any time.
5
After completion of the first and second steps in the Restructuring, the parties concluded that they needed another agreement to complete the Earn-In Agreement and the restructuring process. They therefore entered into the so-called "entrust shareholding" agreements with respect to each of the first and second steps; they made these agreements effective as of the effective date of the first and second steps. These agreements recited that a third-party would maintain the governance rights and economic benefits of the Liuzhou BCT Shareholders during the period of the Earn-In Agreement. The two entrust shareholding agreements were identical except that the third party in the first was Mr. Ping Ki Yue and in the second was Ms. Zhang. The first agreement had been supplanted by the second even at the time it was signed. Under the second agreement, Ms. Zhang, as owner of the shares, had all voting power and, upon notice from her with respect to a matter, the former shareholders might choose to express a preference as to how she would vote the shares; the agreement also confirmed that the former shareholders’ economic interest in the shares was preserved as in the Earn-In Agreement.
These agreements were in fact never utilized and the parties determined that they had been mistaken in their decision to create them and that the Earn-In Agreement expresses entirely the parties’ relationship with respect to the shares and with each other. Representatives of the Liuzhou BCT Shareholders hold the majority of seats on the board of the Company. The Earn-In Agreement reflects the intent and purpose of the parties in undertaking and accomplishing the Restructuring. And following the accomplishment of the third step in the Restructuring, the Earn-In Agreement is the operative agreement for all purposes with respect to the relationship of the Liuzhou BCT Shareholders to the Company. The parties having recognized the mistake, for all of these reasons, the entrust shareholding agreements were rescinded as of their effective dates and the Earn-In Agreement governs the rights of the Liuzhou BCT Shareholders with respect to the shares held by Ms. Zhang.
Under the Earn-in Agreement, Ms. Zhang has legal title to the shares and there are no limits on her voting rights with respect to the shares. Under the Earn-In Agreement, the Liuzhou BCT Shareholders have the right to obtain the economic benefits of the shares by purchasing the shares upon the Company's attaining the low financial thresholds in the agreement which trigger their purchase rights. Ms. Zhang also has no authority to transfer the shares. This can only be done with the agreement of the Liuzhou BCT Shareholders. Therefore, were the Company not to attain the thresholds that have been placed in the Earn-In Agreement, we anticipate that the agreement would be further modified to establish thresholds that were or are attainable.
On December 30, 2009, the goal of the Restructuring was realized when we entered into and completed a share exchange agreement with Ingenious. At that time we were controlled by our then president and CEO, Lisa Lopomo, who owned 54.5% of our common stock. Pursuant to the share exchange agreement, we acquired 100% of the equity of Ingenious in exchange for the issuance of an aggregate of 32,000,000 shares of our common stock to Ms. Zhang and to certain Liuzhou BCT Shareholders. As of the date of this annual report, Ms. Zhang owns 58.9% of our outstanding common stock all of which is subject to the provisions of the Earn-In Agreement. As a result of this transaction, we are a holding company which, through our direct and indirect ownership of Ingenious, Forever Well, Liuzhou BCT, Hefeng Pharmaceutical and BCT Retail, now has operations based in the PRC.
Private Placement
Simultaneously with the closing of the Share Exchange, we completed the initial closing of the private placement of approximately $6.3 million or 632.3 Units (the “Private Placement”). Each Unit consists of (i) 3,937 shares of common stock, and (ii) a warrant to purchase 1,968 shares of common stock at an exercise price of $3.81 per share (the “Investor Warrants”). Upon the initial closing of the Private Placement, we issued an aggregate of 2,489,370 shares of our common stock and warrants exercisable for 1,224,368 shares of our common stock at an exercise price of $3.81 per share. In addition, in connection with the initial closing of the Private Placement, we issued warrants to the Co-Placement Agents (the “Agent Warrants”) that are exercisable for 248,937 shares of common stock at an exercise price of $3.05 per share, on a cash or cashless basis. The closing of the Share Exchange was a condition precedent to the closing of the Private Placement.
6
On February 1, 2010, we completed the second closing of the Private Placement of approximately $2.6 million or 261.61 Units with the issuance of a total of 1,029,970 shares of our common stock and Investor Warrants exercisable for 514,933 shares of our common stock at an exercise price of $3.81. In connection with the second closing of the Private Placement, we issued Agent Warrants to the Co-Placement Agents that are exercisable for 102,997 shares of common stock at an exercise price of $3.05 per share, on a cash or cashless basis.
Additional Provisions Relating to the Private Placement
Registration Rights
Pursuant to the Subscription Agreement, on or prior to March 3, 2010 (the “Registration Statement Filing Date”), we were required to file with the Securities and Exchange Commission (the “SEC”) a registration statement (the “Registration Statement”) under the Securities Act of 1933, as amended (the “Securities Act”), (i) registering for resale by the Investors (a) the shares of common stock issued to the investors in the Private Placement (the “Investors”) and (b) the shares of common stock underlying the Investor Warrants; and (ii) registering for resale for the Co-Placement Agents of the Private Placement and other agents, the shares of common stock underlying the Agent Warrants (all of the foregoing securities being collectively referred to herein as the “Registrable Securities”). We were required to use our best efforts to have the Registration Statement declared effective prior to the 150th day following February 1, 2010, provided, however, that in the event of a “full review” by the SEC, which we received, we were given an additional 30 days to have the Registration Statement declared effective prior to the 180th day following February 1, 2010 (the “Target Effective Date”).
In the event that (i) the Registration Statement has not been (x) filed on or prior to the Registration Filing Date or (y) declared effective by the SEC on or before the Target Effective Date; and (ii) the Registrable Securities included in such Registration Statement are not saleable under Rule 144, we were required to pay to each Investor as liquidated damages, a cash payment equal to 1% of the aggregated amount invested by such Investor in the Private Placement for the first 30 days and 1% of the aggregated amount vested by such Investors in the Private Placement for every 30-day period thereafter until the Registration Statement has been filed and/or declared effective, or such proportionate percentage for any period less than 30 days. The Registration Statement was declared effective after the Target Effective Date and, as a result, we paid the liquidated damages to the Investors.
Make Good Escrow
In addition, pursuant to the Subscription Agreement, at the closing of the Private Placement, Xiaoyan Zhang, our CFO placed 4,000,000 shares of our common stock owned by her (the “Make Good Shares”) in an escrow account administrated by an escrow (the “Make Good Escrow Agent”). If we failed to achieve a performance target of $26,000,000 recurring operating net income under the U.S. GAAP before any extra-ordinary gain and excluding any non-cash expenses for our fiscal year ending December 31, 2010 (the “Performance Target”), the Make Good Escrow Agent would distribute 1,000,000 Make Good Shares to all Investors on a pro rata basis for every $1,000,000 shortfall under the Performance Target. The Make Good shares attach solely to the Investors and not to the securities issued in the Private Placement. Any Make Good Shares released to Investors would not be included in the Registration Statement relating to the resale of the shares issued in the Private Placement. Because the Company met the Performance Target, however, the Make Good Shares will be returned to Ms. Zhang. As the Company has met the 2010 performance target, the 4,000,000 Make Good Shares have been returned to Ms. Zhang by the Make Good Escrow Agent.
Anti-Dilution Protection
As a result of anti-dilution protection included in the Subscription Agreement, we may be obligated to issue additional shares of common stock to the Investors. Pursuant to the Subscription Agreement, the Investors have certain anti-dilution protection from February 1, 2010 until the date that is the earlier of: (i) March 17, 2010, the effectiveness of the original Registration Statement relating to the resale of the shares issued in the Private Placement, or (ii) the date on which the shares being registered on the Registration Statement may be sold under rule 144 (the “Anti-Dilution Period”). During the Anti-Dilution Period, if we sell additional shares of common stock (subject to certain exceptions) at a price per share of less than $2.54 per share (“Additional Shares”), then we are obligated to issue to each Investor that is still a holder of shares issued in the Private Placement, that number of shares of common stock, equal to the difference between (i) the aggregate purchase price paid by such Investor for each share of common stock underlying Units purchased in the Private Placement divided by the Weighted Average Adjusted Price, less (ii) the number of shares of common stock underlying Units actually purchased in the Private Placement by such Investor.
7
The “Weighted Average Adjusted Price” equals the quotient obtained by dividing (i) an amount equal to the sum of the aggregate purchase price of all of the shares of common stock underling Units sold in the Private Placement plus the aggregate consideration received by us for such issuance of Additional Shares during the Anti Dilution Period,; by (ii) an amount equal to the sum of the aggregate number of shares of common stock underlying Units sold in the Private Placement plus the aggregate number of Additional Shares sold by us during the Anti-Dilution Period.
Placement Agency Agreement
We entered into a placement agency agreement (the “Placement Agent Agreement”) with the Co-Placement Agents on October 21, 2009 whereby the Co-Placement Agents received as compensation for acting as placement agent in the Private Placement (i) a total cash fee and a non-accountable marketing allowance in the amount of approximately $0.86 million; and (ii) Agent Warrants to purchase up to 302,521 shares of common stock. Pursuant to participating agent agreements by and among Charles Vista, LLC, May Davis and American Capital, Charles Vista, LLC received as compensation for acting as a sub-agent in the Private Placement (i) a cash fee in the amount of approximately $0.22 million; and (ii) Agent Warrants to purchase up to 49,413 shares of common stock at an exercise price of $3.65 per share. The Co-Placement Agents were responsible for raising the minimum offering amount of $5,820,000 of Units and were compensated as set forth above. The funds in connection with the Private Placement were held with Signature Bank, acting as escrow agent, and were released to us upon the consummation of each closing under the Subscription Agreement.
Agreement to Sell Preferred Shares
On January 18, 2011, the Company entered into a Series A Convertible Preferred Shares Purchase Agreement (the “Preferred Purchase Agreement”) with Milestone Longcheng Limited (“Milestone”) pursuant to which Milestone agreed to purchase 9,375,000 shares of the Company’s Series A Convertible Preferred Shares, par value $.001 per share (the “Preferred Shares”). The sale transaction was completed and Milestone purchased the Preferred Shares on February 28, 2011, for an aggregate purchase price of $30 million less expense reimbursement of up to $400,000 for certain reasonable legal and other expenses incurred by Milestone in connection with the transaction. The Preferred Shares carry a dividend of 5% and are convertible initially into an equal number of shares of our Common Stock at an initial conversion price of $3.20 per share. All conditions to closing the transaction were completed on or prior to February 28, 2011, including the completion of the amendment of our Certificate of Incorporation to increase our authorized capital to 170 million shares of capital stock consisting of 150 million shares of Common Stock (an increase of 50 million shares) and 20 million shares of blank-check preferred stock which our board of directors will have the authority to issue (the “Amendment”) and the filing of the Amendment and the filing of the certificate of designation adopting the terms of the Preferred Shares (the “Certificate of Designation”). All necessary board and stockholder action to approve adoption of the Amendment were taken and the Amendment was complete upon its filing with the Delaware Secretary of State on February 28, 2011, which was 20 days after the mailing to our stockholders of an information statement pursuant to Section 14(c) of the Exchange Act. The board also approved the Certificate of Designation, subject to the filing of the Amendment, and the Certificate of Designation was also filed on February 28, 2011.
Under the Certificate of Designation, the holders of Series A Preferred have voting rights with the Common Stock as well as separate voting rights with respect to certain extraordinary transactions. At any annual or special meeting of the shareholders, the holders of the Preferred Shares shall be entitled to the number of votes equal to the number of Common Shares into which such Preferred Shares could be converted at the record date for determination of the shareholders entitled to vote on such matters, or, if no such record date is established, at the date such vote is taken, and such votes will be counted together with all other shares of the Company having general voting power and not counted separately as a class. After issuance, the Preferred Shares represent 18.5% of the outstanding share capital of the Company on a fully-diluted basis. These shares, together with the shares of Common Stock owned by Ms. Zhang (representing approximately 44.3% of the outstanding share capital after this transaction on a fully-diluted basis) hold the majority voting interest in the Company.
8
The holders of the Company’s Common Stock are junior to the holders of the Series A Preferred with respect to dividend rights, rights on other distributions and rights upon liquidation or dissolution of the Company. Additionally, the holders of the Series A Preferred have preemptive rights in connection with the issuance of new securities by the Company, pursuant to which the holders of the Series A Preferred have the right to purchase a number of the new securities equal to their percentage ownership of Common Shares (calculated on an as converted and fully-diluted basis) multiplied by the number of new securities to be issued.
The Preferred Shares became convertible into Common Stock immediately upon their issuance. The initial conversion price of $3.20 per share is subject to adjustment for dilutive transactions, stock splits and other reclassifications and, to the extent provided in a performance measure, for the 2011 financial performance of the Company. The Preferred Shares will be automatically converted upon consummation of a public offering, of Common Stock in an amount of $60 million at a time when the Company’s market capitalization is $300 million or greater, it is listed on a national exchange and the offering price is at least two times the then applicable adjusted conversion price.
In connection with this transaction, the Company and Milestone also entered into a registration rights agreement and, with certain of the Company’s principal stockholders, a shareholders agreement. The registration rights agreement provides for registration under the Securities Act of 1933, under various circumstances, of the shares of Common Stock into which the Preferred Shares can be converted. Under the Preferred Purchase Agreement, Milestone (or successor holders of the Preferred Shares) has the right to name one director and to recommend an additional independent director to the Company’s board of directors. The Preferred Purchase Agreement also provides that Milestone (or successor holders) will have consent rights with respect to certain operating matters. The shareholders agreement provides limitations on the rights of the principal shareholders to engage in competing businesses in the event that they are no longer employed with the Company and also provides a process for their sale of their shares, including first offering them to the holders of the Preferred Shares and co-sale rights of the holders.
In the event that the outstanding Preferred Shares constitute at least five percent (5%) of the outstanding Common Stock on an as converted and fully-diluted basis, the Company cannot take any of the following actions without the approval of the holders of a majority of the outstanding Preferred Shares: (i) adopt any amendment to the Certificate of Incorporation or By-laws of the Company or the Certificate of Designation that adversely alters the rights or privileges of the Series A Preferred; (ii) authorize or issue any class of shares with powers, rights, preferences or privileges that are senior to or on a parity with the Preferred Shares; (iii) approve or affect any merger, acquisition, consolidation, scheme of arrangement, reclassification of share capital, recapitalization, or any other similar transaction involving the Company or any of its subsidiaries, provided that any of the above actions would require shareholders’ approval; (iv) sell all or substantially all of the assets of the Company or any of its subsidiaries; (v) increase or decrease the authorized number of the board of directors to more than seven (7), except for a further increase as may be required under the listing requirements of a securities exchange; and (vi) pass any resolution or take any steps to have the Company or any of its subsidiaries dissolved, wound up or liquidated.
The holders of the Preferred Shares may require the Company to redeem all or any portion of the outstanding Preferred Shares upon the occurrence of certain events, including: (i) any change of control of the Company; (ii) a sale of all or substantially all of the assets of the Company or any subsidiary; (iii) any consolidation or merger or acquisition or sale of voting securities of the Company resulting in the holders of the issued and outstanding voting securities of the Company immediately prior to such transaction beneficially owning or controlling less than a majority of the voting securities of the continuing or surviving entity immediately following such transaction; (iv) any tender offer, exchange offer or repurchase offer for any Common Stock; (v) the cessation of listing of the Common Stock on an internationally recognized exchange; (vi) it has been three years since the initial issuance of the Preferred Shares; (vii) Mr. Huitian Tang ceases providing services to the Company or any its subsidiaries by reason of resignation, discharge, death, disability, retirement or otherwise; (viii) the failure to pay dividends or any other amounts due with regard to the Preferred Shares; (ix) failure to deliver Common Stock upon conversion of the Preferred Shares; (x) the breach of any covenant or representation or warranty contained in the Certificate of Designation, the purchase agreement or the related transaction documents; (xi) a default in making payment of any indebtedness of the Company allowing the holder of such indebtedness to cause such indebtedness to become due and payable prior to the stated maturity; (xii) if it becomes unlawful or unenforceable for the Company or certain shareholders (as the case may be) to perform or comply with any of its obligations under the Certificate of Designation, the purchase agreement or any related document; (xiii) the Company or any of its subsidiaries suspend or cease all or a substantial part of its business, or disposes of or threatens to dispose of the whole or a substantial part of its business or assets; (xiv) the bankruptcy or insolvency of the Company or its subsidiaries or the commencement of any action or proceeding relating to the bankruptcy or insolvency of the Company or its subsidiaries; (xv) the company, its subsidiaries or any of its respective management commits or is suspected of committing fraud or a violation of applicable laws; or (xvi) any event that has an adverse material effect on the Company. The redemption price is an amount expressed as a compounded rate of return from the time of issuance; this rate is 15% or 25% depending upon the event which causes the redemption.
9
There is no sinking fund with respect to the Preferred Shares. The Preferred Shares have a preference in liquidation over the Common Stock and are entitled to the greater of their stated value ($3.20 per share as adjusted for stock splits and other subdivisions or combinations) plus accrued and unpaid dividends or their value as Common Stock on a fully converted basis. The Preferred Shares also have certain information rights with respect to Company information.
The principal agreements with respect to this transaction have been filed as exhibits to our report on Form 8-K describing the transaction and filed with the SEC on January 18, 2011.
Contractual Arrangements with Property Management
We do not hold a 100% direct ownership interest in BCT Retail due to the restriction of foreign investment in pharmacy chains with 30 or more drugstores. We have entered into contractual arrangements with Property Management pursuant to which the shareholders of Property Management pledged to us their equity interests in BCT Retail and provide us with the ability to effectively control BCT Retail. The directors of Liuzhou BCT, Huitian Tang, Jinghua Li, Youru Jing, Chunlin Liu, Wende Wei and Bangfu Wan have an aggregate 67.2% interest in the registered share capital of Property Management, and thus controlled BCT Retail prior to the Proxy Agreement being entered into. In addition, the directors of Liuzhou BCT are the same as the directors of BCT Retail. We have been advised by our PRC legal counsel that under PRC corporate law we do not own 100% of BCT Retail. As a result of the related party control, however, as well as the contractual arrangements meeting the provisions regarding consolidation of entities controlled by contract set forth in FASB ASC 810-10-15-18 through 810-10-15-22, BCT Retail is effectively, although not legally, 100% owned by Liuzhou BCT, and therefore, BCT Retail has been included in our consolidated group.
The contractual agreements entered into by us and Property Management include:
Share Transfer Agreement. Under this agreement dated April 1, 2008 by and between Liuzhou BCT, which held 100% equity interests of BCT Retail, and Property Management, Property Management acquired from Liuzhou BCT 51% equity interests in BCT Retail. The total amount of transfer price was RMB153,000, which was 51% of the registered share capital of BCT Retail. Within twenty (20) business days from the date when Property Management paid the transfer price, all the parties were to amend the articles of association of BCT Retail and register such equity transfer with the competent Authority of Industry and Commerce. Such amendment was made and equity transfer was registered on June 18, 2008.
Shares Pledge Agreement. Under this agreement dated May 3, 2008 and amended on May 19, 2010 among Liuzhou BCT and Property Management, Property Management pledged all of their equity interests in BCT Retail to Liuzhou BCT to guarantee its obligations to repay a loan in the amount of RMB153,000 made from Liuzhou BCT to Property Management. The loan must be repaid by December 31, 2015 and may only be paid by transfer by Property Management of its equity interest in BCT Retail to Liuzhou BCT. During the term of the agreement, Liuzhou BCT has the right to receive any dividends that would have been paid by BCT Retail to Property Management. The May 19, 2010 amendment was made to: (i) correct the name of Party B from the Shareholders of Property Management to Property Management, (ii) to clarify that the loan could only be paid off by the transfer of Property Management’s interest in BCT Retail to Liuzhou BCT and (iii) to clarify that Liuzhou BCT had the right to any dividends or distributions that BCT Retail would have otherwise made to Property Management during the term of the Share Pledge Agreement. We intend to extend the agreement prior to its expiration on December 31, 2015.
10
Share Repurchase Agreement. Under this agreement dated July 31, 2008 by and between Liuzhou BCT and Property Management, Liuzhou BCT was granted a preemption right to repurchase the 51% equity interests in BCT Retail held by Property Management. The term of the preemption right is two years from the date that Property Management deregisters the pledge as a result of paying off loans under the related share pledge agreements. The repurchase price shall be equal to 51% of the registered capital of BCT Retail at the time of the repurchase.
Shares Pledge Agreement. Under this agreement dated March 31, 2009 and amended on May 19, 2010 among Liuzhou BCT and Property Management, Property Management pledged all of its equity interests in BCT Retail to Liuzhou BCT to guarantee its obligations under a loan in the amount of RMB 1.377 million made from Liuzhou BCT to Property Management. The loan was used by Property Management to increase its registered share capital in BCT Retail and to maintain its 51% interest. The loan must be repaid by December 31, 2015 and may only be paid by transfer by Property Management of its equity interest in BCT Retail to Liuzhou BCT. During the term of the agreement, Liuzhou BCT has the right to receive any dividends that would have been paid by BCT Retail to Property Management. The May 19, 2010 amendment was made to: (i) correct the name of Party B from the Shareholders of Property Management to Property Management, (ii) to clarify that the loan could only be paid off by the transfer of Property Management’s interest in BCT Retail to Liuzhou BCT and (iii) to clarify that Liuzhou BCT had the right to any dividends or distributions that BCT Retail would have otherwise made to Property Management during the term of the Share Pledge Agreement. We intend to extend the agreement prior to its expiration on December 31, 2015.
Agreement terminating the Shares Pledge Agreements. Under this agreement, dated March 2, 2010 by and between Liuzhou BCT, the shareholders of Property Management and Property Management, Share Pledge Agreements entered into on May 3, 2008 and March 31, 2009 between Liuzhou BCT and the shareholders of Property Management were terminated because they were entered into by error and were duplicative of the Shares Pledge Agreements entered into by Property Management on such dates as described above.
Proxy Agreement. Under this agreement, dated May 19, 2010 by and between Liuzhou BCT and Property Management, Property Management agreed to grant an irrevocable proxy to Liuzhou BCT to appoint the directors, officers and management of BCT Retail and to vote all shares of capital stock of BCT Retail. The Proxy Agreement was entered into in order to make the contractual relationship between the parties consistent with past practices and understandings between the parties as it relates to the transfer of 51% of the equity interest of China BCT to Property Management in April 2008. The directors of Liuzhou BCT, Huitian Tang, Jinghua Li, Youru Jing, Chunlin Liu, Wende Wei and Bangfu Wan have an aggregate 67.2% interest in the registered share capital of Property Management, and thus controlled BCT Retail prior to the Proxy Agreement being entered into.
Overview
We are engaged in pharmaceutical distribution, retail pharmacy and manufacturing of pharmaceuticals through our two wholly-owned subsidiaries Liuzhou BCT and Hefeng Pharmaceutical, and BCT Retail, a retail company that we control through a series of contractual arrangements, each of which is located in Guangxi province, China. Since January 2, 2008, we have three operating segments based on our major lines of businesses: pharmaceutical distribution, retail pharmacy and manufacturing pharmacy. For additional information regarding our segments please refer to our financial statements.
|
●
|
Pharmaceutical distribution
|
Pharmaceutical distribution is our principal business. We conduct our wholesale business through Liuzhou BCT by purchasing pharmaceutical products from suppliers and then distributing them to our wholesale customers, including hospitals, retail drug stores, other pharmaceutical wholesalers, clinics, medical centers, and individuals. Our pharmaceutical distribution business is focused on the market of Guangxi province, which includes major cities such as Nanning, Liuzhou and Guilin and which has a population of approximately 50 million people. We operate a large regional wholesale network in Guangxi Province supported by strategically placed warehouse facilities. For the year ended December 31, 2011, revenue generated from our pharmaceutical distribution segment was $190.7 million, or 74.0% of our total revenues for the year.
11
We distribute over 8,000 products from nearly 4,000 suppliers through our wholesale distribution in compliance with PRC regulations. Hefeng Pharmaceutical which is one of our wholly-owned subsidiaries is also one of our suppliers. In 2011, revenue derived from the distribution of third-party products constituted 99.99% of our pharmaceutical distribution segment revenue. The terms of our distribution agreements vary between supplier and vary in terms of payment period, arrangement of delivery, pricing and quality requirements. The general payment terms vary from advance deposit, to cash on delivery, to payment ranging from one month to six months from delivery, and the payment can be settled by means of bank collection, remittance, bills payable, postal check. The delivery is either to our warehouse, railway station, or prescribed destination within Liuzhou City. The quality of drugs supplied is in accordance with the prescribed national standard requirement. Our top 10 suppliers in our pharmaceutical distribution segment accounted for 28% of our purchases in 2011.
PRC rules and regulations require most public hospitals and healthcare institutions to purchase medicines from pharmaceutical distributors through a centralized tendering process, which includes the implementation of government-mandated price controls. The manufacturers of provincial catalog medicines that are on the hospitals’ formularies are invited to bid and participate in the centralized tendering process, which they must do directly. The bidding process covers multiple categories of medicines used by the hospitals. A duly organized committee of pharmaceutical and clinical medical experts is responsible for bid evaluations. Selection is based on a number of factors, including bid price, quality, clinical effectiveness, and manufacturer’s reputation and service. The supply of a particular type of medicine is generally made on a non-exclusive basis by multiple manufacturers and distributors. We typically advise and assist pharmaceutical manufacturers in the hospital tendering process and distribute products of pharmaceutical manufacturers upon purchase orders being made by the hospitals after the bidding process.
The Guangxi centralized-online tendering system was started in 2006, and in 2009 the tendering started to be applied also under the New Rural Co-operative Health Insurance Plan. At the first tendering in 2009 we were awarded distribution rights for six counties and townships under the New Rural Co-operative Health Insurance Plan, including Yizhou, Lipu, Gongchen, Luzhai Laibin and Heshan, and were selected as one of two exclusive distributors for these territories.
Liuzhou BCT’s GSP Certificate, which is a certification that drugstores in China are required to obtain was issued on September 18, 2009 and will expire on September 17, 2013, subject to renewal for an additional five-year term.
|
●
|
Retail Pharmacy
|
Established in 2001, BCT Retail operates a large regional retail network in Guangxi province, consisting of 194 directly owned retail stores in Guangxi province under the registered name “Baicaotang 百草堂.” BCT Retail’s GSP Certificate was issued on February 18, 2009 and will expire on February 17, 2014, subject to renewal for an additional five-year term. Our retail stores provide convenient, high quality and professional pharmaceutical services, and supply a wide variety of medicines, including western medicine, TCM, Chinese herbal medicine, roughly processed Chinese herbal medicine, family planning products, and seasonal medicine. For the year ended December 31, 2011, revenue generated from our retail pharmacy segment was $53.3 million, or 20.7% of our revenues for the year.
Among the 194 stores, there are 21 stores that are medi-care qualified stores, where customers are able to make their purchase either by cash or by using their medi-care insurance card for payment. With respect to medi-care insurance card payments, we issue an invoice to the national medi-care for reimbursement for those drugs included under the medi-care insurance catalogue. Only medi-care card payments from these 21 stores are entitled to reimbursement. The medi-care insurance catalogue used are provincial-based and developed and based on the national insurance catalogue. The medi-care insurance program is also known as National Medical Insurance Program.
12
The National Medical Insurance Program is funded primarily by provincial governments and, to a lesser degree, by program participants and their employers. The program has two types of accounts: individual accounts and social pool accounts. Each participant has an individual account that holds all contributions from the participant and part was contributed from his or her employer. National medicine catalog of the National Medical Insurance Program provides guidance to what extent the purchases of these medicines are reimbursable. The implementation of the National Medical Insurance Program is delegated to provincial governments, each of which has established its own medicine catalog. The medicine catalog comprises two lists of drugs which are named as Tier A and Tier B. People can consume and reimburse the drugs on these two lists by means of the insurance card at the medi-care qualified retail stores. When the customers purchase the drugs at the medi-care qualified stores listed under Tier B, they need to pay 20% co-payment by themselves and 80% is deducted from their individual account under the insurance card, whereas 100% is deducted from their individual account for the drugs listed under Tier A. From July 2009, onward, such policy was cancelled and the cost of drugs listed under Tier B can be deducted from the card in full should the balance of the individual account on the card exceed the cost of drugs purchased. If there is not a sufficient balance on the insurance card to cover the cost of the drug, the customer must personally pay the difference at the time of purchase. Our retail stores participate in both of these two tiers of drugs. Thus, subject to an individual having an adequate balance on their card for Tier B drugs, there is no difference between the treatment of Tier A and Tier B drugs from July 2009 onwards.
In order to get reimbursement from the National insurance program, we have to extract the total amount of sales derived from the amount spent by insurance card under our billing system. Our system is linked with the one at the National program. Each month, we only need to reconcile our record with the system and issue the invoice to the National program for reimbursement approval. Then the money will be remitted to our designated account subject to an individual having an adequate balance on their card for Tier B drugs.
The retail price under the medi-care qualified stores is subject to price controls administered by the Price Control Office under the National Development and Reform Commission. Our retail price for Tier A and Tier B drugs under qualified stores is fixed at an agreed ceiling of an agreed percentage over the prescribed one issued by the Guangxi Price Control Office. The prescribed drug prices issued by the Guangxi Price Control Office are in accordance with the price upon the provincial collective tender result which is held annually.
As for the New Rural Cooperative Medicare Plan, the participants in the scheme are people living in rural areas. The program also has individual accounts in which more subsidies are contributed by the provincial government and no employers are involved. It resembles the schemes under the National Medical Insurance Program in which people consume and reimburse the drugs by their individual account. The transaction is not recorded by a card; we instead login their account under the system of the New Rural Cooperative plan to check their balance to ensure that the limit of the account has not been reached.. Unlike National Medical Insurance Program, the eligible person is entitled to consume the drugs under the list of Basic Drugs Catalogue rather than the Tier A and Tier B catalogue.
The retail prices under the New Rural Cooperative Medicare Plan are subject to price controls administered by the Price Control Office under the National Development and Reform Commission. The retail prices under the list of Basic Drugs Catalogue are fixed at an agreed ceiling of percentage over the prescribed one issued by the Guangxi Price Control Office. The prescribed drugs price issued by the Guangxi Price Control Office are in accordance with the price upon the provincial collective tender result which is held annually. In order to get reimbursement from the New Rural Cooperative Medicare Plan, our stores have to extract the sales figures from the billing system and issue the reimbursement notes with the original vouchers and invoices to the relevant Committee for approval. Then the Committee remits the money to the designated account opened by the stores.
|
●
|
Manufacturing of Pharmaceuticals
|
Hefeng Pharmaceutical has a manufacturing facility located on approximately 40,000 square meters of land, and manufactures four types of products:
|
●
|
A Chinese herbal medicine abstraction unit for raw material and medicine paste with 670 tons of annual abstraction capacity (Maximum daily unit production: 2.5 tons per day; maximum days of operation per year: 270 days);
|
|
●
|
A granular formulation unit with an annual production capacity of 0.25 billion packages (Maximum daily unit production: 768,960 packages per day; maximum days of operation per year: 324 days);
|
|
●
|
A pill formulation unit with an annual production capacity of 0.36 billion pills (Maximum daily unit production: 1,252,800 pieces per day; maximum days of operation per year: 288 days); and
|
|
●
|
A liquid formulation unit with an annual production capacity of 0.1 billion injections (Maximum daily unit production: 347,500 pieces per day; maximum days of operation per year: 288 days).
|
13
The above capacity figures have been derived by our production department. The maximum days of operation is determined after taking into account the days of repairs, sterilization and rinsing process particular to each of the above production lines.
Hefeng Pharmaceutical produces and sells pharmaceutical products under the registered name “Asio (亚太)” including: traditional anti-inflammatory and antibacterial drugs, cancer treatment drugs, cardio-vascular disease drugs and hepatitis drugs.
Hefeng Pharmaceutical’s best-selling products include:
|
●
|
Levodopa is used to treat the stiffness, tremors, spasms, and poor muscle control of Parkinson’s disease.;
|
|
●
|
Tabellae Sarcandrae, a TCM protected drug, has similar anti-inflammatory and antibacterial effects as antibiotics in Western medicine;
|
|
●
|
Rotandine Sulfate is a non-prescription analgesic drug. Used for headaches, menstrual pain, and aiding sleep;
|
|
●
|
Corydalis Saxicola Bunting (Yanghuanglian) has been demonstrated to possess many pharmacological activities, including antibacterial, antiviral and anticancer activities; and
|
|
●
|
Ethacridine Lactate Injection is used for second trimester pregnancy termination from week 12-26 at hospitals.
|
In addition, Hefeng Pharmaceutical collaborates with several renowned medical research universities in China to continuously improve its raw material abstraction efficiency and production process, and to develop alternative formulas for existing drugs. For the year ended December 31, 2011, revenue generated from our manufacturing segment was $13.6 million, or 5.3% of our total revenues for the year.
Hefeng Pharmaceutical’s GMP Certificate was issued on July 14, 2009 and will expire on July 13, 2014, and its GMP Certificate for Small Volume Parental Solution was issued on July 10, 2006 and was originally supposed to expire on July 9, 2011, but the expiration date was extended until December 31, 2013; both certificates are subject to renewal of an additional five-year term. The renewal process requires us to apply through the State Food and Drug Administration and furnish an application form together with relevant supporting documents, such as our product license, business registration certificate, a summary of our management over drugs manufacturing, a self quality control report, an organization chart, education graphic data, a floor plan, assembly line, production flow and control points, inspection detail over key processes and a list of products produced. The Food and Drugs Administration will consider and issue the notice of acceptance upon the formality check and then carry out inspection on our technical know-how. Thereafter, GMP inspection unit will undertake an on-site inspection and issue an on-site inspection report in compliance with the GMP inspection standards. The State Food and Drug Administration then reviews the on-site inspection report and announces the result of inspection to us. The State Food and Drug Administration will issue the GMP certificate renewal if the reports are satisfactory.
The following table sets forth a breakdown of our external segment revenue after elimination of inter-segment sales, and each segment revenue item as a percentage of our total revenue, as well as our inter-segment sales for the year ended December 31, 2011 and 2010. For the year ended December 31, 2011, we had approximately $39.8 million of inter-segment revenue, which includes approximately $38.6 million in sales from our pharmaceutical distribution segment to our retail pharmacy segment, and approximately $1.2 million in sales from our manufacturing segment to our pharmaceutical distribution pharmacy segment. External segment revenue refers to segment revenue after inter-segment elimination.
14
|
Year ended December 31,
|
||||||||||||||||
|
2011
|
2010
|
|||||||||||||||
|
’000
|
% of total sales revenue
|
’000
|
% of total sales revenue
|
|||||||||||||
|
External Segment revenue
|
||||||||||||||||
|
Pharmaceutical distribution
|
$
|
190,663
|
74.0
|
$
|
145,390
|
72.4
|
||||||||||
|
Retail pharmacy
|
53,266
|
20.7
|
44,594
|
22.2
|
||||||||||||
|
Manufacturing pharmacy
|
13,558
|
5.3
|
10,829
|
5.4
|
||||||||||||
|
$
|
257,487
|
100.0
|
$
|
200,813
|
100.0
|
|||||||||||
|
Inter-segment revenue
|
$
|
39,824
|
-
|
$
|
31,012
|
-
|
||||||||||
Our Products
Products Offered by Hefeng Pharmaceutical
Manufacturing both Chinese medicine and Western medicine, Hefeng Pharmaceutical maintains valid production licenses for 76 drugs. Below is the description of the five (5) best-selling drugs.
| 1. Levodopa |
|
|
Levodopa is used to treat the stiffness, tremors, spasms, and poor muscle control of Parkinson’s disease. Levodopa is also used to treat these same muscular conditions when they are caused by drugs such as chlorpromazine (Thorazine), fluphenazine (Prolixin), perphenazine (Trilafon), and others. As traditional Chinese medicine has become more and more popular worldwide, especially in South-east Asian countries whose cultures are similar to that of the Chinese, we have exported roughly processed Levodopa raw material to Japan and Thailand.
|
 |
15
|
2. Tabellae Sarcandrae
|
|
|
Tabellae Sarcandrae, a TCM protected drug, has similar anti-inflammatory and antibacterial effects as anti-biotics in Western medicine. Tabellae Sarcandrae possesses marked inhibition effect to auricular inflammation in mice caused by croton oil, footpad inflammation in rats caused by carragheenin and granuloma in mice by cotton ball. It could also relieve obvious abdominal pain caused by acetic acid and inhibit bacterial growth. The TCM protection is valid from December 19, 2006 to August 1, 2012 and is renwable.
|
 |
|
3. Rotandine
|
|
|
This product is non-prescription analgesic drug. Used for headaches, menstrual pain, and aiding sleep.
|
 |
|
4. Corydalis Saxicola Bunting (Yanhuanglian)
|
|
|
Corydalis Saxicola Bunting is an important component in various prescriptions in TCM. Yanhuanglian has been demonstrated to possess many pharmacological activities, including antibacterial, antiviral and anticancer activities. The active ingredients are dehydrocavidine, coptisine, dehydroapocavidine and tetradehydroscoulerine. Systemic clearance of the four active alkaloids in plasma was over 93% of hepatic blood flow, indicating they may be quickly eliminated via hepatic clearance. Less than 10% of the drug was excreted via urine following intravenous and oral administration, suggesting that these four alkaloids may undergo significant metabolism in the body or the drug may be excreted via routes other than urine. Intravenous administration of Yanhuanglian is the most common clinical practice, because it can improve absorption of the four active alkaloids into systemic circulation.
We are the sole licensed producer for this drug in China, and the market demand for this drug has been extremely strong due to the effectiveness of the drug to treat hepatic diseases.
|
 |
|
5. Ethacridine Lactate Injection
|
|
|
This family planning drug is very popular in China; it’s used for second trimester pregnancy termination from week 12-26 at hospitals. We are one of three licensed producers in China.
|
 |
Products and services offered by BCT Retail
Our retail chain stores provide our customers with high-quality, professional and convenient pharmaceutical services and a wide variety of medicines, including Western medicine, TCM, raw materials of dried herbal products, roughly processed herbal medicine, family planning products, as well as convenient seasonal and promotional items. A typical retail chain drug store carries approximately 2,800 to 3,300 different products. Management regularly reviews and refines the product selection in order to respond to change in demographics, lifestyles, shopping habits and product preferences of our customers.
Our product selection is designed to offer choices and convenience to our customers and to achieve high gross margins for us. We offer our customers a broad range of choices in two respects. First, we offer a wide range of complementary products in each therapeutic category so that customers have more choices to suit their needs. For example, a customer looking for a cough remedy will be able to find a wide variety of choices including different OTC drugs, nutritional supplements and herbal products. Second, for products with the same therapeutic purpose, we offer choices in each of the high, medium and low price ranges to suit the needs of customers with different spending power.
16
|
●
|
Packaged Western and TCM. We offer approximately 2,750 packaged drugs including prescription and OTC drugs. We accept prescriptions only from licensed healthcare providers and do not prescribe medications or otherwise practice medicine. Our in-store pharmacists verify the validity, accuracy and completeness of all prescription drug orders. We ask all prescription drug customers to provide us with information regarding drug allergies, current medical conditions and current medications.
|
|
●
|
Chinese Herbal Medicine. We offer approximately 450 types of various drinkable herbal remedies and packages of assorted herbs for making soup, which are used by consumers as health supplements. Herbal products typically have higher margins than prescription and OTC drugs.
|
|
●
|
Family Planning Products. We offer approximately 40 family planning products, which include family care products such as portable medical devices for family use, birth control and early pregnancy test products and convenience products. Our family planning products also include seasonal and promotional items tailored to local consumer demand for convenience and quality. We believe offering these products increases customer visits by increasing the shopping convenience for our customers.
|
Our wholesale business provides BCT Retail with the majority of the pharmaceutical products sold in our retail drugstores. Approximately 95% of the packaged Western medicine and TCM, 100% of the Chinese Herbal Medicine and 100% of the family planning products are supplied by our wholesale business.
Besides providing procurement to our retail business, the majority of the sales revenue of our wholesale business arises from supplying pharmaceutical products to hospitals, clinics and healthcare centers at provincial, city, county and district levels. In addition, our wholesale business also exchanges our products with other wholesale networks to obtain products that we do not carry. Further, our wholesale business also distributes our products to other retail networks.
|
●
|
Increasing coverage of social medical insurance in China
|
The National Medical Insurance Program (“National Program”), which was introduced in 1999, is the largest medical insurance program in China. The National Program is funded with varying levels of contributions from the PRC Government, individual program participants and their employers. The National Program provides guidance on which prescription and over-the-counter medicines are included in the program and to what extent the purchases of these medicines are reimbursable.
We believe that only a small percentage of the Chinese population can afford commercial insurance plans. Therefore, the National Program coverage is expected to expand in the future. Provincial and municipal authorities who are responsible for administering social medical insurance funds to cover such reimbursements have been gradually increasing funding in recent years. According to the PRC Ministry of Labor and Social Security, total funding under the national insurance program reached RMB225.7 billion, or $28.9 billion, in 2008, representing an increase of 29.2% from 2007. The availability of funding is expected to increase significantly in the near future, primarily as a result of increased financial and policy support from various levels of the PRC Government.
As a major portion of sales from our pharmaceutical distribution segment are derived from hospitals, the increase in coverage of social medical insurance and the roll-out of the New Rural Cooperative Medicare Plan has increased the demand for drugs, including those we distribute, by hospitals as more patients visit hospitals for treatment, knowing that the costs related to the treatment will be reimbursed under the social medical insurance plan. In addition, we also believe that our retail business segment will benefit if the social medical insurance plan covers a larger population base.
17
|
·
|
Increasing access to healthcare in rural areas
|
At the fifth meeting of the tenth National People’s Congress held in March 2007, the PRC Government announced its goal to accelerate the reform and development of healthcare services in the PRC and focus on building a basic healthcare system that covers both rural and urban areas. The PRC Government’s plans include providing expanded healthcare services for its rural citizens and establishing comprehensive community healthcare service centers that would provide basic medical treatment and pharmaceutical services, as well as upgrading existing class-two hospitals and state owned medical facilities. The public health service centers would be allocated based on demand and population. In addition, the PRC Government has actively promoted the implementation of the New Rural Cooperative Medical Insurance Scheme (“New Rural Insurance Scheme”), which seeks to provide healthcare services to the vast rural areas of China. The program extends to cover approximately 2,729 counties in the PRC, which account for 95.4% of the total number of counties in the PRC. In addition, the program covers approximately 814 million rural residents, which accounts for approximately 91.5% of the total population engaged in the agricultural industry in China as of December 31, 2008.
Because we operate in Guangxi Province, which has a large number of small cities and rural areas, we believe that the New Rural Insurance will have a positive impact on the demand for our products in all of our segments.
|
●
|
PRC Healthcare Reform Plan
|
In September 2008, the State Council published a draft plan to ease the difficulties and minimize the costs for PRC citizens to obtain proper healthcare treatment. On March 17, 2009, the PRC Government issued the Opinion on Deepening the Healthcare System Reform (the “Opinion”). The State Council subsequently released the Notice on Important Implementing Plans for the Healthcare System Reform 2009-2011 (the “Implementing Plan”). The goal of the healthcare reform plan is to establish a basic, universal healthcare framework to provide Chinese citizens with safe, efficient, convenient and affordable healthcare. The Opinion calls for healthcare reform to be carried out in two steps:
|
●
|
Step One aims to increase the accessibility while reducing the cost of healthcare. During this phase, the PRC Government will build up a network of basic healthcare facilities, expand coverage of the public medical insurance system to cover 90% or more of the population, and reform the drug supply and public hospital system.
|
|
●
|
Step Two, which we expect to take place between 2012 and 2020, envisions the establishment of a universal healthcare system. The entire population should be covered by public medical insurance; drugs and medical services should be accessible and affordable to citizens in all public healthcare facilities.
|
While the PRC Government has neither provided a concrete timetable nor steps to implement certain tasks, such as the public hospital reform, it has released execution guidance for other tasks. Most notably, the PRC Government announced it would spend an additional approximately RMB 850 billion, or $125 billion from 2009 to 2011 on the healthcare industry. A significant portion was to be expended to establish a basic healthcare medical insurance regime, which was expected to cover over 90% of the national population by the end of 2011, mainly through the Urban Worker Program, Urban Resident Program and the New Rural Insurance Scheme. The PRC Government further announced that the annual subsidy for each participant will be increased from approximately RMB 40, or $5.90 to approximately RMB 120, or $17.60 for Urban Resident Program participants, and from approximately RMB 80, or $11.76 to approximately RMB120 or $17.60 for New Rural Insurance Scheme participants, starting from 2010. The reform plan will also raise the cap on claim payments from four times the local average annual income to six times such income. Another significant part of the spending plan focuses on healthcare facilities. The PRC Government originally planned 29,000 new rural clinics in 2009. From 2010 to 2012, the PRC government plans to build an additional 5,000 rural clinics, 2,000 county-level hospitals and 2,400 urban community clinics in under-developed areas. This substantial increase in healthcare spending is expected to expedite the growth of the healthcare industry in China.
18
Under the healthcare reform plan, the additional funding for the healthcare industry will primarily target four fundamental healthcare systems in China:
|
●
|
The public health services system. This system focuses on preventing disease and promoting health as a complementary alternative to medical treatment. The public health services system will provide services such as immunizations, regular physical check-ups (for senior citizens over 65 years of age and children under three years of age), pre-natal and post-natal check-ups for women, prevention of infectious or chronic diseases and other preventative and fitness activities.
|
|
●
|
The public medical insurance system. This system covers drugs and medical treatments for the majority of the population. The healthcare reform plan will retain the framework of the current public medical insurance schemes under the National Program, but will expand them to cover more of the population and increase the scope of treatments, raise the cap on claim payments and cover more claims at higher percentages.
|
|
●
|
The public healthcare delivery system. One of the primary goals of the Implementing Plan is to build more healthcare facilities and to improve the training of healthcare professionals. Beyond additional public wellness centers, the reform plan aims to place a medical clinic in every village and a hospital in every county by 2011. In addition, the PRC Government will encourage private investors to establish public non-profit hospitals.
|
|
●
|
The drug supply system. This system regulates pricing and how drugs will be procured, prescribed and dispensed in healthcare facilities. The healthcare reform plan will focus on pricing, procurement, prescription and dispensing of essential drugs.
|
Although the healthcare reform plan is expected to benefit our pharmaceutical distribution, retail pharmacy and other business operations and improve our competitive position, the full effect of the healthcare reform plan on our operations is as yet unclear.
Industry Overview
We operate in the large and growing pharmaceutical wholesale and retail industry in China, which we believe offers compelling industry fundamentals and benefits from favorable demographics. With approximately one-fifth of the world’s population and one of the world’s fastest growing economies, China presents significant potential for the retail drugstore industry.
Market Overview
In China, retail pharmaceutical and other healthcare related products may be purchased at either hospital pharmacies or non-hospital drugstores, including independent drugstores and drugstore chains. Historically, sales by hospital pharmacies accounted for a larger percentage of retail sales of pharmaceutical products in China. This is because out-patients typically purchase their prescription drugs at hospital pharmacies in accordance with doctors’ prescriptions. However, if a medical condition can be treated with OTC drugs, many Chinese people typically choose to purchase OTC drugs from non-hospital drugstores instead of consulting a doctor in a hospital for prescription medicines.
Fragmentation of the Pharmaceutical Chain Store Industry and the Trend for Consolidation
The drugstore industry in China is highly fragmented. Retail pharmacies in China include chain drugstores, individual stores, and OTC counters in retail chain stores and supermarkets. While pharmacy chain stores and retail chain stores with OTC counters are expanding quickly, neither format has developed a nationwide presence in China. The NDRC reported that as of December 31, 2004, 7,445 pharmaceutical product wholesalers, 1,410 pharmacy chain stores and 58,065 individual pharmaceutical product retailers have obtained Good Supply Practices certification. According to a White Paper entitled “Status Quo of Drug Supervision in China” dated July 18, 2008 and issued by the PRC Information Office of the State Council, there were more than 340,000 retail pharmaceutical stores in China in 2007. (See http://former.sfda.gov.cn/cmsweb/webportal/W205/A64028182.html). Given the level of fragmentation and increased regulatory requirements, the Company believes retailers with an effective regional presence and a strong reputation are likely to thrive.
19
Non-Pharmaceutical Sales Opportunity at Retail Pharmacies
We believe drugstore non-pharmaceutical merchandise, combined with prescription and non-prescription drugs, provides customers with a complete wellness solution. Non-pharmaceutical merchandise includes nutrition supplements, beauty, cosmetics and fragrance products, personal care products, as well as consumable, seasonal, promotional and other non-prescription products.
Challenges for the Drugstore Industry in China and Increased Competition
While the Chinese economy in general and the drugstore industry in particular have grown significantly in the past decade, such growth may not continue in the future. The drugstore industry in China faces a number of challenges, including:
|
●
|
Competition in the retail drugstore market in China may also intensify;
|
|
●
|
Industry reforms aimed to meet China’s commitments under WTO may foster increased competition from multinational pharmacy chains at the expense of China-based pharmacy chains; and
|
|
●
|
Current PRC laws and regulations limit any foreign investor’s ownership of drugstores to 49.0% if the foreign investor owns interests in more than 30 drugstores in China that sell a variety of branded pharmaceutical products sourced from different suppliers. If this restriction is relaxed or eliminated, there may be increasing competition from large foreign drugstore chains which intend to enter into the drugstore industry in China.
|
The growth profile of Guangxi province is based on the following three factors:
|
●
|
According to data published by the National Bureau of Statistics, Guangxi Province’s GDP was RMB957 billion ($152 billion) in 2010. GDP per capita in Guangxi Province was RMB20,759 ($3,295) in 2010 as compared with GDP per capita between RMB39,906 ($6,334) to RMB74,538 ($11,831) in the coastal regions (such as, Fujian Province ($6,334), Guangdong Province ($6,995), Jiangsu Province ($8,356), Shandong Province ($6,485), Shanghai ($11,831), and Zhejiang Province ($8,079)). In 2010 Guangxi Province’s GDP growth rate was 14.2% as compared to an average GDP growth rate of 10.62% in the coastal regions in 2010. In 2010 Guangxi Province had a population of 46.10 million. In 2010 the population nature growth rate of Guangxi Province was 8.65 ‰ as compared with a population nature growth rate averaged at 4.67 ‰ in the coastal regions. (See http://www.stats.gov.cn/tjsj/ndsj/2011/indexce.htm).
|
|
●
|
The World Bank projected that the inflation rate in China in 2011 should moderate after having risen in March 2011 (World Bank “China Quarterly Update” from April 27, 2011)
(See http://www-wds.worldbank.org/external/default/WDSContentServer/WDSP/IB/2011/04/29/000356161_ 20110429003758/Rendered/PDF/614000Replacem1Quarterly1April12011.pdf); and
|
|
●
|
the general pharmaceutical industry growth rate resulting from the RMB850 billion healthcare reform bill passed by the Chinese government.
|
Manufacturing Facility
We have land use rights to the 40,000 square meters of land on which our manufacturing facilities are located that are granted and allocated to us by the government. According to Chinese law, the government owns all the land in China and companies or individuals are authorized to use the land only through land use rights granted or allocated by, or leased from, the PRC government. We currently have adequate manufacturing capacity for our marketed products.
20
Our production plant maintains Good Manufacturing Practice (“GMP”) certification authorized by the national accreditation bodies of the PRC. A GMP-certified facility operates under the GMP parameters prescribed by the institution granting such certification. GMP parameters are operating standards that are formed to ensure product quality, by regulating the manufacturing space, the storage warehouse for raw materials and finished products, and laboratory areas of the production facility. Hefeng Pharmaceutical operates our production line and holds a general GMP Certificate that was issued on July 14, 2009 and will expire on July 13, 2014, and also holds a GMP Certificate for Small Volume Parental Solution that was issued on July 10, 2006 and was originally supposed to expire on July 9, 2011, but the expiration date was extended until December 31, 2013. The GMP Certificates cover all products that we manufacture, as well as some products that we do not presently manufacture. Both GMP Certificates will be subject to renewal for an additional five-year term.
Raw Materials
We require a supply of quality raw materials to manufacture our products. Historically, we have not had difficulty obtaining raw materials from suppliers. Currently, we rely on numerous suppliers to deliver our required raw materials. We typically enter into one-year contracts with numerous suppliers in China to secure a steady supply of raw materials throughout the year.
Target Market
Our business operations are located in Guangxi province which hosts many second and third-tier cities with less competition in the market of manufacture and distribution of pharmaceutical products. Through our experience in operating in such a business environment, we have accumulated extensive business operating experience in developing a market in second- and third-tier cities and rural areas, and have built a strong reputation and brand name awareness in Guangxi province. Moreover, we have not only gained valuable experience in operational management, but also built up a strong sales network in Guangxi provision. With the brand name and leading position we have established in Guangxi province, we will continue building and expanding our retail and wholesale business in the second- and third-tier cities and the rural areas in Guangxi province through our current retail stores and the new stores that we may acquire in the future. Based on continued forecasted growth in Guangxi province, we may apply the business model we have established in Guangxi province to our business expansion in the second- and third-tier cities and the rural areas of our contiguous provinces, such as Yunnan or Huainan provinces.
Marketing and Sales
We manufacture and market 19 products, consisting of prescription and over-the-counter pharmaceuticals. Our pharmaceutical products are marketed to hospitals, clinics, pharmacies and retail stores. We maintain 22 sales offices throughout Guangxi Province and employ approximately 53 sales and marketing professionals. Where appropriate, we leverage the synergies between complementary products and distribution channels to accelerate the market penetration of our new products.
With respect to our retail stores, our marketing and promotion strategy is to build brand recognition, increase customer traffic to our stores, attract new customers, build strong customer loyalty, maximize repeat customer visits and develop incremental revenue opportunities. We work with vendors to organize promotional campaigns, and vendors typically assign sales persons to our retails stores for these activities. We also hold promotional functions, including price reductions and free gifts, during major Chinese holidays. We also place advertisements in local newspapers regarding our promotions.
21
Competition
We believe that we are well positioned to compete in the fast-developing Chinese pharmaceutical market with our strong brand, diverse product portfolio, research and development capabilities, established sales and marketing network and favorable cost structure. We believe that competition and leadership in our industry are based on managerial and technological expertise, and the ability to identify and exploit commercially viable products. Other factors affecting our competitive position include time to market, patent position, product efficacy, safety, convenience, reliability, availability and pricing.
Retail Pharmacy
The pharmaceutical industry in China is intensely competitive, rapidly evolving and highly fragmented. In many large cities in China, we need to not only compete with other retail drugstores, but also face increasing competition pressure from discount stores, convenience stores and supermarkets. In order to maintain our competitive position in the market, we have increasingly diversified products and services by offering some non-drug products that are provided in regular convenience stores. In addition, we also increased our competitiveness through careful selection of store location, merchandise, and services.
With the continuous consolidation of the pharmaceutical industry and opening of new drugstore chains in large cities, we will face more competition in the industry. However, in many of our targeted second- and third- tier cities and rural areas, we are facing less competition because major drugstore chains have not entered into the market. We are in a good position to establish our standing and reputation in these targeted markets. In addition, the pharmaceutical industry has entrance barriers for new entrants due to the requirements for such resources as capital, brand name and management expertise. Further, PRC laws and regulations limit a foreign investor’s ownership in retail drugstores to the maximum of 49.0% if such investor holds ownership interest in more than 30 drug stores that sell a variety of branded drugs sourced from different suppliers. This limitation, together with the complexity of the Chinese market, creates a barrier for foreign retail drugstore chain operators to enter into the PRC market. As a result, currently we do not face notable competition from foreign owned drugstore chains.
Because our network covers many cities and areas, and many drugstore groups are regional, our competitors vary from region to region. Each region can have its own, among others, distinct demographics, local regulations and shopping style. We do not consider any individual regional drugstores as our major competitor, but we compete with them on an aggregate basis. Our main competitors in Guangxi province are Sinopharma Liuzhou Branch and Sinopharma Nanning Branch, Liuzhou Medical and Pharmaceutical Limited on wholesale side; Shenzhen Accordance Pharm. Chain Store Inc., and Hunan Laobaixing Pharmacy Chain on retail side.
Manufacturing of Pharmaceutical Products
In the pharmaceutical manufacturing business, we compete in general with Harbin Pharmaceutical Group Co. Ltd., Sixth Pharma Factory, Guangdong Boluo Xianfeng Pharmaceutical Group and Jiangsu Chia Tai Tianqing Pharmaceutical.
We also compete with other manufacturers in each specific drug category. For instance, although we are the sole authorized producer of Corydalis Saxicola Bunting (Yanhuanglian), which is the preferred drug treating chronic hepatitis A, B and C, there are drugs that have a similar medical effect for treating hepatitis.
The following table lists the primary competitors for our best selling products produced by Hefeng Pharmaceutical:
|
Our Product
|
Competitor’s Product - Company
|
|
|
Levodopa
|
Guangxi Napo Pharmaceutical Co. Ltd
Guangxi Lingyun Pharmaceutical Co. Ltd
|
|
|
Tabellae Sarcandrae
|
Shanghai Xingcheng Jiahua Pharmaceutical Co. Ltd
Jiangxi Tianshi Chinese Medicine Co. Lt
Zhejiang Guojing Pharmaceutical Co. Ltd
|
|
|
These companies rely solely on their in-house sales force. We believe that our wholesale network enables us to gain market share more quickly.
|
||
|
Rotandine Sulfate Injection
|
Guangdong Boluo Xianfeng Pharmaceutical Group Ltd
Guangxi Nanning Baihui Pharmaceutical Group Ltd
|
|
|
Corydalis Saxicola Bunting (Yanhuanglian)
|
Haimingwe Interferon - Qindao Haier Pharmaceutical
Handadang - Lianyungang Shentiantang Pharmaceutical Group
Tianqing Fuxing Marine and Glucose Injection - Jiangsu Chia-tai Tiangqing Pharmaceutical
|
|
|
We are the only manufacturer of our Yanhuanglian product. These competitors produce alternative products.
|
||
|
Ethacridine Lactate Injection
|
Jiangsu Tianmiao Disainuo Pharmaceutical Co. Ltd
Qinghai Pharmaceutical Manufacturing Co. Ltd
|
|
|
We believe we are the leading supplier of this product in Guangxi Province because these competitors have not been able to sustain their production from time to time.
|
22
Pharmaceutical Distribution
There are relatively well capitalized and established players in the pharmaceutical distribution business, such as Sinopharm Group Co. Ltd, Liuzhou Medical and Pharmaceutical Limited and Jointtown Pharmaceutical group, which have built an intensive nationwide network while our distribution business is relatively regionally strong. Jointtown Pharmaceutical Group does not have any subsidiaries in Guangxi Province. Sinopharm Group has two subsidiaries in Guangxi Province, in Liuzhou Branch and Nanning. Sinopham specializes in large volume, low margin bulk sales to regional distributors, while we specialize in higher margin end customers, including direct sales to hospitals.
Government Regulation
We are subject to various Chinese laws and regulations pertaining to the pharmaceutical industry. We have attained certificates, permits, and licenses required for the operation of a pharmaceutical enterprise and the manufacturing of pharmaceutical products in China.
In 1998, the PRC State Food and Drug Administration (“SFDA”) introduced the GMP Certificate in order to promote quality and safety of pharmaceutical production. Good Manufacturing Practices were revised in July and October, 2004. We are required to meet GMP standards in order to continue manufacturing pharmaceutical products and health foods. For each new product, we prepare documentation of pharmacological, toxicity, pharmacokinetics and drug metabolism studies in addition to providing samples of the drug. The documentation and samples are then submitted to the provincial food and drug administration. This process typically takes approximately three months. After the documentation and samples have been approved by the provincial food and drug administration, the provincial administration submits the approved documentation and samples to the SFDA. The SFDA examines the documentation and tests the samples and presents the findings to the New Drug Examination Committee for approval. If the application is approved by the SFDA, the SFDA will issue a clinical trial license to the applicant for clinical trials. This clinical trial license approval typically takes one year, followed by approximately two years of trials, depending on the category and class of the new drug. The SFDA then examines the documentation from the trial and, if approved, issues the new drug license to the applicant. This process usually takes eight months. The entire process takes anywhere from three to four years.
The GMP certificate is valid for a term of five years, the pharmaceutical products production permits are subject to renewal every five years, and the health food production permits are valid for three-year terms, and each must be renewed before its expiration, if applicable. If our GMP certificate expires without renewal, we will not be able to continue manufacturing pharmaceutical products, which will cause our production operations to be terminated.
In addition, a distributor of pharmaceutical products in China must obtain a pharmaceutical distribution permit from the relevant provincial or local SFDA branches. The distribution permit is granted if the relevant SFDA provincial branch receives satisfactory inspection results of the distributor’s facilities, warehouse, hygiene environment, quality control systems, personnel and equipment. A pharmaceutical distribution permit is valid for five years.
The SFDA applies Good Supply Practice (“GSP”) standards to all pharmaceutical wholesale distributors as well as to retail to ensure the quality of distribution in China. The currently applicable GSP standards require pharmaceutical distributors to implement controls on the distribution of medicine, including standards regarding staff qualifications, distribution premises, warehouses, inspection equipment and facilities, management and quality control. A certificate for GSP standards, or GSP certificate, is valid for five years, except for a newly established pharmaceutical distribution company, for which the GSP certificate is valid for only one year. If our GSP certificate expires without renewal, we will not be able to continue distributing pharmaceutical products, which will cause our wholesale and retail distribution to be terminated.
23
Competitive Advantages
As a leading pharmaceutical distributor in the region, we are well-positioned to benefit from the strong growth, consolidation, and regulatory reform in the PRC pharmaceutical and healthcare industry.
The PRC healthcare market is one of the fastest-growing healthcare markets in the world, driven by China’s rapidly growing economy, rising living standards, increased health consciousness, large aging population and proactive government policies. Furthermore, the PRC Government recently announced a reform plan to spend RMB850 billion on healthcare in addition to the regular healthcare budget from 2009 to 2011, in order to increase the availability of healthcare, basic medicines and health insurance coverage for people in China. As a comparison, in 2007, the total healthcare expenditure in China was approximately RMB1.1 trillion, of which approximately RMB230 billion was government spending, according to the Ministry of Health. The healthcare reform plan is expected to accelerate growth in the PRC pharmaceutical industry not only by the increased government spending, but also by the expected increases in private healthcare spending stimulated by larger government subsidies to PRC residents, as per capita healthcare spending remains much lower than in developed countries. We are well-positioned to capture business opportunities resulting from this fast growing market.
Building up a modernized logistic center, a streamlined supply chain and increased capital entrance barrier for smaller competitors to further strengthen our leading position and differentiate in the region.
In addition, China’s pharmaceutical distribution market is highly fragmented and is characterized by inefficient supply chains. The highly fragmented pharmaceutical distribution industry has recently commenced a process of consolidation, which has led to an increase in market share of the pharmaceutical distributors in the market, we expect the PRC pharmaceutical distribution market to continue to consolidate into one with larger and more efficient distributors. In addition, we expect the healthcare reform plan to promote further consolidation, as it calls for reducing the number of layers between manufacturers and consumers of medicines. We believe that we have the scale, industry standing, brand and financial strength to compete effectively during this process of consolidation. The PRC Government has also adopted measures to raise the operating standards of pharmaceutical companies and promote the quality of distribution of pharmaceutical products in China, in order to ensure a stable supply of safe, effective medicines at reasonable prices. We believe we will benefit from future regulatory reforms, which require pharmaceutical manufacturers and distributors to implement more stringent standards on the manufacturing and distribution of pharmaceutical products. Unlike smaller distributors, we have a large-scale distribution network, high quality equipment and facilities, leading management and qualified personnel, which are required to satisfy the higher standards. These attributes also provide us with a competitive edge over our competitors.
We plan to invest in a modernized logistics center. We estimate the costs of the center to be approximately RMB 150 million. We have not committed to specific timing for our implementation of this advanced logistics center. The timing will depend on the availability of funding, which may come from cash generated from operations, private or public financing or bank loans.
24
Leading Product and Brand Name in Diversified Area
Over the years, we have developed and introduced a number of pharmaceutical products under the brand name Asio (亚太), which we believe are the leading products in their respective market segments, including Levodopa, which treats the stiffness, tremors, spasms, and poor muscle control of Parkinson’s disease; Hydroxycamptotbecine Injection, which we believe is one of the best choices to fight cancers of various kinds with minimal side effects; Tabellae Sarcandrae, which is called Chinese anti-biotic and has similar anti-inflammatory and antibacterial effects as anti-biotics used in Western medicine; Corydalis Saxicola Bunting (Yanhuanglian), which treats hepatitis, liver cirrhosis, liver ascites and liver cancer; and Yinge Tongmai Tea, which improves cardio-vascular condition and blood circulation, and can be taken as a normal healthy drink on a daily basis. As a result, “Asio (亚太)” has become a widely recognized brand name for our self-developed drugs in China. Among our five best-selling drugs, we are the sole producer of Corydalis Saxicola Bunting (Yanhuanglian) and Hydroxycamptotbecine Injection.
In addition, our sales and marketing teams are specialized in promoting products in different therapeutic categories. The teams have strong relationships with healthcare executives, doctors and pharmacies in their respective target markets and possess extensive sales and marketing experience in promoting prescription and non-prescription pharmaceutical products.
Strong Research and Development Capability
Our research and development department relies upon a research institute jointly formed by us and several renowned Chinese medicine universities, including China Medicine University located in Nanjing China, which focuses on utilizing Guangxi’s natural herbal medicine resources to produce drugs that meet the demand of the Chinese domestic market. Currently we pay for such research and development on a project by project basis, which helps us to reduce costs related to an in-house research and development team and to have access to the latest advances in this area. In 2010 and 2011 we did not spend any amount on research and development.
Guangxi is a province with a wealth of natural resources for developing traditional Chinese medicine. In addition, they also used great efforts to discover advanced production process of Levodopa and Corydalis Saxicola Bunting (Yanhuanglian), to develop better understanding of the composition of Hydroxycamptotbecine and its derivatives, to develop key technology of figure print quality control, and to research the production technology of herbal medicine in limestone mountainous areas.
Currently, we are working with the research team for the development of Corydalis Saxicola Bunting Injection Powder by means of vein injection on the foundation of our current Corydalis Saxicola Bunting Injection. Pre-clinical research is now undertaken by the research team in respect of the production tactics, quality standard research, samples inspection, stability testing, package screening process development and the pre-requisite research work required before the application of clinical research. No exact timetable is available for the launch of this new drug in the market.
Extensive Wholesale and Retail Distribution Network and Medi-Care Qualified Stores
We operate wholesale and retail distribution networks covering major cities, townships, and counties of Guangxi province. Our wholesale network offers more than 8,000 pharmaceutical products to our clients. Further, BCT Retail offers more than 3,200 pharmaceutical products in 194 retail stores, holding the leading market position in many major cities of Guangxi province. Among the 194 stores, 21 stores are covered by a medi-care insurance plan for citizens and employees. We’ve also signed an MOU with local authorities to be authorized to open 30 retail stores under the New Rural Cooperative Health Issuance Plan.
Economic Scope and Integration Ability
We believe we have the potential to expand our scale by acquiring more wholesale and retail networks. By acquisition of a production facility in 2008, we have established a full-integrated operation. Established in the early 1970s, our production facility has accumulated a large reserve of production licenses, established an experienced management team, formed a strong research and development team, and developed solid relationships with local raw material providers. This integration enables us to enjoy economies of scale and also provides us with an ability to increase our operating efficiency in order to sustain strong profit margins and favorable growth rates.
25
Experienced Management Team with Proven Track Record
Over the past decade, Mr. Huitian Tang, Chairman of our board of directors and other members of our senior management team have provided successful leadership in our operations and increased our revenue and profit. Many members of our senior management team have worked with us since our inception or otherwise have broad experience in the retail industry, and have developed extensive expertise in operating a national chain of drugstores, which is important to our future development. The Chief Operating Officer of Liuzhou BCT, Jinghua Li, has extensive experience in chain store retailing, gained from his four years service with Meidong Bio-technology (AOB), a publicly held multinational pharmaceutical corporation. In addition, the majority of our mid-level managers and managers of our regional operations and stores have been with us for many years. These managers have obtained extensive experience through our internal management training system and real practice in managing retail stores and distribution centers.
Well-Established Good Supply Practices (GSP) Control System
Our logistics system is established in line with GSP. We also have a full-time GSP supervision body comprised of professionals with professional pharmacist qualification or pharmacist-in-charge titles. They are familiar with national laws and regulations on medicine quality, and have extensive knowledge and practical experience in the implementation of GSP in the enterprise.
Professional storage, advanced transportation equipment, comprehensive management system and detailed flow records are integrated with hardware and software of the pharmaceutical business. The medicine warehouse occupies an area of 4,800 square meters, including warehouses of room temperature (0-30°C), cool (<20°C) and cold (2-8°C), stock control and subsidiary operation area, chemical laboratory and has advanced instruments and facilities.
GSP, which can be called a total quality control system for the pharmaceutical industry, is strictly observed from purchase to sale to storage, forming a secure tunnel for the medicine transportation and guaranteeing medicine quality.
Growth Strategy
Refining Retail and Expanding Sales Networks Targeting the Second and Third Tier Cities and Rural Markets
We believe that refining and expanding our existing retail networks in desirable geographic markets is essential to our competitiveness and our ability to increase our profitability. Due to constraints of resources (e.g. lack of registered pharmacists and medi-care qualifications), we are attempting to consolidate our existing retail networks and resources to transform our retail pharmacy chain into a retail chain of larger pharmacy stores with built-in TCM clinics. With larger in-store floor space, we are able to provide more varieties of products within the larger store areas and free medical consultations also guide customers to more sales of our TCM drugs.
We believe that a strong brand name is critical to obtaining customers’ trust in our business, as well as building customer loyalty and increasing customer visits to our stores. As a result, we intend to continue promoting aggressively and effectively both our brand name and our private label products. Specifically, we will continue to deploy the following marketing and promotional initiatives:
1. adopting advanced category management by focusing on seasonal and cross-merchandising, and offering a wider selection of products;
2. offering services that are carefully tailored to our customers’ healthcare needs, including integrated health programs focused on health supplements, weight management, diabetes, infant care and birth control;
26
3. enhancing our customer loyalty by organizing community-based activities and targeted promotion programs;
4. using data mining techniques to tailor relevant promotional offers to our target customers, especially our loyalty members;
5. enlarging the number of our promotional partners and developing additional promotional campaigns with these partners; and
6. advertising our brand and private label products in selected newspapers that service our targeted cities.
Invest in More Advanced Logistics and Information Management Systems to Improve Cost and Operating Efficiencies
We intend to invest in an advanced logistic center. In Liuzhou city first, we plan to establish regional and provincial logistics facilities equipped with leading technology and information systems. We believe that these improvements will help to provide a direct and high-speed information exchange channel that connects us to our customers and suppliers throughout China, covering each stage of our pharmaceutical distribution operations, including electronic order entry, invoice preparation, purchasing, inventory tracking, GSP-certified warehousing and logistics and delivery arrangements. Once these improvements are completed, we believe that we will be able to shorten delivery lead-times, increase responsiveness to customer demands and reduce distribution and selling expenses.
We have not adopted a specific timetable for implementing the logistic center project, and the expansion plan with respect to our retail stores is our first priority to be carried out in the short term. The timing of the capital investment of the modernized logistic center is subject to two principal factors:
|
●
|
We believe that we must have sufficient scale to justify the investment in modernizing our logistic center. In order to optimize the return on our investment in a modernized logistics center, we will continue to analyze the return on our potential investment and the timing of the modernization.
|
|
●
|
The timetable for the enforcement by the Chinese FDA on requiring modernized logistic centers to be built in order to qualify as a distributor participating in the centralized bid tendering process. We intend to closely monitor the relevant policies, in order to make timely plans for implementing the modernized logistic center. Currently, we do not have an indication of when such a requirement will be put in place as the policy is still in the discussion stage.
|
In addition, the timing will depend on the availability of funding, which may come from cash generated from operations, private or public financing or bank loans.
Continue to Build Upon Our Integrated Business Platform in Order to Enhance the Synergies between Our Pharmaceutical Distribution, Retail Pharmacy and Other Businesses
We will continue to build upon our integrated business platform in order to enhance the synergies that arise from our pharmaceutical operations spanning the distribution, retail and manufacturing of pharmaceutical and healthcare products and expand our reach to end-customers. We plan to leverage our existing businesses to capitalize on particular opportunities that may arise and create efficiencies and cost savings in our business operations. We also plan to utilize our extensive distribution network to provide reliable supply channels for our retail drug stores which, in turn, will sell the pharmaceutical and healthcare products supplied by our distribution network. Further, we intend to utilize our pharmaceutical manufacturing operations to produce private-label products for our retail pharmacy operations. We expect to maintain the flexibility to reallocate manufacturing capacity of our products in response to potential changes in supply and demand, as well as to control inventory in a way that enables us to meet expected demand for our products. With operations in multiple segments of the pharmaceutical industry, we are able to maintain control over the quality, supply chain management and marketing of our products. We believe that our integrated business platform maximizes the synergies between our businesses by optimizing our operational efficiency while reducing the costs of our customers, allowing us to further solidify our leadership in the PRC pharmaceutical distribution industry.
27
Intellectual Property
We believe our rights to our trade names and trademarks are the most important factors in implementing our marketing strategy. Our company’s name, Baicaotang (百草堂), means “Place for all kind of Chinese Herbal Medicines” in Chinese. We registered “Baicaotang (百草堂)” as the trademark for our business group, and Asio (亚太) as the trademark for the medicines that our production plant produces. The extension of the Baicaotang (百草堂) trademark was granted on May 5, 2008 and expires on July 13, 2018. The Asio (亚太) trademark was granted on August 15, 2005 and expires on August 15, 2015.
Under the PRC law, we have the exclusive right to use a trademark for products and services for which the trademark has been registered with the State Administration for Industry and Commerce. We use our trademarks throughout our operations and are presently in the process of transferring trademark registrations within our corporate structure to better align registration with usage. Trademark registration is valid for 10 years, starting from the day the registration is approved. For renewal of registration, an application shall be made within 6 months before the expiration of such 10 years. If no application is made within that period, an extension of 6 months may be granted. We also rely on trade secrets to protect our know-how and other proprietary information.
One of our products, Tabellae Sarcandrae, has protection as a Traditional Chinese Medicine which was granted on December 19, 2006 and expires on August 1, 2012 and is renewable. During the period of the protection, only the company which has been granted the right of protection can manufacture the specified product during the protection period. When we undertake the renewal process, we apply to the local authority and then to the Ministry of Public Health which assigns the National Committee on the Assessment of the Protected TCM Products to inspect our facilities and announce whether their report satisfies the renewal criteria. In order to apply for this certificate, we need to provide certain information such as the GMP certificate, research data, details of the protected drugs, and a safety assessment.
We currently have no patents or pending patent applications.
Employees
As of March 30, 2012, we had 1,559 full-time employees and no part-time employees.
ITEM 1A. RISK FACTORS
An investment in our common stock involves a high degree of risk. You should carefully consider the risks described below and the other information contained in this report before deciding to invest in our common stock.
Risks Relating to Our Business
OUR OPERATING HISTORY MAY NOT SERVE AS AN ADEQUATE BASIS TO JUDGE OUR FUTURE PROSPECTS AND RESULTS OF OPERATIONS.
Our operating history may not provide a meaningful basis on which to evaluate our business. We cannot assure you that we will maintain our profitability or that we will not incur net losses in the future. We expect that our operating expenses will increase as we expand. Any significant failure to realize anticipated revenue growth could result in significant operating losses.
WE MAY BE UNABLE TO COMPETE SUCCESSFULLY AGAINST NEW AND EXISTING COMPETITORS.
The major market for our products and business operations is the cities, villages and towns of Guangxi province. We may face increasing competition in the future as we expand our business in Guangxi province and our adjacent provinces. As we expand our operations in wholesale and retail distribution and manufacture of pharmaceutical products, we will encounter competition from other companies existing in our target markets, such as Sinopharma and Liuzhou Medical and Pharmaceutical Limited in the pharmaceutical distribution area, Shenzhen Accordance Pharmacy Chain Store and Hunan Laobaixing Pharmacy Chain in the retail area and Harbin Pharmaceutical Group, Guangdong Boluo Xianfeng Pharmaceutical Group and Jiangsu Chia Tai Tianqing Pharmaceutical and Sixth Pharma Factory in the manufacturing area, and may face future competition from new foreign and domestic competitors entering the pharmaceutical promotion and distribution market in China. Our current and future competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional and distribution activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. Competition could reduce our market share or force us to lower our prices to unprofitable levels.
28
OUR CURRENT BUSINESS OPERATIONS RELY HEAVILY UPON MR. HUITIAN TANG, OUR CHAIRMAN AND CHIEF EXECUTIVE OFFICER, AND ADDING OTHER KEY EMPLOYEES ARE ESSENTIAL TO GROWING OUR BUSINESS.
We have been heavily dependent upon the expertise and management of Mr. Huitian Tang, our Chairman and Chief Executive Officer, and his continued services. The loss of Mr. Tang’s services could seriously interrupt our business operations. Although we have entered into an employment contract with Mr. Tang, pursuant to which Mr. Tang agrees to serve as our full time Chief Executive Officer, and Mr. Tang has not indicated any intention of leaving us, the loss of his service for any reason could have a very negative impact on our ability to fulfill our business plan. In addition, we face competition for attracting skilled personnel. If we fail to attract and retain qualified personnel to meet current and future needs, this could slow our ability to grow our business, which could result in a decrease in market share.
THE LIMITED PUBLIC COMPANY EXPERIENCE OF OUR MANAGEMENT TEAM COULD ADVERSELY IMPACT OUR ABILITY TO COMPLY WITH THE REPORTING REQUIREMENTS OF U.S. SECURITIES LAWS.
Our management team has limited public company experience, which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley Act of 2002. Our senior management has never had sole responsibility for managing a publicly traded company. Such responsibilities include complying with federal securities laws and making required disclosures on a timely basis. Our senior management may not be able to implement programs and policies in an effective and timely manner that adequately respond to such increased legal, regulatory compliance and reporting requirements, including the establishing and maintaining internal controls over financial reporting. Any such deficiencies, weaknesses or lack of compliance could have a materially adverse effect on our ability to comply with the reporting requirements of the Securities Exchange Act of 1934 (the “Exchange Act”), which is necessary to maintain our public company status. If we were to fail to fulfill those obligations, our ability to continue as a U.S. public company would be in jeopardy in which event you could lose your entire investment in our company.
IF WE NEED ADDITIONAL CAPITAL TO FUND OUR GROWING OPERATIONS, WE MAY NOT BE ABLE TO OBTAIN SUFFICIENT CAPITAL AND MAY BE FORCED TO LIMIT THE SCOPE OF OUR OPERATIONS.
In connection with our growth strategies, we may experience increased capital needs and accordingly, we may not have sufficient capital to fund our future operations without additional capital investments. Our capital needs will depend on numerous factors, including (i) our profitability; (ii) the release of competitive products by our competition; (iii) the level of our investment in research and development; and (iv) the amount of our capital expenditures, including acquisitions. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
If we cannot obtain additional funding, we may be required to: (i) limit our investments in research and development; (ii) limit our marketing efforts; and (iii) decrease or eliminate capital expenditures. Such reductions could materially adversely affect our business and our ability to compete.
29
Even if we do find a source of additional capital, we may not be able to negotiate terms and conditions for receiving the additional capital that are acceptable to us. Any future capital investments could dilute or otherwise materially and adversely affect the holdings or rights of our existing shareholders. In addition, new equity or convertible debt securities issued by us to obtain financing could have rights, preferences and privileges senior to our common stock. We cannot give you any assurance that any additional financing will be available to us, or if available, will be on terms favorable to us.
IF WE FAIL TO INCREASE OUR BRAND RECOGNITION, WE MAY FACE DIFFICULTY IN OBTAINING NEW CUSTOMERS AND BUSINESS PARTNERS.
We believe that establishing, maintaining and enhancing our brand in a cost-effective manner is critical to achieving widespread acceptance of our current and future services and is an important element in our effort to increase our customer base and obtain new business partners. We believe that the importance of brand recognition will increase as competition in our market develops. Some of our potential competitors already have well-established brands in the pharmaceutical promotion and distribution industry. Successful promotion of our brand will depend largely on our ability to maintain a sizeable and active customer base, our marketing efforts and our ability to provide reliable and useful services at competitive prices. Brand promotion activities may not yield increased revenue, and even if they do, any increased revenue may not offset the expenses we incur in building our brand. If we fail to successfully promote and maintain our brand, or if we incur substantial expenses in an unsuccessful attempt to promote and maintain our brand, we may fail to attract enough new customers or retain our existing customers to the extent necessary to realize a sufficient return on our brand-building efforts, in which case our business, operating results and financial condition would be materially adversely affected.
AS A DISTRIBUTOR AND MANUFACTURER OF PHARMACEUTICAL PRODUCTS, WE ARE EXPOSED TO INHERENT RISKS RELATING TO PRODUCT LIABILITY AND PERSONAL INJURY CLAIMS.
Pharmacies are exposed to risks inherent in the manufacturing and distribution of pharmaceutical and other healthcare products, such as with respect to improper filling of prescriptions, labeling of prescriptions, adequacy of warnings, unintentional distribution of counterfeit drugs. In addition, product liability claims may be asserted against us with respect to any of the products we sell and as a retailer, we are required to pay for damages for any successful product liability claim against us, although we may have the right under applicable PRC laws, rules and regulations to recover from the relevant manufacturer for compensation we paid to our customers in connection with a product liability claim. We may also be obligated to recall affected products. If we are found liable for product liability claims, we could be required to pay substantial monetary damages. Furthermore, even if we successfully defend ourselves against this type of claim, we could be required to spend significant management, financial and other resources, which could disrupt our business, and our reputation as well as our brand name may also suffer. We, like many other similar companies in China, do not carry product liability insurance. As a result, any imposition of product liability could materially harm our business, financial condition and results of operations. In addition, we do not have any business interruption insurance due to the limited coverage of any business interruption insurance in China, and as a result, any business disruption or natural disaster could severely disrupt our business and operations and significantly decrease our revenue and profitability.
THE RETAIL PRICES OF SOME OF OUR PRODUCTS ARE SUBJECT TO CONTROL, INCLUDING PERIODIC DOWNWARD ADJUSTMENT, BY PRC GOVERNMENTAL AUTHORITIES.
A number of our pharmaceutical products, primarily those included in the national and provincial medical insurance catalogs, are subject to price controls in the form of fixed retail prices or retail price ceilings. Approximately 60% to 70% of our total retail sales are subject to these price controls. In addition, the retail prices of these products are also subject to periodic downward adjustments as the PRC governmental authorities seek to make pharmaceutical products more affordable to the general public. Since May 1998, the relevant PRC governmental authorities have ordered price reductions of thousands of pharmaceutical products. The latest price reduction occurred in October 2008 and affected 1,357 different pharmaceutical products. Any future price controls or government mandated price reductions may have a material adverse affect on our financial condition and results of operations, including significantly reducing our revenue and profitability.
30
THE AVERAGE NUMBER OF DAYS DURING WHICH OUR ACCOUNTS RECEIVABLE ARE OUTSTANDING HAS INCREASED WHICH MAY NEGATIVELY AFFECT OUR BALANCE SHEET AND OUR ABILITY TO COLLECT OUR RECEIVABLES AND FUND OUR OPERATIONS.
Across all of our pharmaceutical, distributing and manufacturing segments the numbers of days during which our receivables are outstanding has increased significantly from 2009 to 2011. In the pharmaceutical distribution segment we are obtaining a greater percentage of revenue from hospitals and community health centers who traditionally have a slower payment cycle. In our manufacturing segment, we have also experienced an increase in the days during which our receivables are outstanding, because we have extended more generous credit terms to certain of our customers based upon their history with us and in view of the overall financial crisis. If this slower time to collection should result in an inability to collect a greater percentage of our receivables than previously, the result would be a decrease in the cash available to fund our operations as well as an increase in reserves for receivables which would reduce their value on our balance sheet.
WE MAY BE SUBJECT TO FINES AND PENALTIES IF WE FAIL TO COMPLY WITH THE APPLICABLE PRC LAWS AND REGULATIONS GOVERNING SALES OF MEDICINES UNDER THE PRC NATIONAL MEDICAL INSURANCE PROGRAM.
Eligible participants in the PRC national medical insurance program, mainly consisting of urban residents in China, are entitled to buy medicines using their medical insurance cards in an authorized pharmacy, provided that the medicines they purchase have been included in the national or provincial medical insurance catalogs. The pharmacy, in turn, obtains reimbursement from the relevant government social security bureaus. Moreover, the applicable PRC laws, rules and regulations prohibit pharmacies from selling goods other than pre-approved medicines when purchases are made with medical insurance cards, as well as providing cash to the customer for the medical insurance card. We have established procedures to prohibit our drugstores from selling unauthorized goods to customers who make purchases with medical insurance cards. However, we cannot assure you that those procedures will be strictly followed by all of our employees in all of our stores. If we fail to observe the above laws, rules and regulations with respect to purchases made with medical insurance cards, we may be fined and our qualification for selling medicines included in the national or provincial medical insurance catalogs may be withdrawn by competent authorities.
OUR RETAIL OPERATIONS REQUIRE A NUMBER OF PERMITS AND LICENSES IN ORDER TO CARRY ON THEIR BUSINESS.
Drugstores in China are required to obtain certain permits and licenses from various PRC governmental authorities, including GSP certification. We are also required to obtain food hygiene certificates for the distribution of nutritional supplements and food products other than medicine. We cannot assure you that we can maintain all required licenses, permits and certifications to carry on our business at all times, and from time to time we may have not been in compliance with all such required licenses, permits and certifications. Moreover, these licenses, permits and certifications are subject to periodic renewal and/or reassessment by the relevant PRC governmental authorities and the standards of such renewal or reassessment may change from time to time. We intend to apply for the renewal of these licenses, permits and certifications when required by then applicable laws and regulations. Any failure by us to obtain and maintain all licenses, permits and certifications necessary to carry on our business at any time could have a material adverse effect on our business, financial condition and results of operations. In addition, any inability to renew these licenses, permits and certifications could severely disrupt our business, and prevent us from continuing to carry on our business. Any changes in the standards used by governmental authorities in considering whether to renew or reassess our business licenses, permits and certifications, as well as any enactment of new regulations that may restrict the conduct of our business, may also decrease our revenue and/or increase our costs and materially reduce our profitability and prospects. Furthermore, if the interpretation or implementation of existing laws and regulations changes or if new regulations come into effect requiring us to obtain any additional licenses, permits or certifications that were previously not required to operate our existing businesses, we cannot assure you that we may successfully obtain such licenses, permits or certifications.
31
WE MAY HAVE DIFFICULTY DEFENDING OUR INTELLECTUAL PROPERTY RIGHTS FROM INFRINGEMENT RESULTING IN LAWSUITS REQUIRING US TO DEVOTE FINANCIAL AND MANAGEMENT RESOURCES THAT WOULD HAVE A NEGATIVE IMPACT ON OUR OPERATING RESULTS.
We regard our trademarks and other similar intellectual properties as critical to our success. We rely on trademark and other similar intellectual properties, as well as confidentiality and license agreements with certain of our employees, customers and others to protect our proprietary rights. We have received trademark registration for certain of our products in the PRC. No assurance can be given that our licenses will not be challenged, invalidated, infringed or circumvented, or that our intellectual property rights will provide competitive advantages to us. There can be no assurance that we will be able to obtain a license from a third-party technology that we may need to conduct our business or that such technology can be licensed at a reasonable cost.
Presently, we sell our products mainly in PRC. To date, no trademark or patent filings have been made other than in PRC. To the extent that we market our products in other countries, we may have to take additional action to protect our intellectual property. The measures we take to protect our proprietary rights may be inadequate and we cannot give you any assurance that our competitors will not independently develop formulations and processes that are substantially equivalent or superior to our own or copy our products.
WE MAY BE ADVERSELY AFFECTED BY COMPLEXITY, UNCERTAINTIES AND CHANGES IN CHINESE REGULATION OF DRUGSTORES AND THE PRACTICE OF MEDICINE.
The Chinese government regulates drugstores, including foreign ownership, and the licensing and permit requirements. These laws and regulations are relatively new and evolving, and their interpretation and enforcement involve significant uncertainty. As a result, in certain circumstances it may be difficult to determine what actions or omissions may be deemed to be a violation of applicable laws and regulations. Issues, risks and uncertainties relating to Chinese government regulation of the industry include the following:
|
●
|
We only have 49% ownership interest in BCT Retail. We are not able to own 100% interest in BCT Retail due to the restriction of foreign investment in pharmacy chains with 30 or more drugstores and foreign ownership of medical practices. If the Chinese government challenges our control of BCT Retail through contractual relationships, our business could be harmed; and
|
|
●
|
Uncertainties relating to the regulation of drugstores, including evolving licensing practices, means that permits, licenses or operations at our company may be subject to challenge. This may disrupt our business, or subject us to sanctions, requirements to increase capital or other conditions or enforcement, or compromise enforceability of related contractual arrangements, which could materially and adversely affect our business, financial condition and results of operations.
|
THERE IS NO ASSURANCE THAT WE WILL PAY DIVIDENDS TO SHAREHOLDERS IN THE FUTURE.
Although Liuzhou BCT, our wholly owned subsidiary, declared and paid to its then existing shareholders cash dividends in the amount of $6,940,000 and $2,044,056 for the years ended December 31, 2008 and 2007, respectively, our board of directors does not intend to distribute dividends in the near future. The declaration, payment and amount of any future dividends will be made at the discretion of the board of directors, and will depend upon, among other things, the results of our operations, cash flows and financial condition, operating and capital requirements, and other factors as the board of directors considers relevant. There is no assurance that future dividends will be paid, and, if dividends are paid, there is no assurance with respect to the amount of any such dividend.
32
WE MAY INCUR SIGNIFICANT COSTS TO ENSURE COMPLIANCE WITH UNITED STATES CORPORATE GOVERNANCE AND ACCOUNTING REQUIREMENTS.
We may incur significant costs associated with our public company reporting requirements, costs associated with newly applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the Securities and Exchange Commission. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these newly applicable rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
Risks Related to Our Corporate Structure
WE CONTROL BCT RETAIL THROUGH A SERIES OF CONTRACTUAL ARRANGEMENTS, WHICH MAY NOT BE AS EFFECTIVE IN PROVIDING CONTROL OVER THE ENTITY AS DIRECT OWNERSHIP AND MAY BE DIFFICULT TO ENFORCE.
We operate our retail pharmacy business in the PRC through BCT Retail which holds the licenses, approvals and assets necessary to operate our retail pharmacy business in the PRC. We have a 49% minority equity ownership interest in BCT Retail and rely on contractual arrangements with Property management that allow us to substantially control and operate BCT Retail. These contractual arrangements may not be as effective as direct ownership in providing control over BCT Retail because BCT Retail or its shareholders could breach the arrangements.
Our contractual arrangements with BCT Retail are governed by PRC law and provide for the resolution of disputes through arbitration in the PRC. Accordingly, these contracts would be interpreted in accordance with PRC law and any disputes would be resolved in accordance with PRC legal procedures. If BCT Retail or its shareholders fail to perform their respective obligations under these contractual arrangements, we may have to
|
●
|
incur substantial costs to enforce such arrangements; and
|
|
●
|
rely on legal remedies under PRC law, including seeking specific performance or injunctive relief, and claiming damages.
|
The legal environment in the PRC is not as developed as in the United States and uncertainties in the Chinese legal system could limit our ability to enforce these contractual arrangements. In the event that we are unable to enforce these contractual arrangements, our business, financial condition and results of operations could be materially and adversely affected.
IF THE PRC GOVERNMENT DETERMINES THAT THE CONTRACTUAL ARRANGEMENTS THROUGH WHICH WE CONTROL BCT RETAIL DO NOT COMPLY WITH APPLICABLE REGULATIONS, OUR BUSINESS COULD BE ADVERSELY AFFECTED.
Although we believe our contractual relationships through which we control BCT Retail comply with current licensing, registration and regulatory requirements of the PRC, we cannot assure you that the PRC government would agree, or that new and burdensome regulations will not be adopted in the future. If the PRC government determines that our structure or operating arrangements do not comply with applicable law, it could revoke our business and operating licenses, require us to discontinue or restrict our operations, restrict our right to collect revenues, require us to restructure our operations, impose additional conditions or requirements with which we may not be able to comply, impose restrictions on our business operations or on our customers, or take other regulatory or enforcement actions against us that could be harmful to our business.
Risks Relating to the People’s Republic of China
THE SALES PRICES OF SOME MEDICINES ARE CURRENTLY CONTROLLED BY THE CHINESE GOVERNMENT AND THAT MAY ADVERSELY AFFECT OUR BUSINESS.
Prices paid by end consumers for many of our medicines are regulated by PRC’s State Development and Reform Commission. PRC justifies its need to control the drug prices on the basis that, at present, only employees at state or private companies have health insurance. About 900 million rural Chinese people and 35 million urban unemployed Chinese people lack insurance coverage and cannot afford expensive drugs. Our future profitability might suffer if a significant portion of our revenues were to be derived from products whose final selling prices were state-controlled and if those prices were held at levels close to or below our cost of sales.
33
SALES OF OUR PRODUCTS COULD BE HARMED BY THE WIDESPREAD PRESENCE OF COUNTERFEIT MEDICATION IN PRC NEGATIVELY IMPACTING OUR PROFITABILITY.
Chinese counterfeiting of pharmaceuticals and other products affecting public health has grown in tandem with counterfeiting and piracy of goods such as brand-name clothing, compact discs and computer software. This situation negatively affects China Baicaotang and other major domestic and foreign drug manufacturers in PRC, especially for products marketed through the over the counter rather than hospital channel. With the expansion of our business and increased recognition of our brand name, such risks may increase. Currently, active pharmaceutical ingredients are governed only by chemical regulations. Our ability to increase sales as rapidly as we would like, and our profitability, could be affected if this problem persists or worsens.
SUBSTANTIALLY ALL OF OUR OPERATING ASSETS ARE LOCATED IN CHINA AND SUBSTANTIALLY ALL OF OUR REVENUE WILL BE DERIVED FROM OUR OPERATIONS IN CHINA SO OUR BUSINESS, RESULTS OF OPERATIONS AND PROSPECTS ARE SUBJECT TO THE ECONOMIC, POLITICAL AND LEGAL POLICIES, DEVELOPMENTS AND CONDITIONS IN CHINA.
The PRC’s economic, political and social conditions, as well as government policies, could impair our business. The PRC economy differs from the economies of most developed countries in many respects. China’s GDP has grown consistently since 1978 (National Bureau of Statistics of China). However, we cannot assure you that such growth will be sustained in the future. If, in the future, China’s economy experiences a downturn or grows at a slower rate than expected, there may be less demand for spending in certain industries. A decrease in demand for spending in certain industries could impair our ability to remain profitable. The PRC’s economic growth has been uneven, both geographically and among various sectors of the economy. The PRC government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall PRC economy, but may have a negative effect on us. For example, our financial condition and results of operations may be hindered by PRC government control over capital investments or changes in tax regulations.
The PRC economy has been transitioning from a planned economy to a more market-oriented economy. Although in recent years the PRC government has implemented measures emphasizing the use of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of productive assets in China is still owned by the PRC government. In addition, the PRC government continues to play a significant role in regulating industry development by imposing industrial policies. It also exercises significant control over PRC economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies.
IF THE MINISTRY OF COMMERCE, OR MOFCOM, CHINA SECURITIES REGULATORY COMMISSION, OR CSRC, OR ANOTHER PRC REGULATORY AGENCY, DETERMINES THAT MOFCOM AND CSRC APPROVAL OF OUR MERGER WAS REQUIRED OR IF OTHER REGULATORY OBLIGATIONS ARE IMPOSED UPON US, WE MAY INCUR SANCTIONS, PENALTIES OR ADDITIONAL COSTS WHICH WOULD DAMAGE OUR BUSINESS.
On August 8, 2006, six PRC regulatory agencies, including the MOFCOM and the CSRC, promulgated the Rules on Acquisition of Domestic Enterprises by Foreign Investors, or the M&A Rules, a new regulation with respect to the mergers and acquisitions of domestic enterprises by foreign investors that became effective on September 8, 2006. Article 11 of the New M&A Rules requires when a domestic company, enterprise or natural person uses an offshore company legally established or controlled by the domestic company, enterprise or natural person to engage in the merger and acquisition of a related domestic company, the application must be submitted to MOFCOM for approval. Article 40 of the New M&A Rules requires that an offshore special purpose vehicle formed for overseas listing purposes and controlled directly or indirectly by PRC companies or individuals should obtain the approval of the CSRC prior to the listing and trading of such offshore special purpose vehicle’s securities on an overseas stock exchange, especially in the event that the offshore special purpose vehicle acquires shares of or equity interests in the PRC companies in exchange for the shares of offshore companies. Article 39 of the New M&A Rules defines an offshore special purpose vehicle as an offshore company directly or indirectly controlled by a PRC domestic company or natural person for the purpose of the offshore listing of their equity interests in the domestic company. On September 21, 2006, the CSRC published on its official website procedures and filing requirements for offshore special purpose vehicles seeking CSRC approval of their overseas listings.
34
We believe, based on the opinion of our PRC legal counsel, Broad & Bright Law Firm, that MOFCOM and CSRC approvals were not required for our merger transaction or for the listing and trading of our securities on a trading market because we are not an offshore special purpose vehicle that is directly or indirectly controlled by PRC companies or individuals. Although the merger and acquisition regulations provide specific requirements and procedures, there are still many ambiguities in the meaning of many provisions. Further regulations are anticipated in the future, but until there has been clarification either by pronouncements, regulation or practice, there is some uncertainty in the scope of the regulations and the regulators have wide latitude in the enforcement of the regulations and approval of transactions. If the MOFCOM, CSRC or another PRC regulatory agency subsequently determines that the MOFCOM and CSRC approvals were required, we may face sanctions by the MOFCOM, CSRC or another PRC regulatory agency. If this happens, these regulatory agencies may impose fines and penalties on our operations in China, limit our operating privileges in China, delay or restrict the repatriation of the net proceeds (after the payment of fees and expenses in connection with the Private Placement) received by us from the Private Placement into China, restrict or prohibit payment or remittance of dividends paid by Liuzhou BCT, or take other actions that could damage our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our securities.
THE NEW M&A REGULATIONS ESTABLISH MORE COMPLEX PROCEDURES FOR SOME ACQUISITIONS OF CHINESE COMPANIES BY FOREIGN INVESTORS, WHICH COULD MAKE IT MORE DIFFICULT FOR US TO PURSUE GROWTH THROUGH ACQUISITION IN CHINA.
The New M&A Rules establish additional procedures and requirements that could make some acquisitions of PRC companies by foreign investors, such as ours, more time-consuming and complex, including requirements in some instances that the approval of the Ministry of Commerce shall be required for transactions involving the shares of an offshore listed company being used as the acquisition consideration by foreign investors. In the future, we may grow our business in part by acquiring complementary businesses. Complying with the requirements of the New M&A Rules to complete such transactions could be time-consuming, and any required approval processes, including obtaining approval from the Ministry of Commerce, may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business or maintain our market share.
IF THE PRC IMPOSES RESTRICTIONS DESIGNED TO REDUCE INFLATION, FUTURE ECONOMIC GROWTH IN THE PRC COULD BE SEVERELY CURTAILED WHICH COULD HURT OUR BUSINESS AND PROFITABILITY.
While the economy of the PRC has experienced rapid growth, this growth has been uneven among various sectors of the economy and in different geographical areas of the country. Rapid economic growth often can lead to growth in the supply of money and rising inflation. In order to control inflation in the past, the PRC has imposed controls on bank credits, limits on loans for fixed assets and restrictions on state bank lending. Imposition of similar restrictions may lead to a slowing of economic growth, a decrease in demand for our products and generally damage our business and profitability.
FLUCTUATIONS IN EXCHANGE RATES COULD HARM OUR BUSINESS AND THE VALUE OF OUR SECURITIES.
The value of our ordinary shares will be indirectly affected by the foreign exchange rate between U.S. dollars and RMB and between those currencies and other currencies in which our sales may be denominated. Because substantially all of our earnings and cash assets are denominated in RMB and the net proceeds (after the payment of fees and expenses in connection with the Private Placement) received by us from the Private Placement will be denominated and our financial results are reported in U.S. dollars, fluctuations in the exchange rate between the U.S. dollar and the RMB will affect the relative purchasing power of these proceeds, our balance sheet and our earnings per share in U.S. dollars following the offering. In addition, appreciation or depreciation in the value of the RMB relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations. Fluctuations in the exchange rate will also affect the relative value of any dividend we issue that will be exchanged into U.S. dollars as well as earnings from, and the value of, any U.S. dollar-denominated investments we make in the future. Since July 2005, the RMB has not been pegged to the U.S. dollar. Although the People’s Bank of China regularly intervenes in the foreign exchange market to prevent significant short-term fluctuations in the exchange rate, the RMB may appreciate or depreciate significantly in value against the U.S. dollar in the medium to long term. Moreover, it is possible that in the future PRC authorities may lift restrictions on fluctuations in the RMB exchange rate and lessen intervention in the foreign exchange market.
35
Very limited hedging transactions are available in China to reduce our exposure to exchange rate fluctuations. To date, we have not entered into any hedging transactions. While we may enter into hedging transactions in the future, the availability and effectiveness of these transactions may be limited, and we may not be able to successfully hedge our exposure at all. In addition, our foreign currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert RMB into foreign currencies.
EXCHANGE CONTROLS THAT EXIST IN THE PRC MAY LIMIT OUR ABILITY TO UTILIZE OUR CASH FLOW EFFECTIVELY.
We are subject to the PRC’s rules and regulations on currency conversion. In the PRC, the State Administration for Foreign Exchange, or SAFE, regulates the conversion between Renminbi and foreign currencies. Currently, foreign investment enterprises, or FIEs, are required to apply to the SAFE for “Foreign Exchange Registration Certificates for FIEs.” As a result of our ownership of Liuzhou BCT, Liuzhou BCT is a FIE. With such registration certificates, which need to be renewed annually, FIEs are allowed to open foreign currency accounts including a “current account” and “capital account.” Currency conversion within the scope of the “current account,” such as remittance of foreign currencies for payment of dividends, can be effected without requiring the approval of the SAFE. However, conversion of currency in the “capital account,” including capital items such as direct foreign investment, loans and securities, still require approval of the SAFE. Further, any capital contributions to Liuzhou BCT by its offshore shareholder must be approved by the Ministry of Commerce in China or its local counterpart. We cannot assure you that the PRC regulatory authorities will not impose further restrictions on the convertibility of the Renminbi. Any future restrictions on currency exchanges may limit our ability to use our cash flow for the distribution of dividends to our shareholders or to fund operations it may have outside of the PRC.
In August 2008, SAFE promulgated Circular 142, a notice regulating the conversion by FIEs of foreign currency into Renminbi by restricting how the converted Renminbi may be used. Circular 142 requires that Renminbi converted from the foreign currency-dominated capital of a FIE may only be used for purposes within the business scope approved by the applicable government authority and may not be used for equity investments within the PRC unless specifically provided for otherwise. In addition, SAFE strengthened its oversight over the flow and use of Renminbi funds converted from the foreign currency-dominated capital of a FIE. The use of such Renminbi may not be changed without approval from SAFE, and may not be used to repay Renminbi loans if the proceeds of such loans have not yet been used. Violations of Circular 142 may result in severe penalties, including substantial fines as set forth in the SAFE rules.
PRC REGULATIONS RELATING TO THE ESTABLISHMENT OF OFFSHORE SPECIAL PURPOSE COMPANIES BY PRC RESIDENTS MAY SUBJECT OUR PRC RESIDENT SHAREHOLDERS TO PERSONAL LIABILITY AND LIMIT OUR ABILITY TO INJECT CAPITAL INTO OUR PRC SUBSIDIARIES, LIMIT OUR PRC SUBSIDIARIES’ ABILITY TO DISTRIBUTE PROFITS TO US, OR OTHERWISE ADVERSELY AFFECT US.
SAFE issued a public notice in October 2005, or the SAFE notice, requiring PRC residents to register with the local SAFE branch before establishing or controlling any company outside of China for the purpose of capital financing with assets or equities of PRC companies, referred to in the notice as an “offshore special purpose company.” PRC residents that are shareholders of offshore special purpose companies established before November 1, 2005 were required to register with the local SAFE branch before March 31, 2006.The failure of our beneficial owners to timely amend their SAFE registrations pursuant to the SAFE notice or the failure of future beneficial owners of our company who are PRC residents to comply with the registration procedures set forth in the SAFE notice may subject such beneficial owners to fines and legal sanctions and may also limit our ability to contribute additional capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to distribute dividends to our company or otherwise adversely affect our business.
36
BECAUSE CHINESE LAW GOVERNS MANY OF OUR MATERIAL AGREEMENTS, WE MAY NOT BE ABLE TO ENFORCE OUR RIGHTS WITHIN THE PRC OR ELSEWHERE, WHICH COULD RESULT IN A SIGNIFICANT LOSS OF BUSINESS, BUSINESS OPPORTUNITIES OR CAPITAL.
Chinese law governs many of our material agreements, some of which may be with Chinese governmental agencies. We cannot assure you that we will be able to enforce any of our material agreements or that remedies will be available outside of the PRC. The system of laws and the enforcement of existing laws and contracts in the PRC may not be as certain in implementation and interpretation as in the United States. The Chinese judiciary is relatively inexperienced in enforcing corporate and commercial law, leading to a higher than usual degree of uncertainty as to the outcome of any litigation. The inability to enforce or obtain a remedy under any of our future agreements could result in a significant loss of business, business opportunities or capital.
BECAUSE OUR FUNDS ARE HELD IN BANKS IN UNINSURED PRC BANK ACCOUNTS, THE FAILURE OF ANY BANK IN WHICH WE DEPOSIT OUR FUNDS COULD AFFECT OUR ABILITY TO CONTINUE IN BUSINESS.
Funds on deposit at banks and other financial institutions in the PRC are often uninsured. A significant portion of our assets are in the form of cash deposited with banks in the PRC, and in the event of a bank failure, we may not have access to our funds on deposit. Depending upon the amount of money we maintain in a bank that fails, our inability to have access to our cash could impair our operations, and, if we are not able to access funds to pay our suppliers, employees and other creditors, we may be unable to continue in business.
OUR BUSINESS COULD BE SEVERELY HARMED IF THE CHINESE GOVERNMENT CHANGES ITS POLICIES, LAWS, REGULATIONS, TAX STRUCTURE OR ITS CURRENT INTERPRETATIONS OF ITS LAWS, RULES AND REGULATIONS RELATING TO OUR OPERATIONS IN CHINA.
Our business is located in Guangxi province, China and virtually all of our assets are located in China. We generate our sales revenue only from customers located in China. Our results of operations, financial state of affairs and future growth are, to a significant degree, subject to China’s economic, political and legal development and related uncertainties. Our operations and results could be materially affected by a number of factors, including, but not limited to
|
●
|
Changes in policies by the Chinese government resulting in changes in laws or regulations or the interpretation of laws or regulations;
|
|
●
|
Changes in taxation;
|
|
●
|
Changes in employment restrictions;
|
|
●
|
Import duties; and
|
|
●
|
Currency revaluation.
|
Over the past several years, the Chinese government has pursued economic reform policies including the encouragement of private economic activities and greater economic decentralization. If the Chinese government does not continue to pursue its present policies that encourage foreign investment and operations in China, or if these policies are either not successful or are significantly altered, then our business could be harmed. Following the Chinese government’s policy of privatizing many state-owned enterprises, the Chinese government has attempted to augment its revenues through increased tax collection. It also exercises significant control over China’s economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Continued efforts to increase tax revenues could result in increased taxation expenses being incurred by us. Economic development may be limited as well by the imposition of austerity measures intended to reduce inflation, the inadequate development of infrastructure and the potential unavailability of adequate power and water supplies, transportation and communications. In addition, the Chinese government continues to play a significant role in regulating industry by imposing industrial policies.
37
THE CHINESE LAWS AND REGULATIONS WHICH GOVERN OUR CURRENT BUSINESS OPERATIONS ARE SOMETIMES VAGUE AND UNCERTAIN AND MAY BE CHANGED IN A WAY THAT HURTS OUR BUSINESS.
China’s legal system is a civil law system based on written statutes, in which system decided legal cases have little value as precedents, unlike the common law system prevalent in the United States. There are substantial uncertainties regarding the interpretation and application of Chinese laws and regulations, including but not limited to the laws and regulations governing our business, or the enforcement and performance of our arrangements with customers in the event of the imposition of statutory liens, death, bankruptcy and criminal proceedings. The Chinese government has been developing a comprehensive system of commercial laws, and considerable progress has been made in introducing laws and regulations dealing with economic matters such as foreign investment, corporate organization and governance, commerce, taxation and trade. However, because these laws and regulations are relatively new, and because of the limited volume of published cases and judicial interpretation and their lack of force as precedents, interpretation and enforcement of these laws and regulations involve significant uncertainties. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively. We are considered an FIE under Chinese laws, and as a result, we must comply with Chinese laws and regulations. We cannot predict what effect the interpretation of existing or new Chinese laws or regulations may have on our business. If the relevant authorities find us to be in violation of Chinese laws or regulations, they would have broad discretion in dealing with such a violation, including, without limitation: levying fines; revoking our business and other licenses; requiring that we restructure our ownership or operations; and requiring that we discontinue any portion or all of our business.
A SLOWDOWN OR OTHER ADVERSE DEVELOPMENTS IN THE CHINESE ECONOMY MAY MATERIALLY AND ADVERSELY AFFECT OUR CUSTOMERS’ DEMAND FOR OUR SERVICES AND OUR BUSINESS.
All of our operations are conducted in China and all of our revenues are generated from sales to businesses operating in China. Although the Chinese economy has grown significantly in recent years, such growth may not continue. We do not know how sensitive we are to a slowdown in economic growth or other adverse changes in the Chinese economy which may affect demand for our products. A slowdown in overall economic growth, an economic downturn or recession or other adverse economic developments in China may materially reduce the demand for our services and in turn reduce our results of operations.
FAILURE TO COMPLY WITH THE U.S. FOREIGN CORRUPT PRACTICES ACT AND CHINESE ANTI-CORRUPTION LAWS COULD SUBJECT US TO PENALTIES AND OTHER ADVERSE CONSEQUENCES.
Our executive officers, employees and other agents may violate applicable law in connection with the marketing or sale of our products, including China’s anti-corruption laws and the U.S. Foreign Corrupt Practices Act, or the FCPA, which generally prohibits United States companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. In addition, we are required to maintain records that accurately and fairly represent our transactions and have an adequate system of internal accounting controls. Foreign companies, including some that may compete with us, are not subject to these prohibitions, and therefore may have a competitive advantage over us. The PRC also strictly prohibits bribery of government officials. However, corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in the PRC.
While we intend to implement measures to ensure compliance with the FCPA and Chinese anti-corruption laws by all individuals involved with our company, our employees or other agents may engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations. In addition, our brand and reputation, our sales activities or the price of our ordinary shares could be adversely affected if we become the target of any negative publicity as a result of actions taken by our employees or other agents.
38
THE IMPLEMENTATION OF THE NEW PRC EMPLOYMENT CONTRACT LAW AND INCREASES IN THE LABOR COSTS IN CHINA MAY HURT OUR BUSINESS AND PROFITABILITY.
A new employment contract law became effective on January 1, 2008 in China. It imposes more stringent requirements on employers in relation to entry into fixed-term employment contracts, recruitment of temporary employees and dismissal of employees. In addition, under the newly promulgated Regulations on Paid Annual Leave for Employees, which also became effective on January 1, 2008, employees who have worked continuously for more than one year are entitled to paid vacation ranging from 5 to 15 days, depending on the length of the employee’s service. Employees who waive such vacation entitlements at the request of the employer will be compensated for three times their normal daily salaries for each vacation day so waived. As a result of the new law and regulations, our labor costs may increase. There is no assurance that disputes, work stoppages or strikes will not arise in the future. Increases in the labor costs or future disputes with our employees could damage our business, financial condition or operating results.
UNDER THE PRC EIT LAW, WE, INGENIOUS AND/OR FOREVER WELL MAY BE CLASSIFIED AS A “RESIDENT ENTERPRISE” OF THE PRC. SUCH CLASSIFICATION COULD RESULT IN TAX CONSEQUENCES TO US, OUR NON-PRC RESIDENT SHAREHOLDERS, INGENIOUS AND FOREVER WELL.
On March 16, 2007, the National People’s Congress approved and promulgated a new tax law, the PRC Enterprise Income Tax Law, or “EIT Law,” which took effect on January 1, 2008. Under the EIT Law, enterprises are classified as resident enterprises and non-resident enterprises. An enterprise established outside of China with “de facto management bodies” within China is considered a “resident enterprise,” meaning that it can be treated in a manner similar to a Chinese enterprise for enterprise income tax purposes. The implementing rules of the EIT Law define “de facto management bodies” as a managing body that in practice exercises “substantial and overall management and control over the production and operations, personnel, accounting, and properties” of the enterprise; however, it remains unclear whether the PRC tax authorities would deem our managing body as being located within China. Due to the short history of the EIT Law and lack of applicable legal precedents, the PRC tax authorities determine the PRC tax resident treatment of a foreign company on a case-by-case basis.
If the PRC tax authorities determine that we, Ingenious and/or Forever Well are a “resident enterprise” for PRC enterprise income tax purposes, a number of PRC tax consequences could follow. First, we, Ingenious and/or Forever Well could be subject to the enterprise income tax at a rate of 25 percent on our, Ingenious’ and/or Forever Well’s worldwide taxable income, as well as PRC enterprise income tax reporting obligations. Second, under the EIT Law and its implementing rules, dividends paid between “qualified resident enterprises” are exempt from enterprise income tax. As a result, if we, Ingenious and Forever Well are treated as PRC “qualified resident enterprises,” all dividends paid from Liuzhou BCT to us (through Forever Well and Ingenious) should be exempt from PRC tax.
Finally, the new “resident enterprise” classification could result in a situation in which a 10 percent PRC tax is imposed on dividends we pay to our non-PRC stockholders that are not PRC tax “resident enterprises” and gains derived by them from transferring our common stock, if such income is considered PRC-sourced income by the relevant PRC authorities. In such event, we may be required to withhold a 10 percent PRC tax on any dividends paid to non-PRC resident stockholders. Our non-PRC resident stockholders also may be responsible for paying PRC tax at a rate of 10 percent on any gain realized from the sale or transfer of our common stock in certain circumstances. We would not, however, have an obligation to withhold PRC tax with respect to such gain.
39
Moreover, the State Administration of Taxation (“SAT”) released Circular Guoshuihan No. 698 (“Circular 698”) on December 15, 2009 that reinforces the taxation of non-listed equity transfers by non-resident enterprises through overseas holding vehicles. Circular 698 addresses indirect share transfers as well as other issues. Circular 698 is retroactively effective from January 1 2008. According to Circular 698, where a foreign (non-PRC resident) investor who indirectly holds shares in a PRC resident enterprise through a non-PRC offshore holding company indirectly transfers equity interests in a PRC resident enterprise by selling the shares of the offshore holding company, and the latter is located in a country or jurisdiction where the effective tax burden is less than 12.5 percent or where the offshore income of his, her, or its residents is not taxable, the foreign investor is required to provide the PRC tax authority in charge of that PRC resident enterprise with certain relevant information within 30 days of the transfer. The tax authorities in charge will evaluate the offshore transaction for tax purposes. In the event that the tax authorities determine that such transfer is abusing forms of business organization and a reasonable commercial purpose for the offshore holding company other than the avoidance of PRC income tax liability is lacking, the PRC tax authorities will have the power to re-assess the nature of the equity transfer under the doctrine of substance over form. A reasonable commercial purpose may be established when the overall international (including U.S.) offshore structure is set up to comply with the requirements of supervising authorities of international (including U.S.) capital markets. If the SAT’s challenge of a transfer is successful, it may deny the existence of the offshore holding company that is used for tax planning purposes and subject the seller to PRC tax on the capital gain from such transfer. Since Circular 698 has a short history, there is uncertainty as to its application. We (or a foreign investor) may become at risk of being taxed under Circular 698 and may be required to expend valuable resources to comply with Circular 698 or to establish that we (or such foreign investor) should not be taxed under Circular 698, which could have a material adverse effect on our financial condition and results of operations (or such foreign investor’s investment in us).
If any such PRC tax applies, a non-PRC resident investor may be entitled to a reduced rate of PRC tax under an applicable income tax treaty and/or a deduction against such investor’s domestic taxable income or a foreign tax credit against such investor’s domestic income tax liability (subject to applicable conditions and limitations). In the case of a U.S. Holder (as below), if a PRC tax applies to dividends paid on our common stock, or to gain from the disposition of our common stock, such tax should be treated as a foreign tax eligible for a deduction from such holder’s U.S. federal taxable income or a foreign tax credit against such holder’s U.S. federal income tax liability (subject to applicable conditions and limitations). In addition, the U.S. Holder should be entitled to certain benefits under the Agreement between the Government of the United States of America and the Government of the People’s Republic of China for the Avoidance of Double Taxation and the Prevention of Tax Evasion with Respect to Taxes on Income (the “U.S.-PRC Tax Treaty”), including the treatment of any such income as arising in the PRC for purposes of calculating such foreign tax credit, if such holder is considered a resident of the United States for the purposes of the U.S.-PRC Tax Treaty. Prospective investors should consult with their own tax advisors regarding the applicability of any such taxes, the effects of any applicable income tax treaties, and any available foreign tax credits. For purposes of this annual report, a “U.S. Holder” is a beneficial owner of our common stock that is for U.S. federal income tax purposes, (i) an individual citizen or resident of the United States; (ii) a corporation (or other entity treated as a corporation) that is created or organized (or treated as created or organized) in or under the laws of the United States, any state thereof or the District of Columbia; (iii) an estate whose income is includible in gross income for U.S. federal income tax purposes regardless of its source; or (iv) a trust if (i) a U.S. court can exercise primary supervision over the trust’s administration and one or more U.S. persons are authorized to control all substantial decisions of the trust, or (ii) it has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
IT MAY BE DIFFICULT TO AFFECT SERVICE OF PROCESS AND ENFORCEMENT OF LEGAL JUDGMENTS UPON OUR COMPANY AND OUR OFFICERS AND DIRECTORS BECAUSE THEY RESIDE OUTSIDE THE UNITED STATES.
As our operations are presently based in PRC and a majority of our directors and all of our officers reside in PRC, service of process on our company and such directors and officers may be difficult to effect within the United States. Also, our main assets are located in PRC and any judgment obtained in the United States against us may not be enforceable outside the United States.
40
Risks Associated with our Common Stock in General
OUR SHARES OF COMMON STOCK HAVE LITTLE TRADING AND THERE CAN BE NO ASSURANCE THAT THERE WILL BE AN ACTIVE MARKET FOR OUR SHARES OF COMMON STOCK EITHER NOW OR IN THE FUTURE.
Our shares of common stock have little trading, and the price if traded may not reflect our value. There can be no assurance that there will be an active market for our shares of common stock either now or in the future. The market liquidity will be dependent on the perception of our operating business and any steps that our management might take to bring us to the awareness of investors. There can be no assurance given that there will be any awareness generated. Consequently, investors may not be able to liquidate their investment or liquidate it at a price that reflects the value of the business. If a more active market should develop, the price may be highly volatile. Because there may be a low price for our shares of common stock, many brokerage firms may not be willing to effect transactions in the securities. Even if an investor finds a broker willing to effect a transaction in the shares of our common stock, the combination of brokerage commissions, transfer fees, taxes, if any, and any other selling costs may exceed the selling price. Further, many lending institutions will not permit the use of such shares of common stock as collateral for any loans.
WE MAY BE SUBJECT TO THE PENNY STOCK RULES WHICH WILL MAKE THE SHARES OF OUR COMMON STOCK MORE DIFFICULT TO SELL.
We may be subject now and in the future to the SEC’s “penny stock” rules if our shares of common stock sell below $5.00 per share. Penny stocks generally are equity securities with a price of less than $5.00. The penny stock rules require broker-dealers to deliver a standardized risk disclosure document prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer must also provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information must be given to the customer orally or in writing prior to completing the transaction and must be given to the customer in writing before or with the customer’s confirmation.
In addition, the penny stock rules require that prior to a transaction, the broker dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. The penny stock rules are burdensome and may reduce purchases of any offerings and reduce the trading activity for shares of our common stock. As long as our shares of common stock are subject to the penny stock rules, the holders of such shares of common stock may find it more difficult to sell their securities.
OUR SHAREHOLDERS WILL EXPERIENCE DILUTION AS A RESULT OF THE CONVERSION OF OUR CLASS A WARRANTS OR ISSUANCE OF SECURITIES IN FUTURE FINANCINGS.
As of the date hereof, we have Investor Warrants and Agent Warrants outstanding which are exercisable for 2,111,235 shares of common stock. To the extent such Warrants are exercised, there will be further dilution. In the event that any future financing should be in the form of securities convertible into, or exchangeable for, equity securities, investors may experience additional dilution upon the conversion or exchange of such securities. In addition, the issuance and sale of the Preferred Shares, when converted, will result in the issuance of an additional 9,375,000 shares of common stock.
There are additional authorized but unissued shares of our common stock (and also, after amendment of our certificate of incorporation as described above, additional but unissued shares of our preferred stock) that may be later issued by our management for any purpose without the consent or vote of the stockholders. Our current shareholders may be further diluted in their percentage ownership in the event additional shares are issued by us in the future.
OUR COMMON STOCK IS SUBJECT TO PRICE VOLATILITY UNRELATED TO OUR OPERATIONS.
The market price of our common stock could fluctuate substantially due to a variety of factors, including market perception of our ability to achieve our planned growth, quarterly operating results of other companies in the same industry, trading volume in our common stock, changes in general conditions in the economy and the financial markets or other developments affecting our competitors or us. In addition, the stock market is subject to extreme price and volume fluctuations. This volatility has had a significant effect on the market price of securities issued by many companies for reasons unrelated to their operating performance and could have the same effect on our common stock.
41
WE CANNOT ASSURE YOU THAT OUR COMMON STOCK WILL BECOME LIQUID OR THAT IT WILL BE LISTED ON A SECURITIES EXCHANGE.
Currently, we are quoted on the OTC Bulletin Board, where an investor may find it difficult to obtain accurate quotations as to the market value of our common stock. In addition, if we fail to meet the criteria set forth in SEC regulations, by law, various requirements would be imposed on broker-dealers who sell its securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling our common stock, which may further affect its liquidity.
OUR CHIEF FINANCIAL OFFICER OWNS A SUBSTANTIAL PORTION OF OUR OUTSTANDING COMMON STOCK, WHICH WILL ENABLE HER TO INFLUENCE MANY SIGNIFICANT CORPORATE ACTIONS AND IN CERTAIN CIRCUMSTANCES MAY PREVENT A CHANGE IN CONTROL THAT WOULD OTHERWISE BE BENEFICIAL TO OUR SHAREHOLDERS.
After taking into account the sale of the Preferred Shares, Ms. Zhang controls approximately 41.3% of the total voting rights of our capital stock on a fully-diluted basis and the holders of the Preferred Shares have approximately 17.23% of the voting rights of our capital stock on a fully-diluted basis, so that together these parties control a majority of the voting rights. This could have a substantial impact on matters requiring the vote of the shareholders, including the election of our directors and most of our corporate actions. This control could delay, defer or prevent others from initiating a potential merger, takeover or other change in our control, even if these actions would benefit our shareholders and us. This control could adversely affect the voting and other rights of our other shareholders and could depress the market price of our common stock.
IF WE FAIL TO MAINTAIN AN EFFECTIVE SYSTEM OF INTERNAL CONTROLS, WE MAY NOT BE ABLE TO ACCURATELY REPORT OUR FINANCIAL RESULTS OR PREVENT FRAUD.
Since we operated as a private enterprise without public reporting obligations prior to the Share Exchange, we have committed limited personnel and resources to the development of the external reporting and compliance obligations that would be required of a public company. If our financial reporting systems or procedures fail, we may not be able to provide accurate financial statements on a timely basis or comply with the Sarbanes-Oxley Act of 2002 as it applies to us. Any failure of our ability to provide accurate financial statements could cause the trading price of our common stock to decrease substantially.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not Applicable.
ITEM 2. PROPERTIES
All land in the PRC is owned by the government and cannot be sold to any individual or entity. Instead, the government grants or allocates landholders a “land use right,” which we sometimes refer to informally as land ownership. There are four ways of acquiring land use rights in the PRC:
|
●
|
Grant of the right to use land;
|
|
●
|
Assignment of the right to use land;
|
|
●
|
Lease of the right to use land; and
|
|
●
|
Allocation of the right to use land.
|
42
Granted land use rights are provided by the government in exchange for a grant fee, and carry the rights to pledge, mortgage, lease and transfer the land within the term of the grant. Land is granted for a fixed term, generally 70 years for residential use, 50 years for industrial use, and 40 years for commercial and other use. The term is renewable in theory. Unlike in western nations, granted land must be used for the specific purpose for which it was granted.
Allocated land use rights are generally provided by the government for an indefinite period (usually to state-owned entities) and cannot be pledged, mortgaged, leased, or transferred by the user unless otherwise approved by the competent government authorities. Allocated land can be reclaimed by the government at any time. Allocated land use rights may be converted into granted land use rights upon the payment of a grant fee to the government.
Our land use rights are set forth below:
Liuzhou BCT
Liuzhou BCT owns a 2,753.5 square meter land use rights, the term of which expires on November 14, 2053, located at No. 102, Chengzhan Road, Liuzhou City, Guangxi Province, PRC, as its corporate headquarter. In addition, Liuzhou Baicaotang also owns other properties listed below:
|
1.
|
Approximately 321.7 square meter land use rights, the term of which expires on March 8, 2047, located at Building 2, 197 No. 3 Middle Road, Liuzhou City, Guangxi province;
|
|
2.
|
Approximately 10.7 square meter land use rights, the term of which expires on December 28, 2044, located at No. 1-3 XingLong Building, Zhongshan Middle Rd., Liuzhou City, Guangxi province;
|
|
3.
|
Approximately 75.2 square meter land use rights, the term of which expires on August 4, 2043, located at Floor 1, 197 No. 3 Middle Road, Liuzhou City, Guangxi province;
|
|
4.
|
Approximately 346.85 square meter land use rights, located at No. 10 Shizi Rd., Luzhai Town, Luzhai County, Guangxi province;
|
|
5.
|
Approximately 5,655.6 square meter land use rights, the term of which expires on November 14, 2053, located at No. 6 Changfeng Rd. Liuzhou City, Guangxi province;
|
|
6.
|
Approximately 886.7 square meter land use rights, located at No. 4 Changfeng Rd., Liuzhou City, Guangxi province;
|
|
7.
|
Approximately 1,308.5 square meter land use rights, the term of which expires on August 4, 2053, located at No. 4 Changfeng Rd., Liuzhou City, Guangxi province;
|
|
8.
|
Approximately 1,084.5 square meter land use rights, the term of which expires on November 14, 2053, located at No. 4 Changfeng Rd., Liuzhou City, Guangxi province;
|
|
9.
|
Approximately 1,558.05 square meter land use rights, the term of which expires on May 28, 2057, located at Desheng Village, Litang Town Bingyang County, Guangxi province;
|
|
10.
|
Approximately 380.6 square meter land use rights, the term of which expires on August 4, 2043, located at No. 15 May First Rd,, Liuzhou City, Guangxi province.
|
Hefeng Pharmaceutical
Hefeng Pharmaceutical owns a 44,982.18 square meter business facility located at 3 Development District, Donglan County, which is used as its principal executive offices and plant.
43
BCT Retail
The principal executive offices of BCT Retail are located at No. 102, Chengzhan Road, Liuzhou City, Guangxi Province, PRC. The office space that BCT Retail is using is owned by Liuzhou BCT. BCT Retail has used the office space free of charge and without any lease agreement. BCT Retail currently operates 194 retail chain stores. Most of the chain stores managed by BCT Retail are located in the following towns, counties and municipal cities: Liuzhou City, Ronghui County, Sangjiang County, Liujiang County, Nandan County, Yongfu County, Bama County, Binyang County, Yongan County, Laibin City, Zhaoping Country and Rongan County.
BCT Retail's retail chain stores range in size from 40 square meters to 300 square meters. Except for three retail stores which are owned by Liuzhou BCT and used by BCT Retail free of charge and without any lease agreement, all other chain stores are leased. The monthly rents of the chain stores range from approximately $150.00 to $4,500.00, and most of the leases have a three year term. All the lease agreements have similar terms and provisions.
ITEM 3. LEGAL PROCEEDINGS
We are currently not involved in any litigation that we believe could have a material adverse effect on our financial condition or results of operations. To our knowledge, there is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of our executive officers or any of our subsidiaries, threatened against or affecting our company, our common stock, any of our subsidiaries or any of our companies or their subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
| ITEM 5. MARKET FOR COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is currently traded on the OTCBB under the trading symbol “CNBI.OB”. Prior to March 3, 2010, our common stock was not traded on the OCTBB.
The following table sets forth the high and low bid information for our common stock for the period from January 1, 2010 through December 31, 2011. The OTC Bulletin Board quotations reflect inter-dealer prices, are without retail markup, markdowns or commissions, and may not represent actual transactions.
|
Common Stock
|
||||||||
|
High
|
Low
|
|||||||
|
First quarter 2010 (commencing on March 3, 2010)*
|
$
|
3.00
|
$
|
2.60
|
||||
|
Second quarter 2010
|
$
|
4.23
|
$
|
3.05
|
||||
|
Third quarter 2010
|
$
|
4.00
|
$
|
2.71
|
||||
|
Fourth quarter 2010
|
$
|
3.31
|
$
|
2.44
|
||||
|
First quarter 2011
|
$
|
2.95
|
$
|
1.70
|
||||
|
Second quarter 2011
|
$
|
2.61
|
$
|
1.25
|
||||
|
Third quarter 2011
|
$
|
2.45
|
$
|
1.50
|
||||
|
Fourth quarter 2011
|
$
|
2.40
|
$
|
1.01
|
||||
*Trading commenced on March 3, 2010.
As of March 30, 2012, there were 126 holders of record of our common stock.
44
Dividends
During the fiscal years ended December 31, 2008 and 2007, Liuzhou BCT declared and paid to its original shareholders cash dividends in the aggregate amount of $6,940,000 and $2,044,056, respectively. No dividend was paid in 2009, 2010 or 2011.
The declaration or payment of any future cash dividend will be at the discretion of the Board and will depend upon the earnings (if any), capital requirements and financial position of the company, general economic conditions, and other pertinent factors. It is our present intention not to declare or pay any cash dividends in the foreseeable future, but rather to reinvest earnings (if any), in our business operations. Pursuant to the Preferred Purchase Agreement with Milestone, we must obtain Milestone’s prior consent to the declaration of any dividends with respect to our common stock for so long as Milestone owns 5% of the Preferred Shares issued pursuant to the Preferred Purchase Agreement.
Securities authorized for issuance under equity compensation plans
|
Plan Category
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights
|
Weighted-average exercise price of outstanding options, warrants and rights
|
Number of securities remaining available for future issuances under equity compensation plans (excluding securities reflected in first column)
|
|||||||||
|
Equity compensation plans approved by security holders
|
-
|
$
|
-
|
-
|
||||||||
|
Equity compensation plans not approved by security holders
|
3,215,000
|
$
|
2.69
|
2,726,167.5
|
||||||||
|
Total
|
3,215,000
|
$
|
2.69
|
2,726,167.5
|
||||||||
On June 27, 2010, our board of directors adopted the China BCT Pharmacy Group, Inc. 2010 Omnibus Securities and Incentive Plan (the “Plan”) for the benefit of our employees, nonemployee directors and consultants and the employees, nonemployee directors and consultants of its affiliates, for purposes of assisting us to attract, retain and provide incentives to key management employees and nonemployee directors of, and non-employee consultants to, us and our affiliates, and to align the interests of such individuals with those of our stockholders. The aggregate number of shares of common stock that may be issued under the Plan shall not exceed 12.5% of the total shares of common stock which are issued and outstanding and shares of common stock issuable upon the conversion of any outstanding shares of preferred stock of the Company.
The Company’s 2010 Omnibus Securities and Incentive Plan is described in more detail under the heading “2010 Omnibus Securities and Incentive Plan” in Item 11 below.
Recent Sales of Unregistered Securities
On February 28, 2011, the Company consummated a transaction with Milestone, pursuant to which Milestone purchased 9,375,000 Preferred Shares, par value $.001 per share in a private placement transaction, for an aggregate purchase price of $30 million in cash less expense reimbursement of up to $400,000 for certain reasonable legal and other expenses incurred by Milestone in connection with the Transaction. The sale of the Series A Convertible Preferred Shares was completed pursuant to an agreement between the Company and Milestone, dated January 18, 2011. The securities issued to Milestone became immediately convertible into Common Stock of the Company upon issuance. The initial conversion price of $3.20 per share is subject to adjustment for dilutive transactions, stock splits and other reclassifications and, to the extent provided in a performance measure, for the 2011 financial performance of the Company. The securities will be automatically converted upon consummation of a public offering, of common stock in an amount of $60 million at a time when the Company’s market capitalization is $300 million or greater, it is listed on a national exchange and the offering price is at least two times the then applicable adjusted conversion price. The issuance of securities was exempt from registration pursuant to Section 4(2) of the Securities Act since the issuance of the securities did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the limited number of persons involved in the transaction and the manner of the offering. We believe that the sole investor was a sophisticated and an accredited investor.
Issuer Purchases of Equity Securities
None.
45
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATA
Not applicable.
| ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion should be read along with our financial statements and notes thereto. This section includes a number of forward-looking statements that reflect our current views with respect to future events and financial performance. Forward-looking statements are often identified by words like believe, expect, estimate, anticipate, intend, project and similar expressions, or words which, by their nature, refer to future events. You should not place undue certainty on these forward-looking statements. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from our predictions.
Business Review
We are engaged in pharmaceutical distribution, retail pharmacy and manufacture of pharmaceuticals through our two subsidiaries Liuzhou BCT and Hefeng Pharmaceutical, and BCT Retail, a retail company that we control through a series of contractual arrangements, each of which is located in Guangxi Province, China.
We have integrated operations in the following three business segments.
Pharmaceutical Distribution Segment:
We provide a comprehensive offering of pharmaceutical and healthcare products, including branded and generic prescription medicines, over-the counter medicines, Western and Chinese medicines, as well as personal care products and medical supplies, Chinese herbs, and medical instrument from manufacturers and suppliers through distribution to our customers, including hospitals, retail drug stores, other pharmaceutical wholesalers, clinics, medical centers, and individuals located mainly in Guangxi Province except for the other pharmaceutical wholesalers. Over 8,000 products are distributed in compliance with China’s regulations over the pharmaceutical industry. For the year ended December 31, 2011, our pharmaceutical distribution segment accounted for approximately 74.0% of our total revenue after elimination of inter-segment sales.
Revenue derived from Chinese herbal medicine, family planning products, medical instruments, packaged Chinese medicine drugs, and packaged Western medicine drugs constituted 1.4%, 0.1%, 0.1%, 46.9%, and 51.5% of our pharmaceutical distribution segment’s total revenue in 2011, respectively. The terms of our distribution agreements vary between supplies and vary in terms of payment period, arrangement of delivery, pricing and quality requirements. The general payment period terms vary from advance deposit, to cash on delivery, and to payment ranging from one month to six months from the date of delivery, and the payment can be settled by means of bank collection, remittance, bills payable, and postal check. Our top 10 suppliers in our pharmaceutical distribution segment accounted for 28% of our purchases in 2011.
46
Retail Pharmacy Segment:
BCT Retail operates a large regional retail pharmacy network of stores in Guangxi province, consisting of 194 directly owned retail stores in Guangxi province under the registered name “Baicaotang 百草堂.” Our retail stores provide convenient, high-quality, and professional pharmaceutical services, and supply a wide variety of medicines for selling prescription medicines, over-the-counter medicines, Chinese herbal medicine, roughly processed Chinese herbal medicine, family planning products, and other pharmaceutical products and healthcare products. Revenue from the retail pharmacy segment is generated by cash sales or medi-card reimbursement from the national insurance plan. There is no difference in the sales price of our products in our medi-care qualified stores between cash and medi-card payment. Prior to July 2009, co-payment was collected with respect to certain payments by medi-card. Since July 2009, due to a change in the national insurance plan, no co-payments are required under the national insurance plan. For the year ended December 31, 2011, our retail pharmacy segment accounted for approximately 20.7% of our total revenue after elimination of the inter-segment sales.
The following table sets forth the breakdown of accounts receivable for our retail pharmacy segment as of December 31, 2011 and 2010:
|
(in thousands)
|
As of December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Payor
|
||||||||
|
National Program
|
$
|
609
|
$
|
349
|
||||
Our billing system does not currently have the capacity to generate an aging schedule for all of our receivables. Nevertheless, we are able to create aging schedules by reference to data from our accounting system. The payment from the National Program is made on a lump sum basis after the invoices are approved. Our system is linked with the one at National Program. At the end of each month, we reconcile our records with those of the National Program and send invoices to it for reimbursement. Once amounts are confirmed by the National Program, such amounts due for the current month are available for reimbursement. No allowance for bad debts has been made as medi-card reimbursement under the national insurance program is assured.
Manufacturing Segment:
The manufacturing facility located in Donglan District, Guangxi province is on approximately 40,000 square meters of land of which we own the use rights. Hefeng Pharmaceutical has four product processing units: (1) Chinese herbal medicine abstraction unit for raw material and medicine paste with 670 tons of annual abstraction capacity; (2) granular formulation unit with an annual production capacity of 0.25 billion packages; (3) pill formulation unit with annual production capacity of 0.36 billion pills, and (4) liquid formulation unit with an annual production capacity of 0.1 billion injections. We manufacture and sell both the generic and clinic drugs all over the China. For the year ended December 31, 2011 our manufacturing segment accounted for approximately 5.3% of our total revenue after elimination of the inter-segment sales.
Growth Strategy
We expect to focus on obtaining more contracts for the distribution side of our business. As the centralized bidding for the 2011 contract closed in the second quarter of 2011, so far, to our best knowledge, we have won 2,000-3,000 more product distribution rights with regard to our hospital and clinic clients, which represents approximately a 50% increase compared to our product distribution rights in 2010. In addition, we hope to increase the number of bids awarded to us for counties and cities under the New Rural Cooperate Medicare from the 6 counties and cities that were awarded to us in last year’s bidding to 22 counties, cities and regions.
We continue to focus on rapidly growing our retail pharmacy networks by refining and expanding our existing retail networks in selected markets. We have been consolidating our existing pharmacy stores into larger stores with built-in TCM clinics; we are targeting to have about 4 to 5 such stores built for each larger city and at least one such store built for each county in Guangxi. We have been exploring new and more profitable business models in the retail segment to boost same store sales and increase gross margin as we have experienced increasingly intensified competition from small neighborhood drug stores. In addition, we have also come to recognize limitations of resources (e.g. lack of registered pharmacist, medi-care qualification, quality staff and administrative efforts), which limits our ability to increase the number of stores. We re-decorated and opened our first 3,000 square feet pharmacy stores with built-in TCM clinics in December 2011 where we provide around 5,400 type of products (including drugs, medical instruments, foods, convenience goods and personal care products, etc). Within these pharmacy stores we built at least 3 consultation rooms for TCM doctors to provide free consultations to increase in-store TCM sales and attract more in-store traffic. We re-opened the first drug store with built-in TCM clinic in December 2011, the second such store in March 2012 and we expect to open another 3 to 4 such stores in April 2012. In addition, we have been undergoing a process of applying for a license to supply a chain of National Medicine House facilities; these facilities are in size between community hospitals and clinics. With that license we will be able to provide a wide range of medical services at such locations (but not including injections).
47
We intend to expend a total of RMB 75 million, or approximately $12 million for the re-decorating and re-opening of 20 to30 larger TCM clinic built-in chain stores by the end of 2012. We will put priority focus on quality rather than quantity of the stores that we are going to operate. In addition, we may invest in a location for a logistics center, but wait to build the center until our group revenue reaches $500 million, to achieve economy of scale. We intend to finance the above capital investment either from our own sources of funds or through increased debt facilities with banks.
Principal Factor Affecting Our Operating Results
A number of our pharmaceutical products, primarily those included in the national and provincial medical insurance catalogs, are subject to price controls in the form of fixed retail prices or retail price ceiling controls administered by the Price Control Office under the National Development and Reform Commission, or the NDRC, and provincial price control authorities. Approximately 60% to 70% of our total retail sales are subject to these price controls. The retail prices of these products are also subject to periodic downward adjustments as the PRC governmental authorities seek to make pharmaceutical products more affordable to the general public. Any future price controls or government mandated price reductions may cause potential variability of our earnings and cash flows.
RESULTS OF OPERATIONS
The following table sets forth the key components of our results of operations for the periods indicated.
|
|
Year ended December 31,
|
|||||||||||||||
|
|
2011
|
2010
|
||||||||||||||
|
|
’000 |
% of total sales
|
’000 |
% of total sales
|
||||||||||||
|
Sales revenue
|
$ | 257,487 | 100.0 | $ | 200,813 | 100.0 | ||||||||||
|
Cost of sales
|
195,670 | 76.0 | 151,988 | 75.7 | ||||||||||||
|
Gross profit
|
61,817 | 24.0 | 48,825 | 24.3 | ||||||||||||
|
Operating expenses
|
||||||||||||||||
|
Administrative expenses
|
12,407 | 4.8 | 8,097 | 4.0 | ||||||||||||
|
Selling expenses
|
7,210 | 2.8 | 5,350 | 2.7 | ||||||||||||
|
Total operating expenses
|
19,617 | 7.6 | 13,447 | 6.7 | ||||||||||||
|
|
||||||||||||||||
|
Income from operations
|
42,200 | 16.4 | 35,378 | 17.6 | ||||||||||||
|
Non-operating income (expense)
|
||||||||||||||||
|
Interest income
|
60 | - | 11 | - | ||||||||||||
|
Other income
|
159 | 0.1 | 169 | 0.1 | ||||||||||||
|
Change in fair value of warrant liabilities
|
1,082 | 0.4 | 582 | 0.3 | ||||||||||||
|
Other expenses
|
(503 | ) | (0.2 | ) | (463 | ) | (0.2 | ) | ||||||||
|
Finance costs
|
(892 | ) | (0.3 | ) | (878 | ) | (0.4 | ) | ||||||||
|
Exchange income (loss)
|
121 | - | (24 | ) | - | |||||||||||
|
Total non-operating income (expense)
|
27 | - | (603 | ) | (0.3 | ) | ||||||||||
|
Income before income taxes
|
42,227 | 16.4 | 34,775 | 17.3 | ||||||||||||
|
Income taxes
|
(11,036 | ) | (4.3 | ) | (9,086 | ) | (4.5 | ) | ||||||||
|
Net income
|
31,191 | 12.1 | 25,689 | 12.8 | ||||||||||||
|
Other comprehensive income
|
||||||||||||||||
|
Foreign currency translation adjustments
|
4,481 | 1.7 | 2,317 | 1.2 | ||||||||||||
|
Total comprehensive income
|
$ | 35,672 | 13.9 | $ | 28,006 | 13.9 | ||||||||||
48
The table below sets forth a breakdown of our external segment revenue after elimination of inter-segment sales, and each segment revenue item as a percentage of our total revenue, as well as our inter-segment sales for the years ended December 31, 2011 and December 31, 2010. For the year ended December 31, 2011, we had approximately $39.8 million of inter-segment revenue, which includes approximately $38.6 million in sales from our pharmaceutical distribution segment to our retail pharmacy segment, and approximately $1.2million in sales from our manufacturing pharmacy segment to our distribution pharmacy segment. External segment revenue refers to segment revenue after inter-segment elimination.
|
Year ended December 31,
|
||||||||||||||||
|
2011
|
2010
|
|||||||||||||||
|
’000
|
% of total sales revenue
|
’000
|
% of total sales revenue
|
|||||||||||||
|
External Segment revenue
|
||||||||||||||||
|
Pharmaceutical distribution
|
$
|
190,663
|
74.0
|
$
|
145,390
|
72.4
|
||||||||||
|
Retail pharmacy
|
53,266
|
20.7
|
44,594
|
22.2
|
||||||||||||
|
Manufacturing
|
13,558
|
5.3
|
10,829
|
5.4
|
||||||||||||
|
$
|
257,487
|
100.0
|
$
|
200,813
|
100.0
|
|||||||||||
|
Inter-segment revenue
|
$
|
39,824
|
$
|
31,012
|
||||||||||||
Sales Revenue
During the year ended December 31, 2011, we had sales revenue of $257.5 million, as compared to sales revenue of $200.8 million during the year ended December 31, 2010, an increase of $56.7 million or approximately 28.2%. This increase was mainly attributable to an increase in sales revenue of $45.3 million from our pharmaceutical distribution operations. Sales revenue derived from our pharmaceutical distribution segment amounted to $190.7 million, which accounted for 74.0% of our total sales revenue. In addition, the respective increase of $8.7 million and $2.7 million of our retail and manufacturing segments also contributed to the increase in the sales revenue during the period.
Pharmaceutical Distribution Segment
We derive the majority of our revenue from our pharmaceutical distribution segment. We distribute a comprehensive offering of pharmaceutical and health care products, including branded and generic prescription medicines, over-the-counter medicines, Western and TCM, as well as personal care products and medical supplies.
The following table sets forth revenue from our pharmaceutical distribution operations by category of customers:
|
Year ended December 31
|
||||||||||||||||
|
2011
|
2010
|
|||||||||||||||
|
‘000
|
% of sales
|
‘000
|
% of sales
|
|||||||||||||
|
Hospitals
|
$
|
124,715
|
65.4
|
$
|
100,706
|
69.3
|
||||||||||
|
Other drug stores
|
29,613
|
15.5
|
8,459
|
5.8
|
||||||||||||
|
Clinics and health care centre
|
19,529
|
10.3
|
21,793
|
15.0
|
||||||||||||
|
Distributors and others
|
16,806
|
8.8
|
14,432
|
9.9
|
||||||||||||
|
Total
|
$
|
190,663
|
100.0
|
$
|
145,390
|
100.0
|
||||||||||
49
Revenue from our pharmaceutical distribution segment increased by 31.1% from $145.4 million for the year ended December 31, 2010 to $190.7 million for the year ended December 31, 2011. The increase was the result of increase of volume of sales by approximately $46.6million after offset resulting from the reduction in price levels by approximately $1.3 million. The price change was mainly attributed to the change of price levels paid by hospitals as a result of PRC Government-mandated collective tender process. Further, there were price reductions offerered to the drug stores. The increase in sales revenue from our pharmaceutical distribution segment was due primarily to the increase of $24.0 million in sales to hospitals and $21.2 million in sales to the other drug stores. The increase in sales to hospitals was the result of an increase in the quantity and range of products sold to our existing hospital clients, which, in turn, was attributable to the increase in coverage by the national insurance plan. Further, we have entered into separate distribution agreements with six government-owned hospitals since the third quarter of 2010; our share of purchases granted by these large hospitals has been increased with these new agreements. The increase in sales to other drug stores was the result of the increase in the quantity and range of products to the existing and new stores in the county area and the price reduction offered to these stores. We are able to offer the price reduction as we deal more directly with the pharmaceutical manufacturers rather than with the distributors allowing us to acquire the drugs at a lower price. Further we offer a variety of drugs to the stores to meet the needs of these stores more fully. There was a slight reduction in the sales derived from the health care centres, due to the changes in the townships’ distribution plan from the Basic Drugs Catalogue Plan, which only became effective after the third quarter of 2011. We believe we are only at the early stages of developing this market. Under this new plan, we now have distribution rights to 22 townships compared to the 6 that we previously had.
Revenue from our retail pharmacy segment increased by $9 million from $44.6 million for the year ended December 31, 2010 to 53.6 million for the year ended December 31, 2011. The increase was the result of the reduction in average price level by $1.8 million and an increase in sales volume by approximately$7.2 million. The price change was the result of the increase of cost of merchandise and in the price level of the national insurance catalogue which our retail prices are sold by reference to. The slight increase in our retail prices was the result of the adjustment of our selling prices in accordance with the market and our cost of merchandise. The increase in sales volume was primarily attributed to the sales which resulted from the opening of 27 stores during the period. In addition, revenues derived from 38 stores which were opened since the second half of 2011 also contributed to the growth of the sales. Further, we offer free Chinese medical counseling services and we have developed a membership card program, which also drove the sales revenue increase.
The following table sets forth revenue from our retail pharmacy segment by existing stores and new stores opened during each year:
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
‘000
|
‘000
|
|||||||
|
Existing stores (1)
|
$
|
49,390
|
$
|
33,191
|
||||
|
New stores (2)
|
3,876
|
|
11,403
|
|||||
|
Total (include closed stores)
|
$
|
53,266
|
$
|
44,594
|
||||
|
No. of stores
|
194
|
170
|
||||||
(1) Includes sales derived from the stores which were closed during the fiscal period ended 2011. 14 store were closed during the fiscal period ended 2011 and the sales derived from this said period were $1.73 million, while corresponding sales for the period ended 2010 were $2.18 million approximately.
(2) Represents the 27 stores opened during the year ended December 31, 2011.
50
Manufacturing Segment
Revenue from our manufacturing pharmacy segment increased by $2.8 from $10.8 million for the year ended December 31, 2010 to $13.6 million for the year ended December 31, 2011. The increase was attributed to the change in volume of sales and not affected by price factors.
Cost of Sales
Cost of sales was $195.7 million for the year ended December 31, 2011 as compared to $152.0 million for the year ended December 31, 2010. Our cost of sales consists of the cost of merchandise and raw materials and other costs. Other costs include direct labor, depreciation and other costs. The increase was primarily due to an increase in the costs of purchasing merchandise following the increase in our revenue.
Gross Profit
Gross profit was $61.8 million for the year ended December 31, 2011 as compared to $48.8 million for the year ended December 31, 2010, representing an increase of $13.0 million or approximately 26.6%. The gross profit margin was 24.0% and 24.3% for the years ended December 31, 2011 and December 31, 2010, respectively. The gross profit margin was relatively stable; we maintain the margin between our cost of purchasing pharmaceutical products from our suppliers and our prices of pharmaceutical products sold to our hospital, pharmaceutical distributor and other customers. For hospital customers, we establish a pricing range aligned with our suppliers through the PRC Government-mandated collective tender process. For other pharmaceutical product distributors, we arrange three party negotiations with distributors and suppliers. The slight decrease in profit margin was mainly attributed to the increase in the portion of sales derived from our distribution segment which has lower profit margin than the other segment.
Pharmaceutical Distribution Segment
The gross profit margin for our pharmaceutical distribution segment was approximately 18.6% and 19.4% for the year ended December 31, 2011 and 2010, respectively. The cost of sales includes only the cost of merchandise and the slight reduction was due to an increase of the portion of sales to other retail stores in which those sales had a lower gross profit margin than sales to hospital customers.
Retail Pharmacy Segment
The gross margin for our retail pharmacy segment was approximately 33.4% and 31.1% for the years ended December 31, 2011 and 2010, respectively. The cost of sales includes only the cost of merchandise, and we adjust the retail price in accordance with the cost of merchandise. The slight increase in gross profit margin wasattributable to the sales of higher profit margin products.
Manufacturing Segment
The gross profit margin for our manufacturing segment was approximately 62.7% and 63.0% for the years ended December 31, 2011 and 2010, respectively. The cost of sales consists of cost of merchandise and raw materials and other costs. Other costs include direct labor, depreciation and miscellaneous costs. There was no material price change for the cost of sales and our prices, and the gross profit was stable for each of our products manufactured.
Selling and Administrative Expenses
Selling and administrative expenses totaled $19.6 million for the year ended December 31, 2011, as compared to $13.4 million for the year ended December 31, 2010, representing an increase of $6.2 million or approximately 46.3%.
Selling Expenses
Selling expenses increased by 33.3% from $5.4 million for the year ended December 31, 2010 to $7.2 million for the year ended December 31, 2011. The increase was primarily due to the increase in our marketing staff’s wages and salaries, payment for staff welfare, commission and transportation costs in connection with our increased sales and marketing activities. The percentage of our selling expenses to our total revenue was relatively stable and was 2.8% and 2.7% for the years ended December 31, 2011 and 2010, respectively.
51
Pharmaceutical Distribution Segment
The selling expenses of our pharmaceutical distribution segment increased by 26.1% from $2.3 million for the year ended December 31, 2010 to $2.9 million for the year ended December 31, 2011. The increase was primarily due to the increase in our transportation and loading charges by $0.5 million in connection with our increase in sales volume.
Retail Pharmacy Segment
The selling expenses of our retail pharmaceutical distribution segment increased by 52.2% from $2.3 million for the year ended December 31, 2010 to $3.5 million for the year ended December 31, 2011. The increase was primarily due to the increase in our salaries and staff welfare to retail staff by $1.1 million in connection with our increase in the number of staff upon the opening of 27 stores and those acquired during last fiscal year 2010. Further, the increase in average wages also attributed to the increase.
Manufacturing Segment
The selling expenses of our manufacturing segment increased by 14.3% from $0.7 million for the year ended December 31, 2010 to $0.8 million for the year ended December 31, 2011. The increase was primarily due to the increase in commission paid to regional sales representatives by $0.1 million upon the increase in sales.
Administrative Expenses
Administrative expenses increased by 53.1% from $8.1 million for the year ended December 31, 2010 to $12.4 million for the year ended December 31, 2011. The increase in administrative expenses for the year ended December 31, 2011 was primarily due to an increase in management staff cost and legal and consultancy fees and expenses in connection with public company compliance and SEC requirements by $1.2 million and the increase of share based compensation of $0.4 million. In addition, there was an increase in rental expenditures resulting from the opening of new stores and the amortization of the post-acquisition costs of stores, which was approximately $0.5 million and $0.8 million respectively. The increase was further attributed to the increase in provision for doubtful debts by $0.5 million. The percentage of our administrative expenses to our total revenue increased from 4.0% in 2010 to 4.8% in 2011.
Pharmaceutical Distribution Segment
The administrative expenses of our pharmaceutical distribution segment increased by 33.3% from $3.6 million for the year ended December 31, 2010 to $4.8 million for the year ended December 31, 2011. The increase was primarily due to the increase in our provision for doubtful debts by $0.5 million upon the increase in the size of accounts receivables. Further, there was an increase in professional fees by $0.2 million, entertainment expenses by $0.1 million, repair expenses by $0.1 million. In addition, salaries, post-employment benefits to our staff also increased by $0.1 million upon the expansion of our business and the salary increment in line with the inflation in PRC.
Retail Pharmacy Segment
The administrative expenses of our retail pharmaceutical distribution segment were increased by 141.7 % from $1.2 million for the year ended December 31, 2010 to $2.9 million for the year ended December 31, 2011. The increase was primarily due to amortization of the post-acquisition expenses by $0.8 million in respect of stores acquisitions which took place in our previous fiscal year. In addition, there was an increase in rental charges by $0.5 million upon the increase in the number of new stores opened during the year and those acquired during our last fiscal year. Further, the increase was impacted by the disposal of fixed assets of $0.2 million resulting from store closings.
52
Manufacturing Segment
The administrative expenses of our manufacturing segment were $0.6 million and $0.7 million for the years ended December 31, 2011 and 2010, respectively and there were no material changes during the fiscal years.
Change in Fair Value of Warrants
For the year ended December 31, 2011, we incurred a non-cash gain of $1.1 million, which resulted from the change in fair value of warrants issued to investors in conjunction with the Company’s issuance of warrants in December 2009 and February 2010 pursuant to provisions of FASB ASC Topic 815, “Derivative and Hedging”. The accounting treatment of the warrants resulted from a provision providing anti-dilution protection to the warrant holders.
Income before Income Tax
As a result of the foregoing, our income before income tax increased by 21.3% to $42.2 million for the year ended December 31, 2011 compared to $34.8 million for the same period in 2010. The percentage of our income before income tax to total revenue decreased slightly from 17.3% in 2010 to 16.4% in 2011 and was mainly attributed to the increase in our staff costs related to expanding our business as well as the incurrence of costs in compliance as a result of being a public company. Further there is the occurrence of charges related to the change in fair value of warrants and share based compensation.
Pharmaceutical Distribution Segment
Our income before income tax from distribution operations increased by 24.6% from $23.2 million for the year ended December 31, 2010, to $28.9 million for the year ended December 31, 2011. The profit margin decreased to 15.2% from 16.4%, for the reasons explained above.
Retail Pharmacy Segment
Our income before income tax from the retail pharmacy segment increased by 6.7% from $8.9 million, for the year ended December 31, 2010, to $9.5 million, for the year ended December 31, 2011. The profit margin decreased to 17.8% from 19.9%. The reduction in profit margin is due to the incurrence of the post-acquisition expenses for the acquisition of stores.
Manufacturing Segment
Our income before income tax from manufacturing pharmacy segment operations increased by 29.6% from $5.4 million for the year ended December 31, 2010 to $7.0 million for the year ended December 31, 2011. The profit margin increased to 51.8% from 49.9%.
Net Income
As a result of the above factors, we had net income of $31.2 million for the year ended December 31, 2011 as compared to $25.7 million for the year ended December 31, 2010, representing an increase of $5.5 million or approximately 21.4%. For the year ended December 31, 2011, our net income was impacted by a non-cash gain of $1.1 million as a result of change of fair value of warrant liabilities and non-cash expenses from share-based compensation of $1.6 million. Excluding this $0.5 million net of non-cash charges, our net income for the year ended December 31, 2011 would have been $31.7 million, representing an increase of 19.6% from the same period in 2010.
53
Earnings per Share
For the fiscal year ended December 31, 2011, our basic earnings per share was $0.72, representing an increase of 7.5%, compared to the same period in 2010. For the year ended December 31, 2010, our basic earnings per share was $0.67.
Liquidity and Capital Resources
Liquidity is a measurement of our ability to meet potential cash requirements, including ongoing commitments to repay borrowings, fund operations, acquisitions, and expansion. In addition to cash on hand, our primary sources of funds for liquidity during 2011 consisted of cash generated from operations and proceeds received from placement of preferred stock. As of December 31, 2011, $9.0 million of our bank indebtedness was due within one year and $0.2 million was due beyond one year. During the year ended December 31, 2011 we did not experience any difficulties in renewing our bank loans with our lenders.
Restricted Cash and Cash equivalents
Our restricted cash consists of collateral we provide for bills payable. As of December 31, 2011 and December 31, 2010, our restricted cash was approximately $1.1 million and $1.3 million respectively. As of December 31, 2011 and 2010 we had cash of $31.5 million and $20.2 million, respectively, excluding restricted cash.
We believe that our existing sources of liquidity, along with cash expected to be generated from our operating services will be sufficient to fund our operations, anticipated capital expenditures, working capital and other financing requirements (other than acquisitions) for at least the next twelve months. We will continue to monitor our expenditures and cash flow position.
|
For the Year
|
For the Year
|
|||||||
|
Ended
|
Ended
|
|||||||
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
’000
|
‘000
|
|||||||
|
Net cash (used in) /provided by operating activities
|
$
|
(5,781)
|
$
|
15,889
|
||||
|
Net cash provided by (used in) investing activities
|
(7,444)
|
(9,169)
|
||||||
|
Net cash provided by (used in) financing activities
|
22,810
|
(528)
|
||||||
|
Foreign currency translation
|
1,738
|
661
|
||||||
|
Net increase in cash and equivalents
|
11,323
|
6,853
|
||||||
|
Cash and cash equivalents, beginning of year
|
20,157
|
13,304
|
||||||
|
Cash and cash equivalents, end of year
|
$
|
31,480
|
$
|
20,157
|
||||
Operating Activities
We primarily derive our cash flow from operating activities from the sale of our products and services in our three segments. Our cash used in operating activities is primarily from the purchase of raw materials and products, distribution and selling expenses and general administrative expenses and taxes. Cash flows from our operations can be significantly affected by factors such as the timing of payment of accounts receivables and the payment of our accounts payables to suppliers.
Cash from operating activities is primarily affected by our pharmaceutical distribution segment, which has a high demand on working capital while the retail pharmacy segment generates primarily cash and a minority of accounts receivable pending reimbursements for medi-card payments.
Cash used in operating activities was $5.7 million for the year ended December 31, 2011 compared to $15.9 million provided for fiscal 2010, representing an increase of $21.6 million or approximately 35.8%. Operating cash flows for 2011 reflects primarily net cash receipts derived from business operations. The reduction in net cash from operating activities was primarily attributable to the slowdown of accounts receivable collection, which increased from 95 days to 133 days. However, this slowdown of payment of accounts receivable has no impact on our need to continue our existing pattern of payment of our accounts payable, which we maintain according to historic patterns in order to secure the constant supply of the drugs and the price we get from the vendors. The effect of this is, to some extent, offset by the contribution of the cash inflow from the retail segment.
54
For our pharmacy distribution segment, accounts receivable turnover days for the year ended December 31, 2011 were 170 compared to 121days in 2010. The increase in turnover days was attributable to several factors. First, as our primary sales were derived from hospitals and these sales increased, we tended to extend and grant longer credit terms to them. Hospitals and most of the community service centres, which are owned by the PRC government, have comparatively longer payment cycles. In the PRC, all hospitals and the community health care centers are owned or controlled by the PRC government. We believe that it is highly unlikely that a liquidation of one of our hospital and community health care center customers will occur, and that the recovery of accounts receivable from them are highly secure. Therefore, it is our customary practice to grant a longer credit period. There have been no instances of write-offs of any receivables owed by a hospital or the community health care centers. In addition to this, our distributor customers still take a high percentage of receivables even though the percentage of sales dropped. In fact, it is our practice to offset the account receivable and account payable for each distributor. It is common that the distributors act as both the vendor and customers of the company. We are allowed to offset the amount yearly upon the mutual consent over the amounts owed by and to the parties. As we still owe money to these distributors, we continuously verify the balances owed between us. As of December 31, 2011, there were a number of debts remaining to be offset, resulting in a relatively high balance of receivables from distributors. Further, as the sales derived from other retail drug stores grew rapidly by 234% from 2010, we, to some extent, allowed a longer credit period to them during the 2011 year for some sales, which previously were on terms of cash on delivery in the prior year.
For our retail segment, accounts receivable turnover days remained unchanged: 3 days for both the years ended December 31, 2011 and December 31, 2010. We generally receive cash from the customer at the retail store except for our medi-care qualified stores. The accounts receivable comprised only the medi-card reimbursement under the national program.
For our manufacturing segment, the accounts receivable turnover days were 134 days in 2011 compared to 124 days in 2010. The increase was attributed to the extension of a longer credit period to particular customers, which were newly developed in 2011.
Investing Activities
Our cash flow from investing activities primarily consists of purchases of property, plant and equipment involving mainly the openings of new retail locations and increase in long-term deposits. Cash used in investing activities was $7.4 million for 2011, compared to $9.2 million of cash used in investing activities for 2010, representing a decrease of $1.8 million. The reduction in cash used was primarily due to lack of drug store acquisitions compared to the year ended December 2010.The effect of the reduction was offset partly by the increase in purchase of property, plant and equipment of $3.5 million.
Financing Activities
Our cash from financing activities is derived primarily from placements of preferred stock and bank loan activities. Cash provided by financing activities was $22.8 million for 2011, compared to $0.6 million used in financing activities for 2010, an increase of cash from financing activities by $23.4 million. The increase of cash from financing activities was primarily due to the placement of preferred stock of $29.5 million while only $2.3 million was received from the private placement of our securities for the year ended December 31, 2010. However, the increase was offset by the increase in net repayment of bank loans during the year. The net repayment was $2.0 million for the year ended December 31, 2011 while only $0.3 million had been repaid for the year ended December 31, 2010.
55
Working Capital
Our working capital as of December 31, 2011 was $110.0 million. Our working capital is critical to our financial performance. We must maintain sufficient liquidity and financial flexibility to continue our daily operations. Our sales practices with hospitals in our pharmaceutical distribution segment, which have a longer term of payment when compared with other customers and the increase in the proportion of our sales to hospitals have resulted in a significant demand for working capital.
The following table sets forth accounts receivable from our pharmaceutical distribution operations by category of customers:
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
‘000
|
‘000
|
|||||||
|
Hospitals
|
$
|
73,984
|
$
|
45,476
|
||||
|
Other drug stores
|
19,971
|
2,971
|
||||||
|
Clinics and health care centre
|
11,634
|
8,434
|
||||||
|
Distributors and others
|
7,245
|
7,548
|
||||||
|
Total
|
$
|
112,834
|
$
|
64,429
|
||||
In order to satisfy and fully implement our growth strategies, we may need to raise capital, through equity or debt financing, either in a private placement or a public offering. However, there are no assurances that such financing will be available or available on terms acceptable to us. To the extent that we require additional financing in the future and are unable to obtain such additional financing, we may not be able to fully implement our growth strategies.
Borrowings and Credit Facilities
The short-term bank borrowings outstanding as of December 31, 2011 were $7.2 million while long term bank borrowings outstanding as of December 31, 2011 were $2.0 million. The loans are from various financial institutions and represent the maximum amount of each facility except for a bills payable facility. The short term loans bore an average interest rate of 7.73% per annum, which was adjusted currently or quarterly in accordance with the loan rate of the People’s Bank of China. These loans do not contain any financial covenants or restrictions. The loans are secured by the land use rights, property, plant and equipment of the Company and properties from related-parties.
The short-term borrowings have one year terms and expire at various times throughout the year. These facilities contain no specific renewal terms.
The long-term borrowings have three to seven years terms. Part of the borrowings mature at various times over the next years and part of the borrowings are repayable by monthly installments within the four years before maturity. The loans bear an interest rate from 7.68% to 8.32% which were adjusted currently on an annual basis in accordance with the loan rate of the People’s Bank of China
These loans do not contain any financial covenants or restrictions. The loans are secured by the land use rights, property, plant and equipment of the Company and properties from related-parties.
In addition to bank borrowings mentioned above, we have trade credit facilities secured by land use rights, property and equipment of the Company.
Critical Accounting Estimates
This section should be read together with the Summary of Significant Accounting Policies included as Notes to the consolidated financial statements included in our financial statements. Our consolidated financial information has been prepared in accordance with generally accepted accounting principles in the United States, which requires us to make judgments, estimates and assumptions that affect (1) the reported amounts of our assets and liabilities, (2) the disclosure of our contingent assets and liabilities at the end of each fiscal period and (3) the reported amounts of revenues and expenses during each fiscal period. We continually evaluate these estimates based on our own historical experience, knowledge and assessment of current business and other conditions, our expectations regarding the future based on available information and reasonable assumptions, which together form our basis for making judgments about matters that are not readily apparent from other sources. Since the use of estimates is an integral component of the financial reporting process, our actual results could differ from those estimates. Some of our accounting policies require a higher degree of judgment than others in their application.
56
When reviewing our financial statements, you should consider (1) our selection of critical accounting policies, (2) the judgment and other uncertainties affecting the application of those policies, and (3) the sensitivity of reported results to changes in conditions and assumptions. We believe the following accounting policies involve the most significant judgment and estimates used in the preparation of our consolidated financial statements.
Goodwill
We account for goodwill in accordance with the provisions of FASB ASC 805, “Goodwill and Intangible Assets” (“FASB ASC 805”). We conduct impairment tests on an annual basis and, in addition, if we notice any indication of impairment, we conduct such test immediately. The application of the impairment test requires judgment, including the identification of reporting units, assignment of assets and liabilities to reporting units and the determination of the fair value of each reporting unit. Fair value is primarily determined by computing the future discounted cash flows expected to be generated by the reporting unit that are believed to be reasonable under current and forecasted circumstances, the results of which form the basis for making judgments about carrying values of the reported net assets of our reporting units. We conducted an impairment test as of December 31, 2011 and recorded impairment loss of $37,355.
Long-lived Assets
Long-lived assets are tested for impairment in accordance with FASB ASC 360-10-45 “Impairment or Disposal of Long-Lived Assets. We periodically evaluate potential impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The application of the impairment test requires judgment inclusive of the future cash flow attributable from the use of the asset. If the asset is determined not to be recoverable, it is considered to be impaired and the impairment is recognized.
Depreciation
Property, plant and equipment are depreciated on a straight-line basis over their estimated useful lives. We determine the estimated useful lives, residual values and related depreciation charges for our property, plant and equipment. This estimate is based on the historical experience of the actual useful lives and residual values of property, plant and equipment of similar nature and functions. We will revise the depreciation charge where useful lives and residual values are different from those previously estimated, or we will write-off or write-down technically obsolete or non-strategic assets that have been abandoned or sold.
Allowances for Doubtful Accounts
We establish the general provisioning policy to make allowance equivalent to 40% of gross amount of accounts receivable due between half and one year and 100% of gross amount of accounts receivable due over 1 year. Additional specific provision is made against accounts receivable whenever they are considered to be doubtful. We make judgments about the customer’s ability to pay its outstanding invoices on a timely basis and whether its financial position might deteriorate significantly in the future affecting its capability for payment.
Allowances for Inventories
In assessing the ultimate realization of inventories, we make judgments as to future demand requirements compared to current or committed inventory levels. We estimate the demand requirements based on market conditions, forecasts of demand by our customers, sales contracts and orders in hand. As of December 31, 2011, 0.6% of our inventory will expire within 1-6 months, 4.2% will expire within 7 to 9 months time, 10% will expire within 10 to 12 months time, 85.2% of inventory will expire in over 1 year. We estimate the extent of provision to be made should the inventory fall within 6 months before the expiration upon assessing the capability of the sales campaign and the possible return of drugs to the vendors. As for the drugs which will expire in over half a year’s time, we judge that the drugs are still marketable and estimate the provision upon the future demand and the current inventory level.
57
Recognition of Revenue
Revenue is recognized when the following criteria under FASB ASC 605 “Revenue Recognition” are met :
|
1)
|
Persuasive evidence of an arrangement exists;
|
|
2)
|
Delivery has occurred or services have been rendered;
|
|
3)
|
The seller’s price to the buyer is fixed or determinable; and
|
|
4)
|
Collectability is reasonably assured.
|
We base our estimates on historical results, taking into consideration the type of customer, the type of transaction and the specifics of each arrangement.
Pharmaceutical Distribution Segment
We sell a range of pharmaceutical products to various customers and the majority of revenue results from the contracts signed with various distributors and government-owned hospitals. Revenue from pharmaceutical distribution operations is recognized when substantially all of the usual risks and rewards of ownership of the inventory have been transferred to the buyer. Generally, this occurs when the inventory is shipped to the customer. Currently, we do not have any sales on consignment and we do not use consignment sales as our sales method. In the absence of the right of return clause, we estimate that the product return was insignificant other than for returns because of damage arising from delivery. We are not obligated to accept the return should the inventory kept by the customers be excessive or expire without being sold by them. We do accept return of inventory damaged during delivery. We grant credit to customers with proven payment records. Collectibility is assessed by background checks for new customers.
Retail Pharmacy Segment
We operate a chain of retail stores for selling the pharmacy products. Revenue from operation of these stores is recognized when sales occur. We estimate that no significant post-delivery obligations exist. Retail sales are paid in cash or medi-insurance card, in which the reimbursement is assured as it is run by the national government agency. No return is allowed after sales.
Manufacturing Segment
The revenue recognition criteria used with our manufacturing pharmacy segment are the same as under our pharmaceutical distribution segment except that the only customers are distributors.
During the fiscal year ended December 31, 2011, $1,223,172 worth of goods was returned as a result of damage. We have not sold goods to customers as a result of incentives. We do not grant sales discount or any allowances to customers if they don’t re-sell their goods before the date of expiration. No return of goods is allowed except when the goods are damaged during the delivery process. As the return of goods is not allowed, we assess and estimate only the return arising from damage during the delivery process and the estimate of return is negligible. Thus, we do not assess any return derived from the levels of inventory in the distribution channel, estimated shelf life, and the introduction of new products as these factors are not relevant to us for the estimate of return.
Off Balance Sheet Arrangements
We have no off balance sheet arrangements.
58
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and supplementary data required by this item are included in Part IV, Item 15 of this Report.
| ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
On July 15, 2010, we engaged PKF, Certified Public Accountants, A Professional Corporation, Glendale, California, a member firm of PKF International Limited network of legally independent firms (“PKF California”), as our principal accountant, and dismissed PKF, Certified Public Accountants, Hong Kong, China, a member firm of PKF International Limited network of legally independent firms (“PKF HK”), from that role.
Pursuant to Item 304(a) of Regulation S-K under the Securities Act of 1933, as amended, and under the Securities Exchange Act of 1934, as amended:
|
(a)
|
(i)
|
PKF HK was terminated as our independent registered public accounting firm effective on July 15, 2010.
|
|
|
(ii)
|
For the our most recent fiscal years, PKF HK’s reports on the financial statements did not contain any adverse opinions or disclaimers of opinion, and were not qualified or modified as to uncertainty, audit scope, or accounting principles, other than for a going concern.
|
||
|
(iii)
|
The termination of PKF HK and engagement of PKF California was approved by our audit committee.
|
||
|
(iv)
|
We and PKF HK did not have any disagreements with regard to any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure for the audited financials for our two most recent fiscal years, and subsequent interim period through the date of dismissal, which disagreements, if not resolved to the satisfaction of PKF HK, would have caused it to make reference to the subject matter of the disagreements in connection with its reports.
|
||
|
(v)
|
During two most recent our fiscal years, and subsequent interim period through the date of dismissal, we did not experience any reportable events.
|
||
|
(vi)
|
The reports of PKF HK on our financial statements did not contain an adverse opinion or a disclaimer of an opinion, and were not qualified or modified as to uncertainty, audit scope or accounting principles.
|
||
|
(b)
|
(i)
|
On July 15, 2010, we engaged PKF California to serve as our independent registered public accounting firm.
|
|
|
(ii)
|
Prior to engaging PKF California, we had not consulted PKF California regarding the application of accounting principles to a specified transaction, completed or proposed, the type of audit opinion that might be rendered on our financial statements or a reportable event, nor did we consult with PKF California regarding any disagreements with our prior auditor on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of the prior auditor, would have caused it to make a reference to the subject matter of the disagreements in connection with its reports.
|
||
|
(iii)
|
We did not have any disagreements with PKF California and therefore did not discuss any past disagreements with PKF California.
|
||
|
(c)
|
PKF HK furnished us with a letter addressed to the SEC stating that it agrees with the statements made by us regarding PKF HK.
|
59
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures.
We maintain “disclosure controls and procedures,” as such term is defined under Exchange Act Rule 13a-15(e), that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosures. In designing and evaluating the disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives and in reaching a reasonable level of assurance our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
We have carried out an evaluation as required by Rule 13a-15(d) under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2011. Based on an evaluation of our disclosure controls and procedures as of December 31, 2011 covered by this report (and the financial statements contained in the report), our Chief Executive Officer and Chief Financial Officer have determined that our current disclosure controls and procedures were effective.
Internal Controls Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Securities Exchange Act of 1934, as amended. Our Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements in accordance with U.S. generally accepted accounting principles.
Because of its inherent limitations, a system of internal control over financial reporting can provide only reasonable assurance with respect to consolidated financial statement preparation and presentation and may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that degree of compliance with the policies or procedures may deteriorate.
In connection with the filing and preparation of this annual report and after the implementation of the remediation program described above, management assessed the effectiveness of our internal control over financial reporting as of December 31, 2011. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control — Integrated Framework. Based on management’s assessment using those criteria, management concluded that our internal control over financial reporting was effective as of December 31, 2011.
Changes in Internal Control over Financial Reporting. There were no changes in our internal control over financial reporting that occurred during the fourth quarter of fiscal year 2011 that have materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
60
ITEM 9B. OTHER INFORMATION
None
PART III
ITEM 10. DIRECTORS AND EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table sets forth the name and age of officers and directors as of March 30, 2012 . Our executive officers are elected annually by our board of directors. Our executive officers hold their offices until they resign, are removed by the board, or his or her successor is elected and qualified.
Directors and Executive Officers
|
Name
|
Age
|
Position
|
||
|
Huitian Tang
|
50
|
Chairman & Chief Executive Officer
|
||
|
Xiaoyan Zhang
|
38
|
Director & Chief Financial Officer
|
||
|
Kam-Cheung Chin
|
56
|
Independent Director
|
||
|
Simon Choi
|
51
|
Independent Director
|
||
|
Manwai Chiu
|
50
|
Independent Director
|
||
|
Yunli Lou
|
44
|
Director
|
Mr. Huitian Tang
Mr. Tang was appointed as our CEO on December 30, 2009 and as our Chairman on January 14, 2010. Mr. Tang is a registered pharmacist and has been the president of Liuzhou BCT since 2001. In 1993, he was hired as the general manager by Liuzhou BCT (f/k/a Guangxi Liuzhou Wholesaler) and was promoted to president of the company in 2001. Mr. Tang has over 25 years experience in the traditional Chinese medicine industry. As the president of Liuzhou BCT, Mr. Tang has been responsible for the formulation of strategies, decision-making on investment projects and development directions on the operations and overall business management, and led us successfully through the privatization process in 2001. Prior to his employment with Liuzhou BCT in 1993, Mr. Tang was employed by Guangxi Jinchengjiang Medicine Wholesaler Group from 1983 to 1993 where he was deputy general manager. In July 1983, Mr. Tang received a Bachelor of Chinese Pharmacy from Guangxi Chinese Medicine University. As our CEO, we believe that Mr. Tang’s extensive experience in the traditional Chinese medicine industry and his essential knowledge of the Company’s operations provide him with significant insights into our business which make him qualified to be the Chairman of our board of directors.
Ms. Xiaoyan Zhang
Ms. Zhang joined Liuzhou BCT in May 2008 as our Corporate Strategy VP and was appointed as our CFO on December 30, 2009 and as a director on January 14, 2010. Prior to joining Liuzhou BCT, from 2006 to 2008 she was a corporate finance advisor to First Asia Finance Group, in Hong Kong. Ms. Zhang is an Associate Member of CPA Australia. She received a Masters degree in accounting from Curtin University of Technology, Australia in 2007, an MBA in International Business from CMSD Switzerland in 2001 and a BA (Honors) in Marketing from Portsmouth University, UK in 2004. We believe that Ms. Zhang brings extensive finance experience and vital operational experience to the board of directors.
Mr. Kam-Cheung Chin
Mr. Chin currently has been a solo practicing accountant since August, 2005, and has served as an independent director of Jiahua Stores Holdings Limited, a company listed on the Hong Kong Stock Exchange, since May, 2007. Mr. Chin previously served as the Financial Controller and Company Secretary of Wing Shing International Holdings Ltd.(currently known as Petroasian Energy Holdings), a Hong Kong Stock Exchange listed company, from September 2004 to July 2005. Mr. Chin was a solo practicing accountant from June, 2003 to September 2004, and was the Assistant Manager and Manager of Raymond S.W. Ho & Co., Certified Public Accountants from October, 2000 to May, 2003. Mr. Chin has been an Associate of Chartered Institute of Management Accountants of the United Kingdom since 1986 and has been a Fellow since 1993. Mr. Chin has also been a qualified member of Hong Kong Institute of Certified Public Accountants (the “HKICPA”) since 1987 and has held a Practicing Certificate since 2003. Mr. Chin received his Graduate Diploma of Computing from a long distance program from Deakin University of Australia in 2001 and his Master of Business Administration degree from a special long distance program jointly provided by Deakin University and Association of Professional Engineers, Scientists and Managers Australia (APESMA) in 1998. Mr. Chin received Higher Diploma in Accountancy from Lingnan College in Hong Kong in 1982. Mr Chin has significant experience in various roles as an independent director and a financial controller for various companies. He has served on the board of Jiahua Stores Holdings Limited, a company listed Hong Kong Stock Exchange and worked as financial controller for Petroasian Energy Holdings, also a company listed Hong Kong Stock Exchange. We believe that this experience qualifies him to serve on our board of directors.
61
Mr. Simon Choi
Mr. Choi was appointed as our director on June 7, 2010. Mr. Simon Choi has been International Legal Counsel for TCL, a global TV manufacturer, since February 2005. From 1998 to 2005, Mr. Choi was the Legal Counsel and Company Secretary of Simatelex, a Hong Kong OEM small electrical appliances manufacturer, where he advised on a variety of corporate legal matters. Earlier in his career, Mr. Choi was an Assistant Solicitor of Hastings & Co, Solicitors, where he specialized in banking, corporate finance, general commercial and PRC practice. Mr. Choi is also an active PRC legal advisor to the Hong Kong Electrical Appliances Manufacturers Association, a major trade association in Hong Kong. Mr. Choi received an LLB from Peking University in 1991, an LLM from London University in 1992 and a CPEC in Law from Hong Kong University in 1994. Mr. Choi was admitted as a Solicitor of the Supreme Court of England and Wales in 1998 and as a member of the Institute of Linguists in 1996. We believe that Mr. Choi’s diverse legal background and familiarity with PRC law provides him with a unique and valuable legal perspective from which he helps advise the board of directors.
Mr. Manwai Chiu
Mr. Chiu was appointed as our director on June 7, 2010. Mr. Man Wai Chiu has served as the Chief Operating Officer of Deer Consumer Products, Inc. since May 2007, where he manages sales, sourcing, shipping and accounting. From April 1997 to May 2007, he held various positions at Hamilton Beach Proctor-Silex, Inc., most recently as the Sourcing Director. Mr. Chui received his Bachelor of Laws from the University of London and MBA from Charles Sturt University in Australia. We believe that Mr. Chiu brings vital management and corporate strategy experience to the board of directors.
Ms. Yunli Lou
Ms. Yunli Lou was appointed as our director on March 17, 2011. Ms. Lou was nominated and elected as a director in accordance with the terms of the Preferred Purchase Agreement with Milestone on January 18, 2011 in connection with a sale and purchase of 9,375,000 shares of the Company’s Series A Convertible Preferred Shares. In accordance with the Preferred Purchase Agreement and in connection with the closing thereunder, the Board approved the increase in the size of the board of directors from five to seven members. Since March 2007, Ms. Lou has served, and continues to serve as a director of VisionChina Media Inc. and Ms. Lou has been a managing director of Milestone Capital Partners Limited since 2007, responsible for the firm’s overall management, investor relations as well as deal sourcing and execution. Ms. Lou has been a managing partner of Milestone Capital Management Limited since 2002. Before founding Milestone Capital in 2002, Ms. Lou was a vice president of Merrill Lynch’s direct investment group, where she was responsible for the firm’s investment activities in China. Prior to joining Merrill Lynch in 1995, Ms. Lou worked in the corporate finance division of Goldman Sachs in New York and Hong Kong. Ms. Lou received a bachelor’s degree in economics from Harvard University in 1992. We believe that Ms. Lou’s business and finance experience qualify her to serve on our board of directors.
62
Board Composition
Our board of directors is currently composed of six members. All actions of the board of directors require the approval of a majority of the directors in attendance at a meeting at which a quorum is present. A quorum is a majority of the board of directors.
Family Relationships
There are no family relationships between any of our directors or executive officers and any other directors or executive officers.
Corporate Governance
Code of Ethics
We adopted our Code of Business Conduct and Ethics on June 9, 2010. This Code of Business and Ethics, in accordance with Section 406 of the Sarbanes-Oxley Act of 2002 and Item 406 of Regulation S-K, constitutes our Code of Ethics for our principal executive officer, our principal financial and accounting officer and our other senior financial officers. The Code of Ethics is intended to promote honest and ethical conduct, full and accurate reporting, and compliance with laws as well as other matters. A copy of our Code of Ethics is available online on our website at http://is.stockpr.com/chinabct/governance-documents. A printed copy of the Code of Ethics may be obtained free of charge by writing to China BCT Pharmacy Group, Inc., No. 102 Chengzhan Road, Liuzhou City, Guangxi province, China.
Director Independence
We use the definition for independence set forth in Rule 5605(a)(2) of the NASDAQ Marketplace Rules to determine that we have a majority of the board of directors comprised of “independent” directors, and to determine that the committees of our board of directors are comprised of “independent” directors. Based on those standards set forth in Rule 5605(a)(2), the board of directors has determined that Kam-Cheung Kim, Simon Choi and Man Wai Chiu are independent directors.
Committees of the Board of Directors
Audit Committee
Our Audit Committee consists of Kam-Cheung Kim, Simon Choi and Man Wai Chiu, each of whom is independent. The Audit Committee assists the board of directors oversight of (i) the integrity of our financial statements, (ii) our compliance with legal and regulatory requirements, (iii) the independent auditor’s qualifications and independence, and (iv) the performance of our internal audit function and independent auditor, and prepares the report that the Securities and Exchange Commission requires to be included in our annual proxy statement. The Audit Committee operates under a written charter. Mr. Chin is the Chairman of our Audit Committee.
The board of directors determined that Mr. Lee possesses accounting or related financial management experience that qualifies him as financially sophisticated within the meaning of Rule 4350(d)(2)(A) of the Nasdaq Marketplace Rules and that he is an “audit committee financial expert” as defined by the rules and regulations of the SEC.
Nominating and Corporate Governance Committee
The purpose of the Nominating and Corporate Governance Committee is to assist the board of directors in identifying qualified individuals to become members of our board of directors, in determining the composition of the board of directors and in monitoring the process to assess board effectiveness. Each of Kam-Cheung Kim, Simon Choi and Man Wai Chiu are members of the Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee operates under a written charter. Mr. Choi is the Chairman of the Nominating Committee.
63
Compensation Committee
The Compensation Committee is responsible for overseeing and, as appropriate, making recommendations to the board of directors regarding the annual salaries and other compensation of our executive officers and general employees and other policies, and for providing assistance and recommendations with respect to our compensation policies and practices. Each of Kam-Cheung Kim, Simon Choi and Man Wai Chiu are members of the Compensation Committee. The Compensation Committee operates under a written charter. Mr. Choi is the Chairman of the Compensation Committee.
Involvement in Certain Legal Proceedings
There have been no events under any bankruptcy act, no criminal proceedings and no judgments, injunctions, orders or decrees material to the evaluation of the ability and integrity of any director, executive officer, promoter or control person of our Company during the past ten years.
Conflicts of Interest
Ms. Lou, one of our directors, is a managing partner of Milestone Capital Management Limited, an affiliate of Milestone. After taking into account the sale of the Series A Convertible Preferred Shares to Milestone, Ms. Zhang, one of our directors and our chief financial officer, controls approximately 47.3% of the total voting rights of our capital stock and Milestone owns approximately 19.72% of the voting rights of our capital stock, so that together these parties control a majority of the voting rights. This could have a substantial impact on matters requiring the vote of the shareholders, including the election of our directors and most of our corporate actions. This control could delay, defer or prevent others from initiating a potential merger, takeover or other change in our control, even if these actions would benefit our shareholders and us. This control could adversely affect the voting and other rights of our other shareholders and could depress the market price of our common stock.
Certain potential conflicts of interest are inherent in the relationships between our officers and directors, and us.
From time to time, one or more of our affiliates may form or hold an ownership interest in and/or manage other businesses both related and unrelated to the type of business that we own and operate. These persons expect to continue to form, hold an ownership interest in and/or manage other businesses which may compete with ours with respect to operations, including financing and marketing, management time and services and potential customers. These activities may give rise to conflicts between or among the interests of us and other businesses with which our affiliates are associated. Our affiliates are in no way prohibited from undertaking such activities, and neither we nor our shareholders will have any right to require participation in such other activities.
Further, because we intend to transact business with some of our officers, directors and affiliates, as well as with firms in which some of our officers, directors or affiliates have a material interest, potential conflicts may arise between the respective interests of us and these related persons or entities. We believe that such transactions will be effected on terms at least as favorable to us as those available from unrelated third parties.
With respect to transactions involving real or apparent conflicts of interest, we have adopted policies and procedures which require that: (i) the fact of the relationship or interest giving rise to the potential conflict be disclosed or known to the directors who authorize or approve the transaction prior to such authorization or approval, (ii) the transaction be approved by a majority of our disinterested outside directors, and (iii) the transaction be fair and reasonable to us at the time it is authorized or approved by our directors.
ITEM 11. EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
We strive to provide our named executive officers with a competitive base salary that is in line with their roles and responsibilities when compared to peer companies of comparable size in the same or similar locality.
It is not uncommon for companies with operations primarily in China to have base salaries and bonuses as the sole and only form of compensation. Since the establishment of our Compensation Committee in June 2010, our Compensation Committee has been delegated the authority to make decisions regarding salary and compensation for our executive officers. The base salary for our executive officers is compared to similar positions within comparable peer companies and with consideration of the executive’s relative experience in his or her position. Based on an evaluation of available information with respect to the base salaries of executives of our competitors, we believe that the base salary and bonus paid to our named executive officers is in line with our competitors. Base salaries are reviewed periodically and at the time of promotion or other changes in responsibilities.
64
As indicated under the heading “2010 Omnibus Securities and Incentive Plan” below, in 2010, the board of directors implemented a more comprehensive compensation program appropriate for executives of a public company, which takes into account other elements of compensation, including without limitation, short and long term compensation, cash and non-cash, and other equity-based compensation such as stock options. We believe that such compensation programs are comparable to our peers in the industry and will retain and attract talented individuals.
Summary Compensation Table— Fiscal Years Ended December 31, 2011 and 2010
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to the named person for services rendered in all capacities during the noted periods.
|
Name and Principal Position
|
Year Ended December 31
|
Salary
($)
|
Bonus
($)
|
Stock Awards ($)
|
Option Awards ($) (3) (4)
|
Non-Equity Incentive Plan Compensation Earnings ($)
|
Non- Qualified Deferred Compensation Earnings ($)
|
All Other Compensation
($) (5)
|
Total ($)
|
|||||||||||||||||||||||||
|
Huitian Tang
|
2011
|
216,982
|
70,000
|
-
|
1,337,990
|
-
|
-
|
6,220
|
1,631,192
|
|||||||||||||||||||||||||
|
Chairman and CEO (1)
|
2010
|
139,200
|
-
|
-
|
969,370
|
-
|
-
|
-
|
1,108,570
|
|||||||||||||||||||||||||
|
Xiaoyan Zhang
|
2011
|
156,641
|
54,000
|
-
|
1,054,758
|
-
|
-
|
2,619
|
1,268,018
|
|||||||||||||||||||||||||
|
CFO and Director (2)
|
2010
|
109,092
|
-
|
-
|
805,070
|
-
|
-
|
-
|
914,162
|
|||||||||||||||||||||||||
|
(1)
|
Represents amounts paid to Mr. Tang as CEO of the Company and as a director of Liuzhou BCT. The amounts have been converted into US dollars by using average exchange rates for the periods
|
|
(2)
|
Represents amounts paid to Ms. Zhang as CFO of the Company.
|
|
(3)
|
The options granted to each of Mr. Tang and Ms. Zhang in 2010 vested and became fully exercisable as a result of the Company achieving after-tax net income of at least $26 million for the 2010 fiscal year. On December 16, 2011, the Company granted Mr. Tang and Ms. Zhang 762,500 options each, which options have an exercise price of $2 per share, 50% of which vested and became immediately exercisable and 50% of which vest on December 31, 2012. Additionally, the Company granted 1,245,000 options to Mr. Tang and 830,000 options to Ms. Zhang on December 16, 2011 pursuant to the Company’s 2010 Omnibus Securities and Incentive Plan.
|
|
(4)
|
Represents the aggregate grant date fair value of option awards made during each of 2010 and 2011 computed in accordance with FASB ASC Topic 718. Fair values were estimated using the binomial model. For a description of the assumptions used to determine these amounts, see Note 22 in the Notes to the Consolidated Financial Statements filed herewith for the period ended December 31, 2011.
|
|
(5)
|
Represents the social security contributions and the value of benefit in kinds made during the periods.
|
2010 Omnibus Securities and Incentive Plan
On June 27, 2010, our board of directors adopted the China BCT Pharmacy Group, Inc. 2010 Omnibus Securities and Incentive Plan (the “Plan”) for the benefit of our employees, nonemployee directors and consultants and the employees, nonemployee directors and consultants of its affiliates, for purposes of assisting us to attract, retain and provide incentives to key management employees and nonemployee directors of, and non-employee consultants to, us and our affiliates, and to align the interests of such individuals with those of our stockholders. Accordingly, the Plan provides for the granting of distribution equivalent rights, incentive stock options, non-qualified stock options, performance share awards, performance unit awards, restricted stock awards, restricted stock unit awards, stock appreciation rights, tandem stock appreciation rights, unrestricted stock awards or any combination of the foregoing, as may be best suited to the circumstances of the particular employee, director or consultant as provided in the Plan.
65
The following is a summary of the material provisions of the Plan and is qualified in its entirety by reference to the complete text of the Plan, a copy of which is filed as an exhibit to the Company’s Current Report on Form 8-K, filed on June 29, 2010.
Administration. The Plan is administered by the compensation committee of the board of directors, which consists of three members of the board of directors, each of whom is a “non-employee director” within the meaning of Rule 16b-3 promulgated under the Exchange Act and an “outside director” within the meaning of Code Section 162(m). Among other things, the compensation committee has complete discretion, subject to the express limits of the Plan, to determine the directors, employees and nonemployee consultants to be granted an award, the type of award to be granted, the number of shares of common stock subject to each award, the exercise price of each option and base price of each stock appreciation right, the term of each award, the vesting schedule for an award, whether to accelerate vesting, the value of the shares of common stock underlying the award, and the required withholding, if any. The compensation committee may amend, modify or terminate any outstanding award, provided that the participant’s consent to such action is required if the action would materially and adversely impair the participant’s rights or entitlements with respect to that award. The compensation committee is also authorized to construe the award agreements, and may prescribe rules relating to the Plan. Notwithstanding the foregoing, the compensation committee does not have any authority to grant or modify an award under the Plan with terms or conditions that would cause the grant, vesting or exercise to be considered nonqualified “deferred compensation” subject to Code Section 409A.
Grant of Awards; Shares Available for Awards. The Plan provides for the grant of options, stock appreciation rights, performance share awards, performance unit awards, distribution equivalent right awards, restricted stock awards, restricted stock unit awards and unrestricted stock awards to directors, officers, employees and nonemployee consultants of us or our affiliates. The aggregate number of shares of common stock that may be issued under the Plan shall not exceed 12.5% of the total shares of common stock which are issued and outstanding and shares of common stock issuable upon the conversion of any outstanding shares of preferred stock of the Company. If any award expires, is cancelled, or terminates unexercised or is forfeited, any shares subject thereto is again available for grant under the Plan.
Currently, there are 7 employees and directors who would be entitled to receive stock options and/or restricted shares under the Plan. Future new hires and additional consultants would be eligible to participate in the Plan as well. The number of options and/or restricted shares to be granted to executives and directors cannot be determined at this time as the grant of stock options and/or restricted shares is dependent upon various factors such as hiring requirements and job performance.
Stock Options. Options granted under the Plan may be either “incentive stock options” (“ISOs”), which are intended to meet the requirements for special federal income tax treatment under the Code, or “nonqualified stock options” (“NQSOs”). Options may be granted on such terms and conditions as the compensation committee may determine; provided, however, that the per share exercise price under an option may not be less than the fair market value of a share of the underlying shares of common stock on the date of grant and the term of the option may not exceed 10 years (110% of such value and 5 years in the case of an ISO granted to an employee who owns (or is deemed to own) more than 10% of the total combined voting power of all classes of our capital stock or our parent or subsidiary). ISOs may only be granted to employees. In addition, the aggregate fair market value of shares of common stock covered by ISOs (determined at the time of grant) which are exercisable for the first time by an employee during any calendar year may not exceed $100,000. Any excess is treated as a NQSO.
Stock Appreciation Rights. A stock appreciation right (“SAR”) entitles the participant, upon exercise, to receive an amount, in cash or stock or a combination thereof, equal to the increase in the fair market value of the underlying shares of common stock between the date of grant and the date of exercise. SARs may be granted in tandem with, or independently of, options granted under the Plan. A SAR granted in tandem with an option (i) is exercisable only at such times, and to the extent, that the related option is exercisable in accordance with the procedure for exercise of the related option; (ii) terminates upon termination or exercise of the related option (likewise, the option granted in tandem with a SAR terminates upon exercise of the SAR); (iii) is transferable only with the related option; and (iv) if the related option is an ISO, may be exercised only when the value of the stock subject to the option exceeds the exercise price of the option. A SAR that is not granted in tandem with an option is exercisable at such times as the compensation committee may specify.
66
Performance Shares or Performance Unit Awards. Performance share or performance unit awards entitle the participant to receive cash or shares of common stock upon attaining specified performance goals. In the case of performance units, the right to acquire the units is denominated in cash values.
Distribution Equivalent Right Awards. A distribution equivalent right award entitles the participant to receive bookkeeping credits, cash payments and/or common stock distributions equal in amount to the distributions that would have been made to the participant had the participant held a specified number of our shares of common stock during the period the participant held the distribution equivalent right. A distribution equivalent right may be awarded as a component of another award, where, if so awarded, such distribution equivalent right will expire or be forfeited by the participant under the same conditions as under such other award.
Restricted Stock Awards or Restricted Stock Unit Award. A restricted stock award is a grant or sale of shares of common stock to the participant, subject to our right to repurchase all or part of the shares at their purchase price (or to require forfeiture of such shares if purchased at no cost) in the event that conditions specified by the compensation committee in the award are not satisfied prior to the end of the time period during which the shares subject to the award may be repurchased by or forfeited to us. A restricted stock unit entitles the participant to receive a cash payment equal to the fair market value of a share of common stock for each restricted stock unit subject to such restricted stock unit award, if the holder satisfies the applicable vesting requirement.
Unrestricted Stock Awards. An unrestricted stock award is a grant or sale of shares of common stock to the participant that is not subject to transfer, forfeiture or other restrictions, in consideration for past services rendered to us or an affiliate or for other valid consideration.
Change-in-Control Provisions. In connection with the grant of an award, the compensation committee may provide that, in the event of a change in control, such award will become fully vested and immediately exercisable.
Amendment and Termination. The compensation committee may adopt, amend and rescind rules relating to the administration of the Plan, and amend, suspend or terminate the Plan, but no such amendment or termination will be made that materially and adversely impairs the rights of the participant with respect to any award without the participant’s consent, other than amendments that are necessary to permit the granting of awards in compliance with Code Sections 162(m) and/or 409A. We have attempted to structure the Plan so that remuneration attributable to options and other awards will not be subject to the deduction limitation contained in Code Section 162(m).
Employment Agreements
In 2010, we entered into employment agreements with Huitian Tang, CEO, and Xiaoyan Zhang, CFO. The employment agreements were negotiated on our behalf by Mr. Tang and were approved by our board of directors on May 18, 2010. At that time our board of directors consisted only of Mr. Tang and Ms. Zhang. The following is a summary of the material provisions of the employment agreements for each of Mr. Tang and Ms. Zhang and is qualified in its entirety by reference to the complete text of such employment agreements, copies of which are filed as exhibits to Company’s Current Report on Form 8-K, filed on May 21, 2010.
Huitian Tang
On January 1, 2008, Liuzhou BCT entered into an employment agreement with Mr. Huitian Tang pursuant to which Mr. Tang was hired as president of Liuzhou BCT and received a salary of $27,027 per year in 2008 and 2009. That agreement expired on December 31, 2009. From January 1, 2010 to May 18, 2010 we had an oral agreement with Mr. Tang pursuant to which we agreed to pay him 90,000 Hong Kong Dollars ($11,688) per month and a discretionary bonus based upon our 2010 financial performance On May 18, 2010, we and Liuzhou BCT entered into a new employment agreement with Mr. Tang to employ him as our CEO for a term from January 1, 2010 to January 1, 2012, pursuant to which we have agreed to pay him RMB 79,600 ($11,600) per month (or RMB 955,200 ($139,200) per year) and a bonus based upon our 2010 financial performance and for 2011, upon our 2011 financial performance. We believe Mr. Tang deserved this increase in salary because of the Company’s growth over the past several years under Mr. Tang’s leadership and to provide Mr. Tang with a more competitive base salary that is in line with his role and responsibilities at the Company. The agreement provides that if we achieve an after-tax net income of at least $26 million for the 2010 fiscal year, Mr. Tang’s options which may be exercised for 590,000 shares of our common stock will vest and become exercisable, as discussed more specifically below under the heading “Stock Option Agreements.” Because the Company met the $26 million after-tax net income standard, the options vested and became exercisable in March of 2011. The Company may, in its discretion, pay a bonus to Mr. Tang, the details of which will be set forth by the Company.
67
The agreement may be terminated upon mutual agreement between the Company and Mr. Tang in writing. In addition, the Company shall have the right to unilaterally terminate the agreement under certain circumstances, including, among other things, (i) serious violations of the labor laws or the rules or regulations of the Company; (ii) causing serious damage to the interests of the Company; or (iii) Mr. Tang is criminally prosecuted under the law.
The Company may terminate the agreement by serving 30 days' prior written notice to Mr. Tang or giving Mr. Tang one month’s salary in lieu of notice in any one of the following circumstances: (i) where Mr. Tang, after undergoing a legally prescribed period of medical treatment and recuperation for an illness or a non-work-related injury, remains unable to carry out his job responsibilities; (ii) where Mr. Tang is unable to fulfill the duties of his position to the standards required under the terms of the agreement; or (iii) where the agreement cannot be performed due to any major changes of any objective circumstances, which includes, but is not limited to, a merger of the Company into another business entity, or sale or transfer by the Company of a substantial portion of the assets it owns to third parties, a material adjustment in operative policy, or a declaration of bankruptcy, dissolution or liquation by the Company.
Mr. Tang may terminate the agreement during the term upon 30 days prior written notice to the Company. In addition, Mr. Tang may terminate the agreement in certain circumstances, including if (i) the Company fails to pay the social insurance premiums for Mr. Tang in accordance with the law; (ii) the Company forces Mr. Tang to work by means of violence or intimidation; or (iii) the Company fails to pay labor remuneration in full and on time or fails to provide the labor protection or working conditions as agreed under the agreement.
On May 18, 2010, we also entered into a non-disclosure and non-competition agreement with Mr. Tang. Pursuant to this agreement, Mr. Tang agreed to maintain the confidentiality of all confidential information as defined in the agreement and not disclose any of such confidential information without our prior written consent. Mr. Tang also agreed that during the course of his employment and for a period of 24 months immediately following the termination of his relationship with us for any reason, Mr. Tang will not (i) serve as a partner, employee, consultant, officer, director, manager, agent, associate, investor, or otherwise for, (ii) directly or indirectly, own, purchase, organize or take preparatory steps for the organization of, (iii) build, design, finance, acquire, lease, operate, manage, invest in, work or consult for or otherwise affiliate with, any business, in competition with or otherwise similar to our business. The foregoing covenant covers Mr. Tang’s activities in (a) the People's Republic of China, (b) Taiwan, (c) Hong Kong, (d) Macao, (e) the United States of America, and (f) all other countries and/or regions of the world. In addition, Mr. Tang agreed that for a period of 24 months immediately following the termination of his relationship with us for any reason, Mr. Tang shall not solicit any of our employees to leave their employment, either for himself or for any other person or entity. The description of the non-disclosure and non-competition agreement with Mr. Tang is qualified in its entirety by reference to the complete text of such agreement, a copy of which is filed as an exhibit to the Company’s registration statement on July 29, 2010.
Xiaoyan Zhang
Beginning on January 1, 2010, we had an oral agreement with Xiaoyan Zhang to employ her as our CFO, pursuant to which we have agreed to pay her HKD70,000 ($9,091) per month (or HKD840,000 ($109,092) per year) and a discretionary bonus based upon our 2010 financial performance. On May 18, 2010, we and Forever Well entered into a new employment agreement with Ms. Zhang to employ her as our CFO for a term from January 1, 2010 to January 1, 2012, pursuant to which we have agreed to pay her HKD70,000 ($9,091) per month (or HKD840,000 ($109,092) per year) and a bonus based upon our 2010 financial performance and for 2011, upon our 2011 financial performance. The agreement provides that if we achieve an after-tax net income of at least $26 million for the 2010 fiscal year, Ms. Zhang shall receive vested options which may be exercised for 490,000 shares of our common stock, as discussed more specifically below under the heading “Stock Option Agreements.” Because the Company met the $26 million after-tax net income standard, the options vested and became exercisable in March of 2011. The Company may, in its discretion, pay a bonus to Ms. Zhang, the details of which will be set forth by the Company.
68
The agreement may be terminated upon mutual agreement between the Company and Ms. Zhang in writing. In addition, the Company shall have the right to unilaterally terminate the agreement under certain circumstances, including, among other things, (i) serious violations of the labor laws or the rules or regulations of the Company; (ii) causing serious damage to the interests of the Company; or (iii) Ms. Zhang is criminally prosecuted under the law.
The Company may terminate the agreement by serving 30 days' prior written notice to Ms. Zhang or giving Ms. Zhang one month’s salary in lieu of notice in any one of the following circumstances: (i) where Ms. Zhang, after undergoing a legally prescribed period of medical treatment and recuperation for an illness or a non-work-related injury, remains unable to carry out her job responsibilities; (ii) where Ms. Zhang is unable to fulfill the duties of her position to the standards required under the terms of the agreement; or (iii) where the agreement cannot be performed due to any major changes of any objective circumstances, which includes, but is not limited to, a merger of the Company into another business entity, or sale or transfer by the Company of a substantial portion of the assets it owns to third parties, a material adjustment in operative policy, or a declaration of bankruptcy, dissolution or liquation by the Company.
Ms. Zhang may terminate the agreement during the term upon 30 days prior written notice to the Company. In addition, Ms. Zhang may terminate the agreement in certain circumstances, including if (i) the Company fails to pay the social insurance premiums for Ms. Zhang in accordance with the law; (ii) the Company forces Ms. Zhang to work by means of violence or intimidation; or (iii) the Company fails to pay labor remuneration in full and on time or fails to provide the labor protection or working conditions as agreed under the agreement.
On May 18, 2010, we also entered into a non-disclosure and non-competition agreement with Ms. Zhang. Pursuant to this agreement, Ms. Zhang agreed to maintain the confidentiality of all confidential information as defined in the agreement and not disclose any of such confidential information without our prior written consent. Ms. Zhang also agreed that during the course of her employment and for a period of 24 months immediately following the termination of her relationship with us for any reason, Ms. Zhang will not (i) serve as a partner, employee, consultant, officer, director, manager, agent, associate, investor, or otherwise for, (ii) directly or indirectly, own, purchase, organize or take preparatory steps for the organization of, (iii) build, design, finance, acquire, lease, operate, manage, invest in, work or consult for or otherwise affiliate with, any business, in competition with or otherwise similar to our business. The foregoing covenant covers Ms. Zhang’s activities in (a) the People's Republic of China, (b) Taiwan, (c) Hong Kong, (d) Macao, (e) the United States of America, and (f) all other countries and/or regions of the world. In addition, Ms. Zhang agreed that for a period of 24 months immediately following the termination of her relationship with us for any reason, Ms. Zhang shall not solicit any of our employees to leave their employment, either for herself or for any other person or entity. The description of the non-disclosure and non-competition agreement with Ms. Zhang is qualified in its entirety by reference to the complete text of such agreement, a copy of which is filed as an exhibit to the Company’s registration statement on July 29, 2010.
Stock Option Agreements
On June 27, 2010, we entered into a stock option agreement with Mr. Tang which provides for the grant to Mr. Tang of an option to purchase 590,000 shares of our common stock at an exercise price of $4.00 per share. The option vests and becomes exercisable with respect to all of the shares solely if our after-tax net income for fiscal 2010 equals at least U.S. $26,000,000 (excluding any non-cash expenses) (the “2010 Income Target”), determined on the basis of our audited financial statements for our 2010 fiscal year, as confirmed by our independent auditor in its report on said financial statements (the “Audit Report”). The Company met the 2010 Income Target and, as a result, the option vested and became exercisable on the date on which the Audit Report is dated. If the option had not become exercisable on account of the failure to meet the 2010 Income Target, the option would have terminated on the date on which the Audit Report is dated. The exercise price of the option may be paid for in cash or by a cashless exercise. The option terminates on the earlier of (i) June 27, 2020 or (ii) the date as of which the option has been fully exercised.
69
On December 16, 2011, we entered into a new option agreement with Mr. Tang pursuant to which we made a grant of 762,000 stock options. These stock options have an exercise price of $2.00 per share; 50% of the options vest and become exercisable immediately and the remaining 50% vest and become exercisable on December 31, 2012. These options are not granted pursuant to the Company’s 2010 Omnibus Securities and Incentive Plan, but are granted pursuant to the Board’s authority to grant options.
On June 27, 2010, we also entered into a stock option agreement with Ms. Zhang which provides for the grant to Ms. Zhang of an option to purchase 490,000 shares of our common stock at an exercise price of $4.00 per share. The option vests and becomes exercisable with respect to all of the shares solely if our after-tax net income for fiscal 2010 equals at least the 2010 Income Target, determined on the basis of our audited financial statements for our 2010 fiscal year, as confirmed by our independent auditor in the Audit Report. The Company met the 2010 Income Target and, as a result, the option vested and became exercisable on the date on which the Audit Report is dated. If the option had not become exercisable on account of the failure to meet the 2010 Income Target, the option would have terminated on the date on which the Audit Report is dated. The exercise price of the option may be paid for in cash or by a cashless exercise. The option terminates on the earlier of (i) June 27, 2020 or (ii) the date as of which the option has been fully exercised.
On December 16, 2011, we entered into a new option agreement with Ms. Zhang pursuant to which we made a grant of 762,000 stock options. These stock options have an exercise price of $2.00 per share; 50% of the options vest and become exercisable immediately and the remaining 50% vest and become exercisable on December 31, 2012. These options are not granted pursuant to the Company’s 2010 Omnibus Securities and Incentive Plan, but are granted pursuant to the Board’s authority to grant options.
A copy of the Form of Stock Option Agreement used is filed as an exhibit to the Company’s Current Report on Form 8-K, filed December 19, 2011.
On July 16, 2010, we entered into separate stock option agreements with each of our three independent directors, Messrs. Lee, Choi and Chiu, which agreements provide for the grant to each such individual of an option to purchase 10,000 shares of our common stock at an exercise price of $4.00 per share. Each option vests and becomes exercisable with respect to all of the shares on June 6, 2011. The exercise price of each option may be paid for in cash or by cancellation of existing shares of our common stock held by the independent directors. Each option terminates on the earlier of (i) July 16, 2015 or (ii) the date as of which the option has been fully exercised.
In addition, on December 16, 2011, the Board made grants of stock options to the following employees, executive officers and directors of the Company in the amounts set forth below:
|
Name
|
Award Amount
|
|
Huitian Tang , Chief Executive Officer
|
1,245,000
|
|
Xiaoyan Zhang, Chief Financial Officer
|
830,000
|
|
Simon Cho, director
|
10,000
|
|
James Chiu, director
|
10,000
|
|
Chin-Kam Cheung, director
|
10,000
|
|
Kwokwai Ng, senior staff
|
20,000
|
|
Liyee Yik, senior staff
|
10,000
|
70
These stock options are granted pursuant to the Plan and have an exercise price of $2.00 per share. The first 25% of these options vest and become exercisable on June 30, 2013, the second 25% vest and become exercisable on December 31, 2013, the third 25% vest and become exercisable on June 30, 2014 and the remaining 25% vest and become exercisable on December 31, 2014.
The Board provided the right of the recipients to have a cashless exercise election for their stock options granted above and in the event of a Change in Control of the Company (as defined in the form of stock option agreement attached), then any portion of the above options that has not become vested and exercisable shall immediately vest and become exercisable. A copy of the Form of Stock Option Agreement used was filed as an exhibit to the Company’s Current Report on Form 8-K, filed on December 19, 2011.
Outstanding Equity Awards— Fiscal Year Ended December 31, 2010
The following table sets forth information concerning unexercised options; units of stock that have not vested equity incentive plan awards for each named executive officer outstanding as of the end of the fiscal year ended December 31, 2011.
|
Name and Principal Position
|
Number of Securities Underlying Unerxercised Options
(#)
Exercisable
|
Number of Securities Underlying Unerxercised Options
(#)
Unexercisable
|
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options
|
Option Exercise Price ($)
|
Option Expiration
Date
|
Number of Shares or Units of Stock that have not Vested
|
Market
Value of Shares of Units of Stock that Have not Vested ($)
|
Equity Incentive
Plan Awards:
Number of Unearned Shares, Units or
Other Rights that have not Vested
|
Equity Incentive
Plan Awards:
Market or
Payout Value of Unearned
Shares, Units or other Rights that have not Vested($)
|
||||||||||||||||||||||||
|
Huitian Tang
|
590,000 | (1) | – | – | 4.00 |
6/27/2020
|
– | – | – | – | |||||||||||||||||||||||
| 381,250 | (2) | 381,250 | (2) | – | 2.00 |
12/16/2021
|
– | – | – | – | |||||||||||||||||||||||
| – | – | 1,245,000 | (3) | 2.00 |
12/16/2021
|
– | – | – | – | ||||||||||||||||||||||||
|
Xiaoyan Zhang
|
490,000 | (1) | – | – | 4.00 |
6/27/2020
|
– | – | – | – | |||||||||||||||||||||||
| 381,250 | (2) | 381,250 | (2) | – | 2.00 |
12/16/2021
|
– | – | – | – | |||||||||||||||||||||||
| – | – | 830,000 | (3) | 2.00 |
12/16/2021
|
– | – | – | – | ||||||||||||||||||||||||
|
(1)
|
The options granted to each of Mr. Tang and Ms. Zhang in 2010 vested and became fully exercisable as a result of the Company achieving after-tax net income of at least $26 million for the 2010 fiscal year.
|
|
(2)
|
On December 16, 2011, the Company granted Mr. Tang and Ms. Zhang 762,500 options each, which options have an exercise price of $2 per share, 50% of which vested and became immediately exercisable and 50% of which vest on December 31, 2012.
|
|
(3)
|
On December 16, 2011, Company granted 1,245,000 options to Mr. Tang and 830,000 options to Ms. Zhang on December 16, 2011 pursuant to the Company’s 2010 Omnibus Securities and Incentive Plan.
|
71
Independent Director Agreements
In connection with the appointments of Messrs. Chin, Choi and Chiu to our board of directors, we entered into one year agreements with each of them. Pursuant to these agreements, each of Messrs. Chin, Choi and Chiu will receive a fee of One Hundred Twenty Thousand Hong Kong Dollars (HK$120,000) (US$ 17,647) for the term of the agreement, payable in cash in equal monthly installments. We will also reimburse each independent director for expenses related to his attending meetings of the board, meetings of committees of the board, executive sessions and stockholder meetings.
Director Compensation
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to our non-employee directors for 2011.
|
Name
|
Fees earned or paid in cash ($)
|
Stock
Awards ($)
|
Option Awards ($) (1)(2)
|
Non-Equity Incentive Plan Compensation Earnings ($)
|
Non- Qualified Deferred Compensation ($)
|
Nonqualified deferred compensation earnings
($)
|
All Other Compensation ($)
|
Total ($)
|
||||||||||||||||||||||||
|
Engleong Lee (3)
|
9,032
|
0
|
0
|
0
|
0
|
0
|
0
|
9,032
|
||||||||||||||||||||||||
|
Simon Choi
|
18,065
|
0
|
6,979
|
0
|
0
|
0
|
0
|
25,044
|
||||||||||||||||||||||||
|
Manwai Chiu
|
18,065
|
0
|
6,979
|
0
|
0
|
0
|
0
|
25,044
|
||||||||||||||||||||||||
|
Kam-Cheung Chin (3)
|
9,032
|
6,979
|
16,011
|
|||||||||||||||||||||||||||||
|
(1)
|
We awarded each of Messrs. Choi, Chiu and Chin stock options on December 16, 2011, pursuant to the Company’s 2010 Omnibus Securities and Incentive Plan with an exercise price of $2.00 per share. The first 25% of these options vest and become exercisable on June 30, 2013, the second 25% vest and become exercisable on December 31, 2013, the third 25% vest and become exercisable on June 30, 2014 and the remaining 25% vest and become exercisable on December 31, 2014 and allow each of of Messrs. Choi, Chiu and Chin to purchase 10,000 shares of our common stock at an exercise price of $2.00 per share. Additionally, we awarded Mr. options to purchase 10,000 shares of our common stock at an exercise price of $4 per share upon his appointment as a director of the Company, effective June 8, 2011,which options vest on June 8, 2012.
|
|
(2)
|
Represents the aggregate grant date fair value of option awards made during 2011. The fair value of options granted to the three independent directors were expensed in share-based compensation for the year ended December 31, 2011. Fair values were estimated using the binominal model. The assumptions that were used to calculate fair value of stock options as of July 16, 2011 were:0% dividend yield, expected volatility of 44.38%, risk-free interest rate of 1.97%, an exercise multiple of two, and a post-vesting forfeiture rate of 0%.
|
|
(3)
|
Engleong Lee resigned as a director of the Company upon the expiration of his term on June 8, 2011 and Kam-Cheung Chin was appointed as a director, effective June 8, 2011, to fill the vacancy created by Mr. Lee’s departure.
|
Except as set forth above, our directors are reimbursed for reasonable expenses incurred by them in connection with attending meetings of the board, meetings of committees of the board, executive sessions and stockholder meetings, but they do not receive any other compensation for serving on the board of directors.
Compensation Committee Interlocks and Insider Participation
None of our executive officers or directors serves as a member of the board of directors or compensation committee of any entity that has one or more of its executive officers serving as a member of our Board of Directors or Compensation Committee.
72
Indemnification of Directors and Executive Officers and Limitation of Liability
Delaware General Corporation Law does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the courts of the State of Delaware to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. Our articles of association provide for indemnification of our officers and directors for any liability incurred in their capacities as such, except through their own willful negligence or default.
In so far as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is theretofore unenforceable.
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
Securities Authorized for Issuance under Equity Compensation Plan
Information required by Item 201(d) of Regulation S-K is contained under “Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities - Securities Authorized for Issuance Under Equity Compensation Plans” herein.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding beneficial ownership of our common stock effective March 29, 2012 by (i) each person (or group of affiliated persons) who is known by us to own more than five percent of the outstanding shares of our common stock, (ii) each director, and named executive officer, and (iii) all of our directors and executive officers as a group. As of March 29, 2012, we had 38,154,340 shares of common stock issued and outstanding.
|
Amount and Nature of Beneficial Ownership (1)
|
Percent of Class (2)
|
|||||||||||||||
|
Name and Address of Beneficial Owner
|
Common
Stock
|
Preferred
Stock
|
Common
Stock
|
Preferred
Stock
|
||||||||||||
|
Xiaoyan Zhang (2)
|
23,351,250
|
-
|
59.85
|
%
|
-
|
|||||||||||
|
Huitian Tang (2)
|
3,788,193
|
-
|
9.68
|
-
|
||||||||||||
|
Kam-Cheung Chin
|
-
|
-
|
-
|
-
|
||||||||||||
|
Manwai Chiu
|
10,000
|
-
|
-
|
-
|
||||||||||||
|
Simon Choi
|
10,000
|
-
|
-
|
-
|
||||||||||||
|
Yunli Lou (3)
|
-
|
-
|
-
|
-
|
||||||||||||
|
Yujing Tan (2)
|
2,816,889
|
-
|
7.38
|
%
|
-
|
|||||||||||
|
Jinghua Li (2)
|
3,474,237
|
-
|
9.11
|
%
|
-
|
|||||||||||
|
Milestone Longcheng Limited (4)
|
9,375,000
|
9,375,000
|
19.73
|
%
|
100
|
%
|
||||||||||
|
All directors and executive officers as a group (5 persons)
|
27,159,443
|
-
|
67.89
|
%
|
-
|
|||||||||||
73
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. Unless otherwise noted, the principal address of each of the stockholders, directors and officers listed below is c/o Guangxi Liuzhou Baicaotang Medicine Limited, No. 102 Chengzhan Road, Liuzhou City, Guangxi Province, PRC.
All share ownership figures include shares of our common stock and securities convertible or exchangeable into shares of our common stock within sixty (60) days of March 29, 2012, which are deemed outstanding and beneficially owned by such person for purposes of computing his or her percentage ownership, but not for purposes of computing the percentage ownership of any other person.
(1) Beneficial ownership as reported in the table has been determined in accordance with Securities and Exchange Commission (“SEC”) regulations and includes shares of our common stock that may be issued upon the exercise of stock options that are exercisable and preferred stock that is convertible into common stock within 60 days of March 29, 2012 as follows: Mr. Tang — 971,250 shares, Ms. Zhang — 871,250 shares; Mr. Chiu — 10,000 shares, Mr. Choi — 10,000 shares and all directors and executive officers as a group — 1,862,500 shares; and Milestone — 9,375,000 shares of preferred stock convertible into 9,375,000 shares of common stock. Pursuant to SEC regulations, all shares not currently outstanding that are subject to options exercisable within 60 days are deemed to be outstanding for the purpose of computing “Percent of Class” held by the holder thereof but are not deemed to be outstanding for the purpose of computing the “Percent of Class” held by any other stockholder.
(2) Xiaoyan Zhang is a citizen of Hong Kong and our Chief Financial Officer, Secretary and director and holds the 22,480,000 shares as the legal owner. Pursuant to an Earn-In Agreement dated December 30, 2009, as amended on May 19, 2010, by and among Ms. Zhang and the Liuzhou BCT Shareholders, the Liuzhou BCT Shareholders have a call right to purchase up to 22,480,000 shares of our common stock from Ms. Zhang for a nominal amount per share provided that the Company meets certain performance targets for fiscal 2010 and 2011. For the 2010 and 2011 fiscal years the performance targets for the Company are $26 million and $28 million after tax audited net income, respectively. If the 2010 performance target is met, the Liuzhou BCT Shareholders have the right to acquire 50% of shares held by Ms. Zhang over which they have a call right. If the 2011 performance target is met the Liuzhou BCT Shareholders have the right to acquire the other 50% of the shares held by Ms. Zhang over which they have a call right. The number of shares which can be acquired by the Liuzhou BCT Shareholders under the Earn-In Agreement is in proportion to their former relative ownership interest in Liuzhou BCT. Our Chairman and CEO, Huitian Tang, is one of the Liuzhou BCT Shareholders and has the right to acquire up to 2,241,193 shares of our common stock under the Earn-In Agreement if both performance targets are met. Pursuant to the Earn-in-Agreement, there are no restrictions on Ms. Zhang’s ability to vote these shares. The shares may not be transferred by Ms. Zhang except pursuant to the Earn-in-Agreement and after its expiration only in the manner agreed with the Liuzhou BCT Shareholders. The Liuzhou BCT Shareholders can only obtain legal ownership of the shares under the Earn-In Agreement. Messrs. Tan and Li are Liuzhou BCT Shareholders with rights under the Earn-In Agreement to acquire the number of shares set opposite their names and have the same rights with respect to the shares as Mr. Tang’s rights described above in this footnote and elsewhere in this annual report.
(3) Ms. Lou’s business address is Room 1708 Dominion Centre, 43-59 Queen’s Road East, Wanchai, Hong Kong.
(4) Milestone Longcheng Limited’s address is P.O. Box 957, Offshore Incorporation Center, Road Town, Tortola, British Virgin Islands. Milestone Longcheng Limited is wholly owned by Milestone China Opportunities Fund II, L.P., an exempted limited partnership formed under the laws of the Cayman Islands. The general partner of the Milestone China Opportunities Fund II, L.P. is Milestone Capital Partners Limited, a limited liability company incorporated under the laws of the Cayman Islands. The sole director of Milestone Capital Partners Limited is Cherianne Limited, a company organized under the laws of the British Virgin Islands. Yuen Ho Wan and James Ngai, as all of the directors of Cherianne Limited, share the investment and voting power of Cherianne Limited. Ms. Wan’s and Mr. Ngai’s business address is Room 1708 Dominion Centre, 43-59 Queen’s Road East, Wanchai, Hong Kong.
74
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
Forever Well
On March 28, 2008, Forever Well entered into an agreement with the stockholders of Liuzhou BCT to acquire their entire equity interest in Liuzhou BCT for cash consideration of approximately $1,470,588 (RMB10,000,000) which is the registered and fully paid up capital of Liuzhou BCT.
Liuzhou BCT
On February 8, 2007, Mr. Huitian Tang entered into a loan agreement with Industrial and Commercial Bank of China, Guangxi Branch in the amount of approximately $234,720 (RMB1,600,000), pursuant to which Liuzhou BCT, pledged part of its assets to the bank as security interest for the loan. Mr. Huitian Tang then lent the full amount of the above loan to Liuzhou BCT for working capital. On December 31, 2008, a mutual agreement was signed among Mr. Tang, Industrial and Commercial Bank of China, Guangxi Branch and Liuzhou BCT, pursuant to which Liuzhou BCT assumed the obligation to repay the principal amount and accrued interest from January 1, 2009 onwards.
On February 12, 2007, Youru Jiang, director of Liuzhoug BCT, entered into a loan agreement with Industrial and Commercial Bank of China, Guangxi Branch in the amount of approximately $264,060 (RMB1,800,000), pursuant to which Liuzhou BCT pledged part of its assets to the bank as security interest for the loan. Mr. Jiang then lent the full amount of the above loan to the Liuzhou BCT for working capital. On December 31, 2008, a mutual agreement was signed among Mr. Jiang, Industrial and Commercial Bank of China, Guangxi Branch and Liuzhou BCT, pursuant to which Liuzhou BCT assumed the obligation to repay the principal amount and accrued interest from January 1, 2009 onwards.
On January 15, 2009, Liuzhou BCT entered into a loan agreement with Agricultural Bank of China, Liuzhou Branch in the amount of approximately $660,150 (RMB 4,500,000), pursuant to which both the Baicaotang Property Development Limited and Wuxuan Baicaotang Medicine Limited pledged part of their land and property to the bank as security interest for the loan.
On December 19, 2008, Liuzhou BCT entered into a loan agreement with Liuzhou City Commercial Bank in the amount of approximately $733,500 (RMB 5,000,000), pursuant to which Baicaotang Property Development Limited pledged part of its premises to the bank as security interest for the loan.
On December 29, 2008, Liuzhou BCT entered into a loan agreement with Rural Credence Cooperation of Guangxi in the amount of approximately $514,706 (RMB 3,500,000), pursuant to which Baicaotang Property Development Limited pledged part of its premises to the bank as security interest for the loan.
On January 25, 2010, Liuzhou BCT entered into a loan agreement with Agricultural Bank of China Liuzhou Branch in the amount of approximately $1,290,000 (RMB 8,800,000), pursuant to which Baicaotang Property Development Limited and Liuzhou BCT pledged part of their premises to the bank as security interest for the loan.
On April 30, 2010, Liuzhou BCT entered into a loan agreement with Bank of Communication in the amount of approximately $2,640,600 (RMB 18,000,000), pursuant to which Baicaotang Property Development Limited pledged part of its premises to the bank as security interest for the loan.
On September 19, 2010, Liuzhou BCT entered into a loan agreement with Agricultural Bank of China Liuzhou Branch in the amount of approximately $1,211,200 (RMB 8,00,000), pursuant to which Baicaotang Property Development Limited and Liuzhou BCT pledged part of their premises to the bank as security interest for the loan.
75
In addition, we also entered into the following sales transactions at market prices with related parties pursuant to a standard form of purchase agreement and we have continued to enter into such transactions:
|
Year ended
|
Year ended
|
|||||||
|
December 31,
|
December 31,
|
|||||||
|
Sales of goods to related parties
|
2011
|
2010
|
||||||
|
Liucheng Medicine Limited (1)
|
$
|
48,483
|
$
|
325,881
|
||||
|
Guangxi Tianhu Medicine Limited (2)
|
642,535
|
9,632
|
||||||
|
Guangxi Liuzhou Baicaotang Medicine Limited, Guigang Branch (3)
|
1,065,208
|
3,311,379
|
||||||
|
Wuxuan Baicaotang Medicine Limited (4)
|
4,836
|
29,618
|
||||||
|
$
|
1,761,062
|
$
|
3,676,510
|
|||||
|
(1)
|
The directors of Liuzhou BCT, Huitian Tang, Jinghua Li, Youru Jiang, Chunlin Liu, Wende Wei and Bangfu Wang have an aggregate 34.3% interest in the registered share capital of Liucheng Medicine Limited.
|
|
(2)
|
The directors of Liuzhou BCT, Huitian Tang, Jinghua Li, Youru Jiang, Chunlin Liu, Wende Wei and Bangfu Wang have an aggregate 46.3% interest in the registered share capital of Guangxi Tianhu Medicine Limited.
|
|
(3)
|
The directors of Liuzhou BCT, Huitian Tang, Jinghua Li, Youru Jing, Chunlin Liu, Wende Wei and Bangfu Wang have an aggregate 67.2% interest in the registered share capital of Guangxi Baicaotang Medicine Limited Guigang Branch.
|
|
(4)
|
The directors of Liuzhou BCT, Huitian Tang, Jinghua Li, Youru Jing, Chunlin Liu, Wende Wei and Bangfu Wang have an aggregate 35.3% interest in the registered share capital of Wuxuan Baicaotang Medicine Limited.
|
The amount due from related companies as of December, 31 2011 and 2010 was as follows. Amounts due from related parties represent loans from us to related parties together with amounts representing our accounts receivable from related parties. Such loan amounts are non-interest bearing and have no due date as is often the custom in China.
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||||||||||||||
|
2011
|
2010
|
|||||||||||||||||||||||||||
|
Names of related parties
|
Trade receivable
|
Loan receivable
|
Total
|
Trade receivable
|
Loan receivable
|
Total
|
Remark
|
|||||||||||||||||||||
|
Guangxi Tianhu Medicine Limited
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
504,274
|
$
|
-
|
$
|
504,274
|
(1)
|
|||||||||||||||
|
Wuxuan Baicaotang Medicine Limited
|
-
|
-
|
-
|
56,051
|
393,640
|
449,691
|
(1)
|
|||||||||||||||||||||
|
Guangxi Liuzhou Baicaotang Medicine Limited, Guigang Branch
|
-
|
-
|
-
|
2,598,494
|
-
|
2,598,494
|
(1)
|
|||||||||||||||||||||
|
Property Management
|
-
|
255,169
|
255,169
|
231,610
|
231,610
|
(2)
|
||||||||||||||||||||||
|
Total
|
$
|
-
|
$
|
255,169
|
$
|
255,169
|
$
|
3,158,819
|
$
|
625,250
|
$
|
3,784,069
|
||||||||||||||||
|
(1)
|
See Notes 1, 2, 3 and 4 in the table immediately above.
|
|
(2)
|
The directors of Liuzhou BCT, Huitian Tang, Jinghua Li, Youru Jing, Chunlin Liu, Wende Wei and Bangfu Wang have an aggregate 67.2% interest in the registered share capital of Baicaotang Property management.
|
|
(3)
|
The directors of Liuzhou BCT, Huitian Tang, Jinghua Li, Youru Jing, Chunlin Liu, Wende Wei and Bangfu Wang have an interest over the related company by holding 67.2% of its paid up capital collectively.
|
76
The amounts due to related companies represent loans from related companies at December 31, 2011 and December 31, 2010 was as follows. The amounts are interest free and no interest was paid during the relevant reporting period.
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||||||||||||||
|
2011
|
2010
|
|||||||||||||||||||||||||||
|
Names of related parties
|
Repayment of principal
|
Maximum
balance
|
Outstanding
|
Repayment of principal
|
Maximum
balance
|
Outstanding
|
Remark
|
|||||||||||||||||||||
|
Liucheng Medicine Limited
|
$
|
108,820
|
$
|
109,820
|
$
|
-
|
$
|
18,759
|
$
|
128,579
|
$
|
109,820
|
(1)
|
|||||||||||||||
|
Baicaotang Property Development Limited
|
-
|
37,604
|
37,604
|
-
|
$
|
29,399
|
$
|
29,399
|
(1)
|
|||||||||||||||||||
|
$
|
108,820
|
$
|
147,424
|
$
|
37,604
|
$
|
18,759
|
$
|
157,978
|
$
|
139,219
|
|||||||||||||||||
|
(1)
|
See Notes 1, 2 and 3 in the table immediately above.
|
Transactions with Property Management.
We do not have a 100% direct ownership interest in BCT Retail due to the restriction of foreign investment in pharmacy chains with 30 or more drugstores. We have entered into contractual arrangements with Property Management pursuant to which the shareholders of Property Management pledged to us their equity interests in BCT Retail and provide us with the ability to effectively control BCT Retail. The directors of Liuzhou BCT, Huitian Tang, Jinghua Li, Youru Jing, Chunlin Liu, Wende Wei and Bangfu Wan have an aggregate 67.2% interest in the registered share capital of Property Management.
The contractual agreements entered into by us and Property Management include:
Share Transfer Agreement. Under this agreement dated April 1, 2008 by and between Liuzhou BCT, which held 100% of the equity interests of BCT Retail, and Property Management, Property Management acquired from Liuzhou BCT 51% equity interests in BCT Retail. The total amount of transfer price was RMB153,000, which was 51% of the registered share capital of BCT Retail. Within twenty (20) business days from the date when Property Management paid the transfer price, all the parties were to amend the articles of association of BCT Retail and register such equity transfer with the competent Authority of Industry and Commerce. Such amendment was made and equity transfer was registered on June 18, 2008.
Share Pledge Agreement. Under this agreement dated May 3, 2008 among Liuzhou BCT and Property Management, Property Management pledged all of its equity interest in BCT Retail to Liuzhou BCT to guarantee its obligations to repay a loan in the amount of RMB153,000 made from Liuzhou BCT to Property Management. The loan must be repaid by December 31, 2015 and may only be paid by transfer by Property Management of its equity interest in BCT Retail to Liuzhou BCT. During the term of the Agreement, Liuzhou BCT has the right to receive any dividends that would have been paid by BCT Retail to Property Management.
77
Share Repurchase Agreement. Under this agreement dated July 31, 2008 by and between Liuzhou BCT and Property Management, Liuzhou BCT was granted a preemption right to repurchase the 51% equity interests in BCT Retail held by Property Management. The term of the preemption right is two years from the date that Property Management deregisters the pledge as a result of paying off loans under the related share pledge agreements. The repurchase price shall be equal to 51% of the registered capital of BCT Retail at the time of the repurchase.
Share Pledge Agreement. Under this agreement dated March 31, 2009 among Liuzhou BCT and Property Management, Property Management pledged all of its equity interest in BCT Retail to Liuzhou BCT to guarantee its obligations under a loan in the amount of RMB 1.377 million made from Liuzhou BCT to Property Management. The loan was used by Property Management to increase its registered share capital in BCT Retail and to maintain its 51% interest. The loan must be repaid by December 31, 2015 and may only be paid by transfer by Property Management of its equity interest in BCT Retail to Liuzhou BCT. During the term of the Agreement, Liuzhou BCT has the right to receive any dividends that would have been paid by BCT Retail to Property Management.
Hefeng Pharmaceutical
From 2006 to 2009, Mr. Jinghua Li, the General Manager of Liuzhou Baicaotang, entered into a series of loan agreements with Hefeng Pharmaceutical, pursuant to which Hefeng Pharmaceutical borrowed an aggregate of approximately $586,563 at monthly interest rates ranging from 5.8% to 6.8%. All of the loan agreements have similar terms and provisions.
Liuzhou Retail
On June 18, 2009, Liuzhou Retail entered into a loan agreement with Rural Credence Cooperation of Guangxi in the amount of approximately $1,613,700 (RMB 11,000,000), pursuant to which Baicaotang Property Development Limited pledge part of its premise to the bank as security interest for the loan.
Milestone Private Placement
On February 28, 2011, the Company consummated a transaction with Milestone, pursuant to which Milestone purchased 9,375,000 shares of the Company’s Series A Convertible Preferred Shares, par value $.001 per share in a private placement transaction as further described under the heading “Recent Sales of Unregistered Securities” under Item 5 above. Ms. Yunli Lou, one of our directors and a managing partner of Milestone Capital Management Limited, an affiliate of Milestone, was nominated by Milestone and elected to serve as a director of the Company in accordance with the terms of a Series A Convertible Preferred Shares Purchase Agreement between Milestone and the Company.
Transactions with Promoters and Certain Control Persons
Lisa Lopomo, our former director, loaned the Company $1,000 with zero interest. On November 30, 2006, a total of 1,000,000 shares of Common Stock were issued to Lisa Lopomo at $0.005 per share or $5,000. On January 2, 2007 Lisa Lopomo, paid $5,000 on behalf of the Company for the cost of a mining claim. She was issued 1,000,000 shares of common stock at $.005 per share for a total of $5,000 in exchange for the cash paid out. On July 21, 2007, Lisa Lopomo was issued 1,000,000 shares of common stock in exchange for $5,000, or $0.005 per share.
On December 30, 2009 (the “Closing Date”), we entered into a Share Exchange Agreement (the “Exchange Agreement”) with Ingenious Paragon Global Limited, a British Virgin Islands company ("Ingenious”), and the shareholders of Ingenious named in the Exchange Agreement (the “Ingenious Shareholders”), which owns shares constituting 100% of the issued and outstanding common shares of Ingenious (the “Ingenious Shares”). Pursuant to the terms of the Exchange Agreement, the Ingenious shareholders transferred all Ingenious Shares to the Company in exchange for the issuance of 32,000,000 shares of our common stock, $.001 par value to the Ingenious shareholders. As a result of the Share Exchange, Ingenious became our wholly-owned subsidiary. In connection with the closing of the Share Exchange, Lisa Lopomo cancelled 2,900,000 shares of the common stock that she owned in the Company, and resigned from our board of directors and all office positions that she held in the Company.
78
On October 22, 2009, an Earn-In Agreement was entered into by Xiaoyan. Zhang, our current CFO and certain former Liuzhou BCT shareholders, namely Huitian Tang, our current CEO, Youru Jiang, Chunlin Liu, Wende Wei, Bangfu Wang, Mingan Zhao, Qingqiu Zhang, Xiaojian Yang, Yuangang Meng, Qifeng Jiang, Wenheng He, Gongchun Liu, Junwen Jia, Yujing Tan, Jinghua Li, Yuanjian Ye.
The Earn-In Agreement was amended on December 30, 2009 to extend the performance targets from 2009 and 2010 fiscal years to 2010 and 2011 fiscal years because the 2009 performance target was not going to be met. The amendment to the Earn-In Agreement was made to increase the likelihood that the Liuzhou BCT shareholders would all be able to earn back their shares in the Company, which was the goal and purpose of the reorganization. These amendments were made without additional consideration being paid.
The Earn-In Agreement enables those Liuzhou BCT shareholders to purchase shares of Ingenious (or its public parent company) from Ms. Zhang for a nominal amount per share provided that the Company meets certain performance targets for fiscal 2010 and 2011. For the 2010 and 2011 fiscal years the performance targets for the Company are $26 million and $28 million after tax audited net income, respectively. If the 2010 performance target is met, the Liuzhou BCT shareholders have the right to acquire 50% of shares held by Ms. Zhang over which they have a call right. If the 2011 performance target is met, the Liuzhou BCT shareholders have the right to acquire the other 50% of the shares held by Ms. Zhang over which they have a call right. The number of shares which can be acquired by the Liuzhou BCT shareholders under the Earn-In Agreement is in proportion to their former relative ownership interest in Liuzhou BCT. Ms. Zhang may not transfer the shares during the five-year term of the Earn-In Agreement and in the event that all of the shares have not been delivered to the Liuzhou BCT shareholders, Ms. Zhang can only transfer them as she and the Liuzhou BCT shareholders agree. As the Company has met both of the 2010 and 2011 performance targets, the Liuzhou BCT Shareholders are entitled to the call right to purchase back their shares at any time.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Our independent registered public accounting firm for the audit of our annual financial statements for each of our fiscal year ended December 31, 2011 and December 31, 2010 was PKF, Certified Public Accountants, A Professional Corporation, Glendale, California, USA. The following table shows the fees paid or accrued by us to PKF California for 2011 and 2010, respectively.
|
2011
|
2010
|
|||||||
|
Audit fees
|
$ |
180,000
|
$ | 168,000 | ||||
|
Audit-related fees
|
$ | 0 | $ | 0 | ||||
|
Tax fees
|
$ | 0 | $ | 0 | ||||
|
All other fees
|
$ | 15,999 | $ | 0 | ||||
Audit fees were for professional services rendered for the audit of our company’s annual financial statements, the review of quarterly financial statements and assistance in the preparation of statutory and regulatory filings.
Our audit committee has evaluated and approved in advance the scope and cost of the engagement of an auditor before the auditor rendered audit services.
79
PART IV
| ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
(a) The following documents are filed as part of this Report:
1. Financial Statements.
Incorporated by reference from the financial statements and notes thereto that are set forth in Item 8 of this Annual Report on Form 10-K.
2. Financial Statement Schedules.
No schedules have been filed because they are not applicable or not required, or the information is included in the Consolidated Financial Statements or Notes thereto.
(b) Exhibits.
|
2.1
|
Share Exchange Agreement dated December 23, 2009 (1)
|
|
3.1
|
First Amended and Restated Certificate of Incorporation (2)
|
|
3.2
|
By-laws (3)
|
|
4.1
|
Form of Warrant (1)
|
|
4.2
|
Form of Co-Placement Agent Warrant (4)
|
|
4.3
|
Certificate of Designation of Series A Convertible Preferred Shares (2)
|
|
10.1
|
Subscription Agreement Dated October 23, 2009 (1)
|
|
10.2
|
Co-Placement Agent Agreement Dated October 21, 2009 (5)
|
|
10.3
|
Escrow Deposit Agreement Dated October 21, 2009 (1)
|
|
10.4
|
Earn-In Agreement dated October 22, 2009 (1)
|
|
10.5
|
Share Transfer Agreement dated as of April 1, 2008 (5)
|
|
10.6
|
Shares Pledge Agreement dated May 3, 2008 (1)
|
|
10.7
|
Shares Pledge Agreement dated March 31, 2009 (1)
|
|
10.8
|
Shares Repurchase Agreement dated July 31, 2008 (1)
|
|
10.9
|
Loan Agreement and Pledge Agreement dated December 19, 2008 by and among Liuzhou Baicaotang, Baicaotang Property Development Limited and Liuzhou City Commercial Bank (5)
|
|
10.10
|
Loan Agreement and Pledge Agreement dated December 29, 2008 by and among Liuzhou Baicaotang, Baicaotang Property Development Limited and Rurol Credence Cooperation of Guangxi (5)
|
|
10.11
|
Loan Agreement and Pledge Agreement dated January 8, 2009 by and among Liuzhou Baicaotang, Baicaotang Property Development Limited and Agricultural Bank of China (5)
|
|
10.12
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated February 13, 2006 (5)
|
|
10.13
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated March 27, 2006 (5)
|
80
|
10.14
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated April 6, 2006 (5)
|
|
10.15
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated May 18, 2006 (5)
|
|
10.16
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated December 6, 2006 (5)
|
|
10.17
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated December 29, 2008 (5)
|
|
10.18
|
Loan Agreement dated February 8, 2007 by and between Huitian Tang and Industrial and Commercial Bank of China Guangxi Branch (5)
|
|
10.19
|
Confirmation Agreement dated December 31, 2008 by and among Huitian Tang, Liuzhou Baicaotang and Liuzhou Baicaotang Guigang Branch (5)
|
|
10.20
|
Loan Agreement dated February 12, 2007 by and between Jiang You Ru and Industrial and Commercial Bank of China Guangxi Branch (5)
|
|
10.21
|
Confirmation Agreement dated December 31, 2008 by and among Jiang You Ru, Liuzhou Baicaotang and Liuzhou Baicaotang Guigang Branch (5)
|
|
10.22
|
Lock-Up Agreement, dated December 23, 2009 (5)
|
|
10.23
|
Employment Agreement dated May 18, 2010 by and between Guangxi Liuzhou Baicaotang Medicine, Ltd., China BCT Pharmacy Group, Inc. and Huitian Tang (6)
|
|
10.24
|
Employment Agreement dated May 18, 2010 by and between Forever Well Asia Pacific Limited, China BCT Pharmacy Group, Inc. and Xiaoyan Zhang (6)
|
|
10.25
|
Earn-in Agreement dated December 30, 2009 (7)
|
|
10.26
|
Shares Pledge Agreement dated May 3, 2008 (7)
|
|
10.27
|
Shares Pledge Agreement dated March 31, 2009 (7)
|
|
10.28
|
Termination Agreement dated March 2, 2010 (7)
|
|
10.29
|
Share Transfer Agreement by and between He Wenheng and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.30
|
Share Transfer Agreement by and between Jia Junwen and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.31
|
Share Transfer Agreement by and between Jiang Qifeng and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.32
|
Share Transfer Agreement by and between Jiang Youru and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.33
|
Share Transfer Agreement by and between Li Jinghua and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
81
|
10.34
|
Share Transfer Agreement by and between Liu Chunlin and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.35
|
Share Transfer Agreement by and between Liu Gongchun and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.36
|
Share Transfer Agreement by and between Meng Yuangang and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.37
|
Share Transfer Agreement by and between Tan Yujing and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.38
|
Share Transfer Agreement by and between Ye Yuanjian and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.39
|
Share Transfer Agreement by and between Wang Bangfu and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.40
|
Share Transfer Agreement by and between Wei Wende and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.41
|
Share Transfer Agreement by and between Yang Xiaojian and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.42
|
Share Transfer Agreement by and between Zhang Qingqiu and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.43
|
Share Transfer Agreement by and between Zhao Ming’an and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.44
|
Amendment No. 1 to Earn-in Agreement, by and between Xiaoyan Zhang and the Buyers named thererin, dated May 19, 2010 (5)
|
|
10.45
|
Amended and Restated Shares Pledge Agreement by and between Guangxi Liuzhou Baicaotang Medicine Ltd. and Liuzhou Baicaotang Property Management Ltd, dated May 19, 2010 (5)
|
|
10.46
|
Amended and Restated Shares Pledge Agreement by and between Guangxi Liuzhou Baicaotang Medicine Ltd. and Liuzhou Baicaotang Property Management Ltd, dated May 19, 2010 (5)
|
|
10.47
|
Proxy Agreement by and between Guangxi Liuzhou Baicaotang Medicine Ltd. and Liuzhou Baicaotang Property Management Ltd., dated May 19, 2010 (5)
|
|
10.48
|
Form of sales agreement with related parties (7)
|
|
10.49
|
Independent Director Agreement of Simon Choi (8)
|
|
10.50
|
Independent Director Agreement of Man Wai Chiu (8)
|
|
10.51
|
Independent Director Agreement of Kam-Cheung Chin (13)
|
|
10.52
|
2010 Omnibus Securities and Incentive Plan (9)
|
|
10.53
|
Stock Option Agreement between the Registrant and Huitian Tang made as of June 27, 2010 (9)
|
|
10.54
|
Stock Option Agreement between the Registrant and Xiaoyan Zhang made as of June 27, 2010 (9)
|
82
|
10.55
|
Stock Option Agreement between the Registrant and Eng Leong Lee made as of July 16, 2010 (7)
|
|
10.56
|
Stock Option Agreement between the Registrant and Simon Choi made as of July 16, 2010 (7)
|
|
10.57
|
Stock Option Agreement between the Registrant and Man Wai Chiu made as of July 16, 2010 (7)
|
|
10.58
|
Non-Disclosure and Non-Competition Agreement dated May 18, 2010 by and between Guangxi Liuzhou Baicaotang Medicine, Ltd. and Huitian Tang (7)
|
|
10.59
|
Non-Disclosure and Non-Competition Agreement dated May 18, 2010 by and between Guangxi Liuzhou Baicaotang Medicine, Ltd. and Xiaoyan Zhang (7)
|
|
10.60
|
Asset Transfer Agreement dated February 28, 2010 by and between Liuzhou Wubaitang Medicine Chain Store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited (7)
|
|
10.61
|
Asset Transfer Agreement dated March 29, 2010 by and between Zhaoping County Laobaixin Pharmacy Chain Store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited (7)
|
|
10.62
|
Asset Transfer Agreement dated April 25, 2010 by and between Lai Bing Peng Fei Pharmacy Chain Store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited (7)
|
|
10.63
|
Form of Retail Chain Leasing Contract (7)
|
|
10.64
|
Entrust Shareholding Agreement Between Liuzhou BCT Shareholders and Ms. Xiaoyan Zhang on Ingenious Dated July 21, 2008 (11)
|
|
10.65
|
Series A Convertible Preferred Shares Purchase Agreement, by and between the Company and Milestone Longcheng Limited (10)
|
|
10.66
|
Registration Rights Agreement, by and between the Company and Milestone Longcheng Limited (10)
|
|
10.67
|
Shareholders Agreement, by and among the Company, Milestone Longcheng Limited, certain shareholders of the Company and Mr. Tian Hui Tang as representative for the shareholders (10)
|
|
10.68
|
Form of Stock Option Agreement – Not Granted Pursuant to 2010 Omnibus Securities and Incentive Plan (12)
|
|
10.69
|
Form of Stock Option Agreement Pursuant to 2010 Omnibus Securities and Incentive Plan (12)
|
|
14
|
Code of Ethics and Business Conduct (8)
|
|
21
|
List of Subsidiaries (4)
|
|
31.1
|
Certification pursuant to Rule 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. *
|
|
31.2
|
Certification pursuant to Rule 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. *
|
|
32.1
|
Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. *
|
|
32.2
|
Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. *
|
83
|
101.INS
|
XBRL Instance Document.**
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document.**
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document.**
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document.**
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document.**
|
|
(1)
|
Incorporated by reference to the Company’s Form 8-K filed on December 31, 2009.
|
|||
|
(2)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1/A filed on March 3, 2011.
|
|||
|
|
(3)
|
Incorporated by reference to the Company’s Form SB-2 filed on August 22, 2007.
|
||
|
|
(4)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1 filed on March 3, 2010.
|
||
|
(5)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed on May 27, 2010.
|
|||
|
(6)
|
Incorporated by reference to the Company’s Form 8-K filed on May 21, 2010.
|
|||
|
(7)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed July 29, 2010.
|
|||
|
|
(8)
|
Incorporated by reference to the Company’s Form 8-K filed on June 9, 2010.
|
||
|
|
(9)
|
Incorporated by reference to the Company’s Form 8-K filed on June 29, 2010.
|
||
|
(10)
|
Incorporated by reference to the Company’s Form 8-K filed on January 18, 2011. | |||
| (11) | Incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed October 20, 2010 | |||
| (12) | Incorporated by reference to the Company’s Form 8-K filed on December 19, 2011. | |||
| (13) |
Incorporated by reference to the Company’s Annual Report on Form 10-K filed on March 30, 2012.
|
|||
* Filed herewith.
** Previously furnished with Amendment No. 1 to the Company’s Annual Report on Form 10-K/A filed on April 6, 2012, and incorporated herein by reference.
84
China BCT Pharmacy Group, Inc.
Consolidated Financial Statements
Index to Consolidated Financial Statements
|
Pages
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
Consolidated Statements of Income and Comprehensive Income
|
F-2
|
|
Consolidated Balance Sheets
|
F-3 - F-4
|
|
Consolidated Statements of Cash Flows
|
F-5 - F-6
|
|
Consolidated Statements of Stockholders’ Equity
|
F-7
|
|
Notes to Consolidated Financial Statements
|
F-8 - F-32
|
Report of Independent Registered Public Accounting Firm
To the Directors and Stockholders of
China BCT Pharmacy Group, Inc.
We have audited the accompanying consolidated balance sheets of China BCT Pharmacy Group, Inc. and its subsidiaries (the “Company”) as of December 31, 2011 and 2010, and the related consolidated statements of income and comprehensive income, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company and its subsidiaries as of December 31, 2011 and 2010, and the consolidated results of their operations and their cash flows for each of the two years in the period ended December 31, 2011 in conformity with accounting principles generally accepted in the United States of America.
PKF
Certified Public Accountants
A Professional Corporation
Glendale, California
March 30, 201 2
F-1
China BCT Pharmacy Group, Inc.
Consolidated Statements of Income and Comprehensive Income
(Stated in US Dollars)
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Sales revenue
|
$ | 257,487,138 | $ | 200,813,260 | ||||
|
Cost of sales
|
195,670,634 | 151,988,552 | ||||||
|
Gross profit
|
61,816,504 | 48,824,708 | ||||||
|
Operating expenses
|
||||||||
|
Administrative expenses
|
12,406,676 | 8,097,374 | ||||||
|
Selling expenses
|
7,210,359 | 5,350,258 | ||||||
|
Total operating expenses
|
19,617,035 | 13,447,632 | ||||||
|
Income from operations
|
42,199,469 | 35,377,076 | ||||||
|
Non-operating income (expense)
|
||||||||
|
Interest income
|
60,479 | 11,651 | ||||||
|
Other income
|
159,062 | 168,732 | ||||||
|
Change in fair value of warrant liabilities
|
1,082,202 | 582,226 | ||||||
|
Other expenses
|
(502,729 | ) | (462,989 | ) | ||||
|
Finance costs
|
(892,172 | ) | (878,390 | ) | ||||
|
Exchange income (loss)
|
120,519 | (23,608 | ) | |||||
|
Total non-operating income (expense)
|
27,361 | (602,378 | ) | |||||
|
Income before income taxes
|
42,226,830 | 34,774,698 | ||||||
|
Income taxes
|
(11,036,300 | ) | (9,086,106 | ) | ||||
|
Net income
|
31,190,530 | 25,688,592 | ||||||
|
Other comprehensive income
|
||||||||
|
Foreign currency translation adjustments
|
4,481,290 | 2,317,595 | ||||||
|
Total comprehensive income
|
$ | 35,671,820 | $ | 28,006,187 | ||||
|
Earnings per share: basic and diluted
|
$ | 0.72 | $ | 0.67 | ||||
|
Weighted average number of shares
|
||||||||
|
outstanding: basic
|
38,154,340 | 38,063,507 | ||||||
|
|
||||||||
|
Weighted average number of shares
|
||||||||
|
outstanding: diluted
|
38,156,873 | 38,415,441 | ||||||
|
Reconciliation of net income to income applicable to common stock:
|
||||||||
|
Net income
|
||||||||
|
Less: dividends and accretion on preferred stock
|
3,890,037 | - | ||||||
|
Income applicable to common stock
|
$ | 27,300,493 | $ | 25,688,592 | ||||
See the accompanying notes to consolidated financial statements.
F-2
China BCT Pharmacy Group, Inc.
Consolidated Balance Sheets
(Stated in US Dollars)
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Assets
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$
|
31,479,528
|
$
|
20,157,112
|
||||
|
Restricted cash
|
1,052,096
|
1,334,868
|
||||||
|
Accounts receivable, net
|
118,406,001
|
68,664,308
|
||||||
|
Bills receivable
|
33,052
|
-
|
||||||
|
Amounts due from related companies
|
255,169
|
3,784,069
|
||||||
|
Other receivables, prepayments and deposits
|
5,750,056
|
3,332,747
|
||||||
|
Inventories
|
14,183,052
|
10,776,877
|
||||||
|
Deferred income taxes
|
927,860
|
207,222
|
||||||
|
Total current assets
|
172,086,814
|
108,257,203
|
||||||
|
Property, plant and equipment, net
|
18,097,062
|
14,605,888
|
||||||
|
Land use rights, net
|
13,584,135
|
13,422,048
|
||||||
|
Long-term deposits
|
7,070,400
|
3,482,200
|
||||||
|
Goodwill
|
540,157
|
560,418
|
||||||
|
Other intangible assets, net
|
504,948
|
581,481
|
||||||
|
Deferred income taxes
|
667,509
|
629,798
|
||||||
|
Other investment
|
31,424
|
-
|
||||||
|
Total assets
|
$
|
212,582,449
|
$
|
141,539,036
|
||||
See the accompanying notes to consolidated financial statements.
F-3
China BCT Pharmacy Group, Inc.
Consolidated Balance Sheets (Cont’d)
(Stated in US Dollars)
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Liabilities and stockholders’ equity
|
||||||||
|
Liabilities
|
||||||||
|
Current liabilities
|
||||||||
|
Accounts payable
|
$ | 42,290,191 | $ | 35,497,337 | ||||
|
Bills payable
|
2,104,192 | 2,669,752 | ||||||
|
Other payables and accrued expenses
|
5,490,237 | 4,856,956 | ||||||
|
Amounts due to directors
|
168,246 | 190,484 | ||||||
|
Amounts due to related companies
|
37,604 | 139,219 | ||||||
|
Income tax payable
|
2,726,869 | 2,564,359 | ||||||
|
Secured bank loans
|
9,036,060 | 8,898,218 | ||||||
|
Other loans
|
200,956 | 162,664 | ||||||
|
Retirement benefit costs
|
46,854 | 33,412 | ||||||
|
Total current liabilities
|
62,101,209 | 55,012,401 | ||||||
|
Secured long-term bank loans
|
206,767 | 1,941,606 | ||||||
|
Warrant liabilities
|
190,991 | 1,273,193 | ||||||
|
Retirement benefit costs
|
177,368 | 213,763 | ||||||
|
Total liabilities
|
62,676,335 | 58,440,963 | ||||||
|
Commitments and contingencies (Note 19)
|
||||||||
|
Convertible redeemable preferred stock
|
||||||||
|
Series A convertible redeemable preferred stock: $0.001 par value; 20,000,000 shares authorized; 9,375,000 and zero shares issued and outstanding as of December 31, 2011 and 2010, respectively
|
33,385,903 | - | ||||||
|
|
||||||||
|
Stockholders’ equity
|
||||||||
|
Common stock: $0.001 par value; 150,000,000 and 100,000,000 shares authorized; 38,154,340 shares issued and outstanding as of December 31, 2011 and 2010
|
38,154 | 38,154 | ||||||
|
Additional paid-in capital
|
18,273,766 | 16,633,411 | ||||||
|
Statutory and other reserves
|
6,851,002 | 4,585,854 | ||||||
|
Accumulated other comprehensive income
|
8,909,155 | 4,427,865 | ||||||
|
Retained earnings
|
82,448,134 | 57,412,789 | ||||||
|
Total stockholders’ equity
|
116,520,211 | 83,098,073 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 212,582,449 | $ | 141,539,036 | ||||
See the accompanying notes to consolidated financial statements.
F-4
China BCT Pharmacy Group, Inc.
Consolidated Statements of Cash Flows
(Stated in US Dollars)
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Cash flows from operating activities :
|
||||||||
|
Net income
|
$ | 31,190,530 | $ | 25,688,592 | ||||
|
Adjustments to reconcile net income to net
|
||||||||
|
cash (used in) provided by operating activities :
|
||||||||
|
Depreciation and amortization
|
1,442,311 | 1,188,505 | ||||||
|
Deferred income taxes
|
(726,806 | ) | (91,463 | ) | ||||
|
Loss on sale of property, plant and equipment
|
176,312 | - | ||||||
|
Gain on sale of land use right
|
- | (44,919 | ) | |||||
|
Goodwill impairment
|
37,355 | |||||||
|
Change in fair value of warrant liabilities
|
(1,082,202 | ) | (582,226 | ) | ||||
|
Share-based compensation
|
1,640,355 | 1,253,858 | ||||||
|
Allowance for doubtful accounts
|
367,989 | 78,068 | ||||||
|
Changes in operating assets and liabilities, net of effects of acquisitions
|
||||||||
|
Accounts receivable
|
(44,063,988 | ) | (31,464,010 | ) | ||||
|
Bills receivable
|
(32,552 | ) | - | |||||
|
Other receivables, prepayments and deposits
|
(1,598,394 | ) | (708,878 | ) | ||||
|
Inventories
|
(2,164,927 | ) | 1,273,013 | |||||
|
Accounts payable
|
10,128,630 | 15,365,984 | ||||||
|
Bills payable
|
(653,000 | ) | 350,228 | |||||
|
Other payables and accrued expenses
|
(466,503 | ) | 1,665,804 | |||||
|
Income tax payable
|
55,544 | 1,938,527 | ||||||
|
Retirement benefit costs
|
(31,867 | ) | (22,049 | ) | ||||
|
Total adjustments
|
(36,971,743 | ) | (9,799,558 | ) | ||||
|
Net cash flows (used in) provided by operating activities
|
(5,781,213 | ) | 15,889,034 | |||||
|
Cash flows from investing activities :
|
||||||||
|
Addition of property, plant and equipment
|
(4,071,623 | ) | (450,106 | ) | ||||
|
Payments to acquire retail stores
|
- | (6,037,743 | ) | |||||
|
Proceeds from sale of property, plant and equipment
|
5,921 | - | ||||||
|
Long-term deposits
|
(3,365,600 | ) | (3,379,100 | ) | ||||
|
Proceeds from sale of land use right
|
- | 697,495 | ||||||
|
Payments to acquire intangible assets
|
(18,253 | ) | - | |||||
|
Net cash from acquisition of distribution chains
|
36,502 | - | ||||||
|
Other investment
|
(31,362 | ) | - | |||||
|
Net cash flows used in investing activities
|
$ | (7,444,415 | ) | $ | (9,169,454 | ) | ||
See the accompanying notes to consolidated financial statements.
F-5
China BCT Pharmacy Group, Inc.
Consolidated Statements of Cash Flows (Cont’d)
(Stated in US Dollars)
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Cash flows from financing activities :
|
||||||||
|
Advance/ repayment activities with related companies, net
|
$ | (5,037,674 | ) | $ | 620,549 | |||
|
Net proceeds from placement of preferred stock
|
29,495,866 | - | ||||||
|
Proceeds received from private placement
|
- | 2,315,138 | ||||||
|
Restricted cash
|
330,517 | (138,823 | ) | |||||
|
Proceeds from bank loans
|
9,888,040 | 8,039,160 | ||||||
|
Repayments of bank loans
|
(11,838,221 | ) | (8,329,434 | ) | ||||
|
Repayments of other loans
|
- | (2,238,759 | ) | |||||
|
Repayments to directors
|
(28,689 | ) | (830,557 | ) | ||||
|
Proceeds from other loans
|
- | 35,208 | ||||||
|
Net cash flows provided by financing activities
|
22,809,839 | (527,518 | ) | |||||
|
Effect of foreign currency translation on cash and cash equivalents
|
1,738,205 | 660,892 | ||||||
|
Net increase in cash and cash equivalents
|
11,322,416 | 6,852,954 | ||||||
|
Cash and cash equivalents - beginning of year
|
20,157,112 | 13,304,158 | ||||||
|
Cash and cash equivalents - end of year
|
$ | 31,479,528 | $ | 20,157,112 | ||||
|
Supplemental disclosures for cash flow information :
|
||||||||
|
Cash paid for
|
||||||||
|
- Interest
|
$ | 799,339 | $ | 951,670 | ||||
|
- Income taxes
|
$ | 11,681,301 | $ | 7,255,019 | ||||
| Non-cash financing and investing activities: | ||||||||
|
Dividedns and accretion on preferred stock
|
$ | 3,890,037 | $ | - | ||||
See the accompanying notes to consolidated financial statements.
F-6
China BCT Pharmacy Group, Inc.
Consolidated Statements of Stockholders’ Equity
(Stated in US Dollars)
|
Accumulated
|
||||||||||||||||||||||||||||
|
Additional
|
Statutory
|
other
|
||||||||||||||||||||||||||
|
Common stock
|
paid-in
|
and other
|
comprehensive
|
Retained
|
||||||||||||||||||||||||
|
No. of shares
|
Amount
|
capital
|
reserves
|
income
|
earnings
|
Total
|
||||||||||||||||||||||
|
Balance, January 1, 2010
|
37,089,370 | $ | 37,089 | $ | 14,920,899 | $ | 2,605,901 | $ | 2,110,270 | $ | 33,704,150 | $ | 53,378,309 | |||||||||||||||
|
Net income
|
- | - | - | - | - | 25,688,592 | 25,688,592 | |||||||||||||||||||||
|
Foreign currency translation adjustments
|
- | - | - | - | 2,317,595 | - | 2,317,595 | |||||||||||||||||||||
|
Appropriation to reserves
|
- | - | - | 1,979,953 | - | (1,979,953 | ) | - | ||||||||||||||||||||
|
Share-based compensation
|
- | - | 1,253,858 | - | - | - | 1,253,858 | |||||||||||||||||||||
|
Reclassification
|
- | - | (1,294,142 | ) | - | - | - | (1,294,142 | ) | |||||||||||||||||||
|
Private placement
|
1,029,970 | 1,030 | 1,752,831 | - | - | - | 1,753,861 | |||||||||||||||||||||
|
Shares issued for the payment of placement service
|
35,000 | 35 | (35 | ) | - | - | - | - | ||||||||||||||||||||
|
Balance, December 31, 2010
|
38,154,340 | 38,154 | 16,633,411 | 4,585,854 | 4,427,865 | 57,412,789 | 83,098,073 | |||||||||||||||||||||
|
Net income
|
- | - | - | - | - | 31,190,530 | 31,190,530 | |||||||||||||||||||||
|
Foreign currency translation adjustments
|
- | - | - | - | 4,481,290 | - | 4,481,290 | |||||||||||||||||||||
|
Appropriation to reserves
|
- | - | - | 2,265,148 | - | (2,265,148 | ) | - | ||||||||||||||||||||
|
Share-based compensation
|
- | - | 1,640,355 | - | - | - | 1,640,355 | |||||||||||||||||||||
|
Dividend on preferred stock
|
- | - | - | - | - | (1,250,000 | ) | (1,250,000 | ) | |||||||||||||||||||
|
Accretion of preferred stock to redemption
|
- | - | - | - | - | (2,640,037 | ) | (2,640,037 | ) | |||||||||||||||||||
|
Balance, December 31, 2011
|
38,154,340 | $ | 38,154 | $ | 18,273,766 | $ | 6,851,002 | $ | 8,909,155 | $ | 82,448,134 | $ | 116,520,211 | |||||||||||||||
See the accompanying notes to consolidated financial statements.
F-7
China BCT Pharmacy Group, Inc.
Notes to Consolidated Financial Statements
December 31, 2011 and 2010
|
1.
|
Corporate information
|
|
(i)
|
China BCT Pharmacy Group, Inc. (the “Company”), formerly known as Purden Lake Resource Corp. whose name changed to China Baicaotang Medicine Limited on December 24, 2009 and then to China BCT Pharmacy Group, Inc. on March 25, 2010, was incorporated in the State of Delaware on November 30, 2006 as a limited liability company with authorized capital stock consisting of 100,000,000 shares of common stock, par value $0.001. Prior to the completion of reverse takeover transaction (“RTO”) on December 30, 2009 as mentioned in Note 2 (iv), the Company was a development stage company for acquisition, exploration and development of natural resource properties. Since the completion of the RTO, the Company has engaged in distribution, retail and production of pharmaceutical drugs in the People’s Republic of China (the “PRC”).
|
|
(ii)
|
Forever Well Asia Pacific Ltd. (“Forever Well”) was incorporated in Hong Kong on January 10, 2008 as a limited liability company with authorized, issued, paid up capital of HK$10,000, and 10,000 common shares of HK$1 par value each. The principal activity of Forever Well is investment holding.
|
|
(iii)
|
Ingenious Paragon Global Limited (“Ingenious”) was incorporated in the British Virgin Islands (the “BVI”) on May 29, 2008 as a limited liability company with authorized, issued, paid up capital of $50,000, and 50,000 common shares of $1 par value each. Prior to the completion of RTO on December 30, 2009, the 50,000 common shares were held by Xiaoyan Zhang, Chief Financial Officer of the Company.
|
|
(iv)
|
Guangxi Liuzhou Baicaotang Medicine Co., Ltd. (“Liuzhou BCT”) was established on April 3, 1986 in the PRC as a state-owned enterprise. On June 20, 2001, Liuzhou BCT was transformed into a joint stock enterprise through management buyout by its management and employees. On December 29, 2007, Liuzhou BCT was transformed into a limited liability company. Liuzhou BCT is engaged in the distribution of pharmaceutical drugs in the PRC. Before the acquisition by Forever Well as stated in Note 2(ii), the registered and paid up capital was RMB10,000,000 which were held as to 23.83% by Huitian Tang, 13.44% by Jinghua Li, 10.81% by Wende Wei, 6.81% by Youru Jiang, 6.81% by Chunlin Liu and 6.81% by Bangfu Wang, who are also directors of Ingenious and Liuzhou BCT. The remaining 31.49% was held by nine stockholders, who are employees of Liuzhou BCT.
|
|
(v)
|
Guangxi Liuzhou Baicaotang Medicine Retail Chain Co., Ltd. (“BCT Retail”), a wholly owned subsidiary of Liuzhou BCT, was established on October 30, 2001 with registered and paid up capital of RMB300,000. BCT Retail is engaged in the retail sales of pharmaceutical drugs in the PRC.
|
|
(vi)
|
Guangxi Hefeng Pharmaceutical Co., Ltd. (“Hefeng Pharmaceutical”) was established on September 18, 2000 with registered and paid up capital of RMB5,000,000. Hefeng Pharmaceutical is engaged in the production and sale of drugs for healing of hepatitis, cough, and Parkinson’s disease in the PRC. On December 31, 2007, Liuzhou BCT entered into an agreement with Jinghua Li to acquire his entire interest in Hefeng Pharmaceutical at a consideration of RMB36,340,064 (equivalent to $4,982,223) which was satisfied by issuance of 13.44% shares of Liuzhou BCT. The acquisition was completed on January 2, 2008, which was the date Liuzhou BCT obtained the control over Hefeng Pharmaceutical by appointing directors onto the board of directors of Hefeng Pharmaceutical.
|
F-8
|
2.
|
Reorganization
|
The China BCT Pharmacy Group, Inc, and its subsidiaries Liuzhou BCT, Ingenious, Forever Well, and BCT Retail reorganized their group structure (the “Reorganization”) as follows:
|
(i)
|
Due to certain regulatory restrictions on a wholly owned foreign enterprise or its wholly owned subsidiary to hold over 50% equity interest in any PRC company which operates more than 30 drug stores in the PRC (the “Drug Stores Restrictions”), Liuzhou BCT sold its 51% equity interest in BCT Retail to Liuzhou Baicaotang Property Management Company Ltd. (“Baicaotang Property”), of which the directors of Ingenious are the controlling stockholders of Baicaotang Property, at a consideration of RMB153,000 on April 1, 2008. Afterwards, Baicaotang Property pledged its 51% equity interest in BCT Retail to Liuzhou BCT to secure a loan, amounting to RMB153,000, granted by Liuzhou BCT to Baicaotang Property up to December 31, 2015 for the acquisition of the 51% equity interest in BCT Retail. According to a Repurchase Agreement dated July 31, 2008, Liuzhou BCT was entitled to a preemptive right to repurchase the 51% equity interest in BCT Retail from Baicaotang Property up to the earlier of December 31, 2017 or the removal of the Drug Stores Restrictions. Before the execution of the preemptive right by Liuzhou BCT, the rights and obligations as a stockholder of the 51% equity interest in BCT Retail are still vested in Liuzhou BCT and the appointment of the board of directors and management is controlled by Liuzhou BCT.
|
|
(ii)
|
On March 28, 2008, Forever Well entered into an agreement with the stockholders of Liuzhou BCT to acquire their entire equity interest in Liuzhou BCT at a cash consideration of RMB10,000,000, which is the registered and fully paid up capital of Liuzhou BCT.
|
|
(iii)
|
On June 30, 2008, Ingenious acquired the entire equity interest in Forever Well at a cash consideration of HK$10,000, which is the issued and fully paid up capital of Forever Well.
|
|
|
(iv) On December 23, 2009, the Company entered into a Share Exchange Agreement with the shareholders of Ingenious to acquire 100% of the issued and outstanding common shares in Ingenious by issuing 32,000,000 shares of the Company’s common stock, par value $0.001 per share (the “Exchange Transaction”).
|
Following the completion of the Exchange Transaction on December 30, 2009, 2,900,000 shares of the Company’s common stock of $0.001 each, which were held by the Company’s shareholder, Lisa Lopomo, were cancelled on December 30, 2009.
The Exchange Transaction, which was completed on December 30, 2009, constituted a RTO and thereafter Ingenious became a wholly owned subsidiary of the Company.
|
(iv)
|
On December 23, 2009, the Company’s Chief Financial Officer, Xiaoyan Zhang entered into an Earn-In Agreement with the former stockholders of Liuzhou BCT of which the former stockholders of Liuzhou BCT are given the rights to acquire 22,480,000 common shares (Earn-In Shares) of the Company at $300,000 based on their former respective equity interest in Liuzhou BCT immediately before the acquisition by Forever Well as stated in Note 2 (ii) provided that the Company meets the profit targets, representing audited net income of $26 million for 2010 and $28 million for 2011. The former stockholders are allowed to acquire 50% and 50% of Earn-In Shares upon meeting the profit targets for 2010 and 2011, respectively.
|
Upon the completion of Reorganization on December 23, 2009, the Company, Forever Well, Liuzhou BCT, BCT Retail and Hefeng Pharmaceutical are under common control of Huitian Tang, Jinghua Li, Wende Wei, Youru Jiang, Chunlin Liu and Bangfu Wang, who are the directors of the Company and Liuzhou BCT. The acquisition of Ingenious, Forever Well and Liuzhou BCT has been accounted for as a combination of entities under common control.
F-9
|
3.
|
Acquisition
|
During the year ended 2011, the Company acquired the businesses of two sub-distributors and one vendor engaged in packaging of Chinese traditional herbs. The acquisitions were structured in such a manner that the Company assumed liabilities equal to the estimated fair value of the assets acquired. No additional consideration was paid, or is payable. The following summarizes the major class of assets acquired and liabilities assumed at the date of acquisition:
|
Cash
|
$ | 36,502 | ||
|
Accounts receivable
|
4,737,280 | |||
|
Inventory
|
751,112 | |||
|
Other current assets (a)
|
5,724,522 | |||
|
Property, plant and equipment
|
35,006 | |||
|
Account payables
|
(1,783,102 | ) | ||
|
Other current liabilities (b)
|
(9,501,320 | ) | ||
|
Net assets acquired
|
$ | - |
(a) Includes amounts due from the Company of $5,397,757 that are eliminated in consolidation.
(b) Includes amounts due to the Company of $4,127,756 that are eliminated in consolidation.
In order to complement the existing store network and help the company to establish a presence in new markets, the Company selectively acquired retail drugstores in both rural and urban areas of Guangxi province. The Company targeted retail stores in prime locations with good brand names, well-developed facilities and customer bases. The Company acquired certain chain stores successively during 2010 as follows:
On February 28, 2010, the Company entered an agreement with Liuzhou City Wubaitang Medical Chain Store Co., Ltd. (柳州市伍佰堂医药连锁有限公司) for the purchase of thirty retail chain stores including the leases of the premises, inventories, and furniture, fixtures and equipment. The Company has paid total consideration for the transaction of $2,495,431 (equivalent to RMB16,482,375). The transaction was completed on April 1, 2010.
On March 29, 2010, the Company entered an agreement with Zhaoping County Laobaixin Pharmacy Chain Store Co., Ltd. (昭平县老百姓大药房连锁有限公司) for the purchase of eighteen retail chain stores including the leases of the premises, inventories, and furniture, fixtures and equipment. The Company has paid total consideration for the transaction of $640,206 (equivalent to RMB4,228,573). The transaction was completed on May 1, 2010.
On April 25, 2010, the Company entered an agreement with Lai Bing Peng Fei Pharmacy Chain Store Co., Ltd. (來宾腾飞药业连锁有限公司) for the purchase of eleven retail chain stores including the leases of the premises, inventories, and furniture, fixtures and equipment. The Company has paid total consideration for the transaction of $1,286,301 (equivalent to RMB8,496,044). The transaction was completed on April 1, 2010.
On September 19, 2010, the Company entered an agreement with Guangxi San Le Da Uao Fang Pharmacy Chain Store Co., Ltd. (广西三乐大药房连锁有限责任公司) for the purchase of fifteen retail chain stores including the leases of the premises, inventories, and furniture, fixtures and equipment. The Company has paid total consideration for the transaction of $715,208 (equivalent to RMB 4,723,969). The transaction was completed on October 1, 2010.
On September 20, 2010, the Company entered an agreement with Rong Shui Sian Jiu Zhou Pharmacy Chain Store Co., Ltd. (融水县九州医药连锁有限公司) for the purchase of eighteen retail chain stores including the leases of the premises, inventories, and furniture, fixtures and equipment. The Company has paid total consideration for the transaction of $900,598 (equivalent to RMB5,948,459). The transaction was completed on October 1, 2010.
F-10
The following table summarizes the allocation of the purchase price reflecting the amounts of each major class of assets acquired and liabilities assumed at the date of acquisition of the above acquisitions:
|
Assets
|
|
|||
| Inventory | $ | 3,053,859 | ||
|
Furniture, fixtures and equipment, net
|
2,386,782 | |||
|
VAT Recoverable
|
144,654 | |||
| Goodwill | 452,449 | |||
| Net assets acquired | $ | 6,037,744 | ||
|
Cash paid for acquisition
|
$ | 6,037,744 | ||
As of December 31, 2010, the consolidated balance sheet includes goodwill identified upon the above acquisitions amounting to $452,449 which represents the excess of the purchase price over the fair value of the identifiable net assets acquired.
The Company with advice from an independent appraiser has identified all assets acquired including intangible assets which meet either the separability criterion or the contractual-legal criterion in accordance with FASB ASC 805 as of the date of acquisition. No significant identifiable intangible assets inclusive of trademark, product licenses and customers contracts were identified and recognized.
|
4.
|
Summary of significant accounting policies
|
Basis of consolidation
The consolidated financial statements include the accounts of China BCT Pharmacy Group., Inc. and its subsidiaries; Ingenious, Forever Well, Liuzhou BCT, BCT Retail and Hefeng Pharmaceutical. All significant inter-company accounts and transactions have been eliminated in consolidation.
Use of estimates
The Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) is the exclusive reference of authoritative U.S. generally accepted accounting principles (“GAAP”) recognized by the FASB to be applied by nongovernment entities. Rules and interpretive releases of the Securities and Exchange Commission (“SEC”) under authority of US federal securities laws are also sources of authoritative US GAAP for SEC registrants. The Company’s financial statements are prepared in accordance with US GAAP, which may require the use of management estimates and assumptions. The accounts and estimates include, but are not limited to, the valuation of accounts receivable and inventories, the estimation on useful lives of property, plant and equipment and intangible assets, and goodwill impairment. Actual results could differ from those estimates.
Cash and cash equivalents
Cash and cash equivalents include all cash, deposits in banks and other highly liquid investments with initial maturities of three months or less. As of December 31, 2011 and 2010, cash and cash equivalents were mainly denominated in Renminbi (“RMB”) and United States dollars were placed with banks in the PRC and Hong Kong. For those denominated in RMB, they are not freely convertible into foreign currencies and the remittance of these funds out of the PRC is subject to exchange control restrictions imposed by the PRC government. The remaining insignificant balance of cash and cash equivalents were denominated in Hong Kong dollars.
F-11
Restricted Cash
Restricted cash represents amounts set aside by the Company in accordance with the Company’s debt agreements with certain financial institutions. These cash amounts are designated for the purpose of paying down the principal amounts owed to the financial institutions, and these amounts are held at the same financial institutions with which the Company has debt agreements in the PRC. Due to the short-term nature of the Company’s debt obligations to these banks, the corresponding restricted cash balances have been classified as current assets in the consolidated financial statements.
Allowance for doubtful accounts
The Company establishes an allowance for doubtful accounts based on management’s assessment of the
collectability of accounts receivable. A considerable amount of judgment is required in assessing the amount of the allowance. The Company considers the historical level of credit losses of all segments (pharmaceutical distribution, retail pharmacy and manufacturing) and applies percentages to aged receivable categories. The Company makes judgments about the creditworthiness of each customer based on ongoing credit evaluations, and monitors current economic trends that might impact the level of credit losses in the future. If the financial condition of the customers were to deteriorate resulting in their inability to make payments, a higher allowance may be required.
The Company's general credit sales policy is to provide its customers with credit ranging from one month to six months. Within the period, the Company does not consider the receivables overdue and it does not record any bad debt provision. When sales occur, the Company automatically grants its customers credit for one month to three months. An extended credit period of three to six months may be given upon sales managers’ approvals. Therefore, any receivables within six months do not have any provision for bad debt. Any receivable ranging from six months up to one year, are considered overdue, and will be subject to a bad debt provision of 40%.
As of December 31, 2011, 75% of the Company's accounts receivable was derived from hospitals, community service centers and clinics. In the PRC, all hospitals and community service centers are owned or controlled by the PRC government. The Company believes that it is highly unlikely that a liquidation of one of its hospital customers will occur, and that the recovery of debts from hospitals is highly assured. Therefore, it is within the Company's customary practice to grant a longer credit period to those accounts.
Based on the above assessment, management established the general provisioning policy to make allowance equivalent to 40% of gross amount of accounts receivable due between half and one year and 100% of gross amount of accounts receivable due over 1 year. Additional provisions are made against accounts receivable whenever they are considered doubtful.
Bad debts are written off when identified. The Company extends unsecured credit to customers ranging from three to six months in the normal course of business. The Company does not accrue interest on accounts receivable.
Historically, losses from uncollectible accounts have not significantly deviated from the general allowance estimated by management and no significant additional bad debts have been written off. This general provisioning policy has not changed since establishment and management considers that the aforementioned general provisioning policy is adequate and not too excessive and does not expect it to be changed in the near future.
Inventories
Inventories are stated at the lower of cost or market value. Cost is determined on weighted average basis and includes all expenditures incurred in bringing the goods in a saleable condition to the point of sale. The Company’s inventory reserve requirements generally fluctuate based on projected demands, market conditions and product life cycles. In determining the adequate level of inventories to have on hand, management makes judgments as to the projected inventory demands as compared to the current or committed inventory levels. Inventory quantities and expiration dates are reviewed regularly and provisions for excess or obsolete inventory are recorded based on the expiration dates and the Company’s forecast of future demand and market conditions.
F-12
No provision for excess or obsolete inventory was made for the years ended December 31, 2011 and 2010.
Property, plant and equipment
Property, plant and equipment are stated at cost less accumulated depreciation. Cost of major improvements are capitalized, and cost of normal repairs and maintenance are expensed as incurred.
Depreciation is computed on the straight-line method over the following estimated useful lives:
|
Annual rate
|
Residual value
|
|||||||
|
Buildings and improvements
|
2.54% - 9.84
|
%
|
Nil - 2
|
%
|
||||
|
Plant and machinery
|
7.00% -18.40
|
%
|
Nil - 10
|
%
|
||||
|
Motor vehicles
|
6.00% -18.40
|
%
|
10
|
%
|
||||
|
Furniture, fixtures and equipment
|
6.00% -18.40
|
%
|
10
|
%
|
||||
Cost and related accumulated depreciation of property sold or otherwise disposed are removed from the accounts, and any resulting gain or loss is included in non-operating income (expense).
Goodwill and other intangible assets
Goodwill represents the excess of the purchase price and other acquisition related costs over the estimated fair value of the net tangible and intangible assets acquired. Under FASB ASC 805, goodwill and intangible assets with indefinite useful lives are not amortized, but instead are tested for impairment at least annually. All other intangible assets, pharmaceutical licenses, customer contracts, trademarks, technology know-how and patents, are amortized over their estimated useful lives, which range from one to ten years.
Management assesses potential impairments to intangible assets at least annually, or when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered. Judgments regarding the existence of impairment indicators and future cash flows related to intangible assets are based on operational performance of the acquired businesses, market conditions and other factors. During the year ended December 31, 2011, the Company recorded an impairment loss of $37,355.
Land use right
Land use rights are stated at cost less accumulated amortization. Amortization is computed using the straight-line method over the terms of the leases ranging from forty to seventy years. The leases term are obtained from the relevant PRC land authority.
Impairment of long-lived assets
Long-lived assets are tested for impairment in accordance with FASB ASC 360-10-45 “Impairment or Disposal of Long-Lived Assets”. The Company periodically evaluates potential impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The Company recognizes impairment of long-lived assets in the event that the net book value of such assets exceeds the future undiscounted cash flows attributable to such assets. During the reporting periods, the Company has not identified any indicators that would require testing for impairment.
Revenue recognition
Revenue from sales of the Company’s products in both pharmaceutical distribution and manufacturing segments is recognized upon customer acceptance. This occurs at the time of delivery to the customer, provided persuasive evidence of an arrangement exists, such as a signed sales contract. The significant risks and rewards of ownership are transferred to the customer at the time when the products are delivered and there is no significant post-delivery obligation to the Company. In addition, the sales price is fixed or determinable and the collection is reasonably assured. The Company does not provide its customers with contractual rights of return for products. When there are significant post-delivery performance obligations, revenue is recognized only after such obligations are fulfilled. The Company evaluates the terms of the sales agreement with its customers in order to determine whether any significant post-delivery performance obligations exist. Currently sales under both pharmaceutical distribution and manufacturing segments do not include any terms which may impose any significant post-delivery performance obligations on the Company.
F-13
Revenue from sales of the Company’s products in its retail segment is recognized upon customer acceptance. This occurs at the time when the product is purchased by the customers at company-owned retail stores, and there is no significant post-delivery obligation, and collection is reasonably assured. The Company does not have a return policy allowing customers to return the products sold. When there are any significant post-delivery performance obligations, revenue is recognized only after such obligations are fulfilled. The Company evaluates the rules and regulations relating to the retail sales of drugs in the PRC in order to determine whether any significant post-delivery performance obligations exist. Currently, the rules and regulations relating to the retail sales of drugs in the PRC do not include any provisions which may impose significant post-delivery performance obligations on the Company.
Revenue from the sales of the Company’s products represents the invoiced value of goods, net of the value-added tax (“VAT”). All of the Company’s products that are sold in the PRC are subject to the Chinese value-added tax at a rate of 17 percent of the gross sales price. This VAT collected on sales may be offset by the VAT paid on purchases of raw and other materials that are included in the cost of producing the Company’s finished products.
Advertising, research and development expenses
Advertising and research and development expenses are expensed as incurred.
Retirement benefit costs
Liuzhou BCT adopted a retirement plan to provide eligible employees who were hired prior to April 23, 2002. These eligible employees are entitled to receive certain amounts of retirement benefits upon termination or retirement from the Company. The amounts are based on the employees’ years of service in Liuzhou BCT up to April 23, 2002. The obligation for retirement benefit costs is recorded at the present value of the cost that is expected to settle the obligation and is recognized when the retirement plan is approved. The employees hired after April 23, 2002 are not entitled to this retirement plan.
As of December 31, 2011, the discount rate of 7.73% (2010: 5.81%), which represented the Company’s weighted average borrowing rate of Renminbi loans in the PRC, was adopted to calculate the present value of retirement benefits cost. An analysis of the provision for retirement benefit costs for the years ended December 31, 2011 and 2010 is as follows:
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Balance at beginning of year
|
$
|
247,175
|
$
|
260,478
|
||||
|
Imputed interest
|
17,117
|
17,591
|
||||||
|
Payments
|
(48,984
|
)
|
(39,639
|
)
|
||||
|
Translation adjustments
|
8,914
|
8,745
|
||||||
|
Balance at end of year
|
$
|
224,222
|
$
|
247,175
|
||||
Shipping and handling costs
Shipping and handling costs of $842,878 and $679,185 for the years ended December 31, 2011 and 2010, respectively, are expensed as incurred and are included in selling expenses.
F-14
Vendor allowances
The Company receives allowances from certain vendors. These allowances are received for a variety of buying activities such as the volume purchase allowance associated with the vendor programs. Consideration received from a vendor is a reduction in the cost of the inventory and is recognized as a reduction in the cost of goods sold. The Company also receives promotional allowance funds for specific vendor-sponsored programs per the applicable agreements. These promotional allowance funds are recognized as a reduction in the cost of goods sold as the program occurs.
Store opening costs
Costs incurred in connection with store start-up, such as travel for recruitment, training and setup for new store openings costs are expensed as incurred.
Income taxes
The Company uses the asset and liability method of accounting for income taxes pursuant to FASB ASC 740 "Income Taxes”. Under the asset and liability method of FASB ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statements carrying amounts of existing assets and liabilities and loss carry forwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
Dividends
Dividends are recorded in the Company’s financial statements in the period in which they are declared.
Off-balance sheet arrangements
The Company does not have any off-balance sheet arrangements.
Registration payment arrangement
Contingent obligations to make future payments or otherwise transfer consideration under a registration payment arrangement, whether issued as a separate agreement or included as a provision of a financial instrument or other agreement, are separately recognized and measured when they are probable and reasonably estimable.
Comprehensive income
The Company has adopted FASB ASC 220, “Comprehensive Income”, which establishes standards for reporting and display of comprehensive income (loss), its components and accumulated balances. Components of comprehensive income (loss) include net income (loss) and foreign currency translation adjustments.
Concentrations of credit risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist of cash and cash equivalents, restricted cash, accounts receivable and amounts due from related companies. As of December 31, 2011 and 2010, substantially all of the Company’s cash and cash equivalents and restricted cash were held by major financial institutions located in the PRC, which management believes are of high credit standing. With respect to accounts receivable, the Company extends credit based on an evaluation of the customer’s financial condition. The Company generally does not require collateral for accounts receivable and maintains an allowance for doubtful accounts of accounts receivable.
During the year ended December 31, 2011 and 2010, no single customer accounted for 10% or more of the Company’s consolidated sales and no single customer constituted 10% or more of the Company’s accounts receivable.
F-15
Foreign currency translation
The functional currency of the Company is RMB which is not freely convertible into foreign currencies. The Company maintains its financial statements in the functional currency. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet date. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
Assets and liabilities of the Company’s operations are translated into the reporting currency, United States dollars, at the exchange rate in effect at the balance sheet dates. Revenue and expenses are translated at average rates in effect during the reporting periods. The resulting translation adjustment is reflected as accumulated other comprehensive income, a separate component of stockholders’ equity in the statement of stockholders’ equity.
Basic and diluted earnings per share
The Company reports basic earnings per share in accordance with FASB ASC 260, “Earnings Per Share”. Basic earnings per share is computed using the weighted average number of shares outstanding during the reporting period. The weighted average number of shares of the Company represents the common stock outstanding during the reporting periods. Diluted earnings per share is computed using the weighted average number of common shares outstanding plus the effect of dilutive securities outstanding during the reporting periods.
Fair value of financial instruments
FASB ASC 820 requires the disclosure of the estimated fair value of financial instruments including those financial instruments for which the fair value option was not elected. The carrying amounts of both financial assets and liabilities approximate their fair values due to short maturities or the applicable interest rates approximate the current market rates.
Recent accounting standards
In May 2011, the Financial Accounting Standards Board (“FASB”) issued new accounting guidance that provides a consistent definition of fair value and common requirements of and disclosure about fair value between GAAP and International Financial Reporting Standards (“IFRS”). The guidance states the concepts of highest and best use and valuation premise are only relevant when measuring the fair value of nonfinancial assets. Enhanced disclosure requirements will require companies to disclose quantitative information about unobservable inputs used, a description of the valuation processes used, and a qualitative discussion about the sensitivity of the measurements for recurring Level 3 fair value measurements. For assets and liabilities not recorded at fair value but where fair value is disclosed, companies must report the level in the fair value hierarchy of assets and liabilities. This new guidance is effective for interim and annual periods beginning January 1, 2012, and the Company does not anticipate a material impact on the consolidated financial statements.
In June 2011, the FASB issued ASU 2011-05, Presentation of Comprehensive Income, which requires an entity to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income, or in two separate but consecutive statements. ASU 2011-05 eliminates the option to present components of other comprehensive income as part of the statement of equity. In December 2011, the FASB issued ASU 2011-12, Comprehensive Income(Topic 220): Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income in Accounting Standards Update No. 2011-05 , which defers specific requirements to present reclassification adjustments for each component of accumulated other comprehensive income. ASU 2011-05 will be retroactively effective for the Company in the first quarter of 2012. Since the Company already presents single continuous statements of income and comprehensive income, this adoption will not have a material effect on the consolidated financial statements.
F-16
In September 2011, the FASB issued ASU 2011-08, Intangibles – Goodwill and Other (Topic 350): Testing Goodwill for Impairment, which permits an entity to first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying value as a basis for determining whether it is necessary to perform the two-step goodwill impairment test. ASU 2011-08 will be effective for fiscal years beginning after December 15, 2011, with early adoption permitted. The Company plans to adopt ASU 2011-08 for its annual goodwill impairment test conducted in fiscal 2012.
|
5.
|
Income taxes
|
United States
The Company is subject to the United States of America tax law at tax rates up to 34%. No provision for US federal income taxes has been made as the Company had no taxable income in this jurisdiction for the reporting periods.
BVI
Ingenious was incorporated in the BVI and, under the current laws of the BVI, is not subject to income taxes.
Hong Kong
Forever Well is subject to the Hong Kong Profits tax at a rate of 16.5%. No provision for the Hong Kong Profits tax has been made as the Company had no taxable income in this jurisdiction since its incorporation.
PRC
Corporate income tax (“CIT”) to Liuzhou BCT and BCT Retail, was charged at a rate of 25% of assessable profits.
In accordance with the Circular of the State Council on Policies and Measures Pertaining to the Development of the Western Region (“DOWR”), the companies are entitled to a preferential rate of 15%. They are engaged in the projects listed in Guiding Catalogue, and the revenue derived therefrom accounts for over 70% of total revenue. Hefeng Pharmaceutical met this DOWR requirement. It was approved by the tax authority and was granted a preferential tax rate of 15% for fiscal years from 2003 to 2010. During the year 2011, Hefeng Pharmaceutical is subject to CIT at a rate of 25% under the tax law.
The components of the provision (benefit) for income taxes are:
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Current taxes - PRC
|
$ | 11,763,106 | $ | 9,177,569 | ||||
|
Deferred taxes - PRC
|
(726,806 | ) | (91,463 | ) | ||||
| $ | 11,036,300 | $ | 9,086,106 | |||||
The effective income tax expense differs from the PRC statutory income tax rate of 25% for the years ended December 31, 2011 and 2010 in the PRC as follows :
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Provision for income taxes at PRC
|
||||||||
|
statutory income tax rate
|
$ | 10,556,708 | $ | 8,693,675 | ||||
|
Non-deductible items for tax
|
1,141,006 | 1,012,882 | ||||||
|
Non-assessable items for tax
|
(301,725 | ) | (181,743 | ) | ||||
|
Under provision in prior year
|
26,915 | 73,057 | ||||||
|
Effect on change in tax rate
|
(165,314 | ) | - | |||||
|
Tax holiday
|
- | (538,134 | ) | |||||
|
Others
|
(221,290 | ) | 26,369 | |||||
| $ | 11,036,300 | $ | 9,086,106 | |||||
F-17
During the year ended December 31, 2010, the amount of benefit from tax holiday was $538,134 and the effect on earnings per share was $0.01.
FASB ASC 740-10-25 requires recognition and measurement of uncertain income tax positions using a “more-likely-than-not” approach. Under the new CIT Law effective on January 1, 2008, the Company may be deemed to be a resident enterprise by the PRC tax authorities. If the Company was deemed to be resident enterprise, the Company may be subject to the CIT at 25% on the worldwide taxable income and dividends paid from PRC subsidiaries to their overseas holding companies may be exempted from 10% PRC withholding tax. Except for certain immaterial interest income from bank deposits placed with financial institutions outside the PRC, all of the Company’s income is generated from the PRC operation. Given the immaterial amount of income generated from outside the PRC and the PRC subsidiaries do not intend to pay dividend in the foreseeable future, the management considers that the impact arising from resident enterprise on the Company’s financial position is not significant. The management evaluated the Company’s overall tax positions and considered that no additional provision for uncertainty in income taxes is necessary as of December 31, 2011 and 2010.
Deferred tax assets/ (liabilities) as of December 31, 2011 and 2010 are composed of the followings:
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
The PRC
|
||||||||
|
Current deferred tax assets :
|
||||||||
|
Provision and accrued expenses
|
$ | 742,295 | $ | 131,672 | ||||
|
Allowance for doubtful debts
|
185,565 | 75,550 | ||||||
| $ | 927,860 | $ | 207,222 | |||||
|
Non current deferred tax assets/ liabilities :
|
||||||||
|
Depreciation of property, plant and equipment
|
$ | (147,077 | ) | $ | (32,046 | ) | ||
|
Amortization of land use right
|
538,500 | 483,356 | ||||||
|
Amortization of intangible assets
|
276,086 | 178,488 | ||||||
| $ | 667,509 | $ | 629,798 | |||||
|
6.
|
Earnings per share
|
Basic earnings per share has been computed using the weighted average number of common shares outstanding. Potential dilutive shares are included from the computation of earnings per share as average market price of the Company’s common stock exceeded the weighted average exercise price of such options, warrants, and/or preferred shares.
For the year ended December 31, 2011, dilutive options to purchase 3,660,000 shares (2,533 weighted average shares) were included in the diluted earnings per share calculation, and potentially dilutive options, warrants and preferred shares of 1,125,000, 2,111,235 and 9,375,000, respectively, were excluded from the diluted earnings per share calculation as the average market price of the Company’s common stock did not exceed the weighted average exercise price of such options, warrants and preferred shares, and to have included them would have been anti-dilutive.
F-18
For the year ended December 31, 2010, dilutive warrants to purchase 351,934 shares (351,934 weighted average shares) were included in the diluted earnings per share calculation, and potentially dilutive options and warrants of 1,125,000 and 1,759,301, respectively, were excluded from the diluted earnings per share calculation as the average market price of the Company’s common stock did not exceed the weighted average exercise price of such options and warrants, and to have included them would have been anti-dilutive.
|
7.
|
Accounts Receivable, net
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Accounts receivables
|
$ | 119,010,218 | $ | 68,891,244 | ||||
|
Less : allowance for doubtful accounts
|
(604,217 | ) | (226,936 | ) | ||||
| $ | 118,406,001 | $ | 68,664,308 | |||||
An analysis of the allowance for doubtful accounts for the years ended December 31, 2011 and 2010 is as follows:
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Balance at beginning of year
|
$ | 226,936 | $ | 144,397 | ||||
|
Allowance for doubtful accounts
|
367,989 | 78,068 | ||||||
|
Translation adjustment
|
9,292 | 4,471 | ||||||
|
Balance at end of year
|
$ | 604,217 | $ | 226,936 | ||||
No accounts receivable were pledged as collateral under any loan agreements as of December 31, 2011.
|
8.
|
Other receivables, prepayments and deposits
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Advances to staff
|
$ | 479,332 | $ | 123,500 | ||||
|
Prepayments
|
4,241,956 | 2,820,372 | ||||||
|
Other receivables
|
1,028,768 | 388,875 | ||||||
| $ | 5,750,056 | $ | 3,332,747 | |||||
|
9.
|
Inventories
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Raw materials
|
$ | 1,040,582 | $ | 860,993 | ||||
|
Work-in-progress
|
135,533 | 160,730 | ||||||
|
Finished goods
|
13,006,937 | 9,755,154 | ||||||
| $ | 14,183,052 | $ | 10,776,877 | |||||
F-19
|
10.
|
Goodwill and other intangible assets, net
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Goodwill
|
|
|||||||
|
Acquisition of retail stores
|
$ | 432,189 | $ | 452,450 | ||||
|
Acquisition of Hefeng Pharmaceutical
|
107,968 | 107,968 | ||||||
| $ | 540,157 | $ | 560,418 | |||||
|
Other intangible assets
|
||||||||
|
Pharmaceutical licenses
|
$ | 799,197 | $ | 799,197 | ||||
|
Customer contracts
|
90,729 | 90,729 | ||||||
|
Trademarks, technology know-how and patents
|
118,146 | 99,892 | ||||||
| 1,008,072 | 989,818 | |||||||
|
Accumulated amortization
|
(503,124 | ) | (408,337 | ) | ||||
| $ | 504,948 | $ | 581,481 | |||||
During the years ended December 31, 2011 and 2010, amortization expense was $95,493 and $79,346, respectively.
The estimated aggregate amortization expenses for other intangible assets for the future years are as follows:
|
Year
|
||||
|
2012
|
$ | 92,014 | ||
|
2013
|
90,399 | |||
|
2014
|
82,585 | |||
|
2015
|
79,920 | |||
|
2016
|
79,920 | |||
|
Thereafter
|
80,110 | |||
| $ | 504,948 | |||
|
11.
|
Property, plant and equipment, net
|
|
|
Property, plant and equipment are stated at cost, as follows:
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Buildings and improvements
|
$ | 13,625,189 | $ | 12,750,902 | ||||
|
Plant and machinery
|
1,552,234 | 1,453,435 | ||||||
|
Furniture, fixtures and equipment
|
5,668,331 | 3,055,934 | ||||||
|
Motor vehicles
|
443,048 | 420,683 | ||||||
| 21,288,802 | 17,680,954 | |||||||
|
Accumulated depreciation
|
(4,511,548 | ) | (3,437,366 | ) | ||||
| 16,777,254 | 14,243,588 | |||||||
|
Construction in progress
|
1,319,808 | 362,300 | ||||||
| $ | 18,097,062 | $ | 14,605,888 | |||||
F-20
|
|
(a)
|
An analysis of buildings, plant and machinery pledged to banks for bank loans (Note 16(d) (i)) is as follows:
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Buildings
|
$ | 5,471,223 | $ | 6,669,969 | ||||
|
Accumulated depreciation
|
(1,420,229 | ) | (1,469,600 | ) | ||||
| $ | 4,050,994 | $ | 5,200,369 | |||||
|
|
(b)
|
During the reporting periods, depreciation is included in:
|
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Cost of sales and overhead expense
|
$ | 185,131 | $ | 156,077 | ||||
|
Selling expenses
|
1,970 | 4,881 | ||||||
|
Administrative expenses
|
832,298 | 627,073 | ||||||
| $ | 1,019,399 | $ | 788,031 | |||||
|
12.
|
Land use rights, net
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Land use rights
|
$ | 16,440,340 | $ | 15,848,693 | ||||
|
Accumulated amortization
|
(2,856,205 | ) | (2,426,645 | ) | ||||
| $ | 13,584,135 | $ | 13,422,048 | |||||
The Company has obtained land use rights from the relevant PRC land authority for a period ranging from 40 to 70 years to use the land on which the office premises, production facilities and warehouse of the Company are situated. As of December 31, 2011, and 2010, land use rights with carrying amount of $1,127,907 and $5,239,133, respectively, were pledged to a bank for the loans granted to the Company (Note 16(d) (ii)).
During the years ended December 31, 2011 and 2010, amortization expense was $327,419, and $321,127 respectively.
During the year ended December 31, 2010, land use rights with carrying amounts of $652,576 were sold for $697,495, net of direct costs, resulting in a gain of $44,919.
The estimated aggregate amortization expenses for land use rights for future years are as follows:
|
Year
|
||||
|
2012
|
$ | 328,267 | ||
|
2013
|
328,267 | |||
|
2014
|
328,267 | |||
|
2015
|
328,267 | |||
|
2016
|
328,267 | |||
|
Thereafter
|
11,942,800 | |||
| $ | 13,584,135 | |||
F-21
|
13.
|
Other payables and accrued expenses
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Accrued staff costs
|
$ | 1,948,760 | $ | 1,580,984 | ||||
|
VAT and other tax payables
|
1,570,886 | 1,086,980 | ||||||
|
Other payables
|
874,649 | 428,455 | ||||||
|
Deposits received
|
684,568 | 962,187 | ||||||
|
Accrued audit fee
|
148,220 | 164,448 | ||||||
|
Other accrued expenses
|
263,154 | 178,006 | ||||||
|
Registration payment
|
- | 455,896 | ||||||
| $ | 5,490,237 | $ | 4,856,956 | |||||
|
14.
|
Amounts due to directors
|
These amounts are interest free, unsecured and repayable on demand, except to the amount of $47,136 and $75,700 as of December 31, 2011 and 2010, which bears interest at a rate of 24% and 6.96% per annum, respectively.
|
15.
|
Amounts due from/to related companies
|
The related companies are controlled by certain of the Company’s directors including Huitian Tang, Chairman and Chief Executive Officer. These amounts are interest free, unsecured and repayable on demand.
|
16.
|
Secured bank loans
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Short-term loans - Note 16(a)
|
$ | 7,227,520 | $ | 7,085,520 | ||||
|
Current maturities of long-term bank loan
|
1,808,540 | 1,812,698 | ||||||
| $ | 9,036,060 | $ | 8,898,218 | |||||
|
Long-term bank loans - Note 16(b)
|
$ | 2,015,307 | $ | 3,754,305 | ||||
|
Less: current maturities
|
(1,808,540 | ) | (1,812,699 | ) | ||||
| $ | 206,767 | $ | 1,941,606 | |||||
|
|
(a)
|
The weighted average interest rates for short-term loans as of December 31, 2011 and 2010 were 7.73% and 5.84% per annum, respectively.
|
|
|
(b)
|
The long-term loans as of December 31, 2011 bear interest at variable rates ranging from PRC benchmark rate plus 7.68% per annum.
|
|
|
(c)
|
As of December 31, 2011, the Company’s banking facilities include the following:
|
|
As of December 31,
|
||||||||||||||||
|
2011
|
2010
|
|||||||||||||||
|
Bills
|
Secured
|
Bills
|
Secured
|
|||||||||||||
|
|
Payable
|
Bank Loans
|
Payable
|
Bank Loans
|
||||||||||||
|
Granted
|
$ | 2,985,280 | $ | 9,242,827 | $ | 1,589,700 | $ | 10,839,824 | ||||||||
|
Amount utilized
|
$ | 1,052,096 | $ | 9,242,827 | $ | 1,334,868 | $ | 10,839,824 | ||||||||
|
Unused
|
$ | 1,933,184 | $ | - | $ | 254,832 | $ | - | ||||||||
F-22
Some suppliers request the Company to settle accounts payable by issuance of banker’s acceptance bills, which are interest free with maturity of three months from date of issuance. The Company is required to deposit with the banks equal to 50% of the bills amount at the time of issuance and pay bank charges. These deposits will be used to settle the bills at maturity.
|
|
(d)
|
As of December 31, 2011, the above bank loans were secured by the following:
|
|
|
(i)
|
Property, plant and equipment with carrying value of $4,050,994 (Note 11);
|
|
|
(ii)
|
Land use rights with carrying value of $1,127,907 (Note 12);
|
|
|
(iii)
|
Buildings and land use right owned by related companies which are controlled by certain directors.
|
During the reporting periods, there was no covenant requirement for the banking facilities granted to the Company.
The aggregate maturities of long-term bank loans as of December 31, 2011 are as follows:
|
Year
|
||||
|
2012
|
$ | 1,808,540 | ||
|
2013
|
87,154 | |||
|
2014
|
94,689 | |||
|
2015
|
24,924 | |||
| $ | 2,015,307 | |||
|
17.
|
Other loans
|
|
As of December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Other loans
|
$ | 200,956 | $ | 162,664 | ||||
All other loans are unsecured and repayable on demand. Other loans bear interest at a fixed rate of 2% per month.
|
18.
|
Warrant liabilities
|
As of December 30, 2009, the Company completed a private placement of 2,489,370 shares of common stock and five-year warrants to purchase up to 1,244,368 shares of common stock (“First Batch Warrants”). The stock was purchased at an exercise price of $3.81 per share for gross proceeds of $6,322,952, which includes issuance expenses of $1,016,290. In accordance with FASB ASC 815, these warrants are not considered indexed to the Company’s own stock and should be classified as financial derivative liabilities at fair value for each reporting period. For the year ended December 31, 2009, the fair value of First Batch Warrants was $1,294,142 and the Company considered the amount to be immaterial to the financial statements. Thus, the net proceeds were allocated to First Batch Warrants resulting in the entire amount recorded as equity.
F-23
Upon the completion of a private placement as of February 1, 2010, five-year warrants to purchase up to 514,933 shares of common stock (“Second Batch Warrants”) were issued to investors and classified as financial derivative liabilities at fair value for each reporting period in accordance with FASB ASC 815. The Company determined that the aggregate fair value of warrants issued as of February 1, 2010 was material to the financial statements for the year ended December 31, 2010. Accordingly, a reallocation was made for the fair value of First Batch Warrants as of December 31, 2009 amounting to $1,294,142 from the Company’s equity to warrant liabilities as of February 1, 2010. For the Second Batch Warrants, part of the net proceeds amounting to $561,277, representing the fair value as of February 1, 2010, were allocated to warrant liabilities at initial recognition.
The fair value of the warrants was calculated using the binomial model. The assumptions used to calculate fair value of First Batch Warrants and Second Batch Warrants as of December 31, 2011 are as follows:
|
1.
|
Expected volatility of 38.33% and 38.66% for First Batch Warrants and Second Batch Warrants, respectively.
|
|
2.
|
Expected dividend yield of 0%.
|
|
3.
|
Risk-free interest rate of 0.406% and 0.426% for First Batch Warrants and Second Batch Warrants, respectively.
|
|
4.
|
Expected lives of 3 years and 3.09 years for First Batch Warrants and Second Batch Warrants, respectively.
|
|
5.
|
Exercise price of $3.81 per share.
|
As of December 31, 2011, the fair value of warrant liabilities was $190,991 and the corresponding gain on the change in fair value of warrant liabilities of $1,082,202 was recognized in the Company’s statement of operations for the year ended December 31, 2011.
Warrants issued and outstanding, all of which are exercisable at December 31, 2011, are summarized as follows:
|
Number of shares
|
||||
|
First Batch Warrants
|
1,244,368 | |||
|
Second Batch Warrants
|
514,933 | |||
| 1,759,301 | ||||
|
19.
|
Commitments and contingencies
|
|
a.
|
Capital commitment
|
As of December 31, 2011, the Company had no capital commitments in respect of the acquisition of property, plant and equipment which were contracted for but not provided in the consolidated financial statements.
|
b.
|
Operating lease commitments
|
As of December 31, 2011, the Company had non-cancelable operating leases for its retail stores and future minimum lease payments are as follows:
|
Year
|
||||
|
2012
|
$ | 1,287,908 | ||
|
2013
|
691,703 | |||
|
2014
|
228,195 | |||
|
2015
|
79,309 | |||
|
2016
|
4,792 | |||
| $ | 2,291,907 | |||
F-24
The rental expense relating to the operating leases was $1,324,371 and $824,419 for the years ended December 31, 2011 and 2010, respectively.
|
c.
|
Operating lease arrangement
|
As of December 31, 2011, the Company leases its main facility in the PRC under an operating lease arrangement until 2013. Future minimum lease payments to be received under the non-cancelable operating lease are as follows:
|
Year
|
||||
|
2012
|
$ | 17,466 | ||
|
2013
|
3,896 | |||
| $ | 21,362 | |||
|
d.
|
Employment agreements
|
During the 2nd quarter of 2010, the Company entered into employment agreements with Huitian Tang, the Company’s Chairman and CEO, and Xiaoyan Zhang, the Company’s CFO. The employment agreements were approved by the board of directors and the compensation committee. The following is a summary of the material provisions of the employment agreements for Mr. Tang and Ms. Zhang:
On May 18, 2010, the Company entered into a new employment agreement with Mr. Tang to employ him as CEO for a term from January 1, 2010 to January 1, 2012, pursuant to which he will be paid RMB 79,600 ($11,600) per month (or RMB 955,200 ($139,200) per year) and additional share-based compensation based upon its 2010 financial performance (see Note 22). The agreement may be terminated upon mutual agreement between the Company and Mr. Tang in writing. In addition, the Company shall have the right to unilaterally terminate the agreement under certain circumstances, including among other things, (i) serious violations of the labor laws or the rules or regulations of the Company; (ii) causing serious damage to the interests of the Company; or (iii) Mr. Tang is criminally prosecuted under the law.
In 2011, the Board of Directors authorized and approved to increase Mr. Tang’s annual salary from US$139,000 to US$200,000 starting from January 6, 2011.
The Company may also terminate the agreement by serving 30 days’ prior written notice to Mr. Tang or giving Mr. Tang one month’s salary in lieu of notice in any one of the following circumstances: (i) where Mr. Tang, after undergoing a legally prescribed period of medical treatment and recuperation for an illness or a non-work-related injury, remains unable to carry out his job responsibilities; (ii) where Mr. Tang is unable to fulfill the duties of his position to the standards required under the terms of the agreement; or (iii) where the agreement cannot be performed due to any major changes of any objective circumstances, which includes, but is not limited to, a merger of the Company into another business entity, or sale or transfer by the Company of substantial portion of the assets it owns to third parties, a material adjustment in operative policy, or a declaration of bankruptcy, dissolution or liquation by the Company.
Mr. Tang may terminate the agreement during the term upon 30 days prior written notice to the Company. In addition, Mr. Tang may terminate the agreement in certain circumstances, including if (i) the Company fails to pay the social insurance premiums for Mr. Tang in accordance with the law; (ii) the Company forces Mr. Tang to work by means of violence or intimidation; or (iii) the Company fails to pay labor remuneration in full and on time or fails to provide the labor protection or working conditions as agreed under the agreement.
F-25
On May 18, 2010, the Company entered into a new employment agreement with Ms. Zhang to employ her as CFO for a term from January 1, 2010 to January 1, 2012, pursuant to which we have agreed to pay her HKD70,000 ($9,091) per month (or HKD840,000 ($109,092) per year) and additional share-based compensation based upon its 2010 financial performance (see Note 22).
In 2011, the Board of Directors authorized and approved to increase Ms. Zhang’s annual salary from US$109,092 to US$160,000 starting from January 6, 2011.
The agreement may be terminated upon mutual agreement between the Company and Ms. Zhang in writing. In addition, the Company shall have right to unilaterally terminate the agreement under certain circumstances, including, among other things, (i) serious violations of the labor laws or the rules or regulations of the Company; (ii) causing serious damage to the interests of the Company; or (iii) Ms. Zhang is criminally prosecuted under the law.
The Company may terminate the agreement by serving 30 days’ prior written notice to Ms. Zhang or giving Ms. Zhang one month’s salary in lieu of notice in any one of the following circumstances: (i) where Ms. Zhang, after undergoing a legally prescribed period of medical treatment and recuperation for an illness or a non-work-related injury, remains unable to carry out her job responsibilities; (ii) where Ms. Zhang is unable to fulfill the duties of her position to the standards required under the terms of the agreement; or (iii) where the agreement cannot be performed due to any major changes of any objective circumstances, which includes, but is not limited to, a merger of the Company into another business entity, or sale or transfer by the Company of substantial portion of the assets it owns to third parties, a material adjustment in operative policy, or a declaration of bankruptcy, dissolution or liquation by the Company.
Ms. Zhang may terminate the agreement during the term upon 30 days prior written notice to the Company. In addition, Ms. Zhang may terminate the agreement in certain circumstances, including if (i) the Company fails to pay the social insurance premiums for Ms. Zhang in accordance with the law; (ii) the Company forces Ms. Zhang to work by means of violence or intimidation; or (iii) the Company fails to pay labor remuneration in full and on time or fails to provide the labor protection or working conditions as agreed under the agreement.
|
e.
|
Distribution agreements
|
During 2010, the Company entered into separate distribution agreements with three government-owned hospitals, each for a term of three years, pursuant to which the hospitals agreed to further increase our business with them ranging from 30% to 95% of their respective purchase plans for our drugs. We agreed to use our best efforts to fulfill the orders by distributing the drugs under the statutory requirement of the mandated collective tender plan.
During 2011, the Company entered into separate distribution agreements with another three government-owned hospitals for a term of three years, pursuant to which the hospitals agreed to further increase the Company’s business with them from 30% to 40% of their respective purchase plans for its drugs.
In addition to the existing deposits of RMB 23,000,000 ($3,613,760) paid in 2010, the Company made additional deposits totaling RMB 22,000,000 ($3,456,640) for the security of these new distribution agreements entered in 2011. These hospitals are required to repay the deposit amounts in full when the agreements expire or when terminated, which may be done only under certain circumstances. The Company entered into such agreements to pursue relationships with these large hospitals in order to achieve a higher share of these customers’ purchases.
For the years ended December 31, 2011 and 2010, net sales from these government-owned hospital customers was approximately $38,567,000 and $19,074,000, respectively.
F-26
|
20.
|
Convertible redeemable preferred stock
|
On January 18, 2011, the Company entered into a Series A Convertible Preferred Shares Purchase Agreement (the “Purchase Agreement”) with Milestone Longcheng Limited (“Milestone”) pursuant to which Milestone at February 28, 2011 purchased 9,375,000 shares of the Company’s Series A Convertible Preferred Shares, par value $.001 per share (the “Preferred Shares”), for an aggregate purchase price of $30,000,000. The Preferred Shares carry a dividend of 5% and are convertible initially into an equal number of shares of our Common Stock at an initial conversion price of $3.20 per share.
Upon the consummation of a qualified public offering (as defined in the Purchase Agreement) in which the sale price of the Preferred Shares exceeds the product of 2.0 multiplied by the initial conversion price (as adjusted from time to time as provided in the Purchase Agreement), without the payment of additional consideration thereof, all the Preferred Shares shall be automatically converted into Conversion Shares at the then applicable Conversion Rate.
The holders of the Preferred shares may require the Company to redeem all or any portion of the outstanding Preferred Shares upon the occurrence of certain events, including: (a) any change of control of the Company; (b) any substantial asset sale; (c) any consolidation or merger or acquisition or sale of voting securities of the Company resulting in the holders of the issued and outstanding voting securities of the Company immediately prior to such transaction beneficially owning or controlling less than a majority of the voting securities of the continuing or surviving entity immediately following such transaction; (d) any tender offer, exchange offer or repurchase offer for any common shares; (e) cessation of listing of the common shares (or equity securities of an entity that holds all or substantially all the share capital of the Company) on an internationally recognized exchange; (f) it has been three years since the issue date; (g) cessation of provision of services by Mr. Huitian Tang to the Company or any of its Subsidiaries by reason of resignation, discharge, death, disability, retirement or otherwise.
The redemption price to be paid by the Company for such required redemptions shall be equal to an amount necessary to provide an annual return of fifteen percent (15%) compounded annually on the stated value of the Preferred Shares for the period commencing on February 28, 2011 and ending on the applicable redemption date. The redemption price shall take into account and be credited with any dividends paid during such period.
The conversion price shall be subject to adjustment as follows if any of the events listed below occur prior to the conversion of any Preferred Shares being converted:
|
(a)
|
In the event the Company pays a dividend or makes a distribution on its common shares in common shares, subdivides or reclassifies its outstanding common shares into a greater number of shares, or consolidates or reclassifies its outstanding common shares into a smaller number of shares, the conversion price in effect immediately prior to such event shall be adjusted so that the holders of the Preferred Shares thereafter converted shall be entitled to receive the number of common shares of the Company which they would have owned or have been entitled to receive after the occurrence of such event had the Preferred Shares been converted immediately prior to the occurrence of such event.
|
|
(b)
|
In the event the Company issues common shares, issues rights, options or warrants to subscribe for or purchase common shares, or issues or sells other rights for common shares or securities whether or not convertible or exchangeable into common shares for a consideration per share less than the then effective conversion price on the date the Company issues or sells such securities, then in each such case the conversion price in effect immediately prior to the issuance of such securities shall be reduced, concurrently with the issue of such securities, to the price appropriately readjusted.
|
|
(c)
|
In the event the Company’s net income after tax under U.S. GAAP, but excluding the effects of extraordinary gains, and non-cash expenses relating to options and warrants, and qualified public offering related expenses as and when expensed in the Company’s audited annual financial statements for the twelve-month period ending December 31, 2011, and prepared on a pro forma basis, is less than US$31,200,000, the conversion price shall be adjusted, effective on the date of the issuance of the 2011 annual audited financial statements.
|
|
(d)
|
In the event the Company distributes to all holders of its common shares any share capital of the Company (other than common shares) or evidences of indebtedness or cash or other assets (excluding regular cash dividends or distributions paid from retained earnings of the Company and dividends or distributions referred to in (a) above) or rights, options or warrants to subscribe for or purchase any of its securities (excluding those referred to in (b) above) then, in each such case, the conversion price shall be adjusted.
|
F-27
|
(e)
|
In the event any securities of the Company (other than Preferred Shares) , are amended or otherwise modified by operation of their terms or otherwise in any manner whatsoever that results in the reduction of the exercise, conversion or exchange price of such securities payable upon the exercise for, or conversion or exchange into, common shares or other securities exercisable for, or convertible or exchangeable into, common shares and/or such securities becoming exercisable for, or convertible or exchangeable into more shares or a greater amount of such securities which are, in turn exercisable for, or convertible or exchangeable into, common shares, or more common shares, then such amendment or modification shall be treated as if the securities which have been amended or modified have been terminated and new securities have been issued with the amended or modified terms for purposes of Section (b) above.
|
We classify the Preferred Shares as temporary equity in accordance with ASR 268 (Securities and Exchange Commission, Financial Reporting Codification, Section No. 211, Redeemable Preferred Stocks) because the Preferred Shares contain a conditional obligation that may require us, at the option of the holders, to redeem some or all of these shares by transferring our assets upon the occurrence of certain events that are not solely within the control of the Company.
The initial fair value of the Preferred Shares was $29,495,866 representing the transaction price of $30,000,000, in accordance with the FASB ASC 820-10-30-3, less related issue costs of $504,134.
We evaluated the economic characteristics and risks of the conversion feature as an embedded derivative and determined such economic characteristics and risks are clearly and closely related to the host contract. Therefore, the conversion feature does not meet the requirements of FASB ASC 815-15-25-1.a. for bifurcation and separate fair value accounting.
The Company has elected to adjust the carrying amount of the Preferred Shares at each reporting date by applying periodic accretions, using the interest method, so that the carrying amount will be equal to the possible redemption amount at the earliest determinable redemption date of February 28, 2014. As of December 31, 2011, such accretion amount was $3,890,037 resulting in a carrying amount for the Preferred Shares of $33,385,903 at that date.
|
21
|
Statutory and other reserves
|
Under PRC regulations, Liuzhou BCT, BCT Retail and Hefeng Pharmaceutical may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC GAAP. In addition, these companies are required to set aside at least 10% of their after-tax net profits each year, if any, to fund the statutory reserves until the balance of the reserves reaches 50% of their registered capital. The statutory reserves can be used to make up cumulative prior year losses and are not distributable in the form of cash dividends.
|
22.
|
Stock option arrangements
|
On June 27, 2010, we adopted the China BCT Pharmacy Group, Inc. 2010 Omnibus Securities and Incentive Plan (the “Plan”) for the benefit of our employees, nonemployee directors and consultants and the employees, nonemployee directors and consultants of its affiliates, for purposes of assisting us to attract, retain and provide incentives to key management employees and nonemployee directors, and consultants of ours and our affiliates, and to align the interests of such individuals with those of our stockholders. Accordingly, the Plan provides for the granting of distribution equivalent rights, incentive stock options, non-qualified stock options, performance share awards, performance unit awards, restricted stock awards, restricted stock unit awards, stock appreciation rights, tandem stock appreciation rights, unrestricted stock awards or any combination of the foregoing, as may be best suited to the circumstances of the particular employee, director or consultant as provided in the Plan.
F-28
On June 27, 2010, we granted options under the Plan to two executive employees. The options vest and become exercisable with respect to all of the shares only if our after-tax net income for fiscal 2010 equals at least U.S. $26,000,000 (excluding any non-cash expenses) (the “2010 Income Target”), determined on the basis of our audited financial statements for our 2010 fiscal year, as confirmed by our independent auditor in their report on said financial statements (the “Audit Report”). If the 2010 Income Target is met, the options shall vest and become exercisable on the date on which the Audit Report is dated, and if the options do not become exercisable due to the failure to meet the 2010 Income Target, the option shall terminate on the date on which the Audit Report is dated.
On July 16, 2010, we entered into separate stock option agreements with each of our three independent directors, Messrs. Lee, Choi and Chiu, which agreements provide for the grant to each such individual of an option to purchase 10,000 shares of our common stock at an exercise price of $4.00 per share. Each option vests and becomes exercisable with respect to all of the shares on June 6, 2011. The exercise price of each option may be paid for in cash or by cancellation of existing shares of our common stock held by the independent directors. Each option terminates on the earlier of (i) July 16, 2015 or (ii) the date as of which the option has been fully exercised.
August 18, 2010, we entered into separate stock agreements with two senior staff, which agreements provide for the grant to each such individual of an option to purchase 10,000 and 5,000 shares separately of our common stock at an exercise price of $5.00 per share. Each option vests and becomes exercisable on January 1, 2012 so as they are employed by the company as of December 31, 2011.
On December 19, 2011, we entered into separate stock option agreements with two executive employees, Mr. Tang and Ms. Zhang, and three independent directors, Messrs. Lee, Choi and Chiu, which agreements provide for the grant to the two executive employees of an option to purchase 1,245,000, 830,000 and each of 10,000 for three independent directors of the shares of our common stock at an exercise price of $2.00 per share. 25% of such options shall vest and become exercisable on June 30, 2013, the second 25% of such options shall vest and become exercisable on December 31, 2013, the third 25% of such options shall vest and become exercisable on June 30, 2014 and the remaining 25% of such options shall vest and become exercisable on December 31, 2014, The vesting schedule requires continued employment and/or director’s services through each applicable vesting date as a condition to the vesting of the applicable installment of such options.
On December 19, 2011, we entered into separate stock option agreements with two senior staff, which agreements provide for the grant to the two senior staff to 30,000 shares of our common stock at an exercise price of $2.00 per share. 25% of such options shall vest and become exercisable on June 30, 2013, the second 25% of such options shall vest and become exercisable on December 31, 2013, the third 25% of such options shall vest and become exercisable on June 30, 2014 and the remaining 25% of such options shall vest and become exercisable on December 31, 2014, The vesting schedule requires continued employment’s services through each applicable vesting date as a condition to the vesting of the applicable installment of such options.
Instead of the China BCT Pharmacy Group, Inc. 2010 Omnibus Securities and Incentive Plan (the “Plan”) the Board also has the authority to grant options that are not under the Plan and under that certain Series A Convertible Preferred Shares Purchase Agreement with Milestone Longcheng Limited (the “Milestone”) dated January 18, 2011, as amended, has committed to grant a number of options upon the seating of a director designated by Milestone and such director was seated on March 17, 2011.
Accordingly, on December 19, 2011, the Company granted options to two executive employees. Agreements provide the grant to both individuals of an option to purchase 762,500 shares each of our common stock at an exercise price of $2.00 per share. 50% of such options shall vest and become exercisable immediately and the remaining 50% of such options shall vest and become exercisable on December 31, 2012.
The fair value of options granted to the two executive employees pursuant to the Plan at the date of grant, June 27, 2010 was $1,774,440. Fair values were estimated using the binominal model. The assumptions that were used to calculate fair value of stock options as of June 27, 2010 were: 0% dividend yield, expected volatility of 30%, risk-free interest rate of 4.78% and expected lives of ten years. The Company expensed $565,763 in share-based compensation for the year ended December 31, 2011 upon the achievement of 2010 Income Target (2010: $1,208,677).
F-29
The fair value of options granted to the three independent directors at the date of grant, July 16, 2010 was $41,010 and was expensed in share-based compensation for the year ended December 31, 2010. Fair values were estimated using the binominal model. The assumptions that were used to calculate fair value of stock options as of July 16, 2010 were: 0% dividend yield, expected volatility of 41.8%, risk-free interest rate of 3.427% and expected lives of ten years.
The fair value of options granted to the two senior staff at the date of grant, August 20, 2010 was $15,365. Fair values were estimated using the binominal model. The assumptions that were used to calculate fair value of stock options as of August18, 2010 were: 0% dividend yield, expected volatility of 51.31%, risk-free interest rate of 2.46% and expected lives of 5 years. The Company expensed $11,194 and $4,171 in share-based compensation for the years ended December 31, 2011 and 2010 over the requisite service period ended December 31, 2011 for both options.
The fair value of options granted to the two executive employees and three independent directors pursuant to the Plan at the date of grant at December 19, 2011 was $1,405,976. Fair values were estimated using the binominal model. The assumptions that were used to calculate fair value of stock options as of December 19, 2011 were: 0% dividend yield, expected volatility of 44.38%, risk-free interest rate of 1.97% and expected lives of ten years. The Company expensed $49,871 in share-based compensation for the year ended December 31, 2011 and the remaining compensation cost of $1,356,105 related to non-vested awards will be recognized over the implied remaining requisite service period of January 1, 2012 through December 31, 2014 for options.
The fair value of options granted to the two senior staff pursuant to the Plan at the date of grant at December 19, 2011 was $19,047. Fair values were estimated using the binominal model. The assumptions that were used to calculate fair value of stock options as of December 19, 2011 were: 0% dividend yield, expected volatility of 44.38%, risk-free interest rate of 1.97% and expected lives of ten years. The Company expensed $673 in share-based compensation for the year ended December 31, 2011 and the remaining compensation cost of $18,374 related to non-vested awards will be recognized over the implied remaining requisite service period of January 1, 2012 through December 31, 2014 for options.
The fair value of options granted to the two executive employees other than the Plan at the date of grant was $1,012,854 and was expensed in share-based compensation for the year ended December 31, 2011. Fair values were estimated using the binominal model. The assumptions that were used to calculate fair value of stock options as of December 19, 2011 were: 0% dividend yield, expected volatility of 44.38%, risk-free interest rate of 1.97% and expected lives of ten years.
The company expensed the above options in share-based compensation included in administrative expense in the amount of $1,640,355 and $1,253,858 for the years ended December 31, 2011 and 2010, respectively.
There were no options granted with exercise prices below the market value of the stock at the grant date. All outstanding options at December 31, 2011 had no intrinsic value because the Company’s stock price was lower than all option exercise prices. A summary of the Company’s stock options issued and outstanding as of and for the year ended December 31, 2011, is presented below:
|
Number of options
|
Exercise Price
|
|||||||
|
Stock options granted and outstanding
|
3,660,000 | $ | 2.00 | |||||
|
Stock options granted and outstanding
|
15,000 | $ | 5.00 | |||||
|
Stock options granted and outstanding
|
1,110,000 | $ | 4.00 | |||||
|
23.
|
Defined contribution plan
|
Pursuant to the relevant PRC regulations, the Company is required to make contributions at a rate of 30.5% to employees’ salaries and wages to a defined contribution retirement plan organized by a state-sponsored social insurance plan in respect of the retirement benefits for the Company’s employees in the PRC. The only obligation of the Company with respect to the retirement plan is to make the required contributions under the plan. No forfeited contribution is available to reduce the contribution payable in the future years. The defined contribution plan contributions were charged to the consolidated statements of income. The Company contributed $719,873 and $883,118 for the years ended December 31, 2011 and 2010, respectively.
F-30
24. Segment information
The Company uses the “management approach” in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. Management, including the chief operating decision maker, reviews the operating results solely by monthly net sales of pharmaceutical distribution, retail pharmacy and manufacturing segments and the operating results of the Company. As such, the Company has determined that the Company has three operating segments as defined by FASB ASC 280, “Segments Reporting”: Pharmaceutical distribution, retail pharmacy and manufacturing.
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Sales revenue
|
||||||||
|
Pharmaceutical distribution
|
$ | 190,662,940 | $ | 145,390,262 | ||||
|
Retail pharmacy
|
53,266,572 | 44,593,533 | ||||||
|
Manufacturing
|
13,557,626 | 10,829,465 | ||||||
| $ | 257,487,138 | $ | 200,813,260 | |||||
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Operating income
|
||||||||
|
Pharmaceutical distribution
|
$ | 28,895,330 | $ | 23,085,979 | ||||
|
Retail pharmacy
|
9,480,261 | 8,860,674 | ||||||
|
Manufacturing
|
7,020,705 | 5,401,924 | ||||||
| $ | 45,396,296 | $ | 37,348,577 | |||||
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Depreciation and amortization expenses
|
||||||||
|
Pharmaceutical distribution
|
$ | 593,284 | $ | 543,707 | ||||
|
Retail pharmacy
|
223,356 | 73,545 | ||||||
|
Manufacturing
|
625,671 | 571,253 | ||||||
| $ | 1,442,311 | $ | 1,188,505 | |||||
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Assets
|
||||||||
|
Pharmaceutical distribution
|
$ |
164,525,782
|
$ | 108,182,966 | ||||
|
Retail pharmacy
|
21,748,464 | 16,860,112 | ||||||
|
Manufacturing
|
19,389,356 | 16,386,308 | ||||||
| $ | 205,663,602 | $ | 141,429,386 | |||||
F-31
A reconciliation is provided for unallocated amounts relating to corporate operations which are not included in the segment information.
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Total consolidated net sales
|
$ | 257,487,138 | $ | 200,128,260 | ||||
|
Total profit for reportable segments
|
$ | 45,396,296 | $ | 37,348,577 | ||||
|
Unallocated amounts relating to
|
||||||||
|
operations:
|
||||||||
|
Change in fair value of warrant liabilities
|
1,082,202 | 582,226 | ||||||
|
Interest income
|
42,146 | 8 | ||||||
|
Other income
|
169,580 | 212 | ||||||
|
Share-based compensation
|
(1,640,355 | ) | (1,253,858 | ) | ||||
|
Provision for registration payment
|
- | (455,896 | ) | |||||
|
Other general expenses
|
(2,821,001 | ) | (1,443,464 | ) | ||||
|
Finance costs
|
(2,038 | ) | (3,107 | ) | ||||
|
Income before income taxes
|
$ | 42,226,830 | $ | 34,774,698 | ||||
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Assets
|
||||||||
|
Total assets for reportable segments
|
$ | 205,663,602 | $ | 141,429,386 | ||||
|
Cash and cash equivalents
|
6,918,847 | 109,650 | ||||||
| $ | 212,582,449 | $ | 141,539,036 | |||||
All of the Company’s long-lived assets and net sales classified based on the customers are located in the PRC.
|
25.
|
Related party transactions
|
Apart from the transactions as disclosed elsewhere in these consolidated financial statements, the Company has entered into the following transactions with its related parties:
Sales to related companies of which certain of the Company’s directors have controlling interests.
|
Name of related companies
|
Year ended December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Liucheng Medicine Company
|
$ | 48,483 | $ | 325,881 | ||||
|
Guangxi Tianhu Medicine Limited
|
$ | 642,526 | $ | 9,632 | ||||
|
Wuxuan Baicaotang Medicine Limited
|
$ | 4,836 | $ | 29,618 | ||||
|
Guangxi Liuzhou Baicaotang Medicine Limited, Guigang Branch
|
$ | 1,065,208 | $ | 3,311,379 | ||||
|
26.
|
Subsequent events
|
The Company has evaluated its activities subsequent to December 31, 2011 and has concluded that no subsequent events have occurred that would require recognition or disclosure in the consolidated financial statements.
F-32
SIGNATURES
In accordance with the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized on April 25 , 2012.
|
CHINA BCT PHARMACY GROUP, INC.
|
|
|
/s/ Huitian Tang
|
|
|
By: Huitian Tang
|
|
|
Chief Executive Officer and Director
(principal executive officer)
|
85
EXHIBIT INDEX
|
2.1
|
Share Exchange Agreement dated December 23, 2009 (1)
|
|
3.1
|
First Amended and Restated Certificate of Incorporation (2)
|
|
3.2
|
By-laws (3)
|
|
4.1
|
Form of Warrant (1)
|
|
4.2
|
Form of Co-Placement Agent Warrant (4)
|
|
4.3
|
Certificate of Designation of Series A Convertible Preferred Shares (2)
|
|
10.1
|
Subscription Agreement Dated October 23, 2009 (1)
|
|
10.2
|
Co-Placement Agent Agreement Dated October 21, 2009 (5)
|
|
10.3
|
Escrow Deposit Agreement Dated October 21, 2009 (1)
|
|
10.4
|
Earn-In Agreement dated October 22, 2009 (1)
|
|
10.5
|
Share Transfer Agreement dated as of April 1, 2008 (5)
|
|
10.6
|
Shares Pledge Agreement dated May 3, 2008 (1)
|
|
10.7
|
Shares Pledge Agreement dated March 31, 2009 (1)
|
|
10.8
|
Shares Repurchase Agreement dated July 31, 2008 (1)
|
|
10.9
|
Loan Agreement and Pledge Agreement dated December 19, 2008 by and among Liuzhou Baicaotang, Baicaotang Property Development Limited and Liuzhou City Commercial Bank (5)
|
|
10.10
|
Loan Agreement and Pledge Agreement dated December 29, 2008 by and among Liuzhou Baicaotang, Baicaotang Property Development Limited and Rurol Credence Cooperation of Guangxi (5)
|
|
10.11
|
Loan Agreement and Pledge Agreement dated January 8, 2009 by and among Liuzhou Baicaotang, Baicaotang Property Development Limited and Agricultural Bank of China (5)
|
|
10.12
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated February 13, 2006 (5)
|
|
10.13
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated March 27, 2006 (5)
|
|
10.14
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated April 6, 2006 (5)
|
|
10.15
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated May 18, 2006 (5)
|
|
10.16
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated December 6, 2006 (5)
|
|
10.17
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated December 29, 2008 (5)
|
|
10.18
|
Loan Agreement dated February 8, 2007 by and between Huitian Tang and Industrial and Commercial Bank of China Guangxi Branch (5)
|
|
10.19
|
Confirmation Agreement dated December 31, 2008 by and among Huitian Tang, Liuzhou Baicaotang and Liuzhou Baicaotang Guigang Branch (5)
|
|
10.20
|
Loan Agreement dated February 12, 2007 by and between Jiang You Ru and Industrial and Commercial Bank of China Guangxi Branch (5)
|
86
|
10.21
|
Confirmation Agreement dated December 31, 2008 by and among Jiang You Ru, Liuzhou Baicaotang and Liuzhou Baicaotang Guigang Branch (5)
|
|
10.22
|
Lock-Up Agreement, dated December 23, 2009 (5)
|
|
10.23
|
Employment Agreement dated May 18, 2010 by and between Guangxi Liuzhou Baicaotang Medicine, Ltd., China BCT Pharmacy Group, Inc. and Huitian Tang (6)
|
|
10.24
|
Employment Agreement dated May 18, 2010 by and between Forever Well Asia Pacific Limited, China BCT Pharmacy Group, Inc. and Xiaoyan Zhang (6)
|
|
10.25
|
Earn-in Agreement dated December 30, 2009 (7)
|
|
10.26
|
Shares Pledge Agreement dated May 3, 2008 (7)
|
|
10.27
|
Shares Pledge Agreement dated March 31, 2009 (7)
|
|
10.28
|
Termination Agreement dated March 2, 2010 (7)
|
|
10.29
|
Share Transfer Agreement by and between He Wenheng and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.30
|
Share Transfer Agreement by and between Jia Junwen and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.31
|
Share Transfer Agreement by and between Jiang Qifeng and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.32
|
Share Transfer Agreement by and between Jiang Youru and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.33
|
Share Transfer Agreement by and between Li Jinghua and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.34
|
Share Transfer Agreement by and between Liu Chunlin and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.35
|
Share Transfer Agreement by and between Liu Gongchun and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.36
|
Share Transfer Agreement by and between Meng Yuangang and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.37
|
Share Transfer Agreement by and between Tan Yujing and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.38
|
Share Transfer Agreement by and between Ye Yuanjian and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.39
|
Share Transfer Agreement by and between Wang Bangfu and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.40
|
Share Transfer Agreement by and between Wei Wende and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
87
|
10.41
|
Share Transfer Agreement by and between Yang Xiaojian and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.42
|
Share Transfer Agreement by and between Zhang Qingqiu and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.43
|
Share Transfer Agreement by and between Zhao Ming’an and Forever Well Asia Pacific Limited dated March 18, 2008 (5)
|
|
10.44
|
Amendment No. 1 to Earn-in Agreement, by and between Xiaoyan Zhang and the Buyers named thererin, dated May 19, 2010 (5)
|
|
10.45
|
Amended and Restated Shares Pledge Agreement by and between Guangxi Liuzhou Baicaotang Medicine Ltd. and Liuzhou Baicaotang Property Management Ltd, dated May 19, 2010 (5)
|
|
10.46
|
Amended and Restated Shares Pledge Agreement by and between Guangxi Liuzhou Baicaotang Medicine Ltd. and Liuzhou Baicaotang Property Management Ltd, dated May 19, 2010 (5)
|
|
10.47
|
Proxy Agreement by and between Guangxi Liuzhou Baicaotang Medicine Ltd. and Liuzhou Baicaotang Property Management Ltd., dated May 19, 2010 (5)
|
|
10.48
|
Form of sales agreement with related parties (7)
|
|
10.49
|
Independent Director Agreement of Simon Choi (8)
|
|
10.50
|
Independent Director Agreement of Man Wai Chiu (8)
|
|
10.51
|
Independent Director Agreement of Kam-Cheung Chin (13)
|
|
10.52
|
2010 Omnibus Securities and Incentive Plan (9)
|
|
10.53
|
Stock Option Agreement between the Registrant and Huitian Tang made as of June 27, 2010 (9)
|
|
10.54
|
Stock Option Agreement between the Registrant and Xiaoyan Zhang made as of June 27, 2010 (9)
|
|
10.55
|
Stock Option Agreement between the Registrant and Eng Leong Lee made as of July 16, 2010 (7)
|
|
10.56
|
Stock Option Agreement between the Registrant and Simon Choi made as of July 16, 2010 (7)
|
|
10.57
|
Stock Option Agreement between the Registrant and Man Wai Chiu made as of July 16, 2010 (7)
|
|
10.58
|
Non-Disclosure and Non-Competition Agreement dated May 18, 2010 by and between Guangxi Liuzhou Baicaotang Medicine, Ltd. and Huitian Tang (7)
|
|
10.59
|
Non-Disclosure and Non-Competition Agreement dated May 18, 2010 by and between Guangxi Liuzhou Baicaotang Medicine, Ltd. and Xiaoyan Zhang (7)
|
|
10.60
|
Asset Transfer Agreement dated February 28, 2010 by and between Liuzhou Wubaitang Medicine Chain Store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited (7)
|
|
10.61
|
Asset Transfer Agreement dated March 29, 2010 by and between Zhaoping County Laobaixin Pharmacy Chain Store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited (7)
|
88
|
10.62
|
Asset Transfer Agreement dated April 25, 2010 by and between Lai Bing Peng Fei Pharmacy Chain Store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited (7)
|
|
10.63
|
Form of Retail Chain Leasing Contract (7)
|
|
10.64
|
Entrust Shareholding Agreement Between Liuzhou BCT Shareholders and Ms. Xiaoyan Zhang on Ingenious Dated July 21, 2008 (11)
|
|
10.65
|
Series A Convertible Preferred Shares Purchase Agreement, by and between the Company and Milestone Longcheng Limited (10)
|
|
10.66
|
Registration Rights Agreement, by and between the Company and Milestone Longcheng Limited (10)
|
|
10.67
|
Shareholders Agreement, by and among the Company, Milestone Longcheng Limited, certain shareholders of the Company and Mr. Tian Hui Tang as representative for the shareholders (10)
|
|
10.68
|
Form of Stock Option Agreement – Not Granted Pursuant to 2010 Omnibus Securities and Incentive Plan (12)
|
|
10.69
|
Form of Stock Option Agreement Pursuant to 2010 Omnibus Securities and Incentive Plan (12)
|
|
14
|
Code of Ethics and Business Conduct (8)
|
|
21
|
List of Subsidiaries (4)
|
|
31.1
|
Certification pursuant to Rule 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. *
|
|
31.2
|
Certification pursuant to Rule 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. *
|
|
32.1
|
Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. *
|
|
32.2
|
Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. *
|
|
101.INS
|
XBRL Instance Document.**
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document.**
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document.**
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document.**
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document.**
|
|
(1)
|
Incorporated by reference to the Company’s Form 8-K filed on December 31, 2009.
|
|||
|
(2)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1/A filed on March 3, 2011.
|
|||
|
|
(3)
|
Incorporated by reference to the Company’s Form SB-2 filed on August 22, 2007.
|
||
|
|
(4)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1 filed on March 3, 2010.
|
||
|
(5)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed on May 27, 2010.
|
|||
|
(6)
|
Incorporated by reference to the Company’s Form 8-K filed on May 21, 2010.
|
|||
|
(7)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed July 29, 2010.
|
|||
|
|
(8)
|
Incorporated by reference to the Company’s Form 8-K filed on June 9, 2010.
|
||
|
|
(9)
|
Incorporated by reference to the Company’s Form 8-K filed on June 29, 2010.
|
||
| (10) | Incorporated by reference to the Company’s Form 8-K filed on January 18, 2011. | |||
| (11) | Incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed October 20, 2010. | |||
| (12) | Incorporated by reference to the Company’s Form 8-K filed on December 19, 2011. | |||
| (13) | Incorporated by reference to the Company’s Annual Report on Form 10-K filed on March 30, 2012. | |||
* Filed herewith.
** Previously furnished with Amendment No. 1 to the Company’s Annual Report on Form 10-K/A filed on April 6, 2012, and incorporated herein by reference.
89
