Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - K-V Pharmaceutical Co | d329143d8k.htm |
 K-V Pharmaceutical
Investor Presentation
April 2012
Exhibit 99.1 |
 Safe
Harbor Statement 2
Cautionary Note Regarding Forward-looking Statements
This presentation contains various forward-looking statements within the meaning of the United
States Private Securities Litigation Reform Act of 1995 (the “PSLRA”) and which may
be based on or include assumptions concerning our operations, future results and prospects. Such statements may be identified by the use of words like “plan,”
“expect,” “aim,” “believe,” “project,” “anticipate,”
“commit,” “intend,” “estimate,” “will,” “should,” “could,” “potential” and other expressions that indicate future events and
trends.
All statements that address expectations or projections about the future, including, without
limitation, statements about product launches, governmental and regulatory actions and
proceedings, market position, revenues, expenditures and the impact of the recall and suspension of shipments on revenues, adjustments to the financial
statements, and other financial results, are forward-looking statements.
All forward-looking statements are based on current expectations and are subject to risk and
uncertainties. In connection with the PSLRA’s “safe harbor” provisions, we
provide the following cautionary statements identifying important economic, competitive, political,
regulatory and technological factors, among others, that could cause actual results or events
to differ materially from those set forth or implied by the forward-looking statements and related assumptions. Such factors include (but are not
limited to) the following:
(1) our ability to continue as a going concern;
(2) risks associated with the introduction and growth strategy related to the Company’s
Makena ® product, including: (a) the impact of competitive, commercial payor,
governmental (including Medicaid program), physician, patient, public or political responses and reactions, and
responses and reactions by medical professional associations and advocacy groups, on the
Company’s sales, marketing, product pricing, product access and strategic efforts;
(b) the possibility that the benefit of any period of exclusivity resulting from the
designation of Makena® as an orphan drug may not be realized as a result of the FDA’s
decision to decline to take enforcement action with regards to compounded alternatives;
(c) the Center for Medicare and Medicaid Services’ (“CMS”) policy regarding Medicaid
reimbursement for Makena®, and the resulting coverage decisions for Makena® by
various state Medicaid and commercial payors; (d) the satisfaction or waiver of the terms
and conditions for our continued ownership of the full U.S. and worldwide rights to Makena® set forth in the previously
disclosed Makena® acquisition agreement, as amended; and
(e) the number of preterm births for which Makena® may be prescribed and its safety profile
and side effects profile and acceptance of the degree of patient access to and pricing;
(3) the possibility of delay or inability to obtain U.S. Food and Drug Administration
(the “FDA”) approvals of Clindesse and Gynazole-1 and the possibility that any
product relaunch may be delayed or unsuccessful;
(4) risks related to compliance with various agreements and settlements with governmental
entities including: (a) the consent decree between the Company and the FDA and the
Company’s suspension in 2008 and 2009 of the production and shipment and the nationwide
recall of all of the products that it formerly manufactured, as well as the related material adverse
effect on our revenue, assets and liquidity and capital resources; (b) the agreement between
the Company and the Office of Inspector General of the U.S. Department of Health and Human Services (“HHS OIG”) to resolve the risk
of potential exclusion of the Company from participation in federal healthcare programs; and
(c) our ability to comply with the plea agreement between a now-dissolved subsidiary of the
Company and the U.S. Department of Justice; (5) the availability of raw materials
and/or products manufactured for the Company under contract manufacturing agreements with third parties;
|
 Safe
Harbor Statement (cont.) 3
(6) risks that the Company may not ultimately prevail in or that insurance proceeds
will be insufficient to cover potential losses that may arise from litigation, including:
(a) the series of putative class action lawsuits alleging violations of the federal securities
laws by the Company and certain individuals; (b) product liability lawsuits;
(c) lawsuits pertaining to indemnification and employment agreement obligations involving
the Company and its former Chief Executive Officer ; (d)
the possibility that the pending lawsuits and investigation by HHS OIG regarding potential false claims under the Title 42 of the U.S. Code could result in significant
civil fines or penalties, including exclusion from participation in
federal healthcare programs such as Medicare and Medicaid and the possibility; and
(e) challenges to our intellectual property rights by actual or potential competitors and
challenges to other companies’ introduction or potential introduction of generic or
competing products by third parties against products sold by the
Company; (7) the possibility that our current estimates of the financial effect of previously
announced product recalls could prove to be incorrect; (8) risks related to the Company’s highly leveraged capital structure,
including: (a) the risk that our debt obligations may be accelerated due to our
inability to comply with covenants and restrictions contained in our loan agreements;
(b) restrictions on the ability to increase our revenues through certain transactions, including
the acquisition or in-licensing of products; and (c) risks that present or
future changes in the Board of Directors may lead to an acceleration of the Company’s debt;
(9) the risk that we may not be able satisfy the quantitative listing standards of
the New York Stock Exchange, including with respect to minimum share price and public float;
and
(10) the risks detailed from time to time in the Company’s filings with the SEC. This
discussion is not exhaustive, but is designed to highlight important factors that may impact
our forward-looking statements.
Because the factors referred to above, as well as the statements included under the captions Part I,
Item 7A—“Risk Factors,” Item 2—“Management’s Discussion and Analysis
of Financial Condition and Results of Operations” and elsewhere in our Form 10-K/A for the
fiscal year ended March 31, 2011, as supplemented by our subsequent SEC filings,
could cause actual results or outcomes to differ materially from those expressed in any
forward-looking statements made by us or on our behalf, you should not place undue
reliance on any forward-looking statements.
All forward-looking statements attributable to us are expressly qualified in their entirety by the
cautionary statements in this “Cautionary Note Regarding Forward-Looking
Statements” and the risk factors that are included under the caption Part I, Item
7A—“Risk Factors” in our Form 10-K/A for the fiscal year ended March 31, 2011, as
supplemented by our subsequent SEC filings. Further, any forward-looking statement speaks only as
of the date on which it is made and we are under no obligation to update
any of the forward-looking statements after the date of this Report. New factors emerge from time
to time, and it is not possible for us to predict which factors will arise, when
they will arise and/or their effects. In addition, we cannot assess the impact of each factor on our
future business or financial condition or the extent to which any factor, or combination of
factors, may cause actual results to differ materially from those contained in any forward-looking statements. |
 Strategic Direction
Leading Branded Specialty Pharmaceutical
Company with a Focus in Women’s Health
Synergistic product portfolio
anchored by Makena
®
and
supported by Anti-Infective
Franchise & Evamist
®
Commercialization strategy
with history of building
brands in Women’s Health
4
Nationally deployed sales force
supported by an experienced
commercial organization
Strong compliance and corporate governance |
 Strategic Priorities
1.
Increase
Makena
®
penetration:
Reinforce information highlighting differences between compounded 17P and
FDA-approved
Makena
®
with
physician
community
Grow state Medicaid payer relationships
Leverage
expanded
payer
coverage
of
Makena
®
to
drive
volume
Utilize nationally deployed sales organization led by recently hired National
Sales Director
2.
Return our anti-infective products to market
Working with our contract manufacturers to return products to market during
calendar year 2012
3.
Continue to actively manage our financial position and improve liquidity
5 |
 Key
Recent Events 1
2
3
FDA Approval of
Makena®
and
Product Launch
February/March
2011
Divested generics
business
August
2011
Products:
•
Ramp up Makena®
•
Return
anti-infective
products to market
Financial:
•
Continued efforts to
reduce cost structure
•
Financing initiatives
2012
4
6
October
2011
•
ACOG and SMFM
Clarification Statements
•
Medicaid contract signed
with Florida
November
2011
•
Independent testing
results announced
•
FDA issues update
and commences
review and testing
6
5 |
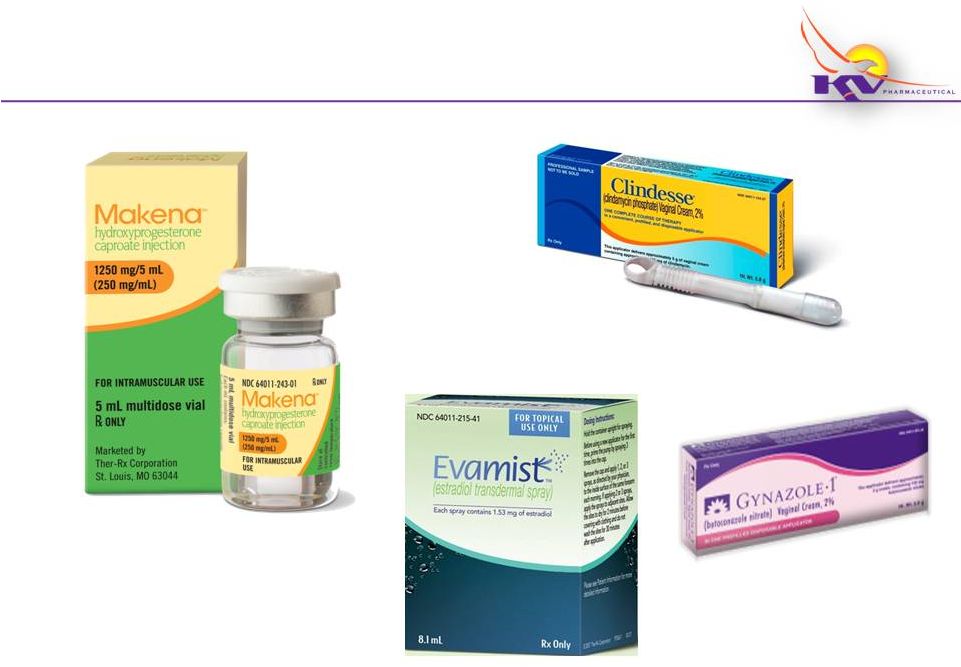 Women’s Health
Product Portfolio Addresses Key Therapeutic Areas
7 |
 Makena
®
Overview
Makena
®
is a progestin indicated to reduce the risk of preterm
birth in women with a singleton pregnancy who have a history
of singleton spontaneous preterm birth
-
Demonstrated efficacy and safety profile
-
Not for multiple pregnancies (twins, etc.)
-
Granted Orphan Drug Exclusivity
-
Patients identifiable based on their obstetric history
-
Many of these patients are highly motivated
to do what
they can to help prevent another preterm birth
Very high cost condition
-
-
Sterile injectable
Sterile injectable
(1)
Behrman
RE,
Butler
AS,
eds.
Committee
on
Understanding
Premature
Birth
and
Assuring
Healthy
Outcomes.
Preterm
Birth:
Causes,
Consequences,
and
Prevention.
Washington,
DC:
National
Academies
Press;
2007.
8
Preterm
birth
costs
average
over
$51,600
(1)
and
can
reach
hundreds of thousands of dollars
Financial burden falls on payors and employers
Administered via weekly IM injections, starting between weeks
16-20 of gestation and continuing through week 37 or delivery,
whichever comes first |
 Makena
®
Market Opportunity
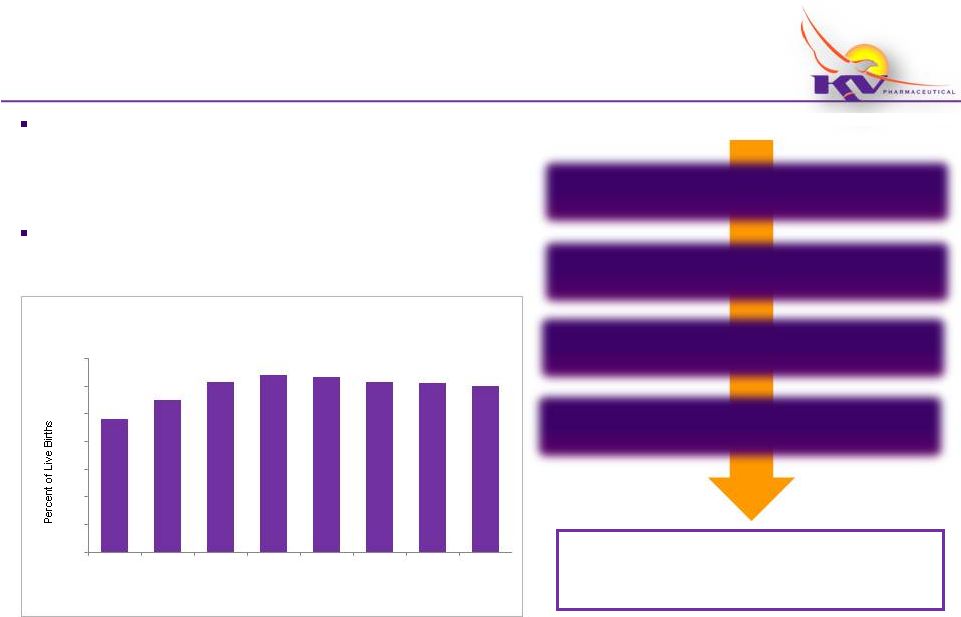
Preterm Birth is Costly and Prevalent
Preterm Birth is a growing concern in the U.S. impacting
+12% of births each year
-
Clinically defined as < 37 weeks
-
Highest risk of PTB are those with a history of PTB
Results
in
$26
billion
in
annual
direct
and
indirect
costs
(2)
-
Emotional costs for families are immeasurable
Makena
®
is an orphan drug with an estimated
patient population of up to
~140,000 annually
~140,000 women with a singleton pregnancy who
have a history of singleton SPTB in U.S. per year
~450,000 singleton preterm births in U.S. per year
~545,000 preterm births in U.S. per year
4.3 million births in U.S. per year
9
Decline in rate of PTB in recent years believed to be primarily attributable
to efforts to reduce elective “C-sections”
prior to 39 weeks
Preterm
Births
in
the
United
States
from
1983–2010
(1)
9.6
11.0
12.3
12.8
12.7
12.3
12.2
12.0
0.0
2.0
4.0
6.0
8.0
10.0
12.0
14.0
1983
1993
2003
2006
2007
2008
2009
2010
(1)
Data highlights preterm birth in general, including recurrent preterm birth.
www.marchofdimes.com. Accessed 09/04/11. (2)
Behrman RE, Butler AS, eds. Committee on Understanding Premature Birth and Assuring Healthy
Outcomes. Preterm Birth: Causes, Consequences ,and Prevention. Washington, DC:
National Academies Press; 2007. Calculations
estimated based on: Petrini et al. Estimated Effect of 17 Alpha-Hydroxyprogesterone
Caproate on Preterm Birth in the United States
Obstetrics and Gynecology VOL. 105, NO.2, FEBRUARY 2001
|
 Recent Makena
®
Events
American College of Obstetricians and Gynecologists (ACOG) and Society for
Maternal-Fetal Medicine (SMFM) issue information update clarifying
misconceptions and
highlighting
inherent
differences
between
Makena
®
and
compounded
17P
Ther-Rx
announces
results
of
independently
commissioned
study
testing
API
and finished products from compounding pharmacies
FDA announces receipt of K-V’s study of API and finished products from
compounding pharmacies
Reviewing the results of K-V testing
Conducting own independent analysis
Pending –
FDA follow-up on independent test results
Signed
Medicaid
contract
with
Florida
–
top
10
state
covering
an
estimated
2
to
3
million lives
3 state contracts in total
20
states
have
reimbursed
Makena
®
10 |
 Makena
®
The Facts…
Exact formulation demonstrated to be safe and
effective in NICHD MFMU Network Study
Manufactured in compliance with FDA’s current Good
Manufacturing Practices Dispensed with complete and
consistent FDA-approved prescribing information
Standardized distribution
National availability
Financial assistance programs with no household
income caps
11
FDA-approved
Makena
®
provides
important
benefits
to patients and healthcare providers
Evidence-
Based
Medicine
Manufacturing
and Safety /
Assurance of
Quality
Distribution
and Support |
 Makena
®
The Facts…
Exclusive sourcing agreement with Organon
API manufactured in FDA approved facilities
Same API manufacturer as NICHD MFMU Network
Study
12
KV
is
fully
committed
to
ensuring
access
to
Makena
®
for
all
eligible patients and to maximizing its value
K-V
Commitment to
FDA
Obligations
FDA Regulated
Supply Chain
Ongoing Phase 3b studies including the largest clinical
trial in preterm birth
1,700
Moms
and
>
500
infants
Enrolled ~500
moms to date |
 Makena
®
The Facts…
Originated primarily from facilities in China
Facilities
were
not
registered
and
not inspected by
the FDA
Unreliable
potency
Unreliable
purity –
unknown identity/toxicity
Outdated
40-year old USP standards
13
Study commissioned by Ther-Rx served as basis for
FDA’s
November
8
th
statement
on
Makena
®
API
Sourcing
Drug
Identity
Drug Purity
and Potency
One
API
sample
was
the
wrong
ingredient
(glucose) |
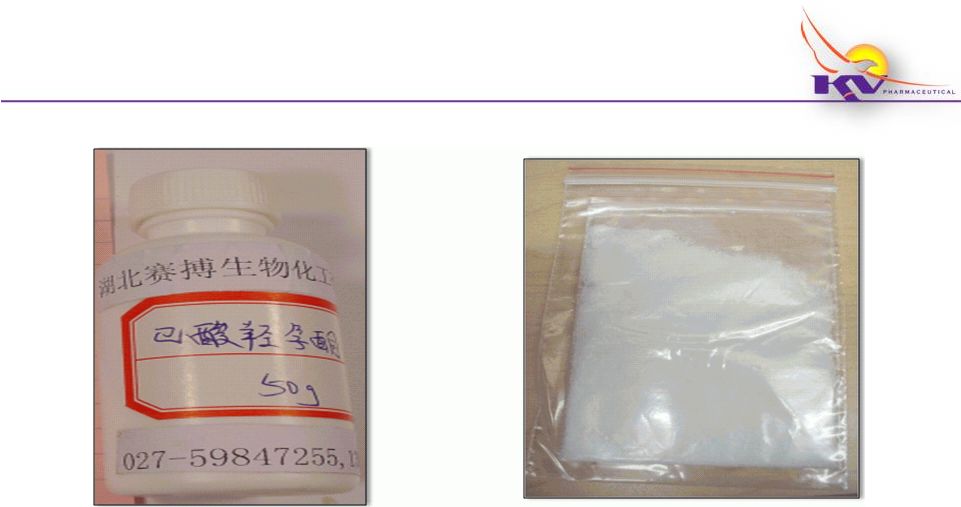 Makena
®
The Facts…
14
Bottle labeled in Chinese as
hydroxyprogesterone caproate
(HPC); upon analysis, the
substance in the bottle was found
to be glucose
Shipment delivered to the U.S. of
API for use in compounded 17P (in
unlabeled bag) from non-FDA
inspected or registered Chinese
manufacturer |
 Why
Makena ®
?
The Facts…
15
Alignment with
Evidence-Based Medicine
Assurance of
FDA’s Quality Standards
National Sales Force Advancing
Education and Awareness
Economic Value
and Commitment to Affordable
Access |
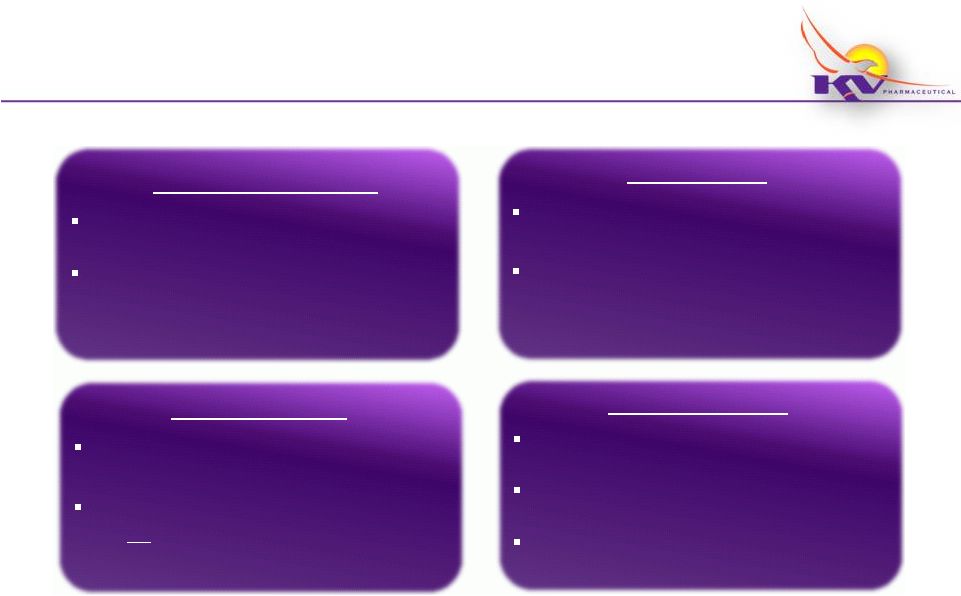 Makena
®
Performance to Date (Launch -
3/31/12)
16
Current Penetration
>3,800 physicians have written
prescriptions
> 4,500 patients have either started
therapy or are pending start
Patient Access
Patient copays averaging $10 per
injection before financial assistance
~ 43% of patients have had a copay
of $0
Unit Volume
~11,300 vials have shipped to
Specialty Pharmacies/Distributors
~9,900 vials have shipped to
patients; ~20% were financially
assisted
Payer Coverage
Over 250 commercial payers have
reimbursed Makena
®
19 of top 20 commercial payers
have reimbursed Makena
®
Medicaid –
20 states have
reimbursed Makena
® |
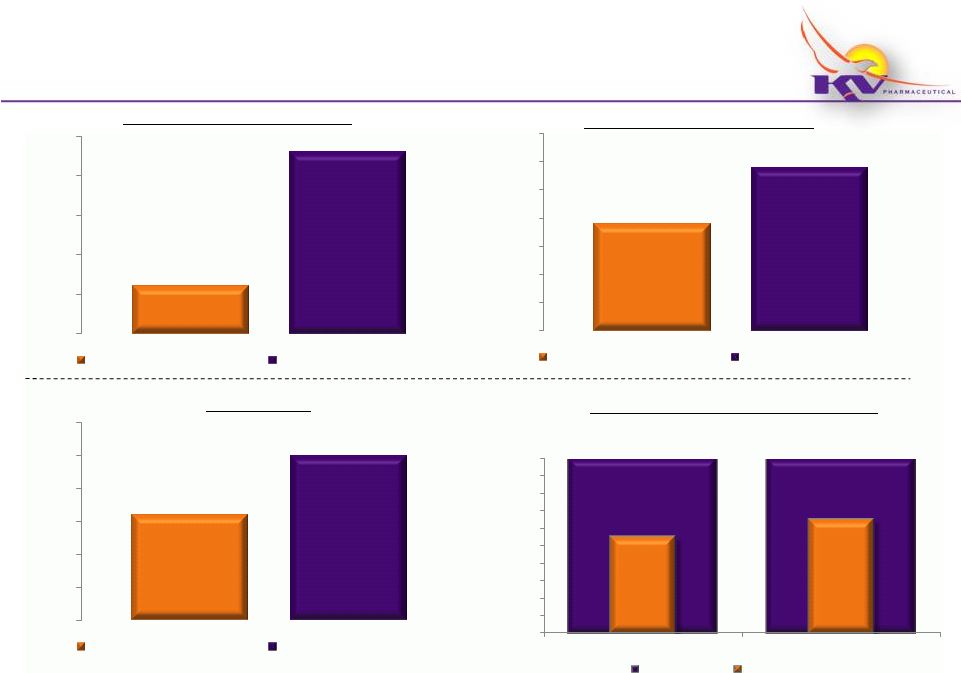 Makena
®
Key Metrics Dashboard
(Fiscal 2012)
17
Vials Shipped to Ther-Rx Customers
Vials Shipped to Physicians/Patients
Net Enrollments
Net Enrollments as a % of Gross Enrollments
~600
~2,300
0
500
1,000
1,500
2,000
2,500
October 2011 -
March 2012
~3,800
~5,800
0
1,000
2,000
3,000
4,000
5,000
6,000
7,000
April 2011 -
September 2011
October 2011 -
March 2012
~1,600
~2,500
0
500
1,000
1,500
2,000
2,500
3,000
April 2011 -
September 2011
October 2011 -
March 2012
~55
%
~65%
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
October
2011
-
March
2012
Gross Enrollments
Net Enrollments
April 2011 -
September 2011
* Product
launch occurred in March 2011; does
not
include
launch
quantities
* Totals shown above are estimated based upon activity through 3/30/12
April 2011 -
September 2011 |
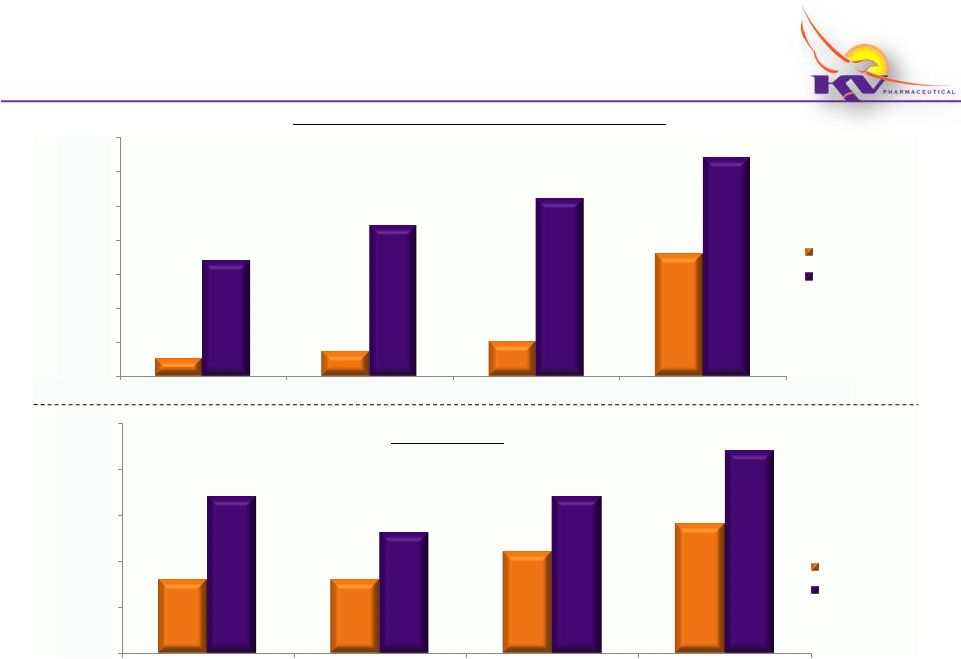 Makena
®
Key Metrics Dashboard
(Fiscal 2012)
Vials Shipped to Customers and Patients
18
Enrollments
~250
~350
~500
~1,800
~1,600
~2,200
~2,600
~3,200
0
500
1,000
1,500
2,000
2,500
3,000
3,500
Q1
Q2
Q3
Q4
Ther-
~800
~800
~1,100
~1,400
~1,700
~1,300
~1,700
~2,200
0
500
1,000
1,500
2,000
2,500
Q1
Q2
Q3
Q4
Rx Customers
Patients / Physicians
Net Enrollments
Gross Enrollments
* Product
launch occurred in March 2011; does
not
include
launch
quantities
* Totals shown above are estimated based upon activity through 3/30/12
|
 Makena
®
: Key Takeaways
19
-
FDA testing is in progress
Company completed independent testing of API and compounded 17P and
provided to the FDA
Efforts supported by clarification statements from the American College of
Obstetricians and Gynecologists (ACOG) and Society for Maternal-Fetal
Medicine (SMFM)
-
Out-of-pocket costs for patients remain in-line or less than the costs
of compounded 17
Successful execution of Makena
®
commercialization strategy generating positive
momentum across key business metrics
Focused on communicating the fundamental quality and safety differences
between FDA-approved Makena
®
and compounded 17P while maintaining an
affordable out-of-pocket cost for eligible patients
|
 20
White House and the F.D.A. Often at Odds
By GARDINER HARRIS
Published: April 2, 2012
Page A1
“
…
to the agency, the only issue was that [Makena] offered guaranteed
safety while those made by pharmacists were riskier. Though FDA
officials were then not aware of safety complaints about the pharmacy
made 17P, they worried about repeated instances over the years when
other pharmacy-made drugs had been found to lack potency or be
contaminated with deadly bacteria …
…
FDA officials said they had often been wrongly accused of
considering price in drug approval deliberations and had always been
able to reply that price was never a factor. ‘We can’t say that
anymore,’ a top F.D.A. official said unhappily.”
|
 Focused on re-building specialty product portfolio targeting critical and unmet
therapeutic needs in the women’s health category, where historically
K-V has played a leadership role
Current strategic priorities include securing FDA approval to re-launch
anti- infective
franchise
and
driving
Evamist
®
volume
Future strategy centered on potential in-licensing, product acquisition
opportunities, and re-launch of other existing products
Beyond Makena
®
21 |
 Other
Women’s Health Products (1)
http://www.cdc.gov/std/stats09/tables/43.html
22
Evamist
®
is a once-daily estrogen spray
indicated for the treatment of moderate to
severe vasomotor symptoms due to
menopause
Competes in the +$300MM Transdermal /
Topical Estrogen Therapy market
Novel delivery mechanism provides an
opportunity to differentiate with patients
and prescribers
Net revenue was ~$8 million through the
first nine months of FY12
Anti-infective Franchise
Clindesse
®
and
Gynazole-1
®
Evamist
®
(estradiol transdermal spray)
Both products offer convenient treatment for
frequently diagnosed conditions
Generated annual combined net sales of
$68MM through the end of 2008
-
An estimated 3.2 million physician office
visits
in
2009
for
women
ages
15
-
44
(1)
Re-launch pursuant to FDA review and
approval
Both products provide high patient satisfaction
and effectiveness
Each became the #1 prescribed branded intra-
vaginal product in its respective category |
 Financial Position
(1)
(in millions)
Expected Cash Position –
3/31/12
Cash and Cash Equivalents
Restricted Cash
Total Cash
Financial
Obligations
–
3/31/12
12% Senior Notes (mature March 2015)
2.5% Convertible Notes (put date May 2013)
Mortgage
Other Material Obligations:
Hologic Milestone Payments
DOJ Settlement
Other Government Settlement
(1) Source: 3Q FY 2012 Form 10-Q
23
40.0 -
$50.0
8.0
48.0 -
$58.0
225.0
200.0
31.0
95.0
18.0
17.0
586.0
$
$
$
$
$
$
$
$
$
$
Total Debt and Other Obligations |
 Reduced Cost Structure
($ in millions)
Operating Costs
Headcount
$ 90 -
100
$ 50 -
60
$ 30 -
40
$ 30 -
35
1,075
675
375
225
24
60 -
70%
~80%
$ 20 -
25
200 -
225
FY 2009
FY 2010
Average Quarterly Cash Costs
FY 2011
FY 2012
Target
% Reduction
2009 -
2012
Divestiture
of
generics
business
reduced
quarterly
costs
by
$8
to
$10
million
and reduced headcount by ~ 150
Continued
focus
by
management
team
to
further
reduce
cash
costs
Resolution
of legacy issues
Consolidation of physical infrastructure |
 Strategic Priorities
1.
Increase Makena
®
penetration:
Reinforce information highlighting differences between compounded 17P and
FDA-approved Makena
®
with physician community
Grow state Medicaid payer relationships
Leverage expanded payer coverage of Makena
®
to drive volume
Utilize nationally deployed sales organization led by recently hired National
Sales Director
2.
Return our anti-infective products to market
Working with our contract manufacturers to return products to market during
calendar year 2012
3.
Continue to actively manage our financial position and improve liquidity
25 |
 26
THE RIGHT DRUG.
THE RIGHT DOSE.
EVERY DOSE.
Only FDA-Approved Makena
®
Delivers … |
 K-V Pharmaceutical
Investor Presentation
April 2012 |
