Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - INFINITY PHARMACEUTICALS, INC. | d338118d8k.htm |
 Building a Fully Integrated Biopharmaceutical
Company
April 2012
Exhibit 99.1 |
 2
•
This presentation contains forward-looking statements within the meaning of The
Private Securities Litigation Reform Act of 1995. •
These statements involve risks and uncertainties that could cause actual results to
be materially different from historical results or from any future results
expressed or implied by such forward-looking statements. •
Such
forward-looking
statements
include
statements
regarding:
the
therapeutic
potential
of
Infinity’s
Hedgehog
pathway,
Hsp90
and
PI3K
inhibitors
and
the
ability
to
achieve
proof
of
concept
in
each
of
these
programs
by
the
end
of
2013;
the
potential
for
data
from
the
ongoing
clinical
trials to lead to multiple registration paths; the potential of saridegib and
Hedgehog pathway inhibition in addressing chondrosarcoma and myelofibrosis;
the potential of IPI-145 and PI3K inhibition in addressing hematologic malignancies and inflammation; the potential of combination
therapy based on retaspimycin HCl in addressing non-small cell lung cancer, the
future market size of non-small lung cancer therapeutics, and the
expectation that Infinity: will report data in the second half of 2012 from the
Phase 2 trial of saridegib in patients with myelofibrosis, the dose-
escalation portion of the Phase 1b/2 trial of retaspimycin HCl in combination with
everolimus in patients with NSCLC, and each of the Phase 1 trials
of
IPI-145;
will
complete
enrollment
in
the
second
half
of
2012
in
the
Phase
2
trial
of
saridegib
in
patients
with
chondrosarcoma
and
the
Phase 2 trial of retaspimycin HCl in combination with docetaxel in patients with
NSCLC; and will commence future clinical development of its product
candidates, including a Phase 2 trial of IPI-145 in inflammation in the second half of this year. Such forward-looking statements also
include
estimates
of
2012
financial
performance
and
the
expectation
that
Infinity
will
have
a
cash
runway
to
support
its
current
operating
plan
through key inflection points.
•
Such statements are subject to numerous factors, risks and uncertainties that may
cause actual events or results to differ materially from the company’s
current expectations. For example, there can be no guarantee that Infinity’s strategic alliance with Mundipharma will continue for its
expected term or that it will fund Infinity’s programs as agreed, that any
product candidate Infinity is developing will successfully complete
necessary preclinical and clinical development phases, or that development of any
of Infinity’s product candidates will continue. Further, there can be
no guarantee that any positive developments in Infinity’s product portfolio will result in stock price appreciation. Management’s expectations
could also be affected by risks and uncertainties relating to: Infinity’s
results of clinical trials and preclinical studies, including subsequent analysis
of existing data and new data received from ongoing and future studies; the content
and timing of decisions made by the U.S. Food and Drug Administration
and other regulatory authorities, investigational review boards at clinical trial
sites and publication review bodies; Infinity’s ability to enroll
patients in its clinical trials; unplanned cash requirements and expenditures, including in connection with business development activities;
development of agents by Infinity’s competitors for diseases in which Infinity
is currently developing its product candidates; and Infinity’s ability to
obtain, maintain and enforce patent and other intellectual property protection for
any product candidates it is developing. •
These
and
other
risks
which
may
impact
management's
expectations
are
described
in
greater
detail
under
the
caption
"Risk
Factors"
included
in
Infinity's annual report on Form 10-K for the year ended December 31, 2011,
filed with the U.S. Securities and Exchange Commission on March 13, 2012.
•
Further, any forward-looking statements contained in this presentation speak
only as of the date hereof, and Infinity expressly disclaims any obligation
to update any forward-looking statements, whether as a result of new information, future events or otherwise.
Forward Looking Statements |
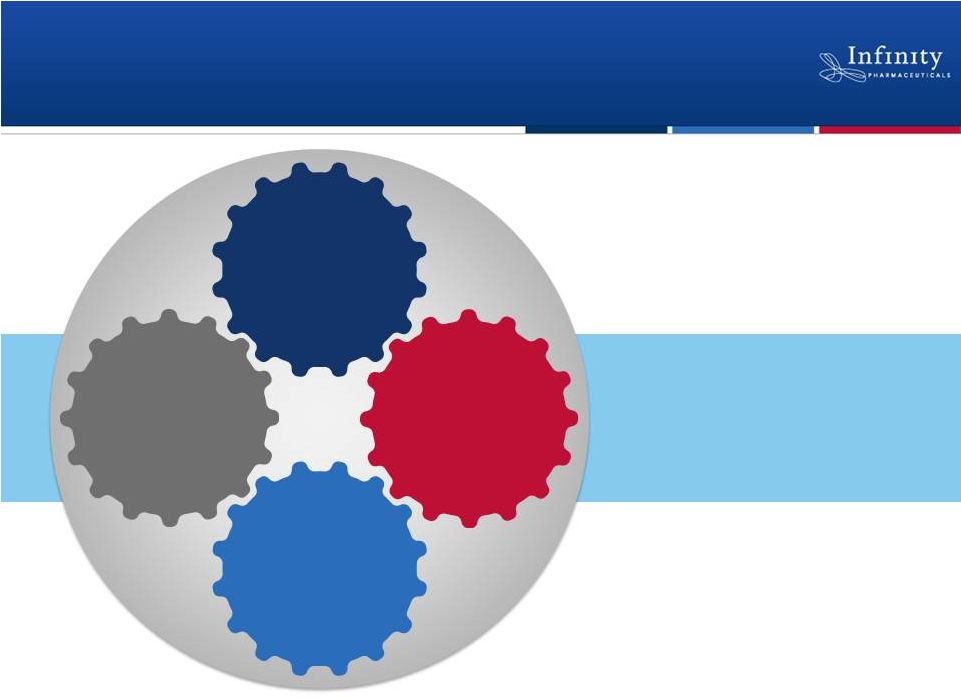 Sustainable model
for value creation
3
Breakthrough
Science
Deep,
Diverse
Pipeline
High-value,
Enabling
Partnerships
Full U.S.
Commercial
Rights
Building a Fully Integrated
Biopharmaceutical Company |
 •
Broad pipeline
–
Six ongoing clinical trials across three novel targets
•
Tailored development strategies
–
Randomized, double-blind, placebo controlled trials based on
compelling scientific or clinical data
–
Single-arm, exploratory trials designed to confirm intriguing
hypotheses with minimal investment
•
Data anticipated from all ongoing trials by YE13
4
Advancing a Diverse Pipeline: R&D Strategy |
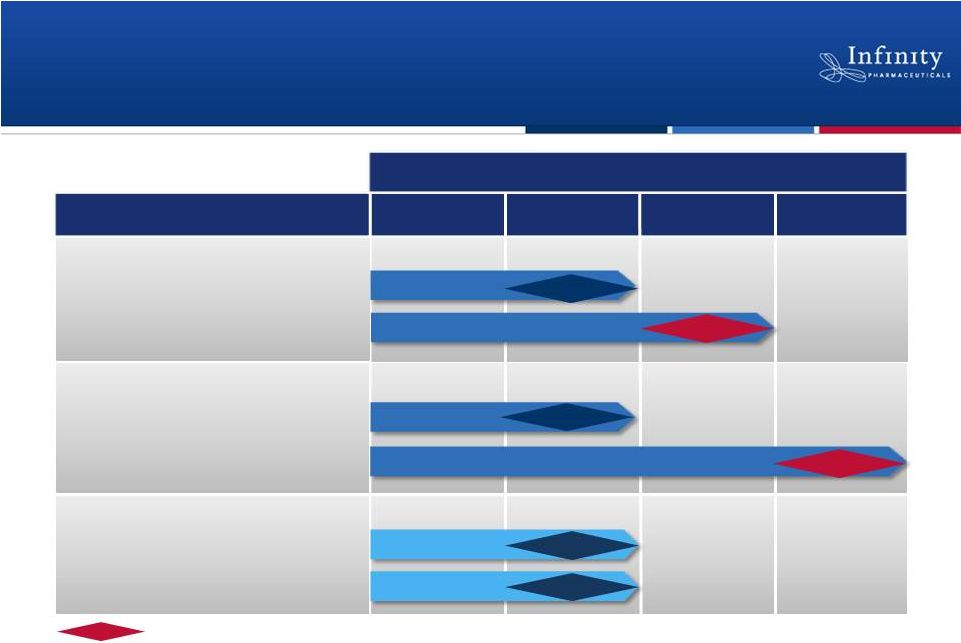 5
1H’12
1H’13
2H’13
2H’12
Hedgehog: Saridegib
Hsp90: Retaspimycin HCl
PI3K: IPI-145
Chondrosarcoma
NSCLC (mKRAS)
Myelofibrosis
NSCLC (Heavy smokers)
Inflammation
Hematologic Malignancies
2012
2013
Randomized, double-blind, placebo-controlled trial.
Data
Data
Data
Data
Data
Data
Promising Pipeline Based on Breakthrough
Science
Phase 1
Phase 1
Phase 2
Phase 2
Phase 1b/2
Phase 2 |
 Saridegib (IPI-926):
Addressing Cancers with High Unmet
Need by Targeting the Hedgehog Pathway |
 Importance of the Hedgehog pathway
7
Saridegib: Establishing a Leadership Position
in Underserved, Life-Threatening Diseases
•
Known to drive a broad range of cancers through multiple mechanisms,
including signaling to
–
Tumor cells directly
–
Tumor microenvironment
–
Tumor progenitor cells
Program status
•
Well-tolerated and clinically active in Phase 1
•
Two Phase 2 trials ongoing exploring distinct biological mechanisms
•
Robust biomarker strategy
•
Disrupts malignant activation of the Hedgehog pathway by
inhibiting the target Smoothened
Saridegib (IPI-926) |
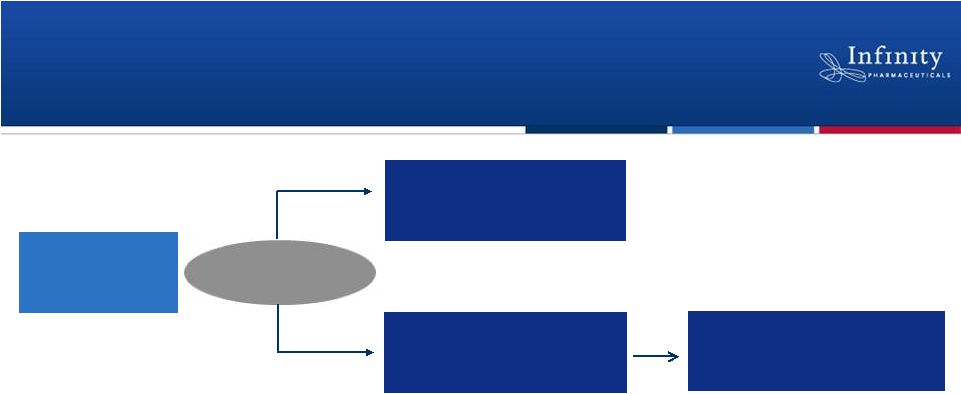 •
Randomized, double-blind, placebo-controlled trial
–
Enrolling patients with metastatic or locally advanced, unresectable
chondrosarcoma
–
Primary endpoint: Progression-free survival
–
Trial design reviewed with FDA and EMA prior to study
•
Anticipate enrollment completion 2H’12 and data 1H’13
~140 Patients
Saridegib 160 mg
(QD)
(N = ~94)
Placebo
(N = ~46)
Progression -
crossover
to
saridegib
8
Saridegib: Global, Randomized Phase 2 Trial in
Chondrosarcoma
2:1
Randomization |
 •
Hedgehog signaling is activated in ~ 70% chondrosarcomas
•
In xenografts derived from primary human chondrosarcoma
tumors, administration of saridegib led to:
–
Down-regulation of the Hedgehog pathway in tumor cells
–
Loss of tumor cells; decreased in tumor growth
9
Read, AACR 2011.
Saridegib
Control
Activated Gli-1 in
Chondrosarcoma
Saridegib in Chondrosarcoma: Strong
Preclinical Rationale
Tumor cells
Cartilage matrix
Calcified matrix
Decreased tumor
cells
Nuclear Gli-1 |
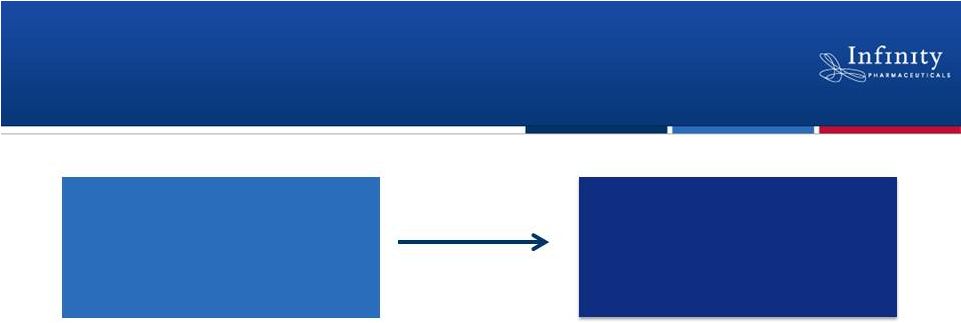 Saridegib 160 mg (QD)
(N = up to 35)
Saridegib 160 mg (QD)
(N = up to 35)
Saridegib 160 mg (QD)
(N = 12)
10
•
Open-label, exploratory trial with option for expansion
–
Primary endpoint: Response rate according to International Working Group
Criteria
–
No spontaneous remissions in this disease
•
First cohort of patients enrolled
•
Data anticipated in 2H’12
Expansion
Saridegib: Phase 2 Trial in Myelofibrosis |
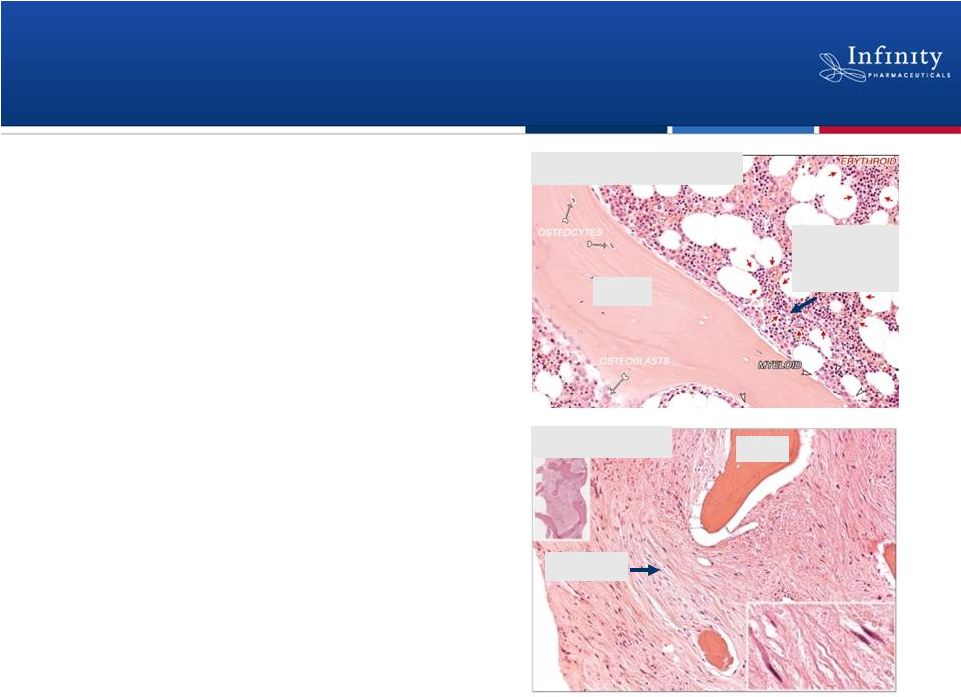 •
Blood cancer characterized by bone
marrow failure and enlarged spleen due
to pathogenic fibrosis
•
In myelofibrosis, Hedgehog ligand
expressed in bone marrow
•
Current treatments target symptoms,
not underlying disease
11
Tkachuk and Hirshmann, Wintrobe’s Atlas of Clinical
Hematology, 2007. Saridegib in Primary Myelofibrosis: Hedgehog Pathway
Plays Key Role in Pathogenic Fibrosis
Healthy Bone Marrow
Myelofibrosis
Fibrosis
Bone
Bone
Red and
white
blood cells |
 *Estimates based upon G7 countries (US, UK, IT, DE, ES, FR, JP)
12
Saridegib: First-in-Class Potential in
Chondrosarcoma and Myelofibrosis
Chondrosarcoma
Myelofibrosis
Market size
Ultra-orphan*
~30,000 patients*
Unmet need
Resistant to radiation
and chemotherapy
Primary treatment option
is surgery
No current treatments
address underlying
cause of disease
Potential for
franchise expansion
Other sarcomas
Other fibrotic diseases
and heme
malignancies |
 Retaspimycin HCl (IPI-504)
Targeting Non-Small Cell Lung Cancer Through
Hsp90 Inhibition |
 Function of Hsp90
•
“Chaperone”
protein necessary for stability
and function of certain ‘client’
proteins,
including oncoproteins
Retaspimycin HCl (IPI-504)
•
Selective, potent, naturally-derived
Hsp90 inhibitor
•
Well-defined and manageable safety
profile with QW dosing
Program status:
•
Well-tolerated and clinically active in
combination with docetaxel in NSCLC
•
Two clinical trials in NSCLC
14
Retaspimycin HCl: Leading the Field in
Hsp90 |
 •
Randomized, double-blind, placebo-controlled trial
•
Anticipate enrollment completion 2H’12 and data 2H’13
15
~200 smokers w/
2
nd
-
or
3
rd
-line
NSCLC
(docetaxel naïve)
Follow-up for OS
Follow-up for OS
Docetaxel +
Retaspimycin HCl
(N = ~100)
Docetaxel +
placebo
(N = ~100)
Retaspimycin HCl: Phase 2 Trial in NSCLC
Patients with a Smoking History
R
Primary endpoint: Overall survival
Secondary endpoints: Predictive biomarkers, progression free
survival,
overall response rate |
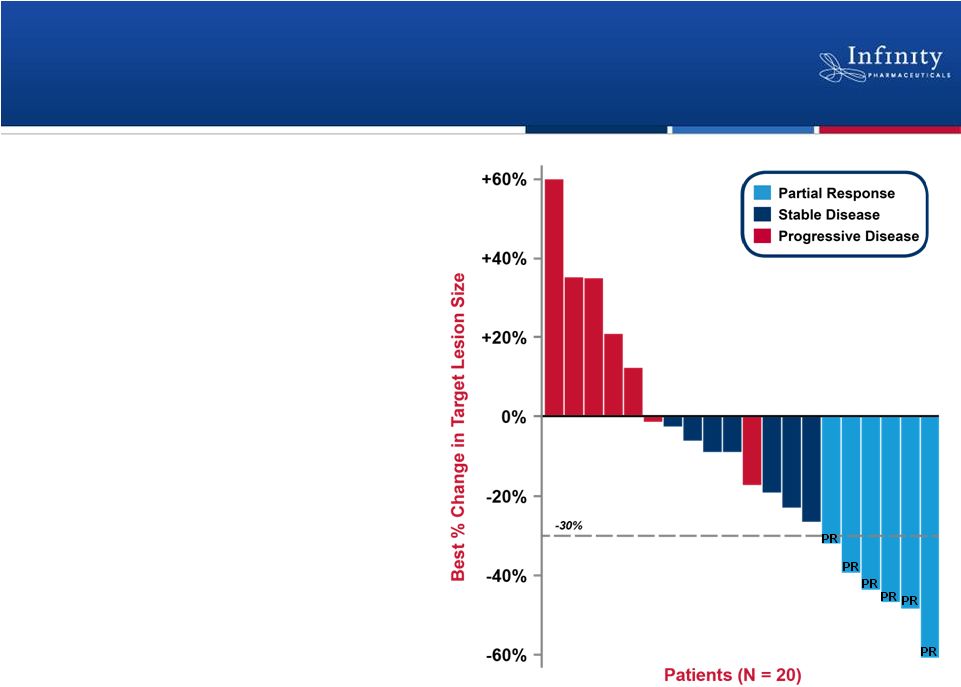 16
Encouraging Phase 1b data
•
Partial response in 6 patients
(ORR = 26%)
•
Stable disease in 7 patients
•
Well-tolerated
–
No unexpected or overlapping
toxicities
–
No dose reductions or
discontinuations due to liver
function tests
–
No ocular toxicities
Riely et al., ASCO 2011.
Retaspimycin HCl Phase 1b Trial: Clinically
Active in Combination with Docetaxel |
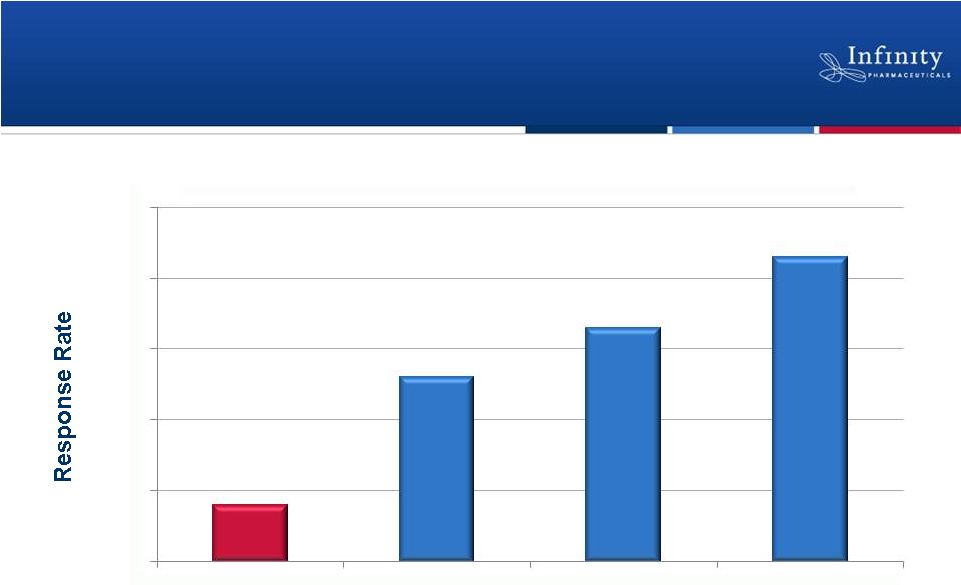 17
*Hanna et al, J Clin Oncol, 22:1589-97.
Retaspimycin HCl: Responses Observed in
Patients with Historically Poor Prognoses
8%
26%
33%
43%
0%
10%
20%
30%
40%
50%
Historical 2nd-line
Docetaxel*
NSCLC patients in
trial (N=23)
Smokers (N=18)
Squamous Cell
Carcinoma (N=7)
Patient Populations
Retaspimycin HCl Plus Docetaxel: Overall Response Rate
|
 Retaspimycin HCl
+ everolimus
(N = 12)
Retaspimycin HCl
+ everolimus
(N = 12)
Determine
recommended
Phase 2 dose in
mKRAS NSCLC
•
Small, open-label exploratory trial with option for expansion
–
Primary efficacy endpoint: Response rate
–
Neither drug active as single agent in these patients
•
Strong preclinical rationale: Evidence of substantial tumor regression in a
NSCLC model*
•
Topline data from dose-escalation portion of trial anticipated 2H’12
18
Retaspimycin HCl
+ everolimus
(N = up to 45)
Retaspimycin HCl
+ everolimus
(N = up to 45)
Expansion
Phase 1b
Phase 2
*De Raedt et al., 2011; Cancer Cell 13;20(3):400-13.
Retaspimycin HCl: Phase 1b/2 Trial in
NSCLC Patients with mKRAS |
 Current NSCLC market is ~ $3.5B and is projected to grow to $9.8B in 2018
References:
Decision
Resources
NSCLC
Pharmacor
Report,
March
2011.
G7
regions:
US,
UK,
IT,
DE,
ES,
FR,
JP;
Roberts
et
al.,
2010;
J
Clin
Oncol
28(31):4769-4777.
Janjigian
et
al.,
2010;
Cancer
116(3):670-675.
19
Retaspimycin HCl Has Significant
Commercial Potential
Patient
Population
% of Overall
NSCLC
Population
Number of
Patients
Number of
Stage IIIb/IV
Patients
Heavy smokers
Squamous cell
KRAS mutant
70%
35%
30%
~291,000
~145,000
~125,000
~183,000
~91,000
~79,000 |
 IPI-145:
Potential Best-in-Class Opportunity for Inflammation
and Hematologic Malignancies |
 21
ADAPTIVE
Graft vs. Host Disease
INNATE
Atherosclerosis
Sepsis
Cancer
Asthma
Crohn’s Disease
Lupus
Rheumatoid Arthritis
The Power of PI3K-
,
Inhibition |
 Function
of
PI3K-
and
PI3K-
•
Involved in key immune cell functions, including cell proliferation,
survival and cellular trafficking
•
Inhibiting both isoforms may be beneficial in both hematologic
cancers and inflammatory conditions
IPI-145
•
Potent, oral inhibitor of both delta and gamma isoforms
•
Active in preclinical models of inflammation
Program status:
•
Phase 1 trial in healthy volunteers completed to enable development
in inflammation
•
Phase 1 in patients with hematologic malignancies ongoing
22
IPI-145: Only PI3K-
Inhibitor in the Clinic
, |
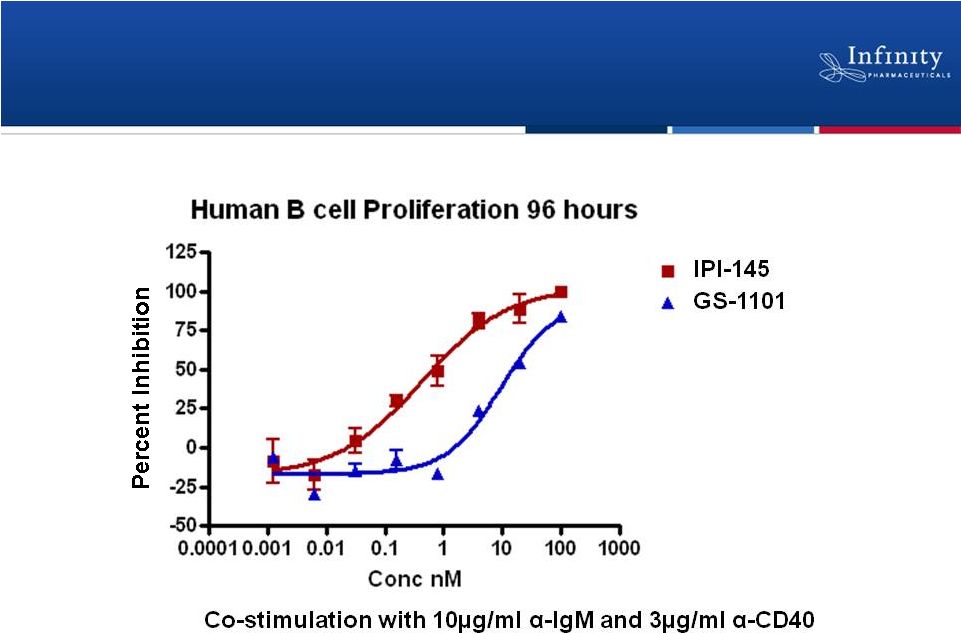 23
In-house data
IPI-145 Is More Potent than Delta-Selective
Inhibitor with Promising Clinical Activity |
 IPI-145
Phase 2 in
Inflammation
24
•
Phase 1 double-blind, randomized, placebo controlled trial
–
Trial completed
–
Data anticipated in 2H’12
•
Phase 2 trial in inflammation expected to begin this year
•
Multiple large commercial opportunities
IPI-145: Dual Clinical Development Paths in
Inflammation and Hematological Malignancies
Phase 1 in
Healthy Subjects
•
Single Ascending Dose
•
Multi Ascending Dose |
 25
•
Phase 1 dose escalation under way
•
Phase 1 data anticipated 2H’12
•
First-in-class and best-in-class commercial opportunities
–
Indications validated with a
-specific inhibitor (e.g., CLL, iNHL, mantle cell)
–
Indications in which a
-specific inhibitor showed little activity (e.g., DLBC, AML, MM)
–
Indications untested by a
-specific inhibitor
IPI-145: Dual Clinical Development Paths in
Inflammation and Hematological Malignancies
IPI-145
Phase 1 in Hematologic
Malignancies
•
Dose escalation
Phase 1 Expansion
in Select Cohorts |
 Strong Financial Foundation to
Reach Key Inflection Points |
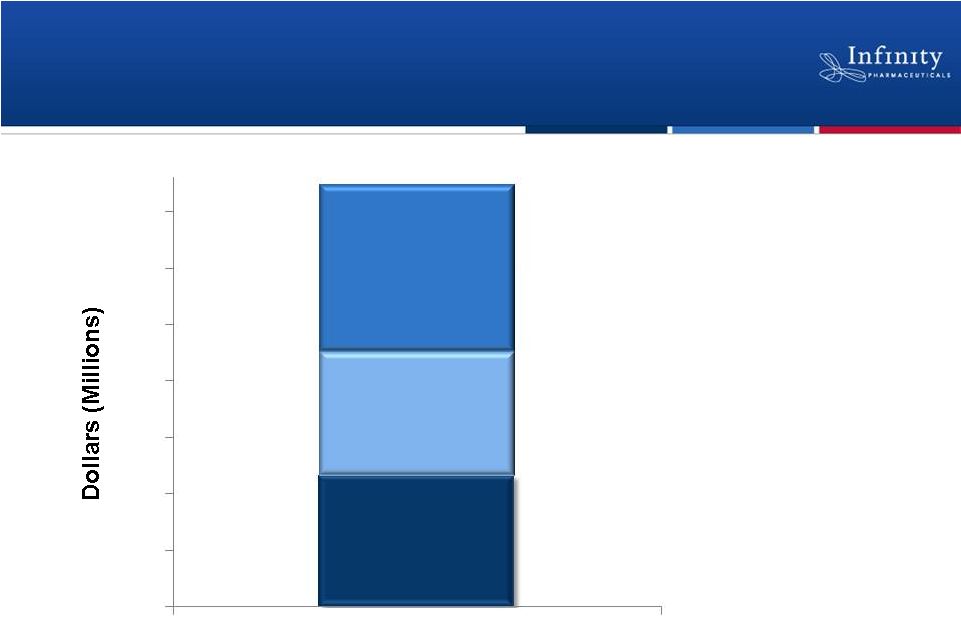 2012 Committed R&D
Funding from Mundipharma
Cash and Investments
Current and Committed Capital*
>$370 Million
*
As of December 31, 2011.
2013 Committed R&D
Funding from Mundipharma
27
Cash Runway Into 2014
$115.9M
$110.0M
$147.5M
0
50
100
150
200
250
300
350 |
 •
Projected 2012 revenue of ~$114M
•
Anticipate year-end cash and investments balance of
$75M
-
$85M
•
Projected
2012
cash
burn
of
$30M
-
$40M
•
Approximately 26.9 million shares outstanding as
of March 31, 2012
28
2012 Financial Guidance |
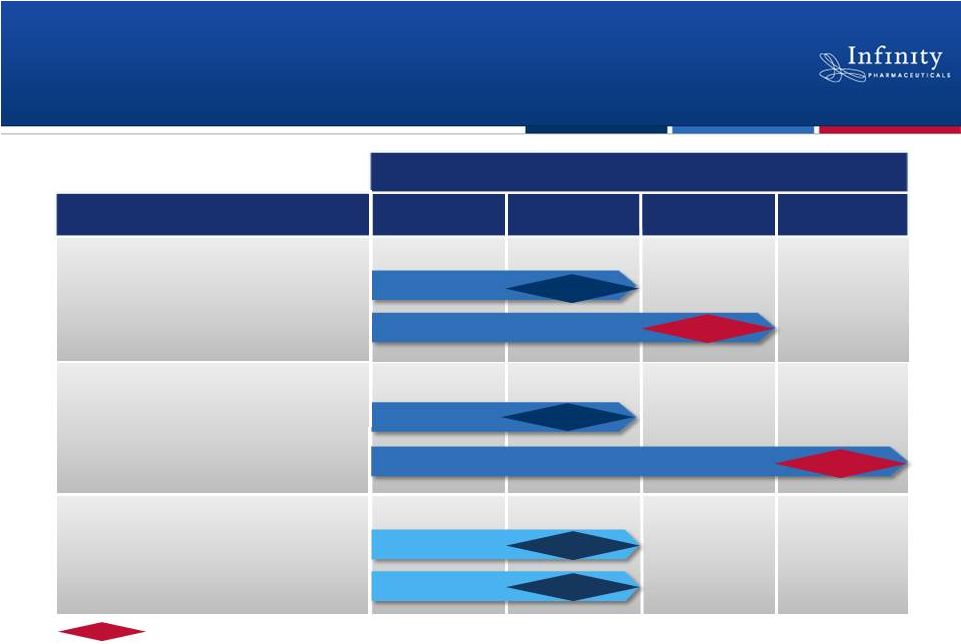 29
1H’12
1H’13
2H’13
2H’12
Hedgehog: Saridegib
Hsp90: Retaspimycin HCl
PI3K: IPI-145
Chondrosarcoma
NSCLC (mKRAS)
Myelofibrosis
NSCLC (Heavy smokers)
Inflammation
Hematologic Malignancies
2012
2013
Randomized, double-blind, placebo-controlled trial.
Promising Pipeline Based on Breakthrough
Science
Phase 2
Phase 1b/2
Phase 2
Phase 1
Phase 1
Data
Data
Data
Data
Data
Data
Phase 2 |
 Building a Fully Integrated Biopharmaceutical
Company
April 2012 |
