Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - BIOLASE, INC | d329291d8k.htm |
 Fred
Furry, COO & CFO NASDAQ: BLTI
www.biolase.com
April 3-4, 2012 •
New York Palace Hotel, New York City
Exhibit 99.1 |
 Safe
Harbor Statement This
presentation
may
contain
forward-looking
statements
that
are
based
on
our
current
expectations,
estimates
and
projections
about
our
industry
as
well
as
management’s
beliefs
and
assumptions.
Words
such
as
“anticipates,”
“expects,”
“intends,”
“plans,”
“believes,”
“seeks,”
“estimates,”
“may,”
“will,”
and
variations
of
these
words
or
similar
expressions
are
intended
to
identify
forward-looking
statements.
These
statements
include
projections
about
our
future
earnings
and
margins
and
speak
only
as
of
the
date
hereof.
Such
statements
are
based
upon
the
information
available
to
us
now
and
are
subject
to
change.
We
will
not
necessarily
inform
you
of
such
changes.
These
statements
are
not
guarantees
of
future
performance
and
are
subject
to
certain
risks,
uncertainties
and
assumptions
that
are
difficult
to
predict.
Therefore
our
actual
results
could
differ
materially
and
adversely
from
those
expressed
in
any
forward-looking
statements
as
a
result
of
various
factors.
The
important
factors
which
could
cause
actual
results
to
differ
materially
from
those
in
the
forward-looking
statements
include,
among
others,
a
downturn
or
leveling
off
of
demand
for
our
products
due
to
the
availability
and
pricing
of
competing
products
and
technologies,
adverse
international
market
or
political
conditions,
a
domestic
economic
recession,
the
volume
and
pricing
of
product
sales,
our
ability
to
control
costs,
intellectual
property
disputes,
the
effects
of
natural
disasters
and
other
events
beyond
our
control
and
other
factors
including
those
detailed
in
BIOLASE’s
filings
with
the
Securities
and
Exchange
Commission
including
its
prior
filings
on
Form
10-K
and
10-Q.
PAGE
2 |
 BIOLASE is Revolutionizing Surgery in Medicine
•
The Er,Cr:YSGG (2780nm) laser crystal has the highest level of absorption in water
in human tissue. •
This absorption creates an expansion and vaporization of the water molecules
causing a biological ablation of human tissue with very little trauma or
bleeding. •
Tooth enamel ~5% water, dentin and bone ~25% water, soft tissue ~80% water, and
the eye ~90% water. How? Through the science of
WaterLase! PAGE 3
Conventional Surgical Devices include the scalpel, high speed
drill, electrosurge, electric bone saws, and heat generating lasers.
…BIOLASE is NOT Just Another Medical Device Company!
|
 In
2011: Single Product Company 1.
Moved from global exclusive distribution model with
Schein to direct sales and multi-distributor model.
2.
Launched iPlus which EQUALS the speed of a
traditional dental drill.
3.
Globalized distribution with approvals in Canada,
Korea, Russia, Taiwan, & others.
4.
Launched Biolase DaVinci digital imaging products.
5.
Grew revenues over 85% on a GAAP basis and 98%
excluding irrevocable purchase orders to Schein and
non-recurring royalty revenue.
6.
Expanded global sales footprint with new offices in
China & India and planned offices in UAE & Brazil.
In 2012: Broaden Platform and Product Portfolio
Becoming a Globally Diversified Platform Company
1.
Diversify revenue base to expand addressable markets
and reduce investor risk.
2.
Launched Waterlase MDX line of all tissue lasers.
3.
Became the exclusive distributor for Cefla’s NewTom line
of cone beam 3D imaging products in North America.
4.
Plan to launch several other new dental products in 2012
and re-launch the iLase with new distribution partners.
5.
Continue clinical development & approval in:
ophthalmology, orthopedics, pain management, podiatry,
and aesthetics.
6.
2012 is a year for execution and resetting investor
expectations …
under promise, over deliver.
PAGE 4 |
 BIOLASE is the World Leader in Laser Dentistry…
…and
the
Dental
Community
is
the
Largest
Medical
Community
in
the
World!
PAGE 5
BIOLASE ~45%
BIOLASE ~45%
Source: iData Research Inc.
US Overall Dental Laser Market:
Hard & Soft Tissue
BIOLASE ~80%
BIOLASE ~80%
Source: Biolase Technology Licensing Reports
US Dental Laser Market:
Hard Tissue |
 Traditional Dentistry
The painting Cavadenti, the Tooth Puller, by Caravaggio, circa 1608
PAGE 6 |
 Medical:
The
Diolase
10
offers
high
intensity
laser
therapy
in
the
palm of your hand for pain management.
Digital
Imaging:
We
offer
Cefla’s
NewTom
cone
beam
3D
imaging
and a full line of Biolase DaVinci Imaging equipment, including
the D3D cone beam as well as x-ray generators and tablets.
Diode
soft-tissue
dental
lasers:
Our
soft
tissue
lasers
include the ezLase total diode solution, which offers
power and portability, and the revolutionary iLase, a
portable diode laser with no foot pedal, power cord, or
external controls.
Current Line of BIOLASE Products
PAGE 7
All-tissue
dental
lasers:
Our
all-tissue
lasers
include
our
new
flagship
laser,
the
revolutionary WaterLase iPlus, and the WaterLase MDX.
|
 Alternative and Advanced Hi-Tech Dentistry
Atraumatic Pediatric Tooth Extraction
The aiming beam on
the WaterLase does
not cut the tissue or
generate any heat.
The tissue is cut
biologically, with little
or no pain, by
energizing water at
the molecular level.
Using BIOLASE WaterLase Systems, including our new WaterLase iPlus System:
PAGE 8
The iPlus features
an intuitive, touch-
based computer
screen, similar to a
tablet personal
computer. It is
designed to allow for
remote servicing and
software upgrades. |
 WaterLase High Speed Cutting
PAGE 9 |
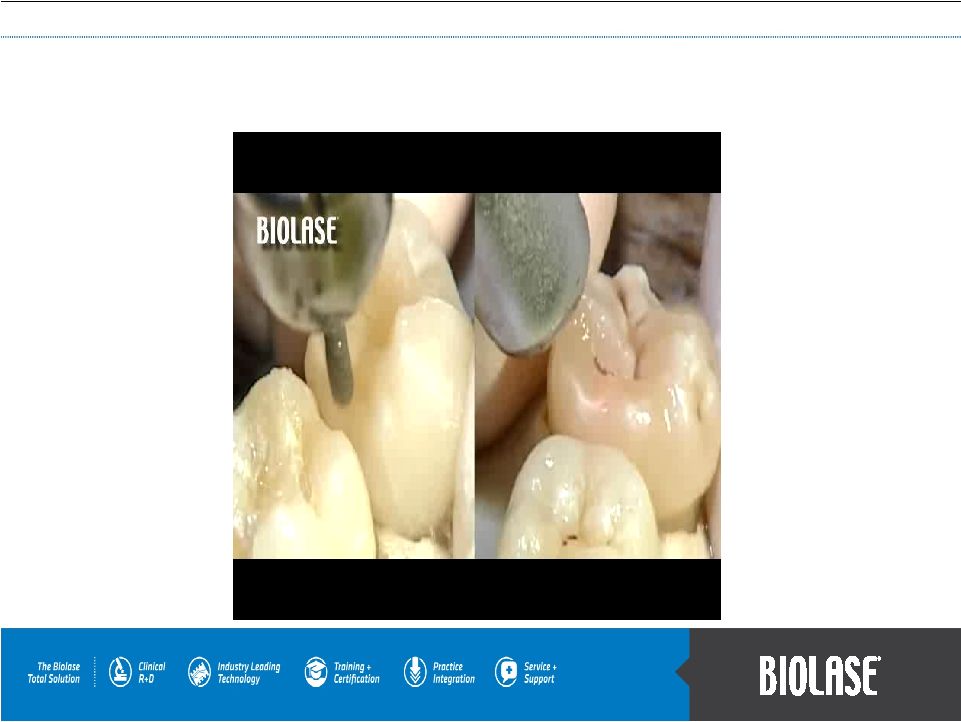 WaterLase Side by Side with Drill |
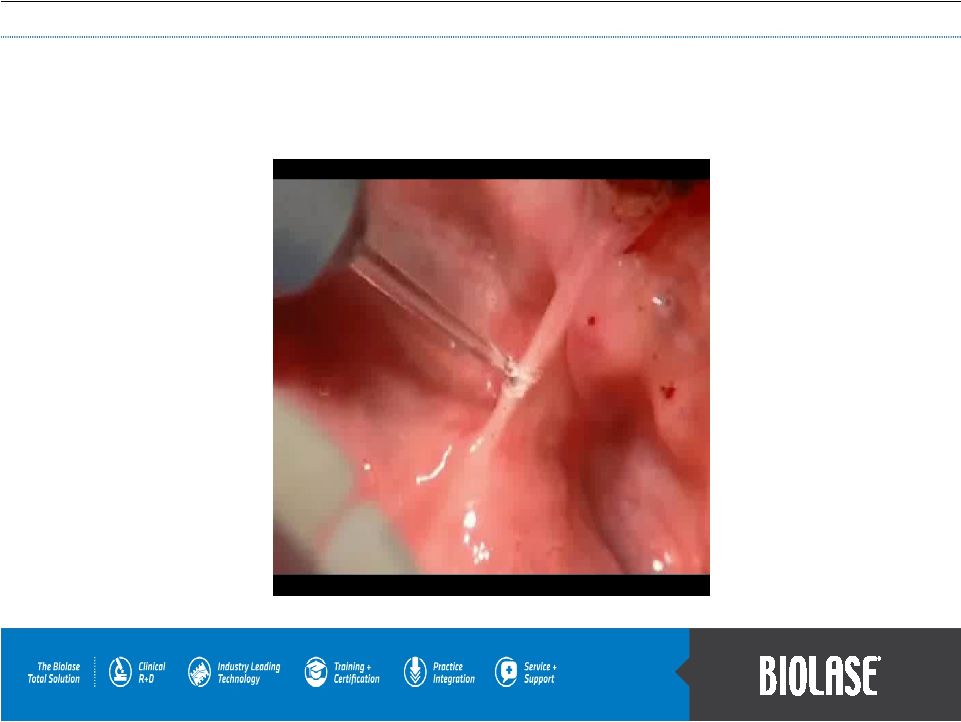 WaterLase Soft Tissue Lingual Frenectomy |
 Why
BIOLASE WaterLase Technology in Dentistry? Traditional
high-speed
drills
work
by
FRICTION
which
creates
HEAT
which
causes
PAIN.
This PAIN
necessitates injections of ANESTHESIA which results in PAIN and
NUMB LIPS.
In addition, the lack of feeling from the numbing injections can
lead to THERMAL DAMAGE
which can lead to PULPAL DEATH and result in a ROOT CANAL.
The traditional high-speed drill
also causes VIBRATION, which causes PAIN
and leads to
MICROFRACTURES. These MICROFRACTURES allow BACTERIA to penetrate the tooth
which causes further DECAY and the FRACTURING of teeth.
High
speed
drills
also
require
the
use
of
BURS
which
even
after
autoclaving
have a 15% chance of carrying pathogenic micro-organisms. Needles
and files can also carry bacteria. That means in traditional dentistry the
patient has a 1 in 6 chance of cross contamination.
WaterLase energy
does not create heat or vibration so there is NO PAIN. Further, the
WaterLase is bactericidal, antiviral and essentially eliminates the risk of cross
contamination.
PAGE 12 |
 Why
Dentists Should Buy a WaterLase System? •
WaterLase technology enables traditional dentists to perform procedures that they
currently refer out to specialists or current WaterLase dentists, for
example: •
Gingivectomy = $160
•
Perio Treatment = $375
•
Hard-tissue Crown Lengthening = $520
•
Herpetic or Aphthous Ulcer = $308
•
Frenectomy = $355
•
With no anesthesia, the WaterLase also increases efficiency and allows the dentist
to work in multiple quadrants in a single visit.
•
This equates to $250-$750 per day in additional revenue. These
procedures are easy to learn and training is included in the cost..
Monthly Lease Payment
New Monthly Revenue Generated
Approx. $1,200
$5,000 –
$15,000
PAGE 13
A dentists’
return on investment can
be between ~500% and ~1,500%!
…the tremendous Return on Investment! |
 $50B to $100B WaterLase Market Opportunity
The WaterLase’s global market opportunity is immense…
because the dental community is the largest medical
community in the world.
•
176,000
dentists
in
the
US
and
Canada.¹
•
Over 1.2 million dentists in 134 countries and growing
rapidly
in
emerging
economies.²
•
19,000 systems in over 11,000 practices.
•
Every 1% of further market penetration, just with the
WaterLase, is equal to an opportunity of well over $600
million in revenues!
Estimated total global market of 1,200,000,
which is growing rapidly due to new markets
such as China, India, and Indonesia.
19,000
3
systems
sold
worldwide.
1. American Dental Association. 2. World Federation of Dentistry. 3.
1998-present over 19,000 BIOLASE systems sold. PAGE 14
Our current market penetration is approximately
3% in the US and slightly less than 1% internationally!
|
 1.
Dental Health Magazine 2. Dental Burs and Endodontic Files: Are Routine Sterilization Procedures Effective?:
Archie Morrison, DDS, MS, FRCD(C); Susan Conrod, DDS JCDA •
www.cda-adc.ca/jcda •
February 2009, Vol. 75,
No. 1. 3. Contaminated dental instruments: Smith A, Dickson M, Aitken J, Bagg
J.J Hosp Infect. 2002 Jul;51(3):233- 5. 4. The antimicrobial
efficacy of the erbium, chromium:yttrium-scandium-gallium-garnet laser with radial emitting
tips on root canal dentin walls infected with Enterococcus faecalis: Wanda Gordon,
DMD, Vahid A. Atabakhsh, DDS, Fernando Meza, DMD, Aaron Doms, DDS, Roni
Nissan, DMD, Ioana Rizoiu, MS and Roy H. Stevens, DDS, MS JADA 2007; 138(7):
992-1002. 5. The Bacterial Challenge: Time to React. A call to narrow the gap between multi-
drug resistant bacteria in the EU and the development of new antibacterial agents.,
ECDC/EMEA Joint Working Group, Stockholm, Sweden, September, 2009.
Warning:
Patients Urged to Ask their Dentists
About the Risk of Cross Contamination!
Cross
contamination
is
a
huge
threat:
1,2,3,4
•
The CDC defines cross-contamination as the
act of spreading bacteria and viruses from one
surface to another. Blood-borne viruses have
the ability to live on objects and surfaces for as
long as a week.
•
Sterilization techniques used for dental burs
used with high speed drill and endodontic files
are not effective. This is a serious health
threat!
•
Many bacteria are becoming more and more
resistant to antibiotics.
5
PAGE 15 |
 The
Bacterial Challenge Antibiotic resistance adversely impacts the health of
millions of patients every year. Of the patients receiving
antibiotics, 50% receive unnecessary or
redundant therapy, resulting in overuse of
antibiotics.¹
Resistance to antibiotics is high among bacteria that cause serious
infections in humans and reaches 25% or more in
several EU Member
States.² Each year, about 99,000 patients in the US die from hospital
acquired infections³
and 25,000 patients in the EU die from an
infection related to multidrug-resistant bacteria.² Mortality rates of one resistant
strain MRSA in the U.S. exceeded the
combined
death
toll
of
AIDS,
tuberculosis,
and
hepatitis
B.¹
1. Centers for Disease Control and Prevention. Delivering safe care for patients:
all healthcare providers play a role., November 15, 2011. Source:
http://www.cdc.gov/getsmart/healthcare/learn-from-others/factsheets/hc_providers.html.
2.
The
Bacterial
Challenge:
Time
to
React.
A
call
to
narrow
the
gap
between
multi-drug
resistant
bacteria
in
the
EU
and
the
development
of
new
antibacterial
agents.,
ECDC/EMEA
Joint
Working
Group,
Stockholm,
Sweden,
September,
2009.
3.
Klevens
RM,
Edwards
JR,
Richards CL, Horan TC. Estimating Health-Care Infections and Deaths in US
Hospitals, 2002. Public Helth Reports 2007; 122: 160-166. |
 WaterLase
MD™ reduced E. faecalis 2.86
times more effectively than NaOCl³
1.
Dental
Burs
and
Endodontic
Files:
Are
Routine
Sterilization
Procedures
Effective?:
Archie
Morrison,
DDS,
MS,
FRCD(C);
Susan
Conrod,
DDS
JCDA
•
www.cda-adc.ca/jcda
•
February
2009,
Vol.
75,
No.
1.
2.
Contaminated
dental
instruments:
Smith A, Dickson M, Aitken J, Bagg J.J Hosp Infect. 2002 Jul;51(3):233-5.
3. The antimicrobial efficacy of the erbium, chromium:yttrium-scandium-gallium-garnet laser with radial emitting tips on root canal dentin walls infected with Enterococcus
faecalis: Wanda Gordon, DMD, Vahid A. Atabakhsh, DDS, Fernando Meza, DMD, Aaron
Doms, DDS, Roni Nissan, DMD, Ioana Rizoiu, MS and Roy H. Stevens, DDS, MS JADA 2007; 138(7): 992-1002. 4. Dentaltown Research: Endodontics;
Survey, October 2011, dentaltown.com.
Burs and Endo Files:
•
15% of “sterilized”
burs and up to 76% of “sterilized”
endodontic
files
carry
pathogenic
micro-organisms.
1, 2
•
Complex and rugged bur surface difficult to sterilize.
•
Autoclaving fails 15% of the time to decontaminate burs
1
•Only 32% of endodontic engine-driven files are replaced
after
every
case
and
36%
after
every
other
case.
4
WaterLase Tips:
•
Smooth tip surface does not harbor debris or bacteria
like abrasive surface of burs or files.
•
YSGG laser energy is bacteriacidal.
3
•
Single-use, disposable tips that work without the need
to contact tissue. Also eliminates accidental sticks with
contaminated burs.
BIOLASE is entering a new dental category utilizing WaterLase technology as
it essentially eliminates the risk of cross contamination and contagion:
PAGE 17
Eliminating the Risk of Cross Contamination |
 Current
Status:
BIOLASE
has
a
tremendous
IP
portfolio
with
285
issued
and
pending
patents, 70% of which are related to our core WaterLase (Er,Cr:YSGG) technology and
medical lasers.
Filing
costs
and
maintenance
are
closely
monitored.
Funding
for
redundant
patents
and
patents with a low-probability for issuance are stopped.
Patent Portfolio
Issued
Pending
Total
U.S.
84
53
137
International
77
71
148
Total
161
124
285
PAGE 18 |
 Patented laser toothbrush for home use:
•
BIOLASE
is
currently
engaged
in
discussions
with
The
Proctor
and
Gamble
Company
(“P&G”)
to
develop
a laser toothbrush for home use using our patents which will whiten teeth,
disinfect teeth and gums, bio- stimulate
teeth
and
gums,
relieve
pain,
and
perform
photodynamic
therapy.
New lasers:
•
BIOLASE introduced the WaterLase MDX line of all-tissue lasers in February 2012
and plans to continue to offer additional all-tissue WaterLase products
in the future that complement the iPlus. •
We are planning to re-launch the iLase, our handheld diode laser with several
new options and applications.
•
We are also currently engineering more powerful diode soft-tissue lasers for
launch in 2012. New Imaging products:
•
In February 2012, BIOLASE became the exclusive North American distributor for
Cefla’s NewTom cone beam
3D
imaging
products,
thereby
complementing
its
large
family
of
BIOLASE
DaVinci
Imaging
products.
Further Opportunities to Expand in Dentistry
PAGE 19 |
 Digital Imaging
Cone
Beam
Computed
Tomography
(CBCT)
3D
Imaging:
We
offer
the
Cefla
NewTom VGi and the BIOLASE DaVinci D3D digital CBCTs. These systems are a
vast improvement over traditional x-ray imaging technologies and offer
tremendous clinical and
diagnostic
advantages
to
dentists.
CBCTs
allow
dentists
to
“see
more”
which,
when
coupled with our WaterLase technology, allows dentists to “do
more.” X-Ray
Generators:
The
BIOLASE
DaVinci
iGen
x-ray
generator
offers
a
unique
ball-
joint and brake assist for optimal positioning.
Portable
X-ray
Tablet:
The
BIOLASE
DaVinci
iTab
x-ray
tablet
allows
for
immediate
access to x-ray images. It offers wireless synchronization to operatory
workstations. Intra-oral
Camera:
The
BIOLASE
DaVinci
iView
is
a
progressive
scan
intra-oral
camera that can take live and still images. This is an excellent tool to
assist the dentist in gaining patient treatment acceptance.
|
 •
We expect WaterLase sales to continue grow in 2012, primarily driven by the iPlus
and complemented by our new line of lower priced WaterLase MDX
lasers. With the MDX, as well as certified, pre-owned MD
Turbos, we can now offer a number of price points to match up with a doctors
specific all-tissue laser needs. •
Disposables and consumables will continue to represent ~20% of total sales.
•
Our BIOLASE DaVinci Imaging and Cefla New Tom digital imaging products
significantly expand our product portfolio and offer dentists a Total
Technology Solution where, when coupled with our industry-leading
WaterLase
technology,
they
can
see
more
and
do
more.
2011 (Projected)
2012 (Projected)
WaterLase Systems
~60%
~60%
Diode Systems
~20%
~15%
Consumables & Other
~20%
~20%
Digital Imaging Products
~0%
~5%
BIOLASE will continue to add new products and diversify in 2012:
Diversification
in 2012
PAGE 21 |
 With
our patented WaterLase and diode technologies, we have created
technological
platforms
that
have
the
ability
to
extend
far
outside
of
dentistry.
We
expect
to
greatly
expand
our
addressable
markets
in
the
coming
years
which, we believe, has the potential to substantially increase our
revenues. Each
of
these
potential
markets
represents
a
multi-billion
dollar
opportunity.
Ophthalmology:
We
currently
hold
14
issued
and
19
pending
U.S.
and
International
patents
in
four patent families in the field of ophthalmology, giving us a wide range of
applications and coverage.
Our
patented
technology
has
the
capability
to
restore
the
elasticity
of
the
eye
and
allow it to return to normal function, eliminating presbyopia. Management has
established a new subsidiary, OCULASE, which will own and develop
BIOLASE’s ophthalmic assets and technologies.
In
2012,
we
expect
approval
to
market
treatments
for
glaucoma
and
dry
eyes.
We
expect approval to market WaterLase technology for presbyopia internationally in
2013 and in the United States in 2014.
2012: Expanding Beyond Dentistry
PAGE 22 |
 Aesthetics:
We
have
various
FDA
approvals
for
applications
in
dermatology,
plastic
surgery,
and
oculoplastics and are currently investigating options for entering these markets in
2012. Pain
Management:
We
anticipate
launching
a
new
deep-tissue
hand
piece
and
upgraded
diode
laser for pain therapy in 2012 which will coincide with a new marketing
campaign. Pain therapy works and we are seeing more high-level
athletes seeking treatment. Podiatry:
We
have
found
that
our
Diolase
10
technology
is
also
very
effective
in
the
treatment
of
nail fungus and we are completing the clinical and regulatory requirements
necessary to enter the market in late 2012.
Orthopedics:
We
are
working
with
several
key
manufacturers
and
universities
to
provide
solutions that are not currently available. We are investigating
opportunities for several orthopedic applications and anticipate filing
several 510(k) applications during 2012. PAGE 23
2012: Expanding Beyond Dentistry (Continued)
|
 Quarterly Revenues (in Millions)
PAGE 24
2004
2005
2006
2007
2008
2009
2010
2011
Q1-Mar
$ 14.5
$ 16.8
$ 16.9
$ 15.1
$ 19.0
$ 6.6
$ 4.4
$ 10.6
Q2-Jun
$ 14.7
$ 14.5
$ 15.9
$ 18.2
$ 18.7
$ 14.3
$ 5.9
$ 12.1
Q3-Sep
$ 12.0
$ 11.7
$ 17.1
$ 12.8
$ 15.3
$ 12.1
$ 6.2
$ 13.1
Q4-Dec
$ 19.1
$ 19.0
$ 19.8
$ 20.8
$ 11.6
$ 10.4
$ 9.7
$ 13.1 |
 Quarterly Non-GAAP Net Income (in Millions)
PAGE 25
2004
2005
2006
2007
2008
2009
2010
2011
Q1-Mar
$ 0.783
$ (4.015)
$ (1.462)
$ (0.890)
$ 0.985
$ (3.763)
$ (4.797)
$ 0.153
Q2-Jun
$ 1.006
$ (6.222)
$ (1.340)
$ (0.089)
$ 1.578
$ 3.020
$ (3.657)
$ 0.069
Q3-Sep
$ (0.962)
$ (4.820)
$ (0.167)
$ (2.765)
$ (3.551)
$ 1.534
$ (2.173)
$ (0.397)
Q4-Dec
$ (23.257)
$ (0.479)
$ (2.466)
$ (0.272)
$ (4.338)
$ (0.888)
$ 0.787
$ (1.260) |
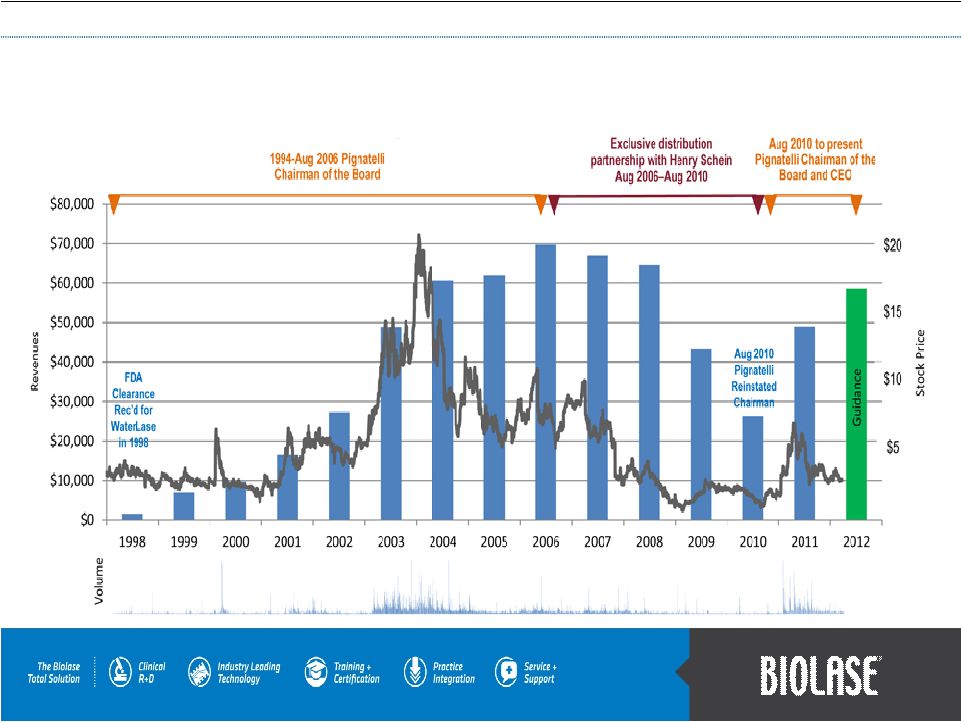 Chart & Financial History |
 Federico
Pignatelli
has
served
as
our
CEO
since
August
2010,
and
as
our
Chairman
of
the
Board
and
CEO
since
September 2010. Mr. Pignatelli is a >5% shareholder and has been active
in the Company as a director and various
other
management
roles
since
1991.
He
also
the
founder,
and
has
served
as
President,
of
the
Art
&
Fashion Group.
Fred
Furry
has
been
our
CFO
since
November
2010
and
our
COO
since
October
2011.
He
has
over
18
years
of
experience specializing in the high-tech and manufacturing industries while
working in public accounting, including
six
years
with
Pricewaterhouse
Coopers.
Mr.
Furry
was
an
audit
partner
with
Windes
&
McClaughry
prior to joining BIOLASE.
Dmitri
Boutoussov
has
over
19
years
developing
laser
technologies
for
dentistry
and
medicine
in
the
U.S.
and
Europe. He joined BIOLASE in 2000 and was promoted to CTO in October
2010. Mr. Boutoussov has authored and co-authored more than 20
U.S. and international patents and patent applications. Bill
Brown
has
more
than
30
years
of
experience
in
global/medical
device
companies.
He
has
been
with
BIOLASE
since 2002 and was promoted to Vice President of Sales and Marketing in March
2012. Rick
Whipp
has
over
30
years
of
experience
in
manufacturing
and
operations.
Mr.
Whipp
joined
BIOLASE
in
July
2011
and
was
promoted
to
Vice
President
of
Operations
in
October
2011.
Prior
to
joining
BIOLASE,
he
spent
13
years with Discuss Dental, currently a division of Philips Electronics.
Ehab Esmail
joined BIOLASE in August 2011 as our Vice President of Global Regulatory,
Clinical, and Quality Affairs. He has more than 18 years of experience
as a specialist in regulatory affairs in the medical device and biologics
industries. PAGE 27
Executive Management |
 BIOLASE Today
Company Headquarters in Irvine, California
PAGE 28
•
We have over 180 employees worldwide.
•
We also have manufacturing capabilities in Floss,
Germany.
•
We have sales offices in the U.S., Floss (Germany),
Madrid (Spain), Shanghai (China), and Mumbai (India),
with expansion planned in Dubai (UAE), and Rio de Janero
(Brazil). We also have training facilities in Charlotte, NC,
and Irvine, CA.
BIOLASE Europe in Floss, Germany
•
Our 57,000 sq. ft. corporate HQ in Irvine houses finance
& administrative, sales, marketing, customer care,
training, manufacturing, and R&D. This facility can
accommodate growth to $250 million.
•
ISO 9001 certified and FDA GMP with clean room
operations. |
