Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REVA Medical, Inc. | d322020d8k.htm |
Exhibit 99.1

Shareholder Newsletter
San Diego, California and Sydney, Australia (Friday 23 March 2012, AEDT) – REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”) is pleased to provide an overview of the Company’s recent activities in the attached newsletter that is being distributed to all shareholders of record as of 21 March 2012.
About REVA
REVA is a development stage medical device company incorporated in Delaware, USA that is focused on the development and eventual commercialisation of its proprietary, bioresorbable stent products. REVA’s lead product, the ReZolve® scaffold, combines REVA’s proprietary stent design with a proprietary polymer that is metabolized and cleared from the body. The ReZolve scaffold is designed to offer full x-ray visibility, clinically relevant sizing and a controlled and safe resorption rate. In addition, by early encapsulation of the stent in the artery tissue coupled with the loss of scaffold structure over time, the ReZolve scaffold may reduce the incidence of late forming blood clots, or thrombosis, a rare but serious problem associated with drug-eluting metal stents currently on the market. REVA will require clinical results and regulatory approval before it can begin selling the ReZolve scaffold.
Forward-Looking Statements
This announcement contains or may contain forward-looking statements that are based on management’s beliefs, assumptions and expectations and on information currently available to management. All statements that are not historical, including those statements that address future operating performance and events or developments that we expect or anticipate will occur in the future, are forward-looking statements. You should not place undue reliance on these forward-looking statements. Although management believes these forward-looking statements are reasonable as and when made, forward-looking statements are subject to a number of risks and uncertainties that may cause our actual results to vary materially from those expressed in the forward-looking statements, including our ability to obtain the regulatory approvals required to market our ReZolve scaffold, our ability to timely and successfully complete our clinical trials, our ability to protect our intellectual property position, our ability to commercialize our
HEAD OFFICE: 5751 Copley Drive, San Diego, CA 92111 Ÿ +1 (858) 966-3000 Ÿ +1 (858)
966-3099 (FAX) Ÿ www.revamedical.com
AUSTRALIAN OFFICE: Level 6, 175 Macquarie Street, Sydney, NSW 2000 Ÿ +61 2 9231
3322 Ÿ +61 9229 2727 (FAX) Ÿ ARBN 146 505 777
REVA Medical, Inc., is a foreign company incorporated in Delaware, USA, whose stockholders
have limited liability.
| REVA Medical, Inc. – ASX Announcement |
products if and when approved, our ability to develop and commercialize new products, and our estimates regarding our capital requirements and financial performance, including profitability. Other risks and uncertainties that may cause our actual results to vary materially from any forward-looking statements are described in the “Risk Factors” section of our Annual Report on Form 10-K filed with the United States Securities and Exchange Commission (the “SEC”) on 28 February 2012. We may update our risk factors from time to time in our periodic reports or other current reports filed with the SEC. Any forward-looking statements in this announcement speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
| United States |
Australia | |
| Investor and Media Enquiries: |
Investor Enquiries: | |
| Cheryl Liberatore |
David Allen or Alan Taylor | |
| Director, Investor Relations and Marketing |
Inteq Limited | |
| REVA Medical, Inc. |
+61 2 9231 3322 | |
| +1 858 966-3045 |
||
| Media Enquiries: | ||
| Katie Mackenzie or Rebecca Wilson | ||
| Buchan Consulting | ||
| +61 3 9866 4722 | ||
HEAD OFFICE: 5751 Copley Drive, San Diego, CA 92111 Ÿ +1 (858) 966-3000 Ÿ +1 (858)
966-3099 (FAX) Ÿ www.revamedical.com
AUSTRALIAN OFFICE: Level 6, 175 Macquarie Street, Sydney, NSW 2000 Ÿ +61 2 9231
3322 Ÿ +61 9229 2727 (FAX) Ÿ ARBN 146 505 777
REVA Medical, Inc., is a foreign company incorporated in Delaware, USA, whose stockholders
have limited liability.
2

REVA designs that disappear
Shareholder update
March 2012
From the CEO
2012 has started out very well and promises to be a pivotal year for REVA Medical as we enroll patients across Brazil and Europe inthe RESTORE clinical trial of our drug-eluting bioresorbable scaffold, ReZolve™, and ultimately in Australia as we expand into ourCE Mark clinical trial. The start of the RESTORE clinical trial in December 2011 marked a significant milestone in our Company’s history. The RESTORE trial will evaluate the safety and performance of ReZolve in 50 patients at multiple leading heart
centers in Brazil and Europe. Data from this trial will become available throughout 2012, beginning with the European Paris Courseon Revascularization (EuroPCR) in May. The first patient was treated by the study’s principal investigator, Dr. Alexandre Abizaid, Chiefof Coronary Interventions at the Instituto Dante Pazzanese de Cardiologia in Sao Paulo, Brazil on December 21, 2011. This firstpatient, now more than two months past implant, is doing well. Dr. Abizaid is a renowned interventional cardiologist and theinstitute is one of the world’s leading heart centers. Dr. Abizaid’s pioneering efforts have been duplicated by leading interventionalcardiologists in Europe that are screening patients and implanting the ReZolve scaffold in strict accordance with theRESTORE trial protocol. Following the point at which we have achieved acceptable results from the pilot study, we plan to initiatea pivotal clinical trial that is designed to provide the data to support CE Mark approval in Europe. So, 2012 is a very important year for REVA in the clinic. As we prepared for the clinic in 2011 we turned our attention to scaling up manufacturing capabilities tomeet clinical trial quantities and to ultimately meet commercial demand. We have hired a Director of Operations who is tasked withtransitioning what we have developed in R&D to standard manufacturing lines and processes. This includes a near doubling ofspace in our San Diego facility that includes the build out of critical lab and clean room assembly spaces. These initiatives, togetherwith technology advances, should help REVA meet its goal to manufacture commercial scale quantities of ReZolve scaffolds. We believe the commercial and clinical communities are very keen to see REVA’s clinical progress, which is under close watch byphysicians, investors and major stent players in the market. Abbott’s bioresorbable scaffold, ABSORB™, is opening up the marketthat REVA is targeting as its data continues to demonstrate the promise of bioresorbable stent technology. Excitement isbuilding among physicians and industry players with a commercial rollout of ABSORB expected to be complete by the end of 2012.We anticipate that the success of Abbott’s product will enable REVA to benefit from accelerated market adoption and a faster path to commercialization in 2014. Bob Stockman, Chairman and CEO From the CEO ReZolve is a fully bioresorbable polymer drugeluting scaffold designed to provide all the proven benefits of a metal drug-eluting stent, with the advantage of dissolving from the body after it is no longer needed, leaving the patient free of a permanent implant. ReZolve’s unique ratcheting geometry called‘Slide & Lock’ is combined with a proprietary polycarbonate material which is visible under x-ray and is natural and safe. Deployed ReZolve Scaffold
ReZolve Scaffold

Investor Relations Update REVA Medical regularly presents at major global healthcare conferences and the company is continuing to build market awareness in the Australian, European and US markets. REVA has recently presented at seven major conferences – the ASX Asia Spotlight Series, the Transcatheter Cardiovascular Therapeutics (TCT) Conference, the Piper Jaffray Health Care Conference, the JP Morgan Healthcare Conference, the Citi Healthcare Conference, as well as Cardiovascular Research Technologies (CRT) and the Local Drug Delivery Meeting and Cardiovascular Course on Revascularization & Molecular Strategies (LDDR) Scientific Conferences. These conferences have enabled REVA to tell its story to scientific, clinical and financial audiences both in the US and internationally. Upcoming REVA Presentations: ASX Spotlight – Small to Mid Caps Sofitel Hotel, New York March 1, 2012 Mediplast Conference Caesar’s Palace, Las Vegas, Nevada March 1, 2012 Bioresorbable Vascular Scaffold Summit Rotterdam, the Netherlands March 8-9, 2012 gi2Summit WTC Hotel, Sao Paulo, Brazil April 11-13, 2012 REVA will be meeting with investors and analysts in Australia in early March and will return to Australia for its AGM 21 May 2012 (AEST). Analyst research Bell Potter Securities and Taylor Collison Limited, both Australian based analysts, currently cover REVA Medical. REVA recently filed its 2011
Annual Financial Report, which is available on the Company website at www.revamedical.com Why a bioresorbable stent? Coronary Artery Disease (CAD) is the leading cause of death in many developed countries and this has historically been treated through open heart surgery, balloon angioplasty (from the late 1970s), bare metal stents (from the early 1990s) and most recently through drug-eluting stents (from 2003 to today). The interventional cardiology market has long sought a solution to the numerous problems that permanent metallic stents, whether bare or drug-eluting, can cause. These problems include the formation of blood clots, the need for long term medicines to prevent clots, and the caging effect permanent stents place on arteries. Now the market is looking towards drug-eluting bioresorbable scaffolds as the next big innovation in interventional medicine for the treatment of CAD because they allow the artery to heal by going away after they are no longer needed. Bioresorbable scaffolds have the potential to offer significant benefits for patients as the risk of clotting may be lowered, which would potentially reduce the need for expensive anti-clotting drugs. In addition, research is being conducted regarding the potential physiologic benefits of bioresorbable scaffolds. “Bioresorbable scaffolds represent an exciting new frontier to the treatment of coronary artery disease due to their potential to return the vessel to normal function after restoring blood flow.” Dr. Alexandre Abizaid, renowned interventional cardiologist and REVA’s principal investigator for the RESTORE trial.
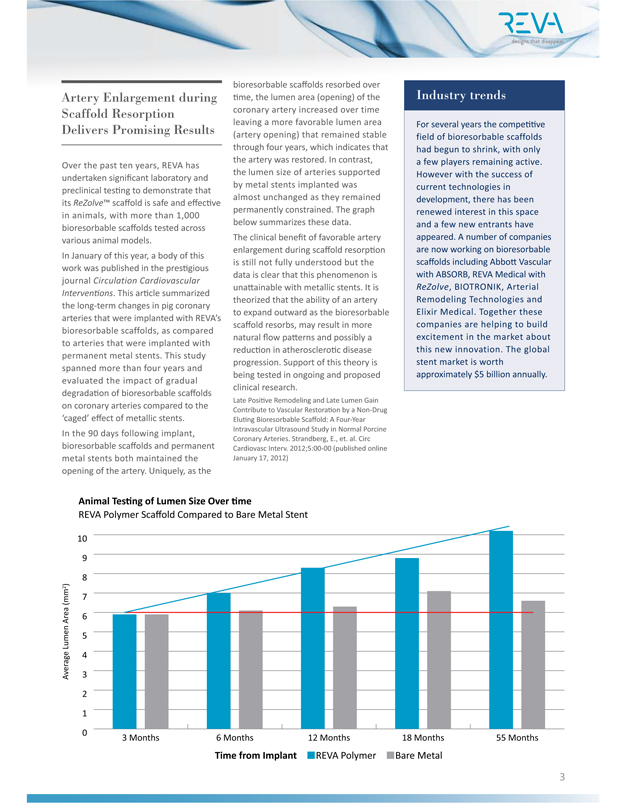
Artery Enlargement during Industry trends Scaffold Resorption Delivers Promising Results Over the past ten years, REVA has undertaken significant laboratory and preclinical testing to demonstrate that its ReZolve™ scaffold is safe and effective in animals, with more than 1,000 bioresorbable scaffolds tested across various animal models. In January of this year, a body of this work was published in the prestigious journal Circulation Cardiovascular Interventions. This article summarized the long-term changes in pig coronary arteries that were implanted with REVA’s bioresorbable scaffolds, as compared to arteries that were implanted with permanent metal stents. This study spanned more than four years and evaluated the impact of gradual degradation of bioresorbable scaffolds on coronary arteries compared to the ‘caged’ effect of metallic stents. In the 90 days following implant, bioresorbable scaffolds and permanent metal stents both maintained the opening of the artery. Uniquely, as the bioresorbable scaffolds resorbed over
time, the lumen area (opening) of the coronary artery increased over time leaving a more favorable lumen area (artery opening) that remained stable through four years, which indicates that the artery was restored. In contrast, the lumen size of arteries supported by metal stents implanted was almost unchanged as they remained permanently constrained. The graph below summarizes these data. The clinical benefit of favorable artery enlargement during scaffold resorption is still not fully understood but the data is clear that this phenomenon is unattainable with metallic stents. It is theorized that the ability of an artery to expand outward as the bioresorbable scaffold resorbs, may result in more natural flow patterns and possibly a reduction in atherosclerotic disease progression. Support of this theory is being tested in ongoing and proposed clinical research. Late Positive Remodeling and Late Lumen Gain Contribute to Vascular Restoration by a Non-Drug Eluting Bioresorbable Scaffold: A Four-Year Intravascular Ultrasound Study in Normal Porcine
Coronary Arteries. Strandberg, E., et. al. Circ Cardiovasc Interv. 2012;5:00-00 (published online January 17, 2012) Industry trends
For several years the competitive field of bioresorbable scaffolds had begun to shrink, with only a few players remaining active. However with the success of current technologies in development, there has been renewed interest in this space and a few new entrants have appeared. A number of companies are now working on bioresorbable scaffolds including Abbott Vascular with ABSORB, REVA Medical with ReZolve, BIOTRONIK, Arterial Remodeling Technologies and Elixir Medical. Together these
companies are helping to build excitement in the market about this new innovation. The global stent market is worth approximately $5 billion annually. Animal Testing of Lumen Size Over time REVA Polymer Scaffold Compared to Bare Metal Stent Average Lumen Area (mm2)
8
9
10
0
1
2
3
4
5
6
7
3 Months 6 Months 12 Months 18 Months 55 Months
Time from Implant
REVA Polymer
Bare Metal

Staff Profile – Jeffrey Anderson
Jeffrey Anderson is the Vice President of Clinical and Regulatory Affairs at REVA Medical. Mr. Anderson oversees the Company’s clinical trial tactics and strategies, where he is intimately involved with each clinical setting and the physicians on whom REVA depends. Mr. Anderson has more than 20 years of experience in the medical device industry and was previously the Vice President of Clinical & Regulatory Affairs and Vice President of Research and Development for Neomend, a private wound care company. Mr. Anderson brings a wealth of cardiovascular experience having worked at Abbott Vascular, Jomed, CRS Clinical Research, and Medtronic. Q&A Can you give us an update on REVA’s pilot clinical trial of the ReZolve™ bioresorbable scaffold? We initiated the RESTORE trial in December of last year at the Instituto Dante Pazzanese de Cardiologia in Sao Paulo, Brazil, under the direction of our Principal Investigator, Dr. Alexandre Abizaid. Since then, we have completed our clinical training for additional centers in Germany and Austria and there are now 11 sites participating in the trial across Germany, Poland and Austria. We intend to enrol 50 patients in this first clinical trial to evaluate the safety of the ReZolve bioresorbable scaffold and have enrolment complete in Q2 2012. When will data from the RESORB trial be available? Patients will be evaluated clinically at 30 days and six months, and will be followed for a period of five years. At 12 months, patients will undergo angiographic and ultrasound imaging to evaluate the performance of the stent. We plan to present 30-day data on a subset of patients at the EuroPCR Conference, to be held in Paris this May. Six month data will be presented on an initial cohort of patients at the Transcatheter Cardiovascular Therapeutics (TCT) Conference in Miami, Florida in October 2012. These clinical evaluations will tell us much about the performance of the device and the condition of the patients’ health. When will REVA initiate its CE Mark trial? We will evaluate data from these first clinical patients to determine when to initiate the larger pivotal trial. We are currently recruiting additional clinical sites for the pivotal trial, including sites in Australia, and plan to initiate enrolment toward the end of 2012, so that we will be in a position to apply for CE Mark approval in late 2013. How has the trial enrolment and patient implants gone to date? REVA, like all companies who conduct clinical trials, does not comment on actual cases or enrolment figures in order to preserve the integrity of the clinical trial. Such data is typically gathered after enough evidence can be compiled and presented as “late breaking news” at major scientific conferences. REVA expects the first such forum will be the disclosure of the one-month clinical evaluation at the Paris Course on Revascularization in May 2012. To date, the Company is pleased with the performance of ReZolve in the cases that have occurred.
United States Investor and Media Enquiries: Cheryl Liberatore Director, Investor Relations and Marketing REVA Medical, Inc. +1 858 966-3045 Australia Investor Enquiries: David Allen or Alan Taylor Inteq Limited +61 2 9231 3322 Media Enquiries: Katie Mackenzie or Rebecca Wilson Buchan Consulting +61 3 9866 4722 Disclaimer: This newsletter contains or may contain forward-looking statements that are based on management’s beliefs, assumptions and expectations and on information currently available to management. All statements that are not historical, including those statements that address future operating performance and events or developments that we expect or anticipate will occur in the future, are forward-looking statements. You should not place undue reliance on these forward-looking statements. Although management believes these forwardlooking statements are reasonable as and when made, forward-looking statements are subject to a number of risks and uncertainties that may cause our actual results to vary materially from those expressed in the forward-looking statements, including our ability to obtain the regulatory approvals required to market our ReZolve scaffold, our ability to timely and successfully complete our clinical trials, our ability to protect our intellectual property position, our ability to commercialize our products if and when approved, our ability to develop and commercialize new products, and our estimates regarding our capital requirements and financial performance, including profitability. Other risks and uncertainties that may cause our actual results to vary materially from any forward-looking statements are described in the “Risk Factors” section of our Annual Report on Form 10-K filed with the United States Securities and Exchange Commission (the “SEC”) on February 28, 2012. We may update our risk factors from time to time in our periodic reports or other current reports filed with the SEC. Any forward-looking statements in this announcement speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
