Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Synthetic Biologics, Inc. | v310344_8k.htm |

NYSE Amex: SYN April 24, 2012

Forward - Looking Statements This presentation includes forward - looking statements on Synthetic Biologics’ current expectations and projections about future events . In some cases forward - looking statements can be identified by terminology such as "may," "should," "potential," "continue," "expects," "anticipates," "intends," "plans," "believes,“ "estimates,” “indicates,” and similar expressions . These statements are based upon current beliefs, expectations and assumptions and are subject to a number of risks and uncertainties, many of which are difficult to predict and include statements regarding our clinical trials, our establishment of partnerships and our execution of our growth strategy . The forward - looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those set forth or implied by any forward - looking statements . Important factors that could cause actual results to differ materially from those reflected in Synthetic Biologics’ forward - looking statements include, among others, a failure of our biologic or drug candidates to be demonstrably safe and effective, a failure to initiate clinical trials and if initiated, a failure to achieve the desired results, a failure to obtain regulatory approval for our biologic and drug product candidates or to comply with ongoing regulatory requirements, regulatory limitations relating to our ability to promote or commercialize our biologic or drug product candidates for the specific indications, a lack of acceptance of our product candidates in the marketplace, a failure of us to become or remain profitable, a failure to establish collaborations, our inability to obtain or maintain the capital or grants necessary to fund the company's research and development activities, a loss of any of our key scientists or management personnel, and other factors described in Synthetic Biologics’ report on Form 10 - K for the year ended December 31 , 2011 , subsequent Form 10 - Qs and any other filings with the SEC . The information in this presentation is provided only as of the date presented, and Synthetic Biologics undertakes no obligation to update any forward - looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law . 2

Corporate Overview • Ticker symbol – NYSE Amex: SYN • Late - stage clinical programs ‒ Oral estriol for multiple sclerosis (MS) ▪ $ 8.75 million in grant funding ‒ Flupirtine for fibromyalgia ▪ $ 17.5 million partnership with Meda AB ‒ Oral zinc acetate for Lou Gehrig’s disease (ALS ) ▪ Phase II/III to begin during 2012 • Preclinical − Synthetic DNA - based therapy ▪ Collaboration Intrexon Corporation ▪ Pulmonary arterial hypertension (PAH ) first indication • Strong, comprehensive IP 3

Recent Milestones 4 Advancing late - stage and preclinical synthetic biologics programs x Completed planned enrollment in Phase II clinical trial for relapses in MS x Initiated Phase II clinical trial for cognitive dysfunction in MS x Strengthened Synthetic Biologics’ team: Jeff Riley, CEO; Evan Ballantyne, CFO; and, Scott L. Tarriff and Nelson K. Stacks, independent board members x Worldwide exclusive channel collaboration with Intrexon Corporation for the development and commercialization of a synthetic DNA - based therapy to treat PAH x Recruited gene medicine industry pioneer, John Monahan, Ph.D., as Sr. VP of R&D

Financial Snapshot • Current Price : $1.57 • 52 Week Range: $0.42 - $ 2.95 • Average Volume (3 months) : 280,594 • Shares Outstanding: ~ 32.8 million • Market Capitalization: ~$51 million • Cap Structure: Straight Common. No Debt. No Preferred. • Cash: ~$6.9 million ( pro forma March 26, 2012) Stock information as of 4/20/2012 5

6 Synthetic Biologics’ Team • Jeffrey Riley, CEO Pfizer, Nichols Institute (Quest), SmithKline Beecham, QIC • C. Evan Ballantyne, CFO Clinical Data, Inc., Avedro , ZymeQuest , ACNielsen, IMS • John Monahan, Sr. VP R&D Avigen, Somatix, Triton Biosciences, Hoffman - LaRoche

Product Pipeline 7

MS: Market Opportunity According to the National Multiple Sclerosis Society − 400,000 MS patients in U.S. − 200 people diagnosed per week in the U.S. − 2.5 million patients worldwide − 1.75 million patients worldwide are women 1 Current estimated sales of injectable disease - modifying therapies 2 Cognitive dysfunction in MS market based on an estimated annual price of $20,000 per patient Patient population and market size 8 Relapsing - Remitting MS • ~60% of MS patients are women originally diagnosed with relapsing - remitting MS • U.S. market potential $8.9 billion 1 Cognitive Dysfunction in MS • ~35% of MS patients are women who are affected by cognitive dysfunction • U.S. market potential $2.8 billion 2

MS: Historical Efficacy of Estriol The “Pregnancy Hormone” – Linked to fetal immune privilege • Hormone produced by placenta during pregnancy • Approved in Europe/Asia for 40 years to treat symptoms of menopause • Never approved in the U.S. for any indication • Pr egnancy I n M ultiple S clerosis (PRIMS) study followed 254 pregnant women with multiple sclerosis (MS) – Relapse rates were significantly reduced by 71% (p < 0.001) through the third trimester of pregnancy from pre - baseline levels – Relapse rates then increased by 120% (p < 0.001) during the first three months post - partum • Trimesta (oral estriol ) developed by Rhonda Voskuhl, M.D., Professor, UCLA Department of Neurology 9

MS: Relapsing - Remitting MS Drug candidate – Trimesta (oral estriol ) • Dr. Voskuhl completed an investigator - initiated, 10 - patient, 22 - month, single - agent, crossover clinical trial evaluating Trimesta – Over 6 months both the number and volume of brain lesions decreased by 82% (p - values < 0.02) * – After 6 months off drug, Trimesta was given for 4 months; the number and volume of brain lesions decreased by 48% (p = 0.04) and 88 % (p = 0.008 ), respectively * – Trimesta generally well - tolerated * Compared to 6 month baseline measurement 10

MS: Relapsing - Remitting MS Phase II Clinical trial status – Trimesta • Randomized, double - blind, placebo - controlled clinical trial 1 of females with relapsing - remitting MS • Comparing relapse rates over 2 years between 8 mg of Trimesta taken daily versus matching placebo with Copaxone® as standard of care for all patients • Enrollment complete: 164 patients at 15 U.S. centers • Trial funded to completion with $ 8 million+ in grants awarded 1 www.clinicaltrials.gov/ct2/show/NCT00451204 11

MS: Cognitive Dysfunction • Dr. Voskuhl of UCLA completed investigator - initiated clinical trial evaluating Trimesta − Demonstrated a 14% improvement in P aced A uditory S erial A ddition T est (PASAT) cognitive testing scores (p = 0.04) in relapsing - remitting MS patients at 6 months of therapy – PASAT is a cognitive test performed in patients with neuro - psychological disorders such as MS Drug candidate – Trimesta (oral estriol ) 12

MS: Cognitive Dysfunction in MS Phase II Clinical trial status – Trimesta • Initiated clinical trial in January 2012 1 − Randomized, double - blind, placebo - controlled clinical trial of female MS patients with cognitive dysfunction at UCLA − Enrolling 64 female MS patients − Patients randomized into treatment ( Trimesta ) and placebo groups − All patients remain on st andard FDA - approved MS treatment − Patients dosed and monitored for one year • Primary endpoint is change in cognition • Majority of the trial is funded by private foundations 1 www.clinicaltrials.gov/ct2/show/NCT01466114 13

Fibromyalgia: Market Opportunity Commercial potential for Effirma (flupirtine) • Condition characterized by widespread pain and stiffness throughout body, accompanied by severe fatigue, insomnia and mood symptoms – Affects 3 - 6% of worldwide population, including ~10 million people in the U.S. 1 • U.S. market potential $6 billion 2 (includes Lyrica ® , Cymbalta ® and Savella ® ) • Flupirtine marketed in Europe since 1984 as pain treatment • Never approved in the U.S . for any indication • Flupirtine has potential to be unique mechanism of action for fibromyalgia − Selective neuronal potassium channel opener with NMDA receptor antagonist properties − Harvard teaching hospital reported encouraging results in human studies 1 According to the National Fibromyalgia Association 2 Based on an estimated annual price of $1,200 per patient 14

Meda AB Corporate Partnership Effirma for fibromyalgia attracted large pharma collaboration • $17.5 million corporate partnership with Meda * – Meda assumes all future development costs – $2.5 million upfront payment (May 2010) – $15 million in future milestone payments – 3.5% future royalties based on net sales • Flupirtine for fibromyalgia in Phase II development 15 * Meda AB is a Swedish - based pharma company with sales operations in more than 50 countries and worldwide sales exceeding $1.8 billion (USD) in 2010.

Lou Gehrig’s Disease: Market Opportunity • Affects motor nerve cells in brain and spinal cord • Progressive loss of muscle strength and coordination • No cure for Lou Gehrig’s disease (ALS) • ALS Association facts − Up to 30,000 people have Lou Gehrig’s disease at any given time in the U.S . − Average lifespan from time of symptom onset 2 - 5 years − ~ 20% of patients live 5 years or more • U.S. market potential $300 million 1 Devastating neurodegenerative disease 1 Based on an estimated annual price of $10,000 per patient 16

Lou Gehrig’s Disease: Prior Zinc Therapy Study • Top - line results from PNA Center for Neurological Research (PNA) Phase I/II study of oral high dose zinc therapy 1 • Top - line results − No safety issues related to zinc therapy − Disease progression slowed in study patients ▪ On average, patients in the study lost 0.37 ALS Functional Rating Score - Revised (ALSFRS - R) points per month ▪ On average, historical controls lost 0.91 ALSFRS - R points per month Positive top - line results 1 www.clinicaltrials.gov/ct2/show/NCT01259050 17

Lou Gehrig’s Disease • Planned double - blind, placebo - controlled adaptive Phase II/III trial − Enroll at least 65 patients at 6 clinical sites − Randomized into treatment or matching placebo arms − All patients remain on RILUTEK® (riluzole) as standard of care − Dosed for at least 6 months with periodic monitoring − If threshold criteria met, a second phase is expected to enroll ~50 additional subjects to receive treatment for 9 months − Conducted by the neurology team at the PNA • Treatment group to receive AEN - 100 – high - dose zinc acetate - based tablet that is gastroretentive and sustained - release • Planned endpoints safety and efficacy of AEN - 100 Clinical trial status – AEN - 100 ( gastroretentive zinc acetate) 18

Next Generation Gene Therapy 19 2012 − Field demonstrating clinical successes − Next generation gene therapies - technology to regulate protein expression 1990 - 2000 − High hopes 2000 - 2004 − Set - backs − Safety concerns 2004 - 2011 − Corporate abandonment of gene therapy

Intrexon Corporation 20 Antibody Discovery More than 350 employees Image courtesy of Intrexon Corporation.

21 Modular Controllable Gene System (RheoSwitch Therapeutic System® + GOI) ECB Injection site RTS® - GOI Activator Ligand (oral dosing) Activator Ligand (small molecule) DNA Delivery GOI GOI GOI GOI RTS® Controlled Protein Expression RheoSwitch Therapeutic System® Protein of Interest GOI: Gene of Interest • Gene Delivery − Complex transgenes manufactured via cGMP methods and administered for episomal delivery using viral and non - viral vector mechanisms • Activator Ligand (AL) − Orally dosed small molecule activator ligand regulates gene expression − Excellent safety profile in humans based on Phase IA pharmacodynamic / pharmacokinetic studies • Controlled Protein Expression − Protein expression only in presence of AL − Expression is dependent on AL dose − Expression is repressed in absence of AL Controlled Expression of Therapeutic Proteins Targeted in vivo gene delivery and expression Images courtesy of Intrexon Corporation. RheoSwitch Therapeutic System®, RTS ® and UltraVector® are registered trademarks of Intrexon Corporation. © 2012 Intrexon Corporation.

22 RheoSwitch Therapeutic System® Synthetic DNA delivered to pulmonary arteries RheoSwitch Therapeutic System® is a registered trademark of Intrexon Corporation.
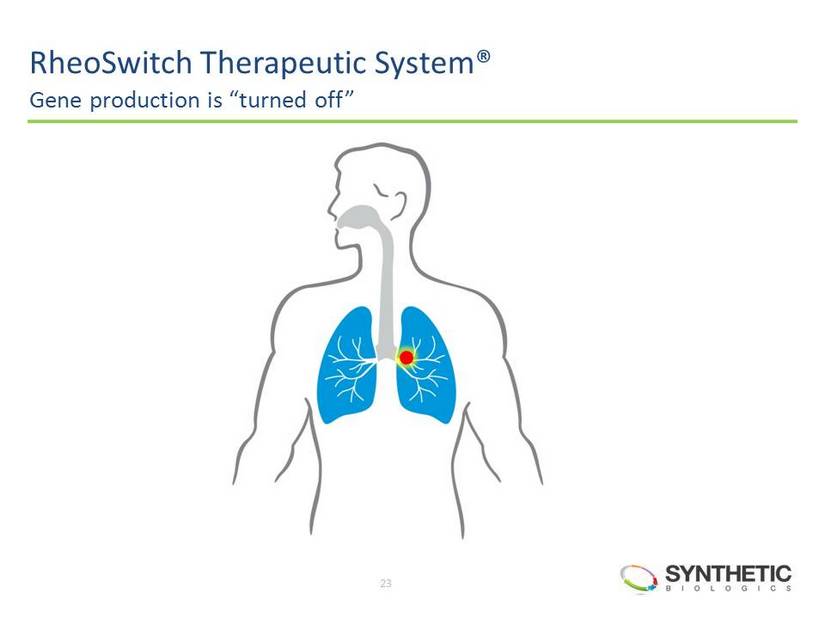
23 RheoSwitch Therapeutic System® Gene production is “turned off”

24 Oral Administration of RheoSwitch ® Activator Ligand “Turns on” the gene production in vivo

RheoSwitch ® Technology RheoSwitch ® control designed to improve safety, Reduce side effects and enhance efficacy On - Off switch with dose adjustment control 25 Image courtesy of Intrexon Corporation. RheoSwitch ® is a registered trademark of Intrexon Corporation.

Anatomy of PAH 26 Normal Artery Flow in the Heart and Lungs of Normal Patient Constricted Artery Flow in the Heart and Lungs of PAH Patient
Current PAH Market 27 • Global PAH market expected to reach $3.6 billion 1 by 2015 • High morbidity and mortality • Three medical therapies − Prostacyclin analogs reserved for more severe stage of disease − Can slow the course and lower the mortality • Prostacyclin analogs have significant systemic side effects that inhibit utility and long - term benefit 1 Source: GlobalData , Pulmonary Arterial Hypertension (PAH) – Drug Pipeline Analysis and Market Forecasts to 2016.

PAH Product Clinical Need/Positioning • Gene therapy is intended to overcome dose - limiting toxicities by generating high levels of prostacyclin at the site of action – the pulmonary arteries • Synthetic DNA - based strategy − Via a single procedure, deliver a vector to the pulmonary arteries − Vector transfers the gene for PGIS, the enzyme that produces prostacyclin − Use the RheoSwitch ® oral activator ligand to regulate the amount of prostacyclin produced in the pulmonary arteries 28

Intellectual Property • Intrexon Corporation ‒ RheoSwitch Therapeutic System® and vector constructs • Synthetic Biologics ‒ Select issued patents ▪ Trimesta (oral estriol ) o U.S. Patent 6,936,599, issued August 30, 2005 ▪ Effirma (flupirtine) o U.S. Patent 6,610,324, issued August 26, 2003 ‒ Select patents pending ▪ Trimesta (oral estriol ) – 11 other patent applications filed ▪ AEN - 100 ( gastroretentive zinc acetate) – once - daily, gastroretentive, sustained - release, high - dose zinc acetate 29

30 Coming Milestones • Initiate Phase II/III clinical trial for Lou Gehrig’s disease later in 2012 • Complete patient enrollment in Phase II clinical trial of females with relapsing - remitting MS • Establish and expand new and existing collaborations in the synthetic biologics area

31 Investment Summary • Three drug candidates for treatment of MS, fibromyalgia and ALS that are either in Phase II clinical development or about to begin Phase II/III clinical development − Together, treatments for these serious conditions represent markets valued at approximately $18 billion − Costs of clinical trials funded in full or in part by foundations, partners, and other outside sources, and/or fully covered by financial reserves • New focus on synthetic DNA - based therapeutics – and its collaboration with Intrexon – provides an early opportunity to participate in what could be the next major wave of biotechnology via synthetic biologics

NYSE Amex: SYN April 24, 2012
